Abstract
The majority of spinal cord injuries (SCIs) in the clinic occur at the lower cervical levels, resulting in both white and gray matter disruption. In contrast, most experimental models of SCI in rodents induce damage in the thoracic cord, resulting primarily in white matter disruption. To address this disparity, experimental cervical SCI models have been developed. Thus, we used a recently characterized model of cervical hemicontusion SCI in adult male rats to assess the potential therapeutic effect of post-SCI administration of 17β-estradiol. Rats received a hemicontusion at the level of the fifth cervical vertebra (C5) followed by administration of 17β-estradiol via a slow release pellet (0.5 or 5.0 mg/pellet) beginning at 30 minutes post-SCI. Behavioral evaluation of skilled and unskilled forelimb function and locomotor function were conducted for 7 weeks after SCI. Upon conclusion of the behavioral assessments, spinal cords were collected and histochemistry and stereology were conducted to evaluate the effect of treatment on the lesion characteristics. We found that post-SCI administration of 17β-estradiol decreased neuronal loss in the ventral horn, decreased reactive astrogliosis, decreased the immune response, and increased white mater sparing at the lesion epicenter. Additionally, post-SCI administration of 17β-estradiol improved skilled forelimb function and locomotor function. Taken together, these data suggest that post-SCI administration of 17β-estradiol protected both the gray and white matter in cervical SCI. Moreover, this treatment improved function on skilled motor tasks that involve both gray and white matter components, suggesting that this is likely a highly clinically relevant protective strategy.
Indexing Terms: estrogen, forepaw function, gray matter sparing, white matter sparing, gliosis
Epidemiological data indicate that 51.0% of spinal cord injury (SCI) patients have injuries in the cervical spine, with the most common neurological level being the fifth cervical vertebra (C5), followed by C4 and C6 (National Spinal Cord Injury Statistical Center, 2006, 2008). Contrastingly, in the preclinical laboratory the majority of SCI models in experimental animals induce injury in the midthoracic region (Noble and Wrathall, 1987; Anderson and Stokes, 1992; Behrmann et al., 1992; Scheff et al., 2003) and it is plausible that important protective effects relevant to cervical SCI may be missed in thoracic models. This is particularly important since preserving the function of just 1–2 segments in the cervical cord would result in a substantial, clinically relevant increase in motor function and independent living, while segmental improvements in the midthoracic cord would not (Van Hedel and Curt, 2006). Another key difference between cervical and thoracic SCIs is that functional deficits after lower cervical SCI are attributable to cellular damage in the gray matter and loss of the functional ascending and descending axonal signals in the white matter (Schrimsher and Reier, 1992; Bunge et al., 1993; el-Bohy et al., 1998; Reier et al., 2002). In contrast, functional deficits in the midthoracic level are mainly attributed to white matter damage (Magnuson et al., 1999; Basso, 2000; Reier et al., 2002). Clearly, differences between injury in the lower cervical and midthoracic spinal cord indicate that improving our understanding of the contribution of gray and white matter to secondary injury and functional recovery is clinically relevant and can be a useful tool in evaluation of potential therapeutics.
Recently, several research teams have developed and characterized rodent models of cervical SCI (Anderson et al., 2005, 2007, 2009; Dunham et al., 2010). These cervical models afford several clinically relevant attributes not available in thoracic models. For example, cervical SCI models in rats enable the evaluation of highly skilled use of prehensile forepaws including tasks such as grasping and reaching, which incorporate independent digit movements that are similar to those used by primates (Whishaw et al., 1992; Whishaw and Gorny, 1994). Additionally, detailed maps of motor neuron pools in the gray matter as well as the specific white matter tracts involved in control of forelimb muscles have been elegantly detailed in rats (Liang et al., 1991; Rouiller et al., 1993; McKenna et al., 2000; Anderson et al., 2005, 2007), making the relationship between tissue preservation and reaching/grasping behaviors after cervical SCI more easily interpreted (Schrimsher and Reier, 1992; Schrimsher and Reier, 1993; Muir et al., 2007; Dunham et al., 2010). There is also a well-established scientific literature that has used functional analysis of skilled forelimb movements in rats in a wide spectrum of neurological disease models (Muir and Webb, 2000; Webb and Muir, 2005). Since both skilled and unskilled forelimb tasks and locomotion relate to tissue damage in the gray and white matter after cervical SCI, we hypothesized that behavior outcome measures from each category could be used to evaluate the protective potential of novel therapeutic compounds.
An emerging and somewhat controversial literature suggests that female hormones are protective in SCI. Upon comparing the effects of SCI in male and female rodents, female rats and mice had less tissue damage and improved hindlimb locomotor function versus male counterparts (Hauben et al., 2002; Farooque et al., 2006). The receptors for 17β-estradiol are present in neurons and glia (Burke et al., 2000; Vanderhorst et al., 2005) in the adult spinal cord and have been shown to be upregulated in lumbar motor neurons after axotomy (Islamov et al., 2003), but the expression remains relatively unchanged in the spinal cord after SCI (Kachadroka et al., 2010). Indeed, 17β-estradiol signaling can affect all cell types in the spinal cord via several signaling mechanisms that can work alone or in concert to reduce secondary damage after SCI. For example, treatment of male mice with 17β-estradiol reduced neutrophil infiltration, as indicated by reduced myeloperoxidase activity, reduced inflammatory cytokines/chemokines, and reduced cyclo-oxygenase-2 expression (Cuzzocrea et al., 2008). Similarly, administration of 17β-estradiol after SCI decreased infiltration of macrophages and microglia, decreased myelin loss, and reduced translocation of the inflammatory transcription factor nuclear factor kappa B (NF-κB) from the cytosol to the nucleus (Sribnick et al., 2005). We have previously demonstrated that 17β-estradiol reduced apoptosis, increased gray and white matter sparing, and promoted functional recovery after SCI in female and reproductively senescent female rats (Chaovipoch et al., 2006) and in adult male rats (Kachadroka et al., 2010; Olsen et al., 2010). Similarly, others have shown in midthoracic models that 17β-estradiol reduced lesion size, reduced apoptotic cell death, and increased expression of the antiapoptotic gene B-cell lymphoma 2 (Bcl-2) via phosphatidylinositol 3-kinase-dependent cAMP response element-binding (CREB) activation (Yune et al., 2004, 2008; Sribnick et al., 2006; Cuzzocrea et al., 2008). However, the protective effects of post-SCI administration have never been evaluated in a cervical model of SCI. Thus, to more completely assess the protective potential of this novel therapeutic intervention the objective of this study was to evaluate the effects of 17β-estradiol administered as a slow-release pellet at a clinically relevant does acutely post-SCI on functional recovery and histopathology in a cervical model of SCI, where both gray and white matter contribute to functional recovery.
Materials and Methods
Experimental groups
Adult, male Sprague–Dawley rats (Harlan, Indianapolis, IN; 275–300 g) were used in all experiments. Animals were group-housed in a temperature- and light-controlled (12/12-hour light/dark cycle) vivarium with standard rat chow and water available ad libitum. All procedures were in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. We evaluated the effects of post-SCI administration of 17β-estradiol on functional recovery and reduction of secondary injury following cervical SCI using 60 rats that were divided into five groups: 1) uninjured laminectomy control (Lam, n = 12); 2) SCI only, (SCI, n = 11); SCI + 17β-estradiol lower dose (E2 0.5, 17β-estradiol administered in a 21-day release 0.5 mg pellet, n = 12); SCI + 17β-estradiol higher dose (E2 5.0; 17β-estradiol administered in a 21-day release 5.0 mg pellet, n = 12); and 5) SCI + 17β-estradiol higher dose + estrogen receptor antagonist (ICI 182,780, ICI, n = 12). It is important to note that all doses of 17β-estradiol evaluated are in a range that is supraphysiological but that is not associated with readily observed untoward effects or overt toxicity (Kachadroka et al., 2010; Numakawa et al., 2011).
One rat in the SCI group was euthanized on post-SCI day 6 due to complications with the urinary bladder and was not included in the final analysis. No other mortality occurred in any treatment group. Experimentation was conducted in five cohorts of 12 animals randomized across experimental groups such that each cohort had animals from each experimental condition. The same experimenter conducted all behavioral analysis for the duration of the study and was naïve to the experimental group of each animal.
Induction of C5 hemicontusion SCI
Hemicontusion SCI at the vertebral level C5 was induced as previously described (Dunham et al., 2010). Briefly, all animals received hemicontusion injury on the side of the spinal cord ipsilateral to the dominant paw. Rats were anesthetized using 4% isoflurane in oxygen followed by a ketamine/xylazine cocktail (100/10 mg/kg, intraperitoneally [i.p.]). The cervical region was shaved and aseptically prepared for surgery and sterile ophthalmic ointment was applied to the eyes. During surgery and for 24 hours thereafter body temperature was maintained with a heating pad. After palpating the vertebral spinous processes to establish landmarks, a midline dorsal skin incision was made between the spinous processes of C2 and T2, the superficial musculature was incised at the midline, and the underlying paravertebral muscles of C4–C6 were removed. A bilateral laminectomy was performed at C5 to expose the dorsal aspect of the spinal cord. The spinal column was stabilized by clamping the vertebral bodies of C2 and T2 using toothed Adson forceps connected to supporting arms and a platform. The spinal cord was aligned under the 0.8-mm impactor tip so that the tip was entirely confined to the side ipsilateral to the dominant paw. Cervical hemicontusion injury was induced using the Infinite Horizon SCI device (Precision Systems and Instrumentation, Lexington, KY) with an intended force of 200 kdyne. Following contusion, musculature was sutured in layers with Vicryl absorbable suture (Vedco, St. Joseph, MO) and skin was sutured using nylon nonabsorbable suture (C.P. Medical, Portland, OR). Immediately after surgery and twice daily for 5 days thereafter, rats received subcutaneous injections of Ringer's solution (3 ml), enrofloxacin (2.5 mg/kg, Bayer HealthCare LLC, Shawnee Mission, KA), and carprofen (5 mg/kg, Pfizer, New York, NY).
Delivery of pharmacological compounds
In the subset of animals that received post-SCI administration of 17β-estradiol, the slow release pellet of either 0.5 or 5.0 mg 17β-estradiol was released over 21 days (Innovative Research of America, Sarasota, FL) and implanted subcutaneously in the midthoracic back at 30 minutes after SCI. This dose range is based on our previous work showing significant cytoprotection after SCI (Kachadroka et al., 2010; Olsen et al., 2010). Moreover, these doses produce 17β-estradiol serum blood levels (Cmax) that we have shown are similar to those in clinically available transdermal delivery systems (Kachadroka et al., 2010).
A subset of animals received the competitive estrogen receptor antagonist ICI 182,780 (Tocris, Ellisville, MO). This steroidal compound is classified as a pure estrogen receptor antagonist that inhibits estrogen receptor alpha and beta (ERα, ERβ) activation and is used both in preclinical and clinical research (Wakeling et al., 1991; Wakeling and Bowler, 1992). The ability of ICI 182,780 to cross the blood-brain barrier (BBB) is controversial, with several studies showing no BBB penetration (Wade et al., 1993a,b; Rivera and Eckel, 2010), while others demonstrate central nervous system (CNS) bioavailability or activity after peripheral administration (Alfinito et al., 2008). To avoid the potential confound of poor BBB penetration, ICI was delivered into the cerebral ventricles in sterile artificial cerebrospinal fluid (containing 150 mM Na+, 3.0 mM K+, 1.4 mM Ca2+, 0.8 mM Mg2+, 1.0 mM P+, and 155 mM Cl-) at a concentration of 600 fmol/day. This was achieved using a brain infusion cannula (Alzet brain infusion kit 2, Durect, Cupertino, CA) implanted into the right lateral ventricle (−2.0 mm anteroposterior, +2.6 mm lateral, 3.00 mm ventrodorsal) and attached to an Alzet osmotic minipump (infusion rate 0.25 μl/h, Durect). The cannula was inserted 7 days prior to SCI and the osmotic minipump was attached to the cannula 20 minutes post-SCI to initiate ICI 182,780 administration 10 minutes prior to administration of 17β-estradiol (i.e., pellet insertion). The ICI administration was terminated at 21 days post-SCI.
Behavioral assessments
Skilled forelimb function: vermicelli handling test
Skilled forelimb function was assessed using a modified version of the vermicelli handling test as described by Allred et al. (2008) and as we have previously described (Dunham et al., 2010). Briefly, animals were individually placed in home-cages and allowed to eat three pieces of 7-cm long uncooked vermicelli (New World Pasta, Harrisburg, PA) for each 10-minute trial. Trials were recorded with a Sony Handycam DCR-DRV280 to allow for slow-motion playback and evaluation of paw adjustments. Animals were assessed prior to SCI and on post-SCI day 6 and then every 7th day thereafter for the duration of the study. Data were recorded as the number of ipsilateral paw adjustments made per vermicelli piece averaged over three trials with adjustments defined as any grasp-regrasp motion or movement of the digits as scored by an evaluator who was naïve to the treatment group of each animal.
Skilled locomotor function: horizontal ladder test
Skilled locomotor function was assessed using the horizontal ladder test as previously described (Soblosky et al., 2001; Gensel et al., 2006; Dunham et al., 2010). The horizontal ladder was 129 cm long and 16.5 cm wide with rungs inserted 2.5, 3.2, or 5.7 cm apart. Rung positioning was varied weekly to prevent animals from learning the rung spacing pattern. The animal was positioned so that as it crossed the ladder the ipsilateral side could be recorded with a Casio Exilim EX-F1 camcorder at 1200 frames/sec to determine fore- and hindpaw placements. Three uninterrupted runs were collected per animal. Animals were assessed prior to SCI and on post-SCI day 8 and then every 7th day thereafter for the duration of the study. The animals' paw placements were analyzed by an evaluator who was naïve to the treatment group of each animal and categorized as: correct placement, touch, slip, or miss as previously described (Gensel et al., 2006; Dunham et al., 2010). The percentage of correct placements [(#correct/(#correct+slip+miss))*100] was analyzed as previously described (Gensel et al., 2006; Dunham et al., 2010).
Unskilled locomotor function: CatWalk gait analysis
The CatWalk gait analysis system (Noldus Information Technology, Leesburg, VA) was used as previously described by Hamers et al. (2006; Koopmans et al., 2005). Briefly, animals traversed a glass walkway (109 × 15 × 0.6 cm) with dark plastic walls (109 × 8 cm) spaced 15 cm length apart in a darkened room. Light from an encased fluorescent bulb was internally reflected within the glass walkway and scattered when the plantar surface of the paw contacted the walkway floor, thereby producing paw prints. Paw prints were recorded by a highspeed CCD camera mounted below the walkway at 50 half-frames/sec and were stored on a computer by the associated CatWalk 7.1 acquisition software. Animals were habituated to the walkway prior to surgical manipulation. A housing tube from the home cage was placed at the end of the walkway to promote uninterrupted walkway crossings, and trials in which the animal stopped or changed direction were excluded from subsequent analysis. Animals were assessed prior to SCI and on post-SCI day 9 and then every 7th day thereafter for the duration of the study. Three uninterrupted trials per day were analyzed and averaged to obtain a daily assessment value. Paw print designations were assigned and data were analyzed using the CatWalk analysis software (v. 7.1) by an evaluator who was naïve to the treatment group of the animal. The parameter analyzed, maximum paw print area, is defined as the area of the ipsilateral forepaw at maximal contact.
Histological analysis
Tissue preparation
On day 50 post-SCI animals were euthanized by an overdose of sodium pentobarbital euthanasia solution (50 mg/kg, i.p., Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) and then perfused intracardially with cold 0.1M phosphate-buffered saline (PBS, pH 7.4) for 5 minutes followed by 4% paraformaldehyde / 0.1M PB (PFA/PB, pH 7.4) for 20 minutes. The injury epicenter was marked with tissue marking dye (Triangle Biomedical Sciences, Durham, NC) and the spinal cord from segments C2–T2 was removed by gentle dissection. Spinal cord tissue was postfixed in PFA/PB at for 24 hours at 4°C and cryoprotected with 10% sucrose/0.1M PB for 1 hour at 4°C followed by 30% sucrose / 0.1M PB for 48 hours at 4°C. Tissue segments (3 mm in length) rostral, caudal, and at the lesion epicenter were blocked, embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA), and stored at −80°C until sectioning. A one-in-ten series of serial 30-μm transverse sections were obtained on a cryostat (Leica Microsystems, Bannockburn, IL) and thaw-mounted onto 1% gelatin-coated slides such that every tenth section was collected throughout the rostral–caudal axis of the epicenter (≈2 mm total tissue).
Cresyl violet histochemistry
Tissue sections were stained for Nissl substance using cresyl violet acetate (Sigma-Aldrich, St. Louis, MO.). Slide-affixed tissue sections were dehydrated through ascending ethanol concentrations (70–100%), cleared with xylene, rehydrated in ethanol, and stained in 0.1% cresyl violet acetate (Sigma-Aldrich) for 5 minutes. Tissue was rinsed in ddH2O and differentiated in 95% ethanol with acetic acid for 2 minutes, dehydrated with alcohol, washed in xylene, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
Luxol fast blue histochemistry
Tissue sections were stained with Luxol fast blue to visualize myelin. Slide-affixed tissue sections were dehydrated in ascending series of ethanol (70–100%), defatted with xylene, and then rehydrated in ethanol. Sections were stained for 6 hours at 30°C with 0.1% Luxol fast blue and 0.05% glacial acetic acid dissolved in 95% ethanol. Slides were then rinsed in 95% ethanol for 1 minute, washed with ddH2O for 30 seconds, then differentiated by 0.5% lithium carbonate for 2 minutes, followed by 70% ethanol for 1 minute. Sections were washed in ddH2O and counterstained with 1% aqueous cresyl violet acetate for 1 minute. The slides were then dehydrated through ascending ethanols, cleared with xylene, and coverslipped with Permount.
GFAP and CD11b immunohistochemistry
Astrocyte activation was assessed using immunohistological analysis of glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA; Table 1) with a goat antirabbit secondary antibody (AlexaFluor 488 Goat Anti-Rabbit IgG, 1:400; Invitrogen, Eugene, OR). Microglial/macrophage activation was assessed using a mouse antirat CD11b primary antibody (Millipore, Temecula, CA; Table 1) and a goat antimouse secondary antibody (AlexaFluor 594 Goat Anti-Mouse IgG, 1:400; Invitrogen). Sections were rinsed in 0.1M PB and endogenous peroxidase activity was blocked for 30 minutes. Sections were rinsed in PB followed by PBS. Background activity was blocked in a solution of 3% normal goat serum, 3% bovine serum albumin, and 0.3% Triton-X in PBS for 1 hour at 37°C. Sections were rinsed in PBS and incubated for 30 minutes at 37°C and then 24 hours at 4°C with primary antibody. Sections were rinsed in PBS and incubated with secondary antibody for 24 hours at 4°C. Slides were then rinsed in PBS followed by PB and air-dried for 1 hour. Slides were then coverslipped with DPX (Sigma-Aldrich).
Table 1.
Primary Antibodies
| Antigen | Immunogen | Manufacturer | Dilution used |
|---|---|---|---|
| Glial fibrillary acidic protein (GFAP) | Purified immunoglobulin fraction | Dako (Carpinteria, CA), rabbit polyclonal, #Z0334 | 1:4,000 |
| CD11b anti-integrin αM, clone OX-42 | Rat peritoneal macrophages | Chemicon/Millipore (Temecula, CA), mouse monoclonal, #CBL1512 | 1:20,000 |
Company, host species, catalog number and dilutions used are listed.
Antibody characterization
See Table 1 for a list of primary antibodies used. GFAP is a 50 kD intracytoplasmic filamentous protein that is commonly used as a specific marker for cells of astrocytic origin. The antibody shows one distinct precipitate (GFAP) in cow brain extract at 50 kD. The expression pattern and cellular morphology in the rat spinal cord seen here are like that reported in previous research (Benton et. al., 2008). The CD11b (anti-integrin aM) antibody has been identified in rat brain extracts by immunoblotting wherein the antibody precipitates three polypeptides of 150, 103, and 95 kDa. This CD11b antibody was used to identify microglial and macrophages and the expression pattern and cellular localization of microglial and macrophages in the rat spinal cord seen here are like that reported in previous research (Silvestroff et al., 2010).
Quantification of Histological Markers
Quantification of stereological probes
Unbiased stereological analysis was conducted on an Olympus BX-51 microscope linked to a MicroFire true color CCD digital camera (Optronics, Goleta, CA) using StereoInvestigator software (MicroBrightField, Williston, VT) at 200–400× magnification. The optical fractionator probe was used to determine the total number of neurons in the ventral horn at the lesion epicenter as previously described (West, 1991; Chaovipoch et al., 2006; Kachadroka et al., 2010; Dunham et al., 2010). For analysis of cresyl violet histochemistry, only neurons with an ovoid, spherical, triangular, or multipolar profile and a soma diameter larger than 10 μm with an intact cell membrane and a clearly defined nucleus were counted. Cells were counted in every tenth section throughout the rostral–caudal extent of the lesion (3 mm total tissue) in the ventral horn. All assessments were performed by an investigator naïve to the treatment of the animal. Estimates of surviving neurons in the contralateral and ipsilateral spinal cord ventral horns were determined independently of each other and were analyzed separately. For quantitative analysis of spared myelin, digital images of tissue processed with histochemistry for Luxol fast blue were captured as described above. Using StereoInvestigator software, contours of the entire tissue section and the intact white matter were placed by an investigator naïve to the treatment group of the animals for all sections in the one-in-ten series throughout the epicenter. The Cavalieri estimator was used to determine the area of intact white matter in each tissue section and the tissue section with the least spared white matter area was designated as the epicenter. The percentage of spared white matter area at the epicenter was expressed as a percentage of the total section area relative the last caudal section in the series to correct for cord collapse at the epicenter and calculated as [total adjusted hemicord area – (gray matter + damaged white matter area)]/total adjusted hemicord area. The percentages of intact white matter in the ipsilateral and contralateral hemicords were determined independently.
Quantitative analysis of fluorescence intensity
GFAP and CD11b relative fluorescence intensity were used to determine the extent of gliosis as previously described (Lin and Cai, 2001; Dunham et al., 2010). Digital images of tissue processed with immunohistochemistry for GFAP were captured with an Olympus BX-51 microscope (100–200×) linked to a MicroFire true color CCD digital camera (Optronics) using StereoInvestigator software (MicroBrightField). Using the Cavalieri probe, four 100 × 200 μm rectangular contours were placed in the ventral white matter and gray matter on the ipsilateral and contralateral hemicord in serial transverse sections throughout the rostral–caudal extent of the lesion epicenter (≈2 mm). The fluorescence intensity per contour was collected and averaged for each hemicord and the fold increase in fluorescence intensity was calculated by dividing the ipsilateral by contralateral values. The section with the highest ipsilateral fluorescence was designated the epicenter and the fluorescence of that section and the serial sections immediately rostral and caudal were averaged to obtain the fold increase in relative fluorescence at the epicenter for each animal. All assessments were conducted by an investigator naïve to the treatment group. All intensity values shown represent raw data, with the pixel intensities within the camera's dynamic range (0–255) without pixel saturation. All imaging parameters including gain, offset, and exposure time were set such that intensity values fell within the middle of the dynamic range to avoid pixel saturation. All images were captured with identical settings. Representative micrographs were collected in MicroBrightField and saved as JPEG images. Digital images were imported into Adobe Photoshop CS3 (v. 10.0, Adobe Systems, San Jose, CA) appropriately sized, tiled, and labeled for presentation in figures.
Statistical analysis
Data were analyzed using SigmaStat Advisory Statistical Software v. 3.5 (Systat, San Jose, CA) on a personal computer with significance set at alpha <0.05. All data are presented as mean ± SEM. Data for histological probes were analyzed using one-way analysis of variance (ANOVA) followed by a Tukey's post-hoc analysis and data for behavioral tests were analyzed using a two-way ANOVA followed by a Tukey's post-hoc analysis with the main effect of injury presented in the figures. Horizontal ladder error scores were analyzed using a Kruskal–Wallis two-way ANOVA on ranks followed by a Dunn's post-hoc analysis.
Results
Injury severity
The actual impact forces and tissue displacement measured by the force transducer of the spinal cord impactor were recorded immediately following injury (Table 2). All animals were impacted with the intended force of 200 kdyne and then randomly assigned to the treatment group. There were no significant differences in impact force or tissue displacement between treatment groups, which suggests that between-group differences in subsequent outcome measures are not attributable to differences in injury severity.
Table 2.
Injury Severity
| Group | n | Impact force (Kdyne) | Tissue displacement (lm) |
|---|---|---|---|
| Laminectomy control (Lam) | 12 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SCI control (SCI) | 11 | 213.3 ± 2.3 | 1224 ± 22.6 |
| SCI + E2 low dose (E2 0.5) | 12 | 211.2 ± 2.9 | 1220 ± 21.8 |
| SCI + E2 high dose (E2 5.0) | 12 | 208.3 ± 2.3 | 1231 ± 25.4 |
| SCI + E2 high dose + ICI 182,780 (ICI) | 12 | 214.3 ± 2.5 | 1222 ± 23.4 |
| All values presented as mean ± SEM |
Actual injury forces and tissue displacement values were obtained with the Infinite Horizons impactor. Mean values (± SEM) are reported according to group.
Effect of post-SCI administration of 17β-estradiol on surviving ventral horn neurons and white matter sparing
To assess the ventral horn neuronal survival, neurons in the ipsilateral and contralateral ventral horns were identified with cresyl violet histochemistry and quantified with stereology in ≈2 mm of tissue extracted from the epicenter of the SCI lesion (vertebral level C5). As seen in representative micrographs, laminectomy controls showed normal spinal cord anatomical structure and had similar numbers of neurons in the ipsilateral and contralateral ventral horns (Fig. 1A). Injured animals showed damage to the spinal cord including cystic cavities and fewer neurons confined to the ipsilateral side (Fig. 1B). Representative higher-magnification micrographs taken from the ipsilateral spinal cord from the SCI (Fig. 1C), lower- and higher-dose 17β-estradiol groups (Fig. 1D,E), and ICI group (Fig. 1F) show cystic cavities and neuronal cell bodies in the ventral horn. As seen in Figure 1G upon quantification with unbiased stereology, it was determined that significantly fewer neurons were counted in the ipsilateral ventral horn in all SCI groups as compared with the neurons counted in the uninjured control group. Significantly greater numbers of neurons were counted in the groups that received either the lower or higher dose of 17β-estradiol as compared with the SCI only group. Additionally, in the group that received coadministration of ICI 182,780 with higher-dose 17β-estradiol (ICI group), significantly fewer neurons were counted in the ipsilateral ventral horn than in the 17β-estradiol lower- or higher-dose groups, suggesting that antagonism of the estrogen receptor blocked the effect of 17β-estradiol on neuronal survival. Neuronal numbers from the contralateral ventral horn were also evaluated and no significant differences were observed between any treatment groups (Fig. 1H).
Figure 1.
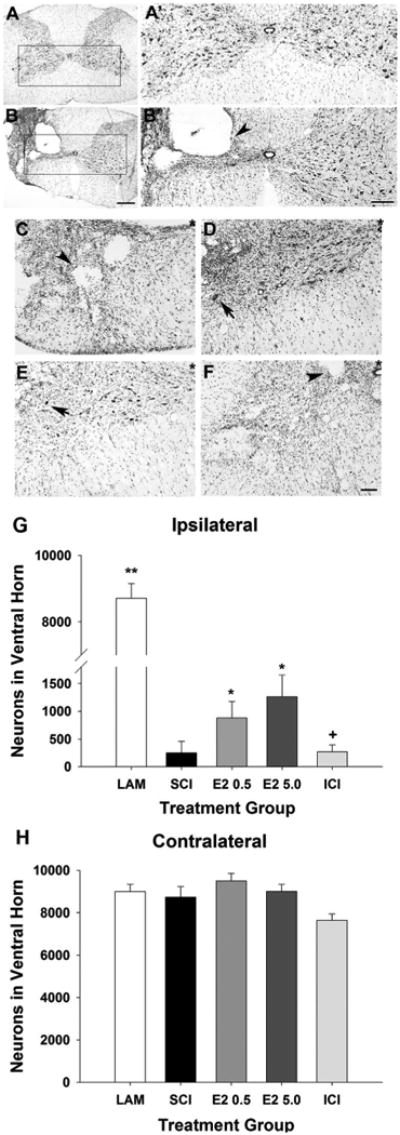
Effect of post-SCI administration of 17β-estradiol on neuronal numbers in the ipsilateral ventral horn. Ventral horn neurons were identified using cresyl violet histochemistry and morphology as described in Materials and Methods. Micrographs of representative transverse sections of spinal cord tissue from uninjured control group (A) and SCI group (B) are shown. Higher-magnification micrographs of the boxed regions are shown in A′ from the uninjured control group and B′ from the SCI group. Cystic cavities (arrowhead) were seen only in spinal cord tissue ipsilateral to the contusion. Higher-magnification representative micrographs of the ipsilateral ventral horns aligned by the central canal (indicated by *) from the SCI (C), E2 0.5 (D), E2 5.0 (E), and ICI (F) treatment groups demonstrate cystic cavities (arrowheads) and neuronal cell bodies (arrow). Quantification of the number of neurons in the ipsilateral ventral horn (G) demonstrated that significantly more neurons were counted in the E2 0.5 and E2 5.0 groups than the SCI group (indicated by *). Significantly less neurons were counted in the ICI group than the E2 0.5 or E2 5.0 groups (indicated by +). Quantification of the number of neurons in the contralateral ventral horn (H) revealed no significant differences between treatment groups. Scale bars = 400 μm in A,B; 200 μm in A′,B′; 50 μm in C–F.
To evaluate white matter sparing at the epicenter of the SCI lesion (vertebral level C5), histochemistry using Luxol fast blue (LFB) was performed to visualize intact myelin (Fig. 2). As seen in the representative micrographs, uniform LFB staining was observed throughout the white matter in uninjured controls and SCI induced a loss of LFB staining in the hemicord ipsilateral to the contusion. Representative higher-magnification micrographs taken from the ipsilateral spinal cord from the SCI (Fig. 2C), higher-dose 17β-estradiol (Fig. 2D), and ICI (Fig. 2E) groups show the border between damaged and intact myelin. The area of intact myelin as a percentage of the adjusted total area (percentage spared white matter area) was calculated for the ipsilateral and contralateral spinal cord. As seen in Figure 2F, we found that the uninjured control group (Lam) had a significantly greater percentage of white matter area as compared with all SCI groups. In the animals that received the higher dose of 17β-estradiol (E2 5.0), the ipsilateral white matter sparing was significantly greater than that of the SCI only group. In the animals that received the lower dose of 17β-estradiol there was a nonsignificant increase in the percentage of white matter area as compared with the SCI-only group. Similarly, in the group that received coadministration of estrogen receptor antagonist ICI 182,780 with 17β-estradiol (ICI), the percentage of white matter was not significantly different from the SCI + E2 higher dose (E2 5.0) group, although a nonsignificant trend toward reduction in white matter area was observed. Contralateral white matter area was quantified and no significant differences between any groups were observed (data not shown). These data indicate that post-SCI administration of the higher dose of 17β-estradiol induces white matter sparing and that this effect is not significantly attenuated by antagonism of the estrogen receptor.
Figure 2.
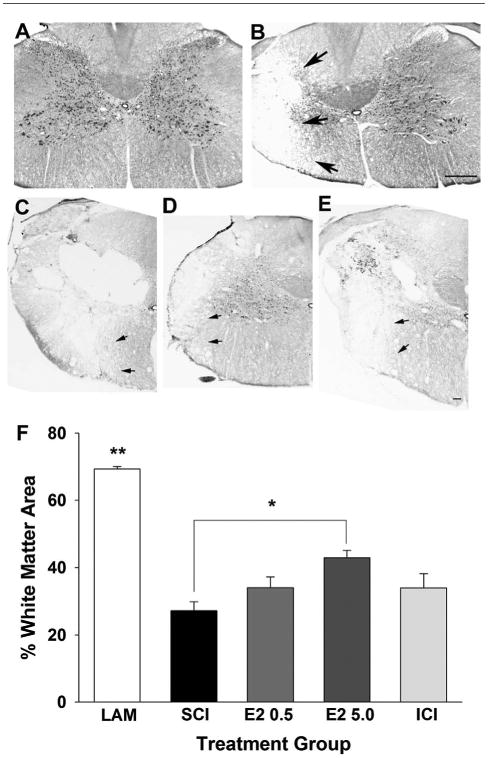
Effect of post-SCI administration of 17β-estradiol on white matter area in the ipsilateral cord. Myelin was identified by Luxol fast blue histochemistry and intact myelin was delineated at the epicenter of the SCI lesion. Micrographs of representative transverse sections of spinal cord tissue from the uninjured control group (A) and the SCI group (B) are shown with arrows depicting the border between intact and damaged myelin. Higher-magnification representative micrographs of the ipsilateral spinal cord aligned by the central canal from the SCI (C), E2 5.0 (D), and ICI (E) groups demonstrate the border of damaged white matter (arrows). The percentage of intact white matter area at the epicenter of the SCI lesion was quantified (F). A greater percentage of intact white matter area was observed in the uninjured control laminectomy group (Lam) than in any other group (indicated by **). Additionally, the E2 5.0 group had a significantly greater percentage of intact white matter as compared with the SCI group (indicated by *). No other statistically significant differences between treatment groups were found. Scale bars = 400 μm in A,B; 150 μm in C–E.
To summarize the effect of post-SCI administration of 17β-estradiol on tissue sparing with regard to motor neuron pools and white matter tracts associated with forepaw function, we selected representative micrographs from the epicenter of the lesion and traced the border between damaged and intact tissue from the SCI and higher-dose 17β-estradiol groups. These tracings were then overlaid on an appropriately scaled anatomical atlas representation of the spinal cord at level C5 depicting motor neurons pools and white matter tracts associated with forelimb function (adapted from Kuchler et al., 2002). As seen in Figure 3, the damaged tissue area was reduced in the higher-dose 17β-estradiol group as compared with the SCI group and this reduction in damaged tissue area included both a reduction in damage in gray matter neuronal pools as well as white matter tracts associated with forelimb function.
Figure 3.
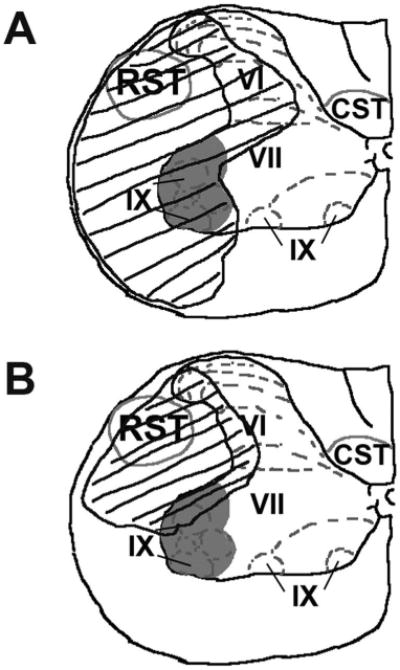
Summary of damage in gray and white matter regions in epicenter of lesion in the ipsilateral spinal cord. The area of damaged tissue was traced in representative micrographs processed with cresyl violet and Luxol fast blue histochemistry using Neurolucida software from the SCI group (A) and the E2 5.0 group (B). The tracings were then fitted onto an anatomical atlas representation of the spinal cord at level C5 depicting motor neuron pools (gray shading) and white matter tracts (gray outline) associated with forelimb function (adapted from Kuchler et al., 2002). The damaged tissue area is depicted by the black outline and hashes. A reduction in damage in gray matter neuronal pools and white matter tracts associated with forelimb function was seen in the group that received post-SCI administration of 17β-estradiol. RST, rubrospinal tract; CST, corticospinal tract. Rexed's laminae VI, VII, and IX are indicated.
Effect of post-SCI administration of 17β-estradiol on microglial activation and reactive astrogliosis
To evaluate microglial activation, immunohistochemistry for CD11b was conducted in adjacent tissue sections from the epicenter of the lesion (vertebral level C5). CD11b is present on the membranes of microglia and macrophages/monocytes and was used to evaluate increased numbers and activation of microglia and macrophages. CD11b immunoreactivity was quantified by measuring normalized ipsilateral fluorescence intensity (ipsilateral/contralateral intensity). Uninjured control tissue showed minimal CD11b immunoreactivity throughout the spinal cord (Fig. 4A). However, after SCI a substantial increase in CD11b immunoreactivity was detected ipsilateral to the contusion (Fig. 4B). Representative higher-magnification micrographs taken from the ipsilateral spinal cord from the SCI (Fig. 4C), higher-dose 17β-estradiol (Fig. 4D) and ICI (Fig. 4E) groups show individual and clusters of CD11b-immunoreactive cells. Upon quantification of CD11b immunoreactivity (Fig. 4F), a significant increase in CD11b immunoreactivity in the SCI group as compared with uninjured controls (Lam) was observed. Significantly reduced CD11b immunoreactivity was observed in the animals that received post-SCI administration of the higher dose of 17β-estradiol as compared with the SCI group. We also found in animals that received coadministration of 17β-estradiol and ICI 182,780 (ICI) significantly greater CD11b immunoreactivity as compared with the group that received the higher dose of 17β-estradiol. Taken together, these data show that post-SCI administration of 17β-estradiol at the higher dose attenuates microglial activation following cervical SCI and implicate the estrogen receptor in this process.
Figure 4.
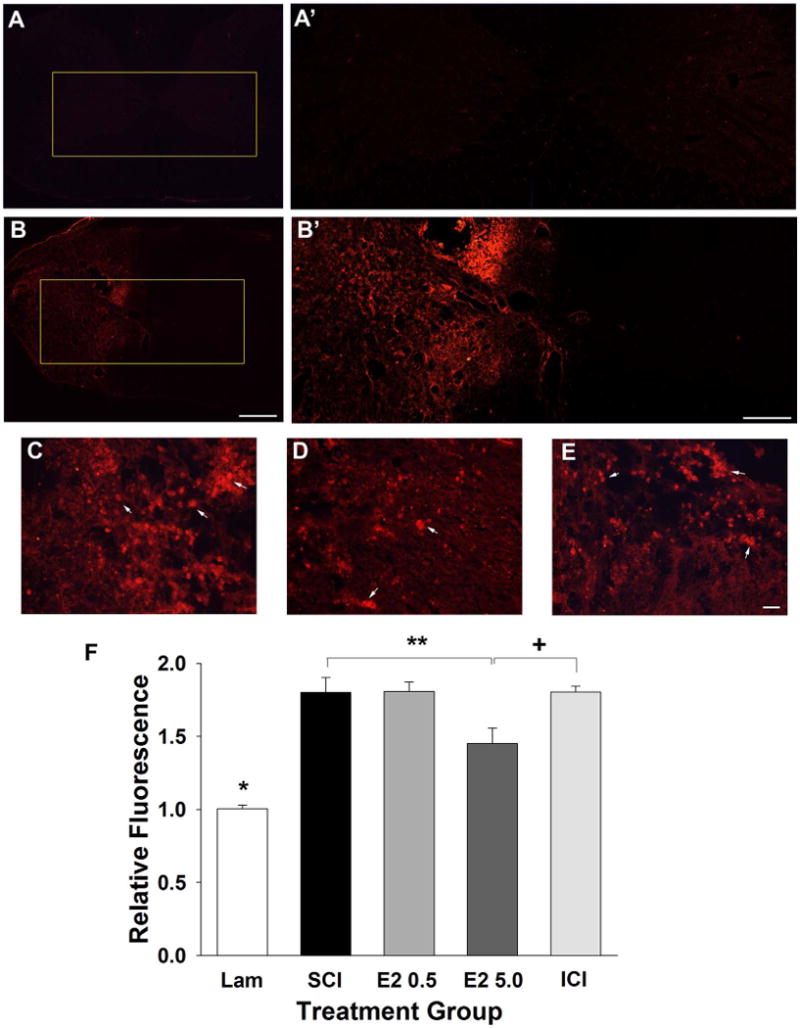
Effect of post-SCI administration of 17β-estradiol on microglial activation in the ipsilateral spinal cord. Microglial activation was evaluated by assessment of CD11B immunoreactivity and micrographs from representative transverse tissue sections at the epicenter of the lesion are shown from the uninjured group (A) and the SCI group (B). Higher magnification micrographs of the boxed regions are shown in A′,B′. Higher-magnification representative micrographs aligned by the central canal from the SCI (C), E2 5.0 (D), and ICI (E) groups demonstrate activated microglial cells (arrows) clustered throughout the ipsilateral spinal cord. Quantification of normalized relative fluorescence intensity of CD11b immunoreactivity is shown in F. The Lam group had a significantly lower relative fluorescence intensity than all SCI groups (indicated by *). A significant difference in fluorescence intensity was found between the SCI and E2 5.0 groups (indicated by **) and between the E2 5.0 and ICI groups (indicated by +). Scale bars = 400 μm in A,B; 200 μm in A′,B′; 20 μm in C–E. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To evaluate the effect of post-SCI administration on reactive astrogliosis, GFAP fluorescent immunohistochemistry was conducted and the extent of astrogliosis at the epicenter of the lesion is reported as normalized ipsilateral fluorescence (ipsilateral/contralateral intensity). As visualized in representative micrographs, modest GFAP immunoreactivity was detected in transverse tissue sections from the uninjured controls (Fig. 5A) and the GFAP immunoreactivity was markedly increased in the ipsilateral hemicord after SCI (Fig. 5B). Representative higher-magnification micrographs taken from the ipsilateral spinal cord from the SCI (Fig. 5C), higher-dose 17β-estradiol (Fig. 5D) and ICI (Fig. 5E) groups show marked GFAP immunoreactivity around cystic cavities. Quantification of relative fluorescence intensity of the GFAP immunoreactivity (Fig. 5F) indicated that there was a significant up-regulation of GFAP immunoreactivity in the SCI group as compared with uninjured control group (Lam). It was found in animals administered the higher dose of 17β-estradiol (E2 5.0) that there was a significant reduction in the GFAP immunoreactivity as compared with the SCI-only group (SCI). Additionally, in animals that received the coadministration of the antagonist of ICI 182,780 with higher-dose 17β-estradiol (ICI) there was a significant increase in GFAP immunoreactivity as compared with the 17β-estradiol higher-dose group, which suggests that antagonism of the estrogen receptor attenuated estrogen-mediated reduction in reactive astrogliosis.
Figure 5.
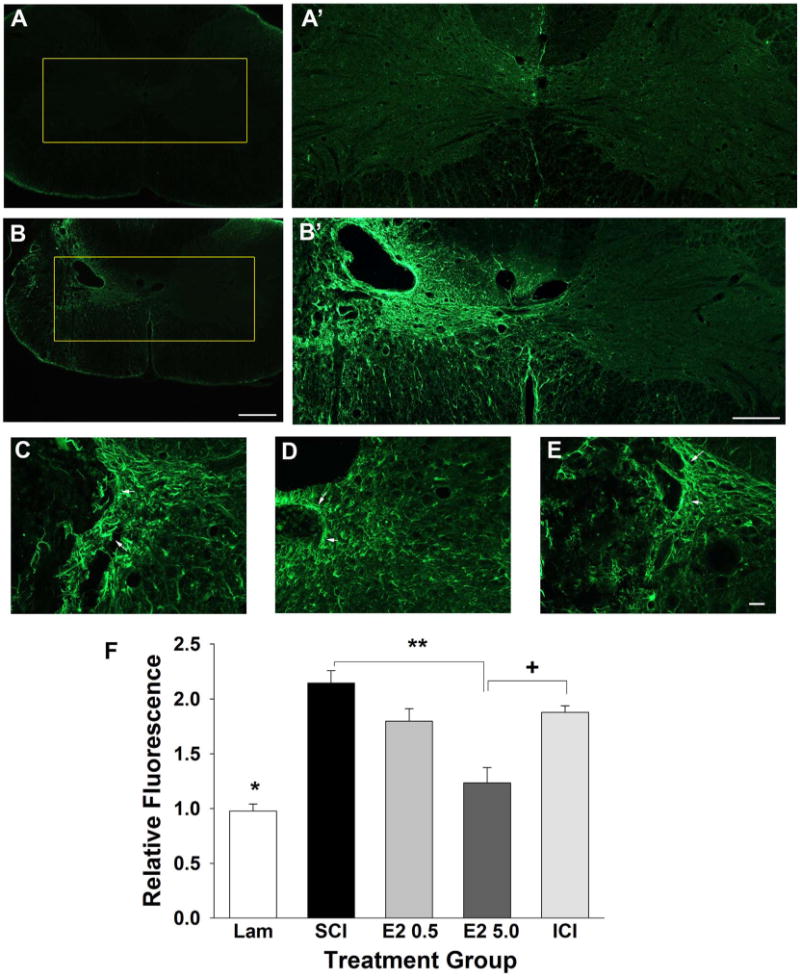
Effect of post-SCI administration of 17β-estradiol on reactive astrogliosis in the ipsilateral spinal cord. Reactive astrogliosis was quantified by GFAP immunoreactivity and micrographs from representative transverse tissue sections at the epicenter of the lesion are shown from the uninjured group (A) and the SCI group (B). Higher-magnification micrographs of the boxed regions are shown in A′ from the uninjured control group and B′ from the SCI group. Higher-magnification representative micrographs aligned by the central canal from the SCI (C), E2 5.0 (D), and ICI (E) groups demonstrate reactive astrogliosis (arrows). Quantification of normalized relative fluorescence intensity of GFAP immunoreactivity (F) demonstrated that the uninjured group had significantly lower intensity than all SCI groups (indicated by *). Also, we found that relative fluorescence intensity was significantly reduced in the E2 5.0 group as compared with the SCI group (indicated by **). The relative fluorescence intensity was significantly increased in the ICI group as compared with the E2 5.0 group (indicated by +). Scale bars = 400 μm in A,B; 200 μm in A′,B′; 20 μm in C–E. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Effects of post-SCI administration of 17β-estradiol on skilled forelimb function
To evaluate the effect of post-SCI administration of 17β-estradiol on skilled forelimb function, the vermicelli handling test was used (Allred et al., 2008; Dunham et al., 2010). This test assesses the ability of an animal to make skilled forepaw manipulations through quantification of forepaw adjustments. As shown in Figure 6A, we found that prior to injury animals made ≈10–12 ipsilateral paw adjustments per pasta piece and animals in the laminectomy control group (Lam) continued to make this number of ipsilateral paw adjustments throughout the duration of the experiment. We also observed that animals in the SCI group exhibited a significant decrease in the number of ipsilateral paw adjustments per piece as compared with the Lam group on all post-SCI days evaluated. The number of adjustments per piece for animals in the lower-dose 17β-estradiol group (E2 0.5) did not differ significantly when compared with the SCI group, but a non-significant increase was observed. However, as seen in Figure 6B, animals that received post-SCI administration of the higher dose of 17β-estradiol (E2 5.0) made significantly more ipsilateral paw adjustments per piece as compared with the SCI group on postinjury weeks 2–7. Also, significant differences were observed between the ICI group and the higher-dose 17β-estradiol groups on post-SCI weeks 2–7 but no differences between the ICI group and SCI were found. Contralateral adjustments and time to eat were also recorded; however, no significant differences were seen between any groups (data not shown). Taken together, these data indicate that higher-dose 17β-estradiol significantly increases skilled ipsilateral forelimb function following injury and that this effect can be blocked by antagonism of the estrogen receptors.
Figure 6.
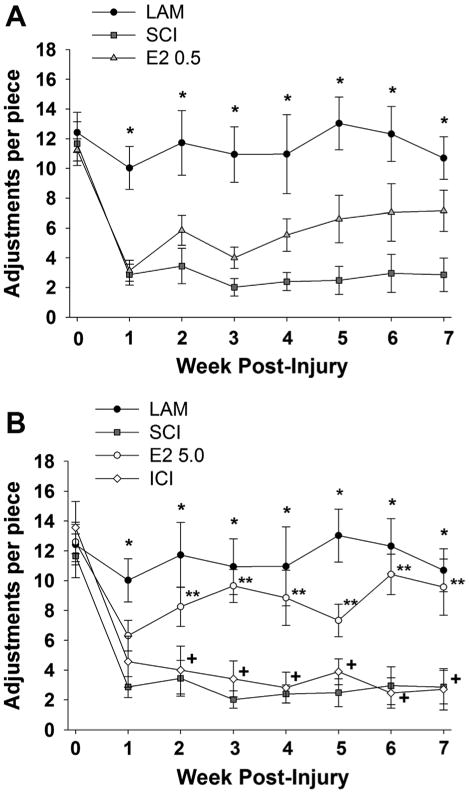
Effect of post-SCI administration of 17β-estradiol on skilled forelimb function. Skilled forelimb function was assessed by the vermicelli handling test. Quantification of the ipsilateral forepaw paw adjustments per piece at baseline (day 0) and on post-SCI days 7–49 are shown for the treatment groups. A: At baseline animals made 10–12 adjustments per pasta piece and animals in the Lam group continued to make this number of adjustments throughout the duration of the experiment. A significant decrease in the adjustments per piece on post-SCI weeks 1-7 was observed in the SCI group compared with the Lam (indicated by *). No significant differences in adjustments per piece were observed between the E2 0.5 group and the SCI only group. B: A significant increase in the adjustments per piece was observed in the E2 5.0 group as compared with the SCI group on post-SCI weeks 2–7 (indicated by **). Animals in the ICI group made significantly fewer adjustments per piece on post-SCI weeks 2–7 as compared with the E2 5.0 group (indicated by +). No significant differences between the SCI and ICI groups were observed. Note that data from Lam and SCI groups are duplicated from (A) for comparison.
Effect of post-SCI administration of 17β-estradiol on skilled locomotor function
To evaluate the effect of post-SCI administration of 17β-estradiol on skilled locomotor behavior, the horizontal ladder test was used (Soblosky et al., 2001; Gensel et al., 2006; Dunham et al., 2010). In this assessment animals traverse a series of rungs spaced at varying intervals and the placement and use of the forepaw on the ladder rungs is evaluated. As described previously (Gensel et al., 2006; Dunham et al., 2010), the percent of correct forepaw placements was calculated. We found that prior to SCI all rats had similar percentages of correct forelimb placements (Fig. 7A). The percent of correct placements was near 100% in the uninjured control group (Lam) on all days evaluated and a significant reduction in the percentage of correct placements was observed in the SCI group at all post-SCI timepoints as compared with the Lam group. In animals that received post-SCI administration of the lower dose of 17β-estradiol (E2 0.5) a significant increase in the percentage of correct placements versus the SCI group at the 2- and 7-week post-SCI evaluation time was found, with a nonsignificant trend toward improvement at all other post-SCI timepoints (Fig. 7A). In animals that received post-SCI administration of the higher dose of 17β-estradiol (E2 5.0) a significant increase in the percentage of correct paw placements on post-SCI week 7 was observed, with a nonsignificant trend toward improvement on all other post-SCI time-points (Fig. 7B). In the group of animals that received coadministration of the higher dose of 17β-estradiol and the estrogen receptor antagonist ICI 182,780 (ICI group), a significant reduction in the percentage of correct paw placements as compared with the 17β-estradiol higher-dose group (E2 5.0) was observed on post-SCI weeks 6 and 7, with a nonsignificant trend toward reduction at all other post-SCI timepoints. Although not robust, collectively these data suggest that post-SCI administration of 17β-estradiol improves skilled locomotor function. Importantly, large within-group variability in the animals' performance was observed at multiple timepoints which potentially diluted possible treatment effects.
Figure 7.
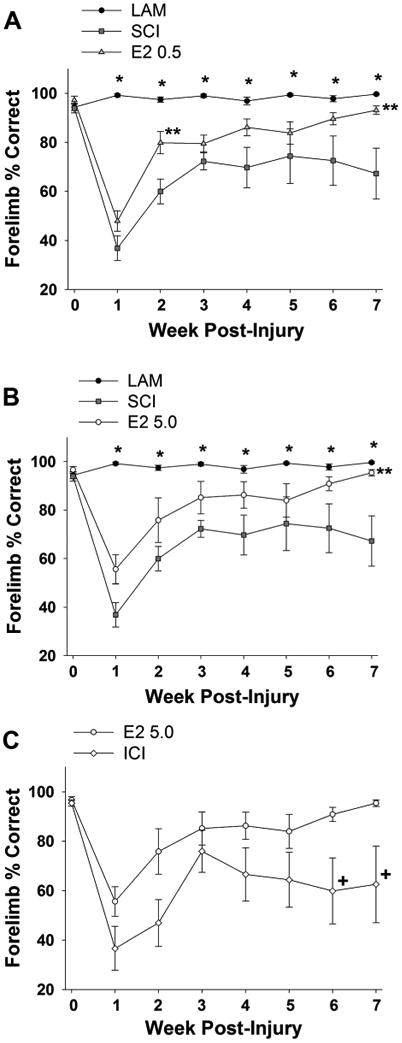
Effect of post-SCI administration of 17β-estradiol on skilled locomotion. Skilled locomotion was assessed by the horizontal ladder test and quantified as percent correct forepaw placement at baseline (day 0) and on post-injury days 7–49. A: At baseline all animals had nearly 100% correct paw placements and animals in the Lam group maintained near this percentage for the duration of the experiment. A significant reduction in the percent correct paw placements was observed in the SCI group versus the Lam group on post-SCI days 7–49 (indicated by *). A significant increase in the percent of correct paw placements was observed in the E2 0.5 group as compared with the SCI group on post-SCI day 14 and 49 (indicated by **). B: A significant increase in the percent correct paw placements was found in the E2 5.0 group as compared with the SCI group on post-SCI day 49 (indicated by **). Note that data from Lam and SCI groups are duplicated from (A) for comparison. C: A significant decrease in the percent correct paw placements was observed in the ICI group as compared with the E2 5.0 group on post-SCI days 42 and 49 (indicated by +). Note that data from the E2 high group are duplicated from (B) for comparison.
Effect of 17β-estradiol on unskilled locomotor function
To evaluate the effect of post-SCI administration of 17β-estradiol on overground locomotion, the CatWalk gait analysis system was used to analyze digitally rendered paw prints as previously described (Gensel et al., 2006; Hamers et al., 2006; Dunham et al., 2010). The mean maximum paw print area of the ipsilateral forepaw was analyzed. As seen in Figure 8, the maximum paw print area was significantly larger for the uninjured control group (Lam) than for any SCI group on all post-SCI timepoints evaluated. In animals that received post-SCI administration of 17β-estradiol in either the lower or higher dose, no significant alterations in the maximum print area were observed as compared with the SCI group, suggesting that post-SCI administration of 17β-estradiol did not alter ipsilateral forepaw usage in unskilled locomotion. Also, in animals that received coadministration of higher dose 17β-estradiol and the estrogen receptor antagonist ICI 182,780 (ICI group) no significant alterations in maximum paw print area as compared with the SCI-only group were found. Other kinematic parameters including regularity index and duty cycle were evaluated without significant differences between treatment groups (data not shown). These data suggest that post-SCI administration of 17β-estradiol did not affect paw usage in this unskilled locomotion task.
Figure 8.
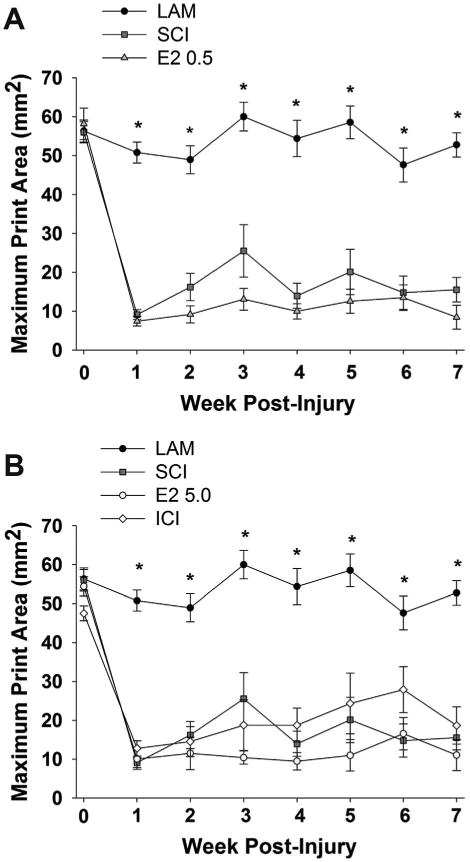
Effect of post-SCI administration of 17β-estradiol on unskilled locomotion. Unskilled locomotion was assessed with the CatWalk gait analysis system and the maximum paw print area for the ipsilateral forepaw was compared between treatment groups at baseline (day 0) and on post-SCI days 7–49. A: A significantly lower maximum print area on all days post-SCI was observed in the SCI group as compared with the Lam group (indicated by *). No significant difference in the maximum paw print area was observed in comparison of the E2 0.5 group and the SCI group. B: No significant differences in the maximum paw print area were observed between the E2 5.0 group and the SCI group on any day. Additionally, no significant differences between the E2 high group and the ICI group were observed on any day. Note that data from Lam and SCI groups are duplicated from (A) for comparison.
Discussion
The goal of this study was to evaluate the effect of post-SCI administration of 17β-estradiol in a newly characterized C5 hemicontusion model (Dunham et al., 2010). We found that post-SCI administration of the higher dose of 17β-estradiol reduced neuronal death in the ventral horn, increased white matter sparing, and reduced gliosis at the lesion epicenter. We also found that the higher dose of 17β-estradiol improved performance on a skilled forepaw test and trended to improve performance in a skilled locomotor task. Thus, we report that post-SCI administration of 17β-estradiol conferred protection in the gray and white matter after a cervical hemicontusion SCI with associated improvements in functional recovery.
Although this is the first report of estrogen-mediated protection in cervical SCI, the protective effects of 17β-estradiol in both the gray and white matter have been both shown in several SCI models conducted at midthoracic levels (Sribnick et al., 2005; Chaovipoch et al., 2006; Kachadroka et al., 2009) as well as disputed (Swartz et al., 2007). However, behavioral recovery on locomotor tasks in midthoracic models of SCI is based on white matter sparing and gray matter preservation does not contribute to functional recovery. Thus, our evaluation of the effects of post-SCI administration of 17β-estradiol on male rats in a model where both gray and white matter sparing contribute to recovery of function more closely mimics the clinical reality than previous work. We found that post-SCI administration of 17β-estradiol induced a significant increase in the number of surviving ipsilateral ventral horn neurons, which is consistent with our previous findings (Chaovipoch et al., 2006; Kachadroka et al., 2010). Intriguingly, we also found that many aspects of estrogen-mediated protection (neuronal survival, reduction in gliosis, and inflammation) could be blocked by coadministration with the estrogen receptor antagonist, which suggests that this is an estrogen receptor-dependent mechanism. Several estrogen receptor-dependent mechanisms of protection have been proposed including activation of the estrogen response element to increase expression of the antiapoptotic bcl-2 gene (Dong et al., 1999). Increased expression of bcl-2 expression by estrogen has been shown to be associated with a reduction of apoptotic cell death after SCI (Yune et al., 2004, 2008; Cuzzocrea et al., 2008; Kachadroka et al., 2009). Activation of estrogen receptors can also induce expression of brain derived-neurotrophic factor (BDNF) (Sohrabji et al., 1995) and increases in BDNF levels have been shown to have protective effects in vitro (Aguirre and Baudry, 2009) and in vivo after SCI (Nakajima et al., 2007). Alternatively, several cytoprotective effects mediated via estrogen receptor-independent mechanisms have been proposed for estrogen-mediated protection including inhibition of L-type calcium channels (Sribnick et al., 2009) or N-methyl-D-aspartic acid (NMDA) receptors (Weaver et al., 1997), antioxidative actions (Winterle et al., 2001; Prokai and Simpkins, 2007), and protection against mitochondrial dysfunction (Nilsen and Diaz, 2003; Simpkins and Dykens, 2008; Arnold and Beyer, 2009). However, our data suggest that the main protective effects are estrogen receptor-mediated.
In addition to the effects of 17β-estradiol on neurons, we found that this treatment increased white matter sparing, which is in agreement with other literature (Sribnick et al., 2005; Chaovipoch et al., 2006; Cuzzocrea et al., 2008; Kachadroka et al., 2010), but the exact mechanisms remain unclear. Following SCI, oligodendrocytes may die from both primary and secondary injury (Beattie et al., 2002; Park et al., 2004; McTigue and Tripathi, 2008). Treatment with 17β-estradiol has also been shown to protect oligodendrocytes against cytotoxicity in vitro (Cantarella et al., 2004; Takao et al., 2004). Activated microglia can induce oligodendrocyte apoptosis (Shuman et al., 1997; Li et al., 2005) and might contribute to the death of oligodendrocytes after SCI. We also report that post-SCI administration of 17β-estradiol significantly reduces the observed injury-induced increase in GFAP reactivity at day 50 post-SCI and that coapplication of an estrogen receptor blocker attenuated this effect. While this finding is consistent with estrogen-mediated protection after SCI, it is in contrast to a previous report that 17β-estradiol significantly increased GFAP expression (Ritz and Hausmann, 2008). This inconsistency may be due, in part, to the duration of estrogen treatment (single injection immediately after SCI vs. continuous release over 21 days), differences in lesion location and severity, or differences in evaluation of gliosis. Lastly, we also report that post-SCI administration of 17β-estradiol significantly attenuates the injury-induced increase in CD11b immunoreactivity that we interpret as a reduction in the inflammatory cascade. Although the role of inflammation in secondary injury and recovery after SCI remains an area of active discussion and research (Alexander and Popovich, 2009), several inflammatory responses are hypothesized to contribute to secondary injury after SCI, and applying antiinflammatory treatments can reduce cell death and improve functional recovery (Stirling et al., 2004; Festoff et al., 2006). Consistent with our finding that post-SCI administration of 17β-estradiol reduces inflammation, other groups have reported antiinflammatory effects of 17β-estradiol in SCI. Cuzzocrea et al. (2008) found that 17β-estradiol treatment pre- and post-SCI reduced neutrophil accumulation and expression of several cytokines including tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 at the injury epicenter. Ritz and Hausmann (2008) revealed that a supraphysiological single dose injection of 17β-estradiol limited lesion spreading and reduced inflammatory cell number at 1 week post-SCI. Sribnick et al. (2005) reported that post-SCI 17β-estradiol treatment reduced the number of macrophages/activated microglia at the injury epicenter as well as caudal penumbra.
With regard to the behavioral assessments evaluated in this study, the data that C5 hemicontusion SCI significantly altered skilled forepaw function is consistent our previous study (Dunham et al., 2010) and with other reports in the literature using this test to assess functional recovery after unilateral middle cerebral artery occlusion, electrolytic somatosensory cortex lesion, or striatal dopamine depleting lesion (Allred et al., 2008). Intriguingly, we also found that the post-SCI administration of the higher dose of 17β-estradiol significantly enhances functional recovery as evaluated by this test. In addition, coadministration of ICI 182,780 with higher-dose E2 attenuated this effect, which suggests that this effect is mediated through estrogen receptor mechanisms. It is notable that this improvement in functional recovery corresponded with the histological findings that higher-dose 17β-estradiol significantly increased the number of surviving ventral horn neurons and reduced white matter damage in regions associated with forelimb function. Gripping and regripping a piece of vermicelli requires digit extension and flexion and injury to the C5/6 spinal cord directly affects extensor muscles that are crucial in skilled manipulations of the forepaw. As the lesion spreads to incorporate motor neuron pools in the adjacent lower cervical spinal cord paw flexors will be affected as well. Therefore, 17β-estradiol-mediated protection as reflected by improvements in skilled paw manipulations is likely due to a combination of protection of motor neurons at the level of the lesion and to the ability of 17β-estradiol to limit secondary damage and subsequent spreading of lesion to the adjacent gray matter.
The clinical relevance of this study stems both from the model used and the therapeutic evaluated. In this study we used a C5 hemicontusion model in adult male rats. This model more closely mimics the majority of clinical SCIs by including both gray and white matter pathophysiology, which both contribute significantly to functional deficits. Using this animal model of SCI, we demonstrate that acute post-SCI administration of a slow-release and clinically relevant supraphysiological dose of 17β-estradiol confers protection and promotes functional recovery after SCI. Since 17β-estradiol is currently approved in several formulations for human use, translation of these findings into clinical trials may yield a promising novel treatment strategy for patients with both gray and white matter injury.
Acknowledgments
The authors thank Nicole Day for the initial behavioral evaluations and Malisa Girard for the final revisions.
Grant sponsor: National Institutes of Health (NIH); Grant number: NS052559 (to C.F; Grant number: K.D.); Grant sponsor: Civitan Emerging Scholar Award (to K.D.); Thailand Commission on Higher Education Staff Development Project (to A.S.); Siriraj Graduate Thesis Scholarship (to A.S.).
Literature Cited
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Alfinito PD, Chen X, Atherton J, Cosmi S, Deecher DC. ICI 182,780 penetrates brain and hypothalamic tissue and has functional effects in the brain after systemic dosing. Endocrinology. 2008;149:5219–5226. doi: 10.1210/en.2008-0532. [DOI] [PubMed] [Google Scholar]
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170:229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TE, Stokes BT. Experimental models for spinal cord injury research: physical and physiological considerations. J Neurotrauma. 1992;9(Suppl 1):S135–S142. [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol. 2009;220:9–22. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Basso DM. Neuroanatomical substrates of functional recovery after experimental spinal cord injury: implications of basic science research for human spinal cord injury. Phys Ther. 2000;80:808–817. [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Minnillo DR, Hagg T, Whittemore SR. Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J Comp Neurol. 2008;507:1031–1052. doi: 10.1002/cne.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Burke KA, Schroeder DM, Abel RA, Richardson SC, Bigsby RM, Nephew KP. Immunohistochemical detection of estrogen receptors alpha in male rat spinal cord during development. J Neurosci Res. 2000;61:329–337. doi: 10.1002/1097-4547(20000801)61:3<329::AID-JNR11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Risuglia N, Lombardo G, Lempereur L, Nicoletti F, Memo M, Bernardini R. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligoden-drocytic cell line: evidence for multiple sites of interactions. Cell Death Differ. 2004;11:503–511. doi: 10.1038/sj.cdd.4401367. [DOI] [PubMed] [Google Scholar]
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Esposito E, Di PR, Muia C, Crisafulli C, Peli A, Bramanti P, Chaudry IH. Effect of 17beta-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29:362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J Biol Chem. 1999;274:32099–32107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- Dunham KA, Siriphorn A, Chompoopong S, Floyd CL. Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J Neurotrauma. 2010;27:2091–2106. doi: 10.1089/neu.2010.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–187. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- Islamov RR, Hendricks WA, Katwa LC, McMurray RJ, Pak ES, Spanier NS, Murashov AK. Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush injury in ovariectomized mice. Brain Res. 2003;966:65–75. doi: 10.1016/s0006-8993(02)04191-4. [DOI] [PubMed] [Google Scholar]
- Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL. Effect of endogenous androgens on 17beta-estradiol-mediated protection after spinal cord injury in male rats. J Neurotrauma. 2010;27:611–626. doi: 10.1089/neu.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Honig WM, Hamers FP, Steinbusch HW, Joosten EA. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J Neurotrauma. 2005;22:214–225. doi: 10.1089/neu.2005.22.214. [DOI] [PubMed] [Google Scholar]
- Kuchler M, Fouad K, Weinmann O, Schwab ME, Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J Comp Neurol. 2002;448:349–359. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J Comp Neurol. 1991;311:356–366. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- Lin J, Cai W. Effect of vimentin on reactive gliosis: in vitro and in vivo analysis. J Neurotrauma. 2004;21:1671–1682. doi: 10.1089/neu.2004.21.1671. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Muir GD, Webb AA. Mini-review: assessment of behavioural recovery following spinal cord injury in rats. Eur J Neurosci. 2000;12:3079–3086. doi: 10.1046/j.1460-9568.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Muir GD, Webb AA, Kanagal S, Taylor L. Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur J Neurosci. 2007;25:1501–1510. doi: 10.1111/j.1460-9568.2007.05411.x. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Kobayashi S, Inukai T, Horiuchi Y, Yayama T, Sato R, Baba H. Rescue of rat anterior horn neurons after spinal cord injury by retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene. J Neurotrauma. 2007;24:703–712. doi: 10.1089/neu.2006.0004. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center NSCISC Annual Report for the Model Spinal Cord Injury Care Systems 2006 2006 [Google Scholar]
- National Spinal Cord Injury Statistical Center Spinal cord injury information network. 2008 http://www.spinalcord.uab.edu/
- Nilsen J, Diaz BR. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci U S A. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. An inexpensive apparatus for producing graded spinal cord contusive injury in the rat. Exp Neurol. 1987;95:530–533. doi: 10.1016/0014-4886(87)90162-2. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H. Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J Toxicol. 2011;2011:40519. doi: 10.1155/2011/405194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and GLT-1: benefit of 17beta-oestradiol treatment. Brain. 2010;133:1013–1025. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excito-toxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, Hubscher C, Johnson R, Schrimsher GW, Velardo MJ. Gray matter repair in the cervical spinal cord. Prog Brain Res. 2002;137:49–70. doi: 10.1016/s0079-6123(02)37007-9. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology. 2010;12:5680–5688. doi: 10.1210/en.2010-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Silvestroff L, Bartucci S, Soto E, Gallo V, Pasquini J, Franco P. Cuprizone-induced demelination in CNP∷GFP transgenic mice. J Comp Neurol. 2010;518:2261–2283. doi: 10.1002/cne.22330. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24:473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89:660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- Van Hedel HJ, Curt A. Fighting for each segment: estimating the clinical value of cervical and thoracic segments in SCI. J Neurotrauma. 2006;23:1621–1631. doi: 10.1089/neu.2006.23.1621. [DOI] [PubMed] [Google Scholar]
- Vander Horst VGJM, Gustafsson J, Ulfhake B. Estrogen receptor-α and -β immunoreactive neurons in the brain-stem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J. ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol. 1992;43:173–177. doi: 10.1016/0960-0760(92)90204-v. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- Wade GN, Blaustein JD, Gray JM, Meredith JM. ICI 182,780: a pure antiestrogen that affects behaviors and energy balance in rats without acting in the brain. Am J Physiol. 1993a;265:1392–1398. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]
- Wade GN, Powers JB, Blaustein JD, Green DE. ICI 182,780 antagonizes the effects of estradiol on estrous behavior and energy balance in Syrian hamsters. Am J Physiol. 1993b;265:1399–1403. doi: 10.1152/ajpregu.1993.265.6.R1399. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Jr, Park-Chung M, Gibbs TT, Farb DH. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Sensorimotor behaviour following incomplete cervical spinal cord injury in the rat. Behav Brain Res. 2005;165:147–159. doi: 10.1016/j.bbr.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B. Arpeggio and fractionated digit movements used in prehension by rats. Behav Brain Res. 1994;60:15–24. doi: 10.1016/0166-4328(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP. Skilled reaching in rats and humans: evidence for parallel development or homology. Behav Brain Res. 1992;47:59–70. doi: 10.1016/s0166-4328(05)80252-9. [DOI] [PubMed] [Google Scholar]
- Winterle JS, Mill T, Harris T, Goldbeck RA. Absolute kinetic characterization of 17-beta-estradiol as a radical-scavenging, antioxidant synergist. Arch Biochem Biophys. 2001;392:233–244. doi: 10.1006/abbi.2001.2431. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/akt-dependent CREB activation. J Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]


