Abstract
Chromogranin A (ChgA) is an acidic protein found in large dense-core secretory vesicles and generally considered to be expressed in all enteroendocrine cells of the gastrointestinal (GI) tract. Here, we characterize a novel reporter mouse for ChgA, ChgA-humanized Renilla reniformis (hr)GFP. The hrGFP reporter was found in the monoamine-storing chromaffin cells of the adrenal medulla, where ChgA was originally discovered. hrGFP also was expressed in enteroendocrine cells throughout the GI tract, faithfully after the expression of ChgA, as characterized by immunohistochemistry and quantitative PCR analysis of fluorescence-activated cell sorting-purified cells, although the expression in the small intestine was weak compared with that of the stomach and colon. In the stomach, hrGFP was highly expressed in almost all histamine-storing enterochromaffin (EC)-like cells, at a lower level in the majority of serotonin-storing EC cells and ghrelin cells, in a small fraction of somatostatin cells, but was absent from gastrin cells. In the small intestine, the hrGFP reporter was selectively, but weakly expressed in EC cells, although not in any peptide-storing enteroendocrine cells. In the colon, hrGFP was exclusively expressed in EC cells but absent from the peptide-storing enteroendocrine cells. In contrast, in the pancreas, hrGFP was expressed in β-cells, α-cells, and a fraction of pancreatic polypeptide cells. It is concluded that ChgA-hrGFP in the GI tract functions as an effective reporter, particularly for the large populations of still poorly characterized monoamine-storing enteroendocrine cells. Furthermore, our findings substantiate the potential function of ChgA as a monoamine-binding protein that facilitates the regulated endocrine secretion of large amounts of monoamines from enteroendocrine cells.
Chromogranin A (ChgA) is an acidic glycoprotein found in large dense-core vesicles of the regulated secretory pathway (1, 2). A number of other vesicular proteins sharing physiochemical properties with ChgA have been identified, giving rise to a family of “granins,” which includes chromogranin B (ChgB), secretogranin II, secretogranin III, HISL-19 antigen (SgIV), 7B2 (SgV), NESP55 (SgVI), VGF nerve factor inducible (VGF, SgVII), and pro-SAAS (SgVIII) (3). Although many different functions have been proposed for granins and granin-derived peptides, their mechanisms of action and physiological importance in most cases still remain to be elucidated (3). ChgA and other granins are thought to act as precursors for smaller peptide fragments; however, potential receptors, downstream signaling pathways, and biological activities remain unknown (3, 4). They have also been implicated in the actual formation of the large, dense-core secretory vesicles and in the sorting of proteins to the regulated secretory pathway (5). Most convincingly, the large, acidic ChgA protein has been proposed to function as an osmotic regulator, binding large amounts of solutes to facilitate the accumulation of monoamines within the dense-core secretory vesicles of the endocrine cells. This function as a storage capacity generating protein has in particular been advocated for ChgA in the adrenaline-storing chromaffin cells of the adrenal medulla (2, 6).
ChgA specifically localizes to peptide hormone and monoamine-storing endocrine cells within the gastrointestinal (GI) tract and was therefore early on accepted as a general marker for enteroendocrine cells as such (7–10), which is still often used as. However, although colocalization of ChgA and enteroendocrine peptide hormones has been shown to a variable degree for nearly all known gut hormones, a large degree of heterogeneity has been noted among species and GI regions (11, 12). Technical variations between studies aside, differential posttranslational processing of the ChgA proprotein in different cell types could contribute to the observed differences in ChgA colocalization (13, 14). In contrast to the inconsistent overlap between ChgA and peptide hormone expression, ChgA has consistently been associated with enteroendocrine cells producing and secreting monoamines, ie, the histamine producing enterochromaffin (EC)-like (ECL) cells of the stomach and the serotonin-producing EC cells, which constitute a major population of enteroendocrine cells throughout the GI tract (11, 12). Notably, gut-derived serotonin has recently been demonstrated to function as a true endocrine factor that modulates adipose and hepatic function (15). Furthermore, the so-called “peripheral serotonin,” which is almost entirely derived from the EC cells, has been shown also to control the functions of brown adipose tissue (16–18).
Several transgenic reporter mice have been generated for individual enteroendocrine cell types based on the transcriptional control elements for peptide hormone precursors, transcription factors and chemosensors (19, 20). However, a reporter mouse based on a general marker protein would enable characterization of the total enteroendocrine cell population. Thus, in the present study, we have generated a transgenic reporter mouse expressing humanized Renilla reniformis (hr) green fluorescent protein (GFP), under the control of the transcriptional control elements for ChgA. The reporter protein was found to be expressed in ChgA storing cells of the adrenal medulla, the pituitary, the endocrine pancreas and throughout the GI tract. Interestingly, in the GI tract, hrGFP was expressed mainly in monoamine-storing cells, which makes the reporter mouse a particularly useful resource for future studies of these important, but still poorly characterized, cells.
Materials and Methods
Production of ChgA-hrGFP transgenic mice
A ChgA-containing bacterial artificial chromosome (BAC) (bMQ421c07) was chosen based on sequence information from www.ensembl.org and obtained from the BACPAC Resources Center at Children's Hospital Oakland Research Institute. The ChgA BAC contained 87 447 base pairs upstream from the ChgA start codon, including the gene Golga5, the entire ChgA coding sequence and 44 312 base pairs downstream from the ChgA stop codon (Figure 1). The coding sequence for hrGFP was inserted into the ChgA BAC, replacing the ChgA start codon and the next 43 base pairs (the rest of exon 1) using ET recombination (21) in EL250 cells kindly provided by Neal Copeland. The BAC construct was diluted in a 10mM Tris-HCL, 0.1mM EDTA, 100mM NaCl buffer to a concentration of 30 ng/μL and delivered to Karolinska Center for Transgenic Technologies for microinjection into pronuclei of oocytes. The next primers were used for genotyping of hrGFP: F 5′-GGCCGCAACTTCCCCAACHGA-3′ and R 5′-TCAGGGTGCGCATGTGGCAG-3′. Animal procedures were conducted in accordance with the Danish Animal Research authorities (personal animal license to KLE 2012-15-2934-00221 issued by the Danish Committee for Animal Research). Founder mice were validated using fluorescence microscopy. The ChgA-hrGFP reporter mouse can be obtained upon request.
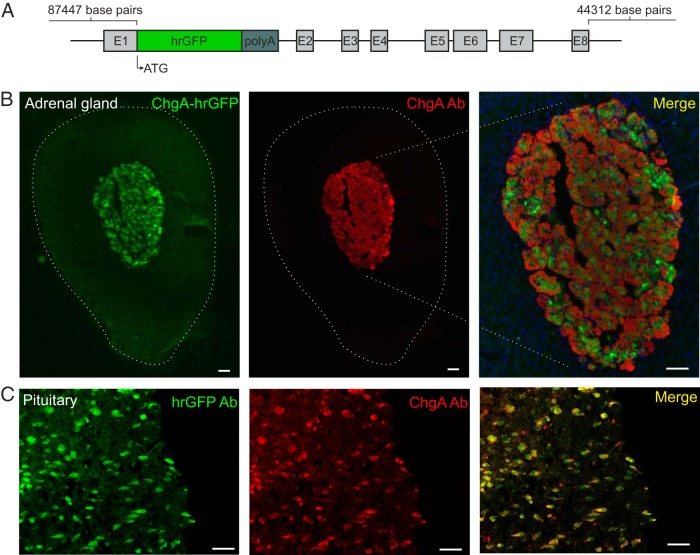
Figure 1. The ChgA-hrGFP construct and ChgA promoter-driven hrGFP expression in the adrenal medulla and pituitary gland.
A, The part of exon 1 after the start codon for ChgA was replaced with the coding sequence of hrGFP followed by a SV polyadenylation signal (polyA). Start codon (ATG) and exons for ChgA are indicated. B, Fluorescence microscopy showing ChgA promoter-driven hrGFP fluorescence (green) and ChgA immunoreactivity (red) in the adrenal gland (Ab from Abcam), and (C) the pituitary gland (Ab from Santa Cruz Biotechnology, Inc). The merged pictures also show DAPI nuclei staining (blue). Scale bars, 50 μm.
Tissue preparation
Mice were euthanized by cervical dislocation and tissues were quickly excised, rinsed with cold PBS and transferred to cold 4% paraformaldehyde for 24 hours of fixation at 4°C. Next, the tissue was transferred to a 20% sucrose solution for 24 hours at 4°C. Tissues were then embedded in Tissue-Tek OCT compound (Sakura Finetek), snap frozen in isopentane, and sectioned perpendicular to the mucosal surface at a 5-μm thickness for gastric tissue and 8 μm for intestinal tissue using a cryostat (CM3050S; Leica) onto SuperFrost plus slides (Thermo Scientific) and air dried for 1 hour in the dark.
Immunohistochemistry
Sections were washed (3 × 2 min) in PBS, preblocked with 2% BSA in PBS and incubated with primary antibodies in blocking buffer (Supplemental Table 1) overnight at 4°C. All colonic sections and duodenal and gastric sections stained with sc-1488 anti-ChgA antibody underwent antigen retrieval (boiled for 15 minutes in 0.01M citrate buffer adjusted to pH 6) before preblocking and incubation with primary antibodies. Tissue sections were then washed and incubated with fluorophore-conjugated secondary antibodies (Supplemental Table 1), diluted 1:200 in blocking buffer for 1 hour at room temperature. After washing, coverslips were mounted with Prolong Gold Antifade Reagent with DAPI (P-36931; Invitrogen). Sections were analyzed with an IX70 Olympus microscope and pictures were captured using a XM10 Olympus camera or, for pancreatic islets, with a Zeiss LSM710 upright laser scanning confocal microscope. Application of pseudo colors and picture merging were done in Adobe Photoshop CS5. For estimations of colocalization, only cells with a visible nucleus were counted. Counting of fluorescent cells was performed using tissue from 3 mice.
Isolation of mucosal cells and fluorescence-activated cell sorting (FACS)
Mice were euthanized by cervical dislocation. The stomach and 8 cm of the proximal small intestine and colon were excised, turned inside out, rinsed with PBS, and inflated with DMEM. The tissues were placed in 50-mL tubes containing 10-mL DMEM with 0.26 Wünsch units of Liberase (Roche) for stomach and colon, and 0.13 Wünsch units for the small intestine, and the tubes were then incubated in a water bath shaking slowly for 20 minutes at 37°C. Every fifth minute, the tubes were taken out and shaken vigorously for 5 seconds. The 20-minute incubation was repeated 2–3 times with fresh enzyme solution. Detached cells were incubated for a second period of 20 minutes at 37°C, and then the suspensions were passed through a 70-μm pore-diameter cell strainer and centrifuged at 1500 rpm for 5 minutes.
The pancreas was cut into small pieces and incubated for 25 minutes in 10-mL DMEM with 0.4-mg/mL collagenase (Sigma) with vigorous shaking every fifth minute. After digestion, all suspensions were passed through a 70-μm pore-diameter cell strainer and centrifuged at 1500 rpm for 5 minutes.
Before sorting, all cells were resuspended and incubated for 3 minutes in 0.05% trypsin-EDTA (15400054; Life Technologies), filtered (70 μm), centrifuged, and resuspended in DMEM with 10% fetal bovine serum and 80-U/mL DNase (D5319; Sigma) and filtered again (35 μm). Cells were sorted based on the intensity of the hrGFP fluorescence (at ∼526 nm) and fluorescence at approximately 576 nm using a MoFlo Astrios. The gates used to separate cells into populations with fluorescence of either low or high intensity were drawn to get pools of similar size that were adequately separated and contained an adequate number of cells for RNA extraction and quantitative PCR (qPCR). The percentages of cells inside each gate (+, relatively low fluorescence intensity; ++, relatively high fluorescence intensity) and number of sorted cells for 3 mice were as follows: 1+: 0.84%, 3906 cells; 1++: 1.14%, 6417 cells; 2+: 0.73%, 3304 cells; 2++: 0.96%, 5875 cells; 3+: 1.03%, 5589 cells; and 3++: 1.05%, 6041 cells.
RNA extraction and qPCR analysis
Total RNA was extracted from FACS-purified cells using QIAzol (79306; QIAGEN), and RT-PCR was performed using SuperScript III Reverse Transcriptase (Invitrogen). Custom-designed StellARray qPCR arrays (LONZA) containing primers for 93 secreted proteins/peptides and granins, the ribosomal subunit 18S, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ-polypeptide (ywhaz), and a control for genomic contamination were assayed on stomach and colon cells, according to the manufacturer's instructions with KAPA SYBR Fast Universal qPCR kit (KAPA Biosystems). Primer target regions have been published before (22). qPCR for gastric, intestinal, and pancreatic marker genes were performed as described in Ref. 23 with primer sequences that have been published (22, 24); insulin, 5′-GTGAAGTGGAGGACCCACAA-3′ and 5′-CAGCTGGTAGAGGGAGCAGA-3′; glucagon, 5′-GGTTGATGAACACCAAGAGGA-3′ and 5′-CAGCATGCCTCTCAAATTCA-3′; pancreatic polypeptide (PP), 5′-CTGGGCCCAACACTCACTAG-3′ and 5′-CGAGAAACAGGGAGAGGCAG-3′; and somatostatin, 5′-GGCTGCCACCGGGAAACAGG-3′ and 5′-AGCTTTGCGTTCCCGGGGTG-3′. Gene expression was quantified relative to the expression of ywhaz (5′-AGACGGAAGGTGCTGAGAAA-3′ and 5′-GAAGCATTGGGGATCAAGAA-3′) using 2(CTywhaz−CTgene). qPCR was run on a Mx3000P (Stratagene) or a LightCycler480 (Roche). qPCR experiments were repeated using 3–6 pools of cells sorted from individual mice.
Data was visualized and tested for significance using GraphPad Prism 6. Statistical significance was tested with Student's t test and defined as P < .05.
Results
Generation of ChgA-hrGFP reporter mice
The construct used to generate a novel transgenic ChgA-hrGFP reporter mouse line is depicted in Figure 1A. Using BAC engineering, the hrGFP coding region was inserted immediately downstream of the ChgA start codon, thereby enabling transcription of the hrGFP to be controlled by ChgA regulatory elements. We obtained 2 potential founder mice in which the transgene was transmitted to offspring; however, we did not determine copy number and insertion site(s). Fluorescence microscopy revealed that hrGFP was expressed in enteroendocrine-like cells in one of the 2 mouse lines (no fluorescence was observed in the other potential founder), which was further characterized using immunohistochemistry and FACS followed by qPCR.
Extra-GI expression of hrGFP
First, we tested whether the hrGFP reporter protein was expressed in the chromaffin cells of the adrenal medulla, in which ChgA was originally identified (25). As shown in Figure 1B, hrGFP fluorescence colocalized with ChgA immunoreactivity in most the cells in the adrenal medulla. hrGFP fluorescence also was observed in the pituitary gland (Figure 1C), in which 94% of the hrGFP fluorescent cells contained ChgA immunoreactivity (445 counted cells), and about 85% of ChgA-immunoreactive cells expressed hrGFP (492 counted cells). The chemical phenotypes of the hrGFP-positive pituitary cells were not further characterized here; however, ChgA has previously been identified in gonadotrophs, thyrotrophs, and a fraction of corticotrophs (26). We did not observe hrGFP fluorescence in the brain, liver, kidney, spleen, testis, or ovary.
Overview of hrGFP expression along the GI tract
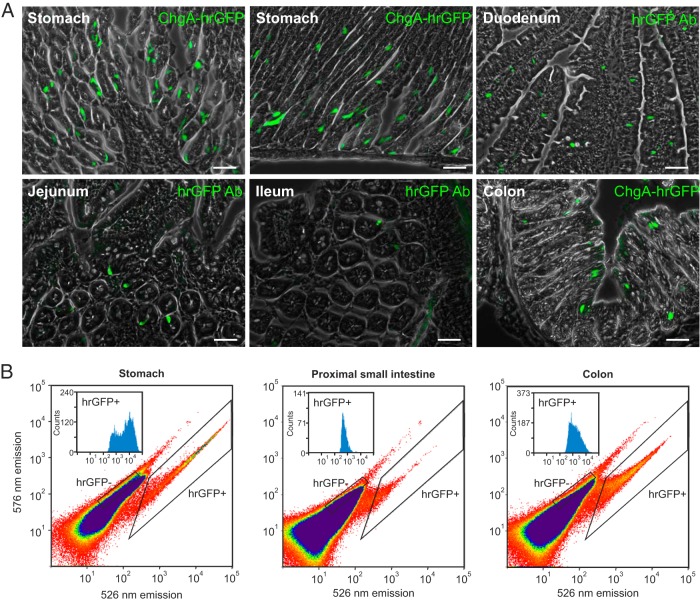
The gastric mucosa of ChgA-hrGFP mice contained abundant intensely fluorescent cells mostly located in the lower half of the gastric glands, as expected for enteroendocrine cells (Figure 2A) (27). In contrast, surprisingly few fluorescent cells were found in duodenal, jejunal, and ileal sections from ChgA-hrGFP mice (Figure 2A). Moreover, the fluorescence was weaker in particular in the distal parts of the small intestine compared with that observed in the stomach, adrenal, and pancreas. In the small intestine, the hrGFP fluorescent cells had the characteristic enteroendocrine morphology, but were too few to represent the entire expected ChgA cell population (28). Anti-hrGFP antibody staining was used to enhance the weak signal, so we were able to visualize more cells in the small intestine. In the colon, hrGFP fluorescence was stronger and was observed in a large number of enteroendocrine-like cells (Figure 2A).
Figure 2. ChgA promoter-driven hrGFP expression in the GI tract.
A, Fluorescence microscopy showing ChgA promoter-driven hrGFP fluorescence in the stomach (no hrGFP antibody), hrGFP-labeled cells in the duodenum, jejunum, ileum, and ChgA promoter-driven hrGFP fluorescence in the colon (no hrGFP antibody). Scale bars, 50 μm. B, Representative FACS diagrams showing the separation of hrGFP negative and positive cells based on fluorescence emission at 526 vs 576 nm after excitation with 488 nm for stomach, proximal small intestine, and colon. In each plot, the distribution of the fluorescence intensity among the cells in the hrGFP-positive pool is shown.
Single-cell suspensions from the stomach, proximal small intestine, and colon from ChgA-hrGFP mice were separated by FACS into hrGFP-positive and hrGFP-negative pools (Figure 2B and Supplemental Figure 1). The hrGFP-positive cells constituted around 3% of the total gastric mucosal cell suspension, around 0.05% of the cell suspension from the proximal small intestine and around 0.4% of the colonic mucosal cell suspension. As observed with fluorescent microscopy, the intensity of the hrGFP fluorescence varied between the tissues. The mean fluorescence intensity was highest in the stomach, 5-fold lower in the colon, and weakest in the proximal small intestine, around 10-fold lower than in the stomach (insets in Figure 2B).
As expected, the ChgA transcript was markedly enriched in all hrGFP-positive pools of cells (Table 1). It was enriched 762-fold in the stomach, 429-fold in the colon, and 66-fold in the proximal small intestine, paralleling the intensity of the hrGFP fluorescence.
Table 1.
Relative mRNA Expression of Selected Genes Within FACS-Separated hrGFP-Positive vs hrGFP-Negative Cells From ChgA-hrGFP Mice
| Cell Type | Gene Family | Gene | n | ΔCT hrGFP+ (CT) | ΔCT hrGFP− (CT) | Enrichment |
|---|---|---|---|---|---|---|
| Stomach | ||||||
| Endocrine | Granins | ChgA | 6 | 74.3 ± 9.19 (20.3) | 0.24 ± 0.08 (28.0) | 762 ± 333 |
| ChgB | 3 | 13.9 ± 1.77 (26.4) | 0.10 ± 0.02 (32.5) | 142 ± 9.78 | ||
| Secretogranin II | 3 | 0.08 ± 0.02 (33.9) | 0.002 ± 0.001 (38.5) | 72.1 ± 42.3 | ||
| Secretogranin III | 3 | 0.41 ± 0.04 (31.5) | 0.02 ± 0.006 (35.5) | 37.3 ± 11.3 | ||
| Secretogranin V | 3 | 1.74 ± 0.23 (29.4) | 0.01 ± 0.002 (35.4) | 139 ± 30.8 | ||
| Vgf | 3 | 1.35 ± 0.21 (29.8) | 0.01 ± 0.006 (36.0) | 190 ± 68.4 | ||
| Prohormone convertases | Prohormone convertase 1/3 | 6 | 0.37 ± 0.03 (27.6) | 0.002 ± 0.0007 (33.8) | 194 ± 68.4 | |
| Prohormone convertase 2 | 6 | 0.52 ± 0.05 (27.1) | 0.02 ± 0.005 (30.7) | 27.3 ± 3.95 | ||
| Peptide hormones | Ghrelin | 3 | 502 ± 58.7 (21.2) | 2.09 ± 0.69 (30.1) | 276 ± 63.1 | |
| Somatostatin | 3 | 184 ± 40.1 (22.7) | 0.53 ± 0.10 (30.1) | 400 ± 167 | ||
| Gastrin | 3 | 6.38 ± 0.42 (27.5) | 6.89 ± 1.38 (26.4) | 0.98 ± 0.14 | ||
| Glucagon | 3 | 0.40 ± 0.1 (31.6) | 0.006 ± 0.002 (36.8) | 82.4 ± 25.1 | ||
| Monoamine production | HDC | 6 | 0.48 ± 0.07 (27.2) | 0.0005 ± 0.0004 (36.1) | 3840 ± 2140 | |
| Tph-1 | 6 | 0.25 ± 0.08 (28.1) | 0.002 ± 0.001 (33.9) | 186 ± 74.9 | ||
| Parietal | H+/K+ ATPase | 6 | 0.33 ± 0.15 (27.7) | 2.56 ± 1.46 (23.7) | 0.18 ± 0.05 | |
| Gastric intrinsic factor | 6 | 3.49 ± 0.64 (24.3) | 3.01 ± 0.61 (23.5) | 1.2 ± 0.14 | ||
| Chief | Pepsinogen F | 6 | 0.24 ± 0.06 (28.2) | 0.45 ± 0.13 (26.2) | 0.55 ± 0.07 | |
| Mucous | Calpain 8 | 6 | 0.003 ± 0.002 (34.6) | 0.009 ± 0.001 (31.9) | 0.92 ± 0.68 | |
| Proximal Small Intestine | ||||||
| Endocrine | Granin | ChgA | 5 | 16.5 ± 4.9 (24.9) | 0.28 ± 0.04 (27.2) | 65.8 ± 22.4 |
| Prohormone convertases | Prohormone convertase 1/3 | 5 | 0.13 ± 0.08 (29.4) | 0.011 ± 0.02 (31.0) | 19.8 ± 10,8 | |
| Prohormone convertase 2 | 5 | 0.017 ± 0.019 (32.3) | 0.003 ± 0.001 (32.9) | 45.4 ± 36.6 | ||
| Peptide hormones | CCK | 5 | 2.25 ± 0.9 (26.7) | 1.36 ± 0.43 (26.7) | 0.79 ± 4.26 | |
| GIP | 5 | 1.49 ± 0.22 (26.7) | 1.79 ± 0.71 (26.4) | 0.83 ± 0.65 | ||
| Monoamine | Tph-1 | 5 | 1.19 ± 0.74 (26.2) | 0.036 ± 0.006 (29.3) | 48.2 ± 26.5 | |
| Enterocytes | Alkaline phosphatase | 5 | 0.74 ± 0.34 (26.8) | 6.69 ± 1.5 (21.7) | 0.26 ± 0.09 | |
| Goblet | Chloride channel calcium-activated 3 | 5 | 0.27 ± 0.05 (28.3) | 1.18 ± 0.25 (24.2) | 0.26 ± 0.05 | |
| Paneth | Lysozyme | 5 | 0.35 ± 0.27 (27.9) | 0.78 ± 0.26 (24.8) | 0.68 ± 0.2 | |
| Colon | ||||||
| Endocrine | Granins | ChgA | 6 | 40.7 ± 9.8 (24.8) | 0.096 ± 0.02 (28.6) | 429 ± 33.7 |
| ChgB | 3 | 30.1 ± 2.9 (27.6) | 0.13 ± 0.03 (30.3) | 260 ± 78.1 | ||
| Secretogranin II | 3 | Not detectable | 0.001 ± 0.00 (37.1) | — | ||
| Secretogranin III | 3 | 0.3 ± 0.14 (35.2) | 0.01 ± 0.003 (35.4) | 44.1 ± 8.2 | ||
| Secretogranin V | 3 | 0.22 ± 0.02 (34.7) | 0.005 ± 0.000 (35.4) | 44.9 ± 4.8 | ||
| Vgf | 3 | 0.57 ± 0.38 (33.9) | 0.007 ± 0.004 (34.9) | 77.2 ± 23.6 | ||
| Prohormone convertases | Prohormone convertase 1/3 | 6 | 0.04 ± 0.008 (33.6) | 0.005 ± 0.001 (31.6) | 10.6 ± 3.3 | |
| Prohormone convertase 2 | 6 | 0.004 ± 0.005 (37.1) | 0.0019 ± 0.016 (34.3) | 3.79 ± 1.79 | ||
| Peptide hormones | GLP-1 | 3 | 0.099 ± 0.03 (36.1) | 0.18 ± 0.05 (29.9) | 0.56 ± 0.29 | |
| PYY | 3 | 0.57 ± 0.38 (33.9) | 0.37 ± 0.14 (28.9) | 1.32 ± 0.4 | ||
| Somatostatin | 3 | 0.20 ± 0.05 (34.9) | 0.03 ± 0.02 (32.3) | 7.64 ± 3.01 | ||
| Monoamine | Tph-1 | 6 | 3.11 ± 1.13 (27.2) | 0.016 ± 0.032 (31.2) | 270 ± 78.5 | |
| Goblet | Chloride channel calcium-activated 3 | 6 | 0.51 ± 0.17 (30.1) | 1.41 ± 0.39 (24.6) | 0.44 ± 0.07 | |
The CT value of each gene has been normalized to the CT value of ywhaz (ΔCT). The mean CT values are listed in parentheses. The enrichment value represents the amount of mRNA in the hrGFP+ cells relative to the amount in the hrGFP− cells. Bold face indicates significant enrichment in hrGFP+ cells; italics indicates significant enrichment in hrGFP- cells assessed by Student's t test.
hrGFP expression in gastric enteroendocrine cells
Colocalization of hrGFP with ChgA
After “antigen retrieval” (see Materials and Methods), we observed an almost total overlap between ChgA antibody staining and the immunohistochemical staining using a hrGFP antibody detecting the hrGFP expression in enteroendocrine-like cells throughout the stomach. Around 98% of the ChgA-immunoreactive cells (589 counted cells) colocalized with hrGFP immunoreactivity, and around 95% of the hrGFP-immunoreactive cells (612 counted cells) stained for ChgA (Figure 3A).
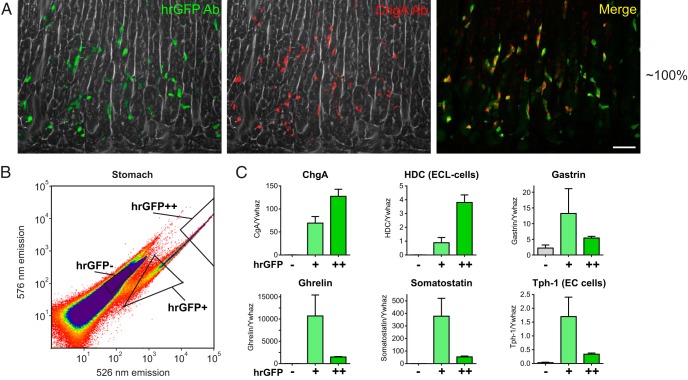
Figure 3. ChgA promoter-driven hrGFP expression in the stomach.
A, Fluorescence microscopy showing hrGFP immunofluorescence and ChgA immunofluorescence (Ab from Santa Cruz Biotechnology, Inc) in the gastric corpus (scale bar, 50 μm). B, FACS diagram showing gates used to sort gastric hrGFP negative cells, positive cells with relatively low fluorescence intensity (hrGFP+), and positive cells with relatively high fluorescence intensity (hrGFP++). C, qPCR analysis of the expression of ChgA, HDC, gastrin, ghrelin, somatostatin, and Tph-1 in the different pools of cells (n = 3).
qPCR analysis of FACS-purified hrGFP cells from the stomach
To identify the cell types labeled by hrGFP in the stomach, cDNA from FACS-purified cells was analyzed for the expression of selected markers for different gastric cell types (Table 1). The 2 enzymes, histidine decarboxylase (HDC) and tryptophan hydroxylase 1 (Tph-1), were both highly enriched in the hrGFP-positive cells (∼3840-fold and ∼190-fold, respectively), indicating that hrGFP is expressed in ECL cells and EC cells. The markers of endocrine cells, prohormone convertase 1/3 and prohormone convertase 2, were also enriched in hrGFP cells (∼190-fold and ∼27-fold, respectively). In contrast, the nonendocrine cell markers H+/K+ ATPase β-subunit, gastric intrinsic factor, pepsinogen F, and calpain 8 were not enriched, as expected (Table 1).
To examine the ChgA-enriched pools of cells for other granins and peptide hormones, the cDNA was analyzed using a qPCR array targeting 93 precursors for secreted proteins and peptides (22). Not only ChgA, but also ChgB, secretogranin II, III, V, and Vgf were enriched in the gastric hrGFP cells, although secretogranin II and III were expressed at rather low levels (Table 1). Among the peptide hormones, mRNA transcripts for both ghrelin and somatostatin were enriched in the hrGFP-positive cells (∼280-fold and ∼400-fold, respectively), whereas gastrin mRNA was equally expressed in the hrGFP cells and in the non-hrGFP cells (Table 1). Among the rest of the examined peptides, the glucagon, angiotensinogen, neurexophilin 1, and neuropeptide y precursors were enriched (>35-fold) and expressed with a mean CT level below 35 in the hrGFP cells (Table 1 and data not shown).
Because the intensity of the hrGFP fluorescence among the hrGFP-positive cells in the gastric samples varied by more than 2 orders of magnitude, we examined whether there was a difference in the expression profiles of the FACS-purified hrGFP-positive pools of gastric cells with the most fluorescence (hrGFP++) compared with the FACS-purified hrGFP-positive pools of gastric cells with the least fluorescence (hrGFP+) (Figure 3B). As expected, the expression of the transcript for ChgA paralleled the intensity of the fluorescence of the hrGFP reporter (Figure 3C, upper left panel). The expression of HDC (ECL cells) was 4.3-fold higher in the most compared with the least fluorescent cells, whereas the expression of Tph-1 (EC cells), ghrelin, and somatostatin was higher in the least fluorescent cells compared with the most fluorescent cells, 5-fold for Tph and 7.1-fold for ghrelin and somatostatin (Figure 3C).
Immunohistochemical characterization of monoamine and peptide hormone expression in gastric hrGFP cells was performed with antibodies against histamine, serotonin, ghrelin, somatostatin, and gastrin (Figure 4). Throughout the gastric epithelium, 87% of the immunoreactive histamine cells (ECL cells, 249 counted cells) expressed the ChgA hrGFP reporter (Figure 4A). Similarly, in the antral mucosa 81% of the immunoreactive serotonin cells (EC cells, 228 counted cells) expressed hrGFP (Figure 4, A and B). Interestingly, in the corpus fundus part of the stomach, immunoreactive serotonin cells located in the upper part of the gastric glands of the oxyntic mucosa did not express hrGFP (Supplemental Figure 1).
Figure 4. Colocalization of ChgA promoter-driven hrGFP expression and hormone immunoreactivity in gastric tissue.
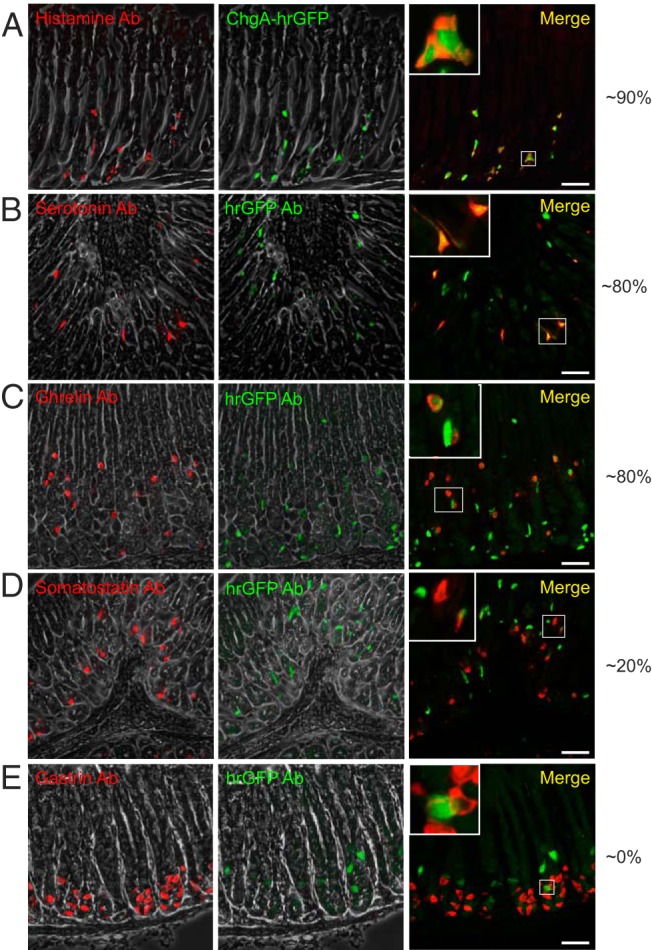
Fluorescence microscopy showing (A) histamine, (B) serotonin, (C) ghrelin, (D) somatostatin, or (E) gastrin immunoreactivity (red) and ChgA promoter-driven hrGFP fluorescence (green) (or hrGFP immunofluorescence for the somatostatin and gastrin staining) in gastric tissue (scale bars, 50 μm). Merged pictures to the right also contain magnifications of selected cells showing colocalization or lack of so. The numbers to the right represent estimates of the percentages of the cell subtypes that colocalize with hrGFP.
Concerning peptide hormone-secreting cells, around 84% of the immunoreactive ghrelin cells in the corpus showed hrGFP fluorescence (376 counted cells), but the hrGFP fluorescence in the ghrelin cells was weak (Figure 4C). Also, a small fraction of the somatostatin-immunoreactive cells, ie, around 18%, coexpressed hrGFP weakly (Figure 4D, 413 counted cells), but no gastrin-immunoreactive cells displayed hrGFP immunoreactivity (Figure 4E), which contrasts with the qPCR detection of gastrin in the weakly hrGFP-positive cells.
In conclusion, in the stomach epithelium, the hrGFP reporter is expressed in most monoamine-storing enteroendocrine cells, ie, histamine-storing ECL cells and serotonin-storing EC cells, as well as in ghrelin cells and a smaller subpopulation of somatostatin cells.
hrGFP expression in enteroendocrine cells of the small intestine
Colocalization of hrGFP with ChgA
Although surprisingly few and only weakly expressing hrGFP-positive cells were observed in the small intestine of the reporter mice, these cells basically all contained immunoreactive ChgA. In early generations of mice, the hrGFP-positive cells constituted up to 80% of the ChgA-immunoreactive cells (Supplemental Figure 2); however, this fraction seemed to markedly decrease in later generations (Figure 5A).
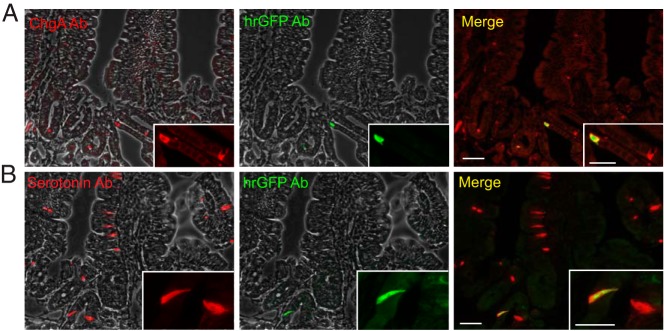
Figure 5. Colocalization of ChgA promoter-driven hrGFP expression and ChgA or serotonin immunoreactivity in duodenal tissue.
Fluorescence microscopy showing ChgA (Ab from Santa Cruz Biotechnology, Inc) (A) or serotonin immunofluorescence (red) and hrGFP immunofluorescence (green) in duodenal tissue (B). Scale bars, 50 and 20 μm.
qPCR analysis of FACS-purified hrGFP cells from the small intestine
As observed in the stomach, enrichment of the transcript for Tph-1 was comparable with that of ChgA, 48- and 65-fold, respectively, in the hrGFP-positive cells (Table 1). Surprisingly, transcripts for intestinal peptide prohormones were not enriched in the hrGFP-positive cells from the small intestine as the enrichment factor for CCK and GIP were 0.79 and 0.83, respectively (Table 1). As expected, nonendocrine markers such as alkaline phosphatase, chloride channel calcium-activated 3 (gob5), and lysozyme were expressed at higher levels in the hrGFP-negative cells (Table 1).
Immunohistochemical analysis of serotonin expression in hrGFP cells in the small intestine
Because Tph-1 as the only marker for enteroendocrine cells was enriched in the FACS-purified hrGFP cells from the proximal small intestine, immunohistochemistry was performed after antigen retrieval with a serotonin-specific antibody. This demonstrated that nearly all hrGFP-immunofluorescent cells also displayed serotonin immunoreactivity, but the fraction of serotonin staining cells that also contained hrGFP-immunoreactive material varied as described above for ChgA, except that the reporter maximally labeled 30% (Figure 5B and Supplemental Figure 2). Because we were surprised that the hrGFP reporter did not label any intestinal enteroendocrine cells expressing peptide hormones, we performed double immunochemical staining experiments on duodenal tissue with a ChgA antibody and antibodies against CCK and GLP-1, respectively (Supplemental Figure 3). We did not observe any cells coexpressing ChgA and CCK or GLP-1 even after antigen retrieval.
In conclusion, in the small intestine, the hrGFP reporter is exclusively expressed in serotonin-storing EC cells, only weakly and only in a smaller fraction, and it is entirely absent from peptide-storing enteroendocrine cells.
hrGFP expression in enteroendocrine cells of the colon
Colocalization of hrGFP with ChgA
In the colon, around 83% (437 counted cells) of the ChgA-immunoreactive cells contained hrGFP, whereas nearly all (98%, 370 counted cells) of the hrGFP-immunoreactive cells also stained for ChgA (Figure 6A).
Figure 6. Colocalization of ChgA promoter-driven hrGFP expression and ChgA or hormone immunoreactivity in colonic tissue.
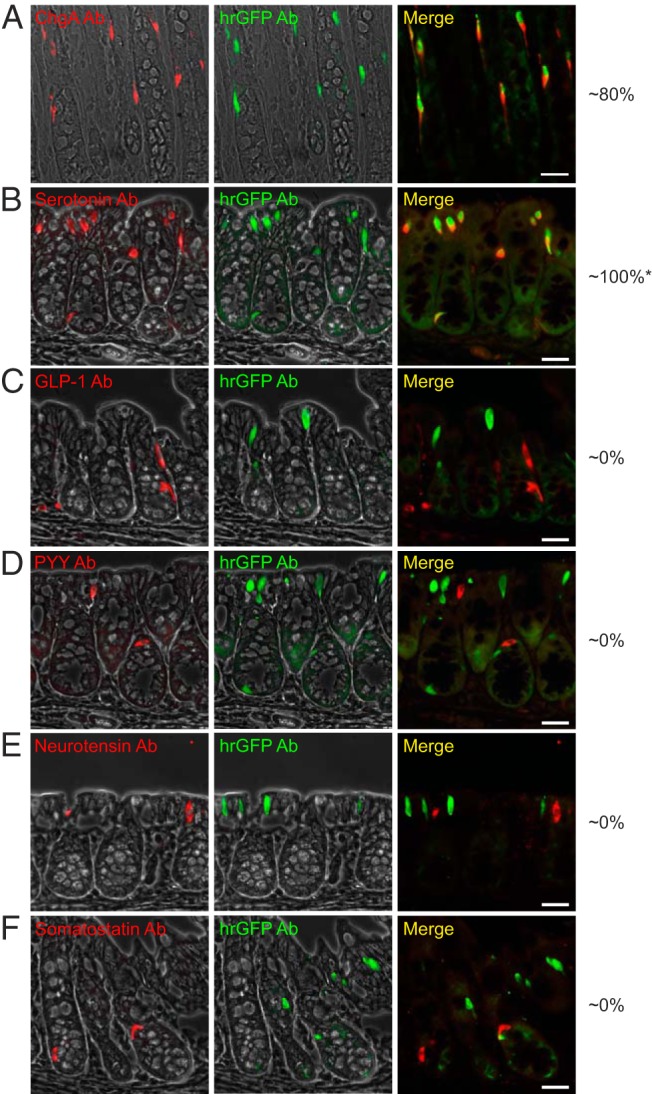
Fluorescence microscopy showing (A) ChgA (Ab from Santa Cruz Biotechnology, Inc), (B) serotonin, (C) GLP-1, (D) PYY, (E) neurotensin, or (F) somatostatin immunofluorescence (red) and hrGFP immunofluorescence (green) in proximal colonic tissue. Scale bars, 25 μm. The numbers to the right represent estimates of the percentages of the cell subtypes that colocalize with hrGFP. *, in later generations of the reporter line, the percentage of reporter-labeled EC cells appeared to decrease to around 50%.
qPCR analysis of FACS-purified hrGFP cells from the colon
As in the stomach, not only ChgA but also several of the other granins were highly enriched in the hrGFP-positive cells from the colon, for example ChgB (260-fold) and secretogranin V (45-fold) (Table 1). As in both the stomach and in the small intestine, Tph-1 was highly enriched in the hrGFP-positive cells from the colon (270-fold) (Table 1). In contrast, none of the transcripts for the peptide hormone precursors were enriched, except for that of somatostatin which, however, only was enriched by approximately 8-fold (Table 1). Interestingly, when testing mRNAs from the FACS-purified hrGFP cells on a specially designed qPCR array for 93 different peptide precursors, we observed that the transcript for the neuropeptide adenylate cyclase-activating polypeptide 1 (encoding PACAP) was highly enriched (∼150-fold) followed by secretin (∼80-fold). Secretin mRNA has been detected in relatively high amounts in the colon before but without the correspondingly high amounts of the peptide product (29), and we were not able to detect any PACAP immunoreactivity in the colonic epithelial cells (data not shown).
Immunohistochemical analysis of peptide and monoamine expression in hrGFP cells in the colon
As expected from the qPCR data, nearly all of the ChgA-immunoreactive cells contained serotonin, whereas the fraction of serotonin-immunoreactive cells that also showed hrGFP immunofluorescence varied from 50%–100% between mice, ie, we observed 100% overlap in mice from earlier generations and 50% overlap in mice from later generations of the reporter line (Figure 6B). No cells immunoreactive for GLP-1, PYY, neurotensin, or somatostatin were found to express hrGFP (Figure 6, C–F).
In conclusion, as in the small intestine, the hrGFP reporter is in the colon exclusively expressed in serotonin-storing EC cells. However, in contrast to the small intestine the ChgA reporter gives a strong fluorescent signal and is present in a large fraction of the EC cells in the colon.
hrGFP expression in the endocrine pancreas
Immunohistochemical analysis of the pancreatic islets
In the pancreas, very clear hrGFP fluorescence was observed in the endocrine islet cells, which also showed ChgA immunoreactivity (Figure 7A). In the islets, all insulin-immunoreactive cells appeared to express hrGFP (Figure 7B), as did the glucagon-immunoreactive cells, although with lower intensity of hrGFP fluorescence (Figure 7C). In contrast, somatostatin-immunoreactive cells did not appear to express the hrGFP reporter protein (Figure 7D). Most PP-immunoreactive cells showed low intensity hrGFP fluorescence, although PP cells with no hrGFP fluorescence were also observed (Figure 7E).
Figure 7. Colocalization of ChgA promoter-driven hrGFP expression and ChgA or hormone immunoreactivity in pancreatic islets.
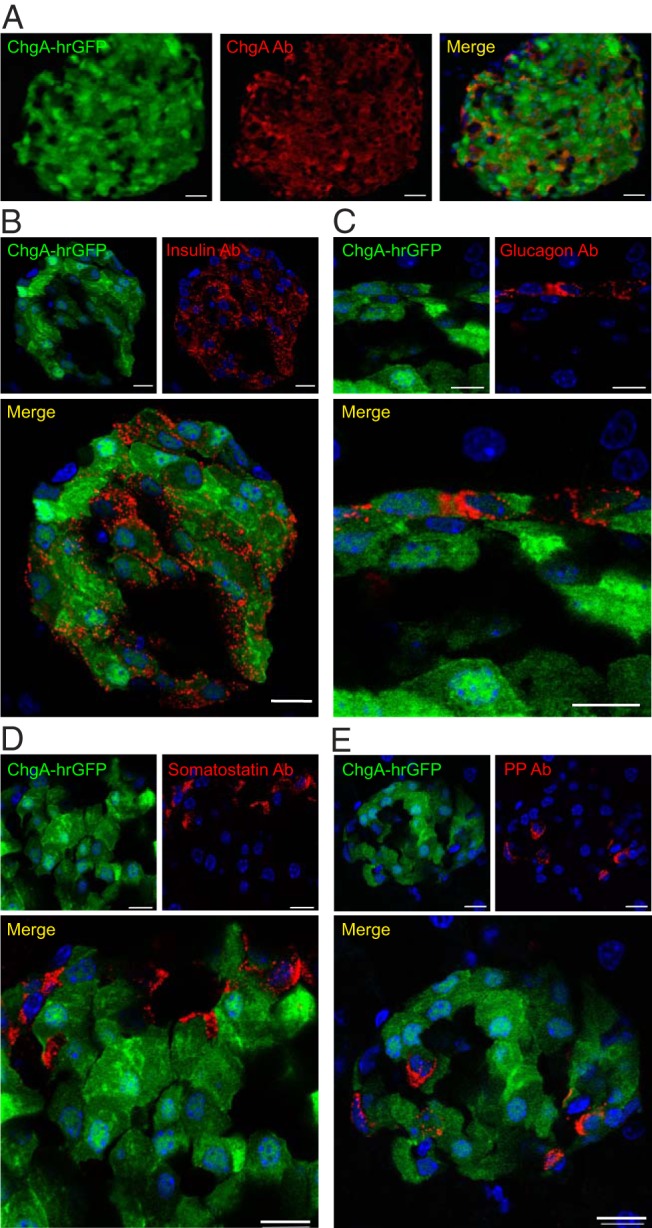
A, Fluorescence microscopy showing ChgA promoter-driven hrGFP fluorescence (green) and ChgA (Ab from Abcam) immunoreactivity (red) in a pancreatic islet (scale bar, 50 μm). Confocal microscopy showing ChgA promoter-driven hrGFP fluorescence (green) and insulin immunoreactivity (red) (scale bars, 10 μm) (B), or glucagon immunoreactivity (red) (scale bars, 5 μm) (C), somatostatin immunoreactivity (red) (scale bars, 5 μm) (D), or PP immunoreactivity (red) (scale bars, 10 μm) (E). Nuclei staining was made with DAPI (blue).
qPCR analysis of FACS-purified hrGFP cells from the pancreas
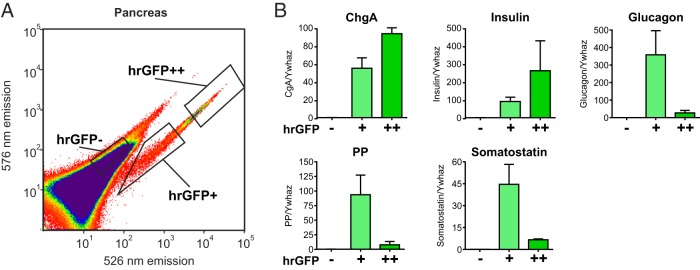
Because the intensity of the hrGFP fluorescence appeared to vary considerably among the pancreatic endocrine cells, we sorted a single-cell preparation of pancreatic cells into populations of hrGFP-positive cells with either high or low fluorescent intensity (Figure 8A). qPCR analysis of these populations confirmed the observations from confocal microscopy, because the transcript for insulin was enriched 2.9-fold in the cells with high fluorescence intensity, whereas the transcripts for glucagon, PP, and surprisingly somatostatin were enriched 13-, 12-, and 7-fold, respectively, in the cells with lowest as compared with highest fluorescence intensity of hrGFP (Figure 8B). The expression of ChgA followed the intensity of the hrGFP fluorescence (Figure 8B, upper left panel).
Figure 8. qPCR analysis of FACS-purified pancreatic hrGFP cells.
A, FACS diagram showing gates used to sort pancreatic hrGFP negative cells, positive cells with relatively low fluorescence intensity (hrGFP+), and positive cells with relatively high fluorescence intensity (hrGFP++) based on fluorescence emission at 526 vs 576 nm after excitation with 488 nm. B, qPCR analysis of the expression of ChgA, insulin, glucagon, PP, and somatostatin in the different pools of cells (n = 3).
We conclude that, in the endocrine pancreas, the hrGFP reporter is strongly expressed in all β-cells and is weakly expressed in nearly all α-cells and in most PP cells but not in somatostatin cells.
Discussion
We have here characterized a novel hrGFP reporter mouse line for ChgA-expressing endocrine cells of the GI tract, islet, pituitary, and adrenal gland. As expected, the ChgA-hrGFP reporter was expressed in cells of the adrenal medulla, where ChgA originally was discovered, as well as in ChgA-positive cells of the pituitary gland and pancreatic islets. Within the GI tract, the hrGFP reporter reliably marked serotonin-storing EC and histamine-storing ECL cells, making the mouse a useful resource for future studies of these otherwise rather elusive endocrine cells.
Fidelity of the ChgA-hrGFP reporter
In the GI tract, the hrGFP fluorescence intensity correlated with the expression level of ChgA in the hrGFP cells, with highest mean intensity and expression in the stomach and colon and lowest mean intensity in the small intestine. The relatively low intensity and number of hrGFP cells in the small intestine was unexpected, because ChgA is generally believed to mark all enteroendocrine cells (7, 30). The enrichment of ChgA in hrGFP-positive cells compared with hrGFP-negative cells was also relatively low in the small intestine, indicating either that the reporter does not label all ChgA-expressing cells, or that the hrGFP fluorescence is too weak to be differentiated from autofluorescence using FACS.
It is not unusual that transgene expression varies between tissues and between cells (31), which might be explained by differences in transgene copy number as well as differences in promoter methylation and silencing (31). The differences in the rate of cell division and renewal of the epithelial lining in the small intestine compared with that of other regions of the GI tract might also affect the expression and the extent of a potential loss of the transgene. Moreover, the genomic insertion site might be unfavorable for hrGFP expression in this region of the GI tract.
We also observed differences between mice with regard to the percentage of EC cells that were labeled by the reporter in the intestines. In line with the issues described above this might be explained by the use of mice from different generations of backcrossing (between 2 and 8). In addition, reporter expression might be balancing at the limit of detection, especially in the small intestine, making subtle changes cause a difference. Although the reporter does not label the entire population of EC cells in the intestines, the expression of hrGFP is, importantly, restricted to EC cells.
ChgA and ChgA-hrGFP as reporters for monoamine vs peptide-storing enteroendocrine cells
The gastric hrGFP cells comprise a mixture of cells with the highest level of ChgA expression in the histamine-storing ECL cells, consistent with the high levels of ChgA-related products secreted from these cells (32), and lower expression in EC cells and ghrelin cells. The coexpression of hrGFP and serotonin was region dependent, ie, surprisingly only the EC cells found in the antrum were labeled. Gastrin expression could be detected in both hrGFP-positive and hrGFP-negative cells using qPCR, but no overlap was observed using immunohistochemistry, although the literature indicates that gastrin cells express ChgA (7, 14). Gastrin cells are predominantly found in the antrum (7), where hrGFP was widely expressed, although mainly in EC and ECL cells. Although ChgA has not typically been shown to colocalize with somatostatin (12), a study applying an antibody specific for a sequence in the C-terminal end of ChgA reported clear staining of somatostatin cells, although this was mostly restricted to the cells of the gastric corpus (14). The antibody we applied was also raised against a C terminus-derived peptide, although it might not be the same. In general, it should not matter for this reporter mouse that ChgA might be cleaved differently in different enteroendocrine cell types (14), as all ChgA-expressing cells should be labeled. We did, however, find few somatostatin cells that coexpressed hrGFP in the stomach, although hrGFP was absent from pancreatic and colonic somatostatin cells.
With the exception of subpopulations of gastric ghrelin and somatostatin cells, hrGFP was not detected in any other peptidergic endocrine cells elsewhere in the GI tract. In the colon, both immunohistochemistry and qPCR analysis of FACS-purified cells showed overlap between serotonin- and hrGFP-expressing cells, but no overlap between hrGFP and peptide hormones. The same was true for the small intestine, where serotonin immunoreactivity was found in hrGFP cells and Tph-1 was enriched in hrGFP cells, whereas for example GIP and CCK were not. These results question the validity of ChgA as a universal marker for enteroendocrine cells, a conclusion also reached by others, although not generally appreciated (11, 12). In these studies, monoaminergic enteroendocrine cells were also consistently found to express ChgA, whereas the ChgA expression varied in enteroendocrine cells containing peptide hormones (11, 12). Nevertheless, in the pancreas we did find strong hrGFP expression in the peptide-producing endocrine islet cells, with the exception of somatostatin cells (Figure 9). Interestingly, ChgA expression appeared to be higher in insulin cells compared with that of glucagon and PP cells.
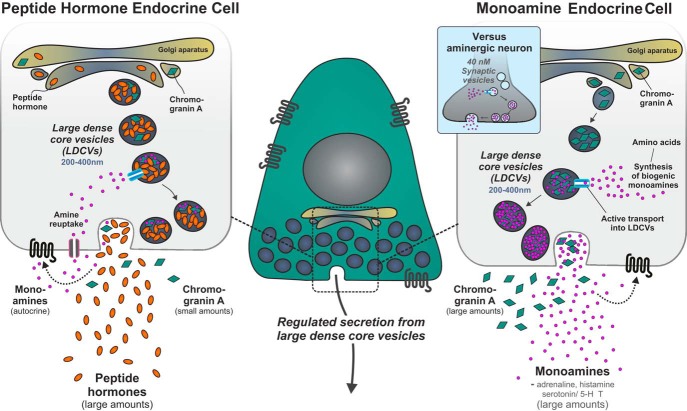
Figure 9. hrGFP marks monoamine endocrine cells, of the adrenal medulla and GI tract, as well as peptide hormone endocrine cells of the pituitary and the pancreas.
ChgA is proposed to mainly play a role as a monoamine binding acidic protein, which allows for the storage of large amounts of the otherwise osmotically active small monoamines in the large dense-core secretory vesicles. In the monoamine storing endocrine cells depicted to the right, the large amounts of monoamines are mainly synthesized from amino acid precursors. In the peptide hormone endocrine cells, which in several cases also store and secrete monoamines albeit in smaller amounts, the monoamines and/or their precursors are mainly taken up from the exterior (they are amine precursor uptake and decarboxylation [APUD] cells). Our results indicate that the amount of ChgA in peptide producing cells in most cases is lower than in the monoamine-secreting cells, maybe because the amount of monoamines stored and released is lower in the peptide producing endocrine cells. As indicated, the monoamines may serve a role in autocrine feedback mechanisms. For comparison, it is indicated that monoamines in nerve terminals are stored in small synaptic vesicles which do not contain ChgA.
The detection in the stomach of enriched, albeit low levels of preproglucagon transcript in hrGFP cells is reminiscent of an older literature on the existence of gastric glucagon, suggesting that this extrapancreatic source of glucagon may contribute to the hyperglucagonemia associated with insulin deficiency (33–35).
ChgA as a monoamine-binding protein in both monoamine and peptide storing endocrine cells?
The apparent selective expression of ChgA in monoaminergic enteroendocrine cells throughout the GI tract suggests that ChgA may serve an important function in these cells. The role of ChgA as an acidic monoamine-binding protein that facilitates the accumulation of large quantities of the otherwise osmotically active adrenaline in the large dense-core vesicles of chromaffin cells of the adrenal medulla is relatively well described (2, 36, 37). We propose that ChgA serves a similar role in the EC and ECL cells of the GI tract, in which it may facilitate the packaging of serotonin and histamine in the secretory granules (37, 38), from which these monoamines can be released to act in a paracrine or even endocrine fashion (Figure 9). Increasing evidence indicates that gut-derived serotonin released from the EC cells acts as a hormone to control metabolism in adipocytes and hepatocytes and perhaps bone (15, 39). Moreover, it was recently reported that peripheral serotonin, which is almost entirely produced by the EC cells of the GI tract, acts as a major regulator of brown adipose tissue function (16–18).
ChgA and/or other granins may also act as monoamine-binding proteins in enteroendocrine and pancreatic endocrine cells, in which the main secretory products are peptide hormones. It is well established that pancreatic and GI tract endocrine cells are amine precursor uptake and decarboxylation (APDU) cells (40), which means that they can store and release monoamines. Some peptide-secreting endocrine cells such as the β-cells of the pancreas store, for instance, serotonin and dopamine (41, 42). These monoamines are packaged into the large dense-core secretory granules and released in parallel with insulin upon stimulation. It has been suggested that serotonin and dopamine may in fact act in an autocrine manner to control insulin secretion through 5-HT and dopamine receptors expressed on the β-cell itself (43). Thus, we propose that ChgA, in analogy with its likely function in the adrenal medulla and in EC and ECL cells, also acts a as a monoamine-binding protein in the secretory granules of peptide-producing cells. However, the amount of ChgA required in the peptide-producing cells may typically be lower than in the specialized monoamine-secreting cells, because the amount of monoamines stored and released is probably relatively lower in the peptide-producing endocrine cells (44). This may partly account for the variable results concerning expression of ChgA and hrGFP in the enteroendocrine cells both in the present study and in the literature.
In conclusion, the ChgA-hrGFP reporter mouse generated in this study in particular enables isolation and characterization of monoamine-secreting endocrine cells from the GI tract and could consequently become an important resource in the elucidation of, eg, the function of the serotonin-secreting EC cell and its increasingly important role in control of metabolism in general. The preferential expression of the ChgA reporter in monoamine-producing cells supports the proposed role of ChgA as a monoamine-binding protein enabling storage and regulated endocrine secretion of large amounts of, eg, “peripheral” serotonin.
Acknowledgments
We thank expert technical assistance from laboratory technicians Heidi M. Paulsen and Lise S. Strange.
The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen. This project was also supported by the UNIK project for Food, Fitness and Pharma from the Danish Ministry of Science, Technology and Innovation. M.S.E. was supported by a PhD scholarship from the Faculty of Health and Medical Sciences, University of Copenhagen, and subsequently by a postdoc fellowship from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen. This project was also supported by the UNIK project for Food, Fitness and Pharma from the Danish Ministry of Science, Technology and Innovation. M.S.E. was supported by a PhD scholarship from the Faculty of Health and Medical Sciences, University of Copenhagen, and subsequently by a postdoc fellowship from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Footnotes
- BAC
- bacterial artificial chromosome
- CCK
- cholecystokinin
- ChgA
- chromogranin A
- ChgB
- chromogranin B
- DAPI
- 4′,6-diamidino-2-phenylindole
- EC
- enterochromaffin
- ECL
- EC like
- FACS
- fluorescence-activated cell sorting
- hrGFP
- humanized Renilla reniformis green fluorescent protein
- GI
- gastrointestinal
- GIP
- glucose-dependent insulinotropic polypeptide
- GLP-1
- glucagon-like peptide 1
- HDC
- histidine decarboxylase
- PP
- pancreatic polypeptide
- qPCR
- quantitative PCR
- Tph-1
- tryptophan hydroxylase 1
- ywhaz
- tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ-polypeptide.
References
- 1. Schneider FH, Smith AD, Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967;31(1):94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borges R, Díaz-Vera J, Domínguez N, Arnau MR, Machado JD. Chromogranins as regulators of exocytosis. J Neurochem. 2010;114(2):335–343. [DOI] [PubMed] [Google Scholar]
- 3. Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32(6):755–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64(22):2863–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106(4):499–509. [DOI] [PubMed] [Google Scholar]
- 6. Helle KB, Reed RK, Pihl KE, Serck-Hanssen G. Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand. 1985;123(1):21–33. [DOI] [PubMed] [Google Scholar]
- 7. Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann NY Acad Sci. 2004;1014:1–12. [DOI] [PubMed] [Google Scholar]
- 8. O'Connor DT. Chromogranin: widespread immunoreactivity in polypeptide hormone producing tissues and in serum. Regul Pept. 1983;6(3):263–280. [DOI] [PubMed] [Google Scholar]
- 9. O'Connor DT, Burton D, Deftos LJ. Chromogranin A: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci. 1983;33(17):1657–1663. [DOI] [PubMed] [Google Scholar]
- 10. Wilson BS, Lloyd RV. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]
- 11. Cetin Y, Müller-Köppel L, Aunis D, Bader MF, Grube D. Chromogranin A (CgA) in the gastro-entero-pancreatic (GEP) endocrine system. II. CgA in mammalian entero-endocrine cells. Histochemistry. 1989;92(4):265–275. [DOI] [PubMed] [Google Scholar]
- 12. Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem. 1997;45(6):815–822. [DOI] [PubMed] [Google Scholar]
- 13. Norlén P, Curry WJ, Björkqvist M, et al. Cell-specific processing of chromogranin A in endocrine cells of the rat stomach. J Histochem Cytochem. 2001;49(1):9–18. [DOI] [PubMed] [Google Scholar]
- 14. Portela-Gomes GM, Stridsberg M. Chromogranin A in the human gastrointestinal tract: an immunocytochemical study with region-specific antibodies. J Histochem Cytochem. 2002;50(11):1487–1492. [DOI] [PubMed] [Google Scholar]
- 15. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16(5):588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crane JD, Palanivel R, Mottillo EP, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider JG, Nadeau JH. Turn up the heat: circulating serotonin tunes our internal heating system. Cell Metab. 2015;21(2):156–158. [DOI] [PubMed] [Google Scholar]
- 18. Carey AL, Kingwell BA. Reducing peripheral serotonin turns up the heat in brown fat. Nat Med. 2015;21(2):114–116. [DOI] [PubMed] [Google Scholar]
- 19. Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;(209):309–335. [DOI] [PubMed] [Google Scholar]
- 20. Engelstoft MS, Egerod KL, Lund ML, Schwartz TW. Enteroendocrine cell types revisited. Curr Opin Pharmacol. 2013;13(6):912–921. [DOI] [PubMed] [Google Scholar]
- 21. Lee EC, Yu D, Martinez de Velasco J, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73(1):56–65. [DOI] [PubMed] [Google Scholar]
- 22. Egerod KL, Engelstoft MS, Grunddal KV, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakata I, Nakano Y, Osborne-Lawrence S, et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155(1–3):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banks P, Helle K. The release of protein from the stimulated adrenal medulla. Biochem J. 1965;97(3):40C–41C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rundle S, Somogyi P, Fischer-Colbrie R, Hagn C, Winkler H, Chubb IW. Chromogranin A, B and C: immunohistochemical localization in ovine pituitary and the relationship with hormone-containing cells. Regul Pept. 1986;16(3–4):217–233. [DOI] [PubMed] [Google Scholar]
- 27. Karam SM, Leblond CP. Identifying and counting epithelial cell types in the “corpus” of the mouse stomach. Anat Rec. 1992;232(2):231–246. [DOI] [PubMed] [Google Scholar]
- 28. Mellitzer G, Beucher A, Lobstein V, et al. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120(5):1708–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78(4):1087–1108. [DOI] [PubMed] [Google Scholar]
- 30. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92(4):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin DI, Whitelaw E. The vagaries of variegating transgenes. Bioessays. 1996;18(11):919–923. [DOI] [PubMed] [Google Scholar]
- 32. Zhao CM, Chen D. The ECL cell: relay station for gastric integrity. Curr Med Chem. 2012;19(1):98–108. [DOI] [PubMed] [Google Scholar]
- 33. Lefebvre PJ, Luyckx AS. Glucose and insulin in the regulation of glucagon release from the isolated perfused dog stomach. Endocrinology. 1978;103(5):1579–1582. [DOI] [PubMed] [Google Scholar]
- 34. Lefebvre PJ, Luyckx AS. Neurotransmitters and glucagon release from the isolated, perfused canine stomach. Diabetes. 1980;29(9):697–701. [DOI] [PubMed] [Google Scholar]
- 35. Blazquez E, Muñoz-Barragan L, Patton GS, Dobbs RE, Unger RH. Demonstration of gastric glucagon hypersecretion in insulin-deprived alloxan-diabetic dogs. J Lab Clin Med. 1977;89(5):971–977. [PubMed] [Google Scholar]
- 36. Dominguez N, Estevez-Herrera J, Borges R, Machado JD. The interaction between chromogranin A and catecholamines governs exocytosis. FASEB J. 2014;28(11):4657–4667. [DOI] [PubMed] [Google Scholar]
- 37. Díaz-Vera J, Camacho M, Machado JD, et al. Chromogranins A and B are key proteins in amine accumulation, but the catecholamine secretory pathway is conserved without them. FASEB J. 2012;26(1):430–438. [DOI] [PubMed] [Google Scholar]
- 38. Cetin Y. Secretin-cells of the mammalian intestine contain serotonin. Histochemistry. 1990;93(6):601–606. [DOI] [PubMed] [Google Scholar]
- 39. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearse AG. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969;17(5):303–313. [DOI] [PubMed] [Google Scholar]
- 41. Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7(5):339–348. [DOI] [PubMed] [Google Scholar]
- 42. Ding WG, Fujimura M, Tooyama I, Kimura H. Phylogenetic study of serotonin-immunoreactive structures in the pancreas of various vertebrates. Cell Tissue Res. 1991;263(2):237–243. [DOI] [PubMed] [Google Scholar]
- 43. Harris PE, Leibel RL. Neurofunctional imaging of β-cell dynamics. Diabetes Obes Metab. 2012;14(suppl 3):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bargsten G, Grube D. Serotonin storage and chromogranins: an experimental study in rat gastric endocrine cells. J Histochem Cytochem. 1992;40(8):1147–1155. [DOI] [PubMed] [Google Scholar]