Abstract
Kisspeptin (Kiss) and G-protein-coupled receptor (Gpr)54 have emerged as key regulators of reproduction. 17β-estradiol (E2)-mediated regulation of these neurons is nuclei specific, where anteroventral periventricular (AVPV) Kiss neurons are positively regulated by E2, whereas arcuate nucleus (ARC) neurons are inhibited. We have generated immortalized Kiss cell lines from male and female adult-derived murine hypothalamic primary culture, as well as cell lines from microdissected AVPV and ARC from female Kiss-green fluorescent protein (GFP) mice. All exhibit endogenous Kiss-1 expression, estrogen receptors (ER)s (ERα, ERβ, and Gpr30), as well as known markers of AVPV Kiss neurons in the mHypoA-50 and mHypoA-Kiss/GFP-4, vs markers of ARC Kiss neurons in the mHypoA-55 and the mHypoA-Kiss/GFP-3 lines. There was an increase in Kiss-1 mRNA expression at 24 hours in the AVPV lines and a repression of Kiss-1 mRNA at 4 hours in the ARC lines. An E2-mediated decrease in ERα mRNA expression at 24 hours in the AVPV cell lines was detected, and a significant decrease in Gpr30, ERα, and ERβ mRNA levels at 4 hours in the ARC cell lines was evident. ER agonists and antagonists determined the specific ERs responsible for mediating changes in gene expression. In the AVPV, ERα is required but not ERβ or GPR30, vs the ARC Kiss-expressing cell lines that require GPR30, and either ERα and/or ERβ. We determined cAMP response element-binding protein 1 was necessary for the down-regulation of Kiss-1 mRNA expression using small interfering RNA knockdown in the ARC cell model. These studies elucidate some of the molecular events involved in the differential E2-mediated regulation of unique and specific Kiss neuronal models.
It is well established that gonadal steroids, including estrogen, androgen and progesterone contribute to the dynamic control of GnRH and gonadotrophin secretion via feedback regulatory loops operating within the hypothalamic-pituitary-gonadal (HPG) axis. Estrogens play a prominent role in reproduction, sexual differentiation, sexual behavior, sexually dimorphic brain development and organization (1, 2), as well as nonreproductive events, such as energy homeostasis, neuronal growth and differentiation, mood and cognition (3, 4). Three estrogen receptors (ERs) mediate the biological actions of estrogens: the 2 nuclear receptors ERα and ERβ and the membrane-based receptor, G-protein-coupled receptor 30 (GPR30, now often referred to as GPER) (5). It has been demonstrated that estrogen is capable of activating both nuclear and membrane bound receptors, where the activated receptors facilitate a multitude of biological effects in many different cell types (5). Studies have demonstrated a bimodal effect of 17β-estradiol (E2) on the hypothalamus, having both positive and negative feedback mechanisms on GnRH neurons (6). Both in vivo and in vitro studies reveal the negative feedback, which inhibits GnRH synthesis and secretion throughout most the female cycle (7–11). Conversely, as E2 levels increase during the late follicular phase, a stimulatory feedback system is provoked, increasing the GnRH pulse frequency and secretion to generate the GnRH surge (12–14).
The GnRH neuronal system acts as a central regulator of reproductive function. Adequate pulsatile GnRH secretion is necessary for the attainment and maintenance of reproduction, where GnRH neurons dictate many of the reproductive activities that cycle throughout reproductive life (15–17). Although knowledge of the GnRH neuronal system has advanced substantially over the past few decades, the stimulatory neuronal systems upstream of GnRH neurons remained rather ambiguous until the discovery of kisspeptin (Kiss) and its receptor, Gpr54 (18, 19). Kiss and Gpr54 have been collectively recognized as indispensable mediators for reproductive development and function, ranging from neonatal sexual differentiation, regulation of GnRH and gonadotropin secretion, the metabolic gating of puberty, and adult fertility (20, 21). Kiss peptides are highly conserved and their expression has been identified in mammalian and nonmammalian vertebrates. Kiss mRNA and protein has been identified centrally in the anterodorsal preoptic area, as well as throughout the hypothalamus, with the 2 major populations located in the arcuate nucleus (ARC) and the anteroventral periventricular (AVPV) region (22–24). Kiss-1 expression in the ARC is inhibited by E2, whereas AVPV Kiss-1 expression is stimulated by E2 (25). In rodents, sheep, and monkeys, ovariectomy and reduction in gonadal steroids generates an increase in ARC Kiss-1 mRNA expression, reversible after E2 treatment (25–29). On the other hand, early postnatal gonadectomy causes a reduction in Kiss-1 expression in the AVPV by 70%–90% at the time of puberty, which persists throughout adulthood (22). Although the regulatory effects of E2 on Kiss-1 expression have been explored in many species, the ERs involved in the differential regulation of Kiss-1 in AVPV and ARC hypothalamic neurons remains to be studied.
E2 regulates gene expression by either binding to nuclear ERs or the membrane-associated G protein-coupled receptor, GPR30. The binding to intracellular ERs (ERα and ERβ) in the nucleus causes a conformational change to the receptors, the release of chaperone proteins to allow them to become active and the dimerization and binding of receptors to specific estrogen-responsive elements (EREs) in the promoter regions of target genes (30, 31). In situations where estrogen-responsive genes do not contain the classical ERE binding sites, ERα and ERβ interact with other DNA-bound transcription factors to regulate gene expression by indirectly binding to the DNA (32). These include the transcription factors specificity protein 1 and activator protein 1 (AP-1) complex (32). AP-1 is a dimeric protein composed of proteins Fos and/or Jun (31). The membrane initiated signaling of E2 accounts for the acute changes in gene expression that cannot be achieved by nuclear E2 signaling. GPR30 or GPER is associated with a Gαs protein that leads to the rapid activation of adenylyl cyclase, production of cAMP and phosphorylation of cAMP response element-binding protein (CREB) (33). E2 can also activate protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phosphatidylinositol-3-kinase (PI3K) via GPR30 and induce the transcription of the immediate early gene c-fos (34). E2 can elicit differential responses through these two pathways depending on the cell type and promoter context.
In the present study, we investigate the differential effects of E2 on gene expression in defined AVPV and ARC hypothalamic Kiss neuronal cell models. Due to the heterogeneous nature and complexity of the hypothalamus, the ability to dissect the molecular events in unique neuronal cell populations is difficult. For this reason, we generated immortalized, clonal adult-derived mouse hypothalamic cell lines. The generation of adult-derived clonal cell lines have been described previously (35), and we have identified 2 specific neuronal models, the mHypoA-50 and mHypoA-55, which exhibit endogenous Kiss expression, among many other potential Kiss models. In the present study, we also report novel adult-derived Kiss cell models from microdissected AVPV and ARC primary cultures of female Kiss-green fluorescent protein (GFP) transgenic mouse hypothalamus (36). These cultures were immortalized and fluorescence-activated cell (FAC) sorted to generate cell models enriched in Kiss-expressing neurons using a technique previously established in our laboratory (37, 38). Specifically, the ARC and AVPV were microdissected from the hypothalamus to generate 2 separate cell models, the mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4. Here, we demonstrate that regulation of the Kiss-1 transcript is differentially responsive to E2 in the ARC vs AVPV neuronal models and also find differential E2-mediated regulation of ERα, ERβ, and Gpr30. We then used selective ER agonists and antagonists in order to determine the receptor subtype responsible for the transcriptional effects in the AVPV vs the ARC. The role of the transcription factor CREB1 was then assessed in the ARC line where GPR30 appeared to play an essential role. We found that the specific ER subtype used is temporally regulated, and unique to the specific population of the Kiss neurons. It is evident that the Kiss system is uniquely modulated by E2 in our selective ARC vs AVPV models of hypothalamic Kiss neurons.
Materials and Methods
Cell culture generation and FAC sorting
The generation of adult-derived clonal cell lines have been described previously (35). Female and male Kiss-GFP transgenic mice generated by Dr Robert Steiner (University of Washington, Seattle, WA) were obtained from The Jackson Laboratory (Kiss-1tm1.1(cre/EGFP)Stei/J; strain (129S6/SvEvTac x C57BL/6NCr)F1) (36, 39). Mice were housed under standard vivarium procedures and were conducted in accordance with the regulations of the Council of Animal Care and approved by the University of Toronto Animal Care Committee. Kiss/GFP transgenic mouse hypothalami were microdissected from 2-month old female or male mice isolating the ARC and AVPV nuclei individually for the female mice or using the entire hypothalamus for the male mice, and placed in Hanks' balanced salt solution. Cells were then immortalized and FAC sorted as previously described (38). Primary hypothalamic cultures were treated with 10-ng/mL recombinant rat ciliary neurotrophic factor for 5–7 days and immortalized using fresh virus-containing medium harvested from confluent culture of ψ2 cells (psitex cells) harboring the intact cDNA sequence for simian virus (SV40) large T-antigen and neomycin resistance gene, as previously described (35, 40). Cells were treated with geneticin (G418) (100 μg/mL) for 3 weeks to select for cells with T-antigen incorporation. Cells were then sorted on a BD FACSAria cell sorter (Becton Dickinson) with a 100-μm nozzle tip and sheath pressure at 20 ψ with a purity greater than 95%. mHypoA-Kiss/GFP-1 and mHypoA-Kiss/GFP-2 (both male) and mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 (both female) immortalized cells were sorted on GFP fluorescence after gating to remove cell aggregates. All fluorescence-activated cell sorting (FACS) was performed in the Faculty of Medicine Flow Cytometry Facility, University of Toronto. These cells represent the entire population of mHypoA-Kiss/GFP neurons from the ARC and AVPV for the female and all Kiss neurons for the male and have not been further subcloned. The mHypoA-50 and mHypoA-55 cell lines were generated as previously described (35, 40), and represent clonal, immortalized Kiss-expressing cell lines originating from hypothalamic primary cultures derived from 2-month-old female mice. Each line has been further characterized as described below.
Cell culture and reagents
mHypoA-50, mHypoA-55, mHypoA-Kiss/GFP-1, mHypoA-Kiss/GFP-2, mHypoA-Kiss/GFP-3, and mHypoA-Kiss/GFP-4 neurons were cultured in monolayer in DMEM 1-mg/mL glucose, supplemented with 5% fetal bovine serum (Sigma-Aldrich) and 1% penicillin/streptomycin (Gibco). Neurons were maintained at 37°C with 5% CO2, a methodology previously described (35, 40). Only the female cell lines were used for further experimentation beyond basic characterization of markers and imaging. Cell lines were imaged using the EVOS cell imaging station (Thermo Fisher Scientific, through Life Technologies, Inc). E2 (Tocris Bioscience) was dissolved in absolute ethanol to a stock concentration of 10mM and stored at −20°C before mRNA studies. During steroid treatment, cells were cultured in phenol red-free DMEM, and treatments were performed in phenol red-free medium (HyClone) supplemented with 5% charcoal-stripped fetal bovine serum (Gemini Bio-products through Cedarlane, Inc) (38). ERα-selective agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT),ER β-selective agonist 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN),ERα antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP dihydrochloride), ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), ER antagonist 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI-182780), and GPR30 antagonist (3aS*,4R*,9bR*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-3H-cyclopenta[c]quinolone (G-15) were obtained from Tocris Bioscience. GPR30 agonist rel-1-[4-(6-bromo-1, 3-benzodioxol-5-yl)-3aR, 4S, 5, 9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G-1) was obtained from Cayman Chemical. All substances were dissolved in dimethyl sulfoxide (Sigma-Aldrich) and stored at a stock concentration of 10μM and were subsequently dissolved in water to a final concentration of 10nM (for the selective ER-agonist treatments) and a final concentration of 1μM (for the ER antagonist treatments), as described in previous publications (38, 41, 42). 1,3,5(10)-estratrien-3,17β-diol 17-hemisuccinate:BSA (E2-BSA) was obtained from Steraloids and dissolved in PBS to a stock concentration of 0.1μM and 1μM. β-Actin antibody was purchased from Sigma-Aldrich, and CREB antibody was purchased from Cell Signaling Technology (via New England Biolabs). Both antibodies were diluted 1:1000 in 5% milk in Tris-buffered saline with 0.1% Tween 20.
One-step RT-PCR
Cells were grown to approximately 80%–85% cell confluence. Total RNA was isolated by the guanidium isothiocyanate phenol chloroform extraction method, followed by quantification using the NanoDrop 2000c spectrophotometer. Subsequently, RNA isolated by the guanidium isothiocynate phenol chloroform extraction method was treated with Turbo deoxyribonuclease (DNase) (Ambion) and amplified. Semiquantitative RT-PCR was performed with the QIAGEN One-Step RT-PCR kit, according to manufacturer's protocol. Briefly, 200 ng of template DNase-treated RNA was combined with 1× 1-step RT-PCR buffer, 0.4mM deoxynucleotide triphosphates, 1-step enzyme mix and 0.6mM both the reverse and forward gene-specific primers. PCR products were separated and visualized on a 2% agarose gel containing 0.5-μg/mL ethidium bromide, and run alongside a 100-bp ladder (Fermentas). Primers were designed using Integrated DNA Technologies PrimerQuest and NCBI Primer-Blast.
Quantitative real-time RT-PCR
mHypoA-50, mHypoA-55, mHypoA-Kiss/GFP-3, and mHypoA-Kiss/GFP-4 neurons were plated in 60-mm culture plates to 80%–85% confluence. The next day, cells were treated with vehicle or E2 and harvested over a 24-hour time course at indicated time points. For the agonist and antagonist studies, neurons were incubated with either dimethyl sulfoxide or indicated treatments, and total RNA was isolated at 4- and 24-hour time points. For E2-BSA experiments, mHypoA-55 neurons were treated with vehicle or E2-BSA, and total RNA was isolated at 4 hours. Total RNA from treated and control cell culture plates were isolated using the guanidium isothiocyanate phenol chloroform extraction method or was isolated with the PureLink RNA kit with on column PureLink DNase (Ambion) (to measure Kiss-1 and ER subtype mRNA expression, because the Kiss-1 transcript appears to be less stable and inconsistent using the standard RNA isolation method). Subsequently, RNA concentration and purity were measured with the NanoDrop 2000c spectrophotometer. Reverse transcription was performed with 2 μg of total RNA that was treated with Turbo DNase (Ambion) before use of the High Capacity cDNA Reverse Transcription kit, according to manufacturer's protocol (Applied Biosystems). A total of 100 ng of cDNA template was amplified using SYBR green PCR master mix for real-time RT-PCR containing, 0.3× SYBR green dye, 1× PCR buffer, 3mM MgCl2, 2mM deoxynucleotide triphosphates, 1× ROX, 6-Carboxyl-X-Rhodamine reference dye, 0.3μM gene-specific primers, and 0.2 U of Platinum Taq DNA polymerase (Invitrogen). Samples were run in triplicate on the Applied Biosystems Prism 7000 real-time PCR machine. In brief, all genes were run on the real-time PCR machine according to the next protocol conditions: 50°C for 2 minutes, 95°C for 10 minutes; 40 cycles for 15 seconds at 95°C, 60°C for 1 minute. When measuring Kiss-1 mRNA, SensiFAST cDNA Synthesis kit purchased from Bioline was used according to manufacturer's protocol to make 1 μg of cDNA from total RNA followed by the use of SensiFAST SYBR Hi-ROX kit purchased from Bioline containing 1× SensiFAST SYBR Hi-ROX Mix and 0.3μM gene-specific primers to amplify 12.5 ng of cDNA template. Parameters for the real-time PCR machine were as follows; 95°C for 2 minutes; 40 cycles for 5 seconds at 95°C, 60°C for 30 seconds, when amplifying Kiss-1 mRNA. Kiss-1: forward TGCTGCTTCTCCTCTGT and reverse ACCGCGATTCCTTTTCC; PCR product length, 132 bp; ERα: forward GAGTGCCAGGCTTTGGGGACTT and reverse CCATGGAGCGCCAGACGAGA; PCR product length, 102 bp; ERβ: forward ATCTGTCCAGCCACGAATCAGTGT and reverse TCTCCTGGATCCACACTTGACCAT; PCR product length, 114 bp; Gpr30: forward GTGGCCAAGCCTCAACACTCAC and reverse GGTGGACAGGGTGTCTGATGTCTG; PCR product length, 103 bp; and Creb1: forward CCACCACCCTCAAGAAGTAATC and reverse GGTAACTGTCCCTAAGGCAATC; PCR product length, 166 bp.
In silico analysis
The web tool Alibaba 2.1 was used to predict transcription factor binding sites for ERs, AP-1, and CREB 2000 base pairs upstream of the 5′ flanking regions of Kiss-1 gene. Alibaba 2.1 is a program that predicts transcription factor binding sites using the TRANSFAC 4.0 transcription factor database.
Knockdown of CREB1
Knockdown of CREB1 was achieved using the TriFECTa kit (Integrated DNA Technologies) containing a negative control Dicer substrate interfering RNA (DsiRNA) sense 5′-CGU UAA UCG CGU AUA AUA CGC GUA T-3′ and antisense 5′-AUA CGC GUA UUA UAC GCG AUU AAC GAC-3′, and 3 DsiRNA duplexes targeted to mouse Creb1: Creb1a sense 5′-GCA AGA GAA UGU CGU AGA AAG AAG A-3′ and antisense 5′-UCU UCU UUC UAC GAC AUU CUC UUG CUG-3′; Creb1b sense 5′-GCC AAA GAA CUA AUA AGA UCC CUA T-3′ and antisense 5′-AUA GGG AUC UUA UUA GUU CUU UGG CAA-3′, and Creb1c sense 5′-GCA AGU ACC AUA UUA GCA ACC AUC A-3′ and antisense 5′-UGA UGG UUG CUA AUA UGG UAC UUG CUU-3′. mHypoA-55 neurons were cultured on 10-cm plates and transfected with 25nM DsiRNA complexed with 25 μL/plate of DharmaFECT Transfection Reagent 3 (Thermo Scientific) for 24 hours. After transfection, the MirVana PARIS kit (Life Technologies) was used to isolate RNA and protein from the same plate, and quantitative RT-PCR and Western blotting were performed, respectively, to determine mRNA and protein expression. The Creb1a DsiRNA duplex was most effective in reducing CREB1 mRNA and protein levels in our cell model and was thus used for all experiments.
Statistical analysis
Data are presented as the mean ± SEM for the number of independent experiments indicated. Data analyses were performed using GraphPad Prism or SigmaStat (Systat Software, Inc). Statistical significance analysis was established using by one- or two-way ANOVA, as indicated, and Bonferroni's post hoc test or Student's t test.
Results
mHypoA-50, mHypoA-55, mHypoA-Kiss/GFP-3, and mHypoA-Kiss/GFP-4 neurons express Kiss-1, as well as ERs, and other relevant peptides and receptors
We have previously reported the generation of an array of adult-derived, clonal hypothalamic neuronal cell lines, which exhibit unique expression profiles of neuropeptides and receptors (35). In order to characterize cell models representative of Kiss-expressing neurons, screening for relevant markers was performed to develop a more thorough gene expression profile of the cell lines. Forty-six adult male and female clonal cell lines were screened for expression of Kiss-1 mRNA, and the Kiss-1 mRNA was detected in a number of the cell lines (data not shown). After initial screening for the Kiss-1 gene, several cell lines with the strongest expression of Kiss-1 were chosen for further investigation. We demonstrate that mHypoA-50 and mHypoA-55 neurons have high expression of Kiss-1 mRNA (Table 1), as well as expression of ERα, ERβ, and Gpr30. Screening for specific Kiss ARC vs AVPV hypothalamic markers was performed in order to further define the cell lines. It has previously been reported that Kiss neurons from the ARC nucleus coexpress substance P (SP), neurokinin B (NKB), and dynorphin (Dyn), whereas Kiss neurons from the AVPV often coexpress tyrosine hydroxylase (TH). We found that SP, NKB, and Dyn were expressed in the mHypoA-55 cell line (Table 1), suggesting that it represents an ARC Kiss neuron. The mHypoA-50 neurons expressed TH, but not SP, NKB, and Dyn, supporting their identity as a putative AVPV Kiss neuron (Table 1).
Table 1.
Characterization of Gene Expression Profiles for the mHypoA-50 and mHypoA-55 Clonal Cell Lines
| Gene | mHypoA-50 | mHypoA-55 |
|---|---|---|
| (AVPV) | (ARC) | |
| Kiss-1 | + | + |
| ERα | + | + |
| ERβ | + | + |
| GPR30 | + | + |
| NKB (Tac 2) | − | + |
| Dyn | − | + |
| SP | − | + |
| TH | + | − |
| Met-enkephalin | + | − |
Quantitative RT-PCR results of relevant reproductive neuropeptides and receptors in the cell lines indicated. +, presence of the gene; −, absence or weak expression of a gene.
To generate adult-derived immortalized murine Kiss cell models specifically from the ARC and AVPV, hypothalami were microdissected from female Kiss-GFP transgenic mice (36, 39). The primary cell cultures were treated with ciliary neurotrophic factor to stimulate cell proliferation to allow the infection with a retrovirus containing the SV40 T-antigen, as previously described (37, 38). The male mHypoA-Kiss/GFP-1 and mHypoA-Kiss/GFP-2 cell models were generated by the same method, however, from the entire hypothalami, because there are minimal Kiss neurons in the AVPV in males. The mHypoA-Kiss-GFP-3 and mHypoA-Kiss/GFP-4 cell lines exhibit neuronal marker expression and Kiss-1 mRNA expression (Table 2), as determined by quantitative real time reverse transcriptase PCR (qRT-PCR). Both models also express the 3 ERs, ERα, ERβ, and Gpr30 (Table 2). To confirm that the mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 cell models were representative of the ARC and AVPV, respectively, screening for Kiss-specific hypothalamic markers was performed. The mHypoA-Kiss/GFP-3 cell model expressed the ARC markers SP and NKB, whereas the mHypoA-Kiss/GFP-4 cell model expressed the AVPV marker TH (Table 2). The expression of Kiss-1 and the ERs indicate that these 4 cell lines are appropriate models for the study of E2 regulatory mechanisms in divergent Kiss ARC vs AVPV neuronal populations. The cell lines exhibit neuronal morphology and unique characteristics in culture (Supplemental Figure 1).
Table 2.
Characterization of Gene Expression Profiles for the mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 Cell Lines
| Gene | mHypoA-Kiss/GFP-4 | mHypoA-Kiss/GFP-3 |
|---|---|---|
| (AVPV) | (ARC) | |
| Kiss-1 | + | + |
| ERα | + | + |
| ERβ | + | + |
| GPR30 | + | + |
| NKB (Tac 2) | − | + |
| Dyn | − | + |
| SP | − | + |
| TH | + | − |
| Met-enkephalin | + | − |
Quantitative RT-PCR results of relevant reproductive neuropeptides and receptors in the cell lines indicated. +, presence of the gene; −, absence or weak expression of a gene.
Comparing male and female Kiss cell models, there is sexual dimorphism between Kiss-1, ERα, ERβ, and GPR30 mRNA expression
Because there is sexual dimorphism between the male and female hypothalami (43), we compared the levels of Kiss-1 and ER receptor expression between the sexes of the Kiss cell models. Because Kiss appears to play a crucial role in the female preovulatory surge, higher levels of Kiss-1 mRNA expression have been reported in females (43–45). The levels of Kiss-1 mRNA expression, were higher in the female Kiss cell models compared with the male lines (Table 3 and Supplemental Figure 2). There also appears to be higher levels of ERβ in the female cell models compared with the male lines. Interestingly, overall the male lines express higher levels of both ERα and GPR30 than the females (Table 4 and Supplemental Figure 2).
Table 3.
Ct Levels for Kiss-1 mRNA and ER Receptors Between Female and Male Kiss Lines
| Histone | Kiss-1 | ERα | ERβ | GPR30 | |
|---|---|---|---|---|---|
| FEMALE | |||||
| mHypoA-50 | 18.3 | 25.6 | 26.3 | 29.4 | 31.5 |
| mHypoA-55 | 18.3 | 25.8 | 26.4 | 29.5 | 30.9 |
| mHypoA-Kiss/GFP-3 | 18.4 | 25.8 | 28.4 | 30.6 | 31.1 |
| mHypoA-Kiss/GFP-4 | 18.2 | 27.4 | 25.7 | 28.5 | 28.1 |
| MALE | |||||
| mHypoA-2/22 | 18.2 | 32.1 | 24.9 | 31.6 | 27.8 |
| mHypoA-2/24 | 18.3 | 30.4 | 25.3 | 30.9 | 28.1 |
| mHypoA-Kiss/GFP-1 | 18 | 27.5 | 25.4 | 30.5 | 29.3 |
| mHypoA-Kiss/GFP-2 | 18.1 | 27 | 25.2 | 28.2 | 29 |
Values represent Ct levels for the corresponding gene. Ct, cycle threshold.
Table 4.
Summary of the E2-Mediated Regulation of ERα, ERβ, and Gpr30 mRNA Expression in the AVPV vs ARC Kiss Cell Models
| ERα | ERβ | Gpr30 | |
|---|---|---|---|
| AVPV | |||
| mHypoA-50 |  |
x | x |
| mHypoA- Kiss/GFP-4 |  |
x | x |
| ARC | |||
| mHypoA-55 | 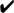 |
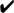 |
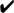 |
| mHypoA- Kiss/GFP-3 | 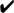 |
 |
 |
 , regulation of the gene by E2; x, absence of regulation of the gene by E2.
, regulation of the gene by E2; x, absence of regulation of the gene by E2.
E2 positively regulates Kiss-1 gene expression in mHypoA-50 and Kiss/GFP-4 neuronal models and negatively regulates Kiss-1 mRNA levels in mHypoA-55 and Kiss/GFP-3 neuronal models
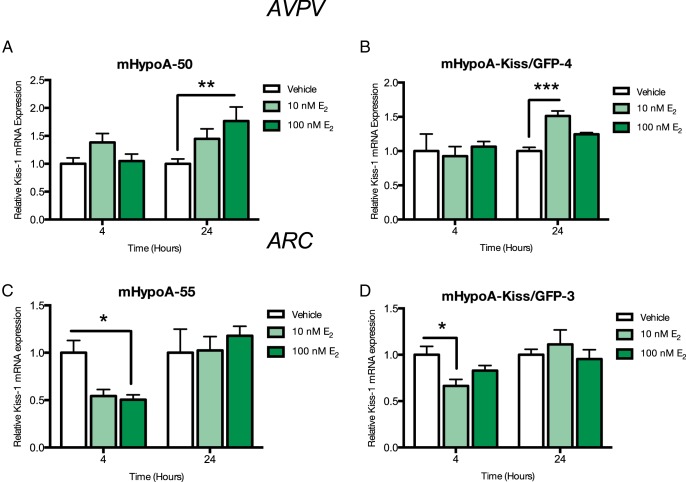
We investigated whether E2-mediated regulation of Kiss expression in the cell models was consistent with in vivo findings. Kiss cell models were treated over a 24-hour time course (1, 2, 4, 8, and 24 h) to determine potential regulation of Kiss-1 expression (Supplemental Figure 3). We detected regulation of Kiss-1 mRNA levels at 4 or 24 hours with E2 and then performed a dose study at these times in the 4 cell lines (10nM and 100nM), followed by quantitative RT-PCR to determine levels of Kiss-1 gene expression. In the AVPV Kiss cell model mHypoA-50, 100nM E2 exposure induced Kiss-1 gene expression at 24 hours (vehicle 1.00 ± 0.87 vs 10nM E2, 1.383 ± 0.161 vs 100nM 1.763 ± 0.253; P < .01) (Figure 1A). There was a significant increase in in Kiss-1 mRNA expression with 10nM E2 at 24 hours in the mHypoA-Kiss/GFP-4 cell model (vehicle, 1.00 ± 0.054 vs 10nM E2, 1.513 ± 0.073; P < .001) (Figure 1B).
Figure 1. Effect of E2 treatment on Kiss-1 mRNA expression in the (A) mHypoA-50, (B) mHypoA-Kiss/GFP-4, (C) mHypoA-55, and (D) mHypoA-Kiss/GFP-3 cell lines.
Cells were treated with vehicle or 10nM or 100nM E2. RNA was harvested at 4 and 24 hours, and changes in Kiss-1 mRNA levels were measured using quantitative RT-PCR. mRNA levels were normalized to control, histone 3a. Data are expressed as mean ± SEM (n = 4–12 independent experiments); *, P < .05; **, P < .01; and ***, P < .001. Statistical significance was determined by two-way ANOVA with Bonferroni's post hoc test.
In the ARC Kiss cell model mHypoA-55, there was a repression of Kiss-1 gene expression at 4 hours with 100nM E2 (vehicle, 1.00 ± 0.132 vs 10nM E2, 0.749 ± 0.072 vs 100nM E2, 0.656 ± 0.044; P < .05) (Figure 1C). Similarly, treatment of the mHypoA-Kiss/GFP-3 neurons with E2 resulted in a down-regulation of Kiss-1 gene expression with 10nM at 4 hours (vehicle, 1.00 ± 0.90 vs 10nM E2, 0.665 ± 0.069 vs 100nM E2 0.829 ± 0.056; P < .05) (Figure 1D). These results illustrate that Kiss gene expression in the 2 divergent neuronal cell models are differentially regulated by E2, and thus we decided to use these models to study the involvement of ERs in the cellular regulation of Kiss-1 mRNA expression in the AVPV compared with the ARC.
E2 down-regulates specific ER subtype mRNA levels in the ARC vs AVPV Kiss neuronal models
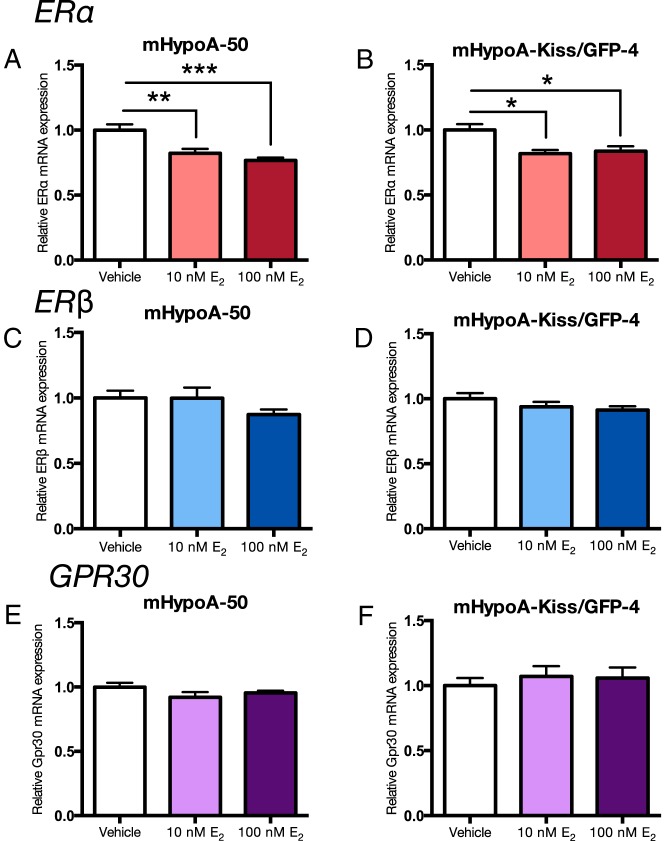
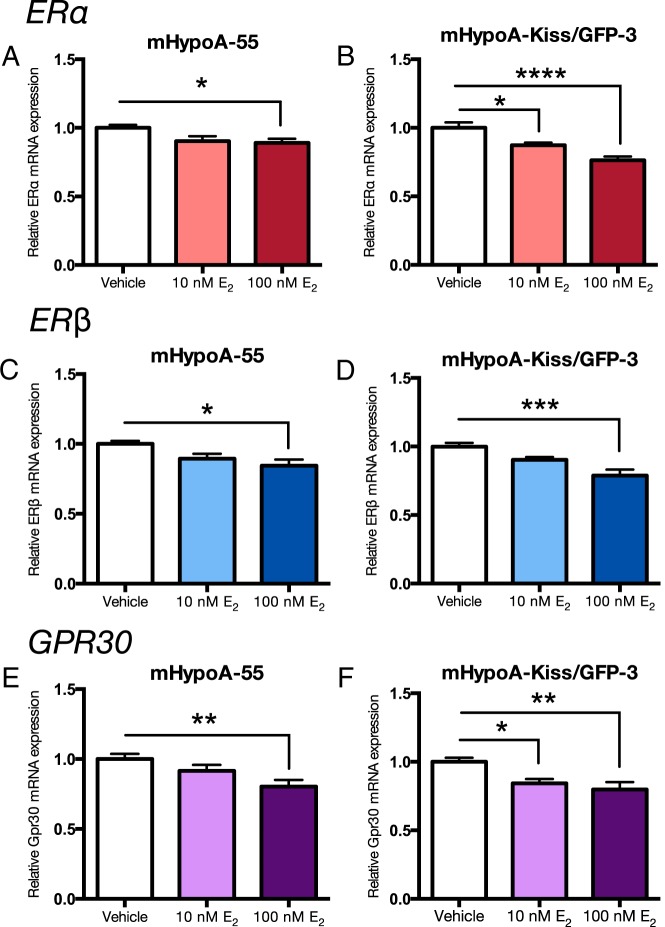
There is some evidence that E2 can regulate the expression of its own receptors. We studied the regulation of the 3 ER subtypes, ERα, ERβ, and Gpr30, in the 4 cell models. Expression of specific ER subtypes was differentially regulated by E2 in the 2 ARC vs AVPV neuronal models. Neurons were treated with 10nM and 100nM E2 or vehicle control over a 24-hour time course, followed by measurement of ERα, ERβ, and Gpr30 mRNA levels. Initial studies demonstrated regulation of the ERs at 4h in the ARC cell models and 24 hours in the AVPV cell models. In the AVPV, mHypoA-50 and mHypoA-Kiss/GFP-4 neurons, E2 repressed the expression of ERα at 24 hours (mHypoA-50; vehicle 1.00 ± 0.043 vs 10nM E2 0.822 ± 0.034, P < .01 vs 100nM E2 0.767 ± 0.021; P < .001 [Figure 2A], mHypoA-Kiss/GFP-4; vehicle 1.00 ± 0.044 vs 10nM E2 0.818 ± 0.028, P < .05 and 100nM E2 0.837 ± 0.036; P < .05 [Figure 2B]), but not ERβ or Gpr30 mRNA levels (Figure 2, C–F). In the ARC mHypoA-55 cell line, ERα, ERβ, and Gpr30 mRNA levels are down-regulated at 4 hours after 100nM E2 treatment; mHypoA-55: ERα (vehicle 1.00 ± 0.022 vs 10nM E2 0.903 ± 0.036 vs 100nM E2 0.890 ± 0.031; P < .05) (Figure 3A), ERβ (vehicle 1.00 ± 0.022 vs 10nM E2 0.894 ± 0.035 vs 100nM E2 0.844 ± 0.043; P < .05) (Figure 3C), and Gpr30 (vehicle 1.00 ± 0.037 vs 10nM E2 0.915 ± 0.042 vs 100nM E2 0.802 ± 0.048; P < .01) (Figure 3E). Similarly, in the mHypoA-Kiss/GFP-3 line, ERα (vehicle 1.00 ± 0.039 vs 10nM E2 0.872 ± 0.018; P < .05 vs 100nM E2 0.763 ± 0.027; P < .0001) (Figure 3B), ERβ (vehicle 1.00 ± 0.027 vs 10nM E2 0.903 ± 0.019 vs 100nM E2 0.788 ± 0.042; P < .001) (Figure 3D), and Gpr30 (vehicle 1.00 ± 0.029 vs 10nM E2 0.843 ± 0.031; P < .05 vs 100nM E2 0.798 ± 0.053; P < .01) (Figure 3F) mRNA expression are repressed at 4 hours. These results demonstrate differential regulation of ERα, ERβ, and Gpr30, by E2 in the AVPV vs ARC hypothalamic cell models (Table 4), which may relate to the divergent regulation of Kiss-1 gene expression in the 2 regions of the hypothalamus.
Figure 2. Effect of E2 treatment on ERα, ERβ, and Gpr30 mRNA expression in the mHypoA-50 and mHypoA-Kiss/GFP-4 AVPV cell lines.
Cells were treated with vehicle or 10nM or 100nM E2. RNA was harvested at 24 hours, and changes in mRNA levels were measured using quantitative RT-PCR. mRNA levels were normalized to control, histone 3a. Data are expressed as mean ± SEM (n = 4–10 independent experiments); *, P < .05; **, P < .01; and ***, P < .001. Statistical significance was determined by one-way ANOVA with Bonferroni's post hoc test.
Figure 3. Effect of E2 treatment on ERα, ERβ, and Gpr30 mRNA expression in the mHypoA-55 and mHypoA-Kiss/GFP-3 ARC cell lines.
Cells were treated with vehicle or 10nM or 100nM E2. RNA was harvested at 4 hours, and changes in mRNA levels were measured using quantitative RT-PCR. mRNA levels were normalized to control, histone 3a. Data are expressed as mean ± SEM (n = 4–10 independent experiments); *, P < .05; **, P < .01; ***, P < .001; and ****, P < .0001. Statistical significance was determined by one-way ANOVA with Bonferroni's post hoc test.
In the mHypoA-50 AVPV cell line, induction of Kiss-1 mRNA expression requires ERα
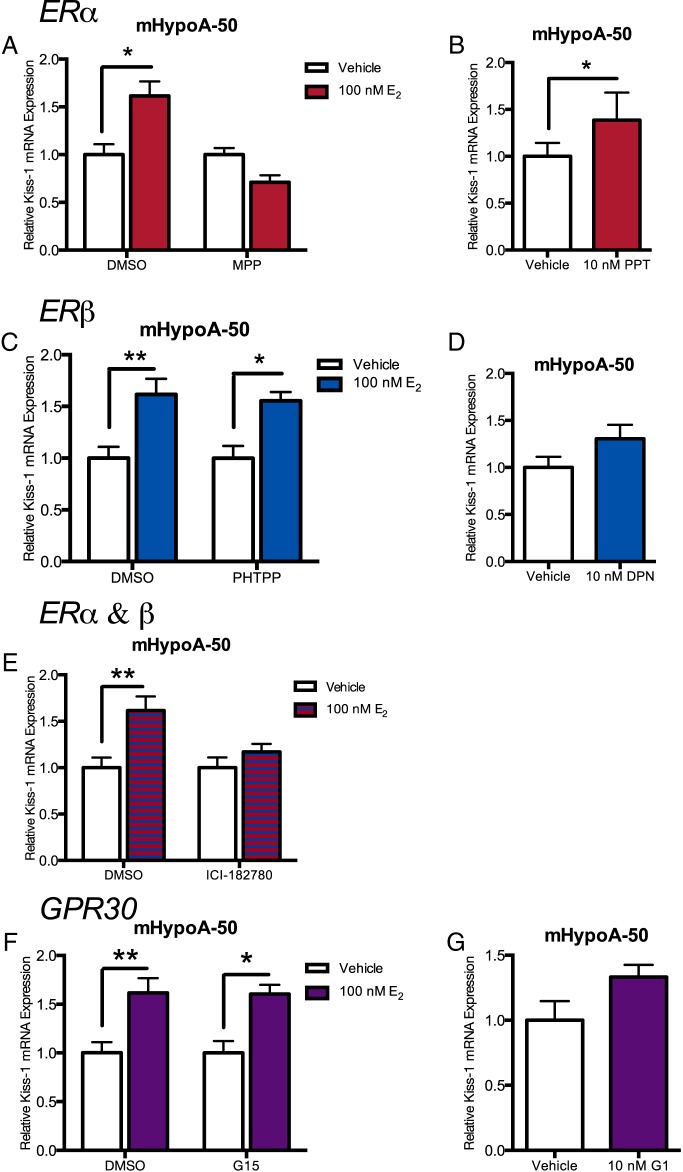
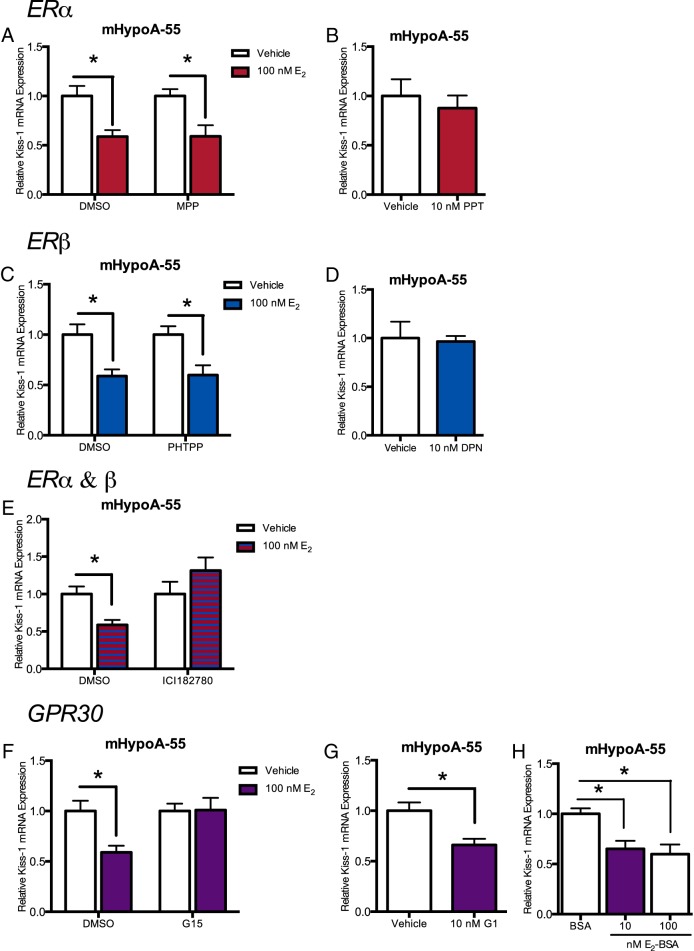
To determine which of the ERs (ERα, ERβ, or GPR30) is required to mediate the regulation of Kiss-1 mRNA expression by E2 observed in the cell models, several selective ER agonists and antagonists were employed. Briefly, neurons were pretreated for 1 hour with vehicle or 1μM 1 of 4 antagonists: ERα antagonist (MPP dihydrochloride), ERβ antagonist (PHTPP), ER antagonist (ICI-182780), and GPR30 antagonist (G-15). Neurons were then treated with either vehicle or E2 (100nM). Additionally, cell lines were also treated with an ERα-selective agonist (PPT), ERβ-selective agonist (DPN), or GPR30 agonist (G-1) to distinguish which receptor was responsible for the changes in gene transcription. RNA was harvested at 24 hours after E2 treatment, the specific time corresponding to the significant changes in gene expression previously observed. The mHypoA-Kiss/GFP-4 cell model did not respond well to the inhibitors and therefore were not used for this experiment.
In the mHypoA-50 cell line, we report that at 24 hours after estrogen treatment, ERα is required for the up-regulation of Kiss-1 mRNA expression. The ERα antagonist, MPP and ER antagonist ICI-182780 both abolished the increase in Kiss-1 mRNA expression (MPP: vehicle 1.00 ± 0.069 vs E2 0.711 ± 0.073, P > .05; ICI-182780: vehicle 1.00 ± 0.111 vs E2 1.171 ± 0.084, P > .05) (Figure 4, A and E); however, ERβ and GPR30 antagonists, PHTPP and G-15, respectively, did not (PHTPP: vehicle 1.00 ± 0.117 vs E2 1.554 ± 0.086, P < .05; G-15: vehicle 1.00 ± 0.120 vs E2 1.604 ± 0.096, P < .01) (Figure 4, C and F). The ERα agonist, PPT, also induced Kiss-1 mRNA expression (vehicle 1.00 ± 0.142 vs PPT 1.385 ± 0.294; P < .05) (Figure 4B); however, the ERβ agonist, DPN, and the GPR30 agonist G-1 did not (DPN: vehicle 1.00 ± 0.113 vs DPN 1.306 ± 0.147, P > .05; G-1: vehicle 1.00 ± 1.46 vs G-1 1.332 ± 0.0943, P > .05) (Figure 4, D and G). This demonstrates that for the induction of Kiss-1 mRNA expression in the mHypoA-50 cell line, ERα is necessary, whereas ERβ and GPR30 are not required.
Figure 4. Effects of ER agonists and antagonists on estradiol-mediated induction of Kiss-1 mRNA expression in mHypoA-50 (AVPV) cell line at 24 hours.
Cells were pretreated for 1 hour in the presence of 1 specific ER antagonist (1μM) or vehicle control followed by treatment with estradiol (100nM), or specific ER agonist (10nM), or vehicle control over a 24-hour time course. Specifically the (A) ERα antagonist MPP, (C) ERβ antagonist PHTPP, (E) ER antagonist ICI-182780, and (F) GPR30 antagonist G-15 were used as pretreatments. The (B) ERα agonist PPT, (D) ERβ agonist DPN, and (G) GPR30 agonist G-1 were used as specific ER agonists. Kiss-1 mRNA expression was determined by quantitative RT-PCR, and levels were normalized to the internal control, histone 3a. Data are expressed as mean ± SEM (n = 4–5 independent experiments); *, P < .05; **, P < .01 Statistical analysis was calculated by two-way ANOVA followed by Bonferroni post hoc test or Student's t test.
In the mHypoA-55 ARC cell line, repression of Kiss-1 mRNA expression requires GPR30 and potentially either ERα or ERβ
In the mHypoA-55 cell line, results demonstrate that at 4 hours, GPR30 and either ERα or ERβ are necessary for the repression of Kiss-1 mRNA expression. The GPR30 antagonist, G-15 and ER antagonist, ICI-182780 both abolished the repression of Kiss-1 mRNA by E2 (G-15: vehicle 1.00 ± 0.073 vs 1.009 ± 0.121, P > .05; ICI-182780: vehicle 1.00 ± 0.164 vs E2 1.315 ± 0.174, P > .05) (Figure 5, E and F), whereas the ERα and ERβ antagonists did not prevent the repression (MPP: vehicle 1.00 ± 0.070 vs 0.590 ± 0.113, P < .05; PHTPP: vehicle 1.00 ± 0.083 vs E2 0.598 ± 0.098, P < .05) (Figure 5, A and C). The GPR30 agonist, G-1 repressed Kiss-1 mRNA expression (vehicle 1.00 ± 0.081 vs G-1 0.660 ± 0.062 P < .05) (Figure 5G). However, neither the ERα agonist, PPT (vehicle 1.00 ± 0.169 vs PPT 0.876 ± 0.128; P > .05) (Figure 5B) nor the ERβ agonist, DPN (vehicle 1.00 ± 0.169 vs DPN 0.965 ± 0.057; P > .05) (Figure 5D) repressed Kiss-1 mRNA expression. These findings suggest that estradiol decreases Kiss-1 mRNA expression, via activation of GPR30 and an ER. Furthermore, although the nuclear ER is required to induce gene expression over a longer period of time, the membrane-bound receptor mediates the earlier and more rapid effects on gene transcription.
Figure 5. Effects of ER agonists and antagonists on estradiol-mediated induction of Kiss-1 mRNA expression in mHypoA-55 (ARC) cell line at 4 hours.
Cells were pretreated for 1 hour in the presence of 1 specific ER antagonist (1μM) or vehicle control followed by treatment with estradiol (100nM), or specific ER agonist (10nM), E2-BSA (10nM and 100nM), or vehicle control over a 24-hour time course. Specifically the (A) ERα antagonist MPP, (C) ERβ antagonist PHTPP, (E) ER antagonist ICI-182780, and (F) GPR30 antagonist G-15 were used as pretreatments. The (B) ERα agonist PPT, (D) ERβ agonist DPN, and (G) GPR30 agonist G-1 were used as specific ER agonists. H, 10nM and 100nM E2-BSA were used as a membrane impermeable form of estradiol. Kiss-1 mRNA expression was determined by quantitative RT-PCR, and levels were normalized to the internal control, histone 3a. Data are expressed as mean ± SEM (n = 3–4 independent experiments); *, P < .05 Statistical analysis was calculated by two-way ANOVA followed by Bonferroni post hoc test or Student's t test.
In the mHypoA-55 ARC cell line, E2-BSA is able to elicit the repression of Kiss-1 mRNA expression, suggesting a membrane initiated mechanism
Because the repression of the Kiss-1 mRNA expression in the mHypoA-55 ARC cell model required the membrane bound receptor, GPR30, a membrane impermeable E2 (E2-BSA) was used to further support these observations. Cells were treated for 4 hours with 10nM and 100nM E2-BSA, and then RNA was harvested and Kiss-1 mRNA expression measured. Both 10nM (BSA 1.00 ± 0.0537 vs 10nM E2-BSA 0.649 ± 0.0796; P < .05) and 100nM (BSA 1.00 ± 0.0537 vs 100nM E2-BSA 0.598 ± 0.0965; P < .05) E2-BSA repressed Kiss-1 mRNA after 4 hours in the mHypoA-55 cell model (Figure 5H). This indicates that estrogen is signaling via receptors from the cell membrane.
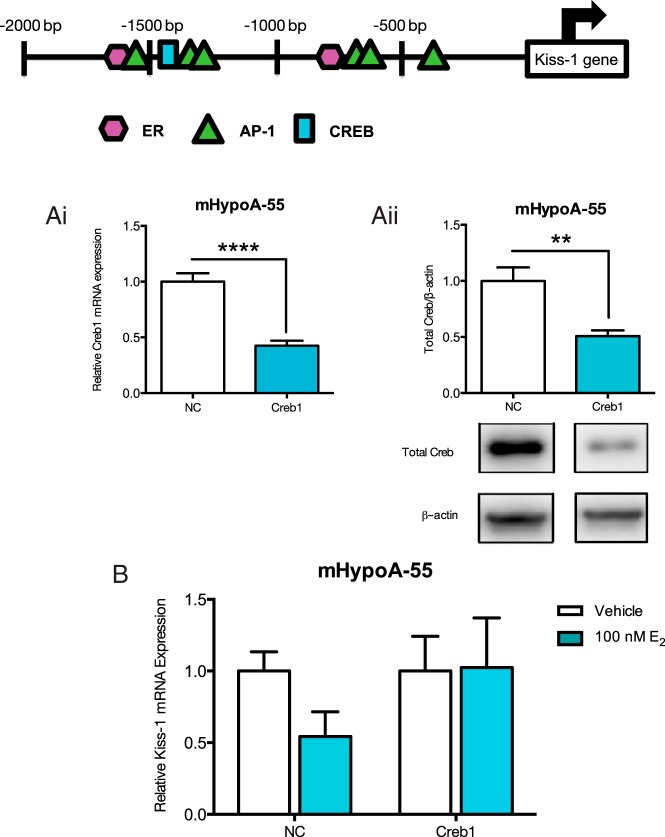
The Kiss-1 promoter contains ER, AP-1, and CRE binding sites
Next, we assessed the 5′ flanking region of the Kiss-1 gene in Mus musculus using a transcription factor binding site analysis program to determine which transcription factors may be binding to the promoter. E2 can change gene expression by ER binding to ERE sites, ER tethering to AP-1 and binding indirectly to AP-1 sites or phosphorylated CREB signaling through CREB binding sites. Therefore, we looked for ERE, AP-1, and CREB binding sites in the 5′ promoter region of the Kiss-1 gene and determined that there were binding sites for all (Figure 6). Therefore, Kiss-1 may be regulated by the induction of either ERα, ERβ, or Gpr30.
Figure 6. Knockdown of CREB1 expression impairs E2-mediated regulation of Kiss-1 mRNA expression in the mHyopoA-55 ARC cell model.
In silico promoter analysis of the 5′ flanking region of Kiss-1 M. musculus gene. The sequence for the 5′ flanking region of the Kiss M. musculus gene was obtained through Ensembl (−2000 bp), then subsequently analyzed by the gene regulation program Alibaba 2.1 (http://www.gene-regulation.com/pub/programs/alibaba2/index.html) for the presence of EREs, AP-1, and CREB binding sites (location of the sites are indicated in the figure). A, The mHypoA-55 cell model was transfected with either 25nM nontargeting negative control siRNA or CREB1 targeting siRNA for 24 hours, then CREB1 mRNA expression was measured by quantitative real-time PCR and protein expression by Western blotting. Ai, CREB1 mRNA expression was reduced by 58% with the CREB1 siRNA. Aii, CREB1 protein expression was reduced by 49% with CREB1 siRNA. B, The mHypoA-55 cell model was treated with 100nM E2 for 4 hours after a 24-hour transfection with either negative control nontargeting siRNA or CREB1 targeting siRNA. RNA was isolated, Kiss-1 mRNA expression was measured using quantitative real-time PCR, and levels were normalized to the housekeeping gene histone 3a. Results are expressed as mean ± SEM (n = 3–4 independent experiments).
In the mHypoA-55 ARC cell line, knockdown of CREB1 attenuates the negative regulatory effects of E2 on Kiss-1 mRNA
Because GPR30 appeared to be involved in the down-regulation of Kiss-1 mRNA expression, we wanted to determine which second messengers may be involved. Using the transcription factor binding program, a CRE binding site was found in the 5′ promoter region of the Kiss-1 gene, suggesting that CREB1 may be involved in the regulation of Kiss-1 mRNA expression by GPR30. We examined whether down-regulation of the CREB1 protein would affect the regulation of Kiss-1 mRNA expression by E2 in the ARC cells. The small interfering RNA (siRNA) method was used to reduce the endogenous levels of CREB1 in the mHypoA-55 cell model. Cells were transfected with negative control or CREB1 siRNA for 24 hours, and then quantitative RT-PCR and Western blotting were used to measure CREB1 mRNA and protein levels. CREB1 siRNA reduced CREB1 mRNA expression by 58% (vehicle siRNA, 1.00 ± 0.076 vs CREB1 siRNA, 0.425 ± 0.045; P < .0001) (Figure 6Ai) and CREB1 protein expression by 49% (vehicle siRNA, 1.00 ± 0.120 vs CREB1 siRNA, 0.508 ± 0.051; P < .01) (Figure 6Aii) both after 24 hours. Reduction of the endogenous levels of CREB1 allowed us to assess the role of this transcription factor in mediating the effects of E2 on Kiss-1 mRNA expression. The knockdown of CREB1 prevented the E2-mediated down-regulation of Kiss-1 in the mHypoA-55 cell model (CREB1 siRNA, vehicle 1.00 ± 0.242 vs 100nM E2 1.025 ± 0.346; P > .05) (Figure 6B). From this siRNA analysis, it appears that CREB1 is necessary for the E2-mediated down-regulation of Kiss-1 mRNA expression in the mHypoA-55 ARC cell model.
Discussion
Several years after its initial discovery as a tumor suppressor gene, Kiss has gained recognition as an important regulator of the HPG axis and reproduction through its potent stimulation of GnRH secretion (27, 43, 46, 47). In vivo animal studies have been essential in developing our current knowledge of Kiss-1 regulation and Kiss secretion by central and peripheral factors during prepubertal, pubertal and adult stages of development. Despite knowledge of Kiss and its significant role in reproduction, studies that focus on regulation of Kiss gene expression are limited. This can be attributed to the lack of a suitable model for in vitro studies. For this reason, we sought to establish adult-derived Kiss-expressing cell models from the mouse hypothalamus.
In the present study, 4 Kiss-expressing cell models were established: 2 from an array of immortalized, clonal, hypothalamic cell lines from postpubertal female adult mouse hypothalamus; and 2 FAC-sorted, immortalized cell lines generated from microdissected ARC and AVPV nuclei from a female Kiss-GFP mouse. The clonal cell lines were screened for expression of Kiss-1 mRNA, and a number of male and female cell lines were found to express Kiss. We detected strong Kiss expression in the mHypoA-50 and the mHypoA-55 cell lines, which were also found to express mRNA of 3 subtypes of ERs. Of interest, screening of our hypothalamic cell lines derived from mouse embryonic hypothalami had nondetectable levels of Kiss-1 mRNA (data not shown), and this expression analysis correlates with in vivo reports where prepubertal Kiss expression is low in comparison with pubertal and adult levels (24, 43). The mHypoA-Kiss/GFP-3 and mHypoA-Kiss/GFP-4 cell models microdissected from the ARC and AVPV regions of the hypothalamus, respectively, also showed expression of Kiss-1 mRNA and ERs. The mHypoA-Kiss/GFP-1 and mHypoA-Kiss/GFP-2 male FAC-sorted, immortalized cell lines and 4 clonal male adult cell models were also screened for Kiss-1 mRNA and the 3 ERs. Overall the female lines had higher expression of Kiss-1 mRNA then the male counterparts which corroborates with previous studies that have demonstrated higher levels of Kiss-1 in females vs males, because it is thought that higher levels are important for the preovulatory surge (43, 44, 45).
Kiss mRNA and protein have been identified in a number of regions from the rodent hypothalamus, including the ARC, AVPV, and periventricular nucleus, with lower expression levels in found in the anterodorsal preoptic area (22, 23, 25, 48). Two major populations of Kiss neurons, in the AVPV and the ARC have been thoroughly explored in vivo (23, 25, 48), and were found to differ in their coexpression profiles with other neuropeptides and neurotransmitters. Most Kiss neurons in the ARC also express Dyn and NKB, thus leading to the acronym KNDy, kisspeptin/neurokinin B/dynorphin, neurons (49). Additionally, ARC Kiss neurons have been found to coexpress SP (50), a peptide belonging to the tachykinin family. On the other hand, Kiss neurons in the AVPV of mice have demonstrated coexpression with met-enkephalin (51, 52), an endogenous opioid peptide, and TH (52, 53), the rate-limiting enzyme necessary for dopamine synthesis. Screening revealed expression of Dyn, NKB, and SP in the mHypoA-55 and mHypoA-Kiss/GFP-3 cell lines, which suggests that they represent populations of ARC Kiss neurons. The lack of NKB, Dyn, and SP expression, and expression of met-enkephalin and TH in the mHypoA-50 and mHypoA-Kiss/GFP-4 cell lines, indicates that they are not representative of populations of ARC Kiss neurons, but rather, that they may represent AVPV Kiss neurons. Further screening revealed expression of ERα and ERβ in all the cell lines, which coincides with findings that reveal nearly all ARC and AVPV (98%–99%) hypothalamic Kiss neurons express ERα mRNA, and 25%–30% express ERβ mRNA (48). The cell models also express the membrane bound ER Gpr30. A comparison of the relative levels of the ERs within the cell models revealed a greater amount of ERα compared with ERβ in all the cell models. Interestingly, the male lines expressed higher levels of ERα and Gpr30, whereas the female lines expressed higher levels of ERβ. Higher levels of ERβ in females have been previously reported in rats (54). This demonstrates that the cell models exhibit the sexual dimorphism of Kiss and ER expression that has been previously reported in vivo.
After detection of ER mRNA in the Kiss neuronal models, we were compelled to investigate the potential responsiveness of our cell lines to E2. Initially, we detected an increase in c-Fos mRNA expression with E2 treatment, establishing that the cell lines are sensitive to E2 stimulation (data not shown). We then found that E2 distinctly regulated Kiss-1 mRNA expression in the AVPV models compared with the ARC models, as has been previously reported (22, 25–29, 48). E2 significantly increased Kiss-1 mRNA in the mHypoA-50 and mHypoA-Kiss/GFP-4 AVPV Kiss cell models while significantly repressing Kiss-1 mRNA in the mHypoA-55 and mHypoA-Kiss/GFP-3 Kiss ARC model. However, the temporal regulation differs substantially between the ARC vs AVPV models, with down-regulation occurring at the 4-hour time point, and the up-regulation occurring at 24 hours. This indicates that there are likely different mechanisms involved in the regulation of Kiss-1 gene expression.
To explore the differential E2-mediated regulation of Kiss, we decided to study the regulation of ERs by E2 between the ARC and AVPV. Estrogens negatively modulate ER levels by the process of ligand-induced down-regulation in certain tissue and cell types. This has been demonstrated in MCF-7 breast cancer cells, rat uterine cells, and the rat hypothalamus (55–60). This negative feedback mechanism is important for limiting the duration of estrogen stimulation on the cell (57). A reduction in the amount of ERs might indicate that the cell was stimulated by E2 and subsequently down-regulated. We measured the levels of ERα, ERβ, and Gpr30 in all cell lines we saw the regulation of Kiss-1 mRNA expression and we found divergent regulation of the ERs by E2 itself depending on whether they were in the AVPV or ARC. In the AVPV cell models, we saw a reduction in ERα after 24-hour E2 treatment but not ERβ or Gpr30, suggesting that ERα may be the prime mediator in Kiss-1 mRNA up-regulation. Meanwhile in the ARC cell models, there was a significant down-regulation of all 3 ERs (ERα, ERβ, and Gpr30) after 4-hour E2 treatment, indicating that all 3 receptors may be involved in the estrogen-mediated down-regulation of Kiss-1 mRNA expression in ARC neurons.
c-fos and CREB could be involved in the regulation of the Kiss-1 gene. The involvement of c-fos and particularly CREB suggests there could be activation of GPR30 to elicit changes in Kiss-1 mRNA expression. However, this is not direct evidence for the role of these ERs and transcription factors in the regulation of Kiss-1 mRNA expression. Therefore, we used selective ER agonists and antagonists to determine the receptors involved in the differential regulation of Kiss-1 mRNA in the AVPV compared with the ARC. Our studies with selective ER agonists and antagonists reveal that E2 mediates its effects on Kiss-1 gene expression through different mechanisms, which are temporally dependent, and contingent on the neuronal population. It has been previously established that the positive regulation of the GnRH/LH surge and ovulation by E2 is dependent on classical ERα ERE-dependent signaling mechanisms but not on ERβ (43, 61, 62). A recent study by Dubois et al (63) demonstrated that E2 could not induce Kiss-1 mRNA expression in Kiss cell-specific ERα knockout mice, and this abolished the GnRH/LH surge showing the necessity of ERα for the induction of Kiss-1 mRNA by E2. Our results are congruent with these findings in the mHypoA-50 cell line, where E2-mediated up-regulation of Kiss-1 mRNA gene expression required ERα, because the ERα agonist PPT was able to increase Kiss-1 mRNA. In contrast, the ERα antagonist PHTPP and the ER antagonist ICI-182780 blocked the E2-mediated increase in Kiss-1 mRNA. Together, it appears that in AVPV Kiss neurons, over a 24-hour period, E2 diffuses into the cell and binds to ERα nuclear receptors, ERα receptors dimerize and bind to ERE sites in the Kiss-1 promoter, and Kiss-1 mRNA expression is up-regulated (Figure 7A).
Figure 7. Diagram of the signaling mechanisms involved in the regulation of Kiss-1 mRNA expression in (A) AVPV and (B) ARC Kiss cell models.
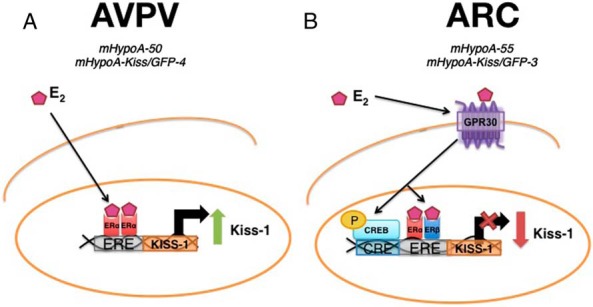
A, In the AVPV, ERα is required for the E2-mediated induction of Kiss-1 mRNA expression. B, In the ARC, GPR30 and either ERα or β are required for the E2-mediated repression of Kiss-1 mRNA expression. CREB1, a transcription factor, is also necessary for the down-regulation of Kiss-1 mRNA expression, possibly via binding to the CRE site in the 5′ promoter region of the Kiss-1 gene.
The mechanisms involved in the reproductive negative feedback by E2 have not been as thoroughly explored as those involved in the positive feedback. A study by Wintermantel et al using ERα and ERβ neuron-specific knockout mice demonstrated that the negative feedback by estrogen was still intact and sufficient to enable basal gonadotropin secretion (61, 63). Other studies suggest that ERα may be the predominant ER isoform mediating the effects for estrogen negative feedback (64–66). Interestingly, several studies suggest that the acute negative regulation by E2 involves ERE-independent signaling which could suggest the involvement of GPR30 (62, 67). Previous reports have also demonstrated the importance of GPR30 in the negative feedback of estrogen on GnRH and LH pulsatility in primate and bovine neurons, respectively (68, 69). As the negative feedback of estrogen on GnRH neurons and the anterior pituitary is facilitated by Kiss neurons, it is conceivable that GPR30 is also involved in the negative regulation of Kiss-1 mRNA expression by E2. In the mHypoA-55 cell line, at 4 hours, GPR30 and either one of the nuclear receptors was required for the repression of Kiss-1 mRNA expression. G-1, the GPR30 agonist, was also able to elicit the repression of Kiss-1 mRNA expression in the ARC. Membrane impermeable, E2-BSA also elicited the same repression of Kiss-1 mRNA expression as E2, suggesting that E2 acts at the membrane of Kiss cells in the ARC as it would with GPR30. Interestingly, only a few studies to date have focused on the role of the membrane-bound receptor GPR30 in the regulation by E2 despite the high expression of Gpr30 in the hypothalamus (70). The negative regulation of Kiss-1 mRNA expression by E2 occurred more rapidly than the positive regulatory effects. This could be explained by actions of E2 at the cell membrane, including E2 activation of GPR30. GPR30 is a G-protein-coupled receptor rapidly activated by estradiol signaling and has been implicated in the transcriptional regulation of genes through a broad range of signaling mechanisms. Activation of GPR30 by E2 was first demonstrated to cause the rapid phosphorylation of MAPKs via transactivation of epidermal growth factor receptors in breast cancer cells (71, 72). It was subsequently demonstrated that E2 stimulation of GPR30 up-regulated nerve growth factor in macrophages through the production of cAMP and up-regulation of c-fos (73). It was also determined that via GPR30, E2 could increase cyclin D2 and B-cell lymphoma-2 expression in keratinocytes by a cAMP-protein kinase A (PKA)-mediated mechanism, resulting in the phosphorylation of CREB (74, 75). GPR30 can activate several pathways including the cAMP-PKA-CREB cascade and c-fos. We determined, there are sites for both CREB and c-fos in the Kiss-1 promoter that could be targets after GPR30 activation by E2. The discovery of the GPR30 specific agonist G-1 demonstrated that Gpr30 transcriptional activity can also involve crosstalk with ERα (76). Our results also demonstrated that one of the ERs was necessary for Kiss-1 mRNA regulation in ARC neurons. Knocking down CREB1 using siRNA prevented the down-regulation of Kiss-1 mRNA expression in the ARC cell model. This suggests that CREB1 is a necessary transcription factor for the down-regulation of Kiss-1 mRNA expression. As GPR30 can cause the induction of c-fos and phosphorylation of CREB1, we could hypothesize that phosphorylated CREB1 and a ER dimer bind to a CRE site in the Kiss-1 promoter to cause the rapid down-regulation of Kiss-1. c-fos may also be involved by binding to an AP-1 site nearby. Future studies will be required to elaborate on the specific transcription factors (ie, c-fos) and pathways involved in the GPR30 membrane initiated down-regulation of the Kiss-1 gene in ARC hypothalamic Kiss neurons; however, it appears that CREB1 and an ER are both essential components in this pathway. From these studies we can conclude that in ARC Kiss neurons, after 4-hour E2 exposure, GPR30 is activated and causes the downstream down-regulation of Kiss-1 mRNA expression. The downstream effectors include ERα and/or ERβ and CREB1 (Figure 7B).
The present study suggests that the mHypoA-50 and mHypoA-Kiss/GFP-4 compared with the mHypoA-55 and mHypoA-Kiss/GFP-3 cell lines are representative of 2 different functional populations of Kiss-expressing hypothalamic neurons. Furthermore, our work demonstrates that estrogen down-regulates expression of ERα, ERβ, and Gpr30, all of which play a pivotal role regulating fertility. We report involvement specific ER subtypes, both temporally and nucleus specific, to mediate changes in gene expression induced by E2. We established that ERα was necessary for Kiss-1 induction in the AVPV population represented by the mHypoA-50 cells and mHypoA-Kiss/GFP-4 cell lines, whereas GPR30 with either ERα or ERβ and CREB1 was necessary for regulation of Kiss-1 in the ARC mHypoA-55 and mHypoA-Kiss/GFP-3 Kiss neuronal models. We have demonstrated the effect of E2 on hypothalamic Kiss-expressing neuronal models, and overall the results suggest that the physiologic effects of E2 on the function of the reproductive axis is mediated in part by the modulation of Kiss-1, Gpr30, ERα, and ERβ expression. Because it is known that Kiss-1 expression is modulated by E2, our findings here may reveal an additional mechanism by which estrogen regulates the HPG axis via alterations in the sensitivity of Kiss neurons to stimuli, subsequently altering their output; a mechanism necessary to maintain GnRH pulsatility and reproductive function.
Acknowledgments
This work was supported by funding from the Canadian Institutes for Health Research and Canada Foundation for Innovation and Canada Research Chairs Program (D.D.B.). Z.F. was supported by a Santalo studentship.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by funding from the Canadian Institutes for Health Research and Canada Foundation for Innovation and Canada Research Chairs Program (D.D.B.). Z.F. was supported by a Santalo studentship.
Footnotes
- AP-1
- activator protein 1
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular
- CREB
- cAMP response element-binding protein
- DPN
- ERβ-selective agonist 2,3-bis(4-hydroxyphenyl)-propionitrile
- DNase
- deoxyribonuclease
- DsiRNA
- Dicer substrate interfering RNA
- Dyn
- dynorphin
- E2
- 17β-estradiol
- E2-BSA
- 1,3,5(10)-estratrien-3,17β-diol 17-hemisuccinate:BSA
- ER
- estrogen receptor
- ERE
- estrogen-responsive element
- FAC
- fluorescence-activated cell
- G-1
- rel-1-[4-(6-bromo-1, 3-benzodioxol-5-yl)-3aR, 4S, 5, 9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone
- G-15
- (3aS*,4R*,9bR*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-3H-cyclopenta[c]quinolone
- green fluorescent protein
- (GFP)
- Gpr
- G-protein-coupled receptor
- HPG
- hypothalamic pituitary gonadal
- ICI-182780
- ER antagonist 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
- Kiss
- kisspeptin
- MPP dihydrochloride
- ERα antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- NKB
- neurokinin B
- PHTPP
- ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- PPT
- 4,4′,4′'-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- ROX
- 6-Carboxyl-X-Rhodamine reference dye
- siRNA
- small interfering RNA
- SP
- substance P
- TH
- tyrosine hydroxylase.
References
- 1. Tena-Sempere M, Barreiro ML, González LC, Pinilla L, Aguilar E. Differential neonatal imprinting and regulation by estrogen of estrogen receptor subtypes α and β and of the truncated estrogen receptor product (TERP-1) mRNA expression in the male rat pituitary. Neuroendocrinology. 2001;74:347–358. [DOI] [PubMed] [Google Scholar]
- 2. Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. [DOI] [PubMed] [Google Scholar]
- 3. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. [DOI] [PubMed] [Google Scholar]
- 4. Labrie F, El-Alfy M, Berger L, et al. The combination of a novel selective estrogen receptor modulator with an estrogen protects the mammary gland and uterus in a rodent model: the future of postmenopausal women's health? Endocrinology. 2003;144:4700–4706. [DOI] [PubMed] [Google Scholar]
- 5. Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. [DOI] [PubMed] [Google Scholar]
- 6. Radovick S, Ticknor CM, Nakayama Y, et al. Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J Clin Invest. 1991;88:1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng Y, Wolfe A, Novaira HJ, Radovick S. Estrogen regulation of gene expression in GnRH neurons. Mol Cell Endocrinol. 2009;303:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roy D, Angelini NL, Belsham DD. Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1–7 GnRH neurons. Endocrinology. 1999;140:5045–5053. [DOI] [PubMed] [Google Scholar]
- 9. Zoeller RT, Seeburg PH, Young WS 3rd. In situ hybridization histochemistry for messenger ribonucleic acid (mRNA) encoding gonadotropin-releasing hormone (GnRH): effect of estrogen on cellular levels of GnRH mRNA in female rat brain. Endocrinology. 1988;122:2570–2577. [DOI] [PubMed] [Google Scholar]
- 10. Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol. 1989;123:375–382. [DOI] [PubMed] [Google Scholar]
- 11. Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136:3604–3610. [DOI] [PubMed] [Google Scholar]
- 12. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. [DOI] [PubMed] [Google Scholar]
- 13. Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. [DOI] [PubMed] [Google Scholar]
- 14. Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. [DOI] [PubMed] [Google Scholar]
- 15. Krey LC, Lieberburg I, Roy E, McEwen BS. Oestradiol plus receptor complexes in the brain and anterior pituitary gland: quantitation and neuroendocrine significance. J Steroid Biochem. 1979;11:279–284. [DOI] [PubMed] [Google Scholar]
- 16. Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. [DOI] [PubMed] [Google Scholar]
- 17. Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131:403–414. [DOI] [PubMed] [Google Scholar]
- 18. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. [DOI] [PubMed] [Google Scholar]
- 19. Lee DK, Nguyen T, O'Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. [DOI] [PubMed] [Google Scholar]
- 20. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. [DOI] [PubMed] [Google Scholar]
- 21. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. [DOI] [PubMed] [Google Scholar]
- 22. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. [DOI] [PubMed] [Google Scholar]
- 23. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. [DOI] [PubMed] [Google Scholar]
- 24. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 26. Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navarro VM, Castellano JM, Fernández-Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. [DOI] [PubMed] [Google Scholar]
- 28. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. [DOI] [PubMed] [Google Scholar]
- 29. Smith JT, Coolen LM, Kriegsfeld LJ, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. [DOI] [PubMed] [Google Scholar]
- 31. Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly MJ, Rønnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. [DOI] [PubMed] [Google Scholar]
- 34. Maggiolini M, Vivacqua A, Fasanella G, et al. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. [DOI] [PubMed] [Google Scholar]
- 35. Belsham DD, Fick LJ, Dalvi PS, et al. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009;23:4256–4265. [DOI] [PubMed] [Google Scholar]
- 36. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McFadden SA, Menchella JA, Chalmers JA, Centeno ML, Belsham DD. Glucose responsiveness in a novel adult-derived GnRH cell line, mHypoA-GnRH/GFP: involvement of AMP-activated protein kinase. Mol Cell Endocrinol. 2013;377:65–74. [DOI] [PubMed] [Google Scholar]
- 38. Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int J Obes (Lond). 2011;35:198–207. [DOI] [PubMed] [Google Scholar]
- 39. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belsham DD, Cai F, Cui H, et al. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. [DOI] [PubMed] [Google Scholar]
- 41. Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. [DOI] [PubMed] [Google Scholar]
- 42. Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen facilitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J Neurosci. 2008;28:6473–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. [DOI] [PubMed] [Google Scholar]
- 45. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. [DOI] [PubMed] [Google Scholar]
- 46. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. [DOI] [PubMed] [Google Scholar]
- 47. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. [DOI] [PubMed] [Google Scholar]
- 48. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 49. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 50. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol. 2010;20:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23:293–301. [DOI] [PubMed] [Google Scholar]
- 54. Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor β in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA. 2002;99:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saceda M, Lippman ME, Lindsey RK, Puente M, Martin MB. Role of an estrogen receptor-dependent mechanism in the regulation of estrogen receptor mRNA in MCF-7 cells. Mol Endocrinol. 1989;3:1782–1787. [DOI] [PubMed] [Google Scholar]
- 56. Martin MB, Saceda M, Lindsey RK. Regulation of estrogen receptor expression in breast cancer. Adv Exp Med Biol. 1993;330:143–153. [DOI] [PubMed] [Google Scholar]
- 57. Borrás M, Hardy L, Lempereur F, et al. Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol. 1994;48:325–336. [DOI] [PubMed] [Google Scholar]
- 58. Saceda M, Lippman ME, Chambon P, et al. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol. 1988;2:1157–1162. [DOI] [PubMed] [Google Scholar]
- 59. Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med. 1991;196:293–300. [DOI] [PubMed] [Google Scholar]
- 60. Lauber AH, Romano GJ, Mobbs CV, Pfaff DW. Estradiol regulation of estrogen receptor messenger ribonucleic acid in rat mediobasal hypothalamus: an in situ hybridization study. J Neuroendocrinol. 1990;2:605–611. [DOI] [PubMed] [Google Scholar]
- 61. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Glidewell-Kenney C, Hurley LA, Pfaff L, et al. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. [DOI] [PubMed] [Google Scholar]
- 65. Cheong RY, Porteous R, Chambon P, Abrahám I, Herbison AE. Effects of neuron-specific estrogen receptor ER α and ER β deletion on the acute estrogen negative feedback mechanism in adult female mice. Endocrinology. 2014;155:1418–1427. [DOI] [PubMed] [Google Scholar]
- 66. Hillisch A, Peters O, Kosemund D, et al. Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol Endocrinol. 2004;18:1599–1609. [DOI] [PubMed] [Google Scholar]
- 67. Arreguin-Arevalo JA, Nett TM. A nongenomic action of estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone in ovariectomized ewes. Biol Reprod. 2006;74:202–208. [DOI] [PubMed] [Google Scholar]
- 68. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rudolf FO, Kadokawa H. Expression of estradiol receptor, GPR30, in bovine anterior pituitary and effects of GPR30 agonist on GnRH-induced LH secretion. Anim Reprod Sci. 2013;139:9–17. [DOI] [PubMed] [Google Scholar]
- 70. Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. [DOI] [PubMed] [Google Scholar]
- 72. Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 73. Kanda N, Watanabe S. 17β-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J Invest Dermatol. 2003;121:771–780. [DOI] [PubMed] [Google Scholar]
- 74. Kanda N, Watanabe S. 17β-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121:1500–1509. [DOI] [PubMed] [Google Scholar]
- 75. Kanda N, Watanabe S. 17β-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123:319–328. [DOI] [PubMed] [Google Scholar]
- 76. Albanito L, Madeo A, Lappano R, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. [DOI] [PubMed] [Google Scholar]