Abstract
Recently, several LH/human chorionic gonadotropin (hCG) receptor-independent activities for hCG have been described, including activation of the TGF-β receptor (TGFβR) by hyperglycosylated hCG and stimulation of trophoblast invasion. Because the hCG concentrations used in these studies have been rather high, reflecting physiological hCG levels in pregnancy, even a minor contamination with growth factors, which act at very low concentrations, may be significant. Several commercial hCG preparations have been found to contain significant amounts of epidermal growth factor (EGF), which we also confirmed here. Furthermore, we found that some hCG preparations also contain significant amounts of TGF-β1. These hCG preparations were able to activate ERK1/2 in JEG-3 choriocarcinoma cells or TGFβR in mink lung epithelial cells transfected with a reporter gene for TGFβR activation. No such activation was found with highly purified hCG or its free β-subunit (hCGβ), irrespective of whether they were hyperglycosylated or not. Taken together, our results suggest that the growth factor contaminations in the hCG preparations can cause activation of TGFβR and, at least in JEG-3 cells, MAPK signaling. This highlights the importance to carefully control for potential contaminations and that highly purified hCG preparations have to be used for biological studies.
Human chorionic gonadotropin (hCG) is a heterodimeric glycoprotein hormone consisting of an α- and a β-subunit (hCGα and hCGβ) that are noncovalently linked. The α-subunit is identical to those in other glycoprotein hormones, ie, LH, FSH, and TSH. Although the free subunits do not activate the LH/hCG receptor (LHCGR) (1, 2), intact hCG binds to the LHCGR in the corpus luteum, stimulating progesterone production, which is essential for successful pregnancy (3). It has been suggested that the action of hCG is modulated by glycosylation, the so called hyperglycosylated hCG (hCG-h) being more active in stimulating trophoblast invasion (4). However, we have not found large differences between different hCG isoforms in this respect (5). Interestingly, hCGβ also stimulated trophoblast invasion, indicating that the activity is not mediated by LHCGR. Indeed, several LHCGR-independent activities for hCG, hCG-h, and hCGβ have recently been described. In addition to proinvasive properties of hCGβ on trophoblast (5) and prostate carcinoma cells (6), free hCGβ has been suggested to stimulate the growth of cancer cells (7, 8). hCG has also been reported to possess proangiogenic activity, which has been suggested to be mediated either by LHCGR (9) or, in case of hCG-h, by the TGF-β receptor (TGFβR) (10). hCG has also been shown to stimulate ERK1/2 phosphorylation, ie, activation of the MAPK signaling pathway, in various cell types, including placental cells (11–14). This appears to be mediated by the LHCGR (12, 15). Because the hCG concentrations used in these studies have been rather high, reflecting physiological hCG levels in pregnancy (16), even a relatively minor contamination with growth factors, which act at very low concentrations, may be significant. We have therefore studied, using both fairly crude and highly purified hCG and hCGβ preparations, whether these are able to activate TGFβR or ERK1/2. Our results suggest that the activation of TGFβR and, at least in JEG-3 cells, MAPK signaling by hCG or its hyperglycosylated form are caused by growth factor contaminations in the hCG preparations used.
Materials and Methods
hCG preparations
Various forms of hCG and hCGβ were purified by immunoaffinity chromatography as described earlier (Table 1) (5, 17). Details of the purification and the carbohydrate structure of most of the hCG preparations used have been described (17). The commercial urinary hCG preparation Pregnyl (Organon) was also used as such or for affinity purification of hCG-h and isolation of hCGβ. For hCGβ isolation Pregnyl (15 × 5000 IU, ie, ∼220 nmol) was dissolved in 4 mL of 4M potassium thiocyanate (KSCN) to dissociate α- and β-subunits, which were then separated by gel filtration chromatography, first using a Sephacryl S-200 column in the presence of 4M KSCN and then, after concentrating the sample (Amicon Ultra 10 kDa concentrator), using Sephacryl S-100M and 0.1M NH4HCO3 for elution. Concanavalin-A (Con-A) Sepharose affinity chromatography purification of hCG from human choriocarcinoma JEG-3 cell (HTB-36; ATCC) conditioned culture medium, containing 5% fetal bovine serum or no serum supplement, was performed as described (10). For Con-A purified hCG, the buffer was changed to DMEM (Lonza). Other hCG preparations were freeze dried in 0.1M ammonium bicarbonate (pH 8) and stored at −20°C until dissolved in cell culture medium for the bioassays.
Table 1.
hCG and hCGβ Preparations Used for TGFβR and ERK1/2 Activation Studies
| hCG (Source) | hCG (nM)a | hCGβ (nM)a | hCG(β)-h (%)a | TGF-β1 (ng/L)b | EGF (ng/L)b | TGFβR activationc | pERKd |
|---|---|---|---|---|---|---|---|
| 1. hCG (PW 6)e | 93.1 | 6.9 | 20 | n.d. | n.d. | n.d. | - |
| 2. hCG (Pregnyl)e | 89.7 | 10.3 | 0 | n.d. | n.d. | n.d. | - |
| 3. hCG (PW 36 + 5)e | 98.7 | 1.3 | 0 | n.d. | n.d. | n.d. | - |
| 4. hCG-h (PW 5)e | 96.5 | 3.5 | ∼100 | n.d. | n.d. | n.d. | - |
| 5. hCG-h (PW 5)e | 92.1 | 7.9 | ∼100 | n.d. | 5.5 | n.d. | - |
| 6. hCG-h (Pregnyl)e | 99.0 | 1.0 | ∼100 | n.d. | 6.2 | n.d. | - |
| 7. hCG-h (TCa)e | 97.0 | 3.0 | 53 | n.d. | n.d. | n.d. | - |
| 8. hCGβ (Pregnyl)g | 1.5 | 98.5 | 7 | n.d.b | 1533 | 11 ± 5 | + |
| 9. hCG(β)-h (JEG-3)e | 52.3 | 47.7 | 51 | n.d. | n.d. | n.d. | - |
| 10. hCGβ-h (TCa)e | 13.0 | 87.0 | 40 | n.d. | 108 | n.d. | + |
| 11. Con-A hCG-h (JEG-3)f | 89.2 | 10.8 | 80 | 81b | n.d. | 101 ± 26 | - |
| 12. Con-A hCG-h (JEG-3)f | 93.2 | 6.8 | ∼100 | 106b | 182 ± 35 | ||
| 13. Pregnyl | 93.9 | 6.1 | 14 | n.d. | 485 | n.d. | + |
PW, pregnancy week; TCa, nonseminomatous germ cell tumor of the testis; JEG-3, conditioned JEG-3 cell culture medium; Con-A, purified by Con-A Sepharose; n.d., the values were below the lowest calibrator.
Molar concentration of hCG and hCGβ, and percent of hCG-h + hCGβ-h (hCG(β)-h) (the percentage of hyperglycosylated forms is indicative as the assay recognizes hCGβ-h with somewhat lower affinity than hCG-h).
TGF-β1 (without acid activation) and EGF concentrations in 100nM hCG + hCGβ. After acid activation, hCGβ (Pregnyl) contained 50.3 ng/L of TGF-β1, and Con-A-purified hCG-h (JEG-3) from cell culture media with (11) and without (12) serum supplement 4570 and 1430 ng/L of TGF-β1, respectively. Samples in which TGF-β1 concentration was below the lowest calibrator (n.d.) even after acid activation were reanalyzed after acid activation at 20-fold higher concentration of hCG + hCGβ and were still found to be below the lowest calibrator. The lowest calibrator in the TGF-β1 assay was 31.2 ng/L and in EGF assay, 3.9 ng/L; 81 ng/L of TGF-β1 corresponds to 3.2pM (assuming a molecular weight of 25 000), whereas 100-ng/L EGF corresponds to 17pM (assuming a molecular weight of 6000).
Activation of TGFβR by different hCG and hCGβ preparations. The values correspond to the signal obtained with TGF-β1 calibrator (ng/L). hCG + hCGβ concentration was 100nM. The lowest TGF-β1 calibrator was 8.3 ng/L. The results show mean ± SD of 2–3 separate assays with duplicate or triplicate samples.
Induction of ERK1/2 phosphorylation by 120nM hCG + hCGβ in JEG-3 cells (Figure 2).
Purified by antibody affinity chromatography.
Purified by Con-A chromatography. The cell culture medium contained (11) or did not contain (12) serum supplement. The preparations are derived from different batches of the cells and were analyzed separately.
Isolated from KSCN treated Pregnyl by gel filtration.
Immunoassays
The content of hCG, hCGβ, and their hyperglycosylated forms was analyzed by specific immunoassays (5). TGF-β1 (with and without acid activation of latent TGF-β) and epidermal growth factor (EGF) were determined by the Human TGF-β1 and EGF Quantikine ELISAs, respectively (both from R&D Systems, Inc).
Detection of ERK1/2 phosphorylation
JEG-3 cells were cultured in EMEM (BE12–125F; Lonza) supplemented with 10% fetal calf serum, 100-IU/mL penicillin, 100-μg/mL streptomycin, and 2mM L-glutamine at 37°C in a humidified atmosphere of 5% CO2 in air. JEG-3 cells were washed with PBS (Lonza) and then incubated in serum-free medium with different hCG and hCGβ preparations (120nM, some of the preparations were also tested with 0.1nM, 1nM, 10nM, and 100nM hCG or hCGβ) or EGF (1 μg/L) (recombinant human EGF; ScienCell Research Laboratories) for 15 minutes at +37°C. Some of the cells were preincubated with 1.4μM EGF receptor (EGFR) inhibitor PD 153035 hydrochloride (Tocris Bioscience) for 15 minutes. The cells were lysed in the presence of Lysis buffer 6 (R&D Systems, Inc) on ice and boiled in reducing sodium dodecyl sulfate sample buffer. Western blotting was carried out essentially as described (18), but the blots were blocked with 5% nonfat dry milk and antibodies to pERK1/2 (phospho-p44/42 MAPK) and ERK1/2 (4376S and 9102S, respectively, both from Cell Signaling Technology) were used.
TGFβR activation
Mink lung epithelial cells stably transfected with a fragment of the plasminogen activator inhibitor-1 (PAI-1) promoter fused to the luciferase gene were provided by D. B. Rifkin (New York University School of Medicine, New York, NY). These cells produce luciferase in response to TGF-β1. The assay for TGFβR activation was performed as described previously (19) in DMEM supplemented with 100-IU/mL penicillin, 100-μg/mL streptomycin, and 2mM L-glutamine. The cells were treated for 18 hours at 37°C with different hCG and hCGβ preparations (100nM, some of the preparations were also tested with 1nM–2000nM hCG). TGF-β1 (recombinant human TGF-β1 [240-B-002] from R&D Systems, Inc) was used as a standard. The specificity was controlled by preincubating the samples for 20 minutes at room temperature with 5-μg/mL neutralizing TGF-β1 antibody (AB-101-NA; R&D Systems, Inc).
Ethical aspects
Informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the Helsinki University Central Hospital.
Results
Con-A purification of hCG
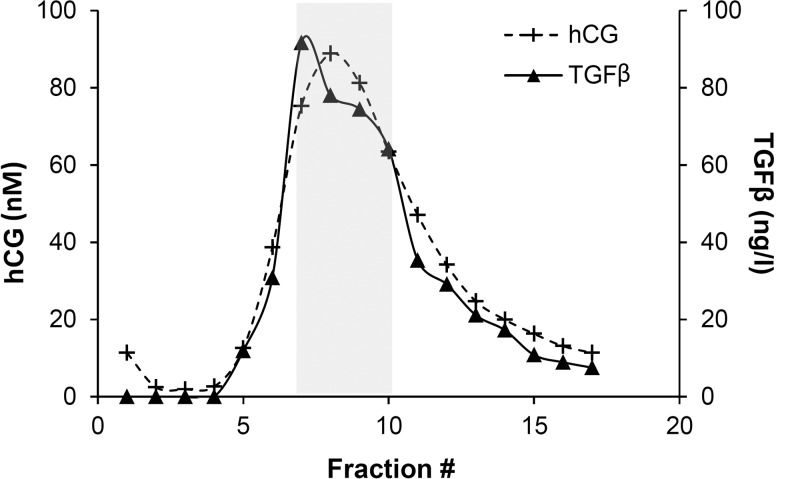
hCG-h was purified from conditioned JEG-3 cell culture medium by Con-A affinity chromatography as described by Berndt et al (10). hCG-h coeluted with TGF-β1 in a single broad peak (Figure 1).
Figure 1. Coelution of hCG and TGF-β1 from Con-A column.
The gray area shows the fractions pooled for the analyses of growth factor contaminations, and TGFβR and ERK1/2 activation (preparation 11, similar results were obtained with cell culture media used for preparation 12).
Growth factor contaminations in hCG preparations
Several different hCG and hCGβ preparations were analyzed for EGF and TGF-β1 contamination at 100nM hCG + hCGβ concentration. hCG and hCGβ purified by immunoaffinity chromatography from urine and conditioned JEG-3 cell culture medium did not contain detectable amounts of TGF-β1 even at 20-fold higher concentrations of hCG (2μM) and after acid activation of latent TGF-β1 (Table 1). However, hCG purified from the JEG-3 cell culture medium with and without serum supplement by Con-A chromatography (preparations 11 and 12 in Table 1) were found to contain 81 and 106 ng/L of TGF-β1 (4.6 and 1.4 μg/L after acid activation), respectively. After acid activation, a preparation of hCGβ (8) isolated from Pregnyl by dissociation of hCGα and hCGβ and separation of these by gel filtration chromatography contained 50.3 ng/L of TGF-β1. The same preparation contained 1.5-μg/L EGF. Most of the immunoaffinity-purified hCG preparations did not contain detectable amounts of EGF. However, hCGβ-h immunopurified from urine of a testis cancer patient (10) contained 108 ng/L of EGF, and very low EGF concentrations (<10 ng/L) were found in some other immunoaffinity-purified hCG preparations (5 and 6). Pregnyl (13) contained significant amount (485 ng/L) of EGF (Table 1).
ERK1/2 activation
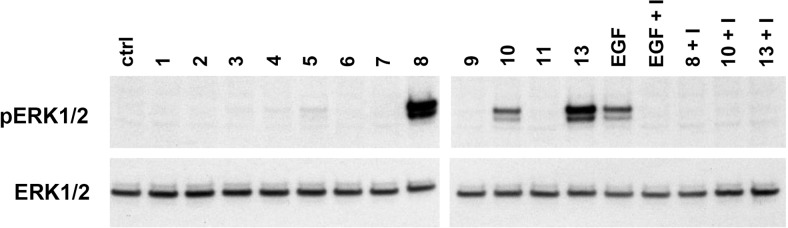
Although hCG or hCGβ preparations (8, 10, and 13), containing more than 100-ng/L EGF, induced phosphorylation of ERK1/2, highly purified hCG or hCGβ preparations did not activate these signaling proteins at any tested concentration (Figure 2). At a concentration of 1 μg/L, EGF used as a control induced clear ERK1/2 phosphorylation. In all cases, ERK1/2 phosphorylation was abolished by addition of the EGFR inhibitor, indicating that ERK1/2 activation was mediated by EGFR activation.
Figure 2. Total ERK1/2 and phosphorylated ERK1/2 levels in JEG-3 cells treated by different hCG and hCGβ preparations (120nM).
The numbers refer to preparation numbers in the Table 1. EGF (1 μg/L) was used as a control. In all cases, the phosphorylation was prevented by EGFR inhibitor PD 153035 hydrochloride (I).
Activation of TGFβR
Highly purified hCG or hCGβ were not able to activate TGFβR in reporter cells. However, Con-A-purified hCG from conditioned JEG-3 cell culture medium with and without serum supplement (11 and 12) activated the TGFβR reporter. The activation corresponded to that obtained with 101 ± 26- and 182 ± 35-ng/L TGF-β1 (n = 3 and 2), respectively, which was in line with the content of TGF-β1 measured without acid activation, ie, the content of active TGF-β1 (81 and 106 ng/L). Addition of TGF-β1 antibody (n = 2) completely abolished the activity of the hCG preparation (12) and TGF-β1 standard containing 142-ng/L TGF-β1.
Discussion
Recently, hCG-h, purified by Con-A affinity chromatography from JEG cells, was reported to possess proangiogenic activity, which is mediated by the activation of TGFβR (10). We used here the same purification method as used by Berndt et al (10) and found that this hCG preparation activated TGFβR. However, this preparation contained TGF-β1 that coeluted with hCG-h and the TGFβR activation was abolished by a neutralizing TGF-β1 antibody. None of the highly purified hCG or hCGβ preparations, including different hCG-h preparations, were found to contain TGF-β1 or to activate TGFβR. Thus, our results do not support the notion that hCG-h activates TGFβR.
EGF has been found to be a contaminant in several commercial hCG preparations, like Pregnyl (21, 22). EGF stimulates the MAPK pathway, including ERK1/2 phosphorylation. Therefore, we addressed ERK1/2 phosphorylation using the same hCG and hCGβ preparations that were used to study TGFβR activation. EGF contamination was more frequently observed than that of TGF-β1. Although some immunoaffinity-purified preparations contained EGF, the preparations that were less extensively purified contained large amounts of EGF and, unlike the hCG and hCGβ preparations that did not contain EGF, they were found to induce ERK1/2 phosphorylation. Thus, it is unlikely that hCG or hCGβ activate ERK1/2 in JEG-3 cells. Contrary to our findings, several papers have described stimulation of ERK1/2 phosphorylation in various cell types by different hCG preparations, including recombinant hCG (11–14). Curiously, in some, but not all, studies, this has been observed only in a relatively narrow range of hCG concentrations, around 0.1nM–10nM, but not with significantly higher or lower concentrations (14). However, we did not observe ERK1/2 phosphorylation in JEG-3 cells treated at a wide concentration range of hCG or hCGβ that did not contain EGF. Although ERK1/2 activation by hCG may take place in some cell lines, one has to be cautious when interpreting results obtained with impure hCG preparations, especially when used at high concentrations. It is noteworthy, that the observed contaminations with TGF-β1, EGF (21, 22), and other factors (23, 24), do not explain all the suggested LHCGR independent activities of hCG and hCGβ, like stimulation of trophoblast invasion (5).
Although the recombinant hCG and hCGβ preparations, usually produced in CHO cells, probably contain less impurities, these may also contain various growth factors unless thoroughly purified. Furthermore, although the potency of recombinant hCG preparations to evoke responses mediated via LHCGR is similar to that observed with human urine-derived hCG preparations (25), their glycan structures are different (17, 26), and as such, recombinant hCG is not suitable for studies aiming at solving the effect of glycans on hCG activity.
In conclusion, our results show that the activation of TGFβR and, at least in JEG-3 cells, MAPK signaling by hCG or hCGβ, or their hyperglycosylated forms observed in earlier studies are caused by growth factor contamination in the preparations used. For studies on growth factor signaling mechanisms it is of utmost importance that potential contaminations are carefully controlled. Highly purified hCG preparations should be used for bioassays, bearing in mind that the glycosylation may be different in recombinant forms. Furthermore, for studies on the activities of different hCG isoforms or subunits, thorough characterization of these is essential.
Acknowledgments
We thank Ms Taina Grönholm and Ms Annikki Löfhjelm for excellent technical assistance.
This work was supported by the grants from Helsinki University Central Hospital, the Finnish Cancer Foundation, the Academy of Finland, Sigrid Jusélius Foundation, Finska Läkaresällskapet, Magnus Ehrnrooth Foundation, K. Albin Johansson Foundation, and Biomedicum Helsinki Foundation.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the grants from Helsinki University Central Hospital, the Finnish Cancer Foundation, the Academy of Finland, Sigrid Jusélius Foundation, Finska Läkaresällskapet, Magnus Ehrnrooth Foundation, K. Albin Johansson Foundation, and Biomedicum Helsinki Foundation.
Footnotes
- Con-A
- concanavalin-A
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- hCG
- human chorionic gonadotropin
- hCG-h
- hyperglycosylated hCG
- LHCGR
- LH/hCG receptor
- TGFβR
- TGF-β receptor.
References
- 1. Catt KJ, Dufau ML, Tsuruhara T. Absence of intrinsic biological activity in LH and hCG subunits. J Clin Endocrinol Metab. 1973;36:73–80. [DOI] [PubMed] [Google Scholar]
- 2. Ryan RJ, Charlesworth MC, McCormick DJ, Milius RP, Keutmann HT. The glycoprotein hormones: recent studies of structure-function relationships. FASEB J. 1988;2:2661–2669. [DOI] [PubMed] [Google Scholar]
- 3. Yoshimi T, Strott CA, Marshall JR, Lipsett MB. Corpus luteum function in early pregnancy. J Clin Endocrinol Metab. 1969;29:225–230. [DOI] [PubMed] [Google Scholar]
- 4. Handschuh K, Guibourdenche J, Tsatsaris V, et al. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-γ. Endocrinology. 2007;148:5011–5019. [DOI] [PubMed] [Google Scholar]
- 5. Lee CL, Chiu PC, Hautala L, et al. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol Cell Endocrinol. 2013;375:43–52. [DOI] [PubMed] [Google Scholar]
- 6. Wu W, Walker AM. Human chorionic gonadotropin β (HCGβ) down-regulates E-cadherin and promotes human prostate carcinoma cell migration and invasion. Cancer. 2006;106:68–78. [DOI] [PubMed] [Google Scholar]
- 7. Gillott DJ, Iles RK, Chard T. The effects of β-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer. 1996;73:323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole LA, Butler S. Hyperglycosylated hCG, hCGβ and hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol. 2012;349:232–238. [DOI] [PubMed] [Google Scholar]
- 9. Berndt S, Blacher S, Perrier d'Hauterive S, et al. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab 2009;94:4567–4574. [DOI] [PubMed] [Google Scholar]
- 10. Berndt S, Blacher S, Munaut C, et al. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013;27:1309–1321. [DOI] [PubMed] [Google Scholar]
- 11. Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knöfler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiraishi K, Ascoli M. Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology. 2007;148:3214–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maymo JL, Perez Perez A, Maskin B, et al. The alternative Epac/cAMP pathway and the MAPK pathway mediate hCG induction of leptin in placental cells. PLoS One. 2012;7:e46216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casarini L, Lispi M, Longobardi S, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One. 2012;7:e46682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirakawa T, Galet C, Ascoli M. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR): a novel experimental paradigm to study the functional properties of the hLHR. Endocrinology. 2002;143:1026–1035. [DOI] [PubMed] [Google Scholar]
- 16. Jauniaux E, Bao S, Eblen A, et al. HCG concentration and receptor gene expression in placental tissue from trisomy 18 and 21. Mol Hum Reprod. 2000;6:5–10. [DOI] [PubMed] [Google Scholar]
- 17. Valmu L, Alfthan H, Hotakainen K, Birken S, Stenman UH. Site-specific glycan analysis of human chorionic gonadotropin β-subunit from malignancies and pregnancy by liquid chromatography–electrospray mass spectrometry. Glycobiology. 2006;16:1207–1218. [DOI] [PubMed] [Google Scholar]
- 18. Mattsson JM, Ravela S, Hekim C, et al. Proteolytic activity of prostate-specific antigen (PSA) towards protein substrates and effect of peptides stimulating PSA activity. PLoS One. 2014;9(9):e107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. [DOI] [PubMed] [Google Scholar]
- 20. Oida T, Weiner HL. Depletion of TGF-β from fetal bovine serum. J Immunol Methods. 2010;362:195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saleh L, Prast J, Haslinger P, Husslein P, Helmer H, Knöfler M. Effects of different human chorionic gonadotrophin preparations on trophoblast differentiation. Placenta. 2007;28:199–203. [DOI] [PubMed] [Google Scholar]
- 22. Yarram SJ, Jenkins J, Cole LA, Brown NL, Sandy JR, Mansell JP. Epidermal growth factor contamination and concentrations of intact human chorionic gonadotropin in commercial preparations. Fertil Steril. 2004;82:232–233. [DOI] [PubMed] [Google Scholar]
- 23. Van Dorsselaer A, Carapito C, Delalande F, et al. Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS One. 2011;6:e17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kauffman HF, Hovenga H, de Bruijn HW, Beintema JJ. Eosinophil derived neurotoxin (EDN) levels in commercial human urinary preparations of glycoprotein hormones. Eur J Obstet Gynecol Reprod Biol. 1999;82:111–113. [DOI] [PubMed] [Google Scholar]
- 25. Stenman UH, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. 2006;12:769–784. [DOI] [PubMed] [Google Scholar]
- 26. Gervais A, Hammel YA, Pelloux S, et al. Glycosylation of human recombinant gonadotrophins: characterization and batch-to-batch consistency. Glycobiology. 2003;13:179–189. [DOI] [PubMed] [Google Scholar]