Abstract
With respect to the high burden of ischemic stroke and the absence of pharmacological treatment for promoting rehabilitation, promising candidates with specific effects on long-term functional recovery are highly desired. Candidates need reasonable experimental paradigms to evaluate the long-term functional outcome focused on ischemia-induced sensorimotor and memory deficits. “Danshen”, a traditional Chinese herb, has long been used to treat coronary and cerebral vascular diseases as well as dementia. Salvianolic acid A (SAA), one of the major active ingredients of Danshen, was demonstrated to be effective in protecting against cerebral ischemic injury. Here, employing an experimental stroke model induced by photothrombosis in the unilateral frontal cortex of rats, we investigated whether SAA has long-term protective effects on ischemia-induced sensorimotor and memory deficits in our behavioral tests. The results indicated that a single SAA treatment improved the cortical ischemia-induced sensorimotor deficits during 15 days’ cylinder test period, and alleviated ischemia-induced sustained spatial memory impairments during the 2 months’ dependent Morris Water Maze (MWM) tests. In addition, either ischemic injury or SAA treatment did not show any changes compared with sham group in other behavioral tests including rotarod tests, swimming speed in MWM tests, open field tests, elevated plus maze tests, treadmill tests and forced swimming tests. The results reveal that the cognitive deficits are not the results of animal’s anxiety or confounding motor impairments. Overall, the present paradigm appears suitable for the preclinical evaluation of the long-term effects of pharmacological treatments on ischemic stroke. Meanwhile, SAA might have therapeutic potential for the treatment of memory deficits associated with ischemic stroke.
Keywords: salvianolic acid A, memory impairments, dementia, ischemic stroke
Introduction
Ischemic stroke, which has many sequelae including cognitive impairments, is one of the leading causes of disability in the world.1,2 However, few effective pharmacological treatments are available for promoting rehabilitation in the clinical practice.3,4 Many previous studies targeting neuroprotective therapy for ischemic stroke are focused on the salvage of the injured tissue and the improvement of functional outcome after stroke onset.4–6 To get more reliable support to confirm whether candidate drugs are beneficial in ischemic stroke, investigation of long-term functional outcome is highly recommended.5
Postischemia neurobehavioral assessments always focus on sensorimotor deficits, and preclinical evaluation of cognitive function is virtually ignored.7–12 Thus, it needs reasonable experimental paradigm employing animal models to evaluate the long-term functional outcome focused on ischemia-induced deficits in cognition as well as sensorimotor. Two commonly used animal models of ischemic stroke, middle cerebral artery occlusion (MCAO) model and the cortical photothrombotic model, are both associated with deficits in sensorimotor and cognitive function.13,14 In previous studies,15,16 the cognitive evolution in Morris Water Maze (MWM) tests after the MCAO surgery are confounded, as it induced severe sensorimotor deficits. In contrast, the model of photothrombosis has the ability to control the size of infarcts in explicit brain regions with distinct functions, which show significant changes in some stroke patients,17 such as the frontal cortex. Therefore, the photothrombosis model provides a reliable platform to detect the effect of agents on ischemia-induced long-term cognition impairments with minor sensorimotor deficits.18–21
Chinese traditional medicinal herbs have gained wider attention due to their safety and effectiveness, which provides us a rich resource for the screening candidates of ischemic stroke. Danshen, the root of Salvia miltiorrhiza (Labiatae), has long been used to treat coronary, cerebral vascular diseases22,23 and dementia.24 Salvianolic acid A (SAA) is one of the major active ingredients of Danshen.25 The cerebral protective effects of SAA in different animal models of ischemic stroke have been examined in previous studies.26–28 Furthermore, it has been proved that a single SAA intraperitoneal injection postischemia induction could improve the 24-h fear memory of mice by using the model of transient global ischemia-reperfusion injury.27 However, there is no study on the long-term protective effects of SAA on deficits in sensorimotor and cognitive function after an ischemic attack.
Thus, to detect the long-term effects of SAA on the functional recovery after cortical photothrombotic injury, we administered a single dose of SAA to rats and monitored appropriate behavioral indices over a period of 2 months. Strikingly, the single SAA treatment significantly reduced sensorimotor deficits and rescued spatial memory impairments induced by ischemic injury.
Materials and methods
Animals
Adult male Sprague-Dawley rats (300–350 g) aged 12–14 weeks, were used in this study. Rats were housed four per cage in a temperature-regulated room maintained at 23°C±1°C, under a 12 h light/12 h dark cycle, with free access to food and water. All experiments complied with the regulations for the administration of affairs concerning experimental animals guidelines, reviewed and approved by the Animal Ethics Committee of Kunming Institute of Zoology, the Chinese Academy of Sciences. All experiments were performed in a fully randomized and blinded fashion.
Photothrombotic ischemia induction
The protocol was similar to previous studies with our minor modifications.21,29–31 Briefly, the rats were anesthetized with pentobarbital sodium salt (dissolved in saline, 80 mg/kg, intraperitoneal injection; Sigma-Aldrich, St Louis, MO, USA) with inhalation of medical oxygen. The rectal temperature was kept at 37°C±1°C by a thermostat-controlled heating pad. Photothrombotic injury was induced in the right frontal cortex. The rats were slowly infused with Rose Bengal (dissolved in saline, 20 mg/kg, intravenous injection; Sigma-Aldrich). Ten min later, a cold white light (YaLan Special Light Factory, Nang Jing, People’s Republic of China) with a 4 mm aperture positioned onto the exposed skull (2 mm anterior to the bregma and 2 mm lateral from the midline), at a distance of 5 mm from the skull, was turned on for 15 min. Sham rats were treated similarly but without the light illumination.
Drug treatments
To detect the effects of SAA on the functional recovery after photothrombosis, there were three experimental groups in the study: the sham group (sham, surgery without illumination and vehicle-treated only, n=9), the ischemia control group (Isch + Saline, illumination and vehicle-treated only, n=9) and the ischemia group treated with SAA (Isch + SAA, illumination and a single treatment of SAA, n=9). A single dose of SAA (2 mg/kg, saline as vehicle, Qing Feng Pharmaceutical Investment Group Co., Ltd., Jiangxi, People’s Republic of China) was administrated by sublingual vein injection 10 min after light illumination. The dose (2 mg/kg) was selected based on previous studies26–28,32 and has been validated.
MWM tests
The MWM tests were conducted using a set of reversal phases at regular time points for up to 2 months, which included four periods: spatial initial training, reversal, double-reversal, and triple-reversal. Following each learning phase, the probe trial (with the platform removed) was administered to assess spatial memory. The protocol was performed similar to those described in previous studies.33–37
Training of the animals in the initial spatial training was started on days 7 until 10 with an additional probe trial on day 11. During the training process, rats were trained for four trials (respectively starting from four different quadrants) with 10 min inter-trial intervals on each day for 4 consecutive days, and during each trial (60 s) the rats were allowed to swim until they found the hidden platform (15×15 cm, submerged 1 cm below the water surface), where they remained for 30 s before being returned to the home cage. Rats that failed to find the hidden platform were guided to the platform and remained there for 30 s. The latency to reach the platform and the average velocity were recorded by EthoVision 8.0 program (Noldus, Wagenigen, the Netherlands). Twenty-four hours after the final training day, the rats were returned to the test process, in which the rats swam in the water pool for 120 s from a novel drop point without the platform. The latency to reach the platform area, the amount of platform crossings and the time spent in the platform quadrant were recorded.
The procedure in the reversal learning task was similar to that of the initial spatial training. The reversal learning task was performed on days 12 until 14, with an additional probe trial on day 15. The hidden platform was moved to the opposite quadrant (eg, from NW to SE). The double-reversal learning protocol was conducted on days 26 until 29, and the hidden platform was moved back to the original goal. Then, the double-reversal probe test was performed on day 30. The triple-reversal learning task was started on days 60 until 63, with the hidden platform moved to the opposite quadrant again, and then a triple-reversal probe test was performed on day 64. MWM test with visible platform was performed on day 65.
Cylinder tests
The forelimb use asymmetry test was analyzed based on the method described in previous studies.7,8,30 Rats were placed in a clear Plexiglas cylinder (20 cm diameter and 30 cm height). Two mirrors were placed behind the cylinder at an angle of 90°, allowing the recording of forelimb movements even when the animals turned away from the camera. The testing sessions were videotaped for 3–10 min to record ~20 movements involving weight-bearing contact with the walls of the cylinder. The following behaviors were scored: 1) the percent use of the ipsilateral, contralateral, and bilateral forelimb at contact with the wall of the cylinder were analyzed; 2) the sliding movements of the impaired forelimb were also scored. Forelimb use asymmetry score was calculated based on the method described in the previous study.8
The cortical injury resulted in significantly increased sliding movements of the impaired forelimb during exploration of the glass cylinder, which is a sensitive marker of impaired forelimb function.30 The frequency of the sliding movements of the contralateral forelimb was calculated using the following formula:
Rotarod tests
An accelerating rotarod test (from 4 to 40 rpm within 5 min; Panlab, Barcelona, Spain) was conducted to assess motor coordination.18 In brief, the rats were placed on the apparatus, and the latency to falling was recorded. An arbitrary time limit of 5 min was set for rats on the apparatus in the training and testing procedures. The rats were pretrained for 3 days before baseline behavioral tests.
Elevated plus maze tests
The rats were placed individually on the central platform of the elevated plusmaze (made of gray Perspex with a 10×10 cm central platform, two open arms of 52×11.5 cm, and two enclosed arms of 52×11.5×42 cm, elevated to a height of 74 cm above the floor; Med Associates, Inc., St Albans, VT, USA) facing a fixed open arm. The rats were allowed to explore freely for 5 min, and the time spent in the open arms was recorded, which was used for the estimation of anxiety-like behaviors.38,39
Sucrose preference tests
The rats were housed singly in individual cages to habituate to the presence of two drinking bottles (one with 200 mL of 1% [w/v] sucrose and the other with 200 mL of tap water) for 1 day. After this acclimation, the rats could freely drink the 2% sucrose solution or tap water for the 24 h test. The positions of the bottles were switched daily; water and sucrose solution intake was also measured daily. Sucrose preference (%) was calculated by dividing the sucrose solution intake by total liquid intake (sucrose solution + tap water intake).40,41
Open field tests
At the start of the test, the rats were placed in the center of a clear Plexiglas box (43 cm length ×43 cm width ×30 cm height; Med Associates, Inc.). The total distance traveled and the number of central zone entries during 30 min was automatically recorded by the Med Associates software.41,42
Forced swimming tests
The rats were placed in the tank (50 cm height ×25 cm diameter, 40 cm height water line) for 15 min; the water temperature was set at 25°C±1°C. The rats were put back in the same tank for a 5 min test 24 h later. The immobility time was recorded.41,43
Treadmill tests
The rats were placed onto a treadmill apparatus (Panlab), which was set at 25 m/min with no incline, for a 5 min test. The running distance was automatically recorded to reflect fatigue resistance ability.41
Histological analysis
The animals were deeply euthanized with pentobarbital sodium salt and perfused intracardially with saline followed by buffered 4% paraformaldehyde. The brains were removed from the cranium. Coronal sections of 40 μm thickness were cut, collected at 0.6 mm intervals, and subsequently stained with Thionine (Sigma-Aldrich) to present Nissl staining.44 The total infarct volumes were calculated by multiplying the infarct area by the distance between the sections and summing together the volumes for each brain.
Statistical analysis
All data are presented as mean ± standard error of the mean. Statistical comparisons between groups were performed depending on the normality of distribution and variances equivalence between groups. In cases of equal variance and normal distribution, the data were analyzed by Student’s t-test, or one- or two-way analysis of variance (ANOVA), including repeated measures ANOVA, according to the experimental design. For comparisons between groups and within days, one-way ANOVA was used, followed by Tukey’s honestly significant difference test for post hoc analysis. If groups of data failed tests for normality and equal variance, results were conducted by nonparametric test (SPSS 12.0). The results were considered significant when P<0.05.
Results
Macroscopic appearance of cortical photothrombotic injury
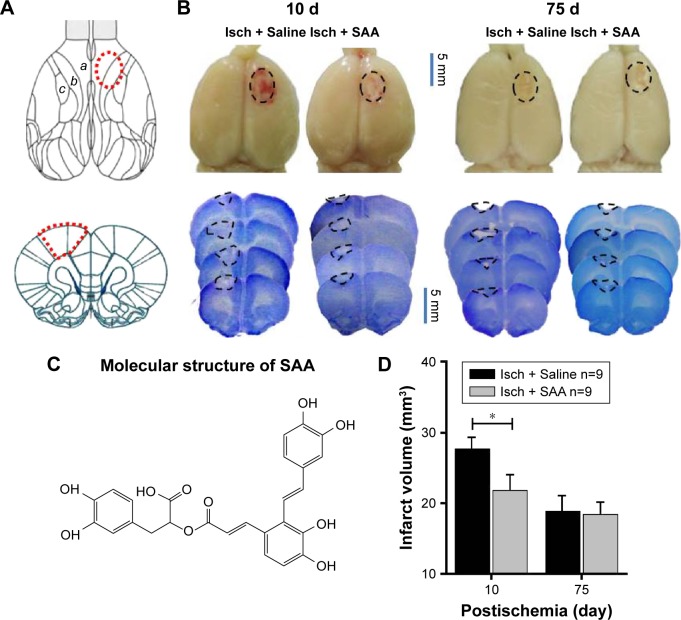
In our paradigm, the location of the cortical injury was in areas 1 and 2 of the frontal cortex (Fr1 and Fr2), part of the forelimb area of the parietal cortex (FL), and extended to the corpus callosum (Figure 1A and B).45 Thus, we used photothrombosis to induce an ischemic injury in the unilateral frontal cortex of rats.
Figure 1.
Macroscopic appearance of cortical photothrombotic injury and the effect of SAA on the injury volume.
Notes: (A) Schematic diagram of the injury locations on the dorsal surfaces and coronal sections of the rat brain; (B) The representative lesion pictures of ischemia treated with Saline and SAA. PFA-perfused brains and coronal section stained with Nissl staining showing a typical infarct in the frontal cortex. The borders of the infarct are marked with dotted lines. Note that all infarcts affected the frontal cortex (Fr1 and Fr2), and part of the forelimb area of the parietal cortex (FL). a, Fr1; b, Fr2; c, FL. (C) Molecular structure of SAA. (D) The effect of SAA on injury volume (mm3). Isch + Saline, ischemia control, n=9; Isch + SAA, ischemia treated with SAA, n=9. Data are given as mean ± SEM. *P<0.05 (Student’s t-test).
Abbreviations: PFA, paraformaldehyde; SAA, Salvianolic acid A; SEM, standard error of the mean.
SAA (structure shown in Figure 1C) treatment protected against photothrombosis-induced histological damage, demonstrated by a reduction in the size of the injury, assessed by Nissl staining, 10 days after photothrombosis (Student’s t-test; t=2.176, P<0.05, Figure 1D). After a series of behavioral tests, the rats were sacrificed to assess the total infarct volumes 75 days after photothrombosis. The core of ischemic injury, which is irreversibly damaged, was not attenuated by SAA (Student’s t-test; t=0.203, P>0.05, Figure 1D).
SAA treatment improved sensorimotor deficits induced by photothrombosis
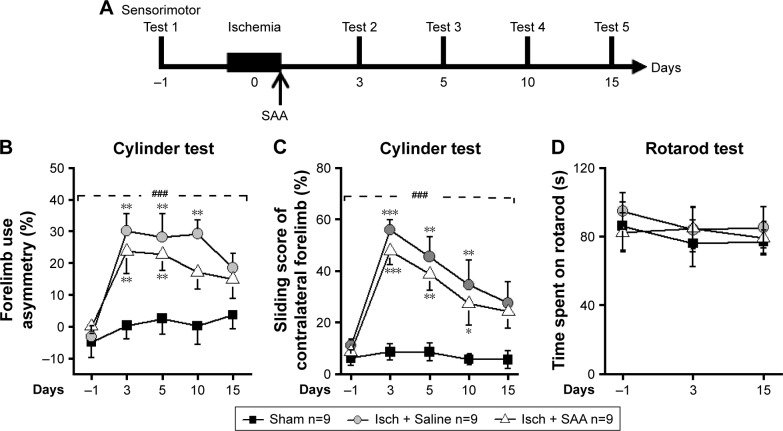
To examine the effect of SAA treatment on sensorimotor deficits induced by photothrombosis, rats were injected with SAA solution shortly after photothrombosis, and sensorimotor tests, including cylinder and rotarod tests (Figure 2A), were performed. Before ischemia, all animals received sensorimotor tests preoperatively to assess the baseline performance (on day −1, Figure 2A) and were divided into three groups randomly. The ischemia groups either treated with SAA or saline showed a significant decrease in use of the impaired forelimb during 15 days’ cylinder test (repeated-measures ANOVA; F(2, 24) =18.520, P<0.001; post hoc: control, P<0.001; SAA, P<0.01, compared with sham; Figure 2B). SAA treatment improved the recovery of forelimb preference (on day 10, one-way ANOVA, F(2, 24) =7.524, P<0.01; post hoc: control, P<0.01; SAA, P>0.05, compared with sham; Figure 2B). In addition, ischemia groups either treated with SAA or saline showed significantly increased sliding movements of the impaired forelimb during exploration of the glass cylinder (repeated-measures ANOVA, F(2, 24) =29.171, P<0.001; post hoc: control, P<0.001; SAA, P<0.001, compared with sham; Figure 2C). SAA treatment did not affect the sliding score of the impaired forelimb (P>0.05, compared with control). Taken together, these findings indicate SAA promotes the recovery of ischemia-induced forelimb preference deficits.
Figure 2.
SAA treatment alleviates sensorimotor deficits following cortical photothrombosis.
Notes: (A) The procedure for the sensorimotor test (sham, n=9; Isch + Saline, ischemia control, n=9; Isch + SAA, ischemia treated with SAA, n=9). (B and C) Compared with sham, rats with photothrombotic injury exhibited mild sensorimotor deficits in the cylinder test. (B) Forelimb use asymmetry score. (C) Sliding movements of contralateral forelimb. (D) Rotarod test. Data are given as mean ± SEM. ###P<0.001 (repeated-measures ANOVA); *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA).
Abbreviations: ANOVA, analysis of variance; SAA, Salvianolic acid A; SEM, standard error of the mean.
The rotarod test was performed to assess deficits in motor function. Performance was similar among three groups (repeated-measures ANOVA, F(2, 24) =0.330, P>0.05, Figure 2D). This result indicates that the focal ischemic injury does not produce confounding motor deficits in the rotarod test.
SAA treatment attenuated spatial memory impairment following cortical photothrombotic injury
To examine the effect of SAA treatment on ischemia-induced memory impairments, rats were injected with SAA solution immediately after photothrombosis and the MWM test was performed (Figure 3A).
Figure 3.
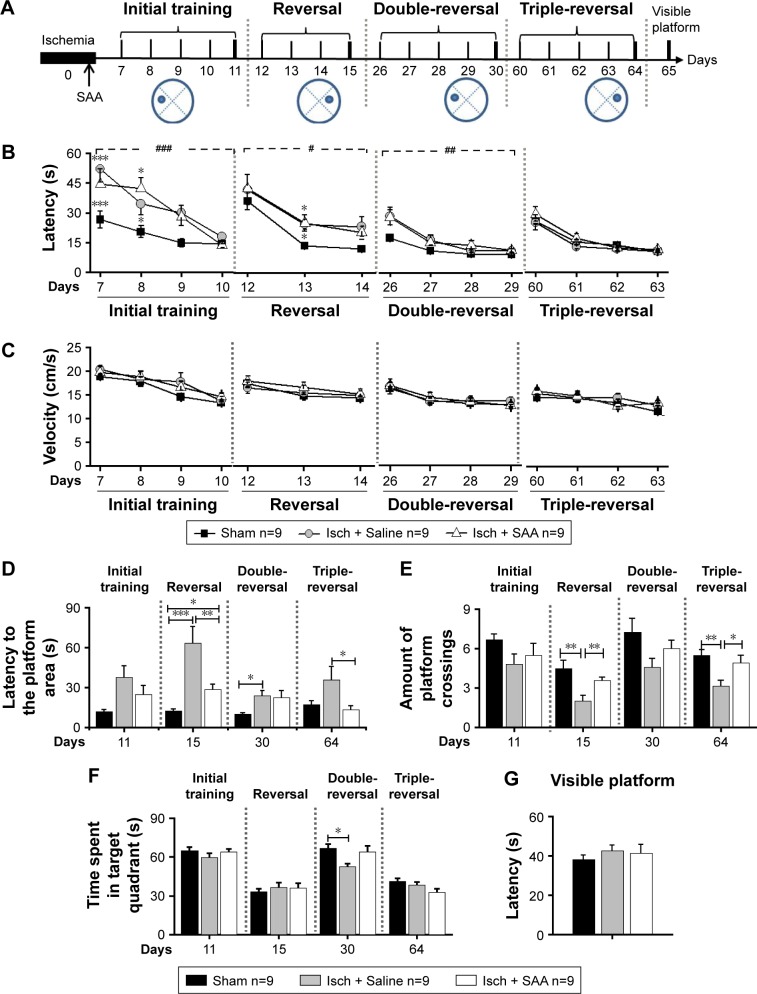
SAA treatment reversed spatial memory impairment in the MWM following cortical photothrombosis.
Notes: (A) The procedure for behavioral study (sham, n=9; Isch + Saline, ischemia control, n=9; Isch + SAA, ischemia treated with SAA, n=9). (B and C) The learning curves in MWM. (B) Latency to find the platform. (C) Velocity. (D–F) Probe test with the platform removed. (D) Latency to reach the platform area, (E) Amount of platform crossings. (F) Time spent in the target quadrant. (G) Visible platform training. Data are given as mean ± SEM. #P<0.05, ##P<0.01, ###P<0.001 (repeated-measures ANOVA); *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA or κ Independent Sample test).
Abbreviations: ANOVA, analysis of variance; MWM, Morris Water Maze; SAA, salvianolic acid A; SEM, standard error of the mean.
Photothrombosis produced learning performance deficits in the initial, reversal, and double-reversal phases, but not in the triple-reversal phase (latency; repeated-measures ANOVA; initial training, F(2, 24) =18.194, P<0.001; post hoc: control, P<0.001; SAA, P<0.001, compared with sham; reversal, F(2, 24) =5.326, P<0.05; post hoc: control, P<0.05; SAA, P<0.05, compared with sham; double-reversal, F(2, 24) =6.120, P<0.01; post hoc: control, P<0.05; SAA, P<0.05, compared with sham; triple-reversal, F(2, 24) =1.023, P>0.05; Figure 3B). SAA treatment had no effect on the learning performance deficits induced by photothrombosis (P>0.05, compared with control).
During each of the learning phases, the swimming speed did not differ among these groups (repeated-measures ANOVA; initial training, F(2, 24) =1.356, P>0.05; sequential reversal, F(2, 24) =0.568, P>0.05; double-reversal, F(2, 24) =0.108, P>0.05; triple-reversal, F(2, 24) =0.634, P>0.05; Figure 3C). In addition, MWM test with visible platform revealed no significant changes in the escape latency (one-way ANOVA, F(2, 24) =0.461, P>0.05, Figure 3G). These results are consistent with our findings from the cylinder and rotarod tests, and together, they show that the rats that underwent photothrombosis only showed mild sensorimotor deficits, with no confounding motor impairments.
To examine the effects of SAA on photothrombosis-induced memory impairments in acute stage, initial and reversal MWM tasks were performed. During the initial probe trial on day 11 after injury, there were no differences in the probe performance among the three groups (one-way ANOVA; the latency to reach the platform area, F(2, 24) =1.871, P>0.05, Figure 3D; the amount of platform crossings, F(2, 24) =0.862, P>0.05, Figure 3E; the time spent in the target quadrant, F(2, 24) =1.628, P>0.05, Figure 3F). However, performance in the reversal (on day 15) probe trial was impaired by photothrombosis, and this impairment was partly ameliorated by SAA treatment (one-way ANOVA, the latency to reach the target area, F(2, 24) =21.162, P<0.001; post hoc: control, P<0.001; SAA, P<0.05, compared with sham; SAA vs control, P<0.01; Figure 3D; the amount of platform crossings, κ Independent Sample test, Kruskal–Wallis H; χ2(8) =11.297, P<0.01; two Independent Sample test, Mann–Whitney, sham vs control, Z=−2.939, P<0.01; SAA vs control, Z=−2.669, P<0.01; sham vs SAA, Z=−0.736, P>0.05; Figure 3E; the time spent in the target quadrant, F(2, 24) =0.474, P>0.05, Figure 3F). Based on these results, we concluded that memory impairments induced by photothrombosis were detectable during the reversal probe test, but not during initial probe test. Furthermore, the protective efficacy of SAA was evident during the reversal probe trial, and the memory impairments were attenuated in the ischemia group treated with SAA.
To examine the effects of SAA on spatial memory impairments in chronic stage, a double-reversal MWM task was performed at 1 month after photothrombosis. The double-reversal probe performance deficits were observed in the ischemia control group, but not in the ischemia group treated with SAA (one-way ANOVA; the latency to reach the platform area, F(2, 24) =4.783, P<0.05; post hoc: control, P<0.05; SAA, P>0.05, compared with sham; SAA vs control, P>0.05; Figure 3D; the amount of platform crossings, F(2, 24) =2.794, P>0.05; Figure 3E; the time spent in the target quadrant, F(2, 24) =4.176, P<0.05; post hoc: control, P<0.05; SAA, P>0.05, compared with sham; SAA vs control, P>0.05; Figure 3F). These results demonstrate that photothrombosis induces sustained memory impairments, which are completely prevented by a single dose of SAA.
Two months after ischemic injury, the triple-reversal MWM task was performed to confirm whether the protective effects of SAA on ischemia-induced memory impairments were long-term. The triple-reversal probe performance deficits were still detectable in the ischemia control group, but there were no performance deficits in the ischemia group treated with SAA (one-way ANOVA; the latency to reach the target area, F(2, 24) =3.675, P<0.05; post hoc: control, P>0.05; SAA, P>0.05, compared with sham; Figure 3D; the amount of platform crossings, F(2, 24) =5.942; P<0.01; post hoc: control, P<0.01; SAA, P>0.05, compared with sham; Figure 3E; the time spent in the target quadrant, F(2, 24) =2.673, P>0.05, Figure 3F). Moreover, the ischemia group treated with SAA took less time to reach the target area (P<0.05) and could more accurately find the removed platform (P<0.05) than the ischemia control group. These results demonstrate that the protective effects of SAA administrated shortly after the focal injury are sustained for 2 months.
Taken together, the photothrombotic injury produced sustained learning and memory impairments. A single dose of SAA effectively attenuated the memory deficits in the reversal trial, and completely prevented the impairment in the double-reversal and triple-reversal trials, although SAA treatment had no effect on learning performance deficits.
The effect of photothrombosis injury and SAA treatment on anxiety- and depression-like behavior
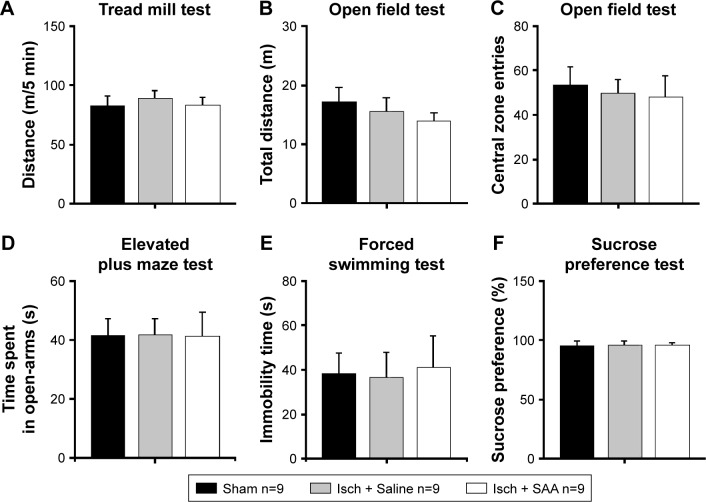
Two months after surgery, we investigated whether the injured rats treated with saline or SAA showed any abnormalities in anxiety- and depression-like behaviors. The endurance capacity in the treadmill test did not differ among the groups (one-way ANOVA, F(2, 24) =0.232, P>0.05, Figure 4A), and no significant difference in locomotor activity was observed, as assessed by total ambulatory distance in the open field test (one-way ANOVA, F(2, 24) =0.653, P>0.05, Figure 4B). The injured rats treated with saline and SAA did not show any anxiety-like behaviors, assessed by the number of central zone entries in the open field test and the elevated plus maze test, compared with the sham group (one-way ANOVA; open field test, F(2, 24) =0.131, P>0.05, Figure 4C; elevated plus maze test, F(2, 24) =0.185, P>0.05, Figure 4D). Both the forced swimming and the sucrose preference tests revealed that the injured groups treated with saline and SAA showed normal levels of depression-like behavior compared with the sham group (one-way ANOVA; forced swimming test, F(2, 24) =0.308, P>0.05, Figure 4E; sucrose preference test, F(2, 24) =0.181, P>0.05, Figure 4F). Injured rats treated with saline or SAA did not exhibit any anxiety or depression-like behaviors, and had normal endurance and locomotor capacities.
Figure 4.
Effects of cortical photothrombotic injury and SAA treatment on anxiety- and depression-like behavior.
Notes: Injured rats treated with saline or SAA had normal endurance capacity in the treadmill test (A) and normal locomotor activity in the open field test (B) as measured by total distance moved in 30 min. Injured rats treated with saline or SAA displayed normal levels of anxiety-like behaviors compared with sham rats as measured by the number of central zone entries in the open field test (C) and the elevated plus-maze test (D). Injured rats treated with saline or SAA exhibited normal levels of depression-like behaviors compared with sham rats as measured by the forced swimming test (E) and the sucrose preference test (F). Sham, n=9; Isch + Saline, ischemia control, n=9; Isch + SAA, ischemia treated with SAA, n=9. Data are given as mean ± SEM.
Abbreviations: ANOVA, analysis of variance; SAA, Salvianolic acid A; SEM, standard error of the mean.
Discussion
In this study, we used an experimental paradigm employing a focal ischemia model of photothrombosis in the unilateral frontal cortex of rats to evaluate the effects of SAA on ischemia-induced changes in a long-term scale. Our results indicated that focal ischemia induction-induced mild somatosensory deficits were maintained for 15 days and sustained cognitive impairments lasted over 2 months. A single SAA injection postischemia conferred long-term protective effects on sustained memory impairments and facilitated the recovery of sensorimotor deficits in our paradigm.
Stroke, especially ischemic stroke, is the most common chronic cause of long-term disability, causing a huge social and economic burden for families and societies.46 However, for a neuroprotective drug, to get beyond the barrier between the successful treatments in animal stroke models and the failed trials in clinical practice is still a big challenge.47 In animal models of ischemic stroke, including MCAO and photothrombosis, many agents show improvements for ischemia-induced injury,6 and most of these studies are focused on the reduction of infarct size at tissue level using some histological methods, such as Nissl staining.12 However, agents which significantly rescue ischemia-induced tissue injury at infarct zones have still failed in clinical trials.4,12 Therefore, it is worthy to explore a strategy to track the effects of drug treatments on functional outcomes in a long-term time scale postischemia induction.
We used the photothrombosis methods to induce ischemic injury in the unilateral frontal cortex of rats. Infarct volume represented by Nissl staining suggested the single SAA injection accelerated the recovery process of ischemic injury without change in the size of core injury 75 days after photothrombosis, compared to the ischemia groups treated with saline. Ischemia injury in the unilateral frontal cortex-induced sensorimotor deficits, which was similar to some previous studies.18,20,48,49 In our study, rats with ischemia treated with saline or SAA showed the same level as sham groups during the rotarod test, which suggested that some basic motor abilities were not affected by ischemic injury. However, for fine motor ability in the cylinder test, rats with ischemic injury in the unilateral frontal cortex showed increased percent of forelimb use asymmetry and sliding score of the impaired forelimb compared to sham groups, and this ability showed spontaneous partial recovery over time. Meanwhile, rats with a single SAA treatment postischemia showed an accelerated tendency of recovery during the cylinder tests, although these rats still revealed dramatic deficits compared to sham groups. These data indicated that ischemic injury in the unilateral frontal cortex could induce mild sensorimotor deficits in rats,18,20,49 which provided us a rationality to further evaluate the effects of SAA treatment on ischemic injury-induced cognitive impairments in a long-term scale.
In clinical practice, 10% of patients develop dementia after a first stroke and up to 30% after recurrent stroke;50 this suggests that the risk of developing dementia is highly related to stroke attacks. Thus, the evaluation of cognitive deficits should be a critical parameter to test the effects of drug treatments on ischemia-induced long-term functional outcome. Here, MWM tasks were selected to evaluate the cognitive deficits, because there are many studies that have proved that the frontal cortex plays key roles in this process, using diverse approaches including photothrombosis.18,20,21 The data of the learning process, including initial training and dependent reversal trainings, showed ischemic injury in the unilateral frontal cortex significantly impaired the learning in MWM tasks from the onset of ischemia injury. However, along the course of rehabilitation, all of the rats with ischemia showed the same level of latency to the platform compared to the sham group 60 days after photothrombosis, and SAA treatment did not change the phenomenon in this process. In contrast, the data of spatial memory tests, especially the latency to the platform area and amount of platform crossings, showed that the ischemia injury impaired the memory representation during MWM tests, which was dramatically rescued by the SAA treatment. The different effects of ischemic injury between the process of reversal learning and memory 2 months after photothrombosis indicated that the ischemia injury only produced transient learning damages while it produced sustained memory impairments. The SAA treatments could significantly improve the impaired memory but not the learning process. In addition, all of these rats showed the same level of swimming speed in the total course of tests and the same ability in MWM test with visible platform. Meanwhile, the ischemic injury or SAA treatment have no effects on the endurance, anxiety- and depression-like behaviors of rats 2 months after photothrombosis using tread mill test, open field test, elevated plus maze test and sucrose preference tests. These results confirmed that the SAA treatment specifically has effects on memory. Thus, the paradigm of long-term functional evaluation could help us to differentiate the exact roles of drug administration in the stages of ischemic injury.
Cognitive deficits in water maze tasks would be confounded by serious sensorimotor deficits in the experimental stroke model, such as MCAO.15,16 In the study, photothrombosis model, which causes minor sensorimotor deficits and long-term cognitive deficits, appears suitable for the preclinical cognitive evaluation of novel candidate drugs on ischemia stroke. However, some previous studies have reported that the model lacks ischemia reperfusion damage,14,51,52 which would limit the degree of preclinical evaluation of drug effects. Maybe another experimental ischemia model, such as microinjection of endothelin-1 in the vicinity of the artery model (EMCAO model), which has a precise location and can lead to long-term mild sensorimotor deficits,53–55 is needed to validate the present paradigm. Moreover, the complicated mechanisms of ischemic stroke damage create a huge challenge during related new-drug development. Many possible targets for the treatment of ischemic stroke, such as glutamate receptor and serotonin-2 receptor,53–55 have been used for new drug development. However, few neuroprotective agents have the same efficacy in clinical practice as they showed in animal models.6 Thus, utilization of different animal ischemia models to investigate the long-term efficacy of candidate drugs on functional outcomes after ischemia damage would be a worthwhile strategy.4
Although the SAA treatment significantly attenuated or improved the focal ischemia of the unilateral frontal cortex-induced sensorimotor and memory impairments, there was still obvious injury at the core of ischemic injury 2 months later. This indicates SAA treatment might affect the functional brain networks, which were dramatically changed by focal ischemia.56,57 Thus, including the underlying molecular mechanisms, we will further investigate the effects of focal ischemia and SAA treatment on the neural networks related to behavioral outcomes in the future.
Conclusion
In our study, we used a paradigm with long-term functional evaluation to examine the effects of SAA treatment on ischemia-induced impairments. The results indicated that the cortical ischemia injury induces mild sensorimotor deficits and sustained memory deficits. The data indicate that the presented paradigm is suited to facilitate preclinical research for the evaluation of long-term effects of pharmacological treatment on ischemic stroke. Meanwhile, this study provides the first evidence that a single treatment of SAA postischemia induction prevents the sustained memory impairments, and further hinted that the early treatment effects of SAA on ischemia are sustained for at least 2 months.
Acknowledgments
This work was supported by National Basic Research Program of China (2013CB835103, 2015CB553502), Strategic Priority Research Program of the Chinese Academy of Science (XDB02020002), National Natural Science Foundation of China (81171294, U1502221, 31371141, 81360679), and Science and Technology Program of Yunnan Province (2013GA003, 2013FA048).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Deniz C, Celik Y, Ozdemir Gultekin T, Baran GE, Deniz Ç, Asil T. Evaluation and follow-up of cognitive functions in patients with minor stroke and transient ischemic attack. Neuropsychiatr Dis Treat. 2016;12:2039–2048. doi: 10.2147/NDT.S102193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arboix A, Alio J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. 2011;9(3):367–379. doi: 10.1586/erc.10.192. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42(1 Suppl):S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 5.Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 6.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 7.Schallert T, Woodlee MT, Fleming SM. Disentangling multiple types of recovery from brain injury. In: Krieglstein J, Klumpp S, editors. Pharmacology of Cerebral Ischemia. Stuttgart: Medpharm Scientific Publishers; 2001. pp. 201–216. [Google Scholar]
- 8.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33(10):2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 9.Hunter A, Hatcher J, Virley D, et al. Functional assessments in mice and rats after focal stroke. Neuropharmacol. 2000;39(5):806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 10.Yu C, Zhou H, Chai AP, Yang YX, Mao RR, Xu L. Whole-scale neurobehavioral assessments of photothrombotic ischemia in freely moving mice. J Neurosci Methods. 2015;239:100–107. doi: 10.1016/j.jneumeth.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Ye R, Kong X, Yang Q, et al. Ginsenoside rd in experimental stroke superior neuroprotective efficacy with a wide therapeutic wind. Neurotherapeutics. 2011;8(3):515–525. doi: 10.1007/s13311-011-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61(5):396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 13.Bacigaluppi M, Comi G, Hermann DM. Animal model of ischemia stroke. Part Two: modeling cerebral ischemia. Open Neurol J. 2010;4:34–38. doi: 10.2174/1874205X01004020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2(3):396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bingham D, Martin SJ, Macrae IM, Carswell HV. Watermaze performance after middle cerebral artery occlusion in the rat: the role of sensorimotor versus memory impairments. J Cereb Blood Flow Metabol. 2012;32(6):989–999. doi: 10.1038/jcbfm.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karhunen H, Pitkänen A, Virtanen T, et al. Long-term functional consequences of transient occlusion of the middle cerebral artery in rats: a 1-year follow-up of the development of epileptogenesis and memory impairment in relation to sensorimotor deficit. Epilepsy Res. 2003;54(1):1–10. doi: 10.1016/s0920-1211(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 17.Sturm W, Longoni F, Weis S, et al. Functional reorganisation in patients with right hemisphere stroke after training of alertness: a longitudinal PET and fMRI study in eight cases. Neuropsychologia. 2004;42(4):434–450. doi: 10.1016/j.neuropsychologia.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Diederich K, Schmidt A, Strecker JK, Schäbitz WR, Schilling M, Minnerup J. Cortical photothrombotic infarcts impair the recall of previously acquired memories but spare the formation of new ones. Stroke. 2014;45(2):614–618. doi: 10.1161/STROKEAHA.113.001907. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt A, Diederich K, Strecker JK, et al. Progressive cognitive deficits in a mouse model of recurrent photothrombotic stroke. Stroke. 2015;46(4):1127–1131. doi: 10.1161/STROKEAHA.115.008905. [DOI] [PubMed] [Google Scholar]
- 20.Rogers DC, Hunter AJ. Photothrombotic lesions of the rat cortex impair acquisition of the water maze. Pharmacol Biochem Behav. 1997;56(4):747–754. doi: 10.1016/s0091-3057(96)00430-3. [DOI] [PubMed] [Google Scholar]
- 21.Silachev DN, Shram SI, Shakova FM, Romanova GA, Myasoedov NF. Formation of spatial memory in rats with ischemic lesions to the prefrontal cortex; effects of a synthetic analog of ACTH(4–7) Neurosci Behav Physiol. 2009;39(8):458–466. doi: 10.1007/s11055-009-9197-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Fang L, Du G, et al. Effects of Danshen on the cardiovascular system. In: Yan X, editor. Dan Shen (Salvia miltiorrhiza) in Medicine. Beijing: People’s Medical Publishing House; 2015. pp. 79–127. [Google Scholar]
- 23.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121(1):9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Hugel HM, Jackson N. Danshen diversity defeating dementia. Bioorg Med Chem Lett. 2014;24(3):708–716. doi: 10.1016/j.bmcl.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Zhang L, Yang X, et al. Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1alpha-Sirt3 axis. Molecules. 2012;17(9):11216–11228. doi: 10.3390/molecules170911216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du G, Zhang J. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ischemia-reperfusion in mice. Chin Med J (Engl) 1997;110(1):65–68. Chinese. [PubMed] [Google Scholar]
- 28.Shang HC, Chao HB, Wang Y, Zhang Bl. Protective effects of salvianolic acid A and B on focal cerebral ischemia in rats. Pharmacol Clin of Chin Materia Med. 2007;23(3):15–17. Chinese. [Google Scholar]
- 29.Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Behavioral effects of photothrombotic ischemic cortical injury in aged rats treated with the sedative-hypnotic GABAergic drug zopiclone. Behav Brain Res. 2005;160(2):260–266. doi: 10.1016/j.bbr.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Shanina EV, Schallert T, Witte OW, Redecker C. Behavioral recovery from unilateral photothrombotic infarcts of the forelimb sensorimotor cortex in rats: role of the contralateral cortex. Neurosci. 2006;139(4):1495–1506. doi: 10.1016/j.neuroscience.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17(5):497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 32.Fan HY, Yang MY, Qi D, et al. Salvianolic acid A as a multifunctional agent ameliorates doxorubicin-induced nephropathy in rats. Sci Rep. 2015;5:12273. doi: 10.1038/srep12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20(12):4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur J Neurosci. 2002;16(3):491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- 35.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh CM, Booth V, Poe GR. Spatial and reversal learning in the Morris water maze are largely resistant to six hours of REM sleep deprivation following training. Learn Mem. 2011;18(7):422–434. doi: 10.1101/lm.2099011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Z, Bai Y, Wu X, et al. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 38.McLelland AE, Martin-Iverson MT, Beninger RJ. The effect of quetiapine (Seroquel™)on conditioned place preference and elevated plus maze tests in rats when administered alone and in combination with (+)-amphetamine. Psychopharmacology(Berl) 2014;231(22):4349–4359. doi: 10.1007/s00213-014-3578-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Yu CL, Wang LP, et al. NMDA and D1 receptors are involved in one-trial tolerance to the anxiolytic-like effects of diazepam in the elevated plus maze test in rats. Pharmacol Biochem Behav. 2015;135:40–45. doi: 10.1016/j.pbb.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 40.First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A. The effects of reboxetine treatment on depression-like behavior, brain neurotrophins, and ERK expression in rats exposed to chronic mild stress. J Mol Neurosci. 2013;50(1):88–97. doi: 10.1007/s12031-012-9872-8. [DOI] [PubMed] [Google Scholar]
- 41.Yu D, Zhou H, Yang Y, et al. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Horm Behav. 2015;69:106–115. doi: 10.1016/j.yhbeh.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Duan T, Tan J, Yuan Q, Cao J, Zhou QX, Xu L. Acute ketamine induces hippocampal synaptic depression and spatial memory impairment through dopamine D1/D5 receptors. Psychopharmacology (Berl) 2013;228(3):451–461. doi: 10.1007/s00213-013-3048-2. [DOI] [PubMed] [Google Scholar]
- 43.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 44.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4(3):e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher NP, Zilles K. Isocortex. In: Paxinos G, editor. The Rat Nervous System. Sydney: Academic Press; 2004. p. 733. [Google Scholar]
- 46.Flynn R, Macwalter R, Doney A. The cost of cerebral ischemia. Neuropharmacol. 2008;55(3):250–256. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Gladstone DJ, Black SE, Hakim AM, Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 48.de Castro JM, Zrull MC. Recovery of sensorimotor function after frontal-cortex damage in rats: evidence that the serial lesion effect is due to serial recovery. Behav Neurosci. 1988;102(6):843–851. doi: 10.1037//0735-7044.102.6.843. [DOI] [PubMed] [Google Scholar]
- 49.Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005;156(1):85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 51.Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8(4):474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- 52.Herz RC, Kasbergen CM, Hillen B, Versteeg DH, de Wildt DJ. Rat middle cerebral artery occlusion by an intraluminal thread compromises collateral blood flow. Brain Res. 1998;791(1–2):223–228. doi: 10.1016/s0006-8993(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 53.Moyanova S, Kirov R, Kortenska L. Multi-unit activity suppression and sensorimotor deficits after endothelin-1-induced middle cerebral artery occlusion in conscious rats. J Neurol Sci. 2003;212(1–2):59–67. doi: 10.1016/s0022-510x(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 54.Moyanova S, Kortenska L, Kirov R, Itzev D, Usunoff K. Ketanserin reduces the postischemic EEG and behavioural changes following Endothelin-1-induced occlusion of the middle cerebral artery in conscious rats. Open Medicine. 2008;3(4):406–416. [Google Scholar]
- 55.Moyanova S, Kortenska L, Mitreva R. Endothelin-1-induced cerebral ischemia: effects of ketanserin and MK-801 on limb placing in rats. Int J Neurosci. 2007;117(9):1361–1381. doi: 10.1080/00207450600938847. [DOI] [PubMed] [Google Scholar]
- 56.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 57.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]