Abstract
Skeletal muscle remodels metabolic capacity, contractile and exercise phenotype in response to physiological demands. This adaptive remodeling response to physical activity can ameliorate/prevent diseases associated with poor diet and lifestyle. Our previous work demonstrated that skeletal muscle-specific transgenic expression of the neuron-derived orphan nuclear receptor, Nor-1 drives muscle reprogramming, improves exercise endurance, and oxidative metabolism. The current manuscript investigates the association between exercise, Nor-1 expression and the role of Nor-1 in adaptive remodeling. We demonstrate that Nor-1 expression is induced by exercise and is dependent on calcium/calcineurin signaling (in vitro and in vivo). Analysis of fatigue-resistant transgenic mice that express Nor-1 in skeletal muscle revealed increased hypertrophy and vascularization of muscle tissue. Moreover, we demonstrate that transgenic Nor-1 expression is associated with increased intracellular recycling, ie, autophagy, involving 1) increased expression of light chain 3A or LC3A-II, autophagy protein 5, and autophagy protein 12 in quadriceps femoris muscle extracts from Tg-Nor-1 (relative to Wild-type (WT) littermates); 2) decreased p62 expression indicative of increased autophagolysosome assembly; and 3) decreased mammalian target of rapamycin complex 1 activity. Transfection of LC3A-GFP-RFP chimeric plasmid demonstrated that autophagolysosome formation was significantly increased by Nor-1 expression. Furthermore, we demonstrated a single bout of exercise induced LC3A-II expression in skeletal muscle from C57BL/6 WT mice. This study, when combined with our previous studies, demonstrates that Nor-1 expression drives multiple physiological changes/pathways that are critical to the beneficial responses of muscle to exercise and provides insights into potential pharmacological manipulation of muscle reprogramming for the treatment of lifestyle induced chronic diseases.
Skeletal muscle is a key metabolic organ comprising approximately 40% of total body mass and accounting for 85% insulin-stimulated glucose removal (1). It is well established that exercise has multiple beneficial effects on human health including the reduced incidence of metabolic disorders such as type 2 diabetes, cardiovascular diseases, sarcopenia, and steatohepatitis (2). Depending on the type of exercise training and the frequency of nervous innervation and metabolic environment, skeletal muscle can accordingly modulate fiber type, fiber mass, and metabolic requirements. This involves a processes of molecular and cellular contractile remodeling and reprogramming after exercise. The molecular mechanisms underlying the adaptions after exercise are incompletely understood; however, the response would require the activation of transcription factors to modulate gene expression.
Nuclear receptors are a family of transcription factors that are known to regulate skeletal muscle physiology and metabolic function (3). Peroxisome proliferator-activated receptor δ (4), Nur77, nerve growth factor IB (5), Nor-1 (6), and Estrogen-related receptor γ (7) have been associated with the regulation of exercise endurance and skeletal muscle fiber type remodeling. We have previously reported that transgenic overexpression of an activated form of the nuclear receptor Nor-1, in the skeletal muscle of mice (Tg-Nor-1) results in a switch to oxidative type IIA/X skeletal muscle fibers and increased exercise endurance (6). This mouse line also exhibited in skeletal muscle, increased numbers of mitochondria and increased expression of mitochondrial/oxidative genes/proteins. Furthermore, the Tg-Nor-1 mouse line was also more resistant to diet-induced weight gain and maintained fasting glucose at normal glycemic levels (8). Gene expression analysis revealed significant increases in genes involved in glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, fatty acid oxidation, and glycogen synthesis in concordance with the lean phenotype (8). Interestingly, we also observed several Z-disc and sarcomeric-binding proteins that modulate fiber type phenotype and endurance, for example α-actinin 3 (8). In conclusion, skeletal muscle-specific Nor-1 expression controls genes and pathways that regulate adiposity, muscle fiber type metabolic capacity, and endurance.
Exercised skeletal muscle activates a cascade of signaling pathways. Neuronal input stimulates calcium ion release to induce contraction and this calcium ion flux has a significant effect on a number of proteins, including calcineurin. Furthermore, skeletal muscle is able to respond concordantly to the type of exercise whether it is aerobic or anaerobic. Aerobic exercise is the activity of the muscle under conditions of high oxygen availability, such as jogging, which uses a high percentage of type I fibers and low levels of maximal contraction strength. Anerobic exercise is the activity of muscle with low availability of oxygen; such as high intensity muscle contractions over short periods of time using complete maximal contraction strength and predominantly type II muscle fibers. Training muscle allows it to have increased capacity in terms of muscle strength, endurance, and size of myofibres.
A particular calcium pathway activated is protein phosphatase 2B or PP2B known as calcineurin, which was initially characterized as an enzyme complex regulated by calcium, comprised of both calcineurin A catalytic and calcineurin B regulatory subunits and also calmodulin (9, 10). This calcium/calmodulin dependent serine/threonine phosphatase functions by dephosphorylating members of the Nuclear factor of activated T-cells (NFAT) family, which results in their translocation out of the nucleus and thus activation of target genes (11, 12). Calcineurin activation in skeletal muscle is known to induce a fiber type shift towards that of slow twitch oxidative and promote differentiation (11, 13). Inversely, chronic inhibition of calcineurin by cyclopsorine A results in a phenotype change towards that of glycolytic skeletal muscle fibers (10). Hypertrophy in skeletal muscle is known to be induced by IGF-1. Furthermore, IGF-1 has been found to mobilize intracellular calcium and thus calcineurin activation playing a vital role in hypertrophic growth (10).
In recent years, autophagy, a key intracellular degradation and recycling mechanism, has been found to be induced after exercise (14, 15). Autophagy is characterized by the formation of a double membrane structure called the autophagosome that engulfs cytoplasmic components for degradation. The autophagosome will then fuse with the lysosome creating an autophagolysosome and initiates cellular degradation. Autophagy forms a key competent of skeletal muscle repair, protein homeostasis and fiber remodeling (16–18). In the context of exercise, the induction of autophagy after exercise appears to mediate exercise-induced adaptations involving enhanced endurance (19, 20), increased angiogenesis (19), enhanced glucose tolerance (14), mitochondrial biogenesis (19, 20), and oxidative metabolism (20).
In the present study, we reveal that Nor-1 expression is involved in multiple physiological changes/pathways that are associated with the beneficial responses of skeletal muscle to exercise (such as hypertrophy, angiogenesis, and autophagy). Moreover, Nor-1 expression is increased after exercise in a Ca2+ and calcineurin-dependent manner suggesting that Nor-1 partly regulates the transcriptional response of skeletal muscle after exercise.
Materials and Methods
Transgenic mice
All animal related procedures were approved by the Animal Experimentation Ethics Committees of the University of Queensland and conformed to the Guidelines for the Care and Use of Experimental Animals described by the National Health and Medical Research Council of Australia. The VP16-activated Nor-1 mouse line (Tg-Nor-1) has been described before (6, 8). Mice were maintained on a 12-hour light, 12-hour dark cycle, with standard mouse chow feed (Meat Free Rat and Mouse; Specialty Feeds) and water provided ad libitum at the University of Queensland Biological Resources animal house facility. All experiments were performed on male mice of 12–17 weeks of age. Experimental mice were backcrossed to the C57BL/6J background for greater than 7 generations, and experiments consist of heterozygous transgenic mice with WT siblings.
Treadmill running and inhibitor pretreatments
WT C57BL/6 mice were run on an Exer 3/6 Treadmill (Columbus Instruments) with mild electrical stimulus at 5% inclination. Mice were exercised at a speed of 10 m/min for 10 minutes, accelerated to 20 m/min over 10 minutes, and followed by another 20 m/min for 20 minutes. This moderate exercise for 40 minutes in total was below exhaustion levels. Control mice were not exercised on treadmill. Mice were left for defined periods before being sacrificed (Figure 1A). In inhibition studies, mice were ip injected with the β-adrenergic antagonist propranolol (3 mg/kg) or the calcineurin inhibitor cyclosporine A 10 mg/kg or vehicle control (saline) 30 minutes before exercise (or rest) as above.
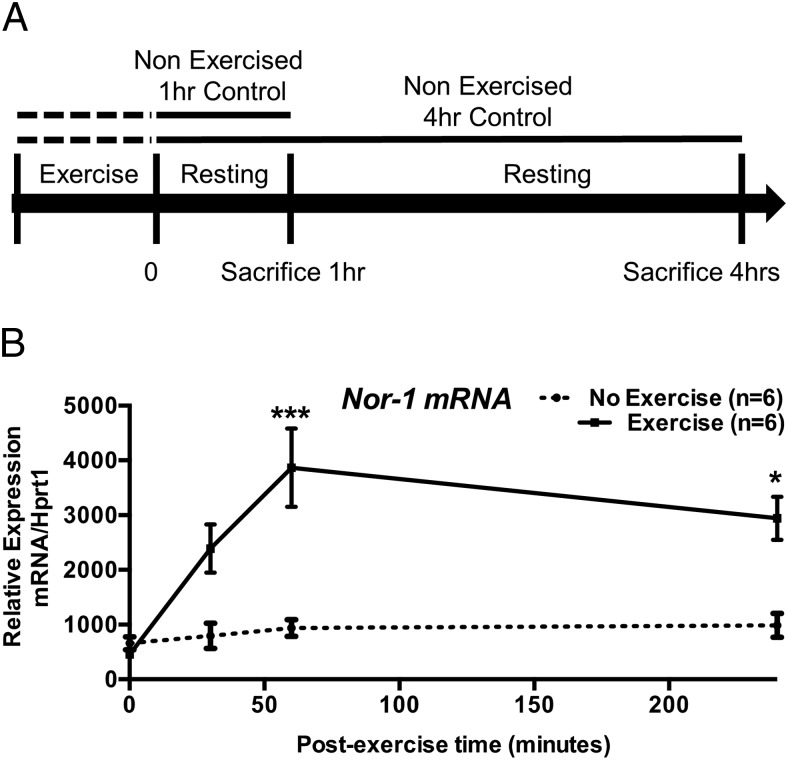
Figure 1. The expression of the mRNA encoding NR4A subgroup is induced after acute exercise in skeletal muscle.
A, Diagram illustrating the exercise protocol for tissues collected at 1 and 4 hours after exercise. B, Nor-1 qRT-PCR was performed on samples collected 0, 0.5, 1, and 4 hours after exercise quadriceps femoris skeletal muscle extracts (n = 6). Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
RNA extraction and cDNA synthesis
All WT and Tg-Nor-1 tissue samples were snap-frozen in liquid nitrogen. RNA extraction and cDNA synthesis were carried out as described (21, 22).
Quantitative real-time PCR
For qRT-PCR, target cDNAs were compared in 25-μL reactions as described (21). Expression levels were normalized to control genes as stated. For TaqMan low-density arrays (TLDAs), the platform was used as previously described (6) in a custom designed format. For the QIAGEN mouse angiogenesis arrays (PAMM-024z), the cDNA levels of target genes were compared by qRT-PCR in 10-μL reactions containing 2× SYBR Green Mastermix. PCR was conducted over 45 cycles of 95°C for 15 seconds and 60°C for 1 minute, proceeded by an initial one cycle of 50°C for 2min and 95°C for 10 minutes on an ABI Real Time ViiA 7 thermocycler sequence detection system (Applied Biosystems). The expression levels of genes were normalized against glucuronidase beta (Gusb), Glyceraldehyde 3-phosphate dehydrogenase (Gapdh), Hypoxanthine phosphoribosyltransferase (Hprt1), Beta-actin (Actb), 18S ribosomal RNA (18S), and Ribosomal protein, large, P0 (Rplp0) established by the ratio of the δCt (cycle threshold) values. The analysis was performed using the StatMiner software package.
Cell culture and in vitro drug treatments
C2C12 mouse myoblasts (American Type Culture Collection) were expanded in growth medium (DMEM [Invitrogen Australia] supplemented with 10% heat-inactivated Serum Supreme [Cambrex Life Sciences]) in 7.5% CO2. Confluent myoblasts were differentiated into multinucleated myotubes using DMEM supplemented with 2% horse serum for 4 days. Authenticity of the C2C12 cell line was confirmed by the ability to phenotypically differentiate into contractile multinucleated myotubes and amplification of mouse- and skeletal muscle-specific mRNA markers. For drug treatment, differentiated C2C12 skeletal muscle myotubes were pretreated for 30 minutes with vehicle control (DMSO, dimethyl sulfoxide), the calcineurin inhibitor cyclosporine A (0.4μM), the calcuim/calmodulin-dependent protein kinase II (CaMKII) inhibitor KN-93 (2μM), the protein kinase C (PKC) inhibitor GF109203X (0.5μM), or the p38 MAPK inhibitor SB202190 (10μM). Cells were then treated with the calcium ionophore A23187 (1μM) or vehicle control (DMSO) for 1 hour and harvested for mRNA.
Western blot analysis
Western blot analysis was carried out as described (23). Blots were probed with anti-GAPDH (1:20 000, 2275-PC-100; Trevigen), anti-LC3A (1:1000, 4599; Cell Signaling), antiautophagy protein (ATG)5 (1:1000, 8540; Cell Signaling); anti-ATG3 (1:1000, 3415; Cell Signaling), anti-Beclin-1 (1:1000, 3495; Cell Signaling), anti-phospho-p70 S6 kinase (p70S6k) Thr389 (1:1000, 9205; Cell Signaling), anti-CREB-regulated transcription coactivator 1 antibody (1:1000, 2501; Cell Signaling), anti-CRTC2 antibody (1:1000, 3826; Cell Signaling), anti-CRTC3 antibody (1:1000, 2768; Cell Signaling), anti-p62 (1:1000, 5114; Cell Signaling), anti-mammalian target of rapamycin (mTOR) (1:500, 2983; Cell Signaling), anti-phospho-mTOR Ser2448 (1:500, 5536; Cell Signaling), antiphospho-mTOR Ser2481 (1:500, 2974; Cell Signaling), anti-Raptor (1:500, 2280; Cell Signaling), anti-phospho-Raptor Ser792 (1:1000, 2083; Cell Signaling), anti-p-p38 MAPK Thr180/Tyr182 (1:1000, 9211; Cell Signaling), anti-p38-MAPK (1:1000, 9212; Cell Signaling), anti-phospho-CREB Ser133 (1:1000, 9191; Cell Signaling), and anti-CREB (1:1000, 9197; Cell Signaling) in tris-buffered saline with 5% skim milk powder (or 3% BSA for anti-phospho proteins). Secondary anti-rabbit horseradish peroxidase (HRP) (1:10 000, 7074; Cell Signaling) and anti-mouse HRP (1:10 000, 32230; Pierce Biotechnology) was used in 5% skim milk powder. Expression signals were detected by ECL Plus Western blotting detection system (Millipore) and visualized using autoradiography by X-OMAT film developer (Kodak). Densitometry analysis was performed using ImageJ (24).
Immunofluorescence
Tibialis anterior muscle was mounted in optimal cutting temperature compound and frozen in melting isopentane cooled in liquid nitrogen; 7-μm transverse sections from the midbelly region of the muscle were cut on a cryostat. Sections were fixed with 4% formaldehyde in PBS for 15 minutes. For LC3a, sections were blocked in 5% goat serum and incubated with anti-LC3a (1:50, 4599; Cell Signaling) overnight and then treated with Alexa Fluor 594 goat anti-rabbit (Invitrogen) at 1:200 for 1 hour. For platelet endothelial cell adhesion molecule (PECAM-1), sections were blocked in 5% rabbit serum and incubated with goat anti-PECAM-1 antibody (M-20 sc-1506; Santa Cruz Biotechnology, Inc) at 1:200 overnight and then treated with Alexa Fluor 488 rabbit antigoat (Invitrogen) at 1:500 for 1 hour (n = 3). For LC3A, immunofluorescence was performed on WT and Tg-Nor-1 muscle sections (n = 5), and photographs are a representative image. No fluorescence was visible in secondary-only controls. Images were taken with an Olympus BX-51 fluorescence microscope. The relative percentage levels of RFP to GFP fluorescent objects were determined using the OBCOL package (25) for ImageJ 1.42q (NIH).
Alkaline phosphatase staining
Tibialis anterior muscle was mounted in optimal cutting temperature compound and frozen in melting isopentane cooled in liquid nitrogen; 7-μm transverse sections from the midbelly region of the muscle were cut on a cryostat. Fresh unfixed sections were stained using the method of McGadey 1970 (26). Images were taken with an Olympus BX-51 fluorescence microscope.
Statistical analysis
Statistical analysis for all non-TLDA quantitative RT-PCR data were performed using Prism 6 (GraphPad Software). Non-TLDA data were analyzed using Student's t test unless otherwise stated. All results are expressed as mean ± SEM. Significant changes in expression of TLDA data were analyzed using the ABI/Integromics StatMiner software package, previously described (22, 27, 28). Differentially expressed genes were identified by Linear models (contained in the LIMMA package for Bioconductor R embedded in StatMiner). Significance is assigned by the application of the Empirical Bayes statistic, described as equivalent to shrinkage of the estimated sample variances towards a pooled estimate, resulting in far more stable inference when the number of arrays is small (29). It returns the empirical Bayes log odds of differential expression (ie, the probability) that a gene is differentially expressed (a higher score represents a more significant result). For example, B statistic of 0 indicates a 50:50 chance of differential expression, B scores more than 0 indicate more than 50:50 chance of differential expression, B less than 0 (−ve scores) reflect odds that a gene is more than likely, not differentially expressed. The B-statistic considers and ranks a proportion of differentially expressed genes (P < .01). Analysis also includes t, the empirical Bayes moderated t statistic (a variant t test), an empirically moderated estimate of standard error. Data are presented as relative quantification (RQ), ie, the calculated fold differences normalized to one of the selected controls (for example 18S rRNA) between the transgenic and the WT littermates. RQ was determined using the equation: RQ = 2 − ΔΔCt (30).
Wheat germ agglutinin (WGA) staining
Tibialis anterior muscle was mounted in optimal cutting temperature compound and frozen in melting isopentane cooled in liquid nitrogen; 7-μm transverse sections from the midbelly region of the muscle were cut on a cryostat. Sections were fixed with 4% paraformaldahyde for 45 minutes at room temperature. Sections were incubated with WGA Alexa Fluor 488 conjugate (Invitrogen) with PBS at a 1:200 concentration for 15 minutes was used to fluorescently stain the cell membranes of the muscle fiber. Slides stained incubated in PBS for 15 minutes were used as negative controls. Images were taken with an Olympus BX-51 fluorescence microscope, and fiber cross-sectional (determined by WGA staining of sarcolemma) was determined by manually tracing the delineated fibers followed by calculation of fiber CSA in ImageJ 1.42q (NIH).
LC3A-GFP-RFP transfection
Coverslips were prepared by etching with concentrated nitric acid overnight. Then, the coverslips were washed with sterile PBS twice and placed into a 6-well plate. Cultured skeletal muscle cells were then transferred and allowed 4–5 hours to attach. Transient transfection was performed with plasmids pSG5-Nor-1 siRNA (small interfering RNA), pSG5-Nor-1 (29), pSG5 (Agilent Technologies) and LC3A-GFP-RFP (Steven Broomfield; Institute for Molecular Bioscience) using Lipofectamine 3000 as manufacturer's protocol (Invitrogen). Passage media were added and incubated overnight. The coverslips were fixed with formalin, washed with PBS and then flipped onto glass slides and sealed. The images were quantified by counting the intensity of the red fluorescence of LC3A-GFP-RFP-probed cells compared with the green fluorescence LC3A-GFP-RFP-probed cells using the values from integrated density in ImageJ 1.42q (NIH) (IntDen, mean gray value × area) generated after setting a empirical cut-off value to remove background noise.
Rapamycin treatment
C2C12 mouse skeletal muscle cells were cultured in 6-well plates and grown to 100% confluence before serum withdrawal and 4-day differentiating into myotubes. The C2C12s myotubes were treated with rapamycin 1 μg/mL (37094; Sigma) overnight and cells harvested for both Western blotting and qRT-PCR using RIPA (Radioimmunoprecipitation assay) buffer and TRI reagent, respectively.
Results
The expression of the mRNA encoding Nor-1 is induced after acute exercise in skeletal muscle
Transgenic overexpression of an activated form of Nor-1 (a member of the NR4A subgroup) in skeletal muscle (6, 8) induced contractile fiber remodeling, oxidative metabolism and increased endurance. To further explore the relationship between the Nor-1 and exercise in skeletal muscle, mice were subject to an acute (but moderate) 40 minutes of treadmill exercise (Figure 1A). After a bout of exercise, the expression of the Nor-1 mRNA from quadriceps femoris tissue was examined at multiple time points (30, 60, and 240 min) using qRT-PCR (n = 5) relative to non-exercised controls (Figure 1B). This revealed that Nor-1 mRNA expression is significantly induced 1 hour after exercise (Figure 1B).
Nor-1 mRNA expression is induced and regulated by calcium/calcineurin signaling
We further explored and investigated the association between exercise and Nor-1 expression and the role of Nor-1 in adaptive remodeling. Upon exercise, skeletal muscle receives multiple sources of stimulus, which can induce changes, such as: motor neuron (electrical) activity, calcium release causing contraction, stretch and strain on muscle, hormonal, heat production, and sympathetic signaling associated with changes in gene expression.
Since skeletal muscle contraction/exercise is mediated by the increases in intracellular calcium ion levels, we examined whether the induction of Nor-1 via exercise is associated with the induction of calcium ion levels. We treated differentiated C2C12 skeletal muscle cells (ie, myotubes) with 1μM calcium ionophore A23187 and observed that Nor-1 mRNA levels were significantly increased after 1 hour of treatment with the calcium ionophore (Figure 2A).
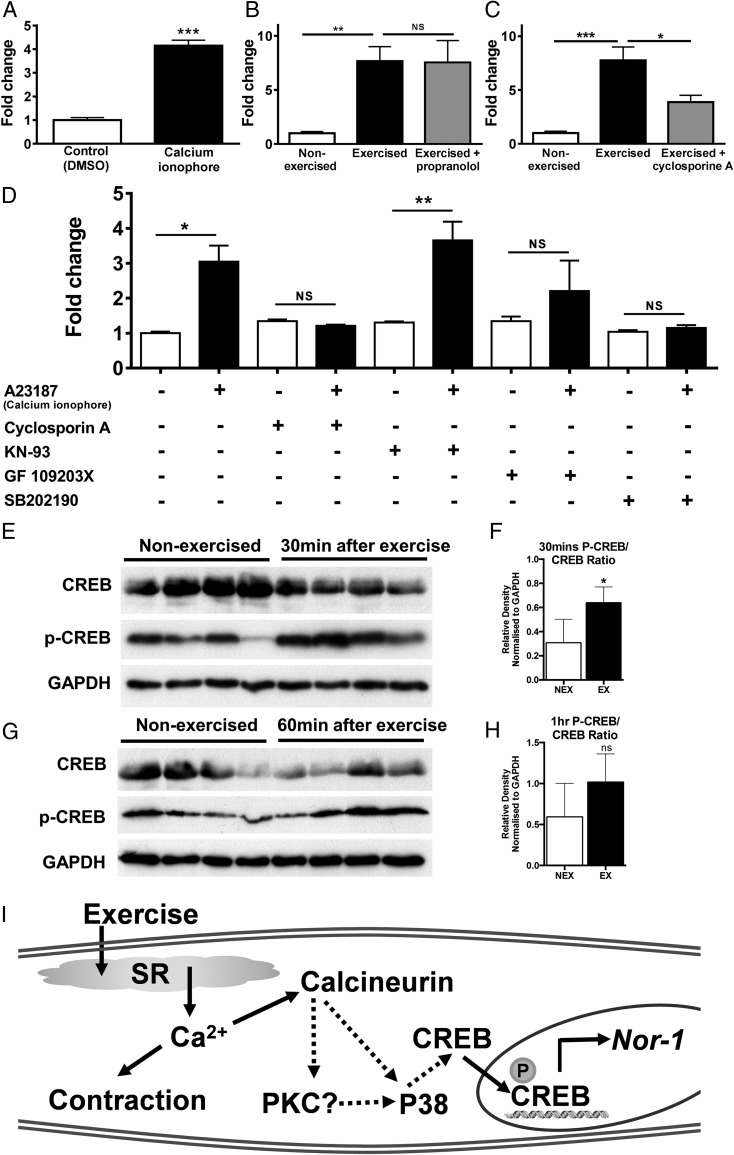
Figure 2. Nor-1 mRNA expression is induced and regulated by calcium/calcineurin signaling.
A, C2C12 skeletal muscle myotubes were treated with 1μM calcium ionophore A23187 or vehicle control (DMSO) for 1 hour. Nor-1 mRNA was measured by qRT-PCR relative to Hprt1 converted as fold change (n = 3). Mice were ip injected with the β-adrenergic antagonist propranolol 3 mg/kg (B), the calcineurin inhibitor cyclosporine A 10 mg/kg (C) or vehicle controls (saline) 30 minutes before 40 minutes of treadmill exercise or 40 minutes of rest for non-exercise controls. One hour after exercise, quadriceps femoris skeletal muscle was removed, mRNA extracted. Nor-1 mRNA was measured by qRT-PCR relative to Hprt1 converted as fold change (n = 5–6). D, C2C12 skeletal muscle myotubes were pretreated for 30 minutes with vehicle control (DMSO), the calcineurin inhibitor cyclosporine A (0.4μM), the CaMKII inhibitor KN-93 (2μM), the PKC inhibitor GF109203X (0.5μM), or the p38 MAPK inhibitor SB202190 (10μM). Cells were then treated with the calcium ionophore A23187 (1μM) or vehicle control (DMSO) for 1 hour. Nor-1 mRNA was measured by qRT-PCR relative to Hprt1 converted as fold change (n = 3) (E and G). Western blot analysis was performed on quadriceps femoris extracts from WT and Tg-Nor-1 skeletal muscle (n = 4) at 30 and 60 minutes after exercise and assayed for phosphorylated CREB (Ser133) and total CREB (F and H). Relative density normalized to GAPDH of Western blotting band intensity. I, Pathways that appear involved in the calcium/exercise dependent induction of Nor-1 mRNA expression. Statistical calculation was performed using a Student's t test or one-way ANOVA with Sidak's multiple comparisons test. P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
Our previous work demonstrated that the expression of Nor-1 and the NR4A subgroup are markedly and transiently induced after acute β-adrenergic signaling (23, 31). Given that exercise activates sympathetic output/β-adrenergic signaling (in addition to contractile signaling via motor neurons), this raises the possibility that sympathetic output during exercise is contributing to the induction of Nor-1 after exercise. In our previous work, we have used the pan β-adrenergic antagonist propranolol to specifically block the induction of Nor-1 after acute β-adrenergic signaling (31). Using this tool, mice were pretreated with propranolol (3 mg/kg) or vehicle control (saline) via an ip injection 30 minutes before exercise. The propranolol did not interfere with the ability of the mice to perform moderate exercise. The administration of the pan β-adrenergic antagonist did not block the exercised-induced induction of Nor-1 mRNA and this suggests that Nor-1 induction is being regulated independently of β-adrenergic signaling (Figure 2B) during the 40-minute acute (moderate treadmill) exercise treatment. As noted in Figure 2A, the calcium ionophore induced Nor-1 expression in vitro. Given that calcium ion levels are induced via exercise it would appear likely that Nor-1 induction by exercise (in vivo) involves calcium signaling. Calcineurin is a key target of exercise-induced calcium signaling in skeletal muscle (32, 33) and has been shown to induce Nor-1 expression in other tissues (34–36), the role of calcineurin was examined using the calcineurin inhibitor, cyclosporine A. Pretreatment of animals (n = 5) with cyclosporine A significantly inhibits and ablates the induction of Nor-1 expression after exercise (Figure 2C). Hence, calcinuerin signaling is necessary for the exercise dependent induction of Nor-1 mRNA.
We used the in vitro skeletal muscle cell culture system to examine the calcium and downstream signaling pathways regulating Nor-1 expression in more detail. Differentiated C2C12 myotubes were pretreated with 4 pathway blocking agents and then treated with the calcium ionophore, A23187, to mimic exercise and induce Nor-1 (Figure 2D). Confirming our in vivo result on cyclosporine A pretreatment with exercise above, we demonstrated that cyclosporine A treatment inhibits the A23187 ionophore induced Nor-1 expression in the cell culture model. Secondly, we demonstrate that pretreatment with the inhibitor SB202190 also inhibits Nor-1 induction after calcium ionophore treatment. However, the PKC inhibitor GF109203X and CaMKII (calmodulin kinase) inhibitor, KN-93, does not inhibit the significant increase in Nor-1 expression (Figure 2D). Thus, the calcium dependent induction of Nor-1 mRNA expression is not regulated by PKC or CaMKII activity signaling.
Moreover, another mediator of calcium signaling, phospho-CREB (Ser133) was found to be increased 30 and 60 minutes after exercise (Figures 2, E and F) however, it did not attain significance 60 minutes after exercise (Figures 2, G and H). This phosphorylation (and therefore activation) of CREB at Ser133 is likely to cause the induction of Nor-1 expression as CREB is known to directly transactivate the Nor-1 promoter which is known to contain characterized CREB-responsive cAMP-response elements (37–39). The cAMP-regulated transcriptional coactivators (CRTC1–CRTC3) are critical coactivators of CREB (40). The expression of these proteins were not changed after 60 minutes of exercise or in Tg-Nor-1 mice (Supplemental Figure 1, A and C). However, Crtc1 expression was induced by the calcium ionophore in C2C12 myotubes (Supplemental Figure 1B).
In summary, Figure 2I shows the likely pathways that maybe involved in the calcium/exercise dependent induction of Nor-1 mRNA expression.
Transgenic Nor-1 expression is associated with increased vascularization in muscle and skeletal muscle hypertrophy
Skeletal muscle is an important metabolic organ, and accounts for approximately 40% of energy expenditure and body mass. Muscle has the capability and plasticity to respond to a pleiotropic range of stimuli, including exercise, and this involves skeletal muscle (fiber type) reprogramming to accommodate the (glycolytic and oxidative) metabolic demands. These exercise dependent events are associated with angiogenic events, such as, increased vascularization required to meet these new muscle physiological demands. The Tg-Nor-1 mice are associated with a dramatic acquisition of type IIA and IIX oxidative fibers, and a decrease in glycolytic type IIB. This phenotype is often linked to increased angiogenic potential with higher levels of angiogenic factors to avoid ischemic damage during oxidative metabolic demands (41).
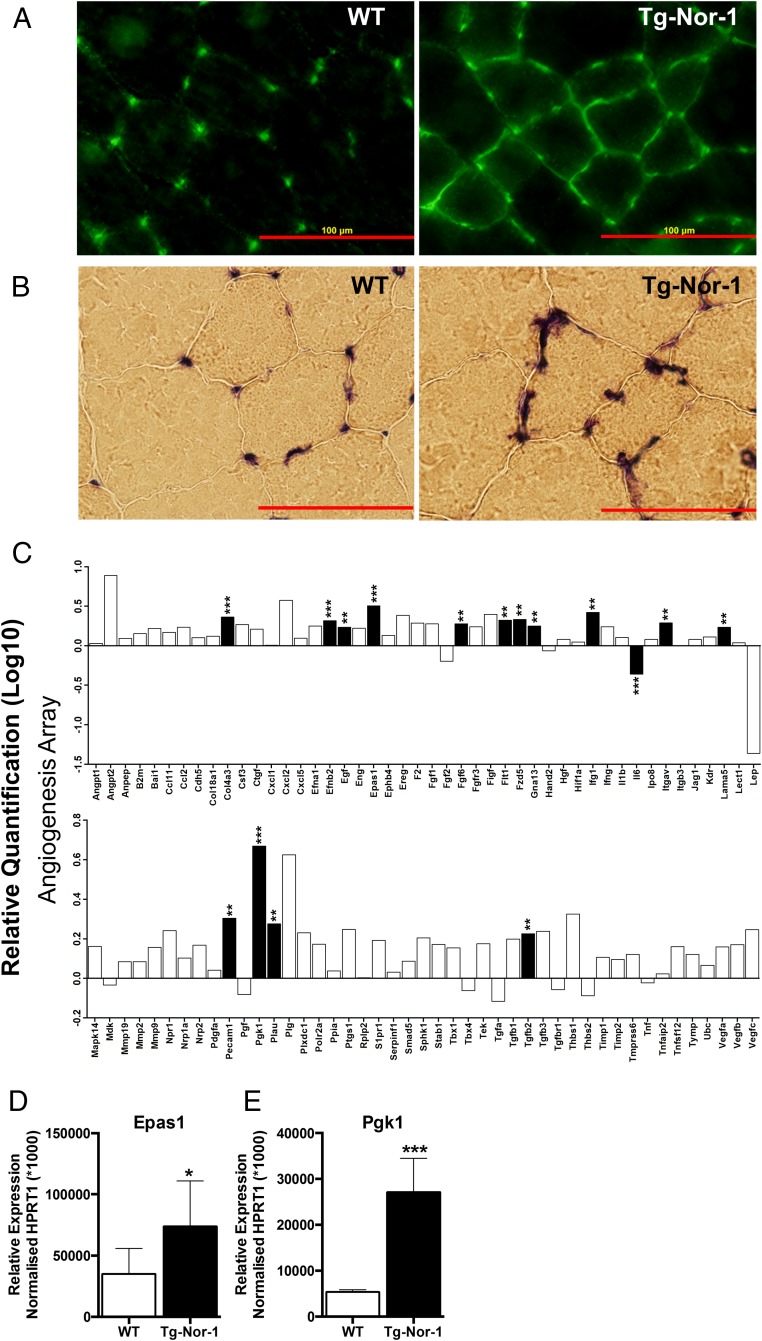
We demonstrate that ectopic transgenic expression of Nor-1 in mice, results in significantly increased vascularization of skeletal muscle, as shown by increased Pecam staining in skeletal muscle from transgenic mice (Figure 3A). Furthermore, the Pecam staining pattern demonstrates that there is increased cross-linking of the blood vessels, highlighted by increased transverse blood vessels compared with parallel blood vessels, respective to the direction of muscle fiber filaments (Figure 3A). This was further confirmed with phosphatase alkaline staining, which was significantly increased (Figure 3B). We used a QIAGEN Angiogenesis Array to investigate the mRNA expression of a number angiogenic genes driving vascular development (Figure 3C). A statistically significant induction of several genes implicated in neovascularization including Epas1, Pecam1, Col4a3, Flt1, and Itgav (Figure 3C). The increased mRNA levels of Epas1 (Figure 3D) and Pgk1 (Figure 3E) were also verified using qRT-PCR.
Figure 3. Transgenic Nor-1 expression is associated with increased vascularization in muscle and skeletal muscle hypertrophy.
A, Tibialis anterior skeletal muscle sections were taken from both WT and Tg-Nor-1 (n = 3). Samples were probed with PECAM antibody and B, stained with alkaline phosphatase. C, A QIAGEN qRT-PCR angiogenesis array RQ (log10) was performed on quadriceps femoris skeletal muscle with cDNA extracted from WT and Tg-Nor-1 mice (n = 4). Data are derived from StatMiner analysis and expressed as fold changes normalized to the mean of geNorm selected most stable controls with the least expression variation, Hprt1 and Gapdh median. D and E, qRT-PCR was performed on chow-fed quadriceps femoris from WT and Tg-Nor-1 mice (n = 4). Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
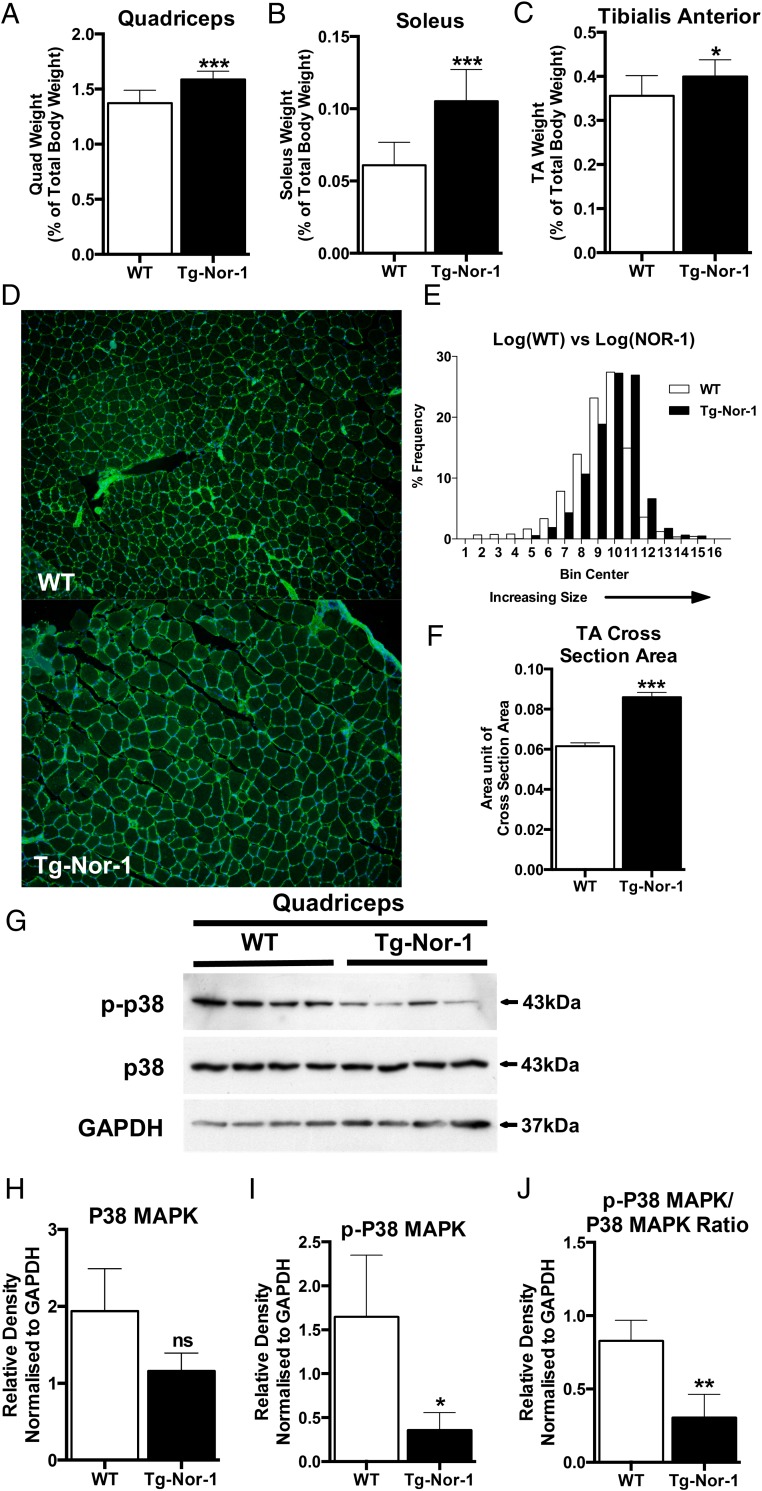
Exercise induced contractile protein remodeling in exercise is also often associated with hypertrophy, and hence we investigated the skeletal muscle of Tg-Nor-1 mice and WT littermates and determined that the quadriceps (Figure 4A), soleus (Figure 4B) and tibialis anterior (Figure 4C) displayed significantly increased mass in Tg-Nor-1 mice. Furthermore, we examined the cross-sectional area of the skeletal muscle fibers by WGA staining of the sarcolemma. This revealed that the area of the Tg-Nor-1 skeletal muscle is significantly increased relative to the WT fibers (Figure 4, D–F)
Figure 4. Transgenic Nor-1 expression is associated with increased skeletal muscle hypertrophy.
Skeletal muscle tissue was surgically removed from WT and Tg-Nor-1 mice and weighed for (A) quadriceps, (B) soleus, and (C) tibialis anterior. Values are expressed as percentage relative to total body weight (n = 10). D, WGA staining of the sarcolemma of tibialis anterior cross-sections from WT and Tg-Nor-1 mice. E, Histogram of distribution of fiber cross-section area sizes (n = 5). F, Average fiber cross-section area sizes in WT and Tg-Nor-1 mice (n = 5). G, Western blot analysis profiling autophagy markers performed on WT and Tg-Nor-1 mice (n = 4) quadriceps femoris tissue. H–J, Relative density normalized to GAPDH of Western blotting band intensity. Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
The gene expression changes potentially involved in skeletal muscle hypertrophy were also investigated using expression profiling of RNA from quadriceps femoris tissue on custom TLDAs designed to identify potential myogenic genes (Supplemental Figure 2). We determined that there were a number of significant changes in genes regulating muscle mass and hypertrophy. Interestingly, we observed a very significantly decreased expression of MuRF1/Trim63 (the atrophy related gene involved in muscle integrity), decreased myostatin, Transforming growth factor, beta receptor II (Tgfbr2), Smad3 and Smad4 expression, coupled to increased Smad7 expression. These changes in gene expression are associated with myostatin signaling and are consistent with hypertrophy, and decreased adiposity (observed in these transgenic mice). These changes in gene expression were associated with the previously reported changes in fiber type associated with increased IIA and IIX, and decreased IIB expression.
The p38 MAPK pathway has been implicated in muscle development, exercise, and calcium/calcineurin signaling, and we observed significant down-regulation in phosphorylated-p38 MAPK compared with total p38 MAPK expression (Figure 4G).
The transgenic Nor-1-dependent endurance phenotype is associated with enhanced autophagic activity; increased expression of LC3A-II, and other markers of autophagosome formation
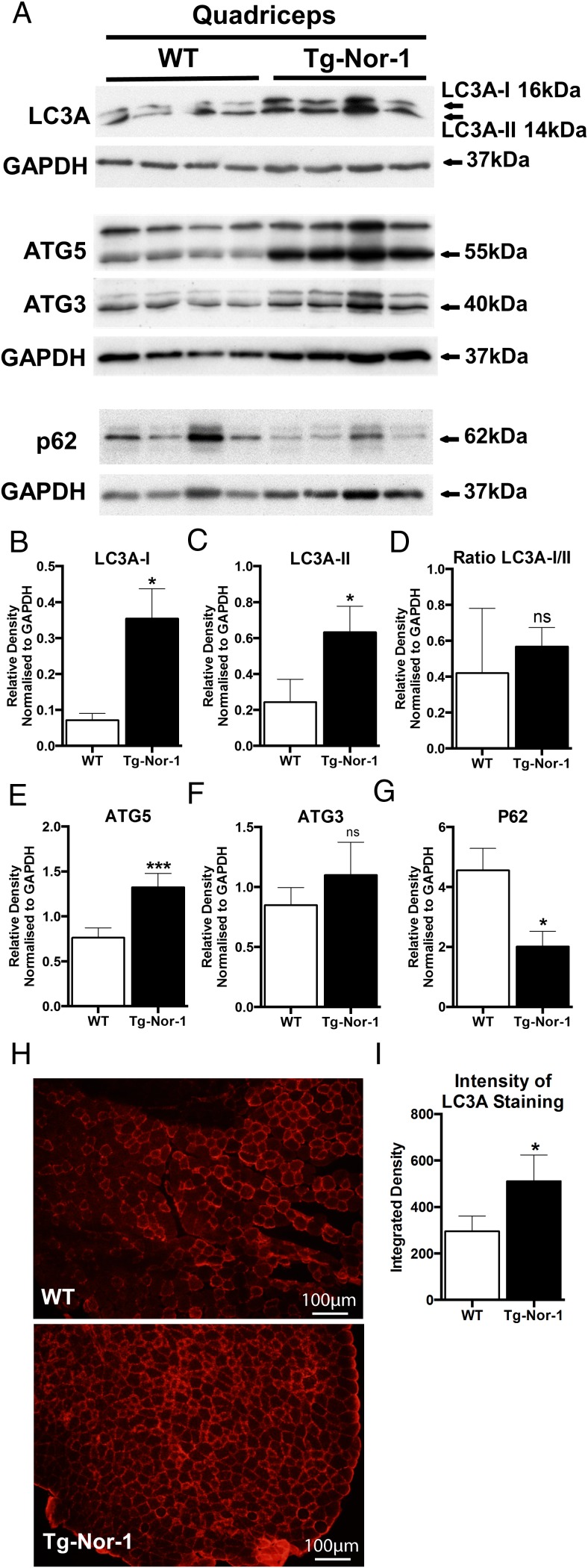
Autophagy is known to be induced after exercise (14, 15) and appears to mediate adaptations after exercise (14, 19, 20). Hence, we investigated whether transgenic Nor-1 expression influenced the autophagic process. We conducted Western blot analysis of cellular extracts from quadriceps femoris tissues (from fasted WT and Tg-Nor-1 mice) with antibodies against critical markers of autophagosome and autophagolysome formation including LC3A, Atg5, Atg3, and p62. Western blotting with an antibody against LC3A identified increased expression of LC3-I and LC3-II (the form of LC3-I covalently linked to phosphatidylethanolamine) (Figure 5A) that is recruited into autophagosome membranes and engaged in binding cargo. We observed similar changes in gastrocnemius tissue from this transgenic line (Supplemental Figure 3). LC3A is a protein that has been identified on the inner and outer membranes of the autophagosome, and the expression of LC3A-II correlates with autophagosome development, and the conversion of LC3A-I to LC3A-II is a reflection of autophagic activity. Moreover, we observed increased levels of Atg5 in quadriceps femoris extracts from Tg-Nor-1 mice relative to WT littermates (Figure 5A). Atg5, an E3 ubiquitin ligase is essential for autophagosome elongation, and necessary for conversion of LC3A-I to LC3A-II. Subsequently, we analyzed the levels of p62, a protein that targets cellular waste products for recycling, ie, identifies cargo for autophagy. Decreased autophagy leads to an accumulation of p62, because the autophagic process does not sequester it. In concordance with increased LC3A-II, we observed decreased expression of p62 in skeletal muscle extracts from Tg-Nor-1 mice relative to WT littermates. Moreover, quantification of Western blot analysis (relative to GAPDH) revealed that the increased expression of LC3A-II, and Atg5, and reduced expression of p62, respectively, were significant (Figure 5, B–E).
Figure 5. Tg-Nor-1 expression is associated with LC3A and autophagy activity.
A, Western blot analysis profiling autophagy markers was performed on WT and Tg-Nor-1 mice (n = 4) quadriceps femoris tissue. B–E, Relative density normalized to GAPDH of Western blotting band intensity. F, Skeletal muscle from WT and Tg-Nor-1 tibialis anterior was stained with LC3A (representative image shown, n = 3). G, The intensity of the fluorescence of the LC3A-probed cells probed cells were quantified using the values from integrated density in ImageJ 1.42q (NIH) (IntDen, mean gray value × area) generated after setting an empirical cut-off value to remove background noise. Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
At the microstructural level, immunofluorescence on tibialis anterior skeletal muscle sections were performed and determined that there was a significant increase in LC3A expression in Tg-Nor-1 muscle sections compared with WT littermates (Figure 5F). The intensity of the fluorescence of LC3A-probed cells was quantified using the values from integrated density in ImageJ 1.42q (NIH) (IntDen, mean gray value × area) were generated after setting an empirical cut-off value to remove background noise. Data were statistically analyzed and found to be significant P < .05 (Figure 5G). The immunofluorescence suggested the WT samples have a baseline level of LC3 expression isolated to a few fibers, whereas the Nor-1 animal sections show autophagy marker activity in nearly every cell.
In summary, we demonstrate that Tg-Nor-1 expression increases the expression of LC3A-II (in accord with increased levels of Atg5) and decreased p62 expression in concordance with increased autophagosome formation, and protein sequestering.
Nor-1 expression regulates components of the mTOR complex 1 (mTORC1) in skeletal muscle
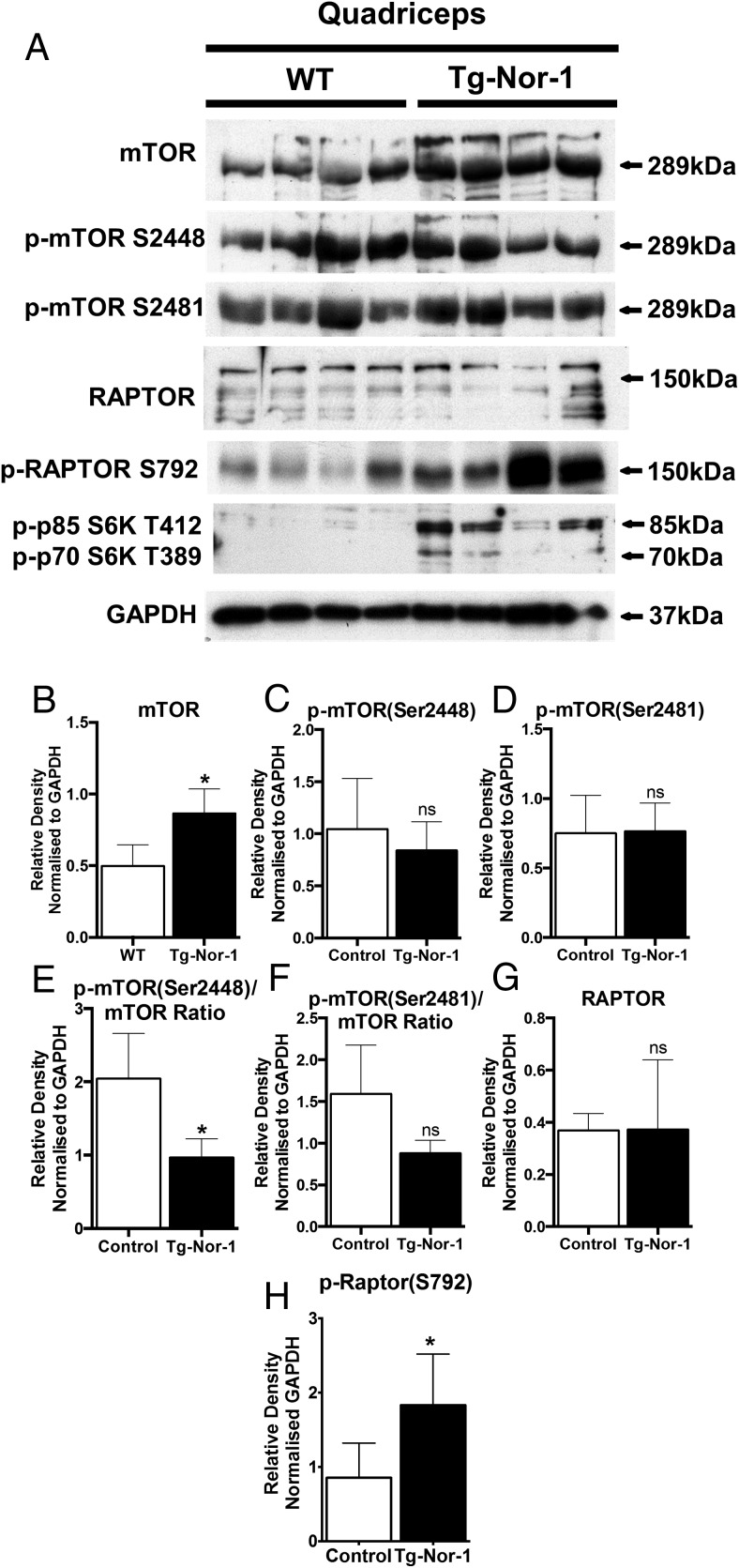
The expression of mTOR and the other components of mTORC1 were investigated by Western blot analysis. The expression of mTOR functions as a major upstream negative regulator of autophagic activity. We observed a significant increase in mTOR expression (Figure 6A). No significant changes were observed in the expression of phosphorylated mTOR Ser2448, phosphorylated mTOR Ser2481, and (total) Raptor (Figure 6, A, C, D, F, and G). However, the increase in mTOR resulted in a relative decrease in the ratio of phosphorylated mTOR Ser2448 relative to total mTOR expression (Figure 6E), this suggested the decreased mTOR activity is in accordance with increased LC3A-II expression, decreased p62 expression and an increase in autophagy. This is further substantiated by significantly increased levels of phosphorylated-Raptor Ser792 expression in muscle extracts from Tg-Nor-1 that is concordant with suppression of mTORC1 activity (42) and enhanced autophagy (Figure 6, A and H). Interestingly, 2 phosphorylation targets of mTOR kinase activity, the S6 kinase isoforms (p70/p85), were both phosphorylated in the Tg-Nor-1 mouse line (Figure 6A).
Figure 6. Nor-1 expression regulates components of mTORC1 in skeletal muscle.
A, Western blot analysis was performed on quadriceps femoris extracts from WT and Tg-Nor-1 skeletal muscle (n = 4) and assayed with mTOR markers. B–H, Relative density normalized to GAPDH of Western blotting band intensity. Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
In summary, the differential expression and phosphorylation status of components in the mTORC are in accord with attenuated mTORC1 activity, and increased expression of autophagy markers (including LC3A-II and Atg5) and increased autophagosome formation.
Rapamycin treatment (ie, inhibition of mTOR activity) increases Nor-1 expression
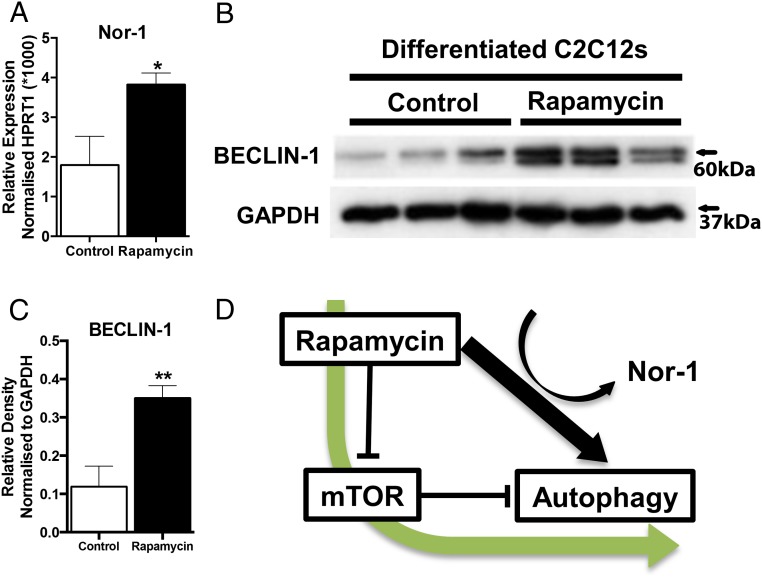
We further explored the relationship between Nor-1 expression and the (negative) regulator of autophagy, mTOR in an in vitro skeletal muscle cell culture model. We treated differentiated C2C12 skeletal muscle cells with the mTOR inhibitor, rapamycin, an agent that targets and binds the cytosolic protein FK-binding protein 12, which directly inhibits mTORC1 (although partially). We determined that rapamycin treatment significantly increased the expression of the mRNA encoding Nor-1 (Figure 7A, and summarized in Figure 7D). This increase in the expression of Nor-1 mRNA was accompanied by the significantly increased protein expression of Beclin-1 in rapamycin treated C2C12 cells (Figure 7, B and C). Beclin-1 is a critical component of a complex necessary for autophagy and this autophagic function of Beclin-1 are robustly conserved during eukaryotic evolution. In summary, the in vitro analysis suggests suppression of mTORC1 activity is associated with increased Nor-1 expression.
Figure 7. Rapamycin treatment and Tg-Nor-1 regulate autophagy activity.
A, RT-PCR was performed on control and rapamycin treated differentiated C2C12's and the expression of Nor-1 was assayed. B, Western blot analysis was used to assay Beclin-1 expression on WT and Tg-Nor-1 (n = 4) quadriceps femoris protein extracts. C, Relative density normalized to GAPDH of Western blotting band intensity. D, Diagram illustrating function of rapamycin, mTOR, and Nor-1 on autophagy. Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
Nor-1 expression increases autophagolysosome formation in skeletal muscle cells; acute exercise induced LC3A-II expression in WT C57BL/6 mice
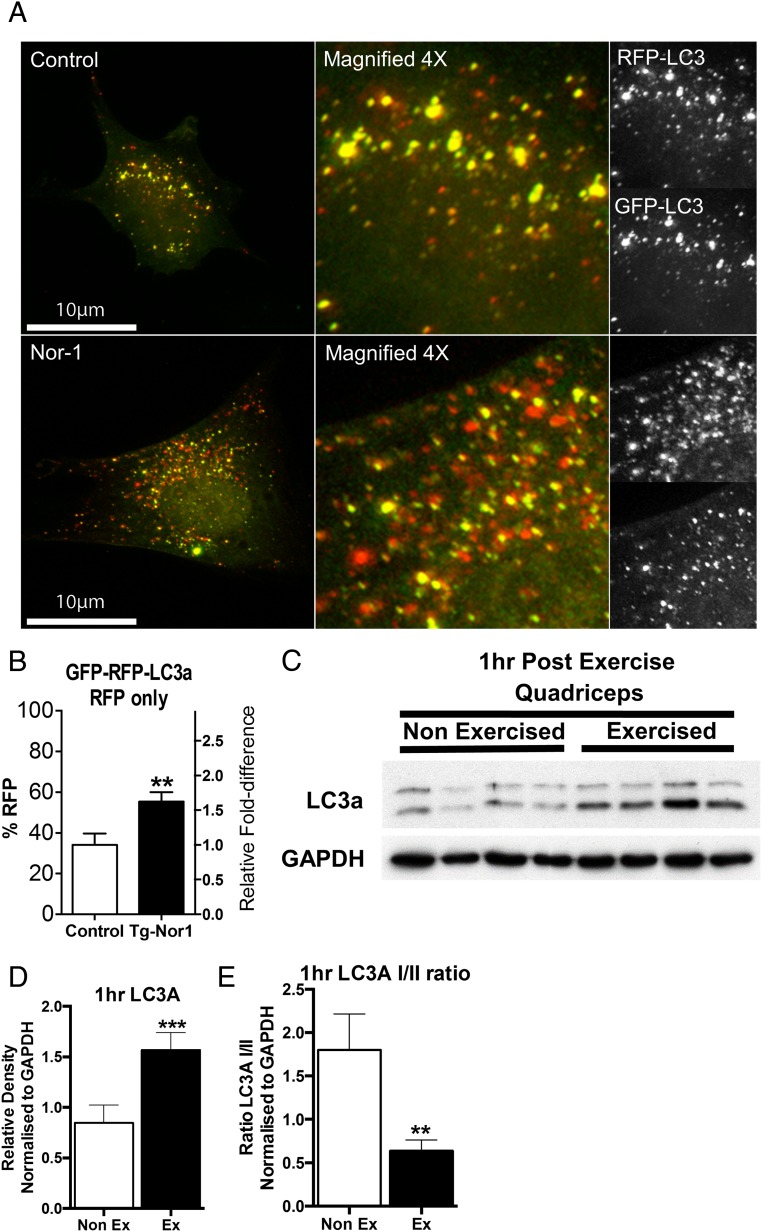
Accordingly, the link between Nor-1 and autophagy was further investigated in skeletal muscle cells (in vitro), using the C2C12 mouse skeletal muscle cell culture system. We used the LC3A-GFP-RFP expression vector that tags LC3A concurrently with both GFP and RFP tags. The fluorescence of GFP is sensitive to pH and thus the acidic environment of the fused lysosome will ablate the GFP fluorescence, whereas RFP is more stable at low pH and will still fluoresce in the acidic environment. Moreover, a reduction in green fluorescently tagged LC3A highlights that the LC3A and autophagosome has fused with the lysosome forming the autophagolysosome and therefore, acidification and degradation of internal components. By examining the amount of RFP fluorescence relative to GFP fluorescence, we could quantify the amount of autophagolysosome formation and thus end state autophagy and sequestered component degradation. The LC3A-GFP-RFP construct was cotransfected with and without Nor-1 into skeletal muscle cells (Figure 8, A and B). We were able to observe autophagic activity acutely regulated by expression of the nuclear receptor Nor-1 (Figure 8A). Furthermore, we were able to examine the extent of lysosomal degradation of internal sequestered components and thus the end state of autophagy activity. We demonstrate that there is a significant increase in red fluorescence in the C2C12 skeletal muscle cells transfected with Nor-1 (Figure 8, A and B).
Figure 8. Nor-1 expression increases autophagolysosome formation in skeletal muscle cells.
A, C2C12 myoblasts were cotransfected with LC3A-GFP-RFP and with either Nor-1 expression vector (Nor-1) or empty vector (control). B, The level of RFP fluorescence as a percentage relative to GFP fluorescence was quantified using OBCOL plugin in ImageJ. C, Western blot analysis was performed on nonexercised and exercised quadriceps femoris muscle extracts 1 hour after exercise (n = 4). D and E, Relative density normalized to GAPDH of Western blotting band intensity. Statistical calculation was performed using a Student's t test, and P values are indicated on graphs as follows: *, P < .05; **, P < .01; ***, P < .001.
Our previous experiments underscored the links between exercise and Nor-1 expression, and transgenic Nor-1 expression and autophagy. We further explore the relationship between exercise and autophagy under physiological conditions that induced Nor-1 expression. We examined mice exercised for 40 minutes and subsequently killed 0, 30, 60, and 240 minutes after exercise (Figure 1A). Cellular extracts from Quadriceps femoris skeletal muscle tissue were subsequently prepared for Western blot analysis. We observed that LC3A-II expression was significantly induced 1 hour after exercise (Figure 8, C and D) and that the LC3A-I to LC3A-II ratio was significantly decreased (Figure 8, C and E). These observations are in agreement with our analysis of the Tg-Nor-1 mice that indicated overexpression of Nor-1 in skeletal muscle induces autophagy and link Nor-1 signaling with exercise-induced autophagy in vitro and in vivo. In summary, we observe that exogenous Nor-1 expression induces autophagolysome formation. These in vitro studies are in concordance with the effects of transgenic Nor-1 expression on autophagy-related protein expression in skeletal muscle in vivo.
Discussion
Exercise is known to induce numerous beneficial health adaptations and a number of nuclear hormone receptors (NRs) have been implicated in mediating the metabolic, contractile, and vascular reprogramming associated with acute and chronic exercise and fatigue resistance. Specifically, we have demonstrated that transgenic expression of the NR, Nor-1, in skeletal muscle improves endurance, that is associated with skeletal muscle fiber type reprogramming (ie, the acquisition of a IIA/IIX phenotype) associated with oxidative metabolism, elevated muscle glycogen levels, and increased mitochondrial density. Therefore, we investigated the association between Nor-1 signaling and exercise in vitro and in vivo and the adaptive reprogramming induced by expression of Nor-1
We demonstrate that Nor-1 expression is induced by 1) in vivo exercise, and 2) in vitro Ca2+ ionophore treatment of cells, and that the in vivo Nor-1 response to a (40 min) acute bout of moderate exercise is dependent on calcium/calcineurin signaling. Analysis of fatigue-resistant Nor-1 transgenic mice revealed the endurance phenotype is associated with increased hypertrophy, vascularization of muscle tissue, and autophagy. Furthermore, additional in vitro and in vivo approaches confirmed the association between Nor-1 expression, autophagy, and exercise providing rigorous evidence for the links between Nor-1 signaling and adaptive reprogramming responses to altered physiological demands.
The involvement of calcium/calcineurin dependent signaling in Nor-1 expression is not entirely surprising. Firstly, we reported that the transgenic Nor-1 fatigue-resistant phenotype is associated with differential expression of sarcomeric and Z-disc-binding proteins, calcineurin-binding proteins (Myoz/calsarcins 1–3, which are involved in muscle regeneration, fiber type remodeling) and several regulators of calcineurin signaling (8). In addition, we showed this Nor-1 expression results in decreased expression of α-actinin 3, and compensatory (increased) expression of α-actinin 2 in accordance with improved endurance (8). Subsequently, Seto et al (43) further confirmed that α-actinin 3 deficiency regulates muscle performance and contractile remodeling by modulating calcineurin signaling via interactions with calsarcins.
Nor-1 transgenic display an increase in mitochondrial number and proteins content (6). In the context of exercise-induced Nor-1 expression mediated by calcium/calcineurin signaling, calcineurin has been implicated as a regulator of exercise-induced mitochondrial biogenesis (33). Although evidence points to PCG-1α (Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha), as a regulator of exercise-induced mitochondrial biogenesis via calcineurin (33), it appears likely that Nor-1 would also be involved in the exercise-induced mitochondrial biogenesis. Interestingly, both Nor-1 and PCG-1α are CREB-responsive transcripts and may both work together to mediate mitochondrial biogenesis.
The increased vascularization was associated with increased levels of several key genes implicated with enhanced angiogenesis/neovascularization including the endothelial genes hypoxia-inducible transcription factor Epas1 and the VEGF (Vascular endothelial growth factor) receptor Flt1. Increased levels of Pecam1 were also observed, but these could simply reflect a greater number of endothelial cells supporting the vascularization. In endothelial cells, Epas1 transcribes both Vegf and the Vegf receptor Flt1 (44), possibly providing the mechanism of enhanced vascularization. However, the link of how ectopic Nor-1 expression in skeletal muscle cells induces Epas1 expression in vascular endothelial cells remains obscure.
Given that the Nor-1 transgenic mouse has a switch towards an oxidative type IIA/IIX fiber phenotype, we would expect that the skeletal muscle fibers and skeletal muscle mass would be reduced as type IIA/IIX fibers are smaller than the predominant type IIB fibers in mice. However, the Nor-1 transgenic appears to have a moderate level of skeletal muscle hypertrophy despite the fiber type change with increased fiber thickness and skeletal muscle mass. The p38 MAPK pathway regulates expression of structural genes, activity of myogenic transcription factors, and chromatin remodeling (45), interesting, it has been shown that elevated p38 MAPK signaling suppresses stemness and renewal of muscle in older mice and that attenuation of the activity rescued impaired satellite cell dependent regeneration of fibers (46). This suggests that Nor-1 drives improved renewal and repair of muscle and the fiber type transitions involved in cell renewal.
In the Tg-Nor-1 mouse line, we identified several protein changes indicative of active autophagy, which included increased expression of LC3A-II (Western blotting and immunofluorescent staining), Atg5 and decreased p62 protein expression. These protein changes were entirely consistent with enhanced autophagy in skeletal muscle (47, 48). Furthermore, immunofluorescent staining of skeletal muscle and quantification revealed significant increases in the expression of LC3A in Tg-Nor-1 mice. This result highlights that the necessary autophagosomal components needed for effective autophagy are up-regulated in the Tg-Nor-1 mice, therefore, suggesting that Tg-Nor-1 have enhanced skeletal muscle autophagy. Moreover, we demonstrated in a cell culture model, that rapamycin treatment of skeletal muscle cells, which activates the autophagic process by attenuating the mTORC induces Nor-1 mRNA expression in differentiated myogenic cells. Additional in vitro experiments demonstrated that exogenous expression of Nor-1 in myogenic cells increases the formation of autophagolysosomes. Finally, we demonstrated that 1 hour after acute exercise in WT (C57BL/6) mice there is a parallel induction of Nor-1 mRNA expression and LC3A-II expression, a critical structural marker of autophagosome development. The activation of autophagy is apparent in many pathophysiological states where there is skeletal muscle atrophy (reviewed in Ref. 16) and this would appear to be in contrast to the observation of both hypertrophy and autophagy in the Tg-Nor-1 mouse line. However, autophagy has been found to be induced after exercise (14, 15). Given that hypertrophy can also occur after exercise, the activation of autophagy would appear compatible with hypertrophy. Furthermore, autophagy appears to be mediate adaptations after exercise involving endurance (19, 20), angiogenesis (19), and metabolic changes (14, 19, 20). In conclusion, our in vitro and in vivo studies demonstrate the association between Nor-1 expression, exercise, and the induction of the autophagic process and highlight the physiological association of skeletal muscle autophagy, exercise and the Tg-Nor-1 phenotype.
The mTOR signaling is key regulator of autophagy and was examined in the Tg-Nor-1 mouse model. mTOR is well known as a master regulator of multiple pathways/processes and including autophagy (49). In our study, the mTOR/mTORC1 activity appeared to be reduced in the Tg-Nor-1 mouse model based on the reduced p-mTOR to mTOR ratio and increased phosphorylation of Raptor at Ser792, which would deactivate the mTORC1 complex. Because mTOR/mTORC1 activity repressed autophagy activity, a reduction in mTOR/mTORC1 activity should therefore enhanced autophagy by derepression (42). Interestingly, 2 phosphorylation targets of mTOR kinase activity, the S6 kinase isoforms (p70/p85) both had increased phosphorylation in the Tg-Nor-1 mouse model which is inconsistent with a reduction in mTOR/mTORC1 activity. Phosphorylation and activation of the S6 kinase isoforms appears to be a major downstream mechanism by which mTOR induces protein synthesis and skeletal muscle hypertrophy (50, 51). Because the S6 kinase isoforms have strikingly increased phosphorylation in the background of reduced mTOR/mTORC1 activity, this would strongly suggest that a non-mTOR kinase is phosphorylating the S6 kinase isoforms. These observations are supported by the study of Eliasson et al (52) that demonstrated that maximal eccentric contractions (ie, exercise that produces greater tension and stretching of the muscle/straight leg to bent leg) led to 2- to 8-fold increases in the phosphorylation of p70S6k and the ribosomal protein S6 that persisted for 2 hours into recovery independent of significant changes in phosphorylation of mTOR or Protein kinase B (Akt). Specifically, maximal eccentric contractions induced an increase in p70S6k phosphorylation on Ser424/Thr421 and Thr389 in muscle (phosphorylation of Ser424/Thr421 is required for phosphorylation of Thr389 necessary for complete activation of p70S6k). In addition, Nader and Esser (53) demonstrated that p70S6k phosphorylation was only observed in response to a growth-inducing stimulus (ie, high-frequency electrical stimulation-driven eccentric exercise associated with hypertrophy). Finally, Parkington et al (54) demonstrated that mTOR phosphorylation in response to exercise occurs in a fiber type-specific manner. The phosphorylation and activation of the S6 kinase isoforms is therefore consistent with our observation of skeletal muscle hypertrophy in the Nor-1 mouse line.
We further explored the role of Nor-1 and the regulation of autophagic events in vitro in the well-characterized C2C12 myogenic cell culture model. Treatment of differentiated C2C12 myotubes cells with rapamycin (that attenuates mTORC1 activity and enhances autophagy) induced Nor-1 mRNA expression. This was coupled to significantly increased expression of Beclin-1 a highly conserved regulator of autophagy (55). This further highlights potential cross talk between mTOR, the nuclear receptor Nor-1 and autophagy. Moreover, we speculate from the in vitro and in vivo results, that Nor-1 is involved in down-regulating mTORC1 activity. The links between Nor-1 expression and autophagic activity in vitro were verified using the LC3A-GFP-RFP chimeric construct that demonstrated Nor-1 expression increased the formation of the autophagolysosome formation, and therefore, acidification and degradation of internal components, ie, thus end state autophagy. These data are in accordance with the increased LC3A-II expression and decreased p62 expression in the skeletal muscle extracts prepared from fasted Tg-Nor-1 mice, and suggest transgenic expression of Nor-1 is directly linked with increased autophagic cycling events.
Finally, to further investigate the link between Nor-1, autophagy and exercise, we examined the expression of Nor-1 and LC3A expression, after 1h exercise in WT C57BL/6 mice. We observed increased expression of Nor-1 mRNA and LC3A-II expression in quadriceps femoris skeletal muscle from exercise vs nonexercised mice. This further validates the association between the fatigue-resistant oxidative type IIA/IIX phenotype in the Tg-Nor-1 mice and enhanced autophagy. Moreover, this is consistent with the literature, which shows that oxidative skeletal muscle fibers display enhanced autophagy compared with glycolytic fibers (19) and LC3A-II levels correlates with the percentage of type IIA fibers (56). It is established that exercise driven autophagy is reputed as being one of the important health improving benefits associated with training. We speculate that Nor-1 signaling is one of the important molecular pathways underlying the regulation of autophagy by exercise, in response to the increased energy demands imposed on this contractile tissue. This further demonstrates that Nor-1 expression mimics and drives the exercise phenotype in the absence of physical activity. This is consistent with very recent studies indicating the NRs PPARα and Farnesoid X receptor (FXR) control autophagy and energy homeostasis in response to the nutrient conditions (57).
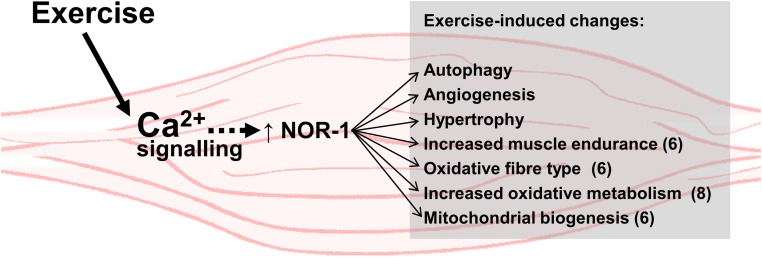
In summary, Nor-1 is an exercise/calcium signaling-induced transcription factor that when overexpressed in skeletal muscle drives a phenotype that mimics physiological changes associated with exercise. These changes (summarized in Figure 9) include 1) striking acquisition of an oxidative type IIA/IIX fiber phenotype; 2) increased mitochondrial density and oxidative enzyme staining/capacity; 3) enhanced markers of autophagy; 4) increased expression of genetic programs driving oxidative metabolism including; 5) increased muscle endurance; 6) skeletal muscle hypertrophy; 7) increased vascularization of skeletal muscle; and 8) resistance to diet-induced obesity. This demonstrates that Nor-1 expression drives, and is associated with the gamut of pathways that are critical responses to physical activity and adaptive responses that improve and prevent pathophysiological diseases associated with sedentary lifestyles and diet-induced chronic diseases. Furthermore, the induction of Nor-1 by exercise suggests that Nor-1 mediates part of the transcriptional response that occurs in skeletal muscle after exercise. Given the scope of the beneficial adaptive responses associated with physical activity, this suggests that activators/agonists of Nor-1 have potential as agents to mimic exercise and potentially help ameliorate obesity, diabetes, and autophagic diseases.
Figure 9. Summary of the suggested functional role of Nor-1 in skeletal muscle in relation to exercise-induced adaptations.
Nor-1 expression is increased after exercise in a calcium ion and calcineurin-dependent manner, and we suggest that this response partly regulates the transcriptional response in skeletal muscle after exercise.
Acknowledgments
G.E.O.M. is a Principal Research Fellow of the National Health and Medical Research Council.
The project was supported by core funding from The Institute for Molecular Bioscience, The University of Queensland. Joel M. Goode is the recipient Australian Postgraduate Award, and Zewen K.Tuong is the recipient of The University of Queensland International Scholarship.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
The project was supported by core funding from The Institute for Molecular Bioscience, The University of Queensland. Joel M. Goode is the recipient Australian Postgraduate Award, and Zewen K.Tuong is the recipient of The University of Queensland International Scholarship.
Footnotes
- ATG
- autophagy protein
- CaMKII
- Calcuim/calmodulin-dependent protein kinase II
- CREB
- cAMP response element binding protein
- Crtc
- CREB Regulated trasncription coactivator
- DMSO
- Dimethyl sulfoxide
- Gapdh
- Glyceraldehyde 3-phosphate dehydrogenase
- GFP
- Green-fluorescent protein
- LC3A
- Microtubule-associated protein-Light chain 3A
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- Nor-1
- Neuron-derived orphan nuclear receptor-1
- NR
- nuclear hormone receptor
- p70S6k
- p70 S6 kinase
- Pecam
- Platelet endothelial cell adhesion molecule
- qRT-PCR
- quantitative Real-Time PCR
- RFP
- Red-fluorescent protein
- NR4A
- Nulcear receptor subfamily 4A
- RQ
- relative quantification
- TLDA
- TaqMan low-density array
- WGA
- wheat germ agglutinin
- WT
- Wild-type.
References
- 1. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. [DOI] [PubMed] [Google Scholar]
- 2. Handschin C, Spiegelman BM. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pearen MA, Muscat GE. Orphan nuclear receptors and the regulation of nutrient metabolism: understanding obesity. Physiology (Bethesda). 2012;27:156–166. [DOI] [PubMed] [Google Scholar]
- 4. Wang YX, Zhang CL, Yu RT, et al. . Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao LC, Wroblewski K, Ilkayeva OR, et al. . Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization. J Lipid Res. 2012;53:2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pearen MA, Eriksson NA, Fitzsimmons RL, et al. . The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol Endocrinol. 2012;26:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rangwala SM, Wang X, Calvo JA, et al. . Estrogen-related receptor γ is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pearen MA, Goode JM, Fitzsimmons RL, et al. . Transgenic muscle-specific Nor-1 expression regulates multiple pathways that effect adiposity, metabolism, and endurance. Mol Endocrinol. 2013;27:1897–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. [DOI] [PubMed] [Google Scholar]
- 10. Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci USA. 2000;97:14632–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002;277:13998–14004. [DOI] [PubMed] [Google Scholar]
- 12. Swoap SJ, Hunter RB, Stevenson EJ, et al. . The calcineurin-NFAT pathway and muscle fiber-type gene expression. Am J Physiol Cell Physiol. 2000;279:C915–C924. [DOI] [PubMed] [Google Scholar]
- 13. Calabria E, Ciciliot S, Moretti I, et al. . NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA. 2009;106:13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He C, Bassik MC, Moresi V, et al. . Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jamart C, Benoit N, Raymackers JM, Kim HJ, Kim CK, Francaux M. Autophagy-related and autophagy-regulatory genes are induced in human muscle after ultraendurance exercise. Eur J Appl Physiol. 2012;112:3173–3177. [DOI] [PubMed] [Google Scholar]
- 16. Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584:1411–1416. [DOI] [PubMed] [Google Scholar]
- 17. Grumati P, Bonaldo P. Autophagy in skeletal muscle homeostasis and in muscular dystrophies. Cells. 2012;1:325–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giordano C, Lemaire C, Li T, Kimoff RJ, Petrof BJ. Autophagy-associated atrophy and metabolic remodeling of the mouse diaphragm after short-term intermittent hypoxia. PLoS One. 2015;10:e0131068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lira VA, Okutsu M, Zhang M, et al. . Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ju JS, Jeon SI, Park JY, et al. . Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. (published online ahead of print March 4, 2016). J Physiol Sci. 2016. doi: 10.1007/s12576-016-0440-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearen MA, Ryall JG, Lynch GS, Muscat GE. Expression profiling of skeletal muscle following acute and chronic β2-adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics. 2009;10:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crowther LM, Wang SC, Eriksson NA, Myers SA, Murray LA, Muscat GE. Chicken ovalbumin upstream promoter-transcription factor II regulates nuclear receptor, myogenic, and metabolic gene expression in skeletal muscle cells. Physiol Genomics. 2011;43:213–227. [DOI] [PubMed] [Google Scholar]
- 23. Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, Nor-1, a target of β-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 2008;149:2853–2865. [DOI] [PubMed] [Google Scholar]
- 24. Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 25. Woodcroft BJ, Hammond L, Stow JL, Hamilton NA. Automated organelle-based colocalization in whole-cell imaging. Cytometry A. 2009;75:941–950. [DOI] [PubMed] [Google Scholar]
- 26. McGadey J. A tetrazolium method for non-specific alkaline phosphatase. Histochemie. 1970;23:180–184. [DOI] [PubMed] [Google Scholar]
- 27. Wang SC, Myers SA, Eriksson NA, Fitzsimmons RL, Muscat GE. Nr4a1 siRNA expression attenuates α-MSH regulated gene expression in 3T3-L1 adipocytes. Mol Endocrinol. 2011;25:291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myers SA, Eriksson N, Burow R, Wang SC, Muscat GE. β-Adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol Cell Endocrinol. 2009;309:101–108. [DOI] [PubMed] [Google Scholar]
- 29. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. [DOI] [PubMed] [Google Scholar]
- 30. Goni R, Garcia P, Foissac ST. The qPCR data statistical analysis. Integrom White Paper. 2009;1–9. [Google Scholar]
- 31. Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE. The orphan nuclear receptor, Nor-1, is a target of β-adrenergic signaling in skeletal muscle. Endocrinology. 2006;147:5217–5227. [DOI] [PubMed] [Google Scholar]
- 32. Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem. 2003;278:44298–44304. [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Roves PM, Huss J, Holloszy JO. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1172–E1179. [DOI] [PubMed] [Google Scholar]
- 34. Youn HD, Sun L, Prywes R, Liu JO. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286:790–793. [DOI] [PubMed] [Google Scholar]
- 35. Lam BY, Zhang W, Enticknap N, Haggis E, Cader MZ, Chawla S. Inverse regulation of plasticity-related immediate early genes by calcineurin in hippocampal neurons. J Biol Chem. 2009;284:12562–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martínez-González J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–618. [DOI] [PubMed] [Google Scholar]
- 37. Nomiyama T, Nakamachi T, Gizard F, et al. . The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem. 2006;281:33467–33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martorell L, Rodriguez C, Calvayrac O, Gentile M, Badimon L, Martinez-Gonzalez J. Vascular effects of thrombin: involvement of Nor-1 in thrombin-induced mitogenic stimulus in vascular cells. Front Biosci. 2008;13:2909–2915. [DOI] [PubMed] [Google Scholar]
- 39. Rius J, Martínez-González J, Crespo J, Badimon L. Involvement of neuron-derived orphan receptor-1 (Nor-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler Thromb Vasc Biol. 2004;24:697–702. [DOI] [PubMed] [Google Scholar]
- 40. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsakas A, Macharia R, Otto A, et al. . Exercise training attenuates the hypermuscular phenotype and restores skeletal muscle function in the myostatin null mouse. Exp Physiol. 2012;97:125–140. [DOI] [PubMed] [Google Scholar]
- 42. Gwinn DM, Shackelford DB, Egan DF, et al. . AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seto JT, Quinlan KG, Lek M, et al. . ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J Clin Invest. 2013;123:4255–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takeda N, Maemura K, Imai Y, et al. . Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95:146–153. [DOI] [PubMed] [Google Scholar]
- 45. Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. [DOI] [PubMed] [Google Scholar]
- 46. Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol. 2010;12:207–209. [DOI] [PubMed] [Google Scholar]
- 48. Pankiv S, Clausen TH, Lamark T, et al. . p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. [DOI] [PubMed] [Google Scholar]
- 49. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bodine SC, Stitt TN, Gonzalez M, et al. . Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. [DOI] [PubMed] [Google Scholar]
- 51. Zanchi NE, Lancha AH Jr. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol. 2008;102:253–263. [DOI] [PubMed] [Google Scholar]
- 52. Eliasson J, Elfegoun T, Nilsson J, Köhnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–E1205. [DOI] [PubMed] [Google Scholar]
- 53. Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985). 2001;90:1936–1942. [DOI] [PubMed] [Google Scholar]
- 54. Parkington JD, Siebert AP, LeBrasseur NK, Fielding RA. Differential activation of mTOR signaling by contractile activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1086–R1090. [DOI] [PubMed] [Google Scholar]
- 55. Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(suppl 1):S137–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tam BT, Pei XM, Yu AP, et al. . Autophagic adaptation is associated with exercise-induced fibre-type shifting in skeletal muscle. Acta Physiol (Oxf). 2015;214:221–236. [DOI] [PubMed] [Google Scholar]
- 57. Lee JM, Wagner M, Xiao R, et al. . Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]