Abstract
Diabetes is characterized by insulin insufficiency due to a relative paucity of functional β-cell mass. Thus, strategies for increasing β-cell mass in situ are sought-after for therapeutic purposes. Pregnancy is a physiological state capable of inducing robust β-cell mass expansion, however, the mechanisms driving this expansion are not fully understood. Thus, the aim of this study was to characterize pregnancy-induced changes in the islet proteome at the peak of β-cell proliferation in mice. Islets from pregnant and nonpregnant littermates were compared via 2 proteomic strategies. In vivo pulsed stable isotope labeling of amino acids in cell culture was used to monitor de novo protein synthesis during the first 14.5 days of pregnancy. In parallel, protein abundance was determined using ex vivo dimethyl labelling at gestational day 14.5. Comparison of the 2 datasets revealed 170 islet proteins to be up regulated as a response to pregnancy. These included several proteins, not previously associated with pregnancy-induced islet expansion, such as CLIC1, STMN1, MCM6, PPIB, NEDD4, and HLTF. Confirming the validity of our approach, we also identified proteins encoded by genes known to be associated with pregnancy-induced islet expansion, such as CHGB, IGFBP5, MATN2, EHHADH, IVD, and BMP1. Bioinformatic analyses demonstrated enrichment and activation of the biological functions: “protein synthesis” and “proliferation,” and predicted the transcription factors HNF4α, MYC, MYCN, E2F1, NFE2L2, and HNF1α as upstream regulators of the observed expressional changes. As the first characterization of the islet-proteome during pregnancy, this study provides novel insight into the mechanisms involved in promoting pregnancy-induced β-cell mass expansion and function.
An increased metabolic demand, such as pregnancy or weight gain, is associated with a compensatory expansion of the β-cell mass in both humans and rodents (1–7). It is well documented that an insufficient compensatory response can manifest into diabetes; women with gestational diabetes are for example at high risk for developing type 2 diabetes later in life (8). Understanding the mechanisms involved in β-cell mass expansion is hence of utmost importance in preventing disease progression, as well as for the development of novel diabetes therapies.
Pregnancy in mice causes a 2- to 4-fold increase in β-cell mass with β-cell proliferation peaking around gestational day 14 (4–6). It is well established that prolactin receptor signaling, activated by the lactogenic hormones prolactin and placental lactogen, is required for β-cell mass expansion during pregnancy (4, 9–11). More recently, it has been shown that epidermal growth factor receptor-mediated signaling and serotonin biosynthesis are crucial mediators of gestational β-cell mass expansion (12, 13).
How these various signaling cues and mediators are integrated to induce β-cell proliferation is incompletely understood, as is the identity of other players in this process. To this end, genome-wide analysis has been performed on isolated islets from pregnant mice (5, 6, 13). However, complementary proteomic efforts are needed to further augment the insight provided by these studies. The requirement is especially underscored by recent findings of posttranscriptional mechanism, such as miRNA regulation, being involved in β-cell adaptation during pregnancy (15).
In the present study, we have mapped the proteome of islets isolated from pregnant mice at day 14.5 and compared it with that of islets from nonpregnant littermates using state-of-the-art quantitative proteomics. We previously developed an in vivo metabolic labeling technique based on pulsed stable isotope labeling of amino acids in cell culture (SILAC) (16–18). By feeding mice SILAC diet, a heavy 13C6 substituted lysine is incorporated into newly synthesized proteins. The ratio between heavy lysine and the naturally abundant 12C6 lysine permits the quantification of de novo protein synthesis. By parallel feeding of pregnant and nonpregnant mice, we identified proteins undergoing increased de novo synthesis in response to pregnancy. To complement our SILAC analysis and to determine the relative protein abundance, we performed an additional experiment using the chemical isotope labeling technique known as dimethyl labeling (19). In this technique heavy and medium isotope labels are chemically linked to the islet peptides ex vivo. Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analysis of the heavy to medium ratios allows relative protein quantification, in this case, between islets from pregnant vs nonpregnant mice. Cross-comparison of the SILAC and dimethyl labeling datasets enabled the identification of islet proteins exhibiting robust regulation during pregnancy-induced β-cell mass expansion. Furthermore, integrative bioinformatic analysis predicted the transcription factors HNF4α, MYC, MYCN, E2F1, NFE2L2, and HNF1α as upstream mediators of gestational β-cell mass expansion.
Results
Identification of islet proteins showing pregnancy-induced increased heavy lysine incorporation
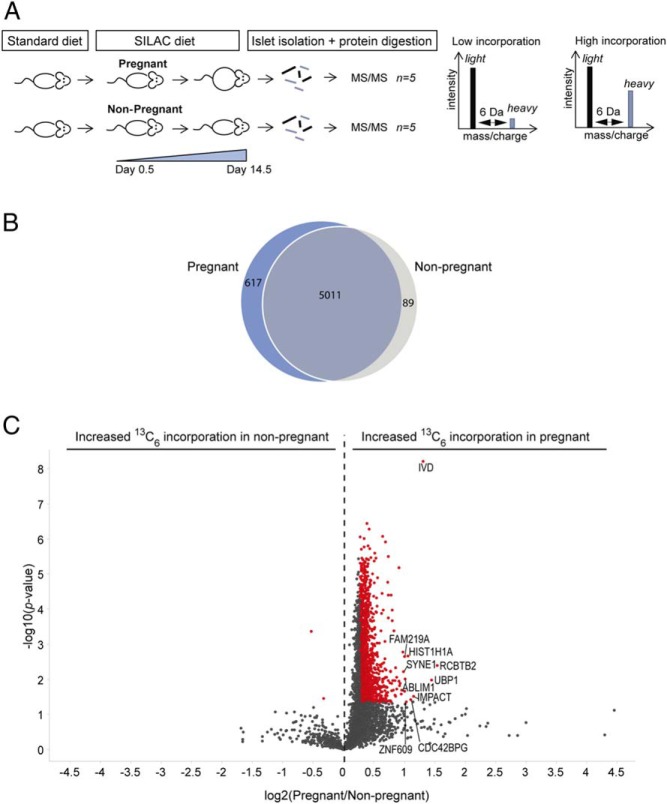
To identify proteins undergoing increased protein synthesis during pregnancy, we employed state-of-the-art in vivo pulsed SILAC labeling. As schematized in Figure 1A, pregnant mice were transferred from standard to SILAC diet upon detection of vaginal mating-plugs taken to indicate pregnancy. Virgin control females were fed a SILAC diet in parallel. After 14.5 days, pancreatic islets were isolated from both groups. Before analysis by LC-MS/MS, proteins were extracted and digested with the lysine specific protease Lys-C. In total, a common group of 5011 proteins showed incorporation of heavy lysine in both pregnant and nonpregnant animals (Figure 1B). To compare de novo protein synthesis between pregnant and nonpregnant mice, the average incorporation ratio (defined as heavy to light) in pregnant was compared with the average incorporation ratio of nonpregnant animals. Presumably caused by increased food consumption during pregnancy, the proteins within the pregnancy group generally showed 4% higher lysine incorporation. To avoid that such increase in incorporation was falsely interpreted as increased proteins synthesis, we subsequently applied the term “increased de novo protein synthesis” to those proteins which showed at least 20% increased heavy lysine incorporation when compared with nonpregnant animals. This lead to the identification of 1359 proteins, which showed significantly increased protein synthesis during pregnancy (Figure 1C red, Table 1, and Supplemental Table 1). The overrepresentation of proteins with a log2-ratio above 0, demonstrates that de novo protein synthesis was increased in islets from pregnant mice (Figure 1C). This is consistent with the previously reported maximal β-cell proliferation being observed at around gestational day 14.5 and is expected in expanding tissues (4–6). To identify how these proteins contributed to gestational islet expansion, we analyzed the proteins using Ingenuity Pathway Analysis (IPA) (QIAGEN). The biological functions “protein synthesis” and “proliferation” were found to be significantly enriched and activated (P values, 2.56E-25 and 2.38E-21; activation scores, 1.7 and 9.0, respectively).
Figure 1. Identification of islet proteins showing pregnancy-induced increased heavy lysine incorporation.
A, Proteins undergoing de novo synthesis in pregnant and nonpregnant animals were labeled in vivo using a SILAC diet. Labeling was initiated when vaginal mating-plugs were detected and continued until day 14.5, where islets were harvested. The protein samples were analyzed individually using LC-MS/MS. For each protein, the heavy to light ratio was calculated and used as a measure of de novo synthesis. B, Venn diagram showing the total number of labeled proteins identified in the pregnant and nonpregnant group. C, The average heavy to light ratio from pregnant animals was compared with the average heavy to light ratio from nonpregnant animals, and analyzed using a 2-sample t test. The log2 (pregnant/nonpregnant) ratios were plotted against the −log10 (P value) in a volcano plot. Significantly regulated proteins are marked in red and the 10 most up-regulated proteins are tagged with gene names.
Table 1.
Top 10 Proteins Showing the Highest Increase in Heavy Lysine Incorporation in Response to Pregnancy
| Gene Name | Protein Name | Fold P/NP | P Value | FDR |
|---|---|---|---|---|
| Rcbtb2 | RCC1 and BTB domain-containing protein 2 | 2.91 | .004 | + |
| Ubp1 | Upstream-binding protein 1 | 2.74 | .011 | + |
| Ivd | Isovaleryl-CoA dehydrogenase, mitochondrial | 2.49 | <.001 | + |
| Impact | Protein IMPACT | 2.22 | .031 | + |
| Cdc42bpg | Serine/threonine-protein kinase MRCKγ | 2.16 | .038 | + |
| Hist1h1a | Histone H1,1 | 2.08 | .002 | + |
| Znf609 | Zinc finger protein 609 | 2.05 | .043 | + |
| Syne1 | Nesprin-1 | 2.02 | .010 | + |
| Ablim1 | Actin-binding LIM protein 1 | 2.01 | .022 | + |
| Fam219a | Protein FAM219A | 2.00 | .002 | + |
P values were calculated using a 2-sample t test. + in the column false discovery rate (FDR) indicates that the proteins retained significance after multiple correction using the Benjamini-Hochberg method with a FDR of 15%.
As shown in Figure 1B, 617 proteins displayed heavy lysine incorporation specifically in the group of pregnant animals. These proteins could be identified exclusively in the pregnant state by chance or they could be proteins that are induced in a response to pregnancy. Because the likelihood of chance identification decreases with the number of exclusive identifications, we present those proteins identified exclusively in minimum 4 out of 5 pregnant animals in Table 2 (all exclusively identified proteins can be found in the full SILAC dataset). Validating the biological relevance of these, the prolactin receptor (PRLR), which is known to be up-regulated in islets during pregnancy (11), was identified in this group. In addition, the pulmonary surfactant-associated protein D (SFTPD), a gene with increased expression in newly formed β-cells (20) and in islets of pregnant mice (21), was also found in this group and was further validated using Western blotting in Supplemental Figure 1.
Table 2.
Proteins Showing Heavy Lysine Incorporation Exclusively in Islets of Pregnant Mice
| Gene Name | Protein Name | Found in n |
|---|---|---|
| Crispld2 | Cysteine-rich secretory protein LCCL domain-containing 2 | 5 |
| Pus7l | Pseudouridylate synthase 7 homolog-like protein | 5 |
| Unknown | UPF0690 protein C1orf52 homolog | 5 |
| Prlr | Prolactin receptor | 4 |
| Sftpd | Pulmonary surfactant-associated protein D | 4 |
| Rbp4 | Retinol-binding protein 4 | 4 |
| Cttnbp2 | Cortactin-binding protein 2 | 4 |
| Stard3 | StAR-related lipid transfer protein 3 | 4 |
| Hltf | Helicase-like transcription factor | 4 |
| Stau2 | Double-stranded RNA-binding protein Staufen homolog 2 | 4 |
| Ttc5 | Tetratricopeptide repeat protein 5 | 4 |
| Wwp2 | NEDD4-like E3 ubiquitin-protein ligase WWP2 | 4 |
| Ehhadh | Peroxisomal bifunctional enzyme; Enoyl-CoA hydratase/3,2-trans-enoyl-CoA isomerase; 3-hydroxyacyl-CoA dehydrogenase | 4 |
| Ccdc22 | Coiled-coil domain-containing protein 22 | 4 |
| Arntl | Aryl hydrocarbon receptor nuclear translocator-like protein 1 | 4 |
| Pygm | Glycogen phosphorylase, muscle form | 4 |
Proteins that showed heavy lysine incorporation exclusively in at least 4 out of 5 pregnant animals are presented. All 617 exclusively identified proteins can be found in the full SILAC dataset Supplemental Table 5 by sorting on ratios NP = 0 and ratios P = 1, 2, 3, 4, 5.
Changes in islet protein expression at day 14.5 of pregnancy
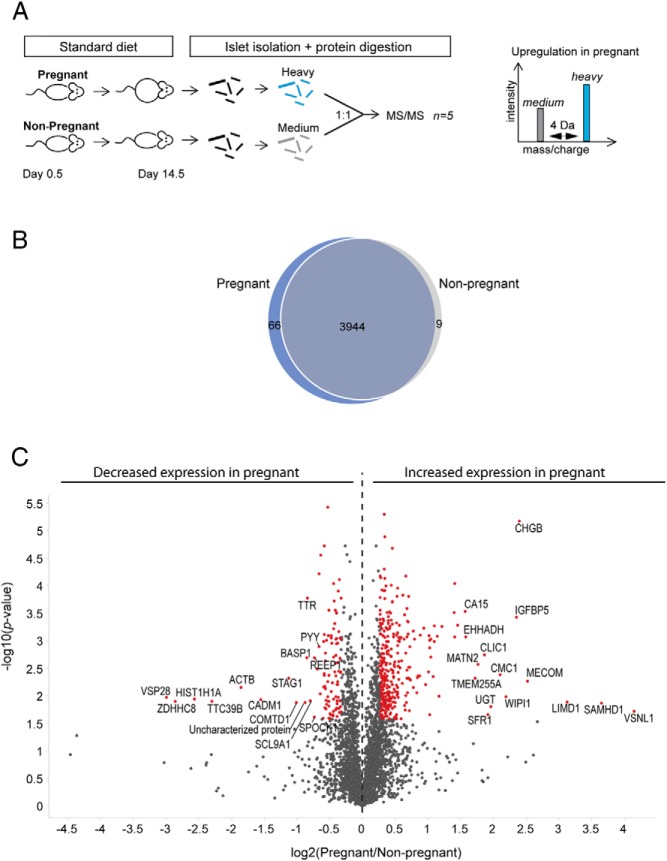
To compare the relative protein abundance between islets from pregnant and nonpregnant animals, we used dimethyl labeling ex vivo. As schematized on Figure 2A, islets were isolated from pregnant and nonpregnant littermates on day 14.5. The islets from each mouse were processed individually and tryptic peptides from pregnant and nonpregnant animals were labeled with heavy and medium labels, respectively. The 5 islet-samples from the pregnant mice were randomly paired with one of the islet-samples from the nonpregnant group before analysis with LC-MS/MS. The mass difference between the heavy and medium label allowed comparison of peptide abundance in the pregnant vs nonpregnant animals. Using this strategy, we identified 3944 islet proteins shared between pregnant and nonpregnant animals (Figure 2B). Among these, 427 proteins were significantly regulated more than 20% (Figure 2C, Table 3, and Supplemental Table 2). Within the group of up-regulated proteins, CHGB, IGFBP5, MATN2, EHHADH, IVD, and BMP1 have previously been associated with pregnancy-induced β-cell mass expansion at the RNA level (5, 6, 13, 21, 22). The confirmation of protein regulation of these candidates additionally validates the robustness of our approach. Furthermore, we found expression of the neuronal Ca2+ sensor visinin-like protein 1 to be increased by 17-fold in islets from pregnant mice. Although this protein has not previously been associated with pregnancy-induced β-cell mass expansion, it has been shown to enhance insulin secretion (23), which is a contributing factor in β-cell adaption to pregnancy (24).
Figure 2. Identification of relative protein abundance in islets at day 14.5 of pregnancy.
A, Relative protein abundance in islets of pregnant and nonpregnant mice at day 14.5 identified using dimethyl labeling ex vivo. Islet peptides from pregnant mice were labeled with heavy labels, whereas those from nonpregnant mice were labeled with medium labels. The peptides were subsequently mixed in a 1:1 ratio and analyzed using LC-MS/MS. For each MS run, the heavy to medium ratio was calculated and used as a measure of fold regulation. B, Venn diagram showing the total number of labeled proteins identified in the pregnant and nonpregnant group. C, The 5 average heavy to medium ratios were compared using a 1-sample t test. Log2(pregnant to nonpregnant) is plotted against −log10(P value) in a volcano plot. Significantly regulated proteins are mark in red, and the 15 most up- and down regulated proteins are tagged with gene names.
Table 3.
Proteins Differentially Regulated at Day 14.5 of Pregnancy
| Gene Name | Protein Name | Fold P/NP | P Value | FDR |
|---|---|---|---|---|
| Vsnl1 | Visinin-like protein 1 | 17.72 | .019 | + |
| Samhd1 | Deoxynucleoside triphosphate triphosphohydrolase SAMHD1 | 12.55 | .014 | + |
| Limd1 | LIM domain-containing protein 1 | 8.71 | .013 | + |
| Mecom; Prdm16 | MDS1 and EVI1 complex locus protein EVI1; PR domain zinc finger protein 16 | 5.74 | .005 | + |
| Chgb | Secretogranin-1; CCB peptide | 5.26 | <.001 | + |
| Igfbp5 | IGF-binding protein 5 | 5.10 | <.001 | + |
| Wipi1 | WD repeat domain phosphoinositide-interacting protein 1 | 4.57 | .011 | + |
| Cmc1 | COX assembly mitochondrial protein homolog | 4.29 | .004 | + |
| Ugt1a9; Ugt2b17; Ugt1a7c; Ugt1a6; Ugt1a2; Ugt1a1 | UDP-glucuronosyltransferase | 3.89 | .016 | + |
| Sfr1 | Swi5-dependent recombination DNA repair protein 1 homolog | 3.77 | .022 | + |
| Clic1 | Chloride intracellular channel protein 1 | 3.64 | .002 | + |
| Matn2 | Matrilin-2 | 3.40 | .003 | + |
| Tmem255a | Transmembrane protein 255A | 3.29 | .005 | + |
| Ehhadh | Peroxisomal bifunctional enzyme | 2.99 | .001 | + |
| Ca15 | Carbonic anhydrase 15 | 2.97 | <.001 | + |
| Vps28 | Vacuolar protein sorting-associated protein 28 homolog | 0.13 | .011 | + |
| Zdhhc8 | Probable palmitoyltransferase ZDHHC8 | 0.14 | .013 | + |
| Hist1h1a | Histone H1,1 | 0.17 | .011 | + |
| Ttc39b | Tetratricopeptide repeat protein 39B | 0.20 | .013 | + |
| Actb | Actin, cytoplasmic 1; Actin, cytoplasmic 1, N-terminally processed | 0.28 | .007 | + |
| Cadm1 | Cell adhesion molecule 1 | 0.34 | .012 | + |
| Stag1 | Cohesin subunit SA-1 | 0.46 | .005 | + |
| Comtd1 | Catechol O-methyltransferase domain-containing protein 1 | 0.50 | .013 | + |
| Unknown | Uncharacterized protein C19orf52 homolog | 0.55 | .013 | + |
| Pyy | Peptide YY | 0.56 | .002 | + |
| Ttr | Transthyretin | 0.56 | <.001 | + |
| Slc9a1 | Sodium/hydrogen exchanger 1 | 0.58 | .012 | + |
| Basp1 | Brain acid soluble protein 1 | 0.60 | .002 | + |
| Spock1 | Testican-1 | 0.60 | .025 | + |
| Reep6 | Receptor expression-enhancing protein 6 | 0.62 | .003 | + |
P values are calculated using a 1-sample t test. Significance was determined after multiple correction using Benjamini-Hochberg with a FDR of 15%. If significance was retained after correction, the proteins are marked with a + in the column FDR.
As for the proteins exhibiting increased de novo synthesis, the significantly regulated proteins showed enrichment and activation of the biological functions “protein synthesis” and “proliferation” (P values, 1.12E-14 and 3.74E-3; activation score, 1.9 and 1.4), when analyzed in IPA. However, in contrast to the set of proteins displaying increased de novo synthesis, significantly regulated proteins also showed mild coactivation of the biological functions “apoptosis” and “cellular death” (P values, 5.27E-4 and 2.24E-5; activation score, 0.2 and 0.5), suggesting that the maximal proliferative capacity is reached at gestational day 14.5.
Integrated transcriptome and proteome profiling of islets during gestation
In vivo pulsed SILAC and dimethyl labeling independently captures distinct aspects of protein expression dynamics. However, proteins showing increased heavy lysine incorporation are nevertheless expected to overlap with proteins that were up regulated at day 14.5. We therefore matched all significantly up regulated proteins in both datasets in order to minimize the risk of identifying false positive proteins. This led to the identification of 170 islet proteins which were significantly increased in both datasets (Figure 3A, * and **, Table 4, and Supplemental Table 3). To examine whether these proteins were also up-regulated at the transcriptional level, we generated a complementary RNA sequencing (RNAseq) dataset from islets isolated at day 14.5 of pregnancy (Supplemental Table 4). Among the proteins with increased heavy lysine incorporation and abundancy in pregnant mice, 52% were also found to be regulated at the RNA level (Figure 3A, **). Three dimensional plotting of the 20 most up-regulated within this group highlights MATN2, IGFBP5, CHGB, and IVD, as well as, the novel candidates CLIC1 and STMN1 as robust players involved in islet expansion (Figure 3B).
Figure 3. Integrated transcriptome and proteome profiling of islets during gestation.
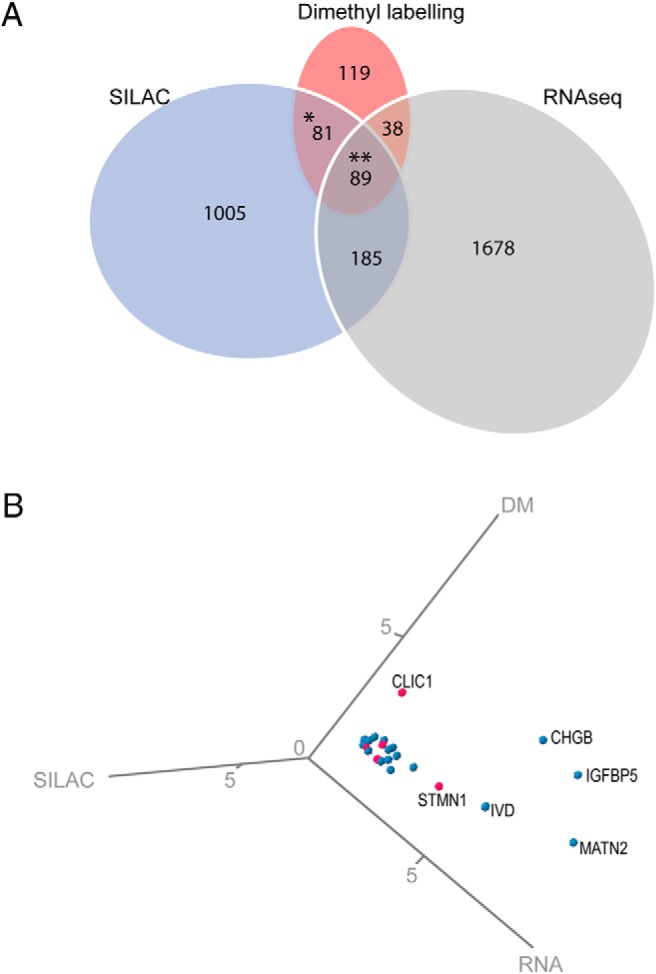
A, Venn diagram showing the overlap between significantly up-regulated candidates across the pulsed SILAC (SILAC), the dimethyl labeling (DM), and RNAseq (RNA) datasets. B, Three dimensional plot of the 20 most up-regulated candidates. The candidates not previously associated with pregnancy-induced β-cell mass expansion are marked in pink.
Table 4.
Proteins Showing Increased Synthesis and Expression in Islets of Pregnant Mice at Day 14.5
| Gene Name | Dimethyl |
SILAC |
RNAseq | Pregnancy Literature | β-Cell Transcriptome | β-Cell Enriched | ||
|---|---|---|---|---|---|---|---|---|
| P/NP | P Value | P/NP | P Value | |||||
| Samhd1 | 12.55 | .014 | 1.27 | .012 | + | Mouse | ||
| Chgb | 5.26 | <.001 | 1.30 | .002 | + | + | + | |
| Igfbp5 | 5.10 | <.001 | 1.47 | .012 | + | + | + | |
| Clic1 | 3.64 | .002 | 1.24 | .012 | + | |||
| Matn2 | 3.40 | .003 | 1.36 | .033 | + | + | + | Mouse |
| Ehhadh | 2.99 | .001 | Excl P | Excl P | + | + | + | |
| Ca15 | 2.97 | <.001 | 1.20 | .028 | ||||
| Ivd | 2.66 | <.001 | 2.49 | <.001 | + | + | + | |
| Bmp1 | 2.14 | .001 | 1.33 | <.001 | + | + | + | Mouse |
| Itga6 | 2.14 | .001 | 1.26 | .004 | + | + | + | Human |
| Stmn1 | 2.11 | .004 | 1.21 | .008 | + | |||
| Enpp2 | 2.06 | .001 | 1.22 | .003 | + | + | + | Mouse |
| Ern1 | 2.06 | .002 | 1.61 | .001 | + | + | + | |
| Mcm6 | 2.04 | .022 | 1.53 | <.001 | + | + | ||
| Dusp23 | 2.03 | .001 | 1.25 | .007 | + | + | + | |
| Ezr | 2.02 | .001 | 1.27 | .001 | + | + | + | Human |
| Prepl | 1.84 | .001 | 1.30 | .019 | + | + | + | Mouse |
| Syne1 | 1.84 | .004 | 2.02 | .010 | + | + | Mouse | |
| Fkbp11 | 1.80 | .004 | 1.54 | <.001 | + | + | + | |
| Hars | 1.78 | .009 | 1.25 | <.001 | + | + | + | Mouse |
| Ppib | 1.66 | .005 | 1.28 | <.001 | + | + | ||
| Erp29 | 1.64 | .019 | 1.29 | <.001 | + | + | + | |
| Srm | 1.63 | <.001 | 1.21 | .001 | + | + | ||
| Aldh9a1 | 1.61 | <.001 | 1.61 | <.001 | + | + | + | |
| Nedd4 | 1.61 | .001 | 1.21 | .005 | ||||
| Hltf | 1.60 | .005 | Excl P | Excl P | + | |||
| Nucb2 | 1.60 | <.001 | 1.35 | <.001 | + | + | + | Mouse |
| Pcsk1 | 1.58 | <.001 | 1.25 | .003 | + | + | + | Both |
| Esyt1 | 1.56 | <.001 | 1.32 | <.001 | + | + | Mouse | |
Proteins that were also significantly up-regulated at the RNA level at day 14.5 are marked with + in RNAseq. Proteins previously associated with islet expansion during pregnancy (5, 6, 13, 21) are marked with + in Pregnancy Literature, whereas the novel proteins are highlighted in bold. Proteins that are expressed at the RNA level in primary human or mouse β-cells (25) are marked with + in β-Cell transcriptome. Similarly, proteins reported to be enriched in β-cells vs non-β-islet cell types (25–28) are marked with species in β-Cell enriched. Excl P indicates proteins with heavy lysine incorporation exclusively in islets from pregnant mice. RNAseq P/NP-values and P values can be found in Supplemental Table 3.
The analyses on whole islet samples comprise detection of protein changes across all islet cell types. To identify genes predominantly expressed in β-cells, we developed a strategy using publicly available datasets. Genes known to be expressed in primary human or mouse β-cells were extracted from Benner et al (Table 4, β-cell transcriptome) (25). In addition, genes reported to exhibit enriched expression in primary β-cells in comparison with other islet cell types in either mice or human were extracted from the literature (Table 4, β-cell enriched) (25–28). Furthermore, our proteome data was compared with published transcriptome data on murine islets at day 12.5–15.5 of pregnancy (Table 4, pregnancy literature) (5, 6, 13, 21), which enabled us to identify proteins not previously associated with β-cell mass expansion during pregnancy (Table 4, bold). Among the newly identified and most regulated proteins (Table 4, bold) CLIC1, STMN1, MCM6, PPIB, NEDD4, and HLTF have been reported to enhance cell proliferation in other tissues (29–34). Although SAMDH1, MCM6, PPIB, HLTF, RPL13A, and ESYT1 are known to be expressed in β-cells; CLIC1 and STMN1 are not found in the β-cell transcriptome (Table 4, β-cell transcriptome) and could hence be up-regulated in either, other islet cell types or in β-cells only during pregnancy. Among the ten most regulated islet proteins, we identified a group, including CHGB, IGFBP5, MATN2, EHHADH, IVD, BMP1, and ITGA6, which were previously shown to be up-regulated at the RNA level in islets in response to pregnancy (Table 4, pregnancy literature) (5, 6, 13, 21). We confirm the biological relevance of these genes by detecting their regulation at the protein level. To validate the protein regulation presented in Table 4, Western blottings were performed for some of the candidates (Supplemental Figure 1).
Prediction of transcription factors mediating gestational β-cell mass expansion
To identify putative transcription factors responsible for the observed pregnancy-induced gene regulations in our 3 datasets, we analyzed all candidates which were significantly up-regulated by more than 20% using 2 complementary bioinformatic approaches. The Upstream Analysis tool in IPA (QIAGEN) provides extended biological insight by aligning the observed expression data to annotated relationships between transcription factors and targets genes. While IPA is mainly literature-based, oPUSSUM-3 is a sequence-based software developed to identify overrepresented transcription factor binding sites within coexpressed genes (35). Exploiting the power of 3 complementary datasets as well as the integration of these 2 softwares, we extracted putative transcription factors consistently enriched across all 3 datasets (Fisher P < .01). The transcription factors HNF4α, MYC, MYCN, NFE2L2, E2F1, and HNF1α passed these stringent criteria, suggesting that they play a central role in mediating pregnancy-induced β-cell mass expansion (Figure 4). Besides being classified as a maturity onset diabetes of the young susceptibility gene, HNF4α, has previously been shown to play a crucial role in β-cell mass expansion, because mice lacking HNF4α in β-cells fail to show β-cell mass adaption during pregnancy (36). The primary biological function of MYC, MYCN, and E2F1 is to promote cell cycle progression and cell growth. Whereas E2F1 knockout mice display impaired β-cell proliferation (37), mice with β-cell specific MYC overexpression force β-cells into cell cycle entry, but the phenotype manifests in acute β-cell loss and diabetes (38). This is consistent with the notion that appropriate β-cell mass expansion relies on mechanisms ensuring survival of replicating β-cells as discussed by Rieck and Kaestner (39). The putative transcription factor NFE2L2 could be one of such prosurvival factors, because it prevents the formation of toxic reactive oxidative species through its antioxidant actions also in β-cells (40). Collectively, the identification of 2 maturity onset diabetes of the young susceptibility genes, HNF4α and HNF1α, suggests that the rodent pregnancy model has translational value to diabetes pathogenesis in human.
Figure 4. Putative transcription factors (TFs) mediating β-cell mass expansion.
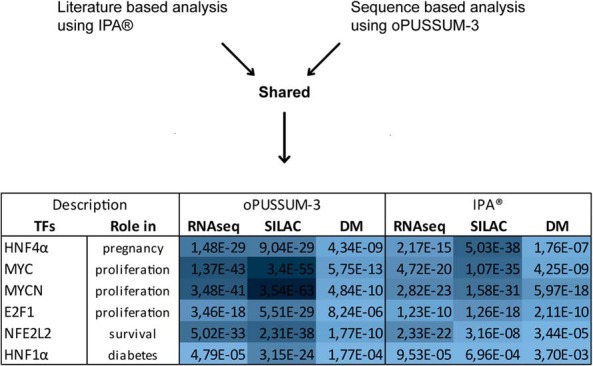
TFs that were overrepresented across all 3 datasets and their respective Fisher's extract test P values generated by oPUSSUM-3 and IPA as indicated. The blue gradient indicates the significance, with darker color highlighting the lowest P values.
Discussion
The aim of this study was to map the islet proteome during β-cell proliferation in vivo. Because pregnancy represents a physiological condition of adult β-cell mass expansion, we compared de novo protein synthesis, protein, and mRNA expression in islets of pregnant and nonpregnant mice. We identified 170 proteins that showed both increased de novo synthesis and increased protein expression at day 14.5 of pregnancy. In addition to known candidates, we identified several proteins which have not previously been associated with β-cell proliferation including SAMHD1, CLIC1, STMN1, MCM6, PPIB, NEDD4, and HLTF. As described in further detail below, most of these proteins are known to enhance cell growth in other tissues.
Together with the nuclear chloride ion channel 1 (CLIC1), we also observed significant up-regulation of CLIC4, CLIC5, and CLIC6 in at least 1 of the 3 datasets, potentially implicating a role for the entire CLIC-family in gestational β-cell mass adaptation. Clic1 is known to possess oncogenic potential and the protein is up regulated in several cancers, including pancreatic cancer (41). Furthermore, Clic6 mRNA is 75-fold enriched in sorted human β-cells when compared with α-cells (28). Notably, it has been suggested that the type 2 diabetes drug metformin targets and inhibits the proliferative effect of CLIC1 (42).
Likewise, we identified Stathmin (STMN1) and Stathmin2 (dimethyl dataset P = .0005; SILAC dataset P = .096) as being up regulated in islets from pregnant mice. Concordantly, these factors are known to play a growth-promoting role in pancreatic cancer (43). Furthermore, Stathmin2 is a selective marker for regenerating axons after nerve injury (44). Due to their secretory functions, neurons and β-cells show high similarity, which could suggest a potential role for the Stathmins, not only in β-cell mass expansion but also in regeneration.
Among the more general cell cycle factors up regulated islets of pregnant mice, we detected the DNA replication licensing factor MCM6, which is essential for cell replication as a key component of the prereplication complex activated by cyclin-dependent kinases (31, 45). This complex consists of MCM2–MCM7, of which all other MCMs were also found up-regulated in at least 2 of our 3 datasets.
Notably, the proteins PPIB and HLTF are associated not only with survival and proliferation but also with STAT5-independent prolactin signaling. PPIB is induced upon endoplasmic reticulum stress where it prevents cell death (46). Furthermore, PPIB has been shown to facilitate nuclear entrance of prolactin and to enhance nuclear prolactin-induced proliferation up to 9-fold (32). The helicase transcription factor, HLTF, is required for cell cycle progression in the developing mouse brain (34) and has been demonstrated to mediate prolactin-induced transcriptional regulation independently of STAT5 (47, 48). Thus, the combined up regulation of PPIB and HTLF proteins in islets of pregnant mice suggests that STAT5-independent functions of prolactin are involved in pregnancy-induced β-cell proliferation.
Among the most strongly regulated proteins, we identified a large protein group, including CHGB, IGFBP5, MATN2, EHHAHD, IVD, BMP1, IGTA6, EZR, and ALDH9A1, which have previously been found to be up-regulated at the RNA level in islets in response to pregnancy (Table 4, Pregnancy Literature) (5, 6, 13, 21). Because the field of proteomics is uniquely positioned in terms of its ability to confirm biological relevance, with proteins being the final determinant in translating a genés function, we believe that our study provides novel insight into the mechanisms mediating pregnancy-induced β-cell mass expansion. Our cross-complementary proteome and transcriptome analyses identified novel and robust factors involved in promoting β-cell mass expansion and function, which might be exploited as therapeutic targets for treating diabetes.
Materials and Methods
Mice
All mouse experiments were performed in accordance with the guidelines and recommendations provided by the Federation of European Laboratory Science Association. Ten-week-old virgin C57BL/6J female mice were mated with C57BL/6J males. The day of appearance of a vaginal mating-plug was designated as day 0.5 of pregnancy. Littermate virgin females were used as controls. For the in vivo pulsed SILAC labeling experiment, pregnant females were transferred from a conventional diet to the 13C6-lysine-substituted “SILAC diet” (Lys(6)-SILAC-Mouse Diet; Silantes) the same day a vaginal mating-plug was detected. The virgin nonpregnant control females were concurrently fed SILAC diet.
Pancreatic islet isolation
The duodenal opening of the common bile duct was clamped and the common bile duct was cannulated to inject 3 mL of ice-cold Liberase TL (Roche)/DNase I (BioNordica) enzyme solution. The inflated pancreata were incubated in 5 mL enzyme solution at 37°C for 18 minutes following shaking until the pancreatic tissue was dissociated. The enzyme action was stopped using serum-containing Hanks buffered salt solution. After washing, the digested pancreata were passed through a 100 μm, then a 70-μm cell strainer retaining islets and allowing multiple washing steps using Hanks buffered salt solution. Islets were handpicked twice before snap freezing. The islet yield was between 150 and 300 islets per mouse.
RNA isolation and sequencing
Total RNA from islets of pregnant and nonpregnant littermates (n = 3) was individually purified using RNeasy Mini kit (QIAGEN). The RNA 6000 Nano Lab Chip kit with a 2100 Bioanalyzer (Agilent Technologies) was used to determine RNA quality. High quality RNA (RNA integrity number > 7) was submitted for RNA sequencing at Beijing Genomics Institute, China.
Protein extraction and dimethyl labeling
For dimethyl labeling islets were lysed on ice using 0.5% RapiGest (Waters Corp) in 50mM triethylammonium bicarbonate buffer for 30 minutes. Lysates were cleared by centrifugation at 16 000g for 10 minutes. Protein concentration were determined using the Bradford protein assay. The protein was reduced by addition of 10mM dithiothereitol and incubation for 30 minutes at 50°C. Alkylation was done in the dark at room temperature by incubation with 45mM chloroacetamide for 2 hours. Digestion was preformed overnight at 37°C using 1 μg of trypsin. Digestion was stopped by incubation with 10% formic acid for 1 hour at 37°C. After centrifugation at 14 000g for 30 minutes, the supernantant was transferred to new tubes and acidified using 1% formic acid. Digested peptides were loaded onto a Sep-Pak C18 column (Waters corporation), which had been prewashed with 100% MeOH and 0.1% formic acid. After washing with 0.1% formic acid, samples from nonpregnant animals were labeled by addition of 5× 1 mL of intermediate labeling reagent (45mM sodium phosphate buffer [pH 7.5], 30mM NaBH3CN, and 0.2% D-formaldehyde), and samples from pregnant animals were labeled by addition of 5× 1 mL of heavy labeling reagent (45mM sodium phosphate buffer [pH 7.5], 30mM NaBH3CN, and 0.2% D-13C-formaldehylde). After additional washing in 0.1% formic acid, the samples were eluted using a solution of 60% acetonitrile and 0.5% acetic acid. The samples were dried down and reconstituted in 40% acetonitrile, 0.1% TFA. The samples from nonpregnant and pregnant animals were mixed 1:1 and fractionated using strong cation-exchange (SCX) fractionation as described below.
For the SILAC experiment proteins were lysed using 0.2% RapiGest in 50mM ammonium bicarbonate. The lysates were cleared by centrifugation at 16 000g for 10 minutes. Proteins were reduced by incubation in 5mM DTT for 30 minutes at 60°C. The proteins were then alkylated in 45mM chloroacetamide for 1 hour in the dark at room temperature. Digestion was preformed overnight at 37°C using 1 μg of Lys-C. Digestions were stopped by adding TFA to 0.5% and incubated for 30 minutes. The samples were centrifuged for 20 minutes at 16 000g, and the supernantant was transferred to a new tube, where acetonitrile was added to reach a final volume of 40%.
For both experiments, the samples were fractionated using SCX fractionation. The SCX microcolumn was made using 6 disks of empore SCX and prepared by washing with 100-μL 100% acetonitrile, 50 μL (pH 4.0), 50-μL (pH 8.5), and 100 μL 0.1% TFA/40% acetonitrile. After loading to the column the sample was eluted at pH 4.0, 4.5, 5.5, 6.5, 8.0, and 11. After elution, the samples were dried down and reconstituted in 100-μL 0.1% formic acid 1% TFA and loaded onto C18 Stagetips as described by Ref. 14.
Nano LC-MS/MS analysis
Before LC-MS/MS the samples were eluted from the C18 Stagetips using 2× 20-μL 40% acetonitrile/0.1% TFA, dried down, and reconstituted in 0.1% formic acid/1%TFA. The samples were loaded onto a 35-cm 75-μm ID Pico Tip emitter (New objective PF360-75-10-N-5) packed with ReproSil-Pur 120 C18-AQ 1.9 um (r119.aq, Dr Maisch). Peptides were separated during a 225-minute gradient with buffer B (90% acetonitrile and 0.1% formic acid) as mobile phase (7%–33%) and buffer A (0.1% formic acid) as stationary phase. The total time of the analysis was 250 minutes, including loading and washing of the column. An EASY LC-1000 system with a flow rate of 250 nL/min coupled to a Q-Exactive instrument was used for the analysis (Thermo Scientific). The data were acquired in a data dependent acquisition manner with a loop count of 12. The resolution in the full scan was 70000, automatic gain control target of 1E6, maximum IT 120 milliseconds, and 1 microscan. The MS/MS scans were recorded with a resolution of 35 000, automatic gain control target of 1E6, maximum IT 60 milliseconds, isolation window 1.2 m/z, normalized collision energy of 25, and underfill ratio 1.2% (intensity threshold of 2E5). The dynamic exclusion was set to 30 seconds.
MS search parameters
The MS-raw files were analyzed using MaxQuant 1.5.0.30. The MS/MS spectra were searched against the Swiss-Prot Mus musculus database (downloaded 8th of April 2014, 16 707 entries) using the MaxQuant associated search engine Andromeda. For the dimethyl labeling experiment, methionine oxidation/acetylation of peptide N termini and cysteine carbamidomethylation were set as variable and fixed modification, respectively. Trypsin/P was defined as the enzyme, and Lys4 and Lys8 were set as medium and heavy labels. Match between runs was used with a time window of 2 minutes. For the SILAC labeling experiment methionine oxidation/acetylation of peptide N termini and cysteine carbamidomethylation were set as variable and fixed modification, respectively. Lys/C was defined as the enzyme, and Lys6 was set as medium label. Match between runs was used with a time window of 0.7 minutes. For both datasets, the precursor mass tolerance for the main database search was 4.5 ppm, and the fragment ion mass tolerance was 20 ppm. Protein and peptide FDR was set at 1% with a minimum of 1 peptide of at least 7 amino acids. Resulting data were analyzed in Perseus 1.5.1.15 and filtered for “discard side directed,” “reverse,” and “potential contaminants.” Heavy to light ratios for SILAC and normalized heavy to medium ratios for dimethyl labeling were log2 transformed. To identify regulated proteins, 1- and 2-sample t tests using Benjamini-Hochberg correction with a FDR 15% were applied for the dimethyl labeling and SILAC, respectively.
Western blotting
Islets from each animal were lysed with 10 μL per 100 islets ice-cold lysis buffer (FNN001; Invitrogen), including protease inhibitor (11836153001; Roche). Lysates were clarified by centrifugation at 10 000g for 10 minutes at 4°C. Total protein was measured using Pierce Micro BCA protein assay (catalog number 23235). A total of 5 μg of protein was loaded on a 10% SDS-PAGE gel and blotted using an iBlot2 device (Invitrogen). The following primary antibodies were used: rabbit-anti-HLTF (1:500, A300-230A; Bethyl Laboratories), rabbit-anti-IVD (1:1000, 10822-1-AP; Proteintech), goat-anti-SFTPD (6 μg/mL, JYD011509A; R&D Systems), rabbit-anti-ALDH9A1 (1:1000; AT12391; Sigma), rabbit-anti-NEDD4 (1:1000, 07-049; Millipore), goat-anti-MCM6 (1:1000, sc-9843; Santa Cruz Biotechnology, Inc). The following secondary antibodies were used in a dilution of 1:5000: horseradish peroxidase (HRP)-linked goat-antirabbit IgG (sc2004; Santa Cruz Biotechnology, Inc) and HRP-linked bovine-antigoat IgG (sc2378; Santa Cruz Biotechnology, Inc).
Luminata Crescendo Western HRP Substrate (WBLUR0500) was used to detect chemiluminescence in a LAS 3000 (Fuji Film). Western blotting quantifications were performed by measuring band intensity with the ImageJ freeware.
Data availability
The full datasets from the dimethyl and SILAC experiment can be found in Supplemental Tables 5 and 6. MS raw files can be provided upon request. The full RNAseq dataset is available in Supplemental Table 7, and the raw files can be obtained through the Gene Expression Omnibus, accession number GSE75074.
Acknowledgments
We thank Mads Larsen for excellent technical assistance.
This work was supported by the Danish Ministry of Higher Education and Science and Novo Nordisk A/S.
Disclosure Summary: S.Hor., J.S.K., C.R., O.D.M., J.N.J., M.G., and J.A.-R. are employees at Novo Nordisk A/S. S.Hoe., P.A.S., J.H.N., and M.K. have nothing to disclose.
Funding Statement
This work was supported by the Danish Ministry of Higher Education and Science and Novo Nordisk A/S.
Footnotes
- FDR
- false discovery rate
- IPA
- Ingenuity Pathway Analysis
- SILAC
- stable isotope labeling of amino acids in cell culture
- LC-MS/MS
- liquid chromatography-mass spectrometry/mass spectrometry
- RNAseq
- RNA sequencing
- TFA
- trifluoroacetic acid
- SCX
- strong cation-exchange
- FDR
- false discovery rate
- HRP
- horseradish peroxidase.
References
- 1. Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol. 1978;85(11):818–820. [DOI] [PubMed] [Google Scholar]
- 2. Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. [DOI] [PubMed] [Google Scholar]
- 4. Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130(3):1459–1466. [DOI] [PubMed] [Google Scholar]
- 5. Rieck S, White P, Schug J, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23(10):1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schraenen A, de Faudeur G, Thorrez L, et al. mRNA expression analysis of cell cycle genes in islets of pregnant mice. Diabetologia. 2010;53(12):2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Søstrup B, Gaarn LW, Nalla A, Billestrup N, Nielsen JH. Co-ordinated regulation of neurogenin-3 expression in the maternal and fetal pancreas during pregnancy. Acta Obstet Gynecol Scand. 2014;93(11):1190–117. [DOI] [PubMed] [Google Scholar]
- 8. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. [DOI] [PubMed] [Google Scholar]
- 9. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29(6):301–307. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of β-cell mass during pregnancy. Endocrinology. 2009;150(4):1618–1626. [DOI] [PubMed] [Google Scholar]
- 11. Møldrup A, Petersen ED, Nielsen JH. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology. 1993;133(3):1165–1172. [DOI] [PubMed] [Google Scholar]
- 12. Hakonen E, Ustinov J, Palgi J, Miettinen PJ, Otonkoski T. EGFR signaling promotes β-cell proliferation and survivin expression during pregnancy. PLoS One. 2014;9(4):e93651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat Med. 2010;16(7):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75(3):663–670. [DOI] [PubMed] [Google Scholar]
- 15. Jacovetti C, Abderrahmani A, Parnaud G, et al. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J Clin Invest. 2012;122(10):3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheean ME, McShane E, Cheret C, et al. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28(3):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaglia T, Milan G, Ruhs A, et al. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest. 2014;124(6):2410–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krüger M, Moser M, Ussar S, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134(2):353–364. [DOI] [PubMed] [Google Scholar]
- 19. Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4(4):484–494. [DOI] [PubMed] [Google Scholar]
- 20. Aye T, Toschi E, Sharma A, Sgroi D, Bonner-Weir S. Identification of markers for newly formed β cells in the perinatal period: a time of recognized β cell immaturity. J Histochem Cytochem. 2010;58(4):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Layden BT, Durai V, Newman MV, et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J Endocrinol. 2010;207(3):265–279. [DOI] [PubMed] [Google Scholar]
- 22. Goyvaerts L, Lemaire K, Arijs I, et al. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes. PLoS One 2015;10(3):e0121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dai FF, Zhang Y, Kang Y, et al. The neuronal Ca2+ sensor protein visinin-like protein-1 is expressed in pancreatic islets and regulates insulin secretion. J Biol Chem. 2006;281(31):21942–21953. [DOI] [PubMed] [Google Scholar]
- 24. Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol. 2007;193(3):367–381. [DOI] [PubMed] [Google Scholar]
- 25. Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse β cells compared to human β cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nica AC, Ongen H, Irminger JC, et al. Cell-type, allelic, and genetic signatures in the human pancreatic β cell transcriptome. Genome Res. 2013;23(9):1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ku GM, Kim H, Vaughn IW, et al. Research resource: RNA-Seq reveals unique features of the pancreatic β-cell transcriptome. Mol Endocrinol. 2012;26(10):1783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54(11):2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valenzuela SM, Mazzanti M, Tonini R, et al. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529(pt 3):541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–250. [DOI] [PubMed] [Google Scholar]
- 31. Wei Z, Liu C, Wu X, et al. Characterization and structure determination of the cdt1 binding domain of human minichromosome maintenance (Mcm) 6. J Biol Chem. 2010;285(17):12469–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rycyzyn MA, Reilly SC, O'Malley K, Clevenger CV. Role of cyclophilin B in prolactin signal transduction and nuclear retrotranslocation. Mol Endocrinol. 2000;14(8):1175–1186. [DOI] [PubMed] [Google Scholar]
- 33. Amodio N, Scrima M, Palaia L, et al. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas. Am J Pathol. 2010;177(5):2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Helmer RA, Foreman O, Dertien JS, Panchoo M, Bhakta SM, Chilton BS. Role of helicase-like transcription factor (hltf) in the G2/m transition and apoptosis in brain. PLoS One. 2013;8(6):e66799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon AT, Arenillas DJ, Worsley Hunt R, Wasserman WW. oPOSSUM-3: advanced analysis of regulatory motif over-representation across genes or ChIP-Seq datasets. G3 (Bethesda). 2012;2(9):987–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta RK, Gao N, Gorski RK, et al. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev. 2007;21(7):756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fajas L, Annicotte JS, Miard S, Sarruf D, Watanabe M, Auwerx J. Impaired pancreatic growth, β cell mass, and β cell function in E2F1(−/−) mice. J Clin Invest. 2004;113(9):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laybutt DR, Weir GC, Kaneto H, et al. Overexpression of c-Myc in β-cells of transgenic mice causes proliferation and apoptosis, downregulation of insulin gene expression, and diabetes. Diabetes. 2002;51(6):1793–1804. [DOI] [PubMed] [Google Scholar]
- 39. Rieck S, Kaestner KH. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21(3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yagishita Y, Fukutomi T, Sugawara A, et al. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2014;63(2):605–618. [DOI] [PubMed] [Google Scholar]
- 41. Lu J, Dong Q, Zhang B, et al. Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol. 2015;32(6):616. [DOI] [PubMed] [Google Scholar]
- 42. Gritti M, Würth R, Angelini M, et al. Metformin repositioning as antitumoral agent: selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget. 5(22):11252–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schimmack S, Taylor A, Lawrence B, et al. Stathmin in pancreatic neuroendocrine neoplasms: a marker of proliferation and PI3K signaling. Tumour Biol. 2015;36(1):399–408. [DOI] [PubMed] [Google Scholar]
- 44. Shin JE, Geisler S, DiAntonio A. Dynamic regulation of SCG10 in regenerating axons after injury. Exp Neurol. 2014;252:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kneissl M, Pütter V, Szalay AA, Grummt F. Interaction and assembly of murine pre-replicative complex proteins in yeast and mouse cells. J Mol Biol. 2003;327(1):111–128. [DOI] [PubMed] [Google Scholar]
- 46. Kim J, Choi TG, Ding Y, et al. Overexpressed cyclophilin B suppresses apoptosis associated with ROS and Ca2+ homeostasis after ER stress. J Cell Sci. 2008;121(pt 21):3636–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helmer RA, Panchoo M, Dertien JS, Bhakta SM, Hewetson A, Chilton BS. Prolactin-induced Jak2 phosphorylation of RUSH: a key element in Jak/RUSH signaling. Mol Cell Endocrinol. 2010;325(1–2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hewetson A, Moore SL, Chilton BS. Prolactin signals through RUSH/SMARCA3 in the absence of a physical association with Stat5a. Biol Reprod. 2004;71(6):1907–1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full datasets from the dimethyl and SILAC experiment can be found in Supplemental Tables 5 and 6. MS raw files can be provided upon request. The full RNAseq dataset is available in Supplemental Table 7, and the raw files can be obtained through the Gene Expression Omnibus, accession number GSE75074.