Abstract
Elevated sympathetic nervous system (SNS) activity aggravates several diseases, including heart failure. The molecular cause(s) underlying this SNS hyperactivity are not known. We have previously uncovered a neurohormonal mechanism, operating in adrenomedullary chromaffin cells, by which circulating catecholamine (CA) levels increase in heart failure: severe dysfunction of the adrenal α2-adrenergic receptors (ARs) due to the up-regulation of G protein-coupled receptor-kinase (GRK)-2, the kinase that desensitizes them. Herein we looked at the potential signaling mechanisms that bring about this GRK2 elevation in chromaffin cells. We found that chronic CA treatment of either PC12 or rat primary chromaffin cells can in itself result in GRK2 transcriptional up-regulation through α2ARs-Gi/o proteins-Src-ERK1/2. The resultant GRK2 increase severely enhances the α2AR desensitization/down-regulation elevating not only CA release but also CA biosynthesis, as evidenced by tyrosine hydroxylase up-regulation. Finally, GRK2 knockdown leads to enhanced apoptosis of PC12 cells, indicating an essential role for GRK2 in chromaffin cell homeostasis/survival. In conclusion, chromaffin cell GRK2 mediates a positive feedback loop that feeds into CA secretion, thereby enabling the adrenomedullary component of the SNS to turn itself on.
Heart failure (HF) is a devastating disease with a significant burden on the public health of the developed world (1). Among its hallmark pathological characteristics is the elevated sympathetic nervous system (SNS) activity/outflow (2). Triggered by the cardiac insult in an effort to maintain cardiac output, SNS hyperactivity becomes maladaptive over time, resulting in increased morbidity and mortality (2). HF-related SNS hyperactivity is evidenced by the elevated levels of circulating catecholamines (CAs), ie, norepinephrine (NE) and epinephrine (Epi). However, the important question of exactly how, at the molecular level, the cardiac insult (eg, myocardial infarction [MI]) stimulates the SNS to increase its outflow to the heart, thereby contributing to the establishment and progression of chronic HF, remains largely unknown.
One candidate for such a molecular culprit appears to be adrenal G protein-coupled receptor (GPCR)-kinase (GRK)-2, which has been documented to be elevated in the adrenal medulla during chronic HF (3). This results in chronically enhanced desensitization and downregulation of α2-adrenergic receptors (ARs), expressed in the plasma membranes of chromaffin cells, wherein they exert autocrine negative feedback control of CA secretion (3). Thus, GRK2 up-regulation in adrenal chromaffin cells results in the excessive CA secretion that accompanies and aggravates chronic HF. In addition, when GRK2 is genetically deleted from birth, specifically from chromaffin cells, the course of HF after experimental MI is much milder, in terms of severity, cardiac function, and overall SNS activity (4). These findings strongly suggest a causal role for adrenal chromaffin cell GRK2 in linking the HF cause/trigger with the ensuing SNS (over)stimulation. Finally, β-blocker (ie, βAR antagonist) treatment during chronic HF, which is known to be beneficial for the failing heart, ameliorates circulating catecholamine levels (in part) via the down-regulation of adrenal GRK2 and the subsequent restoration of the chromaffin cell sympathoinhibitory α2AR function/signaling (5). This suggests that the SNS itself, via certain ARs, may reciprocally regulate adrenal GRK2 levels/activity as well.
In an effort to mechanistically delineate the link between HF and adrenal GRK2 up-regulation, we investigated, in the present study, the effects of CA's on chromaffin cell GRK2. We found that there is an autocrine positive feedback loop operating in adrenal chromaffin cells, in which the secreted CAs, via chronic α2AR activation, cause an up-regulation of GRK2, which, in turn, desensitizes/down-regulates the α2ARs, thereby allowing for continuously more CAs to be secreted.
Materials and Methods
Materials
All pharmacological agents (norepinephrine, nicotine, 5-bromo-6-[2-imidazoline-2-ylamino]-quinoxaline-UK14304, RX821002, pertussis toxin, PD98059, Src-inhibitor-1, etc), including hygromycin B, were from Sigma-Aldrich.
PC12 cell transfection and culture
PC12 cells were purchased from American Type Culture Collection and transfected with the human α2A-AR cDNA (Missouri S&T cDNA Resource Center, Rolla, Missouri) via the Lipofectamine method (Invitrogen), as previously described (6). Culture of PC12 cells was performed under standard protocols, as previously described (6). For α2AR maximal binding capacity determination purposes, a separate flask with transfected cells was harvested for saturation ligand binding using the α2AR-specific antagonist [3H]-RX821002 (specific activity 45–65 Ci/mmol; PerkinElmer), again as previously described (6). To obtain stably transfected clones, the human α2A-AR cDNA was inserted into the pD2500 mammalian stable expression vector (DNA 2.0) and, after the transfection via the Lipofectamine method, selection of stable transfectants was performed with 500 mg/mL Hygromycin B. Radioligand binding confirmed that the transgene (α2AAR) was properly expressed and a stable clone expressing 500 fmol/mg α2AAR of total cellular protein was chosen for further experiments. Finally, for GRK2 small interfering RNA (siRNA)-mediated knockdown, cells were transfected via the Lipofectamine method with rat GRK2-specific siRNA (catalog number AM16708) or scrambled siRNA (negative control, catalog number AM4611), both from Ambion (Life Technologies).
Adrenal chromaffin cell isolation and culture
Chromaffin cells were isolated from adrenal glands excised from adult male Sprague Dawley rats (450–500 g; Harlan), via incubation in a Locke's buffer solution containing 1 mg/mL collagenase (>200 U/mL; Biochrom KG), and cultured as described previously (3, 5). The purity of cultured chromaffin cells was assessed morphologically and by immunoblotting for endogenous expression of tyrosine hydroxylase; purity was greater than 90% in all experiments.
In vitro CA secretion assay
In vitro Epi and NE secretion in response to various treatments was measured in the supernatant of rat primary chromaffin cells by an ELISA, as described previously (3, 5). Cells were pretreated with UK14304 for 30 minutes prior to the nicotine challenge, and the supernatant was collected at 20 minutes after the nicotine application.
RNA isolation and real-time PCR
Total RNA isolation from PC12 cells, reverse transcription to cDNA, and quantitative real-time PCR for the GRK2 gene were carried out as previously described (3). The oligonucleotide primer pairs used were as follows: 5′-CCCTCTCACCATCTCTGAGC-3′ and 5′-CGGTTGGGGAACAAGTAGAA-3′ for rat GRK2 (annealing temperature: 63°C); 5′-GAAGGTTAAGCGGGAAGAGG-3′ and 5′-TCCAGGCGCTTAAAGTTCAT-3′ for rat GRK5 (annealing temperature: 63°C); 5′-GAAGGGCCTCTATGCTACCCA-3′ and 5′-TGGGCGCTGGATACGAGA-3′ for rat tyrosine hydroxylase (TH; annealing temperature: 63°C); and 5′-TCAAGAACGAAAGTCGGAGG-3′ and 5′-GGACATCTAAGGGCATCAC-3′ for rat 18S rRNA (annealing temperature: 60°C). Quantitative real-time PCR was performed using a MyIQ single-color real-time PCR detection system (Bio-Rad Laboratories) using SYBR Green Supermix (Bio-Rad Laboratories) and 100 nM of oligos. Quantification of mRNA included normalization to 18s rRNA levels. Specific PCR products were determined using melting curves and gel electrophoresis as described (3). No bands were seen in the control reactions (in the absence of reverse transcriptase).
Western blotting
Western blots to assess protein levels of GRK2 (antibody sc-562; Santa Cruz Biotechnology) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the loading control (antibody sc-25778; Santa Cruz Biotechnology) were done in protein extracts from PC12 or primary chromaffin cells, as described previously (3). Immunoblots were revealed by enhanced chemiluminescence (Life Technologies) and visualized in the FluorChem E Digital Darkroom (Protein Simple) (7). Densitometry was performed with the AlphaView software (Protein Simple) in the linear range of signal detection (on nonsaturated bands).
Saturation ligand binding
Plasma membranes from rat chromaffin cells were prepared and saturation binding was performed as described (3), using [3H]RX821002 (specific activity 45–65 Ci/mmol; PerkinElmer) because RX821002 is relatively specific for the rat α2ARs (8). Data were analyzed by nonlinear regression analysis using the GraphPad Prism software (GraphPad Software Inc).
Terminal deoxynucleotidyl transferase deoxyuridine 5-triphosphate nick end labeling (TUNEL) assay
The fluorescein-based in situ cell death (TUNEL) detection kit (Life Technologies) was used to perform the TUNEL reaction according to the manufacturer's instructions, essentially as described (9). Briefly, the cells on coverslips were fixed with 4% paraformaldehyde for 1 hour, washed with PBS, and permeabilized with 0.1% Triton X-100, and coverslips were incubated with 50 μL TUNEL reaction mixture in a humidified atmosphere at 37ºC. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole stain.
Statistical analysis
Data are generally expressed as mean ± SEM. An unpaired two-tailed Student's t test and a one- or two-way ANOVA with Bonferroni test were generally performed for statistical comparisons, unless otherwise indicated. For most multigroup statistical comparisons, a Dunnett's test using SAS version 8.2 software was used as well. For all tests, a value of P < .05 was considered to be significant.
Results
CA-induced GRK2 up-regulation via α2ARs in PC12 cells
Previous studies have indicated that, although rat adrenal primary chromaffin cells express (at least) the α2AAR subtype (3), the rat pheochromocytoma-derived chromaffin cell line PC12 does not express any ARs at appreciable levels (10). For this reason and to better simulate the real, normal adrenal chromaffin cells, we used PC12 cells transiently transfected with the human α2AAR subtype in the present study. The α2AAR maximal binding capacity of the cells used in all of the experiments was calculated at approximately 3.0 pmol/mg protein (data not shown). To replicate the in vivo situation immediately after an MI, when levels of circulating NE hitting the chromaffin cells of the adrenal medulla (among other tissues) begin to rise (3), we chronically stimulated the α2AR-expressing PC12 cells in culture with NE for either 6 or 24 hours (applied repeatedly every 6 h). As shown in Figure 1A, either NE treatment led to a significant increase in GRK2 mRNA levels, sustained for up to 24 hours. This increase was strictly α2AR mediated for the following reasons: 1) pretreatment with the α2AR-specific antagonist RX821002 (8) completely abolished NE-induced GRK2 mRNA upregulation and 2) no effect on GRK2 mRNA levels was observed in nontransfected PC12 cells treated with NE (Figure 1A). The latter provides another confirmation of the fact that PC12 cells lack endogenous functional ARs (10). Importantly, similar results were observed in PC12 cells stably transfected with a far lower DNA amount of the human α2AAR-encoding construct to induce only 500 fmol/mg protein of receptor expression, ie, much closer to the physiological, endogenously expressed α2AR levels in chromaffin cells (Supplemental Figure 1). This strongly suggests that the observed NE-induced GRK2 up-regulation in PC12 cells is very likely to be physiologically relevant for adrenal chromaffin cells in vivo.
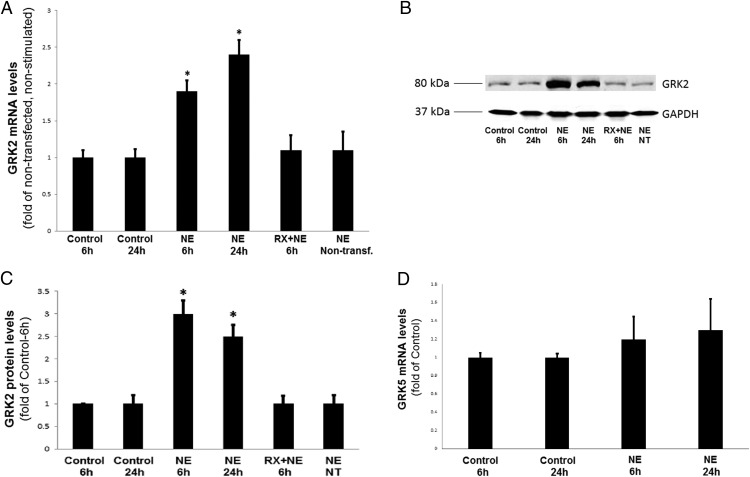
Figure 1. Chronic NE treatment of PC12 cells up-regulates GRK2 but not GRK5.
α2AR-transfected PC12 cells were treated with 10 μM NE or vehicle (control) either once for 6 hours or every 6 hours (repeatedly) for a total period of 24 hours (NE 24 h) in the presence or absence of 10 μM RX821002 (RX + NE). Nontransfected NE-treated cells were also included in the analysis (NE Nontransf., NT). At the end of the indicated treatment periods, cells were harvested and total RNA was isolated to perform real-time PCR for GRK2 mRNA quantitation (A), or protein extracts were prepared to perform Western blotting for GRK2 protein quantitation (B). Blots for GAPDH serving as the loading control are also shown. C, The densitometric quantitation of GRK2 protein induction. *, P < .05 vs control-6 hours (n = 5 independent experiments/condition). D, GRK5 mRNA levels as measured by real-time PCR in α2AR-transfected PC12 cells treated with 10 μM NE or vehicle (control) either once for 6 hours or every 6 hours (repeatedly) for a total period of 24 hours (NE 24 hours). No significant differences were observed among the treatments (n = 5 independent determinations/condition).
The NE-induced increase in GRK2 mRNA levels results in GRK2 up-regulation at the protein level as well (Figure 1, B and C): chronic NE treatment for either 6 or 24 hours (again, repeated every 6 h) led to a significant increase in GRK2 protein levels, which was again absent in RX821002-pretreated (or in nontransfected) PC12 cells, suggesting the increase was α2AR dependent (Figure 1B). Finally, the dose-response experiments with varying concentrations of NE applied for a total period of 24 hours revealed that chronic NE treatment is capable of inducing GRK2 mRNA up-regulation, even at concentrations as low as 100 nM (Supplemental Figure 2). Taken together, these results indicate that chronic NE stimulation of adrenal chromaffin cells up-regulates GRK2 both at the mRNA and protein levels. Of note, the other major GRK isoform expressed in rat adrenal chromaffin cells, GRK5 (3), does not seem to be affected by chronic NE treatment in the α2AAR-expressing PC12 cells, at least at the mRNA level (Figure 1D), suggesting a selective effect of chronic CA stimulation on GRK2 only.
GRK2 up-regulation suppresses the sympathoinhibitory activity of α2ARs in primary chromaffin cells
GRK2 is known to desensitize (and down-regulate) adrenal α2ARs, like many other GPCRs, by phosphorylating the agonist-occupied receptor in its C-terminal tail and/or in its third intracellular loop (11). Thus, we hypothesized that the chronic NE treatment-induced GRK2 up-regulation would enhance chromaffin cell α2AR desensitization, suppressing the ability of these receptors to inhibit CA secretion. To test this possibility, we isolated primary chromaffin cells from adult, healthy rat adrenal glands and cultured them, under standard conditions, for 24 hours with repeated applications of NE (or vehicle) every 6 hours. At 24 hours after the isolation and treatment, we performed in vitro CA secretion experiments using nicotine as the stimulus, in the presence or absence of the α2AR-specific full agonist UK14304 (3) (Figure 2A). Nicotine specifically activates the nicotinic cholinergic receptors (nAChRs) endogenously expressed in chromaffin cells, whose stimulation by acetylcholine (ACh) is the physiological stimulus for adrenal CA secretion (12). Thus, nicotine perfectly emulates the physiologic ACh stimulus for CA secretion and was preferred over ACh in our experiments because it activates only the nAChR, which is not a GRK2 substrate (not a GPCR) (12). In contrast, ACh can also activate muscarinic receptors, which are a GRK2 substrate (they are GPCRs) and have been reported to be present in chromaffin cells affecting CA secretion (12). As shown in Figure 2A, in control (nontreated) primary chromaffin cells, the endogenous α2ARs are capable of blocking nicotine-induced NE (Figure 2A, top panel) or Epi (Figure 2A, bottom panel) secretion upon their agonist activation. In contrast, in chromaffin cells treated with NE for 24 hours, the endogenous α2ARs have essentially lost this ability, ie, they appear severely desensitized (Figure 2A).
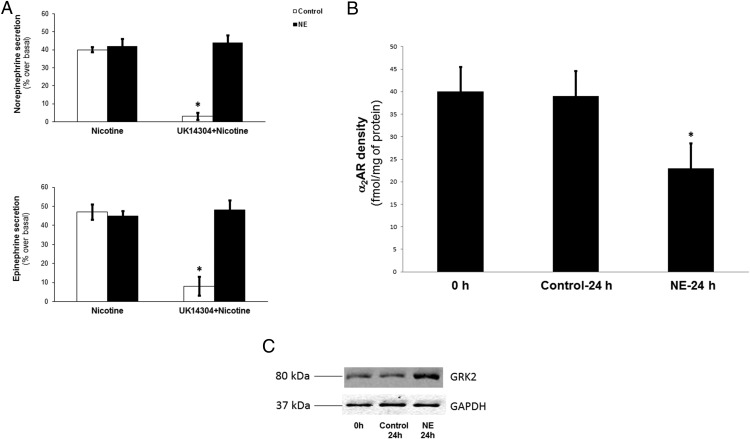
Figure 2. Chronic NE-induced GRK2 up-regulation results in enhanced α2AR desensitization and down-regulation in primary chromaffin cells.
A, In vitro NE (top panel) and Epi (bottom panel) secretion from cultured rat primary chromaffin cells treated with 10 μM NE (NE) or vehicle (control) for 24 hours (repeated application every 6 hours), prior to being placed in the secretion assay buffer. The cells were left to settle in the assay buffer for 30 minutes and then were treated with 10 μM UK14304 for another 30 minutes. At the end of this period, the buffer was changed to wash out UK14304, and then 50 μM nicotine was applied in a fresh buffer, either alone (Nicotine) or together with another 10 μM UK14304 challenge (U-K14304 + Nicotine). No differences were observed between non-UK14304-pretreated cells (data not shown). *, P < .05 vs control/nicotine or NE/UK14304 + nicotine (n = 5 independent experiments). B, Saturation ligand-binding studies using [3H]RX821002 on plasma membrane preparations from these cells. Membrane preparations from cells immediately after isolation (upon plating) were also analyzed for comparison (control 0 h). Nonspecific binding was determined in the presence of 0.4 mM phentolamine. *, P < .05 vs either of the other groups (n = 5 independent experiments/group). Data are expressed as mean ± SEM. C, Western blotting for GRK2 in whole-protein extracts from these cells. Representative blots from three independent experiments are shown, including GAPDH as loading control.
In addition, the total functional α2AR number of the NE-treated cells is significantly reduced compared with control (nontreated) cells or with cells tested immediately upon isolation (ie, before plating and treatment initiation) (Figure 2B). This is not an artifact of cell isolation and culturing for 24 hours because the α2AR densities between the time of isolation and at 24 hours after the culture are essentially identical (Figure 2B). Thus, in addition to being severely desensitized, α2ARs of chromaffin cells chronically treated with NE are also down-regulated. Finally, GRK2 levels are elevated also in these primary chromaffin cells at 24 hours after the NE treatment, compared with control (nontreated) cells or cells at the time of isolation (Figure 2C), ie, similarly to what was found in α2AR-expressing PC12 cells (Figure 1B). This suggests that the up-regulated GRK2 is most likely the culprit for the enhanced α2AR desensitization and down-regulation caused by the chronic NE treatment.
α2ARs induce GRK2 gene transcription in chromaffin cells via Gi/o proteins, Src kinase, and ERK1/2
We also examined the potential signaling pathway(s) underlying this NE-induced GRK2 up-regulation in chromaffin cells. Because the experiments mentioned above indicated a dependence on α2ARs, which primarily (albeit not exclusively) couple to Gi/o proteins (13), we first examined the potential involvement of this type of G proteins. Indeed, pretreatment with the Gi/o protein inhibitor pertussis toxin prevented the NE-induced GRK2 increase both at mRNA and protein levels (Figure 3, A–C), indicating that the signaling pathway is Gi/o protein dependent. Furthermore, the observed increase in GRK2 mRNA levels preceding the increase in protein levels indicates that the effect is pretranslational but does not give any insight into whether it is due to increased gene transcription or mRNA stability (reduced mRNA degradation). To that end, we pretreated the PC12 cells with actinomycin D to block gene transcription (14). NE was unable to up-regulate GRK2 mRNA or protein levels in cells pretreated with actinomycin D, indicating that the NE-dependent GRK2 up-regulation is due to activated gene transcription (Figure 3, A–C).
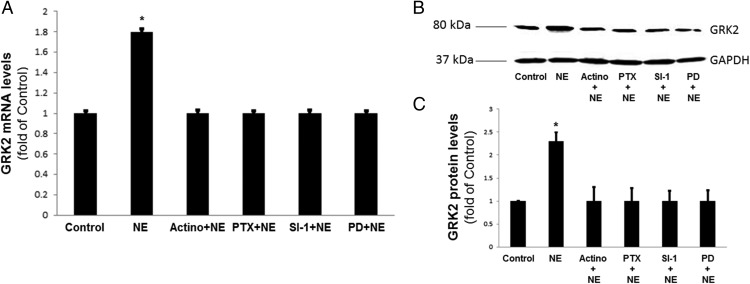
Figure 3. Chronic NE-induced GRK2 up-regulation is mediated transcriptionally by Gi/o proteins and ERKs.
α2AR-transfected PC12 cells were treated with 10 μM NE or vehicle (control) for 6 hours in the presence or absence of 5 μg/mL actinomycin-D (Actino + NE), 100 ng/mL pertussis toxin (PTX + NE), 1 μM Src-inhibtor-1 (SI-1 + NE), or 10 μM PD98059 (PD + NE). At the end of the indicated treatment periods, cells were harvested and total RNA was isolated to perform real-time PCR for GRK2 mRNA quantitation (A) or protein extracts were prepared to perform Western blotting for GRK2 protein quantitation (B). B, Representative Western blots for GRK2, including GAPDH as loading control. C, The densitometric quantitation of GRK2 protein induction. *, P < .05 vs all other groups (n = 5 independent experiments/condition).
Finally, the α2ARs are known in various cell types to activate the ERK1/2 members of the MAPK family through a pathway involving the activated Gi/o protein-derived free Gβγ subunits and the nonreceptor tyrosine kinase Src (15, 16). Activated ERKs then translocate to the nucleus in which they regulate gene transcription, and the GRK2 gene has been reported to be a transcriptional target of μ-opioid receptor-activated ERKs (17). Thus, we tested whether Src kinase and ERK1/2 are involved in the NE-dependent GRK2 up-regulation in PC12 cells, by pretreating the cells with Src inhibitor-1 (18) or with the known ERK activation (MAPK kinase-1) inhibitor PD98059 (19), respectively. Indeed, both pharmacological inhibitors prevented the increases in GRK2 mRNA and protein levels induced by chronic NE treatment (Figure 3, A–C), suggesting that the NE-induced GRK2 gene transcriptional activation proceeds through a Src-ERK1/2 pathway.
Chronic NE-induced GRK2 up-regulation induces CA biosynthesis and promotes cell survival in chromaffin cells
Because chronic CA treatment-induced GRK2 up-regulation results in enhanced CA secretion in chromaffin cells, we tested whether it also affects CA biosynthesis by examining the mRNA levels of TH, the enzyme that catalyzes the rate-limiting step of chromaffin cell CA biosynthesis (3). Indeed, NE treatment of α2AR-expressing PC12 cells for 24 hours led to a significant up-regulation of the TH mRNA levels (Figure 4A). Importantly, this NE-induced TH up-regulation was completely GRK2 dependent because it was abrogated by the siRNA-mediated knockdown of GRK2 (Figure 4A). Thus, chronic NE-induced GRK2 up-regulation enhances not only the secretion but also the biosynthesis of CAs in chromaffin cells, suggesting a tonic regulatory/trophic physiological role for this kinase in these cells. Consistent with this role, GRK2 siRNA-mediated knockdown also leads to significant apoptosis in cultured, untreated α2AR-expressing PC12 cells within 24 hours (Figure 4, B and C). The GRK2 knockdown was confirmed by Western blotting (Supplemental Figure 3, A and B).
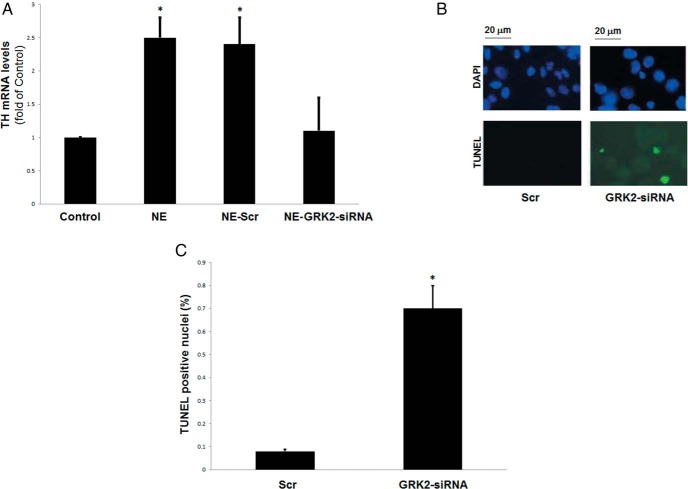
Figure 4. GRK2 promotes chronic NE-induced CA biosynthesis and survival in chromaffin cells.
A, α2AR-expressing PC12 cells were transfected with GRK2- or scrambled (Scr)-siRNA and simultaneously treated with 10 μM NE or vehicle (control) for 24 hours (repeatedly every 6 h). At the end of the treatment period, cells were harvested and total RNA was isolated to perform real-time PCR for TH mRNA quantitation. *, P < .05 vs control (n = 5 independent experiments/condition). B and C, Apoptotic cell death of α2AR-expressing PC12 cells at 24 hours after the transfection with GRK2- or scrambled (Scr)-siRNA, as measured by TUNEL. Representative images of TUNEL-positive nuclei are shown in panel B, and the quantitation in panel C. *, P < .05 vs Scr, (n = 5 fields scanned per group).
Discussion
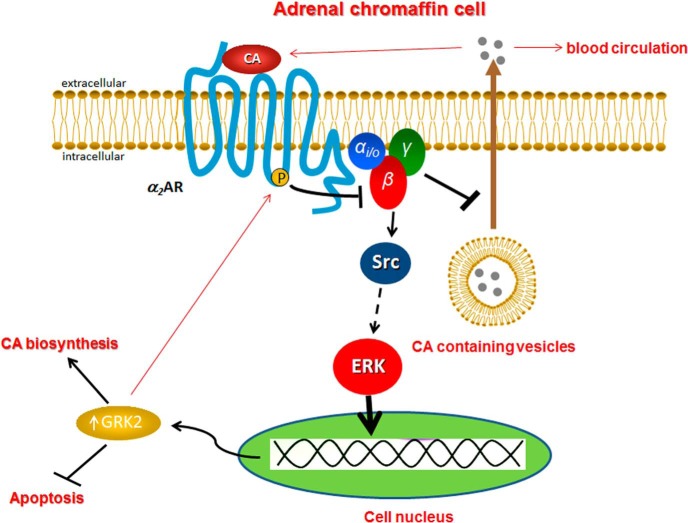
In the present study, we have uncovered a novel signaling pathway in adrenal chromaffin cells, which results in GRK2 transcriptional up-regulation in response to chronic catecholaminergic treatment. Herein we provide evidence, as schematically summarized in Figure 5, that chronic activation of chromaffin cell α2ARs by the CAs stimulates GRK2 gene expression, via the Gi/o protein-Src-ERK pathway. The up-regulated GRK2, in turn, enhances desensitization and down-regulation of the α2ARs so that α2ARs can no longer stimulate GRK2 gene expression, thereby closing a negative feedback loop (with regard to the GRK2 up-regulation). Given, however, that α2ARs normally inhibit CA secretion from the adrenal chromaffin cells, the CA-stimulated GRK2 up-regulation creates another, positive feedback loop at the same time, affecting CA secretion. In other words, the secreted CAs induce the up-regulation of GRK2, which further enhances, over time, their adrenal release via disinhibition of α2AR-dependent sympatholytic function (Figure 5). In addition, CA-stimulated GRK2 up-regulation enhances CA biosynthesis in chromaffin cells because it up-regulates TH and appears also essential for chromaffin cell survival and regular homeostasis (Figure 5). These findings are in complete accordance with in vivo observations in transgenic mice lacking GRK2 specifically in their adrenal chromaffin cells, which display significantly attenuated adrenal hypertrophy and TH expression levels in vivo (4) as well as with the significant adrenal hypertrophy and TH up-regulation exhibited by post-MI HF animals, in which adrenal GRK2 is elevated to chronically enhance CA secretion and circulating CA levels (3).
Figure 5. Schematic illustration of the chronic CA-elicited signaling pathway leading to GRK2 up-regulation in chromaffin cells.
See text for details and molecular acronym definitions.
Furthermore, these findings have obvious pathophysiological implications pertaining to diseases that are characterized by elevated SNS activity/outflow and circulating CAs, such as chronic post-MI HF (2). The biggest question regarding the establishment of SNS hyperactivity in chronic HF is whether the cause is the acute fall in cardiac performance per se or the initial elevation of CAs that occurs in response to it. Our finding that the CAs can stimulate their own secretion from the adrenal medulla via GRK2 up-regulation argues in favor of the latter. It also strongly suggests chromaffin cell GRK2 elevation as the key molecular event linking the commencement of HF with the genesis of SNS hyperactivity. Notably, this signaling mechanism uncovered in the chromaffin cells could very well be operative also in the sympathetic neurons innervating the cardiac muscle, which also express α2ARs and GRK2 (20, 21). Future studies in cardiac sympathetic nerve terminals will be needed to confirm this. However, if this CA-induced GRK2 elevation is a general phenomenon occurring throughout the SNS, then this could provide a molecular explanation for the α2AR down-regulation in the peripheral sympathetic nerves observed in human HF (22, 23) as well as for the therapeutic failure of α2AR-agonist sympatholytic drugs (eg, moxonidine) for human HF treatment (24). In fact, the main reason hypothesized for moxonidine's failure in the Sustained Release Moxonidine for Congestive Heart Failure trial was excessive sympatholysis incompatible with life (25). Our present data suggest the exact opposite may have been the case, ie, chronic α2AR agonism by moxonidine caused excessive SNS activation via GRK2 up-regulation that was too toxic for the failing heart and can cause HF in its own right (26). Thus, α2AR-agonist sympatholytics may not be appropriate for HF therapy anyway because they inadvertently activate the SNS in the long run, via GRK2 up-regulation, rather than suppressing it. In any case, it is quite plausible that CA-induced GRK2 up-regulation may be a general homeostatic cellular mechanism, in addition to CA biosynthetic enzyme up-regulation (27), by which the SNS can stimulate itself in situations in which it is required to respond to, eg, in an acute episode of cardiac decline (myocardial infarction), etc.
Of note, mRNA induction of chromaffin cell GRK2 has also been reported during pressure overload-induced HF, shown to be due to activation of the preganglionic cholinergic (nAChR dependent) nerves ending in the adrenal medulla (28). However, GRK2 protein levels were not examined in that study; neither were specific underlying signaling mechanism(s). Nevertheless, HF-associated chromaffin cell GRK2 up-regulation may also occur in response to nAChR activation as well. Finally, from a therapeutic viewpoint, our findings suggest that pharmacological GRK2 inhibition immediately after a myocardial infarction may not only improve cardiac function and adrenergic reserve by restoring the function and signaling of cardiac βARs (29) but also help keep the SNS at bay, ie, prevent its overstimulation, which is deleterious for the heart in its own right (2).
As far as the signaling pathway mediating GRK2 up-regulation in chromaffin cells is concerned, it is interesting to note that the free Gβγ-subunits of activated Gi/o proteins, known to activate ERKs via Src, are also responsible for the recruitment of GRK2 to the active receptor to phosphorylate it (15). This suggests an alternative route for GRK2 to close the negative feedback loop of its up-regulation in chromaffin cells, ie, in addition to decoupling the α2ARs from Gi/o protein activation (α2AR desensitization), GRK2 may also directly block the Gβγ-dependent Src activation and downstream ERK activation that stimulate its gene expression. It is also worth noting that ERKs have been reported to directly phosphorylate GRK2 at Ser670, resulting in the inhibition of GRK2 activity toward receptors (30). On the other hand, they have been implicated in the transcriptional activation of the GRK2 gene downstream of μ-opioid receptors in a ligand-specific (ligand-biased) manner (17) and, in the present study, they were found to stimulate GRK2 gene expression downstream of α2ARs as well. Therefore, ERK1/2 might exert a temporally bimodal regulation of GRK2 activity in chromaffin cells: acutely (within minutes of stimulation), they inhibit GRK2 via a posttranslational modification (phosphorylation), but in the long term, they stimulate GRK2 by inducing its gene expression. Nevertheless, our present data clearly indicate that chronic catecholaminergic treatment of chromaffin cells leads to ERK-dependent GRK2 up-regulation, which severely impairs α2AR sympatholytic function.
Finally, adrenal chromaffin cells have also been reported to express β2ARs, which may potentiate CA secretion via an autocrine positive feedback loop (12). Chronic CA treatment-induced GRK2 up-regulation would theoretically lead to suppression of this β2AR-mediated loop, curbing (albeit to a limited extent) CA secretion. Although this possibility is worth investigating, it should be pointed out that the β2AR is only one of several chromaffin cell membrane receptors enhancing CA secretion (31); thus, even if true, the physiological significance of this for the total adrenal CA secretion is dubious. Indeed, adrenal GRK2 elevation in vivo leads to overall increased CA secretion with no overt effects on adrenal β2ARs (3).
Notably, the up-regulating effect of CAs on GRK2 in chromaffin cells appears selective for this GRK isoform because the levels of the other major GRK isoform expressed in rat adrenal chromaffin cells, GRK5 (3), are unaltered by a 24-long NE treatment (Figure 1D). This is somewhat expected because GRK5 gene transcription is (in part) induced by the known proinflammatory transcription factor nuclear factor-κB (7), whereas the α2AAR subtype expressed in the PC12 cells of the present study has the smallest nuclear factor-κB-inducing activity, of the three α2AR subtypes, in PC12 cells (32). Thus, this selective up-regulation of GRK2 (but not GRK5) by chronic NE treatment may be α2AR subtype dependent. Alternatively, GRK5 levels may also change by chronic CA treatment but over longer periods of time (beyond 24 h). This apparent difference in the catecholaminergic regulation of GRK5 vs GRK2 levels in chromaffin cells warrants further investigation in the future.
Finally, the fact that GRK2 is up-regulated in chromaffin cells by α2AR signaling, which GRK2 itself blocks (desensitizes the α2ARs), may seem somewhat counterintuitive. However, given that both Gi/o proteins and ERK1/2 induce its elevation (Figure 5), any Gi/o-coupled receptor and any stimulus that activates the ERKs can theoretically cause GRK2 elevation in chromaffin cells. Additionally, it is also possible that β-arrestin signaling, which follows immediately after α2AR desensitization (G protein uncoupling) (2), may also lead to GRK2 up-regulation, a possibility we plan to investigate in the future. Thus, even upon massive α2AR functional desensitization, GRK2 may still remain elevated in adrenal chromaffin cells over time, whereas obviously catecholamine secretion proceeds unabated (because α2ARs are desensitized).
In conclusion, adrenal, and, more specifically, chromaffin cell GRK2 seems to be not only a therapeutic target for sympatholysis in HF (and other diseases accompanied by elevated SNS outflow) but also a molecular master switch for SNS self-activation. By taking advantage of the tight negative regulation GRK2 exerts on α2AR-dependent inhibition of CA secretion, the adrenal gland (and possibly also other components of the SNS) has found in this kinase a very convenient and efficient way to quickly amplify the organismal sympathetic response to physiological and pathological stimuli.
Acknowledgments
We thank Dr X. P. Huang (Florida Atlantic University, Boca Raton, Florida) for excellent technical assistance.
This work was supported in part by Scientist Development Grant 09SDG2010138 from the American Heart Association (American Heart Association National Center) and a Nova Southeastern University's President's Faculty Research and Development Grant (both to A.L.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported in part by Scientist Development Grant 09SDG2010138 from the American Heart Association (American Heart Association National Center) and a Nova Southeastern University's President's Faculty Research and Development Grant (both to A.L.).
Footnotes
- ACh
- acetylcholine
- AR
- adrenergic receptor
- CA
- catecholamine
- Epi
- epinephrine
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- Gi/o
- inhibitory/other G protein
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- HF
- heart failure
- MI
- myocardial infarction
- nAChR
- nicotinic cholinergic receptor
- NE
- norepinephrine
- siRNA
- small interfering RNA
- SNS
- sympathetic nervous system
- TH
- tyrosine hydroxylase
- TUNEL
- terminal deoxynucleotidyl transferase deoxyuridine 5-triphosphate nick end labeling.
References
- 1. Mudd JO, Kass DA. Tackling heart failure in the twenty first century. Nature. 2008;451:919–928. [DOI] [PubMed] [Google Scholar]
- 2. Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. [DOI] [PubMed] [Google Scholar]
- 4. Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rengo G, Lymperopoulos A, Zincarelli C, et al. Blockade of β-adrenoceptors restores the GRK2-mediated adrenal α(2)-adrenoceptor-catecholamine production axis in heart failure. Br J Pharmacol. 2012;166:2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen K, Kassimatis T, Lymperopoulos A. Impaired desensitization of a human polymorphic α2B-adrenergic receptor variant enhances its sympatho-inhibitory activity in chromaffin cells. Cell Commun Signal. 2011;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salazar NC, Vallejos X, Siryk A, et al. GRK2 blockade with βARKct is essential for cardiac β2-adrenergic receptor signaling towards increased contractility. Cell Commun Signal. 2013;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uhlén S, Dambrova M, Näsman J, et al. [3H]RS79948–197 binding to human, rat, guinea pig and pig α2A, α2B- and α2C-adrenoceptors. Comparison with MK912, RX821002, rauwolscine and yohimbine. Eur J Pharmacol. 1998;343:93–101. [DOI] [PubMed] [Google Scholar]
- 9. Bathgate-Siryk A, Dabul S, Pandya K, et al. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams NG, Zhong H, Minneman KP. Differential coupling of α1-, α2-, and β-adrenergic receptors to mitogen-activated protein kinase pathways and differentiation in transfected PC12 cells. J Biol Chem. 1998;273:24624–24632. [DOI] [PubMed] [Google Scholar]
- 11. Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. [DOI] [PubMed] [Google Scholar]
- 12. Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med. 2007;13:503–511. [DOI] [PubMed] [Google Scholar]
- 13. Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326:541–551. [DOI] [PubMed] [Google Scholar]
- 14. Cassé C, Giannoni F, Nguyen VT, Dubois MF, Bensaude O. The transcriptional inhibitors, actinomycin D and α-amanitin, activate the HIV-1 promoter and favorphosphorylation of the RNA polymerase II C-terminal domain. J Biol Chem. 1999;274:16097–16106. [DOI] [PubMed] [Google Scholar]
- 15. Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. [DOI] [PubMed] [Google Scholar]
- 16. Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. [DOI] [PubMed] [Google Scholar]
- 17. Zheng H, Loh HH, Law PY. β-Arrestin-dependent μ-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Csató V, Pető A, Koller A, Édes I, Tóth A, Papp Z. Hydrogen peroxide elicits constriction of skeletal muscle arterioles by activating the arachidonic acid pathway. PLoS One. 2014;9:e103858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vartiainen N, Keksa-Goldsteine V, Goldsteins G, Koistinaho J. Aspirin provides cyclin-dependent kinase 5-dependent protection against subsequent hypoxia/reoxygenation damage in culture. J Neurochem. 2002;82:329–335. [DOI] [PubMed] [Google Scholar]
- 20. Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. [DOI] [PubMed] [Google Scholar]
- 21. Llorente J, Lowe JD, Sanderson HS, et al. μ-Opioid receptor desensitization: homologous or heterologous? Eur J Neurosci. 2012;36:3636–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaye D, Esler M. Sympathetic neuronal regulation of the heart in aging and heart failure. Cardiovasc Res. 2005;66:256–264. [DOI] [PubMed] [Google Scholar]
- 23. Aggarwal A, Esler MD, Lambert GW, Hastings J, Johnston L, Kaye DM. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circulation. 2002;105:1031–1033. [DOI] [PubMed] [Google Scholar]
- 24. Cohn JN, Pfeffer MA, Rouleau J, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail. 2003;5:659–667. [DOI] [PubMed] [Google Scholar]
- 25. Floras JN. The “unsympathetic” nervous system of heart failure. Circulation. 2002;105:1753–1755. [DOI] [PubMed] [Google Scholar]
- 26. Soltysinska E, Olesen SP, Osadchii OE. Myocardial structural, contractile and electrophysiological changes in the guinea-pig heart failure model induced by chronic sympathetic activation. Exp Physiol. 2011;96:647–663. [DOI] [PubMed] [Google Scholar]
- 27. Tomaszek A, Kiczak L, Bania J, et al. Increased gene expression of catecholamine-synthesizing enzymes in adrenal glands contributes to high circulating catecholamines in pigs with tachycardia-induced cardiomyopathy. J Physiol Pharmacol. 2015;66:227–231. [PubMed] [Google Scholar]
- 28. Schneider J, Lother A, Hein L, Gilsbach R. Chronic cardiac pressure overload induces adrenal medulla hypertrophy and increased catecholamine synthesis. Basic Res Cardiol. 2011;106:591–602. [DOI] [PubMed] [Google Scholar]
- 29. Lymperopoulos A, Rengo G, Koch WJ. GRK2 inhibition in heart failure: something old, something new. Curr Pharm Des. 2012;18:186–191. [DOI] [PubMed] [Google Scholar]
- 30. Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274:34531–34534. [DOI] [PubMed] [Google Scholar]
- 31. Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell Mol Neurobiol. 2010;30:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lymperopoulos A, Karkoulias G, Koch WJ, Flordellis CS. α2-Adrenergic receptor subtype-specific activation of NF-κB in PC12 cells. Neurosci Lett. 2006;402:210–215. [DOI] [PubMed] [Google Scholar]