Abstract
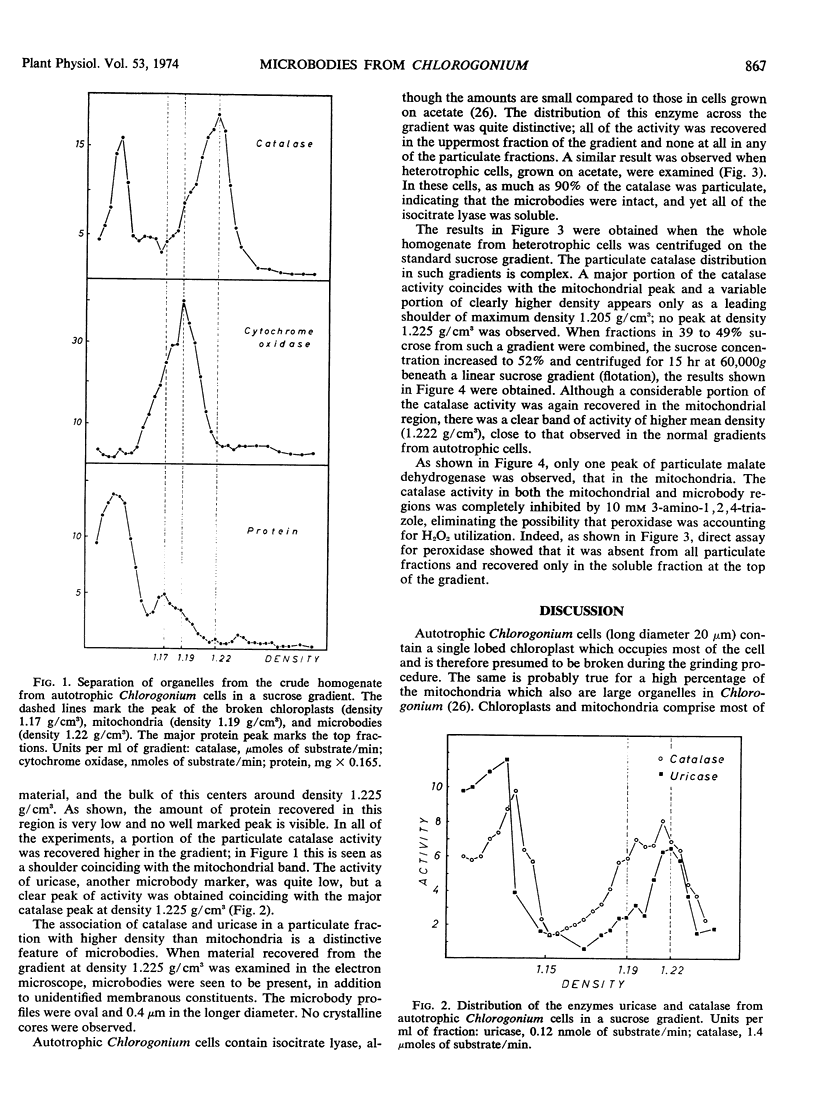
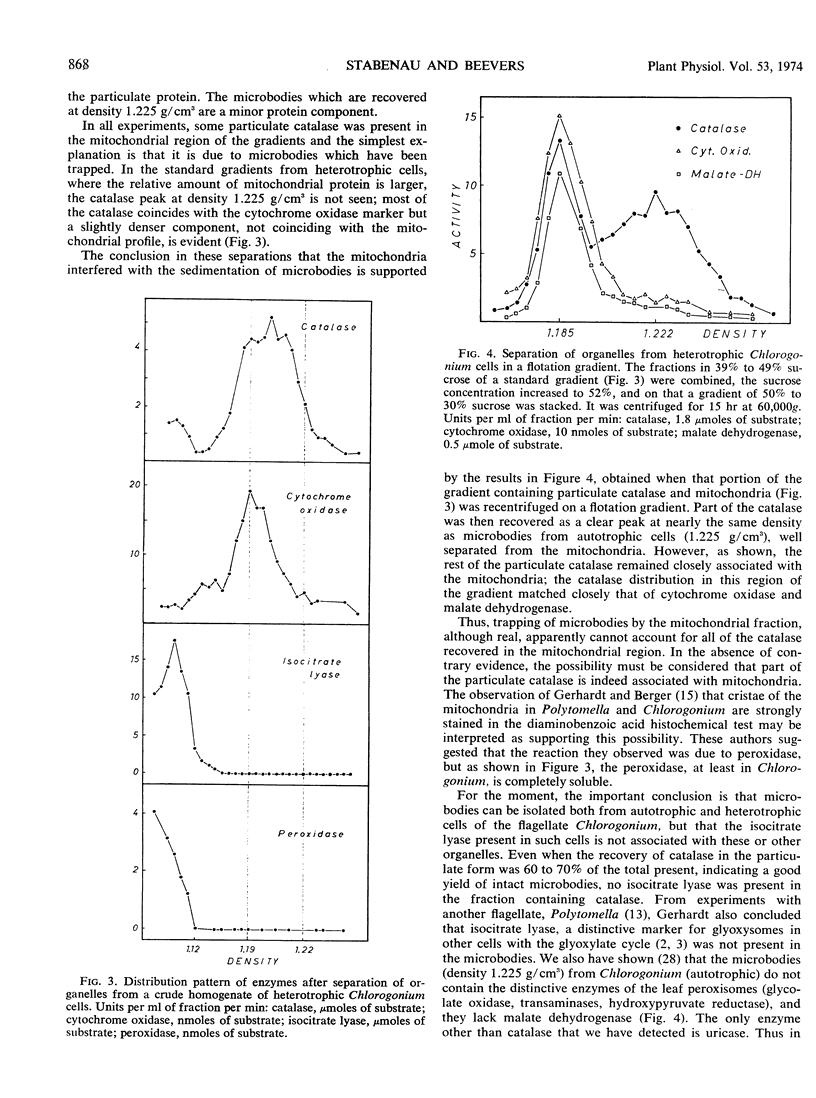
The alga Chlorogonium elongatum was grown autotrophically or heterotrophically on acetate. Cells harvested in the logarithmic phase of growth were disrupted, and the whole homogenates were fractionated on sucrose gradients. Protein and enzyme determinations carried out on the fractions led to the following conclusions. Chloroplast fragments which represent the major portion of particulate protein in autotrophic cells migrate to density 1.17 g/cm3. In heterotrophic cells, mitochondria comprise most of the particulate protein, and these particles accumulate at density 1.19 g/cm3, as shown by a peak of cytochrome oxidase in this region. Part of the catalase and uricase, two marker enzymes for microbodies, were found in the soluble fractions, but 60% or more of these activities were recovered at density 1.225 g/cm3 from autotrophic cells. Electron micrographs showed that in this region there were microbodies with a diameter of 0.4 micrometer. The isolated microbodies contained no isocitrate lyase, a marker enzyme of glyoxysomes. This enzyme was completely soluble and therefore seems not to be associated with organelles in this organism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Schmid G. H., Kowallik W. Enzymic evidence for peroxisomes in a mutant of Chlorella vulgaris. Arch Mikrobiol. 1972;81(3):264–272. doi: 10.1007/BF00412245. [DOI] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch Mikrobiol. 1971;80(3):205–218. [PubMed] [Google Scholar]
- Graves L. B., Jr, Hanzely L., Trelease R. N. The occurrence and fine structural characterization of microbodies in Euglena gracilis. Protoplasma. 1971;72(2):141–152. doi: 10.1007/BF01279047. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Jr, Trelease R. N., Grill A., Becker W. M. Localization of glyoxylate cycle enzymes in glyoxysomes in Euglena. J Protozool. 1972 Aug;19(3):527–532. doi: 10.1111/j.1550-7408.1972.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Trelease R. N., Becker W. M. Particulate nature of glycolate dehydrogenase in euglena: possible localization in microbodies. Biochem Biophys Res Commun. 1971 Jul 16;44(2):280–286. doi: 10.1016/0006-291x(71)90596-1. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Müller M., Moller K. M. Studies on some enzymes of purine metabolism in the amoebae Chaos chaos and Amoeba proteus. C R Trav Lab Carlsberg. 1969;36(24):463–497. [PubMed] [Google Scholar]
- Stewart K. D., Floyd G. L., Mattox K. R., Davis M. E. Cytochemical demonstration of a single peroxisome in a filamentous green alga. J Cell Biol. 1972 Aug;54(2):431–434. doi: 10.1083/jcb.54.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]



