Abstract
Activation of the transcription factor nuclear factor kappa B (NFkB) contributes to β-cell death in type 1 diabetes (T1D). Genome-wide association studies have identified the gene TNF-induced protein 3 (TNFAIP3), encoding for the zinc finger protein A20, as a susceptibility locus for T1D. A20 restricts NF-κB signaling and has strong antiapoptotic activities in β-cells. Although the role of A20 on NF-κB inhibition is well characterized, its other antiapoptotic functions are largely unknown. By studying INS-1E cells and rat dispersed islet cells knocked down or overexpressing A20 and islets isolated from the β-cell-specific A20 knockout mice, we presently demonstrate that A20 has broader effects in β-cells that are not restricted to inhibition of NF-κB. These involves, suppression of the proapoptotic mitogen-activated protein kinase c-Jun N-terminal kinase (JNK), activation of survival signaling via v-akt murine thymoma viral oncogene homolog (Akt) and consequently inhibition of the intrinsic apoptotic pathway. Finally, in a cohort of T1D children, we observed that the risk allele of the rs2327832 single nucleotide polymorphism of TNFAIP3 predicted lower C-peptide and higher hemoglobin A1c (HbA1c) levels 12 months after disease onset, indicating reduced residual β-cell function and impaired glycemic control. In conclusion, our results indicate a critical role for A20 in the regulation of β-cell survival and unveil novel mechanisms by which A20 controls β-cell fate. Moreover, we identify the single nucleotide polymorphism rs2327832 of TNFAIP3 as a possible prognostic marker for diabetes outcome in children with T1D.
Type 1 diabetes (T1D) is a chronic disease that has been globally rising during the past decades (1). Dysregulated blood glucose levels in T1D is a consequence of an inappropriate immune response leading to destruction of the insulin-producing pancreatic β-cells. Genetic predisposition together with environmental factors trigger this autoimmune reaction (2). Production of inflammatory cytokines such as IL-1β, TNF, interferon-γ (IFN-γ), and IL-17 by infiltrating immune cells contribute to β-cell apoptosis at early stages of the disease (2). At later stage, effector T cells induce β-cell demise by different mechanisms (2).
The transcription factor nuclear factor-kappa B (NF-kB) promotes proinflammatory and proapoptotic responses in β-cells upon cytokine exposure (3–5). In vivo, attenuation of inflammatory signaling through β-cell-specific inhibition of NF-κB confers nearly complete protection against multiple low-dose streptozotocin (MLDSTZ)-induced T1D in mice (6). On the other hand, mice with constitutively active NF-κB signaling in β-cells spontaneously develop full-blown immune-mediated diabetes (7). The proapoptotic effects of NF-κB in β-cells are, at least partly due to the induction of FAS receptor (FasR), also known as apoptosis antigen 1 (APO-1 or APT), cluster of differentiation 95 (CD95) FasR (also known as cluster of differentiation 95), production of nitric oxide (NO) via inducible nitric oxide synthase iNOS induction (2) and consequent activation of the endoplasmic reticulum stress response (8). The mechanisms by which endoplasmic reticulum stress contributes to β-cell death are not completely clarified but involve activation of the mitogen-activated protein kinase c-Jun N-terminal kinase (JNK) and the intrinsic pathway of apoptosis (9–11). Although it has been extensively shown that NF-κB has mostly a deleterious role in β-cells, prevention of NF-κB activation in β-cells from nonobese diabetic mice leads to acceleration of the disease (12). This is probably due to reduced expression of NF-κB dependent antiapoptotic proteins, such as X-linked inhibitor of apoptosis protein (XIAP), cellular FLICE-like inhibitory protein (c-FLIP), and A20 (12, 13).
A20, encoded by the gene TNF-induced protein 3 (TNFAIP3), is a cytoplasmic ubiquitin-editing protein that acts as a negative-feedback regulator of NF-κB activation (14). Interestingly, single nucleotide polymorphisms (SNPs) in the TNFAIP3 region were shown to be associated with T1D (15–17). In β-cells, A20 expression is transiently induced by exposure to IL-1β and TNF after NF-κB activation (13). Expression of A20 is also induced in islets after syngeneic and allogeneic islet transplantation (13) and its overexpression protects islets in the early posttransplantation period (18). Moreover, direct transfer of the A20 gene into the pancreas protects mice from MLDSTZ induced diabetes (19). The antiapoptotic effects of A20 in β-cells are often related to its inhibition of NF-κB activation and NO production (20). However, A20 is also protective in cells where NF-κB has mostly an antiapoptotic role (14), indicating that this protein has antiapoptotic functions independent of its inhibitory effects on NF-κB.
In the present study, we further characterized the antiapoptotic role of the A20 protein in pancreatic β-cells. We here showed that besides controlling NF-κB signaling, A20 plays an important role as a negative regulator of cytokine-mediated JNK activation and a positive regulator of v-akt murine thymoma viral oncogene homolog (Akt)-dependent survival pathway in β-cells. Furthermore, we demonstrate that the presence of a SNP of TNFAIP3 (rs2327832) in children with new onset T1D, predicts reduced β-cell function and impaired glycaemic control 12 months after diagnosis. Overall, our studies clarify the molecular mechanisms regulating the antiapoptotic activities of A20 in pancreatic β-cells and unveil the SNP present in the TNFAIP3 locus as a predictor of T1D progression.
Materials and Methods
Materials
Glycogen synthase kinase 3 (GSK3) α/β inhibitor (SB216763; Sigma-Aldrich) and JNK inhibitor (SP600125; Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) (1:1000) and used at 5μM and 25μM, respectively. Recombinant human IL-1β (R&D Systems) was used at 10 U/mL in INS-1E cells and 50 U/mL in rat and mouse islet cells: recombinant rat IFN-γ (R&D Systems) was used at 100 U/mL in INS-1E cells and 1000 U/mL in rat dispersed islet cells, and mouse IFN-γ (PeproTech) was used for 1000 U/mL in mouse islet cells; 1000 U/mL in rat and mouse islet cells recombinant mouse TNF (R&D Systems) was used at 1000 U/mL in INS-1E cells and in rat and mouse islet cells.
Generation of a β-cell-specific A20 knockout mice, islet isolation, islet/cell culture, and NO measurements
Conditional A20 Tnfaip3 knockout mice, in which exons 4 and 5 of Tnfaip3 gene are flanked by 2 LoxP sites, were generated as previously described (21). A20 floxed mice were crossed with RIP-Cre transgenic mice (22) to generate a β-cell-specific A20 knockout mouse (A20β-KO) and backcrossed into the C57BL/6 genetic background for at least ten generations. Littermate mice were used as controls. A20β-KO mice were born showing normal Mendelian segregation and aged normally without any evidence of metabolic defects or pathological signs in the pancreas (data not shown). Male Wistar rats where purchased from Charles River Laboratory) and pancreatic islets were isolated and dispersed as described (23). Rat dispersed islet cells were precultured in complete β-cell medium supplemented with 5% fetal bovine serum (FBS) 4–5 days for recovery. Upon treatment, cells were cultured in β-cell medium without FBS (8). Mouse islets were isolated by collagenase digestion followed by centrifugation over a Histopaque gradient (Sigma-Aldrich NV/SA) (24). Isolated mouse islets were cultured overnight to 5 days with RPMI 1640 supplemented with 10% FBS for recovery. Upon treatment (with cytokines and inhibitors), mouse islets were cultured in β-cell medium supplemented with 1% FBS. Rats and mice were housed and handled according to the Belgian Regulations for Animal Care and with permission from the local Ethical Committee. The rat insulinoma cell line INS-1E (Dr Claes Wollheim, University of Geneva, Geneva, Switzerland) was cultured as described (25). Culture media were collected and nitrite was determined using the Griess method (nitrite is a stable product of NO oxidation) (26).
RNA interference and recombinant adenovirus infection
Small interfering RNAs (siRNAs) (30nM) used are listed in Supplemental Table 1, and the transfections were performed as described (10, 27). Control adenoviruses encoding luciferase and β-galactosidase were purchased from SIRION Biotech. Adenoviruses encoding mouse A20 were prepared as previously described (28). INS-1E cells and rat dispersed islet cells were infected as previously described (10).
Assessment of cell viability
The percentage of viable, apoptotic, and necrotic cells was determined using the DNA-binding dyes propidium iodide (5 μg/mL; Sigma-Aldrich) and Hoechst 33342 (5 μg/mL; Sigma-Aldrich). For INS-1E cells, rat dispersed islet cells, a minimum of 500 cells, were counted in each experimental condition. For mouse islets, the percentage of total dead cells was analyzed on a total of 10 islets/condition. All measurements were performed by 2 independent observers, one of them unaware of sample identity (8).
mRNA extraction and quantitative RT-PCR
Poly(A)+mRNA was isolated and reverse-transcribed as described (8). The real-time PCR amplification reaction was performed using SYBR Green and compared with a standard curve (10). Expression values were corrected for the housekeeping gene glyceraldehyde-3-phosphare dehydrogenase (GAPDH) (3, 8). The cytokines do not modify GAPDH expression in insulin-producing cells (3, 8). All used primers are listed in Supplemental Table 1.
Western blot analysis
INS1-E cells and rat dispersed islet cells were washed with cold PBS and lysed in Laemmli buffer. Mouse islets were washed with cold PBS and lysed with the next lysis buffer:10mM HEPES (pH 8.0), 1.5mM MgCl2, 10mM KCl, 1mM dithiothreitol (DTT), 0.5mM phenylmethylsulfonyl fluoride (PMSF), 0.1% tergitol-type NP-40 (NP-40), 3% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail (Roche). Immunoblot analysis was performed as described (10). The used antibodies are listed in the Supplemental Table 2. Images were acquired by Chemidoc (Bio-Rad) and analyzed using ImageJ software (29). Protein expression was corrected by the values of the loading control proteins tubulin or GAPDH.
NF-κB reporter assay
Cells were cotransfected with the internal control pRL-CMV encoding Renilla luciferase (Promega) and the pNF-κB-Luciferase construct (BD Biosciences). Sample preparation, measurements of luciferase activities were performed as previously described (10).
Study populations from the Hvidoere Study Group on childhood diabetes
The study population is described in Ref. 30. The cohort included 126 girls and 131 boys, 84% white Caucasian, and age at clinical diagnosis 9.1 ± 3.7 years. The study was performed according to the criteria of the Helsinki II Declaration and was approved by the local ethic committee (permit number KA 04010gm) in each Center. Residual β-cell function (C-peptide) was estimated using a liquid-meal Boost test carried out at 1, 6, and 12 months (±1 wk) after diagnosis and analyzed as previously described (30). Hemoglobin A1c (HbA1c) was analyzed centrally by ion-exchange HPLC at onset and 1, 3, 6, 9, and 12 months after diagnosis. A combined expression of insulin dose-adjusted HbA1c (IDAA1c) used to define the partial remission period in children and adolescents with T1D has been defined (31). Genotyping of rs2327832 was done using the KASPar system (KBioscience) and typing of the human leukocyte antigen (HLA)-class II DRB1 locus was performed as described (30).
Statistical analysis
For the in vitro experiments data are presented as means ± SEM. Statistical analysis was performed by Student's t test or one-way ANOVA followed by Bonferroni post hoc analysis. P ≤ .05 was considered statistically significant. For genetic association analysis, the SNP rs2327832 was tested for association with C-peptide (ln-transformed), HbA1c and IDAA1c at 12 months after diagnosis in linear regression models assuming additive allelic effects for the risk allele, except for C-peptide when the alleles were coded according to a dominant model (AA+AG vs GG). The regression models were adjusted for the covariates sex, age group (0–5, 5–10, and >10 y at diagnosis) using a spline model and HLA risk group. The model with C-peptide was further adjusted for baseline C-peptide at 1 month. P ≤ .05 was considered statistically significant. All statistical analyses were performed in SAS version 9.2.
Results
A20 controls pancreatic β-cell apoptosis
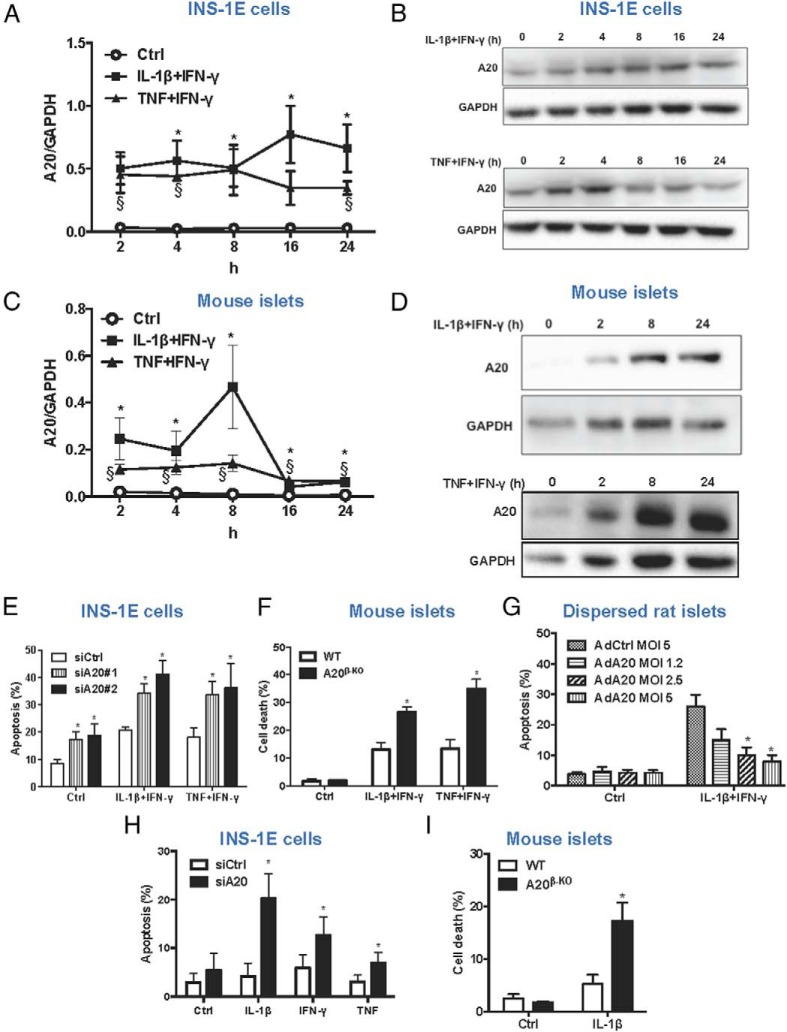
Expression of A20 mRNA and protein were evaluated in INS-1E cells and mouse islets exposed to IL-1β+IFN-γ or TNF+IFN-γ. An early up-regulation of A20 mRNA could be observed in INS-1E cells treated with both cytokine cocktails and expression was maintained up to 24 hours (Figure 1A). Expression of A20 protein followed a similar pattern after exposure to IL-1β+IFN-γ (Figure 1B, upper panel). TNF+IFN-γ induces a peak in A20 protein expression at 4 hours, after which it gradually decreased (Figure 1B, lower panel). Similar results were obtained in primary mouse islets, where cytokine treatment also promotes a potent induction of A20 mRNA and protein expression (Figure 1, C and D).
Figure 1. A20 controls β-cell survival in response to proinflammatory stimuli.
A and B, A20 mRNA (A) and protein expression (B) after treatment of INS-1E cells with IL-1β+IFN-γ or TNF+IFN-γ for different time points, as indicated. C and D, A20 mRNA (C) and protein expression (D) after treatment of mouse islets with IL-1β+IFN-γ or TNF+IFN-γ for different time points, as indicated. A and C, Data are means ± SEM of 3–5 independent experiments. *, P < .05 IL-1β+IFN-γ vs control; §, P < .05 TNF+IFN-γ vs control. B and D, Representative Western blottings of 4–5 independent experiments are shown. E, Quantification of percentage of apoptosis in INS-1E cells transfected with siRNA control (siCtrl), siA20#1, or siA20#2 and treated with IL-1β+IFN-γ or TNF+IFN-γ for 16 hours. *, P < .05 vs siCtrl condition. F, Quantification of percentage of cell death in islets isolated form WT or A20β-KO mice and exposed to IL-1β+IFN-γ or TNF+IFN-γ for 24 hours. *, P < .05 vs WT. G, Quantification of percentage of apoptosis in dispersed rat islets cells infected with the adenoviruses encoding luciferase (AdCtrl) or A20 (AdA20) at different MOIs and exposed to IL-1β+IFN-γ for 24 hours. *, P < .05 vs AdCtrl treated with cytokines. H, Quantification of percentage of apoptosis in INS-1E cells transfected with siCtrl or siA20#2 and treated with IL-1β, TNF, or IFN-γ for 16 hours. *, P < .05 vs siCtrl. I, Quantification of percentage of cell death in islets isolated form WT or A20β-KO mice and exposed to IL-1β for 24 hours. *, P < .05 vs WT. E–I, Data are means ± SEM of 3–6 independent experiments.
Knockdown of A20 increased both basal and cytokine-induced apoptosis in INS-1E cells (Figure 1E and Supplemental Figure 1A). Islets from A20β-KO mice were also more susceptible to cytokine-induced cell death compared with wild-type islets (Figure 1F and Supplemental Figure 1, B and C). No differences in the expression of iNOS mRNA or NO production were observed in INS-1E cells transfected with control or A20 siRNAs (Supplemental Figure 1, D and E). Although the levels of iNOS mRNA were increased in islets from A20β-KO mice as compared with WT, NO levels were similar (Supplemental Figure 1, F and G). To verify the effect of A20 overexpression on β-cell viability and NF-κB regulation, cells were infected with adenovirus encoding the mouse A20 cDNA (AdA20) (Supplemental Figure 2A). A decrease in IL-1β+IFN-γ-induced NF-κB activation and apoptosis was observed in INS-1E cells overexpressing A20 (Supplemental Figure 2, B and C). Moreover, rat dispersed islet cells overexpressing A20 showed a dose dependent protection against IL-1β+IFN-γ-induced apoptosis (Figure 1G). Similar protective results were observed in mouse islets overexpressing A20 (Supplemental Figure 2, D and E).
To further study the role of A20 in β-cell death/survival, INS-1E cells were transfected with control or A20 siRNAs and exposed to IL-1β, TNF, or IFN-γ separately. Knockdown of A20 could sensitize INS-1E cells to apoptosis induced by all of these stimuli (Figure 1H), which was confirmed by Western blotting for cleaved caspase 3 in IL-1β-treated cells (Supplemental Figure 2F). As expected, A20 knockdown potentiated IL-1β- and TNF-induced NF-κB activation, in contrast to IFN-γ, which is not able to activate NF-κB (Supplemental Figure 2G). Consistent with the results in INS-1E cells, IL-1β also strongly induced death of A20β-KO islets (Figure 1I) and dispersed rat islet cells silenced for A20 (Supplemental Figure 2H).
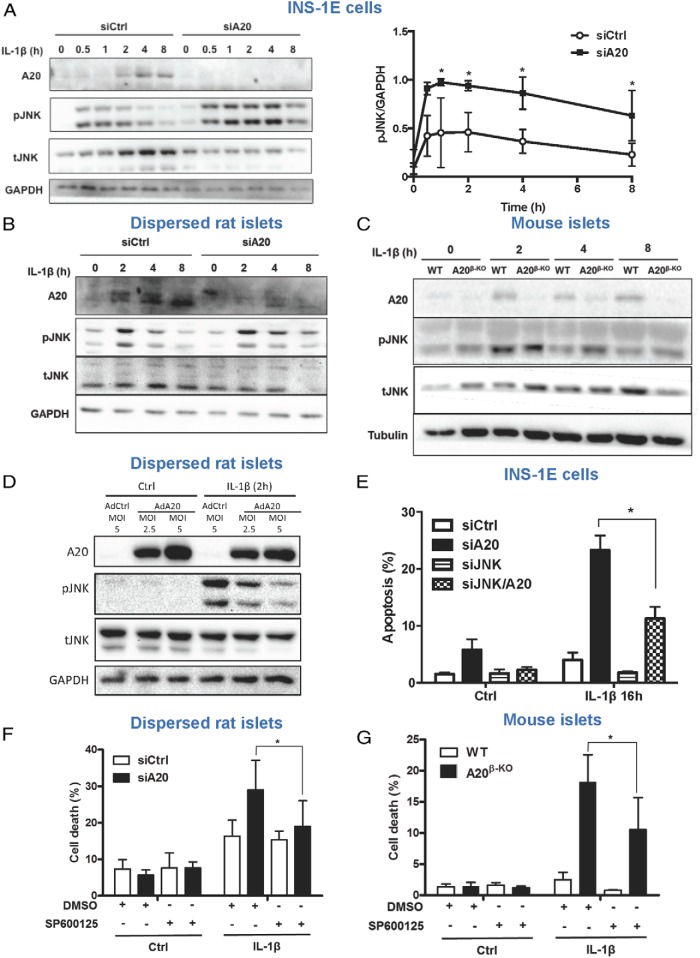
A20 controls cytokine-mediated JNK activation in pancreatic β-cells
A20 suppresses IL-1β-mediated NF-κB activation through interaction with TNF receptor-associated factor 6 (TRAF6) (14), a pathway involved in IL-1β-mediated JNK activation (32). INS-1E cells and dispersed rat islets silenced for A20 showed a stronger and prolonged JNK phosphorylation after IL-1β stimulation than in control conditions (Figure 2, A and B, and Supplemental Figure 3, A and B). IL-1β-induced JNK phosphorylation is also prolonged in islets from A20β-KO mice as compared with WT (Figure 2C). Conversely, overexpression of A20 inhibited JNK phosphorylation induced by IL-1β (Figure 2D and Supplemental Figure 3C). JNK1 knockdown significantly suppressed IL-1β-induced apoptosis and caspase 3 cleavage in A20-silenced INS-1E cells (Figure 2E and Supplemental Figure 3D). Moreover, the JNK inhibitor SP600125 could protect islet cells knocked down or deficient of A20 from IL-1β-mediated cell death (Figure 2, F and G, and Supplemental Figure 3, E and F). Together, these results indicate that A20 controls β-cell apoptosis, at least partially, by counteracting IL-1β-mediated JNK phosphorylation.
Figure 2. A20 controls IL-1β-mediated JNK activation in pancreatic β-cells.
A, Western blot analysis of A20, phospho-JNK (pJNK), total-JNK (tJNK), and GAPDH expression in INS-1E cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points, as indicated. A representative Western blotting (left panel) and quantitative assessment (right panel) are shown. Data are means ± SEM of 4 independent experiments *, P < .05 vs respective siCtrl condition. B, Western blot analysis of A20, pJNK, tJNK, and GAPDH expression in dispersed rat islet cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points as indicated. C, Western blot analysis of A20, pJNK, tJNK, and tubulin expression in islets isolated from WT or A20β-KO mice and exposed to IL-1β for different time points, as indicated. B and C, Representative Western blottings of 3–5 independent experiments are shown. D, Western blot analysis of A20, pJNK, tJNK, and GAPDH expression in dispersed rat islet cells infected with an adenovirus encoding luciferase (AdCtrl) or A20 (AdA20) at the indicated MOIs and exposed to IL-1β for 2 hours. A representative Western blotting of 5 independent experiments is shown. E, Quantification of percentage of apoptosis in INS-1E cells transfected with siCtrl, siA20, siJNK1, or both siA20 and siJNK1 (siA20/JNK) and exposed to IL-1β for 16 hours. *, P < .05 vs siA20. F, Quantification of percentage of cell death in primary dispersed rat islets cells transfected with siCtrl, siA20 and treated with IL-1β, DMSO, and/or JNK inhibitor SP600125 for 24 hours. *, P < .05 vs siA20 treated with IL-1β. G, Quantification of percentage of cell death in islets from WT or A20β-KO mice treated for 24 hours with IL-1β, DMSO, and/or the JNK inhibitor SP600125. *, P < .05 vs IL-1β/DMSO. E–G, Data are means ± SEM of 3–4 independent experiments.
A20 has been shown to inhibit TNF-mediated JNK activation by ubiquitination and degradation of apoptosis signal-regulating kinase 1 (ASK1) (an upstream activator of the JNK pathway) (33). Although, JNK phosphorylation was stronger and prolonged, no change in ASK1 protein levels was observed in TNF-stimulated INS-1E cells silenced for A20 (Supplemental Figure 4A). Although TNF and IL-1β act through different receptors, MAPK activation downstream these cytokines is mediated by a common pathway involving the activation of the transforming growth factor (TGF)-beta activated kinase 1 (TAK1)/transforming growth factor beta-activated kinase-binding protein (TAB) 1/transforming growth factor beta-activated kinase-binding protein 2 complex (32, 34). Consistently, potentiation of IL-1β- and TNF-induced JNK activation in INS-1E cells knockdown for A20 is abolished in conditions of simultaneous knockdown of TAK1 (Supplemental Figure 4, B and C).
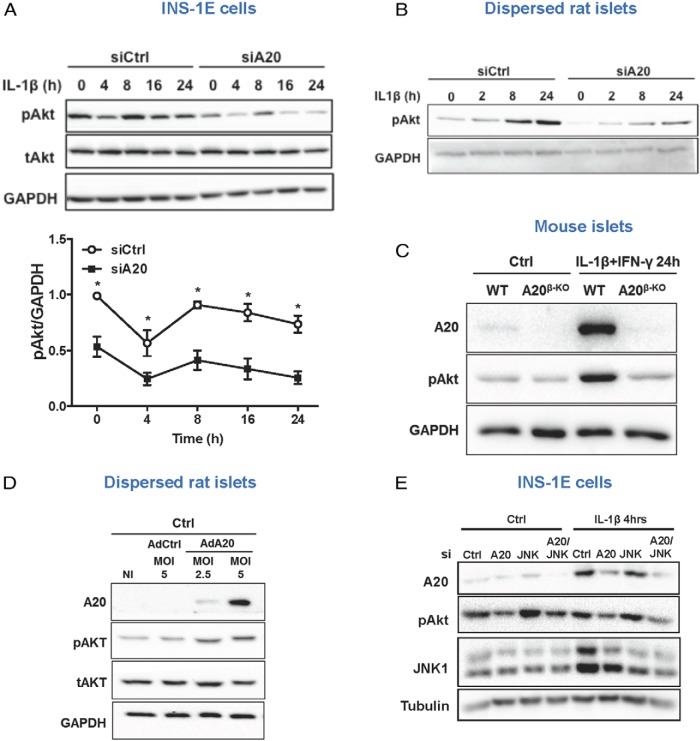
A20 regulates β-cell survival via Akt signaling and inhibition of intrinsic apoptotic signaling
Akt is an important protein controlling β-cell survival (35), and JNK1/2 have been shown to negatively regulate Akt phosphorylation (36). INS-1E cells transfected with control siRNA showed high basal levels of phosphorylated Akt, which were decreased by IL-1β exposure (Figure 3A and Supplemental Figure 5A). A20 silencing strongly decreased the basal levels of phospho-Akt, which were further declined after cytokine treatment (Figure 3A and Supplemental Figure 5A). In dispersed rat islets, the basal levels of phospho-Akt are low and progressively increase after IL-1β exposure (Figure 3B). Nonetheless, the levels of phospho-Akt are significantly lower in rat islets knocked down for A20 as compared with control (Figure 3B). Basal levels of phospho-Akt are also low in mouse islets and increase when cells are exposed to IL-1β alone (data not shown) or in combination with IFN-γ (Figure 3C). Although, IL-1β-induced Akt phosphorylation is not modified in islets from A20β-KO mice as compared with WT (data not shown) a marked decrease in phospho-Akt was observed when these cells were treated with IL-1β+IFN-γ (Figure 3C). In contrast, overexpression of A20 increased phospho-Akt expression in dispersed rat islets (Figure 3D and Supplemental Figure 5B). However, JNK activation did not contribute to the decreased Akt phosphorylation in A20-silenced cells, because similar levels of phospho-Akt were observed in INS-1E cells after double knockdown for A20 and JNK1 (Figure 3E and Supplemental Figure 3G) and in rat dispersed islet cells silenced for A20 and treated with the JNK inhibitor SP600125 (Supplemental Figure 3E).
Figure 3. A20 deletion inhibits Akt phosphorylation in β-cells.
A, Western blot analysis of phospho-Akt (pAkt), total-Akt (tAkt), and GAPDH in INS-1E cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points, as indicated. A representative Western blotting (upper panels) and quantitative assessment (lower panel) are shown. Data are means ± SEM of 4–5 independent experiments. *, P < .05 vs respective siCtrl condition. B, Western blot analysis of pAkt and GAPDH in rat primary cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points as indicated. C, Western blot analysis of A20, pAkt, and GAPDH in islets isolated from WT or A20β-KO mice and exposed to IL-1β+IFN-γ for 24 hours. D, Western blot analysis of A20, pAkt, tAkt, and GAPDH in dispersed rat islet cells infected with adenovirus encoding β-gal (AdCtrl) or A20 (AdA20) at the indicated MOIs. E, Western blot analysis of A20, pAkt, JNK1, and tubulin in INS-1E cells transfected with siCtrl, siA20, siJNK1(JNK), or siA20 and JNK1 (A20/JNK) and exposed to IL-1β for 4 hours. B–E, Representative Western blottings of 3–6 independent experiments are shown.
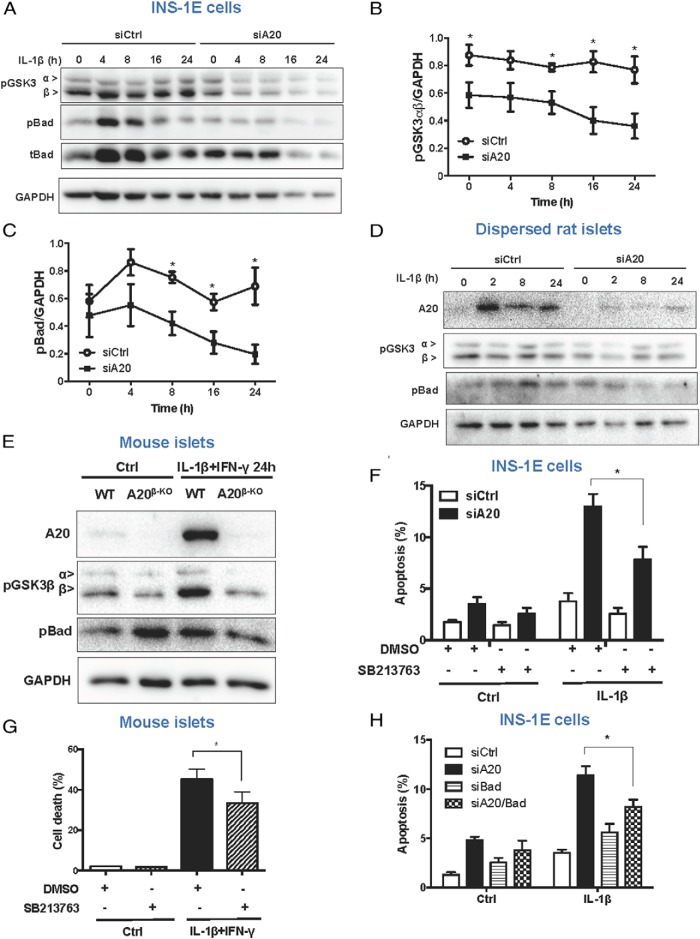
Akt controls survival signals via phosphorylation and inhibition of GSK3β and Bcl-2-associated death promoter (BAD) activities (35, 37, 38). In line with the results for Akt, the levels of phospho-GSK3β and Bad were decreased in INS-1E cells and dispersed rat islets silenced for A20 (Figure 4, A–D, and Supplemental Figure 5C). Decreased GSK3β and Bad phosphorylation were also observed in cytokine-treated islets from A20β-KO mice as compared with WT littermates (Figure 4E). GSK3β inhibition by the chemical compound SB216763 decreased the percentage of apoptotic INS-1E cells in conditions of A20 knockdown and IL-1β stimulation (Figure 4F). Similarly, islets from A20β-KO mice treated with SB216763 were partially protected from IL-1β+IFN-γ-induced death (Figure 4G). As a control for the effects of the SB216763 on GSK3 activity, β-catenin expression was analyzed (Supplemental Figure 5D) (39). Knockdown of both Bad and A20 in INS-1E cells also reduced apoptosis in IL-1β-treated INS-1E cells compared with single A20 knockdown (Figure 4H and Supplemental Figure 5E).
Figure 4. A20 deletion contributes to β-cell apoptosis via inhibition of Akt signaling.
A–C, Western blot analysis (A) and quantification (B and C) for expression of phospho-GSK3α and β (pGSK3), phospho-Bad (pBad), total-Bad (tBad), and GAPDH in INS-1E cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points as indicated. Data are means ± SEM of 5 independent experiments. *, P < .05 vs respective siCtrl condition. D, Western blot analysis of pGSK3, pBad, and GAPDH in dispersed rat islet cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points, as indicated. A representative Western blotting of 5 independent experiments is shown. E, Western blot analysis of A20, pBad, pGSK3, and GAPDH in islets isolated from WT or A20β-KO mice and exposed to IL-1β+IFN-γ for 24 hours. A representative Western blotting of 4 independent experiments is shown. F, Measurement of percentage apoptosis in INS-1E cells transfected with siCtrl or siA20 and treated with IL-1β, DMSO, or the GSK3 inhibitor SB213763, as indicated. *, P < .05 vs siA20 treated with IL-1β. G, Measurement of percentage cell death in A20β-KO mice islets treated with IL-1β+IFN-γ, DMSO, or the GSK3 inhibitor SB213763, as indicated. *, P < .05 vs DMSO IL-1β+IFN-γ condition. H, Measurement of percentage apoptosis in INS-1E cells transfected with siCtrl, siA20, siBad, or both siA20 and siBad (siA20/Bad). *, P < .05 vs siBad. E and F, Data are means ± SEM of 3–7 independent experiments.
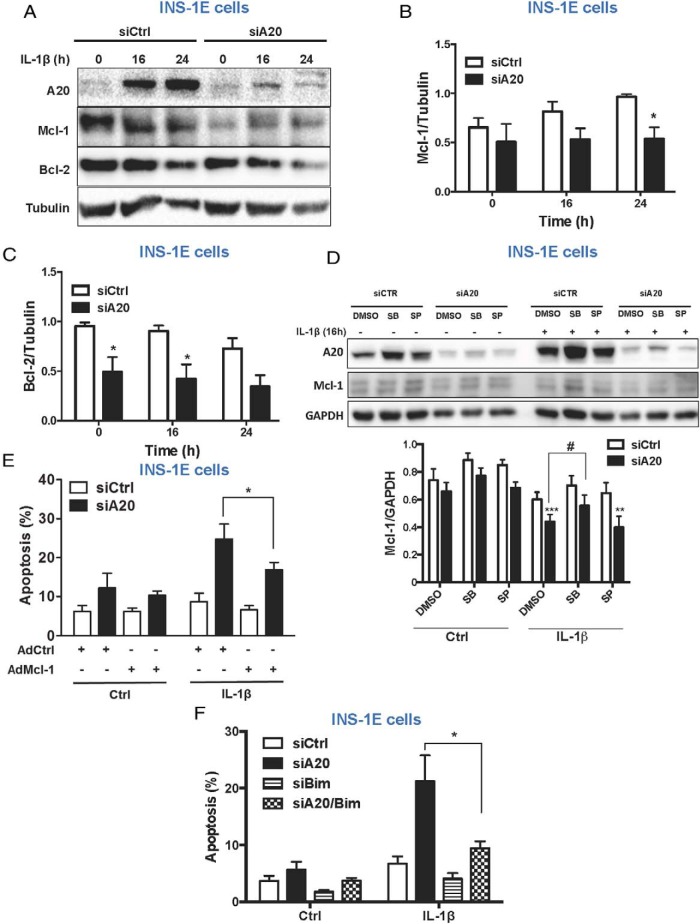
The intrinsic apoptotic pathway, regulated by members of the B-cell lymphoma 2 (Bcl-2) family of proteins, is involved in cytokine-mediated β-cell death (9). Down-regulation of the antiapoptotic protein myeloid leukemia cell differentiation protein (Mcl-1) is an important event in cytokine-mediated apoptosis (40). Because both JNK and GSK3 may phosphorylate Mcl-1 leading to its degradation (41), we analyzed Mcl-1 protein levels in A20-silenced INS-1E cells. Mcl-1 levels were decreased after A20 knockdown in INS-1E cells (Figure 5, A and B). Expression of the Bcl-2 protein was similarly down-regulated in INS-1E cells knocked down for A20 (Figure 5, A and C). Inhibition of GSK3β, but not JNK, partially reverted IL-1β-mediated Mcl-1 down-regulation in A20-silenced INS-1E cells (Figure 5D and Supplemental Figure 5F). Interestingly, overexpression of Mcl-1 partially abrogated cell death in INS-1E cells silenced for A20 and treated with IL-1β (Figure 5E and Supplemental Figure 5G). One of the mechanisms by which Mcl-1 and Bcl-2 prevent apoptosis is through inhibition of the proapoptotic protein Bcl-2-like protein 11 (Bim) (9, 42). Therefore, decreased levels of Bcl-2 and Mcl-1 could increase the levels of free Bim contributing to the activation of the intrinsic apoptotic pathway. Indeed, knockdown of both A20 and Bim efficiently prevented IL-1β-mediated apoptosis in A20-silenced INS-1E cells (Figure 5F and Supplemental Figure 5H).
Figure 5. A20 deletion sensitizes β-cells to intrinsic apoptotic signaling.
A–C, Western blot analysis (A) and quantification (B and C) of A20, Mcl-1, Bcl-2, and tubulin in INS-1E cells transfected with siCtrl or siA20 and exposed to IL-1β for different time points as indicated. Data are means ± SEM of 5 independent experiments. *, P < .05 vs siCtrl. D, Western blot analysis (upper panel) and quantification (lower panel) of A20, Mcl-1, and GAPDH in INS-1E cells transfected with siCtrl or siA20 and treated with IL-1β, DMSO, GSK3 inhibitor SB213763, and/or JNK inhibitor SP600125 for 16 hours, as indicated. Data are means ± SEM of 5 independent experiments. ***, P < .001; **, P < .01 vs siCtrl; #, P < .05 vs siA20. E, Measurement of percentage apoptosis in INS-1E cells infected with the adenovirus encoding β-gal (AdCtrl) or Mcl-1 (AdMcl-1) at MOI 10 followed by transfection with siCtrl or siA20 and treated with IL-1β for 16 hours. Data are means ± SEM of 5 independent experiments. *, P < .05 vs AdCtrl treated with IL-1β. F, Measurement of percentage apoptosis in INS-1E cells transfected with siCtrl, siA20, siBim, or both siA20 and siBim (siA20/Bim). Data are means ± SEM of 4–5 independent experiments. *, P < .05 vs siA20.
A SNP in A20 predicts lower C-peptide and higher HbA1c levels in newly diagnosed T1D patients
We next examined whether a genetic marker of TNFAIP3/A20 (rs2327832) could predict residual β-cell function at the first year after diagnosis of T1D. We genotyped the rs2327832 variant in 257 newly diagnosed children. The risk allele of rs2327832 of TNFAIP3/A20 in a dominant model (AA+AG vs GG) predicted a lower stimulated C-peptide levels 12 months after disease onset (Table 1). The estimate for ln(C-peptide) indicates a 49.8% lower C-peptide levels for the risk allele carriers. Consistently, we found that rs2327832 in an additive fashion predicted a 0.36% higher HbA1c per risk allele at 12 months after diagnosis and a 0.48% higher IDAA1c per risk allele (Table 1).
Table 1.
The TNFAIP3 SNP rs2327832 Predicts Lower C-Peptide, Higher HbA1c, and Higher IDAA1c Levels at 12 Months After Diabetes Onset
| TNFAIP3 SNP | C-Peptide at 12 Months |
HbA1c at 12 Months |
IDAA1c at 12 Months |
|||
|---|---|---|---|---|---|---|
| Effect (SE) | P Value | Effect (SE) | P[v] Value | Effect (SE) | P Value | |
| Rs2327832 (AA + AG vs GG) | −0.69 (0.28) | .02 | 0.36 (0.16) | .02 | 0.48 (0.22) | .03 |
Linear regression models were adjusted for sex, age group, and HLA risk group. The model for C-peptide was also adjusted for baseline C-peptide levels at 1 month. C-peptide levels were ln-transformed.
An attractive hypothesis is that rs2327832 which is located in an intergenic region 215 kb upstream TNFAIP3/A20, affects the expression level of the gene. We therefore examined whether the SNP has a regulatory potential for TNFAIP3/A20 expression. The A>G substitution produced by the SNP overlaps with 10 transcription factor binding motifs (examined by ENCODE and HaploReg). The SNP position overlapped with a region which demonstrated only weak enhancer chromatin states in lymphoblastoid cells (HapMap) and liver tissue, but no significant expression quantitative trait loci signals have been reported for this SNP (Supplemental Table 3).
Discussion
Genome-wide association studies have identified TNFAIP3/A20 as a susceptibility gene for multiple inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, psoriasis, and T1D, indicating an important role for this protein in the control of inflammatory immune responses (14). Besides its antiinflammatory functions, A20 is also recognized as an antiapoptotic protein (14). Although the antiinflammatory role of A20, via its inhibitory effects on NF-κB activation is well described, much less is known about its antiapoptotic effects.
In line with previous studies, we observed that overexpression of A20 prevents NF-κB activation and cytokine-mediated β-cell apoptosis (present data, Ref. 20). However, we have now shown that absence of A20 strongly potentiates NF-κB signaling and sensitizes β-cells to apoptosis induced by different combinations of cytokines and exposure to single cytokines, a condition that does not induce apoptosis in A20 sufficient primary β-cells (present data, Refs. 2, 8). The protective effects of A20 overexpression in β-cells were previously related to a decrease in NF-κB activation and consequent reduction of NO production (13, 20). However, some of our observations suggest that A20 has broader effects in these cells. INS-1E cells silenced for A20, as well as, primary islets from A20β-KO mice were more susceptible to cytokine induced cell death, despite similar NO production in control cells/islets. Moreover, A20 silencing potentiates cell death in conditions where NF-κB activation and NO production are not involved, such as in IFN-γ-treated INS-1E cells. In line with these results, we now show that A20 regulates JNK and Akt signaling in β-cells.
Activation of JNK contributes to cytokine-mediated β-cell apoptosis and decreases islet survival after transplantation (11, 43, 44). In the present study, we observed that knockdown of A20 expression induces a persistent cytokine-mediated JNK activation in INS-1E cells and primary rat and mouse islet cells. A20 was previously shown to suppress TNF-mediated JNK phosphorylation by promoting the ubiquitination and degradation of ASK1 (33). This does not seem to be the case in β-cells, because knockdown of A20 failed to affect ASK1 expression, in both untreated and cytokine-stimulated cells. However, it is conceivable that A20 affects both IL-1β- and TNF-mediated JNK activation in β-cells via indirect inactivation of TAK1, probably as a result of the deubiquitination of TRAF6 or receptor-interacting protein 1 (RIP1), respectively (14). We have previously shown that JNK contributes to cytokine-induced β-cell death by down-regulating the antiapoptotic protein Mcl-1, favoring the activation of the intrinsic mitochondrial apoptotic pathway (40). However, the Mcl-1 down-regulation observed in A20-silenced INS-1E cells seems to be independent of JNK and related to GSK3β phosphorylation (see below). Another mechanism by which JNK activation contributes to cytokine-mediated apoptosis in β-cells is via phosphorylation and activation of the proapoptotic protein Bim (45). In line with these data, we have now shown that knockdown of Bim counteracts the increase in IL-1β-induced apoptosis in A20-silenced cells.
Akt signaling was previously shown to contribute to β-cell survival and proliferation (46–48). We now show that A20 positively regulates Akt signaling in β-cells. Strongly reduced phospho-Akt levels could be observed in INS-1E cells and primary rat and mouse islets knocked down/knocked out for A20. In addition, overexpression of A20 in rat dispersed cells increased Akt phosphorylation. Contrary to previous studies, we could not observe any effect of JNK1 knockdown on Akt phosphorylation (36). The fact that A20 silencing modifies Akt phosphorylation in unstimulated INS-1E cells, a condition where JNK is not activated, is in agreement with a JNK-independent effect of A20 on Akt. A major regulator of Akt signaling in β-cells is insulin via phosphoinositide 3-kinase activation (35). Further experiments are however required to verify whether A20 regulates insulin receptor signaling.
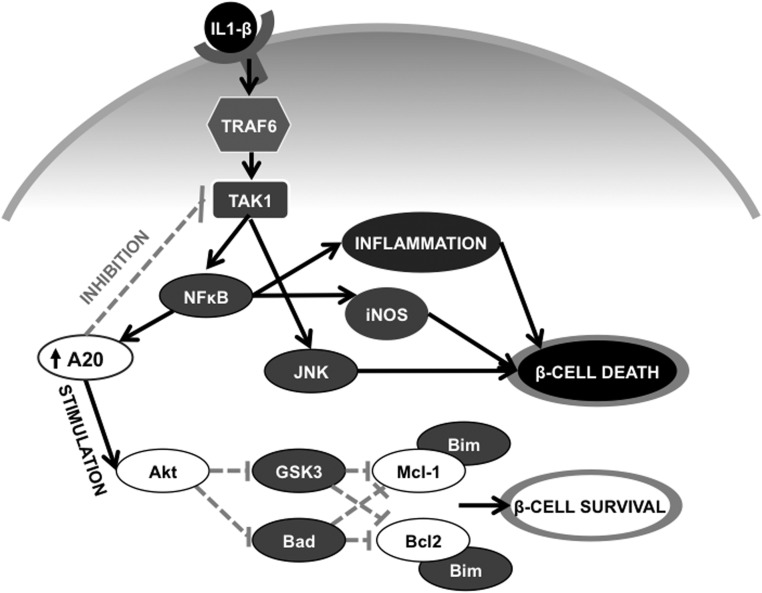
Reduced Akt activation observed in A20-silenced cells was reflected in a decreased phosphorylation of GSK3β and Bad, both direct targets of Akt (35). Inhibition of GSK3β could partially prevent cytokine-mediated death of both INS-1E cells silenced for A20 and islets from the A20β-KO mice. These results are in agreement with previous studies showing a proapoptotic role for this protein in β-cells (37, 46, 49). GSK3β was shown to regulate apoptosis via phosphorylation-mediated degradation of Mcl-1 and Bcl-2 (50, 51), suggesting that the decreased levels of these proteins observed in A20-silenced cells are downstream of GSK3β. In line with this, the GSK3β inhibitor significantly increases Mcl-1 protein expression in A20-silenced INS-1E cells treated with IL-1β. This increased Mcl-1 level may explain the protective effects of the GSK3β inhibitor presently observed. Of note, overexpression of Mcl-1 partially prevented cell death in INS-1E cells treated with IL-1β and silenced for A20 confirming an important role for this protein in the preservation of cell viability. Next to a critical role for GSK3β/Mcl-1, we also suggest Bad to be involved in β-cell apoptosis. Indeed, a reduction in the phosphorylation of Bad could be demonstrated in INS-1E cells silenced for A20, and Bad knockdown reduced apoptosis in response to IL-1β. Bad is a proapoptotic Bcl-2 homology domain 3 (BH3)-only Bcl-2 family member that, when activated, displaces antiapoptotic proteins (eg, Mcl-1 and Bcl-2), releasing the proapoptotic protein Bim leading to the activation of prodeath proteins (eg, BCL2-Associated X (Bax) and BCL2-Antagonist/Killer (Bak)), ultimately inducing caspase 3 activation and apoptosis (9). Our results show that both Bim knockdown and Mcl-1 overexpression prevent IL-1β-induced apoptosis in INS-1E cells silenced for A20, indicating that this apoptotic cascade is activated in these cells. Together, our data indicate that A20 prevents β-cell death by multiple mechanisms: by inhibiting both JNK and NF-κB and by enhancing Akt signaling, A20 protects β-cells from the activation of the intrinsic apoptotic pathway, the major cell death pathway activated by proinflammatory cytokines in β-cells (Figure 6) (9).
Figure 6. A20 prevents β-cell death by multiple ways.
IL-1β activates a signaling cascade involving TRAF6 and TAK1 leading to NF-κB and JNK activation and consequently induction of proinflammatory responses and β-cell death. NF-κB induces the expression of the A20 protein, which inhibits both NF-κB and JNK signaling decreasing cell death. Moreover, A20 favors Akt activation leading to phosphorylation and inactivation of the proapoptotic proteins GSK3β and Bad. GSK3β can phosphorylate the antiapoptotic protein Mcl-1, marking it for proteasomal degradation, whereas GSK3β also targets Bcl-2 for degradation. Decreased Bad phosphorylation and activation, together with a decrease in both Mcl-1 and Bcl-2 expression, promote the activation of proapoptotic Bcl-2 homology domain 3 (BH3) proteins, including Bim, inducing apoptosis. Solid arrows indicate stimulation and doted arrows indicate inhibition.
Although the antiapoptotic effects of A20 in both rodent and human β-cells are evident and consistently described in different studies (present study, Refs. 13, 18, 20), the role of A20 in animal models of T1D is still controversial. A20 overexpression protects against MLDSTZ-induced diabetes (19), however, we have recently shown that specific deletion of A20 in β-cells did not affect diabetes incidence (52). These contradictory results may indicate that in vivo the expression of A20 in β-cells is low and decreasing it further does not accelerate disease development. In agreement with this, our in vitro experiments showed that the levels of the A20 protein induced in β-cells are sufficient to prevent apoptosis when cells are exposed to a single cytokine stimulus (IL-1β alone); however, when cells are exposed to different combinations of cytokines, a condition faced by the β-cells in the in vivo situation (2), the endogenous level of A20 is not sufficient to prevent cell death. Moreover, the lack of an exacerbated diabetic phenotype in A20β-KO mice may also indicate that the absence of A20 expression in β-cells contributes to but is not sufficient to sensitize to apoptosis in vivo. In line with this, we recently showed that the specific inhibition of A20 in intestinal epithelial cells does not lead to spontaneous intestinal inflammation. However, A20 deletion in both myeloid and intestinal epithelial cells leads to the spontaneous development of intestinal pathology (53). The former model is more representative of the situation encountered in diabetic patients carrying the SNP at the TNFAIP3 gene, because the polymorphism may influence A20 expression in all tissues.
The clinical relevance of A20 in T1D is demonstrated by our results showing that T1D children carrying the SNP rs2327832 have reduced residual β-cell function and impaired glycaemic control. Although no expression quantitative trait loci signals have been reported yet for this SNP and the effect of this polymorphism on A20 expression/function still needs to be elucidated, its impact on T1D development in carrier patients is clear. Importantly, however, genetic variants can affect gene expression levels in a tissue-specific manner and the importance of examining the target tissue of interest when mapping SNP effects to gene expression has been demonstrated (54). Hence, to establish whether the SNP has a modulatory effect on gene expression relevant for our study, it will be necessary to examine the effect of rs2327832 on TNFAIP3/A20 expression in human β-cells. It is conceivable that rs2327832 somehow affects A20 expression/function leading to a more active/aggressive immune response due to NF-κB hyperactivation in immune cells combined with a decreased capacity of the β-cells to survive, eventually contributing to the development of T1D. Recent genome-wide association studies have identified a large number of loci affecting T1D risk (16, 17), however, the clinical relevance of these SNPs on disease progression are yet poorly understood. Our studies have now revealed that the rs2327832 of TNFAIP3 can predict glycemic control in recent onset T1D children. These interesting findings should be further addressed in different cohorts of T1D patients.
Overall, our results suggest that besides modulating inflammatory responses, A20/TNFAIP3 controls multiple pathways regulating β-cell survival and may help to predict disease progression in children at T1D onset.
Acknowledgments
We thank our colleagues from the Université Libre de Bruxelles Center of Diabetes Research: Isabelle Millard, Anyishai Musuaya, Stephanie Mertens, Michael Pangerl, and Nathalie Pachera for expert technical assistance and Baroj Abdulkarim for helping with mouse islet isolation. A.K.C. is a research associate from the FNRS. L.C. is a PhD fellow with the Agency for Innovation by Science and Technology (Belgium).
Author contributions: M.F., L.C., C.A.B., E.C.V., F.O., L.B.N., J.S., G.v.L., and A.K.C., contributed to the experimental design of the study; M.F., L.C., C.A.B., D.D., E.C.V., K.M., L.B.N., M.L.A., G.v.L., and A.K.C. carried out experiments and/or helped with data analysis; R.B., F.O., H.B.M., and F.P. contributed with materials and/or data interpretation; M.F., L.C., C.A.B., J.S., G.v.L., and A.K.C. wrote the manuscript; and all the authors reviewed the final version of the manuscript.
Work in A.K.C. group was supported by the Juvenile Diabetes Foundation, New York, Grant 1–2011-589; Actions de Recherche Concertées de la Communauté Française (Belgium); and National Funds from Scientific Research (FNRS) (Belgium, MIS Grant F.4521.11). Work in the G.v.L. and R.B. labs was supported by an Research Foundation Flanders (FWO) Odysseus Grant and by research grants from the FWO (Belgium), the Innovatie door Wetenschap en Technologie (IWT) strategic basic research program, the Belgian Foundation Against Cancer, the Geneeskundige Stichting Koningin Elisabeth (Belgium), the Charcot Foundation (Belgium), the Interuniversity Attraction Poles program (IAP7, Belgium), and the Concerted Research Actions (Belgium) and Group-ID Multidisciplinary Research Partnership (MRP) of the Gent University. Work in the J.S. lab was funded by the Danish Research Council (Denmark) Grant 1331–00163A. The research of F.O. was supported by Fundação de Auxilio a Pesquisa do Estado de São Paulo (Brazil). E.C.V. received a Postdoctoral Fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Wallonie-Bruxelles International (WBI), Belgium/Brazil).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
Work in A.K.C. group was supported by the Juvenile Diabetes Foundation, New York, Grant 1–2011-589; Actions de Recherche Concertées de la Communauté Française (Belgium); and National Funds from Scientific Research (FNRS) (Belgium, MIS Grant F.4521.11). Work in the G.v.L. and R.B. labs was supported by an Research Foundation Flanders (FWO) Odysseus Grant and by research grants from the FWO (Belgium), the Innovatie door Wetenschap en Technologie (IWT) strategic basic research program, the Belgian Foundation Against Cancer, the Geneeskundige Stichting Koningin Elisabeth (Belgium), the Charcot Foundation (Belgium), the Interuniversity Attraction Poles program (IAP7, Belgium), and the Concerted Research Actions (Belgium) and Group-ID Multidisciplinary Research Partnership (MRP) of the Gent University. Work in the J.S. lab was funded by the Danish Research Council (Denmark) Grant 1331–00163A. The research of F.O. was supported by Fundação de Auxilio a Pesquisa do Estado de São Paulo (Brazil). E.C.V. received a Postdoctoral Fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Wallonie-Bruxelles International (WBI), Belgium/Brazil).
Footnotes
- Akt
- v-akt murine thymoma viral oncogene homolog
- DMSO
- dimethyl sulfoxide
- ASK1
- apoptosis signal-regulating kinase 1
- Bad
- Bcl-2-associated death promoter
- Bcl-2
- B-cell lymphoma 2
- Bim
- Bcl-2-like protein 11
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphare dehydrogenase
- GSK3
- glycogen synthase kinase 3
- HbA1c
- hemoglobin A1c
- IDAA1c
- insulin dose-adjusted HbA1c
- IFN-γ
- interferon-γ
- iNOS
- indulcible nitric oxide synthase
- JNK
- c-Jun N-terminal kinase
- Mcl-1
- myeloid leukemia cell differentiation protein
- MLDSTZ
- multiple low-dose streptozotocin
- NF-kB
- nuclear factor-kappaB
- NO
- nitric oxide
- siRNA
- small interfering RNA
- SNP
- single nucleotide polymorphism
- TAK1
- transforming growth factor (TGF)-beta activated kinase 1
- TRAF6
- TNF receptor-associated factor 6
- T1D
- type 1 diabetes
- TNFAIP3
- TNF-induced protein 3.
References
- 1. van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. [DOI] [PubMed] [Google Scholar]
- 2. Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. [DOI] [PubMed] [Google Scholar]
- 3. Cardozo AK, Heimberg H, Heremans Y, et al. . A comprehensive analysis of cytokine-induced and nuclear factor-κ B-dependent genes in primary rat pancreatic β-cells. J Biol Chem. 2001;276:48879–48886. [DOI] [PubMed] [Google Scholar]
- 4. Heimberg H, Heremans Y, Jobin C, et al. . Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes. 2001;50:2219–2224. [DOI] [PubMed] [Google Scholar]
- 5. Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1β by adenoviral gene transfer of an Iκ B repressor. J Biol Chem. 2000;275:36509–36513. [DOI] [PubMed] [Google Scholar]
- 6. Eldor R, Yeffet A, Baum K, et al. . Conditional and specific NF-κB blockade protects pancreatic β cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006;103:5072–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salem HH, Trojanowski B, Fiedler K, et al. . Long-term IKK2/NF-κB signaling in pancreatic β-cells induces immune-mediated diabetes. Diabetes. 2014;63:960–975. [DOI] [PubMed] [Google Scholar]
- 8. Cardozo AK, Ortis F, Storling J, et al. . Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes. 2005;54:452–461. [DOI] [PubMed] [Google Scholar]
- 9. Gurzov EN, Eizirik DL. Bcl-2 proteins in diabetes: mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol. 2011;21:424–431. [DOI] [PubMed] [Google Scholar]
- 10. Allagnat F, Fukaya M, Nogueira TC, et al. . C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ. 2012;19:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of β-cell death. Diabetes. 2001;50:77–82. [DOI] [PubMed] [Google Scholar]
- 12. Kim S, Millet I, Kim HS, et al. . NF-κ B prevents β cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA. 2007;104:1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liuwantara D, Elliot M, Smith MW, et al. . Nuclear factor-κB regulates β-cell death: a critical role for A20 in β-cell protection. Diabetes. 2006;55:2491–2501. [DOI] [PubMed] [Google Scholar]
- 14. Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35:22–31. [DOI] [PubMed] [Google Scholar]
- 15. Fung EY, Smyth DJ, Howson JM, et al. . Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 2009;10:188–191. [DOI] [PubMed] [Google Scholar]
- 16. Bergholdt R, Brorsson C, Palleja A, et al. . Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes. 2012;61:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrett JC, Clayton DG, Concannon P, et al. . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grey ST, Longo C, Shukri T, et al. . Genetic engineering of a suboptimal islet graft with A20 preserves β cell mass and function. J Immunol. 2003;170:6250–6256. [DOI] [PubMed] [Google Scholar]
- 19. Yu LY, Lin B, Zhang ZL, Guo LH. Direct transfer of A20 gene into pancreas protected mice from streptozotocin-induced diabetes. Acta Pharmacol Sin. 2004;25:721–726. [PubMed] [Google Scholar]
- 20. Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C. A20 inhibits cytokine-induced apoptosis and nuclear factor κB- dependent gene activation in islets. J Exp Med. 1999;190:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vereecke L, Sze M, Mc Guire C, et al. . Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. [DOI] [PubMed] [Google Scholar]
- 23. Rasschaert J, Ladrière L, Urbain M, et al. . Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA+ interferon-γ-induced apoptosis in primary pancreatic β-cells. J Biol Chem. 2005;280:33984–33991. [DOI] [PubMed] [Google Scholar]
- 24. Fukaya M, Tamura Y, Chiba Y, et al. . Protective effects of a nicotinamide derivative, isonicotinamide, against streptozotocin-induced β-cell damage and diabetes in mice. Biochem Biophys Res Commun. 2013;442:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678. [DOI] [PubMed] [Google Scholar]
- 26. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. [DOI] [PubMed] [Google Scholar]
- 27. Moore F, Colli ML, Cnop M, et al. . PTPN2, a candidate gene for type 1 diabetes, modulates interferon-γ-induced pancreatic β-cell apoptosis. Diabetes. 2009;58:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortensen HB, Swift PG, Holl RW, et al. . Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual β-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218–226. [DOI] [PubMed] [Google Scholar]
- 31. Mortensen HB, Hougaard P, Swift P, et al. . New definition for the partial remission period in children and adolescents with T1D. Diabetes Care. 2009;32:1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortis F, Miani M, Colli ML, et al. . Differential usage of NF-κB activating signals by IL-1β and TNF-α in pancreatic β cells. FEBS Lett. 2012;586:984–989. [DOI] [PubMed] [Google Scholar]
- 33. Won M, Park KA, Byun HS, et al. . Novel anti-apoptotic mechanism of A20 through targeting ASK1 to suppress TNF-induced JNK activation. Cell Death Differ. 2010;17:1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shim JH, Xiao C, Paschal AE, et al. . TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elghazi L, Bernal-Mizrachi E. Akt and PTEN: β-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdelli S, Bonny C. JNK3 maintains expression of the insulin receptor substrate 2 (IRS2) in insulin-secreting cells: functional consequences for insulin signaling. PLoS One. 2012;7:e35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mussmann R, Geese M, Harder F, et al. . Inhibition of GSK3 promotes replication and survival of pancreatic β cells. J Biol Chem. 2007;282:12030–12037. [DOI] [PubMed] [Google Scholar]
- 38. Mishra R, Barthwal MK, Sondarva G, et al. . Glycogen synthase kinase-3β induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282:30393–30405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coghlan MP, Culbert AA, Cross DA, et al. . Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. [DOI] [PubMed] [Google Scholar]
- 40. Allagnat F, Cunha D, Moore F, Vanderwinden JM, Eizirik DL, Cardozo AK. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to β-cell apoptosis. Cell Death Differ. 2011;18:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. [DOI] [PubMed] [Google Scholar]
- 42. Colli ML, Nogueira TC, Allagnat F, et al. . Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic β cell apoptosis via a Bim / Mcl-1 imbalance. PLoS Pathog 2011;7:e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Noguchi H, Nakai Y, Matsumoto S, et al. . Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant. 2005;5:1848–1855. [DOI] [PubMed] [Google Scholar]
- 44. Noguchi H, Nakai Y, Ueda M, et al. . Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia. 2007;50:612–619. [DOI] [PubMed] [Google Scholar]
- 45. Santin I, Moore F, Colli ML, et al. . PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic β-cell apoptosis via regulation of the BH3-only protein Bim. Diabetes. 2011;60:3279–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3β in mouse insulinoma cells. Diabetes. 2005;54:968–975. [DOI] [PubMed] [Google Scholar]
- 47. Tuttle RL, Gill NS, Pugh W, et al. . Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat Med. 2001;7:1133–1137. [DOI] [PubMed] [Google Scholar]
- 48. Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanabe K, Liu Y, Hasan SD, et al. . Glucose and fatty acids synergize to promote B-cell apoptosis through activation of glycogen synthase kinase 3β independent of JNK activation. PLoS One. 2011;6:e18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy. 2013;9:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. [DOI] [PubMed] [Google Scholar]
- 52. Catrysse L, Fukaya M, Sze M, et al. . A20 deficiency sensitizes pancreatic β cells to cytokine-induced apoptosis in vitro but does not influence type 1 diabetes development in vivo. Cell Death Dis. 2015;6:e1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vereecke L, Vieira-Silva S, Billiet T, et al. . A20 controls intestinal homeostasis through cell-specific activities. Nat Commun. 2014;5:5103. [DOI] [PubMed] [Google Scholar]
- 54. Hernandez DG, Nalls MA, Moore M, et al. . Integration of GWAS SNPs and tissue-specific expression profiling reveal discrete eQTLs for human traits in blood and brain. Neurobiol Dis. 2012;47:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]