Abstract
The human somatostatin receptor 3 (sst3) is expressed in about 50% of all neuroendocrine tumors and hence a promising target for multireceptor somatostatin analogs. The sst3 receptor is unique among ssts in that it exhibits a very long intracellular C-terminal tail containing a huge number of potential phosphate acceptor sites. Consequently, our knowledge about the functional role of the C-terminal tail in sst3 receptor regulation is very limited. Here, we have generated a series of phosphorylation-deficient mutants that enabled us to determine crucial sites for its agonist-induced β-arrestin mobilization, internalization, and down-regulation. Based on this information, we generated phosphosite-specific antibodies for C-terminal Ser337/Thr341, Thr348, and Ser361 that enabled us to investigate the temporal patterns of sst3 phosphorylation and dephosphorylation. We found that the endogenous ligand somatostatin induced a rapid and robust phosphorylation that was completely blocked by the sst3 antagonist NVP-ACQ090. The stable somatostatin analogs pasireotide and octreotide promoted clearly less phosphorylation compared with somatostatin. We also show that sst3 phosphorylation occurred within seconds to minutes, whereas dephosphorylation of the sst3 receptor occurred at a considerable slower rate. In addition, we also identified G protein-coupled receptor kinases 2 and 3 and protein phosphatase 1α and 1β as key regulators of sst3 phosphorylation and dephosphorylation, respectively. Thus, we here define the C-terminal phosphorylation motif of the human sst3 receptor that regulates its agonist-promoted phosphorylation, β-arrestin recruitment, and internalization of this clinically relevant receptor.
Somatostatin-14 (SS-14) is a cyclic peptide that regulates many physiological functions, including the secretion of hormones such as GH, TSH, ACTH, insulin, and glucagon (1). SS-14 is the natural ligand of a family of 5 human G protein-coupled receptors (GPCRs) named somatostatin receptor (sst)1–sst5 (2–4). Because of its short half-life (1–3 min) in human plasma, the clinical utility of this peptide is limited. Therefore, metabolically stable somatostatin analogs have been developed (5–8). In clinical practice, octreotide and lanreotide are used as first-choice medical treatment of neuroendocrine tumors such as GH-secreting adenomas and carcinoids (6, 9). Octreotide and lanreotide bind with high subnanomolar affinity to sst2 only, have moderate affinity to sst3 and sst5 and show very low or absent binding to sst1 and sst4 (10). More recently, the novel multireceptor somatostatin analog, pasireotide (formerly known as SOM230), has been synthesized (7). Pasireotide is a cyclohexapeptide, which binds with high affinity to all ssts except to sst4 (8). Pasireotide has been approved for the treatment of Cushing syndrome and more recently for the treatment of acromegaly (6, 11, 12).
We have recently used phosphosite-specific antibodies to examine agonist-induced phosphorylation of the sst2 and the sst5 (13–16). For the sst2 receptor, we found that SS-14 promotes the phosphorylation of at least 6 C-terminal serine and threonine residues of the sst2 receptor namely, S341, S343, T353, T354, T356, and T359 (13, 17, 18). This phosphorylation is mediated by GPCR kinase (GRK)2 and GRK3 and followed by rapid cointernalization of the receptor and β-arrestin into the same endocytic vesicles (13, 19). Dephosphorylation of sst2 is initiated directly after receptor activation at or near the plasma membrane and is mediated by protein phosphatase 1 (PP1)β (20).
Although there are many studies examining the expression and signaling of sst2 and sst5, there is little knowledge about the functional role of sst3 in human tumors. More recent studies suggest an elevated expression in diverse neuroendocrine-related malignancies such as pancreatic tumors, pheochromocytomas, paragangliomas, gonadotroph adenomas, and lung carcinoids (21–24). Albeit the growing interest in tumoral sst3 receptors, there is still a lack of knowledge about sst3 receptor regulation and signaling. Interestingly, among the sst subtypes only sst2 and sst3 have been suggested to promote apoptosis of tumor cells. Although the clinically value of somatostatin analogs is primarily based on potent antisecretory effects, other positive effects of somatostatin analogs such as tumor shrinkage are poorly understood but could be related in part to induction of tumor cell apoptosis.
In contrast to sst2 and sst5, our knowledge about the functional role of C-terminal phosphorylation of the human sst3 receptor is limited. For the rat sst3, it has been reported that agonist-dependent internalization relies critically on the presence of 4 C-terminal hydroxyl amino acids namely Ser341, Ser346, Ser351, and Thr357 (25). However, the C-terminal regions of the human and the rat sst3 receptor exhibit strikingly different sequences. Nevertheless, it is believed that the presence of the intact C terminus is required for triggering SS-14-induced apoptosis via the sst3 receptor (26).
In the present study, we have examined the primary structure of the human sst3 C-terminal tail. In fact, this 102 amino acids long sequence contains 18 potential phosphate acceptor sites including 12 serine and 6 threonine residues. We have constructed a series of phosphorylation-deficient mutants and generated phosphosite-specific antibodies, which enabled us to provide direct evidence for agonist-selective phosphorylation of the human sst3 receptor. Using these antibodies, we identified kinases and phosphatases required for agonist-dependent phosphorylation and dephosphorylation.
Materials and Methods
Antibodies and reagents
The human hemagglutinin (HA)-tagged sst3 receptor as well as the 317–370 S/T-A, 4 S/T-A, 371–418 S/T-A, and K-R mutants were generated via artificial gene synthesis and cloned into pcDNA3.1 by imaGenes. Phosphosite-specific antibodies were custom generated by Genosphere Biotechnologies. The phosphosite-specific antibodies for the S337/T341-phosphorylated form of sst3 were generated against the following sequence that contained a phosphorylated serine and threonine residue: CRRVR(pS)QEP(pT)VGPP. This sequence corresponds to 333–345 of the human sst3. Phosphosite-specific antibodies for the T348-phosphorylated form of sst3 were generated against the following sequence that contained a phosphorylated threonine residue: CGPPEK(pT)EEED. This sequence corresponds to 343–352 of the human sst3. Phosphosite-specific antibodies for the S361-phosphorylated form of sst3 were generated against the following sequence that contained a phosphorylated serine residue: CDGEE(pS)REGG. This sequence corresponds to 357–365 of the human sst3. The specificity of the antisera was initially tested using dot-blot analysis. For subsequent analysis, antibodies were affinity purified against their immunizing peptide as well as against the nonphosphorylated peptide. The phosphorylation-independent rabbit monoclonal anti-sst3 antibody UMB-5 and the rabbit polyclonal anti-HA antibody were generated and extensively characterized as previously described (17, 27, 28). Anti-GRK2 (sc-562), anti-GRK3 (sc-563), anti-GRK5 (sc-565), anti-GRK6 (sc-566), anti-PP1α (sc-6104), anti-PP1β (sc-6106), and anti-PP1γ (sc-6108) antibodies were obtained from Santa Cruz Biotechnology. SS-14 was obtained from Bachem. Phorbol 12-myristate 13-acetate (PMA) and forskolin were obtained from Sigma-Aldrich. Octreotide, pasireotide, and the sst3 antagonist, NVP-ACQ090 (29), were kindly provided by Dr Herbert Schmid (Novartis). Somatoprim was obtained from DeveloGen. L-796/778 (30), a selective ligand for sst3, was provided by Merck (please see Supplemental Table 1).
Cell culture and transfection
Human embryonic kidney (HEK)293 cells were obtained from the German Resource Centre for Biological Material (DSMZ). AtT20-D16v-F2 cells were obtained from American Type Tissue Culture Collection. HEK293 cells and AtT-20 cells were grown in DMEM supplemented with 10% fetal calf serum. Cells were transfected with plasmids encoding for HA-tagged wild-type or mutant sst3 receptors using Lipofectamine 2000 according to the instructions of the manufacturer (Invitrogen). Stable transfectants were selected in the presence of 400-μg/mL G418. To increase the total number of AtT20 cells stably expressing sst3 or phosphorylation-deficient mutant receptors, we used fluorescence-activated cell sorting (FACS). Trypsinized cells were washed with PBS and transferred into opti-MEM containing an A488-labeled anti-HA antibody at a dilution of 1:1000 (Sigma-Aldrich). After 30 minutes of preincubation at room temperature, cells were centrifuged and the cell pellet was resuspended in FACS buffer (2mM EDTA and 0.5% BSA in PBS). FACS was executed using a BD FACSAria III cell sorter. Approximately 1% of the positive cell population was sorted at an average purity of 85%. Sorted cells were then recultivated. To ensure similar expression levels of wild-type and mutant receptors, stable cells were characterized using radioligand-binding assays, Western blot analysis, surface ELISA, and immunocytochemistry as described previously (19, 31). The level of sst expression was between 800 and 1200 fmol/mg membrane protein for all experiments using stably transfected and between 1500 and 2000 fmol/mg membrane protein for all experiments using transiently transfected HEK293 cells (19, 31) (please see Supplemental Figure 1).
Western blot analysis
HEK293 cells or AtT-20 cells stably expressing HA-tagged human sst3 receptors were plated onto 60-mm dishes and grown to 80% confluence. After indicated treatment with SS-14, L-796/778, octreotide, pasireotide, somatoprim, PMA, or forskolin, cells were lysed in detergent buffer (50mM Tris-HCl, [pH 7.4], 150mM NaCl, 5mM EDTA, 10mM NaF, 10mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) in the presence of protease and phosphatase inhibitors Complete mini and PhosSTOP (Roche Diagnostics) and centrifuged at 16 000g for 20 minutes at 4°C. When indicated, cells were incubated with 10nM or 30nM calyculin A or with 5nM or 50nM okadaic acid for 10 minutes before agonist exposure. All phosphorylation and dephosphorylation assays were performed at physiologic temperature (37°C) or at room temperature (22°C) for the indicated time periods. Glycosylated proteins were partially enriched using wheat germ lectin-agarose beads as described (32–34). When indicated, ssts were isolated using anti-HA antibodies coupled to protein A agarose beads. Proteins were eluted from the beads using SDS-sample buffer for 15 minutes at 60°C and then resolved on 8% SDS-polyacrylamide gels. After electroblotting, membranes were incubated with phosphosite-specific antibodies anti-pS337/pT341, anti-pT348, or anti-pS361 at a concentration of 0.1 μg/mL followed by detection using enhanced chemiluminescence (Amersham). Blots were subsequently stripped and reprobed with anti-sst3 antibody (UMB-5) or anti-HA antibody to confirm equal loading of the gels. Films exposed in the linear range were then densitized using ImageJ 1.45. Data were analyzed using GraphPad Prism 4.0 software.
Analysis of receptor internalization by confocal microscopy
HEK293 cells stably expressing HA-tagged human sst3 receptors or its phosphorylation-deficient mutants were grown on poly-L-lysine-coated coverslips overnight. After the appropriate treatment with 1μM ligands at 37°C, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 30 minutes at room temperature and washed several times. Cells were permeabilized and then incubated with anti-HA antibody followed by Alexa Fluor 488-conjugated secondary antibody (Amersham). Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope (35).
Quantitative internalization assays
Receptor internalization was quantified using a linear surface receptor ELISA that has been characterized extensively (13, 31, 35, 36). Equal numbers of stably transfected HEK293 cells expressing HA-tagged human sst3 receptors or its phosphorylation-deficient mutants were seeded onto poly-L-lysine-treated 24-well plates (200 000 cells per well). The next day, cells were preincubated with 1-μg/mL anti-HA antibody for 2 hours at 4°C. After the appropriate treatment with SS-14 or another agonist (1μM) at 37°C, cells were fixed and incubated with peroxidase-conjugated antirabbit antibody overnight. After washing, plates were developed with ABTS solution and analyzed at 405 nm using a microplate reader. Statistical analysis was carried out with unpaired t test. P < .05 was considered statistically significant.
β-Arrestin-EGFP mobilization assay
Untransfected HEK293 cells were seeded onto 35-mm glass-bottom culture dishes (Mattek). The next day, cells were transiently cotransfected with 0.2 μg β-arrestin-2-EGFP or β-arrestin-1-EGFP and 2μg human sst3 receptor or the phosphorylation-deficient mutants per dish containing 200 000 cells using TurboFect. After 24 hours, cells were transferred onto a temperature-controlled microscope stage set at 37°C of a Zeiss LSM510 META laser scanning confocal microscope. Images were collected sequentially using single line excitation at 488 nm with 515- to 540-nm band pass emission filters. Saturating concentrations of agonists (1μM) were applied directly into the culture medium immediately after the initial image was taken.
Colocalization of receptor and β-arrestin-EGFP
Untransfected HEK293 cells were grown on poly-L-lysine-coated coverslips overnight. The next day, cells were transiently cotransfected with 0.2μg β-arrestin-2-EGFP or β-arrestin-1-EGFP, 1.2μg human sst3 receptor or the phosphorylation-deficient mutants and 0.8μg GRK2 per dish using TurboFect. After 24 hours, cells were preincubated with 1μg/mL anti-HA antibody for 2 hours at 4°C. After the appropriate treatment with 1μM SS-14 at 37°C, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 30 minutes at room temperature and washed several times. Cells were permeabilized and then incubated with chicken anti-GFP antibody (Aves) followed by goat antichicken Alexa Fluor 488-conjugated secondary antibody (Life Technologies) and with donkey anti-rabbit Cy3-conjugated secondary antibody (Jackson ImmunoResearch) for receptor staining. Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope.
Membrane potential assay
AtT-20 cells were plated into poly-L-lysine covered 96-well plates and a number of 30 000 cells per well in a volume of 200 μL. Cells were kept in an incubator for 48 hours to reach a confluency of 80% before experiments. After removal of medium, cells were washed with Hanks' balanced salt solution, buffered with HEPES 20mM (pH 7.4), containing 1.3mM CaCl2, 5.4mM KCl, 0.4mM K2HPO4, 0.5mM MgCl2, 0.4mM MgSO4, 136.9mM NaCl, 0.3mM Na2HPO4, 4.2mM NaHCO3, and 5.5mM glucose. The membrane potential dye (FLIPR Membrane Potential kit BLUE; Molecular Devices) was reconstituted according to manufacturer's instructions. Cells were incubated in 90 μL of Hanks' balanced salt solution/HEPES (vehicle solution) and an equal volume of assay dye solution for 45 minutes at 37°C. Plates were assayed using a FlexStation 3 microplate reader (Molecular Devices). All measurements were conducted at 37°C. Compounds were injected in a volume of 20 μL in a 10-fold concentration referring to the final concentration in the well. A baseline was recorded before agonist injection for 60 seconds. An equal concentration of solvent was used as vehicle control. Cells were exposed to maximum concentration of 0.5% dimethyl sulfoxide (DMSO). For data analysis GraphPad Prism 6 and OriginPro were used using a 4-parameter nonlinear regression for fitting concentration-response curves.
Small interfering RNA (siRNA) silencing of gene expression
Chemically synthesized double-stranded siRNA duplexes (with 3′ dTdT overhangs) were purchased from QIAGEN for the following targets: GRK2 (5′-CCGGGAGATCTTCGACTCATA-3′ and 5′-AAGAAGTACGAGAAGCTGGAG-3′), GRK3 (5′-AAGCAAGCTGTAGAACACGTA-3′ and 5′-GCAGAAGTCGACAAATTTA-3′), GRK5 (5′-AGCGTCATAACTAGAACTGAA-3′ and 5′-AAGCCGTGCAAAGAACTCTTT-3′), GRK6 (5′-AACACCTTCAGGCAATACCGA-3′ and 5′-AACAGTAGGTTTGTAGTGAGC-3′), PP1α catalytic subunit (5′-AAGAGACGCTACAACATCAAA-3′), PP1β catalytic subunit (5′-TACGAGGATGTCGTCCAGGAA-3′ and 5′-GTTCGAGGCTTATGTATCA-3′), PP1γ catalytic subunit (5′-AACATCGACAGCATTATCCAA-3′ and 5′-AGAGGCAGTTGG TCACTCT-3′), and a nonsilencing RNA duplex (5′-GCTTAGGAGCATTAGTAAA-3′ or 5′-AAACTCTATCTGCACGCTGAC-3′). HEK293 cells were transfected with 100nM siRNA for single transfection or double transfection using HiPerFect (QIAGEN). Silencing was quantified by immunoblotting. All experiments showed protein levels reduced by more than or equal to 80% (20).
Results
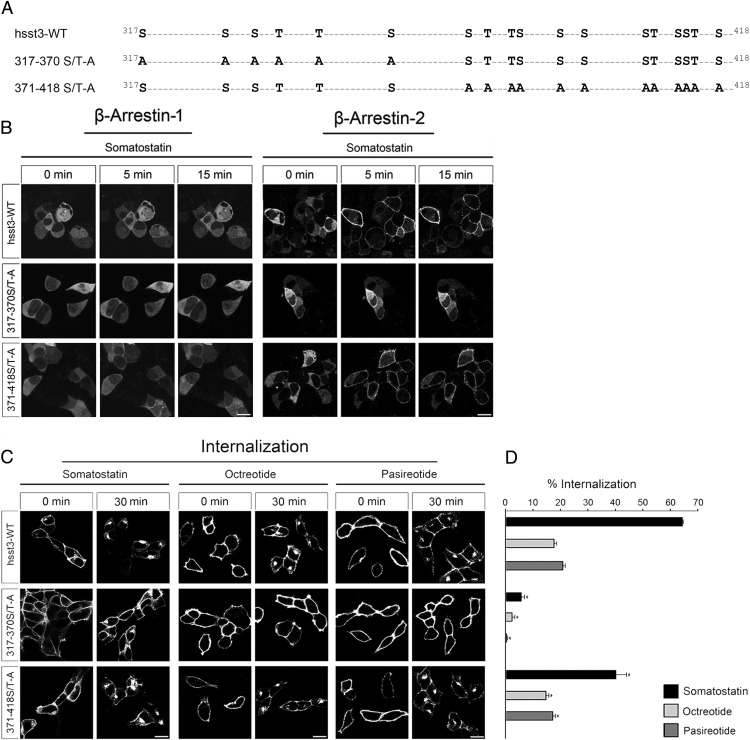
We have recently shown that agonist-induced phosphorylation of sst2 receptor occurs at a specific cluster of serine and threonine residues within the C-terminal tail of the receptor (13, 18, 37). Similar, we found that trafficking of the sst5 receptor is regulated by phosphorylation of C-terminal T333 (14). Nevertheless, little is known about the functional role of the C terminus for sst3 receptor regulation. This is in part due to presence of a huge number of potential phosphate acceptor sites within its very long C-terminal tail. To address this problem, we first generated 2 sst3 receptor mutants, where either all 6 serine and threonine residues present in the proximal part (317–370) or all 12 serine and threonine residues present in the distal part (371–418) of the C terminus were mutated to alanine (Figure 1A). This approach should allow us to determine which part of the sst3 C terminus is crucial for agonist-dependent arrestin recruitment and receptor internalization. Thus, we employed functional β-arrestin-1 and β-arrestin-2 conjugated to enhanced green fluorescent protein to visualize the translocation of arrestin to the plasma membrane in live HEK293 cells transfected with wild-type or mutant sst3 receptors. In the absence of somatostatin, both arrestins were mostly distributed throughout the cytoplasm of the cells (Figure 1B, 0 min). The addition of saturating concentrations of agonist induced a small but clearly detectable redistribution of β-arrestin-1 and a very robust redistribution of β-arrestin-2 from the cytoplasm to the plasma membrane in cells transfected with wild-type sst3 receptor or the 371–418 S/T-A mutant. In contrast, no such translocation was observed in cells transfected with the 317–370 S/T-A mutant. Similar effects were obtained after qualitative and quantitative analysis of receptor internalization (Figure 1, C and D). In fact, confocal microscopic images show that a 30-minute SS-14 exposure of the wild-type sst3 receptor induced a very robust redistribution of the receptor from the plasma membrane into puntate-like structures within the cytososl (Figure 1C). Quantitative analysis using a biochemical ELISA indicated that approximately 65% of all surface receptors were internalized (Figure 1D). A robust receptor internalization was also observed with 371–418 S/T-A mutant with only few receptors remaining at the plasma membrane (Figure 1C). Quantitative analysis revealed that up to 40% of all surface receptors were internalized (Figure 1D). In contrast, in the 317–370 S/T-A mutant receptor endocytosis was nearly undetectable (<5% internalization) (Figure 1, C and D). Both octreotide and pasireotide induced a partial internalization (∼20%) of the wild-type sst3 receptor and the 371–418 S/T-A mutant, but virtually no internalization of the 317–370 S/T-A mutant (Figure 1, C and D). These results clearly indicate that arrestin recruitment and internalization of the sst3 receptor crucially depend on phosphorylation of the proximal part of its C terminus.
Figure 1. Construction of phosphorylation-deficient sst3 receptor mutants.
A, Schematic representation of the human sst3 receptor indicating all serine and threonine sites within the C-terminal tail (317–418). Phosphate acceptor sites mutated to alanine in the 317–370 S/T-A, and 371–418 S/T-A mutants are marked in bold. B, HEK293 cells were transiently transfected with either wild-type hsst3, 317–370 S/T-A or 371–418 S/T-A and β-arrestin-2-EGFP or β-arrestin-1-EGFP. The distribution of β-arrestin-2 and β-arrestin-1 were visualized sequentially in the same live cells before (0 min) and after (5 and 15 min) the addition of 1μM SS-14 to the culture medium. Shown are representative images from 1 of 4 independent experiments performed in duplicate. Scale bar, 15 μm. C, HEK293 cells stably expressing wild-type hsst3, 317–370 S/T-A, or 371–418 S/T-A were treated with 1μM somatostatin, octreotide, or pasireotide for 30 minutes. Cells were then fixed, stained with the anti-HA antibody, and examined by confocal microscopy. Shown are representative images from 1 of at least 3 independent experiments performed in duplicate. Scale bar, 15 μm. D, HEK293 cells stably expressing wild-type or mutant hsst3 receptors were treated for 30 minutes with 1μM agonist. Receptor sequestration was measured by surface ELISA. Data represent receptor internalization in agonist-treated cells as compared with sister cultures receiving 30 minutes of vehicle (100%). Data are presented as mean ± SEM from at least 4 independent experiments performed in quadruplicate. Results were analyzed by unpaired t test vs wild type receiving 30 minutes of vehicle (*, P < .05).
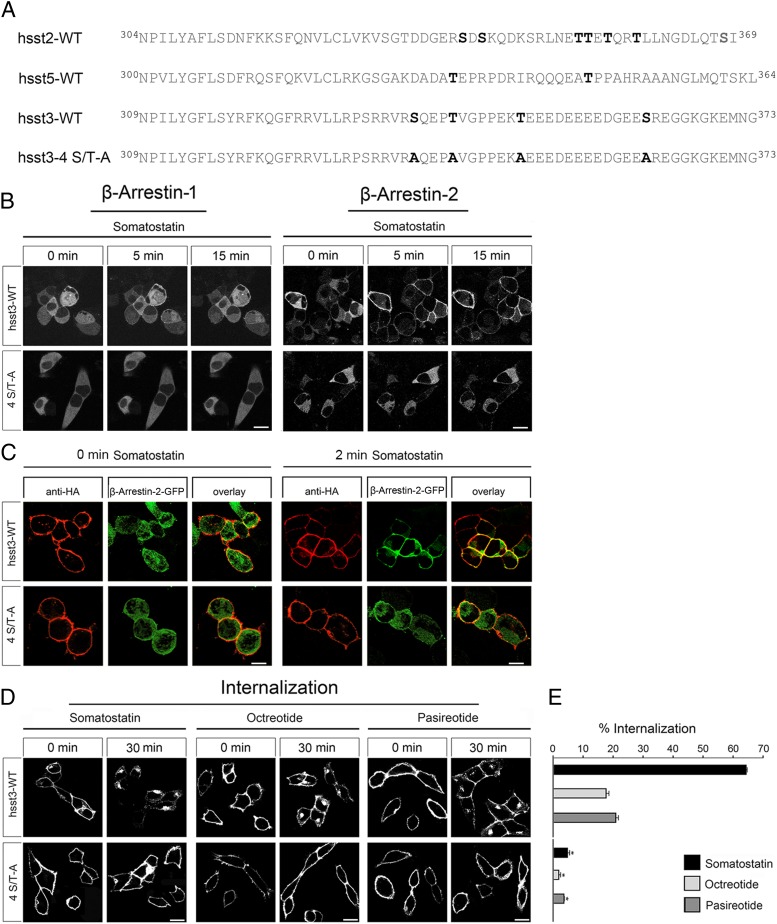
We then aligned the proximal part of the sst3 C terminus with the C-terminal domains of the well-characterized sst2 and sst5 receptors. Figure 2A depicts the amino acid sequences of all 3 receptors beginning with the NPXXY motif which is conserved in GPCRs of the rhodopsin subfamily and marks the end of the seventh transmembrane domain and the beginning of the C terminus. All known phosphorylation sites namely S341, S343, T353, T354, T356, and T359 in sst2 and T333 and T347 in sst5 are depicted in bold. Comparison of these sequences suggests that C-terminal phosphorylation of these receptors occurs preferentially in a certain distance from the plasma membrane. In the corresponding region of the sst3 receptor, 4 potential phosphate acceptor sites namely S337, T341, T348, and S361 are present (Figure 2A). In order to assess their role in sst3 regulation, we generated a receptor with combined mutation of only these 4 serine and threonine residues (4 S/T-A). Interestingly, analysis of the 4 S/T-A mutant revealed no mobilization of β-arrestin-1 or β-arrestin-2 and virtually no internalization (<5%) in response to SS-14 exposure (Figure 2, B–D).
Figure 2. Internalization and β-arrestin recruitment of the sst3 receptor is regulated by 4 C-terminal serine and threonine residues.
A, Schematic representation of the human sst2, sst5, sst3, and sst3-4 S/T-A receptors beginning with the conserved NPXXY motif which marks the end of seventh transmembrane domain and the beginning of the C-terminal tail. Phosphate acceptor sites essential for the agonist-driven internalization of the human sst2 and sst5 receptors are marked in bold. Serine and threonine residues present in the corresponding region of the human sst3 receptor were replaced by alanine resulting in a 4 S/T-A mutant. B, HEK293 cells were transiently transfected with either wild-type hsst3 or 4 S/T-A and β-arrestin-2-EGFP or β-arrestin-1-EGFP. The distributions of β-arrestin-2 and β-arrestin-1 were visualized sequentially in the same live cells before (0 min) and after (5 and 15 min) the addition of 1μM SS-14 to the culture medium. Shown are representative images from 1 of 4 independent experiments performed in duplicate. Scale bar, 15 μm. C, HEK293 cells were transiently transfected with either wild-type hsst3, 317–370 S/T-A, 371–418 S/T-A, or 4 S/T-A and β-arrestin-2-EGFP or β-arrestin-1-EGFP as well as GRK2. The next day, cells were treated with 1μM SS-14 for 0 and 2 minutes, fixed, stained, and examined by confocal microscopy. Shown are representative images from 1 of at least 3 independent experiments performed in duplicate. Scale bar, 15 μm. D, HEK293 cells stably expressing wild-type hsst3 or 4 S/T-A were treated with 1μM somatostatin, octreotide, or pasireotide for 30 minutes. Cells were then fixed, stained with anti-HA antibody, and examined by confocal microscopy. Shown are representative images from 1 of at least 3 independent experiments performed in duplicate. Scale bar, 15 μm. E, HEK293 cells stably expressing wild-type hsst3 or 4 S/T-A receptor were treated for 30 minutes with 1μM agonist. Receptor sequestration was measured by surface ELISA. Data represent receptor internalization in agonist-treated cells as compared with sister cultures receiving 30 minutes of vehicle (100%). Data are presented as mean ± SEM from at least 4 independent experiments performed in quadruplicate. Results were analyzed by unpaired t test vs wild type receiving 30 minutes of vehicle (*, P < .05).
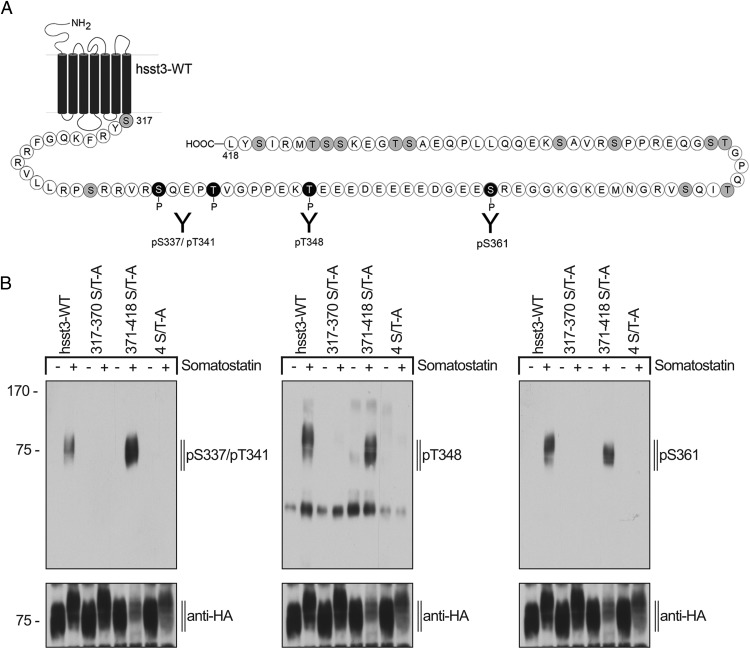
Our results suggest that these 4 sites are most likely to undergo agonist-induced phosphorylation. Consequently, we generated phosphosite-specific antibodies to S337/T341, T348, and S361 (Figure 3A). These antisera were affinity-purified against their immunizing peptides and initially tested in dot-blot assays using the phospho- and the corresponding nonphospho-peptides (data not shown). All antibodies, which clearly detected their respective phospho-peptide and did not cross-react with the corresponding nonphospho-peptide were further characterized in Western blot analysis using HEK293 cells transiently transfected with the human sst3 receptor and its phosphorylation-deficient mutants (Figure 3B). In Western blottings from cells expressing the wild-type sst3 receptor, a band migrating at Mr 65 000–80 000 was detectable which corresponds to the expected molecular size of the sst3 monomer (Figure 3B). The anti-pS337/pT341, anti-pT348 as well as anti-pS361 antibodies detected the phosphorylated form of the sst3 receptor in SS-14-treated cells but not in untreated cells (Figure 3B). As expected, analysis of the 371–418 S/T-A mutant revealed a phosphorylation profile that was similar to that seen with the wild-type sst3 receptor (Figure 3B). Moreover, no phosphorylation signal was detectable in the 317–370 S/T-A or the 4 S/T-A mutant (Figure 3B) indicating that the newly generated anti-pS337/pT341, anti-pT348, and anti-pS361 antibodies specifically detect the phosphorylated form of their targeted receptor. Moreover, the results show that all 4 sites including S337, T341, T348, and S361 undergo agonist-promoted phosphorylation in the presence of the endogenous ligand SS-14.
Figure 3. Generation of phosphosite-specific antibodies for the human sst3 receptor.
A, Schematic representation of the human sst3 receptor indicating all potential phosphate acceptor sites within the C-terminal tail in gray. Epitopes of the phosphosite-specific antibodies are depicted in black. B, HEK293 cells stably expressing wild-type hsst3, 317–370 S/T-A, 4S/T-A, or 371–418 S/T-A were either not exposed or exposed to 1μM SS-14 for 15 minutes at 37°C. The levels of phosphorylated sst3 receptor and phosphorylation-deficient mutants were then determined using the phosphosite-specific anti-pS337/pT341, anti-pT348, or anti-pS361 antibodies. Blots were subsequently stripped and reprobed with anti-HA antibody to confirm equal loading of the gels (anti-HA). Blots shown are representative of 3 independent experiments. The positions of molecular mass markers are indicated on the left (in kDa).
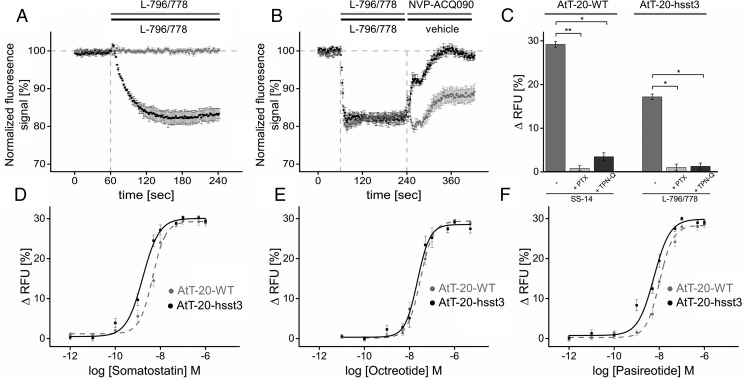
We then examined the ability of sst3 and its phosphorylation-deficient mutants to activate G proteins. Similar to other sst subtypes, sst3 is known to activate G protein-coupled inwardly rectifying potassium (GIRK) channels (38). Measuring GIRK channel activation is therefore a plausible surrogate parameter to assess an agonist-induced sst3-mediated G protein downstream signal. We therefore adapted a fluorescence-based membrane potential assay protocol originally established by Vazquez et al (39) and Walsh (40) in AtT-20 cells. This mouse corticotrope cell line expresses abundantly sst2 and sst5 receptors (41, 42). In wild-type AtT-20 cells, the sst3-selective agonist L-796/778 did not induce a significant change in fluorescence signal even in a saturating concentration of 10μM. However, in cells stably expressing hsst3 L-796/778 induced a fast and robust decrease in fluorescence signal intensity (Figure 4A). This agonist-induced hyperpolarization was efficiently blocked by subsequent application of the sst3-selective antagonist NVP-ACQ090 (Figure 4B). In wild-type AtT-20 cells, the SS-14-induced decrease of fluorescence was completely abrogated by pretreatment with pertussis toxin (PTX), which is indicative for a Gαi protein-mediated process (Figure 4C). Tertiapin-Q (TPN-Q) significantly inhibited both the SS-14- and the L-796/778-induced hyperpolarization, which is indicative for a GIRK-mediated mechanism (Figure 4C). Interestingly, SS-14, octreotide and pasireotide induced a dose-dependent hyperpolarization in wild-type AtT-20 cells (Figure 4, D–F). After coexpression of sst3, however, only SS-14 and pasireotide but not octreotide were able to promote a robust leftward shift of the dose-response curve (Figure 4, D–F).
Figure 4. Membrane potential measurements.
A, Wild-type AtT-20 cells (gray trace) and AtT-20 cells stably expressing hsst3 (black trace) were exposed to a solution containing 10μM sst3-selective agonist L-796/778 to yield a final in-well concentration of 1μM. Changes in fluorescence signal intensity detected for wild-type cells were 0.6 ± 0.7% (mean ± SEM, n = 3, ns), respectively, 17.2 ± 2.3% (mean ± SEM, n = 4; *, P < .05). Shown is 1 representative experiment executed as triplicate (mean ± SD). Data indicated are composed of 3 independent experiments executed as triplicate vs vehicle injection as duplicates. Vehicle induced background changes in membrane potential were subtracted from agonist-induced signals. All values indicated are interpreted at t180sec. B, Inhibition of L-796/778-induced hyperpolarization by sst3-selective antagonist NVP-ACQ090. AtT-20 cells stably expressing wild-type sst3 were primarily exposed to 50μM L-796/778 at t60sec for 180 seconds (black and gray trace) and subsequently exposed to either a 50μM solution of NVP-ACQ090 at t240sec for another 180 seconds (black trace) or vehicle (gray trace). C, PTX and TPN-Q sensitivity of SS-14 and L-796/778-induced changes in membrane potential. Left panel, Exposure of wild-type AtT-20 cells with 10μM SS-14 induced a change of 29.2 ± 0.8% (mean ± SEM, n = 5) of the fluorescence signal (left bar). After pretreatment with 300-ng/mL PTX for 20 hours, signal magnitude significantly decreased to 1.8 ± 0.9% (mean ± SEM, n = 4; **, P < .01) after exposure to 10μM SS-14 (center bar) compared with untreated cells. In cells pretreated with 500nM TPN-Q for 5 minutes before agonist exposure (right bar), signal magnitude significantly decreased to 3.6 ± 0.9% (mean ± SEM, n = 5; *, P < .05). Right panel, AtT-20 cells stably expressing hsst3 were challenged with 10μM 796/778 (left bar) to induce a significant change in fluorescence signal of 17.2 ± 0.6% (mean ± SEM, n = 4). L-796/778 induced a significant decrease of fluorescence intensity in cells pretreated with PTX (1.0 ± 0.8%, mean ± SEM, n = 4; *, P < .05) or TPN-Q (1.3 ± 0.7%, mean ± SEM, n = 4; *, P < .05) compared with the untreated cells. D, Dose-response curves of wild-type AtT-20 cells vs hsst3-expressing cells stimulated with SS-14 in a concentration range from 1pM to 1μM. The EC50 value obtained was 4.5 ± 0.5nM for wild-type cells and 1.7 ± 0.3nM for hsst3-expressing cells. E, Dose-response curves of wild-type AtT-20 cells vs hsst3-expressing cells stimulated with octreotide in a concentration range from 10pM to 5μM with EC50 of 30.5 ± 4.6nM for wild-type cells and 30.5 ± 3.9nM for hsst3-expressing cells. F, Dose-response curves of wild-type AtT-20 cells vs hsst3-expressing cells stimulated with pasireotide in a concentration range from 1pM to 1μM with EC50 of 10.4 ± 0.8nM for wild-type cells and 5.8 ± 1.3nM for hsst3-expressing cells. EC50 values are given as mean ± SD. Results were analyzed by unpaired t test vs wild type.
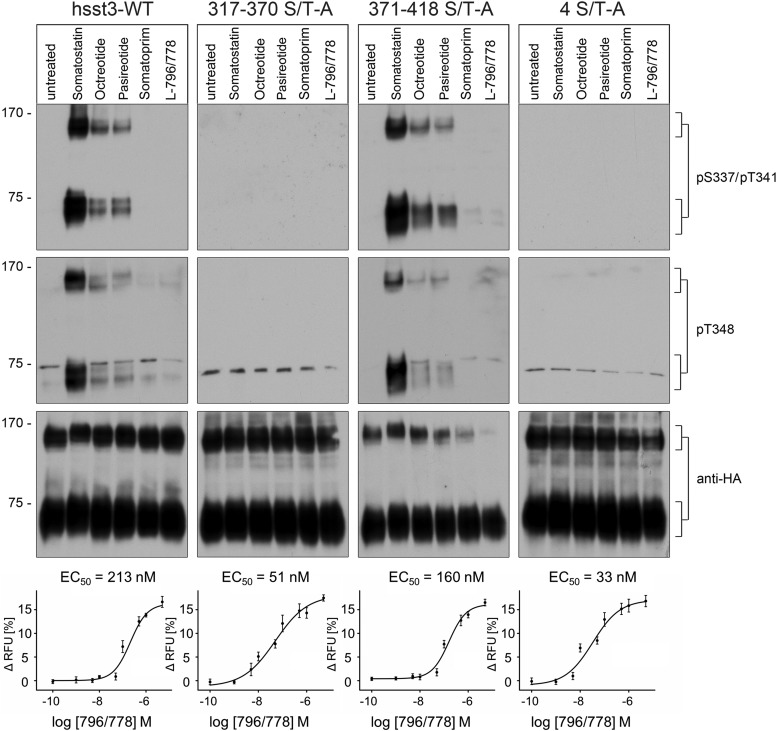
We then analyzed the capacity of clinically relevant somatostatin analogs to induce sst3 phosphorylation using HEK293 cells stably expressing sst3 receptor (Figure 5, left panel). In Western blottings from these cells, 2 broad bands were detectable migrating at Mr 65 000–80 000 and at Mr 130 000–160 000 corresponding to the sst3 monomer and sst3 homodimer, respectively. The anti-pS337/pT341, and the anti-pT348 antibodies detected the phosphorylated forms of sst3 monomers and sst3 dimers in SS-14-treated cells but not in untreated cells. Interestingly, SS-14 induced a very strong phosphorylation signal, whereas the stable somatostatin analogs pasireotide and octreotide promoted a much weaker although clearly detectable sst3 phosphorylation (Figure 5). In contrast, neither somatoprim nor the selective sst3 agonist L-796/778 were able to induce any detectable sst3 phosphorylation (Figure 5). As expected, analysis of the 371–418 S/T-A mutant revealed a phosphorylation profile that was nearly indistinguishable from that observed for the wild-type sst3 receptor (Figure 5, third panel). Moreover, no phosphorylation signal was detectable in the 317–370 S/T-A or the 4 S/T-A mutant (Figure 5, second and fourth panels) indicating that the newly generated anti-pS337/pT341 and anti-p348 antibodies specifically detect the phosphorylated form of their targeted receptor. In addition, the present results suggest an excellent correlation between sst3 receptor phosphorylation and internalization (Figures 1, 2, and 5). We then assessed G protein signaling of all sst3 mutants using the selective sst3 agonist L-796/778 (Figure 5, bottom panel). Although we were unable to detect any differences in Emax values after exposure of saturating concentrations of L-796/778, we found robust differences in EC50 values indicating a leftward shift of the dose-response curves in the phosphorylation-deficient 317–370 S/T-A and 4 S/T-A mutants (Figure 5, bottom panel).
Figure 5. Evaluation of agonist-dependent phosphorylation of phosphorylation-deficient human sst3 receptor mutants.
HEK293 cells stably expressing wild-type hsst3, 317–370 S/T-A, 4S/T-A, or 371–418 S/T-A were treated with 1μM SS-14, octreotide, pasireotide, somatoprim, or L-796/778 for 10 minutes at room temperature. The levels of phosphorylated sst3 receptors were then determined using the phosphosite-specific anti-pS337/pT341 or anti-pT348 antibodies. Blots were subsequently stripped and reprobed with the phosphorylation-independent anti-HA antibody to confirm equal loading of the gels (anti-HA). Blots shown are representative of 3 independent experiments. The positions of molecular mass markers are indicated on the left (in kDa). Dose-response curves of AtT-20 cells stably expressing wild-type hsst3, 317–370 S/T-A, 371–418 S/T-A, or 4S/T-A stimulated with sst3-selective agonist L-796/778. Cells were stimulated with L-796/778 at concentrations ranging from 0.1nM to 5μM to yield a dose-response curve. EC50 values are given as mean ± SD.
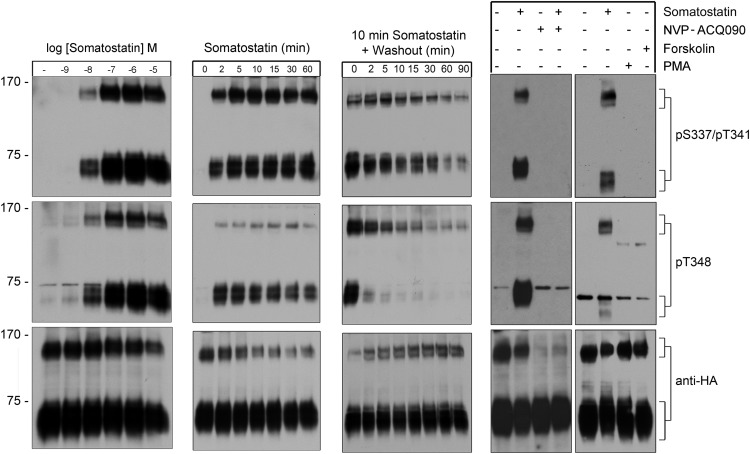
Next, we examined dose- and time-dependent sst3 phosphorylation after exposure to various concentrations of SS-14 ranging from 10−9M to 10−5M. As depicted in Figure 6, left panel, a weak phosphorylation signal was first detectable at concentrations of SS-14 as low as 10nM. Phosphorylation reached a maximum at concentrations between 100nM and 1μM SS-14. In the presence of 1μM SS-14, sst3 phosphorylation became detectable within 2 minutes even at room temperature (∼22°C) (Figure 6, second panel). Maximal sst3 phosphorylation with no further increase was reached after 5 minutes of SS-14 exposure at room temperature (Figure 6, second panel). To examine the time course of sst3 dephosphorylation, we first exposed the cells for 10 minutes to SS-14. The ligand was then washed out and cells were incubated in culture medium in the absence of agonist for different periods of time ranging from 2 to 90 minutes. Under these conditions, dephosphorylation of T348 occurred within 10–15 minutes, whereas dephosphorylation of S337/T341 required more than 30 minutes (Figure 6, third panel). We also observed that SS-14-induced phosphorylation of the sst3 receptor was completely blocked by the specific sst3 antagonist NVP-ACQ090 (Figure 6, fourth panel). This antagonist also inhibited SS-14-mediated arrestin recruitment and receptor internalization (data not shown). Phosphorylation of GPCRs can occur via specific GRKs or second messenger-activated kinases. We therefore treated sst3-expressing HEK293 cells either with the protein kinase A activator forskolin (10μM) or the protein kinase C activator PMA (0.1μM) and examined sst3 phosphorylation. We found that neither PMA nor forskolin produced any detectable phosphorylation of S337/T341 or T348 (Figure 6, right panel).
Figure 6. Agonist-induced phosphorylation and dephosphorylation of the human sst3 receptor.
Left panel, HEK293 cells stably expressing the sst3 receptor were either not exposed or exposed to the indicated concentrations of SS-14 for 10 minutes at room temperature. Second panel, cells were exposed to 1μM SS-14 at room temperature for the indicated time periods. Third panel, cells were exposed to 1μM SS-14 for 10 minutes, washed, and incubated in the absence of agonist for 0, 2, 5, 10, 15, 30, 60, or 90 minutes. Fourth panel left, Cells were incubated with the sst3 antagonist NVP-ACQ090 for 10 minutes before SS-14 exposure at 37°C. Fourth panel right, Cells were exposed to either 1μM SS-14, 0.1μM PMA, or 10μM forskolin for 10 minutes at room temperature. Cells were lysed and immunoblotted with the phosphosite-specific antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-HA antibody to confirm equal loading of the gel (anti-HA). Representative results from 3 independent experiments are shown. The positions of molecular mass markers are indicated on the left (in kDa).
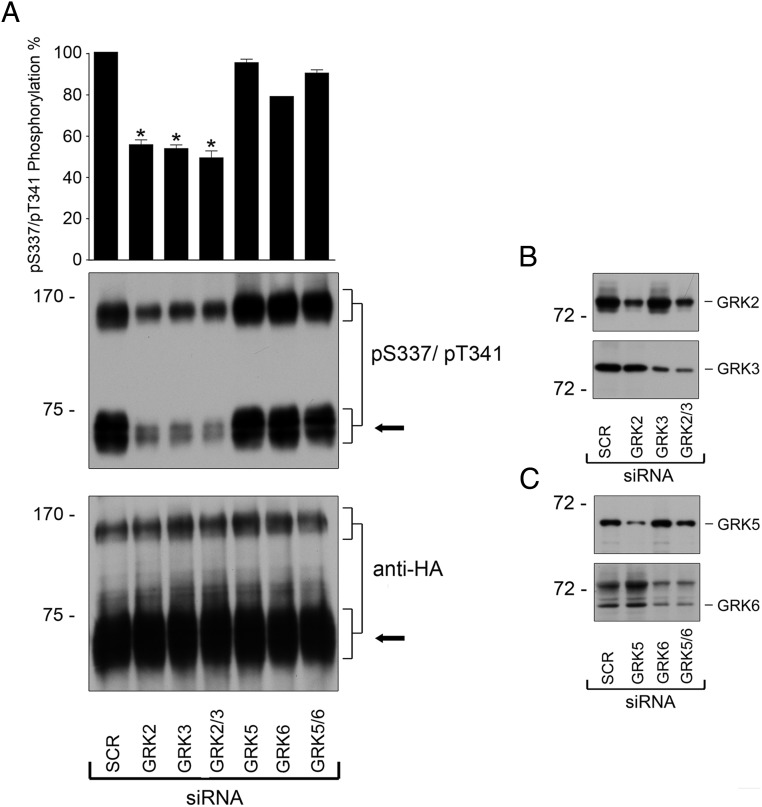
We then elucidated the question, which GRKs are involved in sst3 phosphorylation. We used specific siRNAs to knockdown gene expression of GRK2, GRK3, GRK5, or GRK6. Under these conditions, we observed a robust inhibition of SS-14-induced sst3 phosphorylation after knockdown of GRK2 or GRK3 (Figure 7). Quantitative analysis revealed that phosphorylation was reduced by approximately 40% after knockdown of GRK2 or GRK3. In contrast, no such inhibition was observed after knockdown of GRK5 and/or GRK6 (Figure 7).
Figure 7. GRK2 and GRK3 are responsible for agonist-induced phosphorylation of the human sst3 receptor.
A, HEK293 cells stably expressing the sst3 receptor were transfected with siRNA targeted to GRK2, GRK3, GRK5, and GRK6 or nonsilencing siRNA control (SCR) for 72 hours and then exposed to 1μM SS-14 for 10 minutes (room temperature). Cells were lysed and immunoblotted with anti-pS337/pT341 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-HA antibody to confirm equal loading of the gels (anti-HA). Receptor monomers were quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells. Data correspond to the mean ± SEM from 3 independent experiments. Results were analyzed by two-way ANOVA followed by the Bonferroni post hoc test (*, P < .05). The arrow indicates the position of sst3 monomers that were used for quantification. B and C, siRNA knockdown of GRK2, GRK3, GRK5, and GRK6 was confirmed by Western blotting. The positions of molecular mass markers are indicated on the left (in kDa).
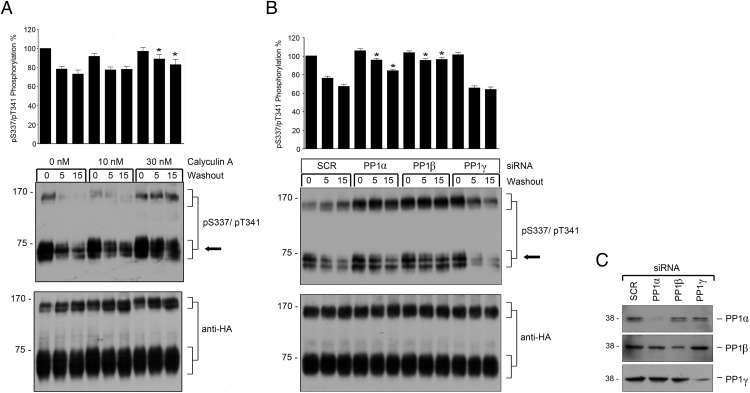
Recently, we identified PP1 as GPCR phosphatase for the sst2 and sst5 using a combination of chemical inhibitors and siRNA knockdown screening (14, 20). We hence used the same approach to identify the GPCR phosphatase responsible for dephosphorylation of sst3. When stably transfected HEK293 cells were exposed to increasing concentrations of protein phosphatase inhibitors, sst3 dephosphorylation was inhibited in a dose-dependent manner only by calyculin A (Figure 8A) but not by okadaic acid (data not shown). Both calyculin A and okadaic acid can effectively block the protein phosphatases PP2, PP4, and PP5 (43). In contrast to okadaic acid, calyculin A is also a potent inhibitor of PP1 activity (43). Thus, the present data suggest that PP1 activity is required for the S337/T341 dephosphorylation. To date, the 3 distinct catalytic subunits α, β, and γ for PP1 are known. To elucidate which of these PP1 isoforms is involved in sst3 dephosphorylation, we performed siRNA knockdown experiments. As depicted in Figure 8B, only a knockdown of PP1α and PP1β resulted in a robust inhibition of sst3 dephosphorylation. These results indicate that PP1 is the GPCR phosphatase responsible for S337/T341 dephosphorylation of sst3.
Figure 8. Calyculin A prevents sst3 dephosphorylation and PP1α and PP1β catalyze this process.
A, HEK293 cells stably expressing sst3 were treated with calyculin A for 10 minutes (37°C) at the indicated concentrations and then exposed to 1μM SS-14 for 10 minutes (37°C) in the presence of calyculin A. Cells were washed 3 times and then incubated for 0, 5, or 15 minutes in the presence of calyculin A but absence of agonist (room temperature). Data correspond to the mean ± SEM from 3 independent experiments. B, HEK293 cells stably expressing the sst3 receptor were transfected with siRNA targeted to PP1α, PP1β, PP1γ, or nonsilencing siRNA control (SCR) for 72 hours and then exposed to 1μM SS-14 for 10 minutes. Cells were washed 3 times and then incubated for 0, 5, or 15 minutes in the absence of agonist. Cells were lysed and immunoblotted with anti-pS337/pT341 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-HA antibody to confirm equal loading of the gels (anti-HA). Phosphorylation of monomers was quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells, which was set at 100%. Data correspond to the mean ± SEM from 4 independent experiments. Results were analyzed by two-way ANOVA followed by the Bonferroni post hoc test (*, P < .05). Note that 1) calyculin A inhibited sst3 dephosphorylation in a dose-dependent manner and 2) transfection with PP1α and PP1β siRNA resulted in a significant inhibition of sst3 dephosphorylation. The arrow indicates the position of sst3 monomers which were used for quantification. C, siRNA knockdown of PP1 was confirmed by Western blotting using subunit-specific PP1 antibodies. The positions of molecular mass markers are indicated on the left (in kDa).
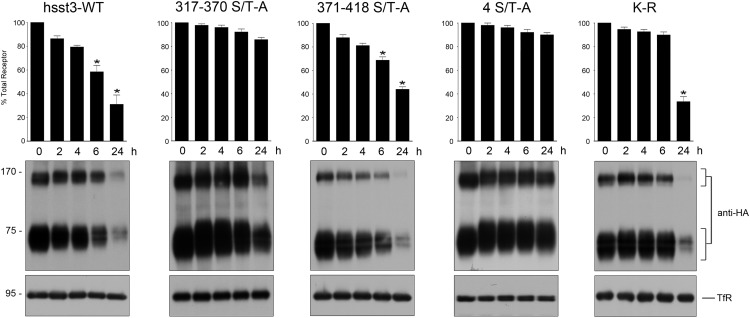
Given the differential trafficking of wild-type and mutant sst3 receptors, we next assessed the effects of the ablation of specific phosphorylation sites on the down-regulation of the receptor proteins during prolonged SS-14 exposure by Western blotting. As shown in Figure 7, we did not observe any detectable change in the level of cellular 4 ST-A or 317–370 S/T-A mutants during the 24-hour treatment period. In contrast, the sst3 receptor and the 371–418 S/T-A mutant underwent pronounced down-regulation, which became clearly detectable after prolonged agonist exposure (Figure 9). Furthermore a mutation of all 7 intracellular lysine residues which are potential acceptor sites for ubiquitination of the sst3 receptor only delays down-regulation during the first 6 hours of SS-14 treatment. After 24 hours SS-14 the K-R mutant was degraded to virtually identical extend as the wild-type sst3 receptor (Figure 9). These results suggest that rapid sst3 down-regulation requires phosphorylation of S337, T341, T348, and S361, whereas ubiquitination appears to be not required.
Figure 9. Phosphorylation is required for sst3 down-regulation.
HEK293 cells stably expressing the wild-type or mutant sst3 receptors were treated with 1μM somatostatin for 0, 2, 4, 6, or 24 hours. Cells were lysed and immunoblotted with the phosphorylation-independent anti-HA antibodies. Blots were stripped and reprobed with the antitransferrin receptor antibody to confirm equal loading of the gel (TfR, transferrin receptor). Receptor monomers were quantified and expressed as percentage of untreated cells. Data correspond to the mean ± SEM from 5 independent experiments. Results were analyzed by two-way ANOVA followed by the Bonferroni post hoc test (*, P < .05). The positions of molecular mass markers are indicated on the left (in kDa).
Discussion
The human sst3 receptor is unique among ssts in that it exhibits a very long intracellular C-terminal tail containing a large number of potential phosphate acceptor sites. Nevertheless, we show that the sst3 receptor is regulated like a prototypical GPCR. In fact, we identify S337, T341, T348, and S361 as primary sites of agonist-induced phosphorylation mediated primarily by GRK2 and GRK3. These 4 sites are all located in the proximal part of the sst3 C-terminal tail. Alignment of the phosphorylation motifs identified for the sst2 and sst5 receptors suggested that GRK2/3-mediated phosphorylation of these receptors occurs preferentially in a defined distance from the NPXXY motif. This knowledge led us to the identification of the sst3 phosphorylation motif with high precision and to successfully generate several novel phosphosite-specific antibodies. The C-terminal regions of the human and the rat sst3 receptor exhibit strikingly different sequences. However, critical sites involved in agonist-induced phosphorylation have previously been identified in a similar region of the rat sst3 receptor namely S341, S346, S351, and T357 (25). In contrast, phosphorylation of the proximal part of the sst3 C-terminal tail or the third intracellular loop appears not to be required for its rapid agonist-dependent regulation.
Generation of these phosphosite-specific antibodies enabled us to demonstrate an excellent correlation between extent and temporal dynamics of C-terminal phosphorylation and the trafficking properties of the human sst3 receptor. We also show that S337/T341 is rapidly phosphorylated and slowly dephosphorylated in a SS-14-dependent manner. In addition, we also show that mutation of these 4 serine and threonine residues is required and sufficient to completely block arrestin recruitment and receptor internalization. Moreover, we identify GRK2 and GRK3 as kinases responsible for phosphorylation and PP1α and PP1β as phosphatases responsible for dephosphorylation.
We have previously shown that analysis of agonist-induced phosphorylation using sst chimeras can provide valuable and previously unappreciated information about multireceptor somatostatin analogs currently under clinical and preclinical examination (18). In fact, pasireotide exhibited potent agonistic activity at the sst5 receptor but only weak partial agonistic properties at sst2 and sst3 receptors. Consequently, pasireotide should be classified as sst5-preferring ligand. Octreotide is a full agonist at the sst2 receptor but exhibited partial agonistic activity at the sst3 receptor and virtually no agonistic activity at the sst5 receptor. Consequently, octreotide should be classified as sst2-preferring ligand. In contrast, somatoprim is unique in that it was a potent agonist at both sst2 and sst5 receptors but no activity at the sst3 receptor. The present results using our newly generated phosphosite specific sst3 antibodies targeting directly the sst3 receptor not only confirm these results but also show that these antibodies can be valuable tools for pharmacological characterization of novel somatostatin analogs.
The human sst3 receptor is also unique among ssts in that it is rapidly down-regulated during prolonged agonist exposure (35). This rapid down-regulation was observed after exposure to both pasireotide and octreotide and, hence, appears to be independent of the nature of the peptide ligand (35). We have previously reported that the sst3 receptor undergoes agonist-induced ubiquitination (19). To test whether this ubiquitination is a precondition for down-regulation, we mutated all 7 lysine residues. However, after 24 hours SS-14 the K-R mutant was degraded to virtually identical extend as the wild-type sst3 receptor. Thus, rapid sst3 down-regulation requires phosphorylation of S337, T341, T348, and S361 and subsequent internalization, whereas ubiquitination appears to be dispensable.
In many systems, rapid down-regulation of GPCRs during continued or repeated drug administration leads to development of tolerance. Based on our down-regulation assays, we would predict that an early loss of clinical response is more likely to occur in tumors with predominant sst3 expression than in sst2- or sst5-expressing tumors. Thus, due to its rapid down-regulation, the sst3 receptor seems to be a less favorable pharmacological target for long-term administration of somatostatin analogs. Nevertheless, we observed that overexpression of sst3 in AtT-20 cells which endogenously express sst2 and sst5 receptors results in a leftward shift of the dose-response curve for pasireotide but not octreotide. Moreover, the sst3 receptor is one of the few GPCRs, which can drive tumor cells apoptosis and the C-terminal tail seems to be required for this activity (3, 26, 44–46). Thus, the sst3 receptor is a potential pharmacological target for diagnostic and acute therapeutic intervention using multireceptor somatostatin analogs (22–24, 47).
In conclusion, we provide direct evidence for agonist selective phosphorylation of C-terminal S337/T341, T348, and S361 of the human sst3 receptor. In addition, we identify GRK2- and GRK3-mediated phosphorylation and PP1α- and PP1β-mediated dephosphorylation as key regulators of rapid phosphorylation and dephosphorylation of the human sst3 receptor.
Acknowledgments
We thank Heidrun Guder, Heike Stadler, and Yvonne Schlenker for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft Grant SCHU924/10–4, the Deutsche Krebshilfe Grant 109952, and a grant from the Dr. Robert Pfleger-Stiftung.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft Grant SCHU924/10–4, the Deutsche Krebshilfe Grant 109952, and a grant from the Dr. Robert Pfleger-Stiftung.
Footnotes
- FACS
- fluorescence-activated cell sorting
- GIRK
- G protein-coupled inwardly rectifying potassium
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- PMA
- phorbol 12-myristate 13-acetate
- PP1
- protein phosphatase 1
- PTX
- pertussis toxin
- siRNA
- small interfering RNA
- SS-14
- somatostatin-14
- sst
- somatostatin receptor
- TPN-Q
- tertiapin-Q.
References
- 1. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2(12):999–1017. [DOI] [PubMed] [Google Scholar]
- 2. Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab. 2010;21(3):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157–198. [DOI] [PubMed] [Google Scholar]
- 4. Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides. 2001;35(1):1–23. [DOI] [PubMed] [Google Scholar]
- 5. Donangelo I, Melmed S. Treatment of acromegaly: future. Endocrine. 2005;28(1):123–128. [DOI] [PubMed] [Google Scholar]
- 6. Oberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology. 2010;139(3):742–753.e741. [DOI] [PubMed] [Google Scholar]
- 7. Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146(5):707–716. [DOI] [PubMed] [Google Scholar]
- 8. Lewis I, Bauer W, Albert R, et al. A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J Med Chem. 2003;46(12):2334–2344. [DOI] [PubMed] [Google Scholar]
- 9. Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev. 2011;32(2):247–271. [DOI] [PubMed] [Google Scholar]
- 10. Colao A, Faggiano A, Pivonello R. Somatostatin analogues: treatment of pituitary and neuroendocrine tumors. Prog Brain Res. 2010;182:281–294. [DOI] [PubMed] [Google Scholar]
- 11. Petersenn S, Schopohl J, Barkan A, et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010;95(6):2781–2789. [DOI] [PubMed] [Google Scholar]
- 12. Ma P, Wang Y, van der Hoek J, et al. Pharmacokinetic-pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin Pharmacol Ther. 2005;78(1):69–80. [DOI] [PubMed] [Google Scholar]
- 13. Pöll F, Lehmann D, Illing S, et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24(2):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrich A, Mann A, Kliewer A, et al. Phosphorylation of threonine 333 regulates trafficking of the human sst5 somatostatin receptor. Mol Endocrinol. 2013;27(4):671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh M, Schonbrunn A. Differential temporal and spatial regulation of somatostatin receptor phosphorylation and dephosphorylation. J Biol Chem. 2011;286(15):13561–13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kao YJ, Ghosh M, Schonbrunn A. Ligand-dependent mechanisms of sst2A receptor trafficking: role of site-specific phosphorylation and receptor activation in the actions of biased somatostatin agonists. Mol Endocrinol. 2011;25(6):1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehmann A, Kliewer A, Schutz D, Nagel F, Stumm R, Schulz S. Carboxyl-terminal multi-site phosphorylation regulates internalization and desensitization of the human sst2 somatostatin receptor. Mol Cell Endocrinol. 2014;387(1–2):44–51. [DOI] [PubMed] [Google Scholar]
- 18. Kliewer A, Mann A, Petrich A, Poll F, Schulz S. A transplantable phosphorylation probe for direct assessment of G protein-coupled receptor activation. PLoS One. 2012;7(6):e39458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Höllt V, Schulz S. Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279(20):21374–21382. [DOI] [PubMed] [Google Scholar]
- 20. Pöll F, Doll C, Schulz S. Rapid dephosphorylation of G protein-coupled receptors by protein phosphatase 1β is required for termination of β-arrestin-dependent signaling. J Biol Chem. 2011;286(38):32931–32936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanakis G, Grimelius L, Spathis A, et al. Expression of somatostatin receptors 1–5 and dopamine receptor 2 in lung carcinoids: implications for a therapeutic role. Neuroendocrinology. 2015;101(3):211–222. [DOI] [PubMed] [Google Scholar]
- 22. Lee M, Lupp A, Mendoza N, et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr Relat Cancer. 2015;22(1):111–119. [DOI] [PubMed] [Google Scholar]
- 23. Elston MS, Meyer-Rochow GY, Conaglen HM, et al. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase-deficient pheochromocytomas and paragangliomas. Hum Pathol. 2015;46(3):390–396. [DOI] [PubMed] [Google Scholar]
- 24. Shahbaz M, Ruliang F, Xu Z, et al. mRNA expression of somatostatin receptor subtypes SSTR-2, SSTR-3, and SSTR-5 and its significance in pancreatic cancer. World J Surg Oncol. 2015;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth A, Kreienkamp HJ, Meyerhof W, Richter D. Phosphorylation of four amino acid residues in the carboxyl terminus of the rat somatostatin receptor subtype 3 is crucial for its desensitization and internalization. J Biol Chem. 1997;272(38):23769–23774. [DOI] [PubMed] [Google Scholar]
- 26. War SA, Somvanshi RK, Kumar U. Somatostatin receptor-3 mediated intracellular signaling and apoptosis is regulated by its cytoplasmic terminal. Biochim Biophys Acta. 2011;1813(3):390–402. [DOI] [PubMed] [Google Scholar]
- 27. Lupp A, Nagel F, Doll C, et al. Reassessment of sst3 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-5. Neuroendocrinology. 2012;96(4):301–310. [DOI] [PubMed] [Google Scholar]
- 28. Otte M, Kliewer A, Schütz D, Reimann C, Schulz S, Stumm R. CXCL14 is no direct modulator of CXCR4. FEBS Lett. 2014;588(24):4769–4775. [DOI] [PubMed] [Google Scholar]
- 29. Troxler T, Hurth K, Schuh KH, et al. Decahydroisoquinoline derivatives as novel non-peptidic, potent and subtype-selective somatostatin sst(3) receptor antagonists. Bioorg Med Chem Lett. 2010;20(5):1728–1734. [DOI] [PubMed] [Google Scholar]
- 30. Rohrer SP, Birzin ET, Mosley RT, et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282(5389):737–740. [DOI] [PubMed] [Google Scholar]
- 31. Pfeiffer M, Koch T, Schröder H, et al. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A). J Biol Chem. 2001;276(17):14027–14036. [DOI] [PubMed] [Google Scholar]
- 32. Schulz S, Pauli SU, Schulz S, et al. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin Cancer Res. 2000;6(5):1865–1874. [PubMed] [Google Scholar]
- 33. Mundschenk J, Unger N, Schulz S, et al. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88(11):5150–5157. [DOI] [PubMed] [Google Scholar]
- 34. Plöckinger U, Albrecht S, Mawrin C, et al. Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J Clin Endocrinol Metab. 2008;93(4):1203–1210. [DOI] [PubMed] [Google Scholar]
- 35. Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab. 2009;94(2):654–661. [DOI] [PubMed] [Google Scholar]
- 36. Nagel F, Doll C, Pöll F, Kliewer A, Schröder H, Schulz S. Structural determinants of agonist-selective signaling at the sst(2A) somatostatin receptor. Mol Endocrinol. 2011;25(5):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lehmann A, Kliewer A, Martens JC, Nagel F, Schulz S. Carboxyl-terminal receptor domains control the differential dephosphorylation of somatostatin receptors by protein phosphatase 1 isoforms. PLoS One. 2014;9(3):e91526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kreienkamp HJ, Hönck HH, Richter D. Coupling of rat somatostatin receptor subtypes to a G-protein gated inwardly rectifying potassium channel (GIRK1). FEBS Lett. 1997;419(1):92–94. [DOI] [PubMed] [Google Scholar]
- 39. Vazquez M, Dunn CA, Walsh KB. A fluorescent screening assay for identifying modulators of GIRK channels. J Vis Exp. 2012(62);pii:3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walsh KB. Targeting GIRK channels for the development of new therapeutic agents. Front Pharmacol. 2011;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cervia D, Nunn C, Fehlmann D, Langenegger D, Schuepbach E, Hoyer D. Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs. Br J Pharmacol. 2003;139(1):109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Günther CaS. Analysis of somatostatin receptor signaling in pituitary cells with a novel membrane potential assay. Mol Endocrinol. 2016;30(4):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9(22):2055–2075. [DOI] [PubMed] [Google Scholar]
- 44. War SA, Kim B, Kumar U. Human somatostatin receptor-3 distinctively induces apoptosis in MCF-7 and cell cycle arrest in MDA-MB-231 breast cancer cells. Mol Cell Endocrinol. 2015;413:129–144. [DOI] [PubMed] [Google Scholar]
- 45. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–299. [DOI] [PubMed] [Google Scholar]
- 46. War SA, Kumar U. Coexpression of human somatostatin receptor-2 (SSTR2) and SSTR3 modulates antiproliferative signaling and apoptosis. J Mol Signal. 2012;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eigler T, Ben-Shlomo A, Zhou C, Khalafi R, Ren SG, Melmed S. Constitutive somatostatin receptor subtype-3 signaling suppresses growth hormone synthesis. Mol Endocrinol. 2014;28(4):554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]