Abstract
Reproductive function is coordinated by kisspeptin (Kiss) and GnRH neurons. Phoenixin-20 amide (PNX) is a recently described peptide found to increase GnRH-stimulated LH secretion in the pituitary. However, the effects of PNX in the hypothalamus, the putative signaling pathways, and PNX receptor have yet to be identified. The mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell lines represent populations of GnRH and Kiss neurons, respectively. PNX increased GnRH and GnRH receptor (GnRH-R) mRNA expression, as well as GnRH secretion, in the mHypoA-GnRH/GFP cell model. In the mHypoA-Kiss/GFP-3 cell line, PNX increased Kiss1 mRNA expression. CCAAT/enhancer-binding protein (C/EBP)-β, octamer transcription factor-1 (Oct-1), and cAMP response element binding protein (CREB) binding sites are localized to the 5′ flanking regions of the GnRH, GnRH-R, and Kiss1 genes. PNX decreased C/EBP-β mRNA expression in both cell models and increased Oct-1 mRNA expression in the mHypoA-GnRH/GFP neurons. PNX increased CREB phosphorylation in both cell models and phospho-ERK1/2 in the mHypoA-GnRH/GFP cell model, whereas inhibiting the cAMP/protein kinase A pathway prevented PNX induction of GnRH and Kiss1 mRNA expression. Importantly, we determined that the G protein-coupled receptor, GPR173, was strongly expressed in both GnRH and kisspeptin cell models and small interfering RNA knockdown of GPR173 prevented the PNX-mediated up-regulation of GnRH, GnRH-R, and Kiss1 mRNA expression and the down-regulation of C/EBP-β mRNA expression. PNX also increased GPR173 mRNA expression in the mHypoA-GnRH/GFP cells. Taken together, these studies are the first to implicate that PNX acts through GPR173 to activate the cAMP/protein kinase A pathway through CREB, and potentially C/EBP-β and/or Oct-1 to increase GnRH, GnRH-R, and Kiss1 gene expression, ultimately having a stimulatory effect on reproductive function.
At the peak of the hypothalamic pituitary gonadal (HPG) axis resides GnRH. GnRH travels to the anterior pituitary to promote the transcription and secretion of LH and FSH (1). A variety of factors regulate GnRH synthesis and secretion, including estrogen (2, 3) and kisspeptin (4, 5). Kisspeptin has emerged as a critical player in the regulation of the reproductive axis, particularly of GnRH neurons. Disabling mutations to the kisspeptin receptor, G protein-coupled receptor (GPR)-54, are associated with hypogonadotropic hypogonadism and disrupted pubertal progression in both humans (6, 7) and rodents (7, 8). Kisspeptin administration stimulates gonadotropin secretion (9) and in GnRH neurons increases c-fos expression and GnRH secretion (10, 11). The two main populations of kisspeptin neurons are located in the arcuate nucleus (Arc) and anteroventral periventricular nucleus (AVPV) of the hypothalamus. The Arc is important for the tonic regulation of GnRH, and the AVPV is involved with the initiation of the preovulatory surge in females (4). Together the kisspeptin and GnRH neurons play an essential role in the regulation of the HPG axis. Determining how central and peripheral signals regulate these neural populations is critical for a full understanding of reproductive physiology.
Using information from the Human Genome Project and a bioinformatics approach, a novel reproductive peptide was recently described and named Phoenixin (PNX) (12). PNX is cleaved from the larger precursor protein, small integral membrane protein 20 (SMIM20), into 14 and 20 amino acid products and is highly conserved across species. PNX immunoreactivity was detected in multiple tissues, including the heart, thymus, stomach, and spleen; however, the highest expression was in the hypothalamus. Within the hypothalamus, PNX is most significantly expressed in the paraventricular and supraoptic nuclei (12). PNX was also found in the median eminence and pituitary, suggesting PNX releases into the hypophyseal portal vessel and transports to the anterior pituitary. The 20-amino acid product was the most abundant SMIM20 product in the hypothalamus. In primary pituitary cultures, PNX increased GnRH-stimulated LH release and increased GnRH receptor (GnRH-R) mRNA. When PNX was endogenously knocked down using small interfering RNA (siRNA) in cycling female rats, the estrous cycle was lengthened by 2.3 days, and there was a reduction in GnRH-R mRNA in the anterior pituitary. From these initial studies, it was hypothesized that PNX is a pituitary priming factor that helps stimulate reproductive function and may help initiate the preovulatory surge (12). It was also determined that there is a high expression of PNX in all spinal segments of the superficial dorsal horn, and PNX-14 was able to suppress visceral pain (13) and can have beneficial effects for anxiety and memory hypothesized to be mediated through the GnRH system (14, 15). The abundance of PNX in multiple regions of the hypothalamus and in the periphery suggests that PNX has unidentified functions.
PNX may therefore have important roles within the hypothalamus to complement its stimulatory action on the gonadotropes. PNX is expressed in the Arc neurons (16) that express kisspeptin and contact GnRH neurons in the medial preoptic area (17, 18), suggesting that PNX may stimulate GnRH neurons. PNX could also act on kisspeptin neurons through autocrine mechanisms or connections with other PNX-expressing neurons of the hypothalamus, but this has yet to be explored.
We therefore sought to determine the hypothalamic role for PNX, specifically on the GnRH and kisspeptin neuronal populations. Because the hypothalamus is a highly heterogeneous region with a complex architecture, it is difficult to investigate these specific nuclei in vivo. To circumvent this issue, our laboratory has generated immortalized cell lines representative of specific populations of the hypothalamus. The mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell lines model GnRH and Arc kisspeptin (Kiss) populations, respectively. These immortalized hypothalamic neurons were isolated from adult transgenic green fluorescent protein (GFP) mice that express the GFP gene under the control of the GnRH or Kiss1 promoters. Immortalized cells were fluorescence activated cell sorted to generate pure populations of GnRH and kisspeptin neurons, as previously described (19–21). Here we demonstrate that PNX directly affected GnRH and GnRH-R mRNA expression as well as GnRH peptide secretion in mHypoA-GnRH/GFP neurons and Kiss1 mRNA expression in the mHypoA-Kiss/GFP-3 neurons.
The receptor for PNX and the signaling pathways that it activates have yet to be identified; however, it has been hypothesized that PNX acts on an orphan GPR (12, 13). GPR173 is part of the highly conserved receptor family known as superconserved receptor expressed in the brain (SREB), and it is also known as SREB3 (22). SREB3 is most highly expressed in the brain and the gonads, suggesting it may be involved in the regulation of the reproductive system (22). It was demonstrated that the GnRH metabolite, GnRH-(1–5) is a ligand for GPR173 (23) and increased GnRH mRNA expression (24) and secretion from GT1–7 neurons (25). Initial ligand binding assays demonstrated that PNX bound to GPR173 (Stein L., Yosten, G.; Samson, W.K., unpublished observations). Similar to GnRH-(1–5), we demonstrated PNX also increased GnRH mRNA and secretion; therefore, we used siRNA to determine whether the down-regulation of GPR173 would reduce the effects of PNX in our hypothalamic cell models. We also explored whether PNX activates the cAMP-protein kinase A (PKA) or MAPK kinase (MEK)-ERK pathways by assessing the phosphorylation of the downstream second messengers cAMP response element binding protein (CREB) and ERK1/2 and used specific inhibitors to block these pathways. PNX-mediated changes to the expression of the transcription factors CCAAT/enhancer-binding protein (C/EBP)-β and octamer transcription factor-1 (Oct-1), known to regulate both GnRH and GnRH-R, were also measured in both the GnRH and Kiss cell models. From these studies we determined that PNX signals through GPR173 to activate the cAMP-PKA pathway and induce the gene expression of reproductive genes. Taken together, these findings provide the first convincing evidence that PNX is involved in the regulation of reproductive peptides at the level of the hypothalamus and GPR173 is the cognate receptor for PNX.
Materials and Methods
Cell culture and reagents
Adult-derived nonclonal hypothalamic cell lines were used to represent GnRH and Arc/AVPV KISS neurons. The mHypoA-GnRH/GFP cell line originated from fluorescence activated cell-sorted immortalized hypothalamic neurons from a GnRH-GFP mouse (26) (The Jackson Laboratory), whereas the mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 cell lines originated from a Kiss-GFP mouse (27, 28) (The Jackson Laboratory), as previously described (19–21). The mHypoA-Kiss/GFP-3 line was isolated through the microdissection of the Arc region, whereas the mHypoA-Kiss/GFP-4 line was from the AVPV region.
The immortalized cell lines were grown in a monolayer in DMEM (Sigma) 1 mg/mL glucose, supplemented with 5% fetal bovine serum (FBS) (Sigma-Aldrich) and 1% penicillin/streptomycin (Gibco). Cells were maintained at 37°C and 5% CO2 as previously described (29, 30).
PNX-20 amide was used because it was the most highly expressed PNX in the hypothalamus (12). PNX-20 amide, KISS-10, and LHRH enzyme-linked immunoassay (EIA) kits were purchased from Phoenix Pharmaceuticals. PNX-20 amide and KISS-10 were dissolved in Hypure water (Hyclone, Fisher Scientific) to 100 μM and 1000 μM (PNX-20 amide only) stock concentrations and stored at −80°C prior to use.
G protein-β antibody was purchased from Santa Cruz Biotechnology. Phospho-CREB (number 9191), phospho-p44/42 MAPK (ERK1/2) (number 9101), CREB, and p44/42 MAPK (ERK1/2) (number 4695) antibodies were purchased from Cell Signaling Technology (via New England Biolabs). Phospho-CREB and CREB were diluted 1:750 and 1:1000, respectively, in 5% BSA in Tris-buffered saline with 0.1% Tween 20 (1× TBST) (Sigma-Aldrich). Phospho-p44/42 MAPK (ERK1/2), p44/42 MAPK (ERK1/2), and G protein-β antibodies were diluted 1:1000 in 5% milk in 1× TBST.
PNX-20 and inhibitor pretreatments
For 24-hour PNX treatments, cells were grown to 75%–80% confluency in 1 mg/mL glucose DMEM supplemented with 5% FBS and 1% penicillin/streptomycin. Media were replaced to 2.5 mL volume the evening before treatments. Treatments were added to each 60-mm plate in 0.5 mL serum-free DMEM to give final concentrations. Concentrations used were determined based on previous work performed with PNX-20 (12).
For Western blot experiments, cells were grown to 90%–95% confluency in 60-mm plates (Sarstedt) and then serum starved in 2.5 mL 1 mg/mL glucose Dulbecco's supplemented with 1% penicillin/streptomycin (Gibco, Life Technologies) DMEM (Sigma) for 4 hours. Next, treatment was added in 0.5 mL serum-free 1 mg/mL glucose DMEM to give the final concentrations of 10 and 100 nM PNX. The cells were then harvested at 0, 5, 15, 30, and 60 minutes.
All inhibitor experiments were performed by pretreating the cells with the inhibitors in 2.5 mL 1 mg/mL low glucose, DMEM supplemented with 5% FBS, and 1% penicillin/streptomycin for 1 hour prior to PNX exposure. Concentrations used were based from previous work in cell models from our laboratory and/or the IC50 values of the inhibitors. Inhibitors included the PKA inhibitors H-89 dihydrochloride (10 μM, IC50 0.14 μM [31]) and Rp-cAMPS, triethylammonium salt (15 μM, IC50 11–16 μM), the protein kinase C inhibitor K-252c (1 μM, IC50 2.45 μM [32]), and the MEK inhibitor U0126 (25 μM, IC50 0.07 μM [33]). All inhibitors were purchased from Tocris Bioscience (R&D Systems Inc). PNX was subsequently added in 0.5 mL serum-free 1 mg/mL glucose DMEM to give the final concentrations of 10 and 100 nM PNX for the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cells, respectively.
For the siRNA experiments, cells were transfected for 48 hours in 2 mL antibiotic-free 1 mg/mL glucose DMEM supplemented with 5% FBS, and then DMEM was replaced with 2 mL antibiotic-free 1 mg/mL glucose DMEM with 10 or 100 nM PNX for the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models, respectively.
cDNA synthesis and quantitative RT-PCR
Total RNA was isolated using the guanidium thiocyanate phenol chloroform extraction method (34) and PureLink RNA isolation kit (Life Technologies). RNA concentration and purity were measured using the Nanodrop 2000c spectrophotometer. Contaminating DNA was then removed using Turbo deoxyribonuclease (Ambion) treatment for 30 minutes at 37°C. Two milligrams of RNA were reverse transcribed using the high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's protocol. Next, 50 ng of cDNA was amplified by real-time PCR with Platinum SYBR Green qPCR SuperMix-UDG with ROX (Life Technologies) and gene-specific primers using an Applied Biosystems 7900 HT real-time PCR machine. The SYBR primers were designed using PrimerBLAST, an online primer design tool (35). Primer sequences, annealing temperatures, and amplicon sizes are listed in Table 1.
Table 1.
List of Primers Used for Quantitative RT-PCR
| Gene Name | Primer Sequence (5′–3′) | Amplicon Size, bp | Annealing Temperature, °C |
|---|---|---|---|
| Histone 3a | Forward, CGC TTC CAG AGT GCA GCT ATT | 72 | 57.5 |
| Reverse, ATC TTC AAA AAG GCC AAC CAG AT | 55.1 | ||
| c-Fos | Forward, CAACGAGCCCTCCTCCGACT | 68 | 60 |
| Reverse, TGCCTTCTCTGACTGCTCACA | 60 | ||
| GnRH | Forward, CGT TCA CCC CTC AGG GAT CT | 51 | 59.8 |
| Reverse, CTC TTC AAT CAG ACT TTC CAG AGC | 55.4 | ||
| SMIM20 | Forward, AGCAGGCTGTAAATCGAGCTGGTA | 146 | 60.1 |
| Reverse, ACTGCGGAGTGCACAGGATAAAGA | 60.3 | ||
| Kiss1-Set 1 | Forward, AAG GAA TCG CGG TAT GCA GA | 191 | 56.6 |
| Reverse, CAG TTG TAG GTG GAC AGG TC | 54.9 | ||
| Kiss1-Set 2 | Forward, TGC TTC TCC TCT GT | 132 | 54.7 |
| Reverse, ACC GCG ATT CCT TTT CC | 54.7 | ||
| GnRH-R | Forward, CAA TGT GTG ACC CAC TGC AGC TTT | 218 | 60.3 |
| Reverse, TTT AGC GTT CTC AGC CGA GCT CTT | 60.4 | ||
| C/EBP-β | Forward, CTG AGC GAC GAG TAC AAG ATG | 186 | 55.1 |
| Reverse, GAA CAA GTT CCG CAG GGT | 55.4 | ||
| Oct-1 | Forward, AGG AGC GAG TCA AGA TG | 132 | 59.8 |
| Reverse, CCA TTG GTT TGT GTG CCT GT | 59.2 | ||
| GPR15 | Forward, AGC TCC TAG CCA TTG TTT CAG GGT | 181 | 60.3 |
| Reverse, AGA CAA GGG CAC AGA CAA CGT ACA | 60.3 | ||
| GPR25 | Forward, TTC TTG CTC CTG ACT GTT CTT GCC | 103 | 59.4 |
| Reverse, AGG GAG AGG TGT TTG AGA TGG TGT | 59.8 | ||
| GPR173 | Forward, CTG GCG AGT GTT TGT GAA AG | 125 | 54.4 |
| Reverse, TCT TGA GGT CCT TGT TAA GCA | 53.7 |
GnRH enzyme-linked immunoassay
mHypoA-GnRH/GFP cells were split into triplicates and grown to 90%–95% confluency in 24-well plates (Sarstedt). Cells were maintained in 500 μL of 1 mg/mL glucose, 5% FBS, 1% penicillin/streptomycin media. For the initial PNX studies without pretreatments, media were replaced with 300 μL treatment media (1 mg/mL glucose, 1% penicillin/streptomycin) containing vehicle, 10, 100, or 1000 nM PNX, or 100 μM sodium nitroprusside (SNP; Sigma-Aldrich). In pretreatment studies, the media were replaced with 300 μL of vehicle or 1000 nM PNX DMEM (1 mg/mL glucose, 5% FBS, 1% penicillin/streptomycin) 24 hours before the 1-hour treatments described above. Cell supernatants were collected in triplicates after a 1-hour period and immediately dried and stored at −80°C. GnRH immunoreactivity was measured using a LH-RH EIA kit (Phoenix Pharmaceuticals; assay range 0–25 ng/mL) following the manufacturer's protocol. Total protein was collected after cell supernatant, isolated, and quantified as described in the section above. Secretion values were normalized to the total protein per treatment.
Protein isolation, SDS-PAGE, and Western blotting
First, plates were washed with cold 1× PBS and then harvested in 1× lysis buffer supplemented with phosphatase inhibitor cocktail, protease inhibitor cocktail, and phenylmethylsulfonyl fluoride (Sigma-Aldrich). The soluble fraction of the lysate was isolated after centrifugation (14000 rpm, 10 min, 4°C). Total protein was quantified using the biocinchoninic acid protein assay kit according to the manufacturer's protocol (Thermo Scientific). Total protein (25–35 μg) was run on a 8% polyacrylamide gel and transferred onto a 0.22-μm polyvinylidene difluoride membrane (Bio-Rad Laboratories). Membranes were blocked with 5% milk in 1× TBST for 1 hour to prevent the binding of nonspecific antibodies and subsequently incubated in primary antibody overnight at 4°C. Primary antibody was either diluted in 5% milk in 1× TBST or 5% BSA (Sigma-Aldrich) in 1× TBST, depending on the antibody (see Cell culture and reagents section). After three washes in 1× TBST, membranes were complexed with secondary horseradish peroxidase antibody for 1 hour and then washed six times in 1× TBST. Secondary antibody was diluted 1:7500 in either 5% milk in 1× TBST or 5% BSA in 1× TBST to match the dilution of the primary antibody. Fluorescence was visualized using the enhanced chemiluminescence select Western blotting detection reagent (GE Healthcare Life Sciences) and captured using a Kodak Image Station 2000R. Western blot experiments were normalized using the relative phosphorylated samples divided by total protein for the specific protein analyzed. These values were normalized to the corresponding vehicle protein levels.
In silico analysis
The web tool Alibaba 2.1 was used to predict transcription factor binding sites for 2500 bp upstream of the 5′ flanking regions of the SMIM20 gene. Alibaba 2.1 is a program that predicts transcription factor binding sites using the TRANSFAC 4.0 transcription factor database (http://www.gene-regulation.com/pub/programs/alibaba2/index.html).
Knockdown of GPR173 by siRNA
The mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cells were plated into six-well plates, and GPR173 mRNA expression was knocked down using the TriFECTa kit (Integrated DNA Technology) containing nontargeting negative control Dicer substrate short-interfering RNA, DsiRNA sense, 5′-CGU UAA UCG CGU AUA CGC GUA T-3′, and antisense, 5′-AUA CGC GUA UUA UAC GCG AUU AAC GAC-3′, and three DsiRNA oligonucleotide duplex sets specifically targeting mouse GPR173 mRNA: GPR173a sense, 5′-GGA GGG CAA AUA GGG UAA ACU CGG G-3′, and antisense, 5′-CCC GAG UUU ACC CUA UUU GCC CUC CCA-3′; GPR173b sense, GGA UGU UUA GGA GGU AUU GGC UUA A-3′, and antisense, 5′-UUA AGC CCA AUA UUU CCU AAA CAU CCA A-3′; and GPR173c sense, 5′-GGA AGC UGC UAU AGG AAU AGA GGA A-3′, and antisense, 5′-UUC CUC UAU UCC UAU AGC UUC CUC-3′. Cells were transfected with 30 nM siRNA complexes for 48 hours using 7.5 μL/well Dharmafect Transfection Reagent 3 (Thermo Scientific). GPR173 mRNA expression was quantified using quantitative RT-PCR 48 hours after the transfection. The GPR173b set of DsiRNAs was the most effective at reducing GPR173 mRNA expression in our cell models and therefore was used in all experiments to reduce endogeneous GPR173 mRNA levels.
Statistical analysis
Data were presented as mean ± SEM and was analyzed using GraphPad Prism Software 6.0 (GraphPad Software Inc). Statistical significance was determined using a Student's t test or a one-way or a two-way ANOVAs where appropriate, followed by either a Bonferroni or Dunnett's Tukey's post hoc test. Statistical significance was assumed when P < .05. All experiments were performed with 3–11 repeats.
Results
PNX increases GnRH and GnRH-R mRNA expression in mHypoA-GnRH/GFP neurons.
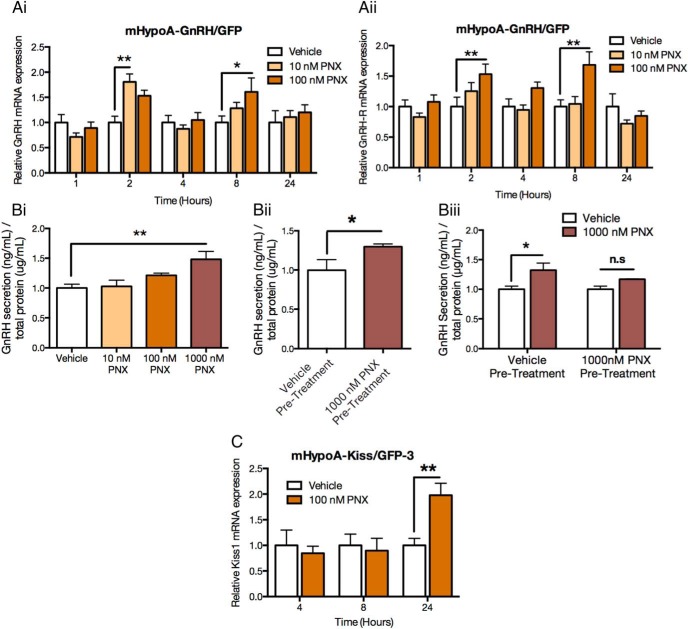
To determine the effect of PNX in the reproductive neurons of the hypothalamus, we first treated a GnRH cell model with PNX. In our preliminary studies, PNX induced the expression of c-fos in the mHypoA-GnRH/GFP cell line, indicating neuronal activation of the GnRH cells (36, 37) and suggesting the presence of a PNX receptor. To examine the specific role of PNX in GnRH neurons, the mHypoA-GnRH/GFP cell line was treated for 1, 2, 4, 8, and 24 hours with 10 or 100 nM of PNX. Changes in GnRH gene expression were assessed using quantitative RT-PCR. Ten nanomoles of PNX increased GnRH mRNA expression at 2 hours (vehicle: 1.000 ± 0.124 vs 10 nM PNX: 1.805 ± 0.160; P < .01), and 100 nM PNX increased GnRH mRNA expression at 8 hours (vehicle: 1.000 ± 0.127 vs 100 nM PNX: 1.606 ± 0.277; P < .05) (Figure 1Ai). Five hundred nanomoles of PNX did not have any effect in the mHypoA-GnRH/GFP neurons (data not shown); thus, 10 and 100 nM were used as the concentrations of PNX for this study. These findings suggest that PNX increases GnRH mRNA expression in an adult-derived GnRH neuronal cell model. Of interest, the GT1–7 cell line was also tested but did not exhibit the regulation by PNX at the times and concentrations tested (Supplemental Figure 1). This may be due to the clonal nature or origin of the GT1–7 cell model, and further studies could be undertaken in this line. The mHypoA-GnRH/GFP line was thus used for all further experiments.
Figure 1. PNX-mediated regulation of reproductive gene mRNA expression and secretion in the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 neuronal cell lines.
A and C, Cells were treated with vehicle (water) or 10 or 100 nM PNX amide over a 24-hour time course. GnRH (Ai), GnRH-R (Aii), and Kiss1 (C) mRNA expression was determined using real-time PCR, and levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 3–11). *, P < .05; **, P < .01. Bi, Cells were treated with vehicle (water) or 10 nM, 100 nM, or 1000 nM PNX for 1 hour. Bii, Cells were pretreated with either vehicle (water) or 1000 nM PNX for 24 hours and then treated for 1 hour with 100 μM SNP. Biii, Cells were pretreated with vehicle or 1000 nM PNX for 24 hours and then treated for 1 hour with vehicle or 1000 nM PNX. Media and total protein were collected, and GnRH levels were measured by a GnRH-specific EIA and normalized to total protein levels. Results are expressed as mean ± SEM (n = 3–4). *, P < .05. Statistical significance was determined by a one-way or two-way ANOVA with Bonferroni's post hoc test or a Student's t test.
Because GnRH-R mRNA expression has previously been shown to increase with PNX treatment and GnRH-R is expressed on GnRH neurons, we studied whether PNX could also regulate GnRH-R in the mHypoA-GnRH/GFP neurons. GnRH-R mRNA expression increased with 100 nM PNX at both 2 (vehicle: 1.000 ± 0.154 vs 100 nM PNX: 1.533 ± 0.164; P < .01) and 8 hours (vehicle: 1.000 ± 0.111 vs 100 nM PNX: 1.684 ± 0.213; P < .01) (Figure 1Aii). This suggests that PNX not only acts at the level of the pituitary but also in hypothalamic GnRH neurons.
PNX increases GnRH secretion in mHypoA-GnRH/GFP neurons
To further understand the function of PNX in GnRH hypothalamic neurons, the effect of PNX on GnRH secretion in the mHypoA-GnRH/GFP cell line was explored. Neurons were treated with vehicle water control, 10 nM, 100 nM, or 1000 nM PNX for 1 hour, and then the media were collected and GnRH protein measured using a GnRH-specific EIA kit. There were no significant changes in GnRH secretion with 10 or 100 nM PNX; however, 1000 nM PNX caused a 50% increase in GnRH secretion after 1 hour (vehicle: 1.000 ± 0.179 vs 1000 nM PNX: 1.479 ± 0.232; P < .01) (Figure 1Bi).
To determine whether GnRH protein levels could be elevated by PNX, the mHypoA-GnRH/GFP cells were pretreated for 24 hours with 1000 nM PNX or water vehicle control. Cells were then incubated for 1 hour in media containing SNP, a nitric oxide donor shown previously to induce the secretion of GnRH in the GT1–7 cell line (20, 38), or water control. The neurons pretreated with PNX increased the amount of GnRH released when stimulated with SNP compared with those pretreated with control media (vehicle pretreatment: 0.996 ± 0.135 vs 1000 nM PNX pretreatment: 1.296 ± 0.035; P < .05) (Figure 1Bii). This suggests the PNX-pretreated cells contained a higher GnRH content than those without PNX pretreatment and that PNX increases GnRH protein synthesis.
Because PNX increased GnRH secretion, we sought to determine whether a 24-hour PNX pretreatment could further increase the amount of GnRH released when stimulated with 1000 nM PNX. The mHypoA-GnRH/GFP neurons were pretreated for 24 hours with 1000 nM PNX and then GnRH secretion was measured after 1 hour of 1000 nM PNX treatment. Neurons pretreated with PNX for 24 hours did not have a significant change in the amount of GnRH released with PNX (PNX + vehicle: 1.000 ± 0.053 vs PNX + PNX: 1.169 ± 0.006; P > .05) compared with controls (vehicle: 1.000 ± 0.053 vs 1000 nM PNX: 1.322 ± 0.120; P < .05) (Figure 1Biii). This suggests that the 24-hour PNX before exposure desensitized the cells to PNX so that reexposure to 1000 nM PNX for 1 hour did not increase GnRH secretion as seen previously.
Taken together, these results suggest that PNX can increase the secretion of GnRH in mHypoA-GnRH/GFP neurons, and a 24-hour pretreatment can also increase the amount of GnRH that is subsequently released when the cells are stimulated by SNP but not by PNX. It appears the pretreatment with PNX may desensitize the cells to further PNX treatment over 24 hours.
PNX increases Kiss-1 mRNA expression in mHypoA-Kiss/GFP-3 cell model
The mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 cell lines from the Arc and AVPV, respectively, were used to assess the potential action of PNX in kisspeptin neurons. Cells were treated with 100 nM PNX and samples taken at 0, 4, 8, and 24 hours to measure the relative changes in Kiss1 mRNA expression. There was a significant increase in Kiss1 mRNA expression in the mHypoA-Kiss/GFP-3 neurons at 24 hours with 100 nM PNX (vehicle: 1.000 ± 0.137 vs PNX: 1.979 ± 0.232; P < .01) (Figure 1C); however, regulation of Kiss1 mRNA did not reach significance in the mHypoA-Kiss/GFP-4 neurons (Supplemental Figure 1); thus, the mHypoA-Kiss/GFP-3 line was used for all further experiments. These results suggest that PNX stimulates kisspeptin neurons to increase the Kiss1 mRNA expression.
PNX decreases C/EBPβ mRNA expression in mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models and increases Oct-1 in mHypoA-GnRH/GFP cells
The GnRH promoter has been well characterized and is regulated by the transcription factors C/EBP-β and Oct-1 (39, 40). Oct-1 also regulates GnRH-R mRNA expression, including the induction by GnRH (41). Therefore, we sought to determine whether PNX might regulate the expression of these transcription factors, which could cause the changes to GnRH, GnRH-R, and potentially Kiss1 mRNA expression. First, the transcription factor binding site program Alibaba 2.1 was used to locate C/EBP-β, Oct-1, and CREB binding sites in GnRH, GnRH-R, and Kiss1 (Figure 2A) gene promoter regions. Multiple binding sites were located for both transcription factors in all three genes, suggesting that C/EBP-β and Oct-1 could be involved in the transcriptional regulation of these genes.
Figure 2. PNX-mediated regulation of the transcription factors C/EBPβ (B) and Oct-1 (C) mRNA expression in mHypoA-GnRH/GFP (Ci) and mHypoA-Kiss/GFP-3 (Cii) neuronal cell lines.
A, The sequence for the 5′ flanking region of the GnRH, GnRH-R, and Kiss-1 mus musculus gene was obtained through Ensembl (2500 bp) and then subsequently analyzed by the gene regulation program Alibaba 2.1 (www.generegulation.com) for the presence of C/EBP-β, Oct-1, and CREB. Cells were treated with vehicle (water) or 10 or 100 nM PNX amide over a 24-hour time course. C/EBPβ and Oct-1 mRNA expression was determined using quantitative real-time PCR, and levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 4–5 independent experiments). *, P < .05; **, P < .01;***, P < .001; ****, P < .0001. Statistical significance was determined by a two-way ANOVA with Bonferroni's post hoc test.
Next, the levels of C/EBP-β and Oct-1 mRNA expression were measured in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell lines after PNX treatment. C/EBP-β decreased in the mHypoA-GnRH/GFP cell model at all time points with 100 nM PNX (1 h [vehicle: 1.000 ± 0.089 vs PNX: 0.626 ± 0.041; P < .01], 2 h [vehicle: 1.000 ± 0.100 vs PNX: 0.446 ± 0.049; P < .0001], 4 h [vehicle: 1.000 ± 0.077 vs PNX: 0.467 ± 0.044; P < .0001], 8 h [vehicle: 1.000 ± 0.050 vs PNX: 0.502 ± 0.068; P < .001], and 24 h [vehicle: 1.000 ± 0.183 vs PNX: 0.514 ± 0.039; P < .001]) and at 4, 8, and 24 hours with 10 nM PNX (4 h [vehicle: 1.000 ± 0.077 vs PNX: 0.629 ± 0.055; P < .01], 8 h [vehicle: 1.000 ± 0.050 vs PNX: 0.656 ± 0.060; P < .01], and 24 h [vehicle: 1.000 ± 0.183 vs PNX: 0.704 ± 0.137; P < .05]) (Figure 2Bi). In the mHypoA-Kiss/GFP-3 cell model, C/EBP-β decreased at 24 hours with 100 nM PNX (vehicle: 1.000 ± 0.066 vs PNX: 0.717 ± 0.049; P < .05) (Figure 2Bii). Oct-1 mRNA expression increased in the mHypoA-GnRH/GFP cell model at 2 hours with 10 nM PNX (vehicle: 1.000 ± 0.067 vs 1.351 ± 0.117; P < .05) and at 8 hours with 100 nM PNX (vehicle: 1.000 ± 0.076 vs 1.372 ± 0.239; P < .05) (Figure 2Ci). There were, however, no significant changes in Oct-1 mRNA expression in the mHypoA-Kiss/GFP-3 cell model (Figure 2Cii). This suggests that PNX can regulate the expression of C/EBP-β and Oct-1 transcription factors in hypothalamic neurons.
PNX increases phosphorylation of CREB and ERK1/2 in mHypoA-GnRH/GFP and CREB in mHypoA-Kiss/GFP-3
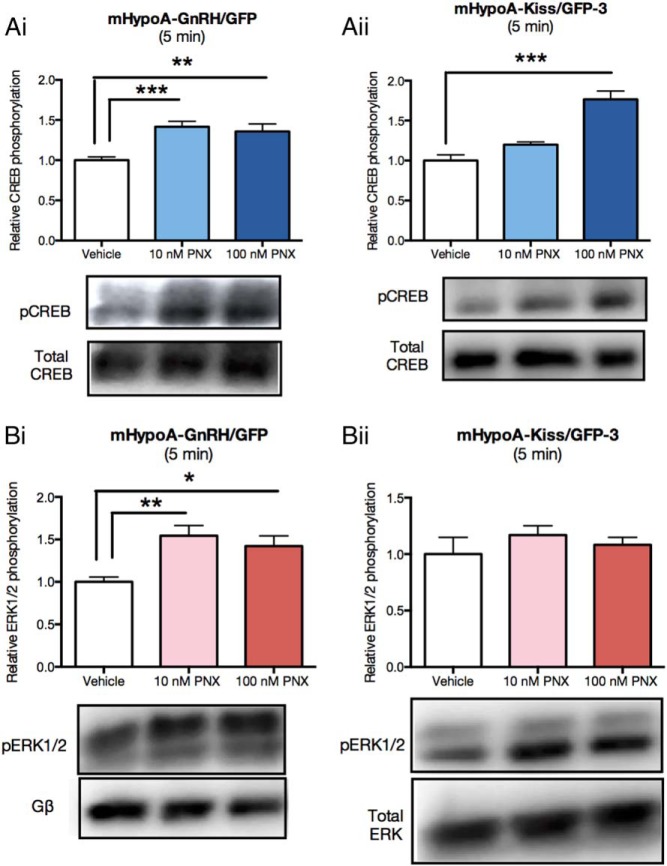
To elucidate possible signaling pathways responsible for mediating mRNA expression and secretion changes by PNX, the phosphorylation of key signaling molecules was measured using Western blotting techniques. Patent data indicated that PNX elevated cAMP levels in rat pituitary adenoma (RC-4B/C) cells (Chang, J-K, Lyu, R-M; phoenixin patent no. US 9018350 B2, Phoenix Pharmaceuticals, 2012), which suggests PNX activated the cAMP-PKA-CREB pathway. However, other pathways may also be activated by PNX including the protein kinase C (PKC)-MAPK-ERK pathway. Therefore, we looked at changes in both the phosphorylation of CREB and ERK1/2 after PNX treatment. Neurons were serum starved for 4 hours to quiesce all signaling events. Cells were then treated with either 10 or 100 nM of PNX and protein isolated after 0, 5, 15, 30, and 60 minutes for Western blotting (only 5 min shown). Both 10 and 100 nM PNX increased the phosphorylation of CREB (vehicle: 1.000 ± 0.041 vs 10 nM PNX: 1.416 ± 0.068; P < .001 vs 100 nM PNX: 1.358 ± 0.092; P < .01) (Figure 3Ai) and ERK (vehicle: 1.000 ± 0.056 vs 10 nM PNX: 1.543 ± 0.121; P < .01 vs 100 nM PNX: 1.421 ± 0.122; P < .05) (Figure 3Bi) after 5 minutes in the mHypoA-GnRH/GFP cells. There was also a significant increase in CREB phosphorylation at 5 minutes in the mHypoA-Kiss/GFP-3 with 100 nM PNX (vehicle: 1.000 ± 0.070 vs 100 nM PNX 1.767 ± 0.078; P < .001) (Figure 3Aii). No significant changes were seen in the phosphorylation of ERK1/2 in the mHypoA-Kiss/GFP-3 (vehicle: 1.000 ± 0.149 vs 10 nM PNX: 1.169 ± 0.083 vs 100 nM PNX: 1.082 ± 0.067; P > .05) (Figure 3Bii). There were also no changes to the phosphorylation of AKT in either the mHypoA-GnRH/GFP or mHypoA-Kiss/GFP-3 cell lines (data not shown). These findings indicate that PNX increases the phosphorylation of CREB in both GnRH and kisspeptin neurons but increases the phosphorylation of ERK1/2 only in the mHypoA-GnRH/GFP cell model. This suggests that signaling can be cell type dependent and that there could be convergence of the adenylate cyclase and MAPK signaling cascades in the GnRH cell line.
Figure 3. PNX regulation of the phosphorylation of CREB (A) and ERK1/2 (B) in the mHypoA-GnRH/GFP (Ai, Bi) and mHypoA-Kiss/GFP-3 (Aii, Bii) neuronal cell lines at 5 minutes.
Cells were serum starved for 4 hours and then treated with either vehicle (water) or 10 or 100 nM PNX amide for 5 minutes. Protein was isolated and relative CREB and ERK1/2 phosphorylation was determined using Western blot. Values were normalized to total protein. Results are expressed as mean ± SEM (n = 4–8). *, P < .05; **, P < .01. Statistical significance was determined by a one-way ANOVA with Dunnett's post hoc test.
PNX requires PKA, but not ERK1/2, to regulate mRNA expression in the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models
To determine whether the cAMP-PKA and/or the MEK-ERK1/2 signaling pathways mediate the regulation of GnRH mRNA expression by PNX in the mHypoA-GnRH/GFP cell models and whether the cAMP-PKA pathway mediates the expression of Kiss1 mRNA by PNX in the mHypoA-Kiss/GFP-3 cell model, we used two PKA-specific inhibitors (H-89 and Rp-cAMPS), a PKC-specific inhibitor (K-252c) and a MEK-specific inhibitor (U0126). Both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models were pretreated with the inhibitors for 1 hour (mHypoA-Kiss/GFP-3 cell model only with PKA inhibitors).
In the mHypoA-GnRH/GFP cell model, this was followed by cotreatment of 10 nM PNX with the inhibitors for 2 hours, and in the mHypoA-Kiss/GFP-3 cell model, it was followed by cotreatment of 100 nM PNX with the inhibitors for 24 hours. These experiments were designed to correspond with the optimal concentration and time conditions for GnRH and Kiss1 mRNA expression. RNA was subsequently harvested and then analyzed using quantitative RT-PCR. In the mHypoA-GnRH/GFP cell model, 10 μM H-89 (vehicle: 1.000 ± 0.121 vs PNX: 0.801 ± 0.171; P > .05) attenuated the PNX-mediated induction of GnRH mRNA expression (vehicle: 1.000 ± 0.114 vs PNX: 2.128 ± 0.293; P < .01) (Figure 4Ai). Similarly, 15 μM Rp-cAMPS (vehicle: 1.000 ± 0.054 vs PNX: 1.053 ± 0.062; P > .05) also attenuated the PNX-mediated induction of GnRH mRNA expression (vehicle: 1.000 ± 0.060 vs PNX: 1.228 ± 0.057; P < .05) (Figure 4Aii). Unlike the PKA inhibitors, both the PKC inhibitor (K-252c) (vehicle: 1.000 ± 0.276 vs PNX: 2.405 ± 0.492; P < .05) (Figure 4Aiii) and the MEK inhibitor (U0126) (vehicle: 1.000 ± 0.204 vs PNX: 1.904 ± 0.204; P < .05) (Figure 4Aiv) did not attenuate the induction of GnRH mRNA expression by PNX.
Figure 4. PNX-mediated regulation of GnRH and Kiss1 mRNA expression in the presence of cAMP/PKA and/or MEK-ERK pathway inhibitors in the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models.
Cells were pretreated with 10 μM H-89 (Ai, Bi), 15 μM Rp-cAMPS (Aii, Bii), 1 μM K-252c (Aiii) or 25 μM U0126 (Aiv) inhibitors for 1 hour and then cotreated with 10 nM PNX for 2 hours. GnRH (A) or Kiss1 (B) mRNA expression was determined using quantitative RT-PCR, and levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 4–10 independent experiments). *, P < .05; **, P < .01. n.s., not significant. Statistical significance was determined by a two-way ANOVA with Bonferroni's post hoc test.
In the mHypoA-Kiss/GFP-3 cell model, because PNX induced the phosphorylation of only CREB and not ERK1/2, only PKA inhibitors were used. Ten micromoles of H-89 (vehicle: 1.000 ± 0.110 vs PNX: 1.208 ± 0.236; P > .05) significantly attenuated the PNX-mediated induction of Kiss1 mRNA expression (vehicle: 1.000 ± 0.101 vs PNX: 1.522 ± 0.145; P < .05) (Figure 4Bi), as did 15 μM Rp-cAMPS (vehicle: 1.000 ± 0.328 vs PNX: 0.662 ± 0.066; P > .05) (Figure 4Bii). Together these results suggest that the cAMP-PKA pathway, but not the MEK-ERK pathway, is essential for the regulation of both GnRH and Kiss1 mRNA expression by PNX in hypothalamic neurons.
GPR173 is strongly expressed in both mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 hypothalamic cell models
Because PNX is a novel neuropeptide, the cognate receptor remains currently unknown; however, it likely is a ligand for a GPCR. A ligand-binding assay for GPCRs was performed for PNX to determine potential receptor candidates (Stein, L., et al, unpublished observations). This assay revealed that PNX bound to three different GPCRs: GPR15, GPR25, and GPR173 (data not shown). We screened for the expression of each of these GPCRs in both our mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models using quantitative RT-PCR. Neither GPR15 nor GPR25 was strongly expressed in the GnRH (Figure 5Ai) and kisspeptin (Figure 5Aii) cell models; however, there was a robust expression of GPR173 in both. The mHypoA-GnRH/GFP cells had higher GPR173 mRNA expression than the mHypoA-Kiss/GFP-3 cell model (GnRH: 1.198 ± 0.097 vs kisspeptin: 0.765 ± 0.128; P < .05) (Figure 5B). GPR173 is most highly expressed in the hypothalamus and also in the gonads (22), which makes it a strong candidate receptor for PNX.
Figure 5. GPR15, GPR25, and GPR173 expression in mHypoA-GnRH/GFP and mHypoA-Kiss/GFP cell models.

mHypoA-GnRH/GFP (Ai) and mHypoA-Kiss/GFP-3 (Aii) cell models were analyzed for GPR15, GPR25, and GPR173 mRNA expression using quantitative real-time PCR. B, The relative amount of GPR173 mRNA expression was compared between the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models. C, mHypoA-GnRH/GFP cells were treated with vehicle (water) or 10 or 100 nM PNX amide over a 24-hour time course. GPR173 mRNA expression was determined using real-time PCR, and levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 3–10). *, P < .05; ***, P < .001. Statistical significance was determined by a Student's t test and a two-way ANOVA with Bonferroni's post hoc test.
PNX regulates the expression of the SREB3/GPR173
Because many neuropeptides are known to regulate the expression of their receptor, including GnRH, which increases the expression of GnRH-R during the estrous cycle (42), we investigated whether PNX also regulates the expression of its receptor, GPR173, in the mHypoA-GnRH/GFP cell model. The mHypoA-GnRH/GFP cells were treated with vehicle or 10 nM or 100 nM PNX and RNA isolated at 1-, 2-, 4-, 8-, and 24-hour time points. Then quantitative RT-PCR was used to analyze GPR173 mRNA expression. At 2 hours, 10 nM PNX significantly increased the GPR173 mRNA expression (vehicle: 1.000 ± 0.082 vs PNX: 1.583 ± 0.194; P < .001) (Figure 5C). This suggests that PNX up-regulates the expression of its receptor in GnRH neurons.
GPR173 mediates the effect of PNX on mRNA expression in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models
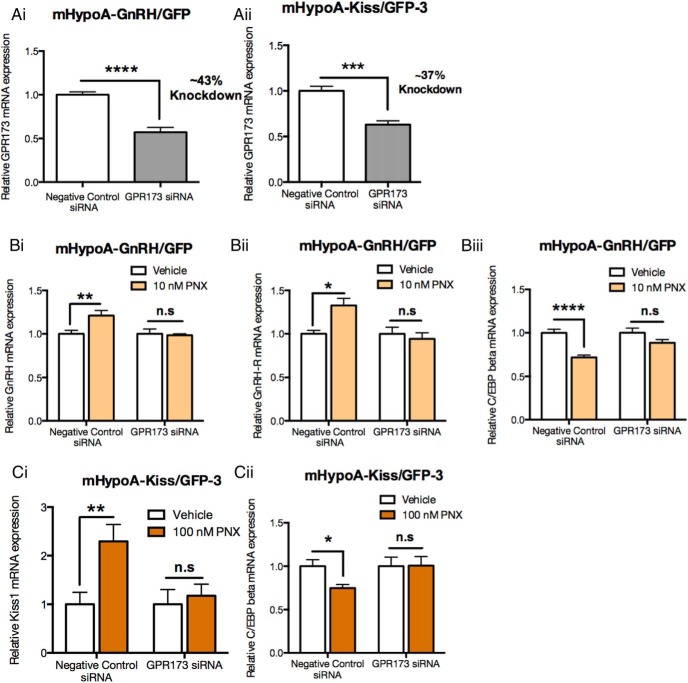
Because it was determined that GPR173 is a potential candidate receptor for PNX and is highly expressed in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models, we examined whether the down-regulation of GPR173 blocks the effects of PNX on mRNA expression in both GnRH and kisspeptin neurons. The siRNA method was used to reduce the endogenous levels of GPR173 in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models. Cells were transfected with 30 nM negative control or GPR173 siRNA for 48 hours, and then quantitative RT-PCR was used to measure GPR173 mRNA levels. GPR173 siRNA reduced GPR173 mRNA expression in the mHypoA-GnRH/GFP cell model by 43% (vehicle siRNA: 1.000 ± 0.034 vs GPR173 siRNA: 0.572 ± 0.054; P < .0001) (Figure 6Ai) and by 37% in the mHypoA-Kiss/GFP-3 cell model (vehicle siRNA: 1.000 ± 0.050 vs GPR173 siRNA: 0.630 ± 0.041; P < .001) (Figure 6Aii), both after 48 hours. Protein levels could not be assessed due to the lack of an appropriate antibody. However, our group previously demonstrated that a 40% reduction in GPR120 mRNA expression resulted in a 75% protein knockdown of GPR120 in the rHypoE-7 cell model when using a similar siRNA protocol (32). The reduction in GPR173 mRNA expression enabled us to assess the role of GPR173 in mediating the effects of PNX on the mRNA expression of reproductive genes in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models.
Figure 6. Knockdown of GPR173 expression impairs PNX-mediated regulation of reproductive genes in the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models.
A, Cell models were transfected with either 30 nM of nontargeting negative control siRNA or GPR173 targeting siRNA for 48 hours, and then GPR173 mRNA expression was measured by quantitative real-time PCR. GPR173 mRNA expression was reduced by 43% in the mHypoA-GnRH/GFP cell model (Ai) and by 37% in the mHypoA-Kiss/GFP-3 cell model (Aii) with the GPR173 siRNA. B, The mHypoA-GnRH/GFP cell model was treated with 10 nM PNX for 2 hours after a 48-hour transfection with either negative control nontargeting siRNA or GPR173 targeting siRNA. RNA was isolated and mRNA expressions of GnRH (Bi), GnRH-R (Bii) and C/EBP-β (Biii) were measured using quantitative real-time PCR. The mHypoA-Kiss/GFP cell model was treated with 100 nM PNX for 24 hours after a 48-hour transfection with either negative control nontargeting siRNA or GPR173 targeting siRNA. The RNA was isolated, and the mRNA expressions of Kiss1 (Ci) and C/EBP-β (Cii) were measured using quantitative real-time PCR. The mRNA levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 4–9 independent experiments). *, P < .05; **, P < .01; ***, P < .001; ****, P < .001. n.s., not significant. Statistical significance was determined by a two-way ANOVA with Bonferroni's post hoc test or a Student's t test.
In the mHypoA-GnRH/GFP cell model, the reduction in GPR173 mRNA expression impaired the PNX-induced increase in GnRH mRNA expression (vehicle: 1.000 ± 0.055 vs PNX: 0.985 ± 0.016; P > .05), unlike in the negative control siRNA (vehicle: 1.000 ± 0.040 vs PNX: 1.210 ± 0.059; P < .01) (Figure 6Bi). Similarly, it impaired the induction of GnRH-R mRNA expression by PNX (vehicle: 1.000 ± 0.076 vs PNX: 0.942 ± 0.070; P > .05), which remained in the negative control siRNA-treated cells (vehicle: 1.000 ± 0.040 vs PNX: 1.327 ± 0.081; P < .01) (Figure 6Bii) and the regulation of C/EBP-β mRNA expression (vehicle: 1.000 ± 0.055 vs PNX: 0.884 ± 0.038), which was significantly reduced in negative control siRNA-treated cells (vehicle: 1.000 ± 0.040 vs PNX: 0.720 ± 0.027; P < .0001) (Figure 6Biii).
In the mHypoA-Kiss/GFP-3 cell model, silencing of GPR173 impaired the induction of Kiss1 mRNA expression by PNX after 24 hours (vehicle: 1.000 ± 0.303 vs PNX: 1.175 ± 0.239; P > .05), which was retained in the negative control siRNA-treated cells (vehicle: 1.000 ± 0.247 vs PNX: 2.294 ± 0.348; P < .01) (Figure 6Ci). The reduction in GPR173 also impaired the reduction in C/EBP-β mRNA expression (vehicle: 1.000 ± 0.105 vs PNX: 1.006 ± 0.106; P > .05), which was present in the negative control siRNA-treated cells (vehicle: 1.000 ± 0.076 vs PNX: 0.748 ± 0.042; P < .05) (Figure 6Cii). Together these results suggest that GPR173 mediates the regulation of reproductive genes by PNX in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 hypothalamic cell models, and GPR173 is the cognate receptor for PNX.
Discussion
PNX is a novel reproductive peptide proposed to be involved in the regulation of the HPG axis, specifically involved in sensitizing the anterior pituitary to GnRH. However, PNX may have other roles in the regulation of reproductive function that have yet to be delineated. Specifically we were interested in determining the role of PNX on GnRH and kisspeptin neuronal populations of the hypothalamus. The siRNA knockdown of PNX in vivo was found to interrupt the normal estrous cycle (12), and this could be due to a disruption of PNX signaling to hypothalamic GnRH and kisspeptin populations as well as the anterior pituitary.
In the present studies, the mHypoA-GnRH/GFP cell line was used as a model for GnRH neurons and the mHypoA-Kiss/GFP-3 cell line as a model kisspeptin neurons (16) to study the role of PNX in reproductive hypothalamic neurons. Through the use of these cell models, it was possible to examine changes to gene expression and peptide secretion and determine the receptor and signaling cascades activated by PNX in GnRH and kisspeptin neurons.
Our initial studies revealed the presence of GnRH, GnRH-R, and SMIM20 mRNA expression in the mHypoA-GnRH/GFP cell lines, making them suitable to study the regulation of GnRH and GnRH-R by PNX. The mHypoA-Kiss/GFP-3 cell line expresses Kiss1 and SMIM20 mRNA. The expression of SMIM20 in these cell lines suggests that PNX is synthesized in these cell types and may exert an autoregulatory effect on these populations if the PNX receptor is present. Autoregulatory mechanisms in GnRH neurons act to regulate both GnRH peptide secretion and mRNA synthesis (43, 44), and therefore, it is plausible that PNX could also have autoregulatory effects in the hypothalamus.
We sought to elucidate the role of PNX in the GnRH cell model. PNX caused a significant increase in GnRH and GnRH-R mRNA as well as GnRH secretion. GnRH mRNA expression increased in a biphasic manner at both 2 and 8 hours. Interestingly, 10 nM PNX increased GnRH mRNA expression at 2 hours but 100 nM PNX was required at 8 hours. This could be the result of receptor desensitization and resensitization. At 8 hours, the PNX receptor could be down-regulated, and therefore, a higher concentration of PNX is required to elicit the same response. When a 24-hour, 1000 nM PNX pretreatment was added prior to the PNX treatment, GnRH secretion was abolished in the mHypoA-GnRH/GFP, suggesting that desensitization is occurring in the PNX receptor. Desensitization of GPCRs is an important physiological feedback mechanism that prevents chronic stimulation of the receptor. It involves the uncoupling of the receptor from the G protein by phosphorylation, internalization of the receptor, and eventual down-regulation of total cellular concentration of receptors (45). The pretreatment of PNX may have desensitized the neurons to further PNX treatment and caused the internalization of GPR173. Dose response does not always correspond with ligand-receptor binding and sensitivity, and there may be other mechanisms involved to elicit the differences in response at 2 and 8 hours. Interestingly, 100 nM PNX was required at 2 hours to stimulate GnRH-R expression. This suggests that different genes are regulated by PNX, depending on the concentration in GnRH neurons.
A 24-hour pretreatment of PNX also increased the amount of GnRH secretion induced by SNP, suggesting that the PNX pretreatment augmented GnRH protein stores. These are the first studies implicating a direct stimulatory role for PNX on GnRH neurons of the hypothalamus. It is widely recognized that GnRH neurons are the convergence point for a wide array of neuropeptides and hormones. The summation of effects on GnRH neurons ultimately determines the output to the gonadotropes. PNX appears to be an additional player in the complex regulation of the GnRH neuron. Immunohistochemistry in the hypothalamus revealed PNX was expressed in multiple regions, including those classically expressing kisspeptin, in the Arc and periventricular region (16). It is well established that many kisspeptin-immunoreactive fibers form close appositions with GnRH neurons in the preoptic area and median eminence (5, 46) to regulate both the transcription (47) and secretion of GnRH and to increase neuronal firing. Mounting evidence suggests kisspeptin can also act through intermediary neurons, such as γ-aminobutyric acid- or neuropeptide Y-expressing cells to exert effects on GnRH neurons (48). PNX could be released from the same synaptic terminals as kisspeptin onto GnRH neurons or interneurons to help stimulate GnRH neurons in concert with kisspeptin.
Interestingly, PNX also appeared to have a role in the regulation of kisspeptin neurons themselves. A cell line microdissected from Kiss-GFP mice, representing the Arc population, was used to examine the effects of PNX on Kiss1 gene expression. PNX increased Kiss1 mRNA expression after 24 hours. Steroidogenic and metabolic regulation of kisspeptin is thought to be part of the environmental control of GnRH neurons (49), and it is plausible that other neuropeptides, such as PNX, may influence these populations. Because kisspeptin is crucial in the development of the preovulatory surge and for the tonic regulation of GnRH and subsequent LH secretion, PNX may stimulate these processes by inducing Kiss1 mRNA expression. Because knockdown of PNX by siRNA prolonged the estrous cycle of female cycling rats (12), it could be hypothesized that there was a disruption to the production of kisspeptin or GnRH. The up-regulation of the GnRH-R mRNA was also evoked only in the female rats (12), suggesting that PNX may be primarily involved in the regulation of the female rather than the male reproductive system. PNX could theoretically be involved in generating the GnRH/LH surge by stimulation of both kisspeptin and GnRH. Because these experiments were performed in vitro, further characterization of PNX expression and colocalization with kisspeptin in vivo is necessary. In vivo experiments would also delineate the effects of PNX on the HPG axis as well as in overall reproductive physiology.
To help elucidate the mechanism through which PNX may increase GnRH-R, GnRH, and Kiss1 mRNA expression, the 5′ flanking region of the genes was examined to look for potential transcription factor binding sites. Both GnRH and GnRH-R are regulated by Oct-1, a POU homeodomain transcription factor. Oct-1 regulates genes in a tissue-specific manner by binding with other transcription factors or tissue-specific coactivators (40, 41). These mechanisms both repress and stimulate GnRH mRNA expression by targeting the GnRH promoter and enhancer in different regions (41). Multiple Oct-1 and C/EBP-β binding sites were found in the GnRH, GnRH-R, and Kiss1 genes. Moreover, PNX reduced the expression of C/EBP-β in both the mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models and increased the expression of Oct-1 in the mHypoA-GnRH/GFP cells. The increases in Oct-1 mRNA expression correlated to those in which GnRH and GnRH-R mRNA expression were induced, which could mean that Oct-1 is involved in the regulation of these genes.
C/EBP-β is a transcription factor required for the nitric oxide-mediated down-regulation of GnRH mRNA expression in the GT1–7 cells (39) and the melatonin-mediated repression of GnRH mRNA expression (50). Therefore, the repression of C/EBP-β mRNA expression by PNX may have increased GnRH mRNA expression by reducing the expression of C/EBP-β that was bound to the GnRH promoter region. C/EBP-β binding domains are also similarly expressed in the GnRH-R promoter, and this transcription factor may be responsible for the induction of the GnRH-R by PNX. C/EBP-β may also be involved in the regulation of Kiss1 mRNA expression in the mHypoA-Kiss/GFP-3 cells because C/EBP-β also was reduced in this cell model. It has yet to be determined, but if C/EBP-β is involved in the repression of Kiss1 mRNA expression via one of the binding domains found in the Kiss1 promoter region, then the repression of C/EBP-β by PNX may be responsible for the induction of Kiss1 mRNA expression, similar to GnRH transcriptional regulation. Future experiments will need to identify the necessity of these transcription factors in the PNX-mediated regulation of these reproductive genes in the GnRH and kisspeptin hypothalamic neurons, but they appear to be logical putative candidate transcription factors.
To further examine the molecular mechanisms mediating the regulation of reproductive genes by PNX in hypothalamic GnRH and kisspeptin neurons, we measured the phosphorylation of two key signaling molecules, CREB and ERK1/2, after exposure to PNX. Preliminary experiments in the patent for PNX indicated that PNX increased cAMP in pituitary adenoma cells (Chang, J-K, Lyu, R-M; phoenixin patent no. US 9018350 B2, Phoenix Pharmaceuticals, 2012). This indicated that the cAMP-PKA pathway is induced by PNX and suggested that CREB, a transcription factor classically phosphorylated downstream of PKA, may be phosphorylated by GPR173 activation. ERK1/2 phosphorylation was also measured because it is a second messenger involved in the signaling of a number of reproductive neuropeptides, including GnRH. PNX increased the phosphorylation of CREB and ERK1/2 in the mHypoA-GnRH/GFP cells but only CREB in the mHypoA-Kiss/GFP-3 cell line. Pathway-specific inhibitors demonstrated that PKA was necessary for the induction of GnRH mRNA expression in the mHypoA-GnRH/GFP cell model and Kiss1 mRNA in the mHypoA-Kiss/GFP-3 cell model. However, blocking signaling of PKC and MAPK did not affect the induction of GnRH mRNA expression by PNX, suggesting that these second messengers are not necessary for the induction of GnRH mRNA expression by PNX. From these studies, it appears that the cAMP-PKA pathway is necessary for the PNX-mediated up-regulation of GnRH and Kiss1 mRNA expression. The phosphorylation of ERK1/2 was not required for PNX-mediated expression of GnRH mRNA, and therefore, the PKC-MAPK signaling cascade is not likely the primary signaling mechanism for PNX. ERK1/2 may, however, be involved the regulation of other genes that were not studied. The phosphorylation of ERK1/2 was specific to GnRH neurons and could be caused by cross talk with cAMP, from the adenylate cyclase pathway to the MAPK pathway. The activation of the cAMP-PKA pathway by PNX suggests that the receptor for PNX is likely a Gαs GPR; however, we cannot rule out the possibility that it may bind with a Gq/11 GPR in GnRH neurons in a cell type-specific manner.
CREB is a transcription factor that is expressed in most hypothalamic neurons and found to be phosphorylated after PNX treatment. It signals by binding to specific Ca2+-cAMP-responsive elements (CREs) in the promoter region of target genes (51). As was determined by promoter analysis in silico and is shown in the literature, the GnRH gene has regions that bind CREB to regulate its transcription (52). Interestingly, mice with GnRH neuron-specific CREB deletion have disrupted estrous cycles, with particularly long diestrous periods and shortened estrous phases (53). A similar dysfunction in estrous cyclicity was observed in PNX siRNA knockdown in rats that also had prolonged diestrous periods (12). If PNX signal transduction relies on the phosphorylation of CREB, CREB knockout animals and PNX knockout animals may display similar phenotypes. The GnRH-R proximal promoter also contains CREB binding sites in both the rat and mouse genes (54). Together these studies suggest PNX may signal through CREB to increase the activity in GnRH neurons. Similarly, in kisspeptin cells, CREB may be important for PNX signal transduction. A CRE was localized on the Kiss1 gene for CREB and CREB-1 regulated transcription coactivator-1 (Crtc1) transcription factors (55). A chromatin immunoprecipitation assays confirmed the binding of CREB and Crtc1 to the Kiss1 promoter in GT1–7 cells (55). Knockdown of Crtc1, a transcription factor that binds with CREB to induce or repress gene expression, caused a significant reduction in Kiss1 mRNA expression in mice (55). The phosphorylation of CREB in the mHypoA-Kiss/GFP-3 cell model in response to PNX could directly induce the transcription of the Kiss1 gene through CREB binding elements.
These are some of the first studies to determine that GPR173 is a receptor for PNX and demonstrate that it does in fact signal through a GPR, as has been previously hypothesized (12, 13). Using a ligand binding assay, three potential GPCR candidates for PNX were determined (Stein et al unpublished observations). GPR15 and GPR25 did not have high expression in our hypothalamic cell models; however, there was robust GPR173 mRNA expression. This corroborates with previous studies, which have identified high expression of GPR173 in the brain (22). GPR173 also is highly expressed in the reproductive system and is known as SREB3 due to the high conservation across species. The high sequence conservation between species suggests GPR173 plays a critical role in the regulation of both the nervous and reproductive systems (22). We next demonstrated that the down-regulation of GPR173 by siRNA in both GnRH and kisspeptin cell models disrupted the PNX-mediated regulation of reproductive genes. This confirms that GPR173 is a receptor for PNX and is necessary for the PNX-mediated regulation of GnRH and kisspeptin neurons. Interestingly, GPR173 had a higher expression in the GnRH cell model compared with the kisspeptin cell model, and therefore, GnRH neurons may be more sensitive to PNX and other ligands for GPR173 than upstream kisspeptin neurons. In corroboration with this possibility, 10 nM PNX increased CREB and ERK1/2 phosphorylation more robustly than 100 nM PNX in the GnRH neurons. Meanwhile, in the mHypoA-Kiss/GFP-3 cell models, only 100 nM PNX was effective at increasing CREB and ERK1/2 phosphorylation. The higher concentration of GPR173 receptors in GnRH neurons may cause them to be more sensitive to stimulation by PNX, whereas the kisspeptin neurons require a higher concentration of PNX to elicit the same response.
GPR173 has already been identified as the receptor for the GnRH metabolite GnRH-(1–5) (23); however, some GPCRs can bind multiple ligands and contain both orthosteric and allosteric sites for ligand binding that produce different effects on the cell. GnRH-(1–5) and PNX share similar functions in the regulation of GnRH neurons, both increase GnRH mRNA expression after 2 hours (24), and increase GnRH secretion (25). Therefore, it is plausible GnRH-(1–5) may also activate the cAMP-PKA pathway to stimulate GnRH mRNA expression and secretion. Larco et al demonstrated that GnRH-(1–5) could inhibit the migration of the premigratory GnRH secreting GN11 cell model (23). Surprisingly, although it appeared GnRH-(1–5) coupled to a GPCR, it did not increase the second messenger cAMP or inositol triphosphate, suggesting it acts independent of these messengers in the premigratory neurons (56). Instead, signaling deviates from classical Gαs and Gq/11 pathways, and GnRH-(1–5) recruits β-arrestin-2 to GPR173 to inhibit cellular migration (56).
β-Arrestin can function as a scaffolding protein to localize the necessary components for cellular migration and therefore may be involved only in the migratory action of GnRH-(1–5) (57). GnRH-(1–5) may signal through different mechanisms, depending on the cell type (premigratory GN11 vs adult derived GT1–7 and mHypoA-GnRH/GFP neurons) and therefore may also require the cAMP-PKA pathway to increase GnRH mRNA expression and secretion. Future studies will have to elucidate the precise signaling mechanisms involved in GnRH-(1–5)-mediated regulation of GnRH mRNA expression and secretion and determine whether it is similar to PNX and determine the relationship between these two functionally related neuropeptides. PNX also increased the expression of GPR173 in the mHypoA-GnRH/GFP cell model. By increasing the concentration of GPR173 in GnRH neurons, PNX may sensitize the neurons to impending stimulation by PNX.
The interaction between PNX and GPR173 needs further characterization. However, having determined the receptor for PNX allows future research that can explore the localization of GPR173 and thus investigate other potential physiological functions for PNX.
In summary, we have demonstrated a role for PNX in the stimulation of both GnRH and kisspeptin neurons of the hypothalamus. PNX appears to stimulate reproductive gene expression in both GnRH- and Kiss1-expressing neurons through GPR173, and a cAMP-PKA dependent pathway, perhaps through the activation of a Gαs protein. This pathway may also involve the regulation transcription factors CREB, C/EBP-β, and Oct-1 (Figure 7). Developing our understanding of PNX and its mechanisms of action could have promising implications for reproductive medicine. Because GnRH agonists are used in reproductive disorders and kisspeptin is now being considered as a therapeutic target (58), it is conceivable that PNX or GPR173 agonists and/or antagonists could eventually be used to alter GnRH or kisspeptin neuronal function.
Figure 7. Summary of the proposed mechanisms involved in the regulation of gene expression by PNX in mHypoA-GnRH/GFP and mHypoA-Kiss/GFP-3 cell models.

GPR173 has been identified as the cognate receptor for GPR173. We report that PNX increases the phosphorylation of CREB, suggesting that it activates a Gαs protein and the cAMP/PKA pathway. PKA was shown to be necessary to induce changes in GnRH and Kiss1 mRNA expression by PNX. This pathway may therefore mediate the changes in mRNA expression by PNX. Dotted line indicates to be elucidated. AC, adenyl cyclase.
Acknowledgments
We thank Jennifer Chalmers and Dr Leigh Wellhauser for their technical assistance and helpful discussions. We also appreciate the collaboration and discussions regarding the putative PNX receptor with Drs Lauren Stein, Gina Yosten, and Willis Samson.
This work was supported by the Canadian Institutes for Health Research and the Canada Foundation for Innovation and Canada Research Chairs Program (to D.D.B.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Canadian Institutes for Health Research and the Canada Foundation for Innovation and Canada Research Chairs Program (to D.D.B.).
Footnotes
- Arc
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- C/EBP
- CCAAT/enhancer-binding protein
- CRE
- cAMP-responsive element
- CREB
- cAMP response element binding protein
- Crtc1
- CREB-1 regulated transcription coactivator-1
- DsiRNA
- Dicer substrate short-interfering RNA
- EIA
- enzyme-linked immunoassay
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- GnRH-R
- GnRH receptor
- GPR
- G protein-coupled receptor
- HPG
- hypothalamic pituitary gonadal
- Kiss
- kisspeptin
- MEK
- MAPK kinase
- Oct-1
- octamer transcription factor-1
- PKA
- protein kinase A
- PKC
- protein kinase C
- PNX
- Phoenixin
- siRNA
- small interfering RNA
- SMIM20
- small integral membrane protein 20
- SNP
- sodium nitroprusside
- SREB
- superconserved receptor expressed in the brain
- TBST
- Tris-buffered saline with Tween 20.
References
- 1. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. [DOI] [PubMed] [Google Scholar]
- 2. Radovick S, Ticknor CM, Nakayama Y, et al. Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J Clin Invest. 1991;88:1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. [DOI] [PubMed] [Google Scholar]
- 4. Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254–255:91–96. [DOI] [PubMed] [Google Scholar]
- 5. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Roux N, Genin E, Carel J-C, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 8. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. [DOI] [PubMed] [Google Scholar]
- 9. Shahab M, Mastronardi C, Seminara SB, et al. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han S-K, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. [DOI] [PubMed] [Google Scholar]
- 12. Yosten GLC, Lyu R-M, Hsueh AJW, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyu R-M, Huang X-F, Zhang Y, et al. Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience. 2013;250:622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang JH, He Z, Peng YL, et al. Phoenixin-14 enhances memory and mitigates memory impairment induced by AŒ≤1–42 and scopolamine in mice. Brain Res. 2015;1629:298–308. [DOI] [PubMed] [Google Scholar]
- 15. Jiang JH, He Z, Peng YL, et al. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res. 2015;286:39–48. [DOI] [PubMed] [Google Scholar]
- 16. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. [DOI] [PubMed] [Google Scholar]
- 17. Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. [DOI] [PubMed] [Google Scholar]
- 18. Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. [DOI] [PubMed] [Google Scholar]
- 19. Dhillon SS, McFadden SA, Chalmers JA, et al. Cellular leptin resistance impairs the leptin-mediated suppression of neuropeptide Y secretion in hypothalamic neurons. Endocrinology. 2011;152:4138–4147. [DOI] [PubMed] [Google Scholar]
- 20. McFadden SA, Menchella JA, Chalmers JA, Centeno M-L, Belsham DD. Glucose responsiveness in a novel adult-derived GnRH cell line, mHypoA-GnRH/GFP: involvement of AMP-activated protein kinase. Mol Cell Endocrinol. 2013;377:65–74. [DOI] [PubMed] [Google Scholar]
- 21. Treen AK, Luo V, Chalmers JA, et al. Divergent Regulation of ER and Kiss genes by 17β-estradiol in hypothalamic ARC versus AVPV models. Mol Endocrinol. 2016;30:217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsumoto M, Saito T, Takasaki J, et al. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun. 2000;272:576–582. [DOI] [PubMed] [Google Scholar]
- 23. Larco DO, Cho-Clark M, Mani SK, Wu TJ. The metabolite GnRH-(1–5) inhibits the migration of immortalized GnRH neurons. Endocrinology. 2013;154:783–795. [DOI] [PubMed] [Google Scholar]
- 24. Wu TJ, Mani SK, Glucksman MJ, Roberts JL. Stimulation of luteinizing hormone-releasing hormone (LHRH) gene expression in GT1–7 cells by its metabolite, LHRH-(1–5). Endocrinology. 2005;146:280–286. [DOI] [PubMed] [Google Scholar]
- 25. Larco DO, Williams M, Schmidt L, et al. Autoshortloop feedback regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by its metabolite, GnRH-(1–5). Endocrine. 2015;49(2):470–478. [DOI] [PubMed] [Google Scholar]
- 26. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. [DOI] [PubMed] [Google Scholar]
- 27. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belsham DD, Cai F, Cui H, et al. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. [DOI] [PubMed] [Google Scholar]
- 30. Belsham DD, Fick LJ, Dalvi PS, et al. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009;23:4256–4265. [DOI] [PubMed] [Google Scholar]
- 31. Roy D, Belsham DD. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1–7 GnRH neurons. Signal transduction mechanisms. J Biol Chem. 2002;277:251–258. [DOI] [PubMed] [Google Scholar]
- 32. Wellhauser L, Belsham DD. Activation of the ω3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation. 2014;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim GL, Dhillon SS, Belsham DD. Kisspeptin directly regulates neuropeptide Y synthesis and secretion via the ERK1/2 and p38 mitogen-activated protein kinase signaling pathways in NPY-secreting hypothalamic neurons. Endocrinology. 2010;151:5038–5047. [DOI] [PubMed] [Google Scholar]
- 34. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. [DOI] [PubMed] [Google Scholar]
- 35. Ye J, Coulouris G, Zaretskaya I, et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. [DOI] [PubMed] [Google Scholar]
- 37. Kovacs KJ. Measurement of immediate-early gene activation-c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. [DOI] [PubMed] [Google Scholar]
- 38. Mahachoklertwattana P, Black SM, Kaplan SL, Bristow JD, Grumbach MM. Nitric oxide synthesized by gonadotropin-releasing hormone neurons is a mediator of N-methyl-D-aspartate (NMDA)-induced GnRH secretion. Endocrinology. 1994;135:1709–1712. [DOI] [PubMed] [Google Scholar]
- 39. Belsham DD, Mellon PL. Transcription factors Oct-1 and C/EBPβ (CCAAT/enhancer-binding protein-β) are involved in the glutamate/nitric oxide/cyclic-guanosine 5′-monophosphate-mediated repression of mediated repression of gonadotropin-releasing hormone gene expression. Mol Endocrinol. 2000;14:212–228. [DOI] [PubMed] [Google Scholar]
- 40. Eraly SA, Nelson SB, Huang KM, Mellon PL. Oct-1 binds promoter elements required for transcription of the GnRH gene. Mol Endocrinol. 1998;12:469–481. [DOI] [PubMed] [Google Scholar]
- 41. Kam K-Y, Jeong K-H, Norwitz ER, Jorgensen EM, Kaiser UB. Oct-1 and nuclear factor Y bind to the SURG-1 element to direct basal and gonadotropin-releasing hormone (GnRH)-stimulated mouse GnRH receptor gene transcription. Mol Endocrinol. 2005;19:148–162. [DOI] [PubMed] [Google Scholar]
- 42. Bauer-Dantoin AC, Hollenberg AN, Jameson JL. Dynamic regulation of gonadotropin-releasing hormone receptor mRNA levels in the anterior pituitary gland during the rat estrous cycle. Endocrinology. 1993;133:1911–1914. [DOI] [PubMed] [Google Scholar]
- 43. Krsmanovic LZ, Stojilkovic SS, Mertz LM, Tomic M, Catt KJ. Expression of gonadotropin-releasing hormone receptors and autocrine regulation of neuropeptide release in immortalized hypothalamic neurons. Proc Natl Acad Sci USA. 1993;90:3908–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Padmanabhan V, Evans NP, Dahl GE, et al. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. [DOI] [PubMed] [Google Scholar]
- 45. Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 46. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. [DOI] [PubMed] [Google Scholar]
- 47. Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parhar I, Ogawa S, Kitahashi T. RFamide peptides as mediators in environmental control of GnRH neurons. Prog Neurobiol. 2012;98:176–196. [DOI] [PubMed] [Google Scholar]
- 50. Gillespie JMA, Roy D, Cui H, Belsham DD. Repression of gonadotropin-releasing hormone (GnRH) gene expression by melatonin may involve transcription factors COUP-TFI and C/EBPβ binding at the GnRH enhancer. Neuroendocrinology. 2004;79:63–72. [DOI] [PubMed] [Google Scholar]
- 51. Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee VHY, Lee LTO, Chow BKC. Gonadotropin-releasing hormone: regulation of the GnRH gene. FEBS J. 2008;275:5458–5478. [DOI] [PubMed] [Google Scholar]
- 53. Kwakowsky A, Herbison AE, Abraham IM. The role of cAMP response element-binding protein in estrogen negative feedback control of gonadotropin-releasing hormone neurons. J Neurosci. 2012;32:11309–11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pincas H, Laverrière JN, Counis R. Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3′,5′-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem. 2001;276:23562–23571. [DOI] [PubMed] [Google Scholar]
- 55. Altarejos JY, Goebel N, Conkright MD, et al. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nat Med. 2008;14:1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larco DO, Semsarzadeh NN, Cho-Clark M, Mani SK, Wu TJ. β-Arrestin 2 is a mediator of GnRH-(1–5) signaling in immortalized GnRH neurons. Endocrinology. 2013;154:4726–4736. [DOI] [PubMed] [Google Scholar]
- 57. DeFea KA. Stop that cell! β-Arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. [DOI] [PubMed] [Google Scholar]
- 58. Jayasena CN, Dhillo WS. Kisspeptin offers a novel therapeutic target in reproduction. Curr Opin Investig Drugs. 2009;10:311–318. [PubMed] [Google Scholar]