Abstract
Interferon-β (IFN-β) is the treatment most often prescribed for relapsing-remitting multiple sclerosis (RRMS). However, 30–50% of MS patients do not respond to IFN-β. In some cases, IFN-β exacerbates MS, and it consistently worsens neuromyelitis optica (NMO). In order to eliminate unnecessary treatment for patients non-responsive to IFN-β, and to avoid possible harm, researchers are identifying biomarkers that predict outcome prior to the initiation of treatment. These biomarkers reveal insights into mechanisms of disease. Recent discoveries on human samples from patients with RRMS, NMO, psoriasis, rheumatoid arthritis, systemic lupus erythematosus and ulcerative colitis, indicate that IFN-β is ineffective and may worsen the clinical status in diverse diseases when a Th17 immune response is prominent.
Relapse-remitting multiple sclerosis (RRMS) and Interferon-β (IFN-β) therapy: Unmet need for rational prescriptions
Type I Interferons (type I IFNs), which include the various IFN-β and IFN-α molecules, were first discovered in virally infected chick embryo cells1. They bestowed cells with a resistance to virus. This pleotropic cytokine family is now known to have anti-viral, anti-tumor and immune-regulatory functions. In autoimmunity and inflammation, type I IFNs possess both pro- and anti-inflammatory functions depending on the context of the particular pathology.
The various forms of recombinant IFN-β are collectively the most commonly prescribed treatment for RRMS. In general, IFN-β therapy is well tolerated and the various approved versions of IFN-β carry a label claiming that they reduce the relapse rate by 30%. Clinicians often refer to patients that remain relapse free for years while on treatment as “super responders”2. The major side effects of IFN-β are moderate to severe flu-like symptoms and the potential for liver damage. But the most troubling problem for IFN-β is that 10–50% of RRMS patients do not respond to treatment3, thus delaying alternative and possibly beneficial treatments. And in some patients, treatment with IFN- β actually induces exacerbations4–6.
We do not fully understand the mode of action of IFN-β. One hypothesis is that multiple sclerosis (MS) is caused by a viral infection7; therefore IFN-β was thought to help attenuate disease by clearing the virus. However, IFN-β successfully reverses experimental autoimmune encephalomyelitis (EAE)8–10, a disease model devoid of a viral pathogenesis. Therefore, the anti-viral effects of IFN-β might not be as essential as its anti-inflammatory properties for the treatment of MS. In MS there is prominent perivascular lymphocytic infiltration and increased immunoglobulin synthesis within the CNS. Now, the favored theory is that IFN-β provides benefit for RRMS through its actions as an “immune modulator”. Several reports have identified potential anti-inflammatory functions of IFN-β that may contribute to its efficacy as a treatment11. These include blockade of lymphocyte trafficking to the CNS, reduction of expression of MHC class II molecules on antigen presenting cells, attenuation of T-cell proliferation and alteration of the cytokine milieu from pro-inflammatory to anti-inflammatory.
In contrast to its role in RRMS, IFN-β has pro-inflammatory functions that contribute to the pathogenesis in other autoimmune diseases including system lupus erythematosus (SLE), neuromyelitis optica (NMO) rheumatoid arthritis (RA) and psoriasis4,12–17. The role IFN-β treatment has in Th1 and Th17 diseases has been reviewed previously18. In this essay we will argue the case that IFN-β is a double-edged sword in autoimmune and inflammatory diseases, where it inhibits symptoms in diseases with a Th1 bias whereas it promotes the pathology in diseases with a predominant Th17 bias. Understanding these differences in the pro- and anti-inflammatory functions of IFN-β will be critical in understanding how this cytokine is therapeutic for RRMS. This understanding of its immune modulatory functions should also provide insights on how to discern which patients will respond to treatment with IFN-β.
Understanding the Mode of Action of IFN-β treatment: Lessons from EAE
Understanding the mechanism whereby IFN-β is effective in RRMS is formidable. Obtaining well-characterized MS tissue at various stages of disease, both before and during treatment is rare. Such specimens are usually limited to the blood. Given the barrier of obtaining such samples from RRMS patients, various models of EAE in rodents, primarily mice, have been used to dissect mechanisms of action for therapy with IFN-β8–10,19,20. However, identifying the mechanism whereby IFN-β attenuates EAE has been complicated for several reasons. First, EAE is a varied collection of models, involving different inciting antigens, different species and different genetic strains within a species. Moreover, so-called ‘active’ EAE is induced with different adjuvants, while no adjuvants are used for inducing ‘passive’ EAE in adoptive transfer protocols21. Analysis of disease mechanism must take into consideration processes occurring in various tissues (including the CNS) and cell types. To fully understand of the mechanism of action of IFN-β, the IFN-β receptor, IFNAR, must be analyzed. IFNAR is expressed in most tissues and cell types including, endothelial cells, neurons, glial cells and immune cells19.
EAE studies showed that Ifnb1 (IFN-β) knockout mice had increased severity of clinical symptoms, demonstrating that endogenously expressed IFN-β acts as a brake on CNS inflammation22. Recently, two exciting sets of experiments described that the inflammatory functions of myeloid cells are targeted and regulated via endogenously expressed type I IFN, and that this interaction attenuates EAE. In a series of elegant experiments utilizing a panel of conditional IFNAR knockout mice, it was demonstrated that local expression of IFN-β is elevated in the CNS during EAE. In direct response to IFN-β, myeloid cells decrease expression of chemokines to inhibit disease19. The role of endogenous type 1 IFN signaling in EAE was also examined in another publication20. Here the authors also demonstrated that IFNAR-deficient mice had defects in myeloid cell function. But, in contrast to the previous study, they concluded that the immunosuppressive effect of type 1 IFN is due to the upregulation of IL-27, which inhibits inflammatory Th17 cells (see Box 1). These two discoveries independently describe two key mechanisms whereby endogenously expressed type 1 IFN suppresses inflammation and autoimmunity in the CNS.
Box. T helper subsets in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis.
RRMS symptoms are initiated by a Th response to myelin in the CNS. Several studies have identified CD4 T cells in the spinal fluid and in brain lesions from MS patients. Moreover, in animal models of MS, collectively called experimental autoimmune encephalomyelitis (EAE), disease is initiated by Th cells21. An ongoing research subject in the MS and EAE fields is to determine the subset of helper T cells critical for the pathogenesis of this disease. Effector Th cells have been categorized into the following subsets based on cytokine secretion and function in immunity: The Th1 subset is driven by IL-12 to secrete IL-2, IFN-γ and TNF and is involved in anti-viral immunity. Th2, driven by IL-4, produce IL-4, IL-5 and IL-13 and have a central role in clearing parasites. The newly discovered Th17 subset develops in the presence of IL-6, TGFβ and IL-23 and secretes IL-17A, IL-17F, IL-22 and defends against bacterial and fungal infections70.
Originally, both RRMS and EAE were regarded as a Th1-mediated disease based on several observations. First, IFN-γ has been found in considerable quantities in the cerebrospinal fluid (CSF) of patients with MS and in spinal cords from mice with EAE71. Second, mice deficient in the ‘master’ transcription factor for Th1 differentiation (T-bet) are resistant to EAE72. Third, myelin-specific Th1 cells transferred into mice produce severe EAE symptoms. And finally, an early clinical trial reported that IFN-γ treatment induced severe relapses in RRMS patients73.
But discoveries using the EAE model catalyzed the discovery of the IL-23–IL17 pathway. Paradoxical data demonstrated that treatment of EAE with of IFN-γ reversed paralysis in mice with EAE. Deletion of the gene encoding IFN-γ, IL-12 or administration of antibodies specific for these cytokines worsened disease74. Given that Th17 differentiation is strongly inhibited by IFN-γ75 and deletion of IL-23 protects mice from EAE, the Th17 population gained notoriety as a highly inflammatory population of T helper cells in neuro-inflammation. In MS, transcriptional profiling and FACS analysis of MS tissue demonstrated the presence of Th17 in lesions and T-cells from blood25. IL-17 and interferon-γ transcripts are prominent in MS lesions. But since the discovery of the Th17 pathway, several papers have questioned the primacy of Th17 in causing inflammation in EAE and MS30. Two papers have provided data that question the pathogenic potential of Th17 cells in neuroinflammation: first, mice with conditional deletion of IL-17 in T cells develop EAE normally; second, experimental uveitis is cured in rats by treatment with recombinant IL-1776. However, it is now generally accepted that both Th1 and Th17 cells, as well as Th2 cells, can induce autoimmune demyelination and may also be pertinent to MS and its variants such as NMO47,77. There may indeed be Th1, Th2 and Th17 variants of autoimmune demyelinating disease.
Other studies have utilized the EAE model to elucidate the mechanism of IFN-β as a treatment, using various active and passive transfer protocols. Two recent papers have used the active EAE model to assess the effects of IFN-β treatment9,10. Active EAE experiments are conducted by immunization of mice with myelin antigens in complete freund’s adjuvant, often along with a boost of pertussis toxin. Procedures that use these complex adjuvants elicit both a Th1 and Th17 response23,24. Depending on the specific experimental conditions the balance between Th1 to Th17 responses is quite variable. In these experiments, the investigators found that IFN-β does moderately attenuate EAE symptoms. This reduction in EAE correlated with decreased amounts of both Th17 and Th1 cells, and upregulation of Th2 and regulatory T cells.
Recently, T-cell culture assays were conducted using both mouse and human cells to determine how IFN-β treatment alters T helper differentiation8. In Th1 conditions, IFN-β directly induced IL-27 production in antigen-presenting cells, which subsequently led to elevated IL-10 secretion in Th1 cells. In addition, treatment with IFN-β during Th17 differentiation was inhibitory. These activities of IFN-β required functional IFN-γ signaling.
The in vitro experiments showed that IFN-β has anti-inflammatory effects that potentially applied to both Th1- and Th17-driven EAE. To directly test this, IFN-β was administered to mice with EAE that was passively induced with either Th1 or Th17 cells reactive to a myelin protein-derived peptide8. The ‘passive’ EAE approach induces disease without adjuvants or use of B. pertussis toxin that can be confounding variables. IFN-β treatment effectively reduced disease symptoms in mice with EAE induced by Th1 cells. In congruence to the in vitro experiments, IFN-β increased IL-10 production by splenocytes in Th1 EAE. In Th17-induced EAE, IFN-β treatment unexpectedly exacerbated disease symptoms. This observation was perplexing, intriguing and altogether a stunning surprise. A popular theory on the mechanism of IFN-β treatment in MS and EAE is that it attenuates disease by inhibiting the differentiation of Th17 cells9,10,20,25,26. IFN-β did indeed inhibit IL-17 production both in vitro and in vivo; yet, IFN-β treatment was ineffective and actually worsened Th17-induced EAE. Only later was it learned that IFN-β also worsens human diseases where Th17 is prominent.
Understanding IFN-β Response in RRMS
The clinical trials that lead to the approval of IFN-β in RRMS patients indicated that some patients do not respond to treatment. One theory that explains the lack of response to treatment is poor bioavailability of IFN-β in these patients. One well known cause for the decreased bioavailability is the development of neutralizing antibodies to IFN-β. Several studies have identified that in some non-responders repeated injections of recombinant IFN-β elicits an antibody response against the cytokine and thereby this antibody neutralizes IFN-β’s beneficial effects27. However, non-responsiveness to IFN-β could be due to other mechanisms that reduce the pharmacodynamic properties of IFN- β, for example: decreased expression of IFNAR or its downstream signaling molecules (STAT1 and STAT2), or increased expression of the IFNAR inhibitors such as the SOCS proteins11,27. To assess IFN-β bioavailability, the standard bioassay is an antiviral cytopathic effect assay which is a sensitive assay to measure interferon activity. Recently, however, molecular assays, such as measuring the inducible expression of the IFN-inducible genes including myxovirus resistance 1 (MxA) and IP10 (CXCL10) and others are showing promise as biomarkers for treatment response28,29.
Beyond simply being ineffective in some patients, there is the real possibility that IFN-β could have an active role in inducing inflammation in non-responders. A recent study using a cDNA array demonstrated that RRMS patients have a heterogeneous molecular response to IFN-β22. Within the patient population, some non-responders had increased transcriptional activity in response to IFN-β, which was qualitatively different to that of the responders. It was concluded that IFN-β could contribute to the disease process. In a separate study of 26 RRMS patients (12 responders and 14 non-responders), a subset of non-responders (6 of 14) was identified that had high serum concentrations of the Th17 cytokine IL-17F before IFN-β therapy was initiated8. In both human and mouse, IL-17F is produced by Th17 cells, suggesting that this group of non-responders has a pathology for their type of disease that is skewed toward a Th17 phenotype30,31. In addition, these subjects also had high endogenous levels of IFN-β compared to responders. In fact, a strong correlation was found between IL-17F and IFN-β concentrations in the serum suggesting there is a tight biological association between these two cytokines. This observation is congruent with the Th17 EAE mice, which are non-responders to IFN-β treatment. Moreover, they are also concordant with the identification of biomarkers to predict the response to IFN-β in RRMS were identified32. In this study, serum levels of type I IFN and the expression of type I IFN-regulated genes were elevated in non-responders prior to initiation of IFN-β therapy compared to responders. This suggested that the non-responders might have a similar disease process to SLE, which also has an activated transcriptional signature of type I IFN12. It was further concluded that type I IFN might contribute to the neuro-inflammation in non-responding MS patients.
Two recent papers have shown that IFN-β directly inhibits IL-17A expression in PBMCs from MS patients, suggesting a mechanism for the positive therapeutic response of IFN-β in RRMS25,26. As already discussed, IFN-β also decreases IL-17A in mouse T cell cultures. Therefore, observations that high levels of IFN-β correlate with high levels IL-17F are quite confounding. A possible difference is that we measured IL-17F and where as the other investigators measured IL-17A. Even though IL-17A and IL-17F are located in the same region on the chromosome (Chr6 in human and Chr1 in mouse), the transcriptional regulation of these genes is quite different33. This phenomenon where IFN-β and Th17 are tightly linked biologically can be explained by two hypotheses. It can be speculated that the non-responders displaying aggressive Th17-mediated disease up-regulate IFN-β to counteract inflammation. Because endogenous IFN-β expression is already high, administering IFN-β treatment would be ineffective. But we propose another theory that extends from the work showing that IFN-β non-responders had high 32, where endogenous IFN-β drives the pro-inflammatory effects during Th17 biased disease. Therefore, not only would IFN-β treatment be ineffective in these patients, it could worsen symptoms. Our mouse studies provide evidence that type I IFN drives inflammation in a Th17 version of adoptively transferred EAE. A detrimental link between Th17 and IFN-β also occurs in other human autoimmune diseases.
The role of IFN-β in autoimmune diseases other than RRMS
RRMS is the one of the few known autoimmune diseases where the anti-inflammatory effects of IFN-β can be harnessed as a therapy. IFN-β was notably ineffective in rheumatoid arthritis (RA) for example34. In fact it is striking that the main treatment for RA, TNF blockade, has been shown to worsen MS symptoms and this drug carries an FDA black box warning against this contra-indication35. However, trials with IFN-β and other type I IFNs have shown some efficacy in ulcerative colitis (UC)36. A recent study of IFN-β treatment of UC assessed the biological differences between responders and non-responders. These authors observed a result stunningly congruent to IFN-β responders vs non-responders in RRMS. Investigators at NIH found that prior to IFN-β therapy in UC, those patients who were non-responders to IFN-β had significantly higher IL-17 production from lamina propria T cells compared to responders36.
In most other autoimmune disorders, including SLE, psoriasis, RA and NMO, type I IFN contributes to the pathogenesis of the disease12–14. It now emerges that Th17 also plays a pivotal role in these diseases37–41. Here we will discuss three of these diseases in the context of Th17 and type I IFN: Neuromyelitis Optica, and Psoriasis.
Neuromyelitis Optica
NMO, also known as Devic disease, is a neuro-inflammatory condition which until recently was considered a variant of RRMS. Unlike MS, the lesions in NMO are rarely associated with the brain. Instead, extensive demyelination is found in the spinal cord and optic nerve of these patients42. Recent developments have show that NMO patients develop antibodies against the aquaporin 4 (AQP4) water channel and assays detecting AQP4-Igs are now used for making the NMO diagnosis43. In addition to the lesion location and auto-antigens targeted in NMO, several groups have observed that the dominant infiltrating cells found in NMO lesions are granulocytes, a cell type which are largely absent in RRMS lesions42,44. Strikingly, levels of IL-17 are elevated in the CSF of patients with NMO compared to RRMS41. The high level of IL-17 in the CNS of NMO patients likely induces the local expression of IL-8, G-CSF and Gro-alpha which in turn would recruit and activate granulocytes45–49. IFN-β has been tried as a therapy for NMO but has had devastating consequences. Not only are NMO non-responders to IFN-β treatment, several case reports have shown that this treatment induces severe relapses and exacerbations in this disease4,15–17.
Psoriasis
In the dermatological disease, psoriasis, keratinocytes proliferate in an abnomal manner in response to a chronic inflammatory reaction. The IL-23–Th17 pathway (See Box) has been implicated in the pathogenesis of psoriasis39,50,51. Genetic studies have identified polymorphisms in IL23A, IL12B and IL23R as risk factors for developing psoriasis52,53. In mice intradermal injections of IL-23 induces inflammation in the epidermis that resembles psoriasis54. Moreover, monoclonal antibody (mAb) therapy that blocks IL-23 signaling have been successful in clinical trials of psoriasis (interestingly this same treatment failed in RRMS)55–57. Other Th17 cytokines indentified in psoriasis include IL-17A, IL-22, IL-8 and also IL-17F39,40,50,51,58. Neutralizing IL-17 with mAb, as with anti-IL-23, is showing promise as a treatment for psoriasis59.
In addition to Th17, type I IFN has also been implicated as an important mediator of inflammation in psoriasis. Like SLE, the transcriptional signature of type I IFN is activated within the psoriatic plaques14. The cellular source of type I IFN is likely plasmacytoid dendritic cells (pDC). Among the first events that occur in this disease, is the recruitment of pDCs to the pre-plaque area where they are activated and secrete large quantities of IFNβ and IFN-α60. Experiments using blocking antibodies and knockout mice have demonstrated that type I IFNs play a pathogenic role in psoriasis14,61. Type I IFN has been identified as a key component for the pathology in several cases of psoriasis that developed in RRMS and Hepatitis C patients who received type I IFN as a treatment for their disease62–69.
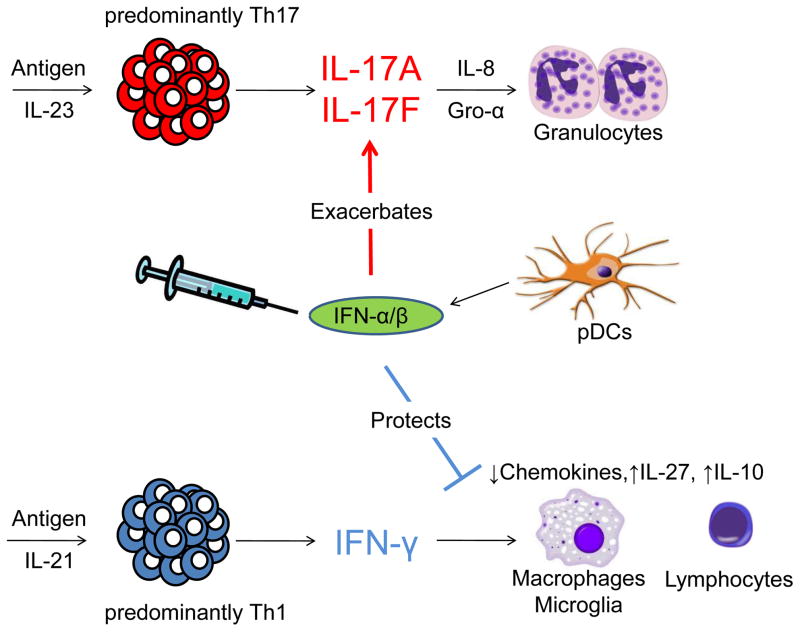
As a whole, the observations encompassing these pre-clinical experiments in mice and clinical studies in human diseases suggest that type I IFN and Th17 are a dangerous combination in autoimmune disease, where endogenous expression or therapeutic administration of IFN-β in conditions with a Th17 bias, only worsens the disease (see Fig). But the question remains, how does IFN-β exacerbate these Th17-mediated diseases? One hypothesis is that type 1 IFN could have a pro-inflammatory effect on granulocytes. In particular, it has been shown that IFN-α instructs neutrophils to release neutrophil extracellular traps (NETS) which contain destructive proteases and cytokines. However, B-cells may also be targeted in these diseases. It has been shown that the B-cell stimulating cytokine BAFF is elevated after in both NMO and MS patients after IFN-β therapy. This effect could promote B-cell mediated pathology which is thought to be of primary importance in NMO.
Figure.
Autoimmune diseases with a predominately IL-23–Th17 response secrete high levels of IL-17A and IL-17F, which signals to the surrounding tissue to up-regulate granulocyte recruitment and activation factors, such as G-CSF, Gro-α and IL-8. Through a currently unknown mechanism, endogenous expression of IFN-β by plasmacytoid DCs or therapeutic administration of IFN-β, worsens Th17 disease. Conversely, predominantly Th1-mediated diseases have high levels of IFN-γ and have a lymphocytic and macrophage infiltrate. In Th1 disease, IFN-β, in concert with IFN-γ, drives anti-inflammatory responses such as upregulation of IL-27, IL-10 or inhibiting chemokine expression by macrophages.
Concluding Remarks
RRMS is a complex disease that has an unpredictable clinical course, with variable pathological patterns. RRMS might not be a single disease, but rather a collection of different syndromes that cause inflammatory demyelination. This heterogeneity is illuminated by the variability in responses to IFN-β. This variability poses a challenge to clinicians and researchers to develop ways to identify responsiveness early after treatment begins, or better yet, before treatment is initiated.
In this article, we have described the discovery of several biological markers that have great potential to address this important health care issue. However, as of publication there is no approved test to determine whether or not an MS patient should take IFN-β, glatiramer acetate or the recent FDA approved oral medication. Therefore, it should be a high priority for agencies to fund research that is geared to identify the mode of the action of these therapies, to discover biomarkers that predict response, and to develop reliable tests for clinical use. This would be a wise investment for the public for it would eliminate the unnecessary treatment of patients who are non-responders, and would streamline the way clinicians treat RRMS patients with these expensive drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 2.Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther. 2007;29(9):2031–2048. doi: 10.1016/j.clinthera.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Rio J, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol. 2006;59(2):344–352. doi: 10.1002/ana.20740. [DOI] [PubMed] [Google Scholar]
- 4.Warabi Y, et al. Interferon beta-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J Neurol Sci. 2007;252(1):57–61. doi: 10.1016/j.jns.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang AG, et al. Early relapse in multiple sclerosis-associated optic neuritis following the use of interferon beta-1a in Chinese patients. Jpn J Ophthalmol. 2006;50(6):537–542. doi: 10.1007/s10384-006-0359-4. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu Y, et al. Development of extensive brain lesions following interferon beta therapy in relapsing neuromyelitis optica and longitudinally extensive myelitis. J Neurol. 2008;255(2):305–307. doi: 10.1007/s00415-007-0730-5. [DOI] [PubMed] [Google Scholar]
- 7.Pender MP. Does Epstein-Barr virus infection in the brain drive the development of multiple sclerosis? Brain. 2009;132(Pt 12):3196–3198. doi: 10.1093/brain/awp312. [DOI] [PubMed] [Google Scholar]
- 8.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Saavedra FM, et al. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol Immunol. 2008;45(15):4008–4019. doi: 10.1016/j.molimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Galligan CL, et al. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult Scler. 2010;16(12):1458–1473. doi: 10.1177/1352458510381259. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste EN, Qin H. Type I interferons as anti-inflammatory mediators. Sci STKE. 2007;2007(416):pe70. doi: 10.1126/stke.4162007pe70. [DOI] [PubMed] [Google Scholar]
- 12.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Pouw Kraan TC, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66(8):1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Fits L, et al. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J Invest Dermatol. 2004;122(1):51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu J, et al. IFNbeta-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology. 2010;75(16):1423–1427. doi: 10.1212/WNL.0b013e3181f8832e. [DOI] [PubMed] [Google Scholar]
- 16.Palace J, et al. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010;67(8):1016–1017. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 17.Uzawa A, et al. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol. 2010;17(5):672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 18.Prinz M, Kalinke U. New lessons about old molecules: how type I interferons shape Th1/Th17-mediated autoimmunity in the CNS. Trends Mol Med. 2010;16(8):379–386. doi: 10.1016/j.molmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Prinz M, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28(5):675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Guo B, et al. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118(5):1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60(1):12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 22.Teige I, et al. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170(9):4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- 23.Tigno-Aranjuez JT, et al. Encephalitogenicity of complete Freund’s adjuvant relative to CpG is linked to induction of Th17 cells. J Immunol. 2009;183(9):5654–5661. doi: 10.4049/jimmunol.0900645. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, et al. Adjuvant treatment suppresses IL-17 production by T cell-independent myeloid sources in nonobese diabetic mice. Mol Immunol. 2010;47(14):2397–2404. doi: 10.1016/j.molimm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Durelli L, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 26.Ramgolam VS, et al. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183(8):5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 27.Hesse D, Sorensen PS. Using measurements of neutralizing antibodies: the challenge of IFN-beta therapy. Eur J Neurol. 2007;14(8):850–859. doi: 10.1111/j.1468-1331.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 28.Serrano-Fernandez P, et al. Time course transcriptomics of IFNB1b drug therapy in multiple sclerosis. Autoimmunity. 2010;43(2):172–178. doi: 10.3109/08916930903219040. [DOI] [PubMed] [Google Scholar]
- 29.Buttmann M, et al. Interferon-beta induces transient systemic IP-10/CXCL10 chemokine release in patients with multiple sclerosis. J Neuroimmunol. 2004;156(1–2):195–203. doi: 10.1016/j.jneuroim.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Haak S, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boniface K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185(1):679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 32.Comabella M, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132(Pt 12):3353–3365. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Rodriguez J, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genovese MC, et al. A randomized, controlled trial of interferon-beta-1a (Avonex(R)) in patients with rheumatoid arthritis: a pilot study [ISRCTN03626626] Arthritis Res Ther. 2004;6(1):R73–R77. doi: 10.1186/ar1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. ( http://www.fda.gov/OHRMS/DOCKETS/ac/01/briefing/3779b2_01_cber_safety%20_revision2.pdf)
- 36.Mannon PJ, et al. Suppression of inflammation in ulcerative colitis by interferon-{beta}-1a is accompanied by inhibition of IL-13 production. Gut. 2010;60(4):449–455. doi: 10.1136/gut.2010.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hamburg JP, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2010;63(1):73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 38.Shah K, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(2):R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujishima S, et al. Involvement of IL-17F via the induction of IL-6 in psoriasis. Arch Dermatol Res. 2010;302(7):499–505. doi: 10.1007/s00403-010-1033-8. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe H, et al. Functional characterization of IL-17F as a selective neutrophil attractant in psoriasis. J Invest Dermatol. 2009;129(3):650–656. doi: 10.1038/jid.2008.294. [DOI] [PubMed] [Google Scholar]
- 41.Ishizu T, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128(Pt 5):988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 42.Lucchinetti CF, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul F, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;4(4):e133. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hengstman GJ, et al. Neuromyelitis optica with clinical and histopathological involvement of the brain. Mult Scler. 2007;13(5):679–682. doi: 10.1177/1352458506070145. [DOI] [PubMed] [Google Scholar]
- 45.Smith E, et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179(12):8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, et al. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46(1):79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroenke MA, et al. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205(7):1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang SC, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 49.Uzawa A, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16(12):1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 50.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 51.Ortega C, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86(2):435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- 52.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair RP, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128(7):1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 56.Kimball AB, et al. Efficacy and safety of ABT-874, a monoclonal anti-interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36-week observation/retreatment and 60-week open-label extension phases of a randomized phase II trial. J Am Acad Dermatol. 2008 doi: 10.1016/j.jaad.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 57.Segal BM, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 58.Coimbra S, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163(6):1282–1290. doi: 10.1111/j.1365-2133.2010.09992.x. [DOI] [PubMed] [Google Scholar]
- 59.Hueber W, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 60.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hida S, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13(5):643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 62.Seckin D, et al. Concomitant vitiligo and psoriasis in a patient treated with interferon alfa-2a for chronic hepatitis B infection. Pediatr Dermatol. 2004;21(5):577–579. doi: 10.1111/j.0736-8046.2004.21512.x. [DOI] [PubMed] [Google Scholar]
- 63.Imafuku S, et al. Ciclosporin treatment of psoriasis in a patient with chronic hepatitis C. Br J Dermatol. 2007;156(6):1367–1369. doi: 10.1111/j.1365-2133.2007.07873.x. [DOI] [PubMed] [Google Scholar]
- 64.Downs AM, Dunnill MG. Exacerbation of psoriasis by interferon-alpha therapy for hepatitis C. Clin Exp Dermatol. 2000;25(4):351–352. doi: 10.1046/j.1365-2230.2000.00655-4.x. [DOI] [PubMed] [Google Scholar]
- 65.Horev A, Halevy S. New-onset psoriasis following treatment with pegylated interferon-alpha 2b and ribavirin for chronic hepatitis C. Isr Med Assoc J. 2009;11(12):760–761. [PubMed] [Google Scholar]
- 66.La Mantia L, Capsoni F. Psoriasis during interferon beta treatment for multiple sclerosis. Neurol Sci. 2010;31(3):337–339. doi: 10.1007/s10072-009-0184-x. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Lerma I, et al. New-onset psoriasis in a patient treated with interferon beta-1a. Br J Dermatol. 2009;160(3):716–717. doi: 10.1111/j.1365-2133.2008.09005.x. [DOI] [PubMed] [Google Scholar]
- 68.Webster GF, et al. Cutaneous ulcerations and pustular psoriasis flare caused by recombinant interferon beta injections in patients with multiple sclerosis. J Am Acad Dermatol. 1996;34(2 Pt 2):365–367. doi: 10.1016/s0190-9622(07)80010-7. [DOI] [PubMed] [Google Scholar]
- 69.Navne JE, et al. Activation of psoriasis in patients undergoing treatment with interferon-beta. Ugeskr Laeger. 2005;167(32):2903–2904. [PubMed] [Google Scholar]
- 70.Harrington LE, et al. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40(2–3):211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 72.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panitch HS, et al. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1(8538):893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 74.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- 75.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 76.Ke Y, et al. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182(5):3183–3190. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wensky A, et al. The role of IFN-gamma in the production of Th2 subpopulations: implications for variable Th2-mediated pathologies in autoimmunity. J Immunol. 2001;167(6):3074–3081. doi: 10.4049/jimmunol.167.6.3074. [DOI] [PubMed] [Google Scholar]