Abstract
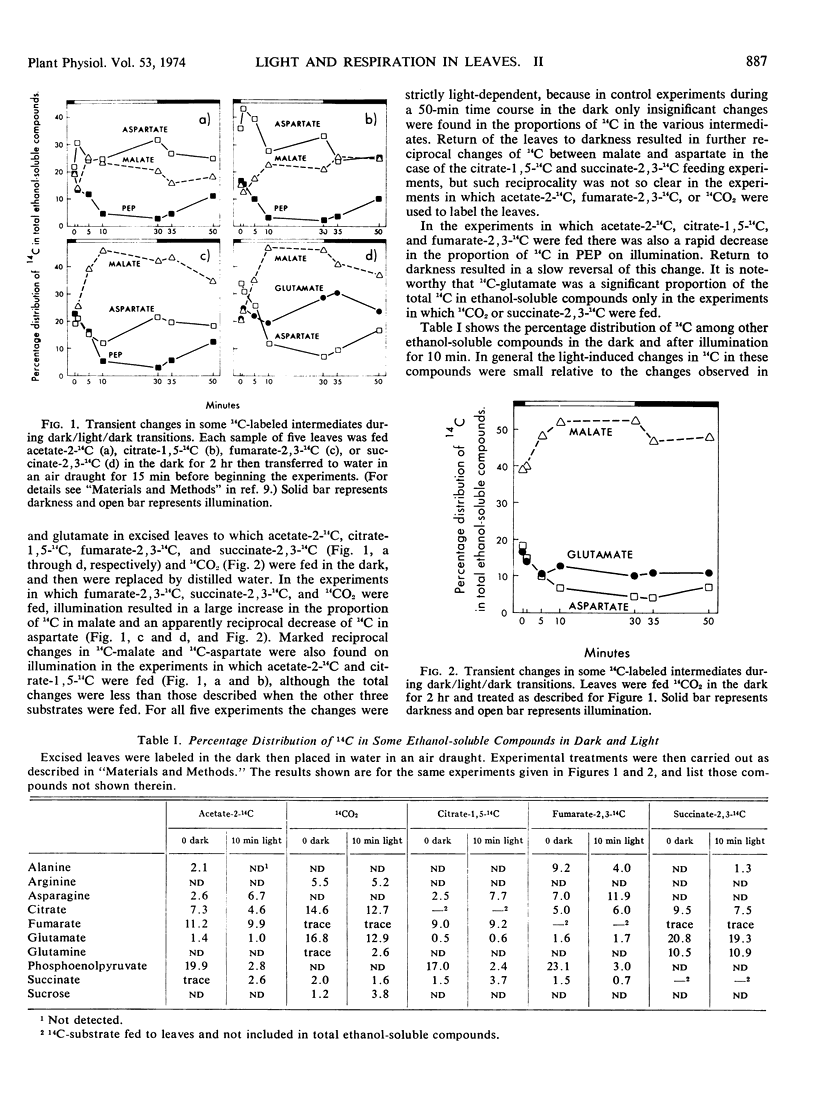
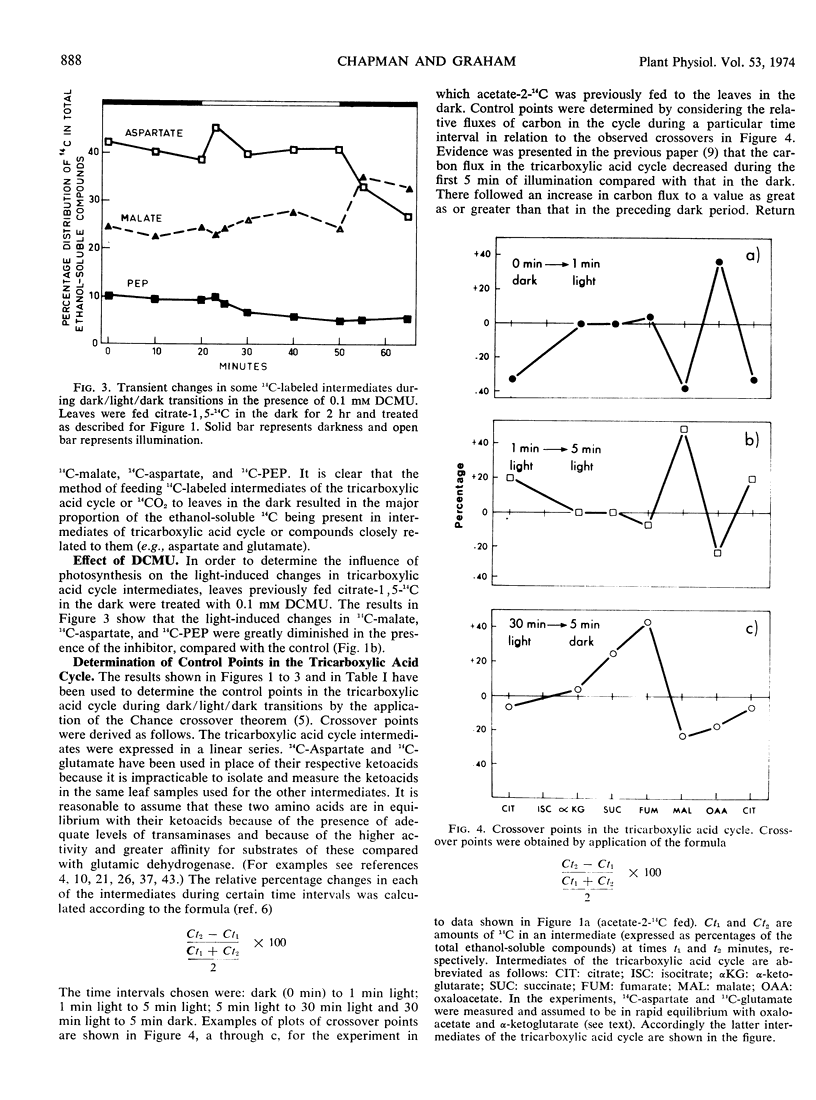
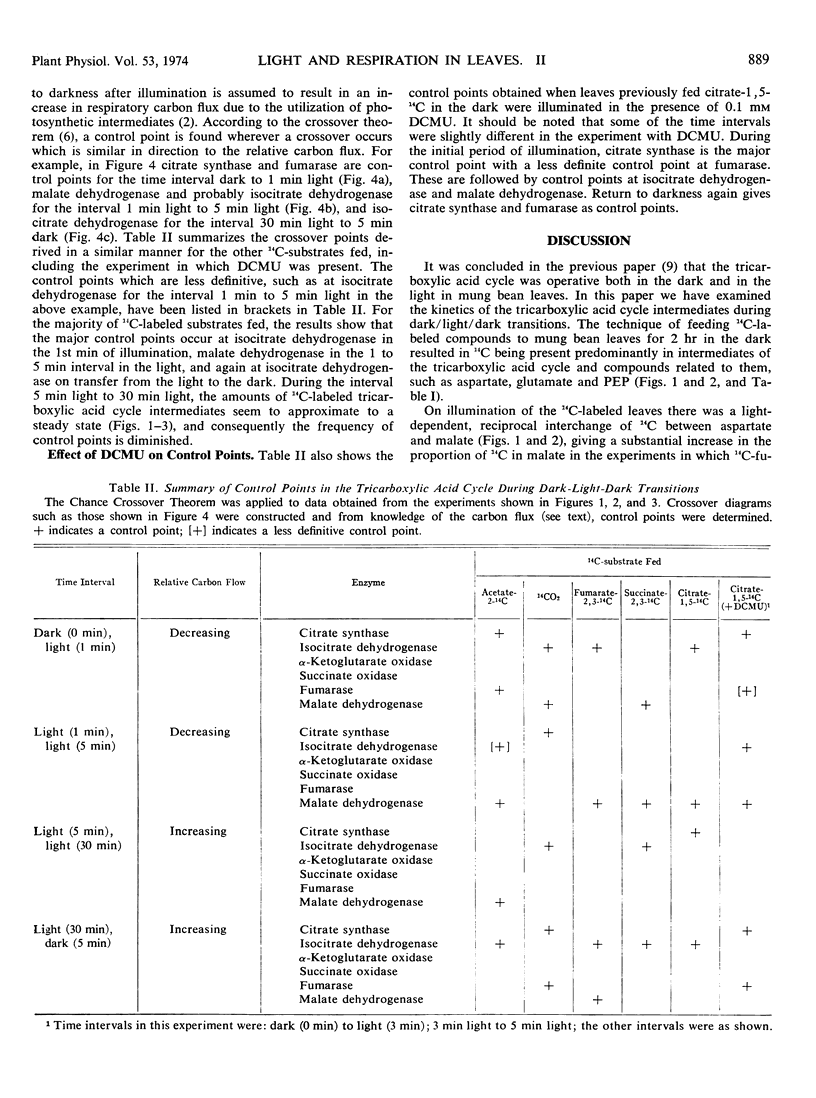
Long term feeding of acetate-2-14C, 14CO2, citrate-1,5-14C, fumarate-2,3-14C, and succinate-2,3-14C to mung bean (Phaseolus aureus L. var. Mungo) leaves in the dark gave labeling predominantly in tricarboxylic acid cycle intermediates. Kinetics of the intermediates during dark/light/dark transitions showed a light-induced interchange of 14C between malate and aspartate, usually resulting in an accumulation of 14C in malate and a decrease of it in aspartate. 14C-Phosphoenolpyruvate also showed a marked decrease during illumination. Changes in other intermediates of the tricarboxylic acid cycle were relatively minor. The kinetic data have been analyzed using the Chance crossover theorem to locate control points during the dark/light/dark transitions. The major apparent control points are located at malate and isocitrate dehydrogenases, and less frequently at citrate synthase and fumarase. These findings are explained in terms of the light-induced changes in adenine nucleotides and nicotinamide adenine dinucleotides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER J., KHAN M. A., SOLOMOS T. MECHANISM OF THE PASTEUR EFFECT. Nature. 1964 Mar 14;201:1126–1127. doi: 10.1038/2011126a0. [DOI] [PubMed] [Google Scholar]
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- Bogin E., Wallace A. The inhibition of lemon citrate-condensing enzyme by ATP. Biochim Biophys Acta. 1966 Oct 17;128(1):190–192. doi: 10.1016/0926-6593(66)90158-5. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R., HOLMES W. F., HIGGINS J. Respiratory enzymes in oxidative phosphorylation. V. A mechanism for oxidative phosphorylation. J Biol Chem. 1955 Nov;217(1):439–451. [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The effect of light on the tricarboxylic Acid cycle in green leaves: I. Relative rates of the cycle in the dark and the light. Plant Physiol. 1974 Jun;53(6):879–885. doi: 10.1104/pp.53.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K. H., Splittstoesser W. E. Glutamate dehydrogenase from pumpkin cotyledons: characterization and isoenzymes. Plant Physiol. 1972 Apr;49(4):550–554. doi: 10.1104/pp.49.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Regulation of the nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase from a higher plant. The effect of reduced nicotinamide adenine dinucleotide and mixtures of citrate and isocitrate. J Biol Chem. 1970 Aug 10;245(15):3751–3754. [PubMed] [Google Scholar]
- GRAHAM D., WALKER D. A. Some effects of light on the interconversion of metabolites in green leaves. Biochem J. 1962 Mar;82:554–560. doi: 10.1042/bj0820554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Chance B. Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun. 1964 Jun 1;16(2):174–181. doi: 10.1016/0006-291x(64)90357-2. [DOI] [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- Harley J. L., Beevers H. Acetate Utilization by Maize Roots. Plant Physiol. 1963 Jan;38(1):117–123. doi: 10.1104/pp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M. J., Brown A. P. Nicotinamide cofactors of intact chloroplasts isolated on a sucrose density gradient. Biochim Biophys Acta. 1969 Jan 14;172(1):116–125. doi: 10.1016/0005-2728(69)90096-6. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M. Differences between the reactivity of endogenous and exogenous adenine nucleotides in mitochondria as studied at low temperature. Eur J Biochem. 1968 Mar;4(1):1–8. doi: 10.1111/j.1432-1033.1968.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of Organic Acids in Corn Roots II. The Cytoplasmic Pool of Malic Acid. Plant Physiol. 1966 Apr;41(4):713–717. doi: 10.1104/pp.41.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of organic acids in corn roots I. Differential labeling of 2 malate pools. Plant Physiol. 1966 Apr;41(4):709–712. doi: 10.1104/pp.41.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- Osmond C. B., Laties G. G. Compartmentation of malate in relation to ion absorption in beet. Plant Physiol. 1969 Jan;44(1):7–14. doi: 10.1104/pp.44.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner P. E., Cohen L. H. Effects of adenosine triphosphate and magnesium ions on the fumarase reaction. J Biol Chem. 1969 Feb 10;244(3):1070–1075. [PubMed] [Google Scholar]
- SANWAL B. D., ZINK M. W., STACHOW C. S. CONTROL OF DPN-SPECIFIC ISOCITRIC DEHYDROGENASE ACTIVITY BY PRECURSOR ACTIVATION AND END PRODUCT INHIBITION. Biochem Biophys Res Commun. 1963 Aug 20;12:510–515. doi: 10.1016/0006-291x(63)90325-5. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T., Beevers H. Compartmentation of Organic Acids in Corn Roots. III. Utilization of Exogenously Supplied Acids. Plant Physiol. 1967 Sep;42(9):1197–1201. doi: 10.1104/pp.42.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R. Chloroplast Isolation in Nonaqueous Media. Plant Physiol. 1959 Jan;34(1):56–61. doi: 10.1104/pp.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGER J. M., SLATER E. C. SYNTHESIS OF GLUTAMATE FROM ALPHA-OXOGLUTARATE AND AMMONIA IN RAT-LIVER MITOCHONDRIA. I. COMPARISON OF DIFFERENT HYDROGEN DONORS. Biochim Biophys Acta. 1963 Oct 1;77:227–245. doi: 10.1016/0006-3002(63)90495-5. [DOI] [PubMed] [Google Scholar]
- WARD P. F., PETERS R. A. The chemical and biochemical properties of fluorocitric acid. Biochem J. 1961 Mar;78:661–668. doi: 10.1042/bj0780661. [DOI] [PMC free article] [PubMed] [Google Scholar]


