Abstract
The liver X receptors (LXRs) are important regulators of lipid, cholesterol, and glucose homeostasis by transcriptional regulation of many key genes in these processes, and the transcriptional activities of LXRs are finely controlled by cooperating with retinoid X receptors and many other coregulators. Here, we report that the LIM protein Ajuba binds to the hinge and the ligand binding domains of LXRα via its C-terminal tandem LIM motifs and enhances LXR target gene expression in liver cells. Depletion of Ajuba in HepG2 cells and in mouse primary hepatocytes decreases LXR target gene expression, whereas stable expression of Ajuba in HepG2 cells results in increased expression of these genes. Mechanistic investigations found that Ajuba selectively interacts with LXRα/retinoid X receptor-γ heterodimer to form a ternary complex, which displays a higher transactivation activity to LXR target genes. Moreover, Ajuba and LXR mutually affect their DNA binding activity at endogenous target chromatins and the cooperation between Ajuba and LXRα is dependent on the functional LXR response elements located in the target promoters. Together, our studies demonstrate that Ajuba is a novel coactivator for LXRs and may play important role in lipid and glucose metabolism.
Liver X receptors (LXRs) are members of the nuclear receptor superfamily and usually form heterodimers with retinoid X receptors (RXRs) (1). LXRs are present in 2 isoforms α and β, encoded by separate genes. LXRα is expressed mainly in liver, intestine, adipose tissues and macrophages, whereas LXRβ is expressed ubiquitously (2). LXRs are ligand-dependent transcription factors and the natural ligands are oxysterols, including 24(S)-hydroxycholesterol, and 22(R) - hydroxycholesterol (3, 4). Two synthetic nonsteroidal compounds, T0901317 (T090) and GW3965, also act as agonists for both LXRα and LXRβ (5).
LXRs play a crucial role in cholesterol, lipid and glucose metabolism by transcriptional regulation of a large pool of genes that control these processes. In liver, LXRs regulate lipid metabolism mainly through induction of sterol regulatory element-binding protein-1C(sterol regulatory element-binding protein [SREBP]-1C), fatty acid synthase, stearoyl-coenzyme A desaturase 1 (SCD-1), and acetyl-coenzyme A carboxylase 1 (6). LXRs exert its transcriptional activities by recruitment of a series of protein regulatory complexes to its target chromatins (7). In general, LXR/RXR heterodimers recruit corepressors such as SMRT and NcoR in the absence of agonists, whereas coactivators such as CBP/p300, SRC-1, and PGC-1α are recruited to LXR/RXR to transactivate its target genes upon ligand stimulation (7–9).
Ajuba belongs to the LIM protein family, which are characterized by tandem homologous C-terminal LIM domains and a unique N-terminal preLIM region rich in glycine and proline residues (10). The LIM motif is a double zinc finger structure and functions as a protein-protein interface (11). Ajuba functions as a scaffold involving assembly of multiple protein complexes to regulate cell adhesion, migration, mitosis, microRNA maturation and transcriptional regulation (10–16). Ajuba functions as a corepressor for Snail by recruiting protein arginine methyltransferase 5, 14–3-3, HDACs, and EZH2 to Snail and as an essential regulator of mesenchymal-epithelial transition and metastasis (12). Ajuba is an important regulator of Hippo pathway by binding to Warts and Lats and preventing their activation by Hpo and MST (10). Moreover, we lately demonstrated that Ajuba contains functional nuclear receptor binding motifs and directly interacts with RARα via these conserved NR boxes, where Ajuba functions as corepressor and negatively regulate RAR signaling (17). However, the binding activities of Ajuba to other types of nuclear hormone receptors are not clear. In this report, we describe that Ajuba enhances LXRα/RXRγ heterodimer formation and plays an important role in transactivating LXRα target genes involved in lipid metabolism in liver cells.
Materials and Methods
Plasmid constructs
Flag-LXRα, Flag-LXRβ and the LXRα truncations were subcloned into pcDNA3.1 vector. The Myc-Ajuba and its mutants plasmids, PCDH-Ajuba, PLKO.1-shAjuba, PLKO.1-shLuc vectors, HA and Flag-tagged RXRα, RXRβ, and RXRγ have been described previously (17, 18). The pGL3-SREBP-1C-Luc reporter and its mutations were kindly provided by Dr Etienne Lefai from Claude Bernard University Lyon 1. The mouse SCD-1 gene promoter from −1147 to +198 containing a functional LXR response element (LXRE) site was amplified by PCR and subcloned into pGL3.0 basic vector. LXRE mutations were made using the site-directed mutation method and verified by DNA sequencing. pGIPZ-shLuc and pGIPZ-shLXRα were obtained from Open Biosystems.
Isolation of mouse primary hepatocytes
Mouse primary hepatocytes were isolated from the livers of 10-week-old male C57BL/6 mice. The mice were anesthetized with 1% pentobarbital sodium and their livers were exposed surgically. The livers were first perfused with Hanks' balanced salt solution (Gibco) and then cut off into a 10-cm dish and perfused with collagenase (Gibco) solution for 30 minutes. Subsequently, the suspensions were filtered through a 100-μm filter (BD Biosciences). After centrifugation at 500 rpm for 5 minutes, hepatocytes were collected and washed twice with PBS. The viability was estimated by trypan blue exclusion assay and was consistently in excess of 85%. Hepatocytes were then seeded onto collagen type-1 (Sigma)-coated 6-well plates. All animal experiments were approved by the Institutional Animal Care and Use Committee of Shanghai in accordance with the National Research Council Guide for Care and Use of Laboratory Animals (SCXK [Shanghai 2007–0005]).
Cell culture, retroviral infection, and luciferase reporter assay
HEK-293T and HepG2 (ATCC) and mouse primary hepatocytes were cultured in DMEM containing 10% Fetal Bovine Serum, 25mM L-glutamine, and penicillin (50 U/mL)/streptomycin (50 μg/mL) at 37°C under 5% CO2 in a humidified chamber. Transient transfections in 293T cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For luciferase assays, transfection conditions were performed in 24-well plates as described previously (12). Cells were harvested 24 hours later for measuring luciferase and β-galactosidase activities. Supernatants containing viruses were generated from 293T cells. After adhesion, HepG2 or mouse primary hepatocytes in 6-well plates were infected with viral supernatants, and maintained in the presence of 1-μg/mL puromycin to select stable cells.
Coimmunoprecipitation (co-IP) and Western blot analysis
co-IP and Western blot analyses were performed as described previously (12, 17). Briefly, HEK-293T cells were transfected with indicated expression plasmids. Cell lysates were prepared 48 hours after transfection in lysis buffer containing 20mM Tris-HCl (pH 8.0), 150mM NaCl, 0.5% NP-40, 2.5mM EDTA, and protease inhibitor mixture. The whole-cell extracts were precleared with protein A/G beads, and co-IP assays were performed with either α-Myc or α-Flag antibodies. Antibodies used in these assays are as follows: mouse anti-Myc (13–2500; Invitrogen), mouse anti-Flag (F3165; Sigma), rabbit anti-Flag (F7425; Sigma), mouse anti-HA (MMS-101P; Covance), mouse anti-LXRα (ab41902; Abcam), rabbit anti-RXRα (sc553; Santa Cruz Biotechnology, Inc), rabbit anti-RXRγ (sc555; Santa Cruz Biotechnology, Inc), rabbit anti-CBP (A-22; Santa Cruz Biotechnology, Inc), and mouse anti-β-actin (60008–1-lg; Proteintech). Rabbit anti-Ajuba was described previously (19).
Quantitative PCR
Total mRNA was isolated using the TRIzol reagent (Ambion) from HepG2 or mouse primary hepatocytes treated with 1μM T090 (Sigma) or corresponding volume of DMSO for 16 hours. The reverse transcription procedure was described previously (17). Quantitative gene expression analysis was performed on an ABI 7500 fast machine with SYBR to estimate the mRNA levels of LXRα target genes. Actin was used as the internal control and the DNA sequences for all primers used were showed as Supplemental Table 1.
Chromatin immunoprecipatation (ChIP)
ChIP experiments were performed in HepG2 stable cell lines. Cells were cross-linked, and the chromatin templates were broken into genomic fragment ranging from 200 to 500 bps by sonication (Bioruptor), and the resulting lysates were precleared by adding protein A/G beads. Immunoprecipitation was carried out using specific antibodies against LXRα, Ajuba, CBP, and RXRγ. Equal amount of rabbit normal IgG was used as control. Quantitative PCR was performed using primer sets flanking the functional LXREs in SREBP-1C and SCD-1 promoters. The primers were showed in Supplemental Table 1.
Statistical analysis
Data are presented as mean ± SD. Statistical significance was determined by the Student's 2-tailed t test. Difference with P < .05 was considered statistically significant.
Results
Ajuba binds LXRα in a ligand dependent manner
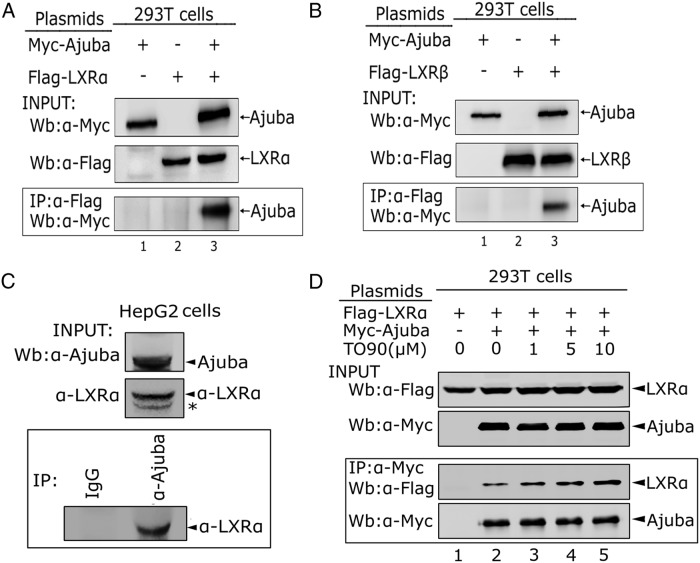
To examine whether Ajuba physically interacts with LXRs, we transiently coexpressed Flag-LXRα, or LXRβ, together with Myc-Ajuba in 293T cells and the resulting cell lysates were immunoprecipitated with Flag antibody. The coeluted proteins were detected by Western blot analysis. Indeed, both LXRα and LXRβ were able to immuoprecipitate Ajuba (Figure 1, A and B). To further validate the interaction between Ajuba and LXRα, we separated cytoplasmic (N) and nuclear (N) fractions from whole cell extracts prepared from 293T cells expressing Ajuba and LXRα, and Western blotting and co-IP assays were performed with nuclear extracts. The results indicated that Ajuba was detected both in cytoplasm and nucleus, whereas LXRα was found in nucleus. Consistently, in the nuclear fraction, Ajuba readily immunoprecipitated LXRα (Supplemental Figure 1). To further confirm the interaction of the endogenous Ajuba and LXRα, we first performed Western blot analysis to examine the expression of LXRα and Ajuba in HepG2 cells and found that both LXRα and Ajuba were readily detected. Whole cell lysates prepared from 2 × 107 of HepG2 cells were incubated with antibody specific to Ajuba or normal rabbit IgG, and the coeluted LXRα proteins were detected by Western blot analysis and found that LXRα interacted with Ajuba at endogenous level (Figure 1C).
Figure 1. Ajuba interacts with LXRα.
A and B, co-IP assays of the interaction between Ajuba and LXRα or LXRβ. Myc-Ajuba, Flag-LXRα, or LXRβ was transiently transfected into 293T cells, and 48 hours after transfection, cells were harvested and whole cells extracts were prepared for co-IP assays. Inputs for all co-IP assays were 5% of total lysates, and the experiments were repeated twice. C, Endogenous Ajuba and LXRα interaction in HepG2 cells. Whole cell extracts prepared from 2 × 107 cells were precleared with protein A/G beads for 2 hours, and co-IP assays were performed using Ajuba antibody and normal rabbit IgG. D, LXR agonist T090 stimulates the interaction between Ajuba and LXRα. Myc-Ajuba and Flag-LXRα proteins were cotransfected into 293T cells; 24 hours after transfection, T090 (1μM, 5μM, 10μM) was added into the culture media for another 24 hours, and whole cell extracts were prepared for co-IP assays. All co-IP assays were repeated at least twice.
To determine whether their interaction is regulated by LXRα agonist, LXRα and Ajuba were coexpressed in 293T cells and were treated with T090, the known LXRα agonist for 24 hours before harvesting. The co-IP assays showed that increasing the doses of T090 from 1μM to 10μM, the coeluted LXRα protein by Ajuba was increased, suggesting that the interaction between LXRα and Ajuba is ligand dependent. Together, these data clearly demonstrate that Ajuba is an LXRα interacting protein.
The LIM region of Ajuba contains dominant binding sites for LXRα
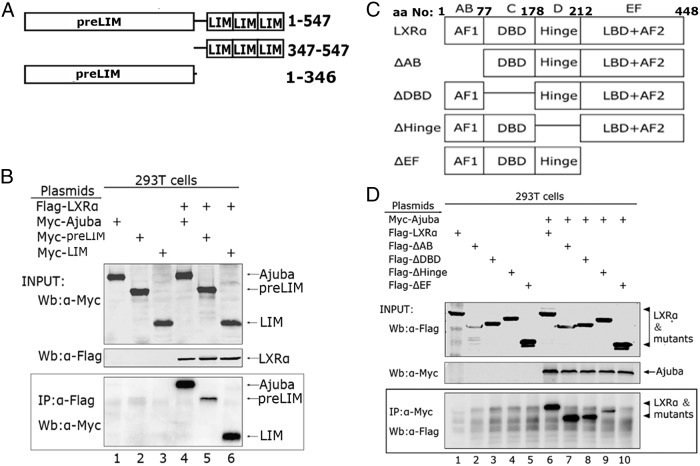
To identify the domains that mediate the interaction between Ajuba and LXRα, we first made truncation mutants of Myc-preLIM and Myc-LIM (Figure 2A). We coexpressed full-length LXRα and Ajuba or its truncation mutants in 293T cells, and co-IP assays were performed with Flag antibody. Notably, the LIM domain alone robustly bound LXRα, whereas the preLIM showed only weak binding activity to LXRα (Figure 2B), indicating the LIM domain is the major binding region for LXRα.
Figure 2. Identification of the binding domains in Ajuba and LXRα.
A, A schematic diagram shows Ajuba truncation mutants. B, Myc-Ajuba and its truncation mutants, together with Flag-LXRα, were transiently coexpressed in 293T cells, and whole cell extracts were prepared, immunoprecipitated with Flag antibody, and Western blotting were performed with Myc antibody. C, A schematic diagram shows the Flag-tagged deletion mutants of LXRα. The mutants were constructed using the method of the PCR-based site directed mutagenesis, and all mutants were confirmed by DNA sequencing. D, Myc-Ajuba and Flag-LXRα, or its deletion mutants, were coexpressed in 293T cells via transient transfection, and co-IP assays were performed with Myc antibody.
Both the hinge (D domain) and the ligand binding domain (EF domain) of LXRα are required for Ajuba binding
To determine the regions for Ajuba binding in LXRα, we coexpressed series of Flag-epitope tagged deletion mutants of LXRα, together with full-length Ajuba in 293T cells (Figure 2C). co-IP assays were carried out with Myc antibody and the coeluted proteins were detected with Flag antibody. Deletion of AB domain, or C domain alone did not apparently affect the binding to Ajuba (Figure 2D, lanes 6–8), whereas deletion of D domain markedly decreased the binding activity (Figure 2D, lane 9); notably, deletion of EF domain, the region responsible for ligand binding and transactivation, abolished the binding to Ajuba (Figure 2D, lane 10). These data indicate that both D (the hinge region) and EF domains in LXRα are required for maximal binding to Ajuba.
The binding of Ajuba to endogenous LXRα target promoters is dependent on LXRα
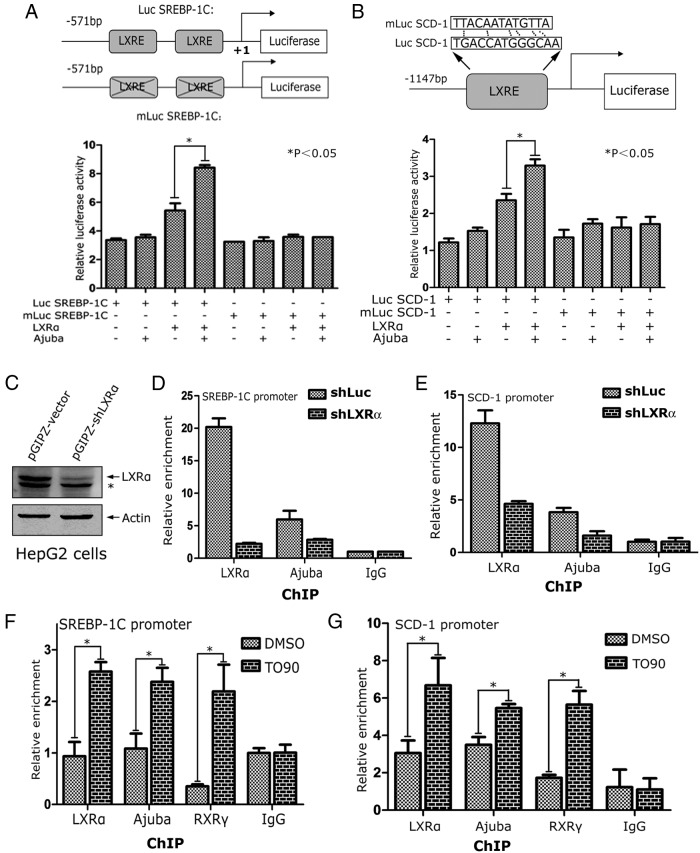
To determine whether Ajuba directly modulates LXRα target genes, we performed luciferase reporter assays using 2 known LXRα target promoters of SREBP-1C and SCD-1 genes, and their respective LXRE mutants (Figure 3, A and B, top panels). These reporters, together with plasmids encoding LXRα and Ajuba were transiently transfected into 293T cells, and the luciferase activity was normalized to β-galatosidase activity. Ajuba by itself did not apparently increase SREBP-1C-Luc or SCD-1-Luc activities; however, when coexpressed with LXRα, Ajuba markedly enhanced the SREBP-1C-Luc or SCD-1-Luc activities (Figure 3, A and B). Conversely, mutation of the functional LXREs located in the promoters of SREBP-1C or SCD-1 resulted in loss of responsiveness of these promoters to LXRα and Ajuba, suggesting that the combination effect of LXRα and Ajuba is dependent on the functional LXREs in the target promoters (Figure 3, A and B).
Figure 3. Ajuba increases LXRα-induced target promoter activities.
A, Coexpression of Ajuba and LXRα-induced SREBP-1C-Luc activity. Human SREBP-1C gene promoter containing 2 functional LXREs (−311/−296 and −260/−245) was inserted into the pGL3 basic luciferase vector to make a SREBP-1C-Luc reporter. Reported assays were performed in 293T cells, and all reporter assays were repeated 3 times in triplicate. Data shown is an average of 3 independent experiments. B, Mouse SCD-1 gene promoter containing a functional LXRE (−1116/−1101) was cloned into pGL3.0 basic luciferase vector to make a SCD-1-Luc reporter and the LXRE was subsequently mutated. The luciferase assays were performed as the same as in A. C–E, Both LXRα and Ajuba bind the LXRE loci of LXRα target genes. ChIP assays in HepG2-vector and HepG2-shLXRα cells were performed using antibodies specific to LXRα and Ajuba. The coeluted DNA fragments were amplified by qPCR using primers flanking the functional LXREs at the promoters of SREBP-1C and SCD-1, the known target genes of LXRα, respectively. F and G, HepG2 cells were treated with DMSO or T090 for 24 hours, and the resulting cells were prepared for ChIP assays. Data shown were an average from 3 independent experiments. *, P < .05.
To further examine the binding activities of LXRα and Ajuba at the endogenous target chromatins, we performed ChIP assays in HepG2-shLuc and HepG2-sh LXRα cells using antibodies specific to LXRα and Ajuba (Figure 3C). The coeluted DNA fragments were amplified using primer sets flanking the functional LXREs at the promoters of SREBP-1C or SCD-1 genes, respectively. Depletion of LXRα in HepG2 cells resulted in significant decrease of the binding activities of LXRα and Ajuba to the promoters of SREBP-1C and SCD-1 genes (Figure 3C).
To determine the effect of ligand stimulation on the binding activities of LXRα and Ajuba at the endogenous target chromatins, HepG2 cells were treated with T090 for 24 hours, and the resulting cells were subjected for ChIP assays. T090 treatment increased the binding of LXRα, Ajuba, and RXRγ at the promoters of SREBP-1C and SCD-1 genes (Figure 3, F and G). Taken together, these results indicate that the binding activity of Ajuba at LXRα target promoters is dependent on the presence of LXRα and ligand stimulation.
Ajuba is required for maximal LXRα target gene expression stimulated by T090
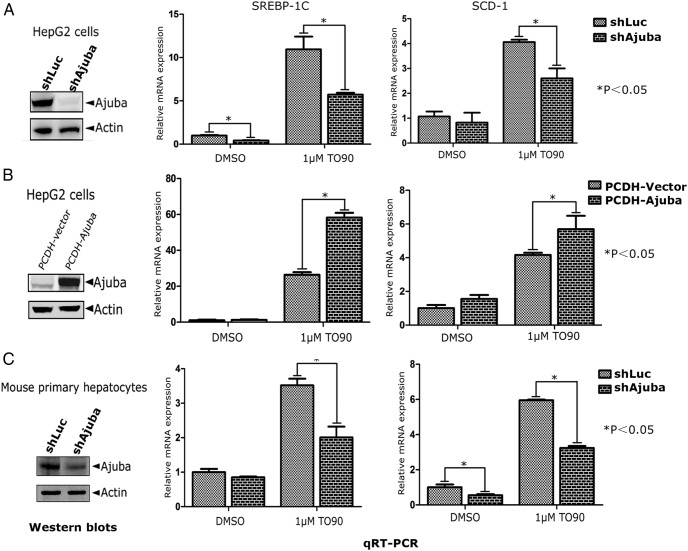
To examine the effect of Ajuba on endogenous LXRα target gene expression, we first depleted Ajuba in HepG2 cells using lentiviral shRNA specifically targeting Ajuba (Figure 4A). The resulting cells were then treated with LXRα ligand T090 (1μM) or DMSO for 24 hours and the total mRNAs were extracted for qRT-PCR analysis of SREBP-1C and SCD-1 expression. Consistently, depletion of Ajuba in HepG2 cells resulted in slightly decreased SREBP-1C and SCD-1 expression in DMSO control cells but markedly dampened the induction amplitudes of these genes by T090 stimulation (Figure 4A). Conversely, stable expression of Ajuba in HepG2 cells resulted in elevated SREBP-1C and SCD-1 expression at DMSO or T090-treated cells (Figure 4B). To extend our observations obtained from HepG2 cells, we isolated mouse primary hepatocytes from the livers of C57BL/6 male mice (10 wk old), and Ajuba expression was depleted using specific shRNA targeting murine Ajuba via viral infections (Figure 4C). Similar to the results observed in HepG2 cells, depletion of Ajuba inhibited T090-stimulated SREBP-1C and SCD-1 expression (Figure 4C). Additionally, we observed similar effects of Ajuba on LXR target genes acetyl-coenzyme A carboxylase 1α and fatty acid synthase (Supplemental Figure 2).
Figure 4. Ajuba enhances LXRα target gene expression in HepG2 cells and mouse primary hepatocytes.
A, Depletion of Ajuba in HepG2 cells using lentiviral shRNA specifically targeting Ajuba. The cells were selected with puromycin for 2 weeks, and the resulting pooled cells were treated with T090 (1μM) or DMSO for 24 hours and total RNAs were extracted, which were subjected for qRT-PCR analysis for SREBP-1C and SCD-1 expression. B, Ajuba was stably expressed in HepG2 cells via viral infection followed by puromycin selection. The expression of Ajuba was verified by Western blotting, and pooled cells were analyzed as in A. C, Mouse primary hepatocytes isolated from C57BL/6 were infected with viral supernatants containing shRNA-specific targeting mouse Ajuba and subsequently selected with puromycin to eliminate noninfected cells. Ajuba protein level was examined by Western blot analysis, and pooled cells were subjected for analysis as same as above. Relative mRNA levels were normalized to β-actin expression. Experiments were repeated 3 times and results shown are averages of 3 independent experiments.
To examine the effect of Ajuba on lipid metabolism in liver cells, we measured triglyceride contents in HepG2-shLuc and HepG2-shAjuba cells treated with DMSO or T090. Depletion of Ajuba decreased triglyceride levels in DMSO or T090-treated cells (Supplemental Figure 3). Taken together, these data demonstrate that Ajuba is an important coactivator for LXRα mediated transcriptional activity.
Ajuba enhances LXRα and RXRγ herterodimer formation
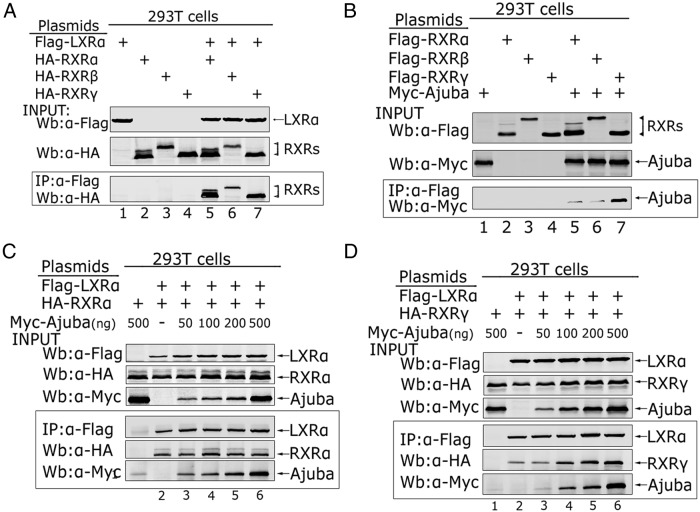
It is known that LXRα forms heterodimers with RXRα, RXRβ, or RXRγ, and that Ajuba selectively binds to RXRγ, we reasoned that Ajuba may preferentially associate with LXRα/RXRγ heterodimer. We first verified the interaction between LXRα and RXRα, RXRβ, or RXRγ by co-IP assays and found LXRα interacted with RXRα, RXRβ, or RXRγ when coexpressed in 293T cells (Figure 5A). Next, we verified the interaction between Ajuba and RXRα, RXRβ, or RXRγ and consistent to previous report (17), Ajuba bound to RXRγ with high affinity while weakly bound to RXRα and β (Figure 5B). To examine whether Ajuba could enhance the binding between LXRα and RXRα or RXRγ, we coexpressed Flag-LXRα and HA-RXRα or HA-RXRγ, together with increasing amount of Myc-Ajuba in 293T cells, and performed co-IP assays with Flag antibody. The coeluted proteins were detected by Western blot analysis with Flag, HA, and Myc antibodies, respectively. LXRα immunoprecipitated RXRα and Ajuba, but increasing amount of Ajuba did not affect the binding between LXRα and RXRα (Figure 5C); strikingly, increasing the amount of Ajuba resulted in LXRα to coimmuoprecipitate more Ajuba and RXRγ (Figure 5D, lanes 3–6), suggesting that Ajuba may facilitate the interaction between LXRα and RXRγ.
Figure 5. Ajuba enhances LXRα/RXRγ formation.
A, Flag-LXRα was coexpressed with HA-RXRα, HA-RXRβ, and HA-RXRγ (1 μg), respectively, in HEK-293T cells via transient transfection using Lipofectamine 2000, and co-IP assays were performed with Flag antibody and Western blottings carried out with HA antibody. Inputs of LXRα and RXRs expression from the 5% lysate were analyzed by Western blotting with indicated antibodies. B, Myc-Ajuba was cotransfected with Flag-RXRα, Flag-RXRβ, Flag-RXRγ (1 μg), respectively, in HEK-293T cells, and co-IP assays were performed with Flag antibody. Ajuba and RXRs expression from the 5% of total lysate was analyzed by Western blotting with indicated antibodies. C and D, Flag-LXRα and HA-RXRα or HA-RXRγ, together with increasing amount of Myc-Ajuba as indicated in the figure, were coexpressed in HEK-293T cells, and co-IP assays were performed with Flag antibody. The coeluted proteins were detected by Western blot analysis with Flag, HA, and Myc antibodies, respectively.
Ajuba is required for LXRα and RXRγ binding at the endogenous target promoters
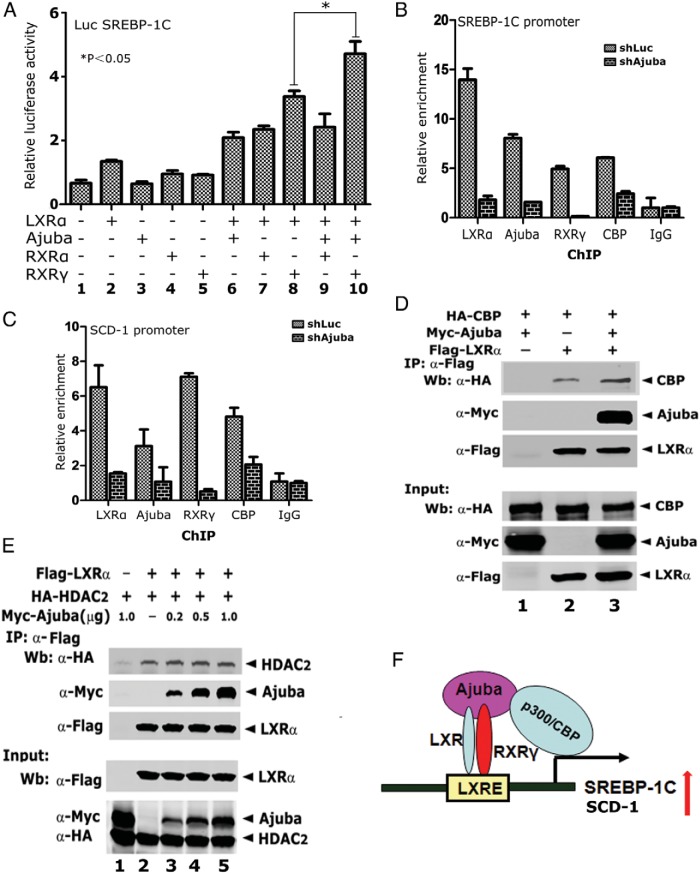
To examine whether the Ajuba, LXRα, and RXRγ ternary complex is preferred in transactivating LXRα target promoter activity, we performed luciferase reporter assays on SREBP-1C promoter in HepG2 cells. As predicted, coexpression of RXRγ and LXRα stimulated stronger SREBP-1C promoter activity than that of coexpression of RXRα and LXRα (Figure 6A, columns 7 and 8); coexpression of Ajuba, RXRγ and LXRα further stimulated SREBP-1C promoter activity, whereas coexpression of Ajuba, RXRα, and LXRα did not (Figure 6A, columns 9 and 10), suggesting that Ajuba, RXRγ, and LXRα ternary complex is more effective in transactivation of LXRα target gene expression.
Figure 6. Depletion of Ajuba results in decreased DNA binding of LXRα and RXRγ in HepG2 cells.
A, Luciferase reporter assays on SREBP-1C promoter in HEK-293T cells. Experiments were done in triplicate and were repeated 3 times. Data shown was from 1 experiment in triplicate. B and C, ChIP assays in HepG2-shLuc and HepG2-shAjuba cells were performed using antibodies specific to LXRα, Ajuba, RXRγ, and CBP. The coeluted DNA fragments were amplified by qPCR using primers flanking the functional LXREs at the promoters of SREBP-1C and SCD-1, respectively. Data shown were an average of 3 independent experiments. *, P < .05. D, Ajuba increases the binding between CBP and LXRα. co-IP assays were performed in 293T cells transiently expressing HA-CBP, Flag-LXRα, and Myc-Ajuba, using Flag antibody. E, Ajuba does not affect the interaction between LXRα and HDAC2. co-IP assays were performed as that in D. Experiments were repeated twice. F, Working model for Ajuba/LXRα/RXRγ ternary complex to control LXR target gene expression. Ajuba enhances LXRα/RXRγ heterodimer formation and also recruits CBP to acetylate histones and activate gene expression.
To further examine the assembly of Ajuba, RXRγ and LXRα ternary complex at endogenous chromatins, we performed ChIP assays in HepG2-shLuc and HepG2-shAjuba cells using antibodies specific to Ajuba, RXRγ, LXRα, and CBP, respectively. The enriched DNA fragments were amplified by qPCR using primer sets flanking the functional LXRE sites of SREBP-1C and SCD-1 promoters. In HepG2-shLuc cells, LXRα, Ajuba, RXRγ, and CBP were found to be associated with SREBP-1C and SCD-1 promoters (Figure 6, B and C). Strikingly, depletion of Ajuba in HepG2 cells resulted in drastically decreased binding of Ajuba, LXRα RXRγ and CBP at the target chromatins (Figure 6, B and C).
To further examine whether Ajuba could enhance the recruitment of CBP to LXRα, we coexpressed Flag-LXRα, HA-CBP, and/or Myc-Ajuba either alone or in combinations, and co-IP assays were performed with Flag antibody. The results showed that expression of Ajuba increased the recruitment of CBP to LXRα (Figure 6D), but did not affect the binding of LXRα and HDAC2 (Figure 6E). Collectively, these data demonstrate that Ajuba is required for LXRα/RXRγ heterodimer occupancy as well as CBP recruiting at the target chromatins.
Discussion
The LIM protein Ajuba is characterized by 3 C-terminal LIM domains and an N-terminal preLIM region and contains both nuclear localization and nuclear exporting signals, which attribute Ajuba the capacity of shuttling between the cytoplasm and nucleus (16). In cells Ajuba functions as a scaffold participating in assembly of many protein complexes involved in cell adhesion, mitosis, microRNA processing, differentiation and transcriptional regulation (19–23). In the nucleus, Ajuba acts as a corepressor for various transcription factors such as Gfi-1, Snail, and ISI-1 by recruiting HDACs and protein arginine methyltransferase 5 to reprogram the histone marks (19, 24, 25).
We previously discovered that Ajuba contains 3 conserved functional NR boxes (LXXLL) mediating a direct interaction with RARα and functions as a typical corepressor for RARα in a ligand-dependent manner (17). Of the 3 NR boxes, 1 is located in the preLIM region, and the other 2 are located in the LIM region, but each of 3 NR boxes by itself is sufficient to bind RARα. However, in this report, we demonstrate that Ajuba binds to LXRα in a different manner: its LIM region is the dominate binding site for LXRα, and the preLIM only weakly binds LXRα; instead of repression, Ajuba functions as an obligate coactivator to enhance LXR target gene expression in liver cells. Although the detailed molecular mechanism by which Ajuba exerts distinct effects on LXRα and RARα is not clear, we postulate that different binding modules may affect Ajuba to further recruit repressors or activators to LXRα or RARα. Additionally, we also examined the interaction between Ajuba and PPARγ and found that the conserved NR boxes of Ajuba do not contribute to the interaction between Ajuba and PPARγ, instead, a region in the preLIM (not the first NR box) is required for the interaction with PPARγ (our unpublished data). Ajuba further recruits p300/CBP to PPARγ and enhances PPARγ target gene expression. The binding activities of Ajuba to nuclear receptors are reminiscence of coactivator of PGC-1, which interacts with PPARα via its NR boxes, but interaction with PPARγ is independent of these NR boxes. We also observed that Ajuba help to recruit CBP to LXR target chromatins evidenced by ChIP assays in HepG2-shAjuba cells (Figure 6). To better understand the interaction between Ajuba and nuclear receptors, a more detailed physical mapping of the binding motifs from these molecules will be helpful to find the “switch” controlling Ajuba molecule to be a corepressor or coactivator.
LXRα usually forms heterodimer with RXRα, RXRβ, or RXRγ to control target gene expression. The dimerization with RXRs may enhance the DNA binding of LXRα, and also help to recruit coregulators such as PGC-1α, SRCs, and p300/CBP (26–28). In mouse liver, LXRα, RXRα and γ are highly expressed, suggesting their predominant roles in transcriptional control of lipid metabolic genes (29–31). In this report, we observed that Ajuba strongly binds RXRγ, and only weakly binds RXRα and β; Ajuba promotes LXRα/RXRγ heterodimer formation, and the Ajuba/LXRα/RXRγ ternary complex displays higher transactivation activity to LXR target promoters. These observations suggest that Ajuba may preferentially affect a subset of gene expression controlled by LXRα/RXRγ. Ajuba can increase the DNA binding and facilitate p300/CBP recruitments, which is evidenced by the ChIP assays in HepG2 cells. Depletion of Ajuba greatly reduced the occupancies of LXRα, RXRγ, and CBP at the target gene promoters.
In summary, our studies have shown that Ajuba functions as coactivator to enhance LXR target gene expression by forming ternary complex with LXR and RXR, suggesting that Ajuba may play important role in regulating LXR-mediated lipid and glucose metabolism and may be a useful target for future therapeutics in the treatment of diabetes and fatty liver disease.
Acknowledgments
We thank Dr Eugene Y. Chin for the gift of the CBP plasmids and Dr Etienne Lefai for the pGL3-SREBP-1C-Luc reporters.
This work was supported by the Ministry of Sciences and Technology of China Grant 2012BAI02B05; National Science Foundation of China Grants 81172028, 81372309, and 81270317; and Shanghai Committee of Science and Technology Grants 13JC1401302, 12PJ1405800, 13ZR1423300, 11410709000, and 124119a5700.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Ministry of Sciences and Technology of China Grant 2012BAI02B05; National Science Foundation of China Grants 81172028, 81372309, and 81270317; and Shanghai Committee of Science and Technology Grants 13JC1401302, 12PJ1405800, 13ZR1423300, 11410709000, and 124119a5700.
Footnotes
- ChIP
- chromatin immunoprecipitation
- co-IP
- coimmunoprecipitation
- LXR
- liver X receptor
- RXR
- retinoid X receptor
- SCD-1
- stearoyl-CoA desaturase 1
- SREBP
- sterol regulatory element-binding protein
- T090
- T0901317.
References
- 1. Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008;59(suppl 7):31–55. [PubMed] [Google Scholar]
- 2. Steffensen KR, Gustafsson JA. Putative metabolic effects of the liver X receptor (LXR). Diabetes. 2004;53(suppl 1):S36–S42. [DOI] [PubMed] [Google Scholar]
- 3. Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045. [DOI] [PubMed] [Google Scholar]
- 4. Janowski BA, Grogan MJ, Jones SA, et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc Natl Acad Sci USA. 1999;96(1):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JM, Gang GT, Kim DK, et al. Ursodeoxycholic acid inhibits liver X receptor α-mediated hepatic lipogenesis via induction of the nuclear corepressor SMILE. J Biol Chem. 2014;289(2):1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wojcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online). 2007;61:736–759. [PubMed] [Google Scholar]
- 7. Hu X, Li S, Wu J, Xia C, Lala DS. Liver X receptors interact with corepressors to regulate gene expression. Mol Endocrinol. 2003;17(6):1019–1026. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28(1):91–106. [DOI] [PubMed] [Google Scholar]
- 9. Nedumaran B, Kim GS, Hong S, et al. Orphan nuclear receptor DAX-1 acts as a novel corepressor of liver X receptor α and inhibits hepatic lipogenesis. J Biol Chem. 2010;285(12):9221–9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20(7):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ 3rd, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev Cell. 2008;14(3):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou Z, Peng H, Ayyanathan K, et al. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol. 2008;28(10):3198–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratt SJ, Epple H, Ward M, Feng Y, Braga VM, Longmore GD. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J Cell Biol. 2005;168(5):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kisseleva M, Feng Y, Ward M, Song C, Anderson RA, Longmore GD. The LIM protein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKI α. Mol Cell Biol. 2005;25(10):3956–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng Y, Longmore GD. The LIM protein Ajuba influences interleukin-1-induced NF-κB activation by affecting the assembly and activity of the protein kinase Cζ/p62/TRAF6 signaling complex. Mol Cell Biol. 2005;25(10):4010–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanungo J, Pratt SJ, Marie H, Longmore GD. Ajuba, a cytosolic LIM protein, shuttles into the nucleus and affects embryonal cell proliferation and fate decisions. Mol Biol Cell. 2000;11(10):3299–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou Z, Peng H, White DE, et al. LIM protein Ajuba functions as a nuclear receptor corepressor and negatively regulates retinoic acid signaling. Proc Natl Acad Sci USA. 2010;107(7):2938–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goyal RK, Lin P, Kanungo J, Payne AS, Muslin AJ, Longmore GD. Ajuba, a novel LIM protein, interacts with Grb2, augments mitogen-activated protein kinase activity in fibroblasts, and promotes meiotic maturation of Xenopus oocytes in a Grb2- and Ras-dependent manner. Mol Cell Biol. 1999;19(6):4379–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ayyanathan K, Peng H, Hou Z, et al. The Ajuba LIM domain protein is a corepressor for SNAG domain-mediated repression and participates in nucleocytoplasmic shuttling. Cancer Res. 2007;67(19):9097–9106. [DOI] [PubMed] [Google Scholar]
- 20. Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114(5):585–598. [DOI] [PubMed] [Google Scholar]
- 21. Marie H, Pratt SJ, Betson M, et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with α-catenin. J Biol Chem. 2003;278(2):1220–1228. [DOI] [PubMed] [Google Scholar]
- 22. Abe Y, Ohsugi M, Haraguchi K, Fujimoto J, Yamamoto T. LATS2-Ajuba complex regulates γ-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580(3):782–788. [DOI] [PubMed] [Google Scholar]
- 23. James V, Zhang Y, Foxler DE, et al. LIM-domain proteins, LIMD1, Ajuba, and WTIP are required for microRNA-mediated gene silencing. Proc Natl Acad Sci USA. 2010;107(28):12499–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montoya-Durango DE, Velu CS, Kazanjian A, et al. Ajuba functions as a histone deacetylase-dependent co-repressor for autoregulation of the growth factor-independent-1 transcription factor. J Biol Chem. 2008;283(46):32056–32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou Z, Peng H, White DE, et al. 14–3-3 binding sites in the Snail protein are essential for Snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res. 2010;70(11):4385–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huuskonen J, Fielding PE, Fielding CJ. Role of p160 coactivator complex in the activation of liver X receptor. Arterioscler Thromb Vasc Biol. 2004;24(4):703–708. [DOI] [PubMed] [Google Scholar]
- 27. Unno A, Takada I, Takezawa S, et al. TRRAP as a hepatic coactivator of LXR and FXR function. Biochem Biophys Res Commun. 2005;327(3):933–938. [DOI] [PubMed] [Google Scholar]
- 28. Mitro N, Mak PA, Vargas L, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445(7124):219–223. [DOI] [PubMed] [Google Scholar]
- 29. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58(4):760–772. [DOI] [PubMed] [Google Scholar]
- 31. Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. [DOI] [PubMed] [Google Scholar]