Abstract
The blood-brain barrier (BBB) or blood-spinal cord barrier (BSCB) formed by capillary endothelial cells provides a physical wall between the central nervous system (CNS) and circulating blood with highly selective permeability. BBB/BSCB disruption by activation of matrix metalloproteinases (MMPs) has been shown to result in further neurological damage after CNS injury. Recently it has been discovered that estrogen attenuates BBB/BSCB disruption in in vitro and in vivo models. However, the molecular mechanism underlying the estrogen-mediated attenuation of BBB/BSCB disruption has not been elucidated fully. In the present study, we found that 17β-estradiol (E2) suppresses nuclear factor-κB-dependent MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene activation in microvessel endothelial bEnd.3 cells subjected to oxygen and glucose deprivation/reperfusion injury. E2 induced the recruitment of ERα and nuclear receptor corepressor to the nuclear factor-κB binding site on the MMPs' gene promoters. Consistently, ER antagonist ICI 182.780 showed opposite effects of E2. We further found that E2 attenuates tight junction disruption through the decreased degradation of tight junction proteins in bEnd.3 cells subjected to oxygen and glucose deprivation-reperfusion injury. In addition, E2 suppressed the up-regulation of MMP expression, leading to a decreased BSCB disruption in the injured spinal cord. In conclusion, we discovered the molecular mechanism underlying the protective role of estrogenin BBB/BSCB disruption using an in vitro and in vivo model. Our study suggests that estrogens may provide a potential therapeutic intervention for preserving BBB/BSCB integrity after CNS injury.
The blood-brain barrier (BBB) or blood-spinal cord barrier (BSCB) is a physical wall between the central nervous system (CNS) and circulating blood, which exhibits highly selective permeability to maintain CNS homeostasis. The BBB/BSCB consists of capillary endothelial cells, pericytes, and astrocytic feet. The plasma membrane and the astrocytic foot are separated by a basal membrane made of collagen IV, fibronectin, and heparin sulfate proteoglycans. Collectively the BBB/BSCB with glia and neurons constitutes the neurovascular unit (1, 2). Endothelial cells are connected by tight junctions, which are composed of a group of integral membrane proteins, Claudin, Occludin, and junction adhesion molecules. Several cytoplasmic accessory proteins including zonula occludens (ZO) and cingulin also are involved in tight junction formation (3). The BBB/BSCB controls tightly the flow of neurotoxic amino acid and prevents the penetration of hazardous macromolecules such as plasminogen into CNS tissue, which would cause eventual cell death (2, 4). The vascular endothelial cells express several transporters that allow the entry of glucose, nucleosides, amine, and ions for CNS homeostasis (5). However, degradation of junctional proteins followed by disruption of the BBB/BSCB occurs frequently in many neurological diseases such as meningitis, epilepsy, Alzheimer's disease, Parkinson's disease, and multiple sclerosis as well as traumatic CNS injury and ischemia (2, 6). The BBB/BSCB disruption causes an increased permeability, blood extravasation, and immune cell infiltration, leading to the detrimental effects on the progression of injury such as excitotoxicity and neuronal loss.
Recently steroid hormones such as glucocorticoids, estrogens, progestogens, and vitamin D have been shown to attenuate BBB/BSCB disruption in in vitro and in vivo models. For example, glucocorticoid treatment induces an increased level of tight junction proteins and improved BBB in vascular endothelial cells (7, 8). In addition, glucocorticoids inhibit BBB disruption and leukocyte infiltration in experimental autoimmune encephalomyelitis (9). In acute ischemic stroke, glucocorticoids in combination with proteasome inhibitor reduce BBB disruption, leading to less brain edema and fewer functional deficits (10). Progesterone inhibits matrix metalloproteinases (MMPs) and inflammatory gene expression, thereby reducing BBB disruption and infarct size after brain injury (11).
Estrogen reduces the BBB/BSCB disruption induced by transient cerebral ischemia in ovariectomized or male rats (12–14). Consistently, agonists for estrogen receptors (ERs) have been found to attenuate brain edema and BBB permeability after traumatic brain injury (15, 16). Tamoxifen, a selective ER modulator, significantly inhibits inflammatory genes including TLR4, IL-1β, TNF-α, and IL-6, resulting in reduced brain edema and BBB impairment in rats after subarachnoid hemorrhage (17). In addition, estrogen prevents the major side effect of tissue plasminogen activator treatment for ischemic stroke, ie, it reduces BBB damage, leading to decreased hemorrhage (18). Several mechanisms for estrogen-mediated inhibition of BBB/BSCB disruption have been suggested. For instance, estrogen suppresses the expression of MMPs, which are responsible for the degradation of tight junction proteins (14, 19–21). Interestingly, the Claudin 5 tight junctional protein is activated directly by estrogen in vascular endothelial cells (22). However, the exact molecular mechanisms behind the protective effects of estrogen in the BBB/BSCB have yet to be uncovered fully. For example, it is unclear whether estrogen acts through a genomic or nongenomic mechanism. If signaling of estrogen is mediated through ERs, which ER (ERα, ERβ) has a vital role in BBB/BSCB integrity? In addition, it remains to be determined what the target genes of estrogen that may regulate vascular endothelial cell permeability and apoptosis are.
In the present study, we investigated the molecular mechanism underlying estrogen-mediated attenuation of BBB/BSCB disruption using brain microvessel endothelial bEnd.3 cells subjected to oxygen-glucose deprivation (OGD)/reperfusion injury, which may mimic pathological situations including free radical production and depletion of oxygen and glucose after CNS injury (23–26). We also examined the protective effects of estrogen on BSCB disruption in male rat after spinal cord injury.
Materials and Methods
Cell culture and OGD/reperfusion injury
Mouse brain microvessel endothelial cell line bEnd.3 was purchased from American Type Culture Collection and maintained in DMEM supplemented with 10% fetal bovine serum and antibiotics. OGD/reperfusion was performed as described previously (26). Briefly, bEnd.3 cells were subjected to 6 hours of OGD followed by 1 hour of reperfusion. In addition, 10 nM 17β-estradiol (E2) (Sigma-Aldrich) or 10 nM ICI 182.780 (Tocris) was treated during OGD/reperfusion injury.
Spinal cord injury
Adult Sprague Dawley rats (male; 230–250 g; Samtako) were subjected to contusion injury (10 g × 25 mm) at the T9-T10 level spinal cord as described previously (27). For the sham-operated controls, animals underwent a T9-T10 laminectomy without weight-drop injury. All surgical interventions and postoperative animal care were performed in accordance with the Guidelines and Polices for Rodent Survival Surgery provided by the Animal Care Committee of the Kyung Hee University.
Estrogen administration
Rat was iv injected with 300 μg/kg of E2 (2-hydroxypropyl-β-cyclodextrin-encapsulated; Sigma-Aldrich) dissolved in sterile 0.1 M PBS immediately after spinal cord injury. A second and third injection was performed at 6 and 24 hours after spinal cord injury with the same dose (28). As a vehicle control, β-cyclodextrin was injected at the same interval.
Evaluation of BSCB permeability
At 24 hours after spinal cord injury, the permeability of BSCB was investigated with Evans blue dye extravasation (28). Briefly, 5 mL of 2% Evans blue dye (Sigma) solution was ip injected. Three hours later, rats were anesthetized and perfused with saline by intracardiac perfusion. The spinal cord segment (3 mm) was removed and homogenized in a 50% trichloroacetic acid solution. After centrifugation, the supernatant was collected and fluorescence was quantified using a spectrophotometer at an excitation wavelength of 620 nm and an emission wavelength of 680 nm. Dye in supernatant was determined from a standard curve plotted using known amounts of dye.
RNA isolation and quantitative PCR analysis
After the OGD/reperfusion injury, total RNA was isolated and real-time PCR analysis was performed as described previously (26). In addition, total RNA was isolated from the spinal cord segment (10 mm), centered at the lesion site. PCR amplification was achieved using the oligonucleotide primers described in Supplemental Table 1.
Promoter reporter assay
Mouse MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene promoters were amplified and cloned into pGL4.15 [luc2P/Hygro] vector (Promega). The primers for cloning are described in Supplemental Table 2. Mutation of estrogen response element (ERE) or nuclear factor-κB (NF-κB) binding site in MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene promoters was performed by PCR using primers described in Supplemental Table 2. Mouse bEnd.3 cells were transfected transiently with MMP gene promoter-driven firefly luciferase (1 μg) in conjunction with a control thymidine kinase promoter-driven renilla luciferase (100 ng). After transfection for 36 hours, cells were subjected to OGD/reperfusion injury and harvested for luciferase activity using the dual-luciferase assay system (Promega) with a Lumat BL 9507 luminometer (Berthold technologies). Renilla luciferase activity served as an internal control for normalizing firefly luciferase activity of the gene promoter.
Western blot analysis and immunoprecipitation
Western blotting and immunoprecipitation were performed as described previously (26). After the OGD/reperfusion injury, cells were harvested for protein extraction. Anti-ERα (1:1000, sc542; Santa Cruz Biotechnology), anti-ERβ (1:1000, sc8974; Santa Cruz Biotechnology), anti-NF-κB-p65 (1:1000, ab7970; Abcam), antinuclear receptor corepressor (N-CoR; 1:5000, cs207360; Millipore), anti-MMP-1 (1:1000, sc241565; Santa Cruz Biotechnology), anti-MMP-2 (1:5000, ab79781; Abcam), anti-MMP-3 (1:5000, ab53015; Abcam), anti-MMP-9 (1:5000, 1AB19016; Millipore), anti-MMP-10 (1:1000, sc26697; Santa Cruz Biotechnology), anti-MMP-13 (1:1000, sc30073; Santa Cruz Biotechnology), and anti-β-actin antibodies (1:5000, YF-MA-10008; Abfrontier) were used. For immunoprecipitation, cell lysates were immunoprecipitated with 1 μg of anti-ERα antibody (sc542; Santa Cruz Biotechnology). Normal IgG (sc2027; Santa Cruz Biotechnology) was used as a control. Western blots were analyzed quantitatively using the ImageJ software (National Institutes of Health, Bethesda, Maryland).
Immunocytochemistry
After the OGD/reperfusion injury, cells were fixed and immunocytochemistry was performed as described previously (26). Anti-ERα (1:100, sc542; Santa Cruz Biotechnology), anti-ERβ (1:100, sc8974; Santa Cruz Biotechnology), anti-Claudin 5 (1:500, ab53765; Abcam), and anti-Occludin (1:500, ab31721; Abcam) were used. Nuclei were identified using 4′,6′-diamino-2-phenylindole (DAPI) staining. Images were acquired with a confocal microscope (Leica TCS SPE; Leica).
RNA interference (small interfering RNA [siRNA])
siRNA against mouse ERα (M-058688-01-0005, Dharmacon), ERβ (M-065564-00-0005; Dharmacon), and NF-κB p65 (M-040776-01-0005; Dharmacon), and N-CoR (1390518; Bioneer) were used. Control siRNA (sc37007; Santa Cruz Biotechnology) was used for a negative control. After 50 μM siRNA transfection in a six-well plate using the X-treamGENE siRNA transfection reagent (Roche) for 48 hours, cells were subjected to OGD/reperfusion injury. The efficiency of knockdown of specific gene was confirmed with real-time PCR.
Chromatin immunoprecipitation (ChIP)
After OGD/reperfusion injury, cells were harvested and ChIP was performed as described previously (26). Soluble chromatin from cells grown in a 10-cm dish was immunoprecipitated with 2 μg of antibodies. Anti-ERα (sc542; Santa Cruz Biotechnology), anti-NF-κB p65 (ab7970; Abcam), anti-N-CoR (cs207360; Millipore), and antiphosphorylated RNA polymerase II antibodies (ab5131; Abcam) were used. Normal IgG (sc2027; Santa Cruz Biotechnology) was used as the ChIP control. Quantitative PCR was performed using the primers described in Supplemental Table 3.
Measurement of transendothelial electrical resistance (TEER)
After the OGD/reperfusion injury, endothelial permeability was determined by measuring TEER as described previously (29). TEER across the monolayers grown on the filter membranes was measured using the Millicell ERS Voltohmmeter (Millipore), and the values are shown as ohms per square centimeter based on culture inserts. The TEER of cell-free inserts was subtracted from that of filters with cells (29).
Statistical analyses
All quantitative data are presented as mean ± SEM for three independent experiments. The differences between two groups were evaluated by a paired t test. Significance values were as follows: *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .005.
Results
E2 suppresses the up-regulation of MMP gene expression in brain microvessel endothelial bEnd.3 cells subjected to OGD/reperfusion injury
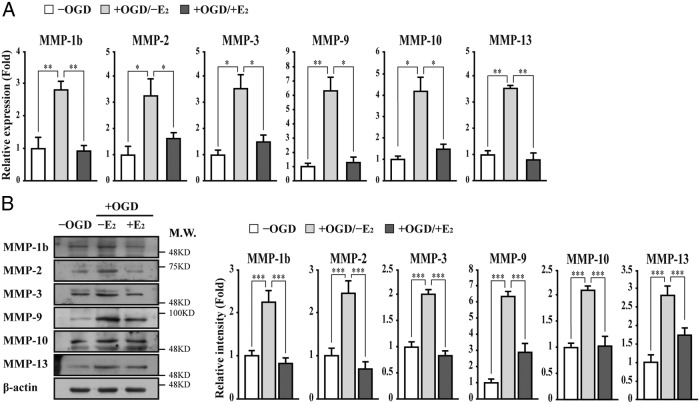
Given that estrogens have beneficial effects on the integrity of the BBB/BSCB and MMPs play critical roles in the disruption of the BBB, we first examined MMPs gene expression in mouse brain microvessel endothelial bEnd.3 cells subjected to OGD/reperfusion injury (26). Among the MMPs we tested, the expression of MMP-1b (interstitial collagenase), MMP-2 (gelatinase A), MMP-3 (stromelysin-1), MMP-9 (gelatinase B), MMP-10 (stromelysin-2), MMP-13 (collagenase-3), MMP-20 (enamelysin), and MMP-21 was up-regulated in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 1A and Supplemental Figure 1). However, E2 treatment suppressed significantly the OGD/reperfusion-induced gene activation of certain MMPs such as MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 (Figure 1A). Western blot analysis further confirmed E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 1B).
Figure 1. E2 suppresses the up-regulation of MMP expression in brain microvessel endothelial bEnd.3 cells subjected to OGD/reperfusion injury.
The expression of MMPs was determined in bEnd.3 cells subjected to OGD/reperfusion injury with or without 10 nM E2. A, The expression of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene is up-regulated in bEnd.3 cells subjected to OGD/reperfusion (−OGD vs + OGD/−E2). However, E2 treatment suppresses OGD/reperfusion-induced up-regulation of the MMP genes (+OGD/−E2 vs +OGD/+E2). Transcripts of MMPs and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. B, Lysates were immunoblotted with anti-MMP-1b, anti-MMP-2, anti-MMP-3, anti-MMP-9, anti-MMP-10, and anti-MMP-13 antibodies, respectively. The anti-β-actin antibody was used as a loading control. Western blots were analyzed quantitatively. All data represent mean ± SEM for three independent experiments. *, P ≤ .05; **, P ≤ .01; ***, P ≤ .005.
ERα mediates E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury
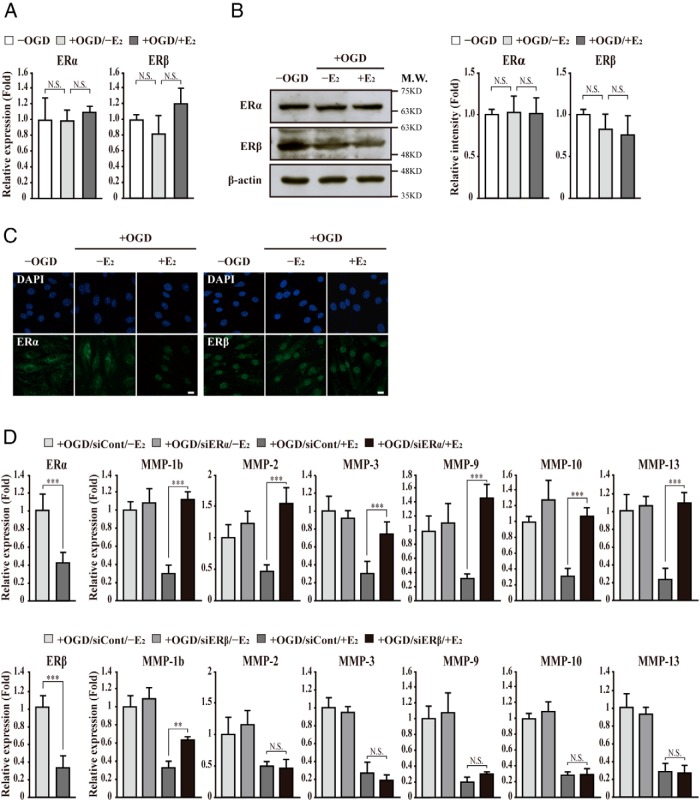
To investigate whether this effect is mediated by ERs, the expression of ERα and ERβ was determined by quantitative PCR, Western blot analysis, and immunocytochemistry in bEnd.3 cells subjected to OGD/reperfusion injury. The expression levels of ERα and ERβ were not significantly changed by E2 treatment (Figure 2, A and B). Translocation of ERα into the nucleus was observed by E2 treatment, whereas ERβ localization in the nucleus was not changed (Figure 2C). To clarify the requirement of ERα or ERβ in the E2-induced suppression of MMP gene activation, we applied RNA interference. Both ERα and ERβ siRNA efficiently depleted transcripts of ERα and ERβ, respectively (Figure 2D). ERα depletion significantly abolished E2-induced suppression of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene activation by OGD/reperfusion injury. In contrast, ERβ depletion showed minimal effects on E2-induced suppression of MMP gene activation except the MMP-1b gene (Figure 2D). These results indicate that ERα may be a major ER that mediates E2-induced suppression of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene activation in bEnd.3 cells subjected to OGD/reperfusion injury.
Figure 2. ERα mediates E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury.
A and B, The expression of ERs was determined in bEnd.3 cells subjected to OGD/reperfusion with or without E2. The expression levels of ERα and ERβ are not changed by E2 treatment in bEnd.3 cells subjected to OGD/reperfusion injury. Transcripts of ERα, ERβ, and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. In addition, lysates were immunoblotted with anti-ERα and anti-ERβ antibodies, respectively. Western blots were analyzed quantitatively. C, Representative photomicrographs of ERα and ERβ in bEnd.3 cells. Cells were immunostained with anti-ERα and anti-ERβ antibodies, respectively (n = 3). E2 induces ERα translocation into the nucleus in bEnd.3 cells subjected to OGD/reperfusion injury. However, ERβ localizes constantly in the nucleus with or without OGD/reperfusion injury. Nuclei were identified using DAPI staining. Scale bar, 20 μm. D, After control (siCont) or ER siRNA (siER) was transfected, the transcripts of ERα, ERβ, MMPs, and GAPDH were determined by quantitative PCR. The ERα and ERβ transcripts were efficiently depleted by ERα and ERβ siRNA, respectively. Depletion of ERα attenuates the E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/siCont/+E2 vs +OGD/siERα/+E2). However, the depletion of ERβ does not affect the E2-induced suppression of MMP gene activation significantly except MMP-1b (+OGD/siCont/+E2 vs +OGD/siERβ/+E2). All data represent mean ± SEM for three independent experiments. **, P ≤ .01; ***, P ≤ .005.
NF-κB p65 is required for both OGD/reperfusion injury-induced MMPs gene activation and E2-induced suppression of MMPs gene expression in bEnd.3 cells
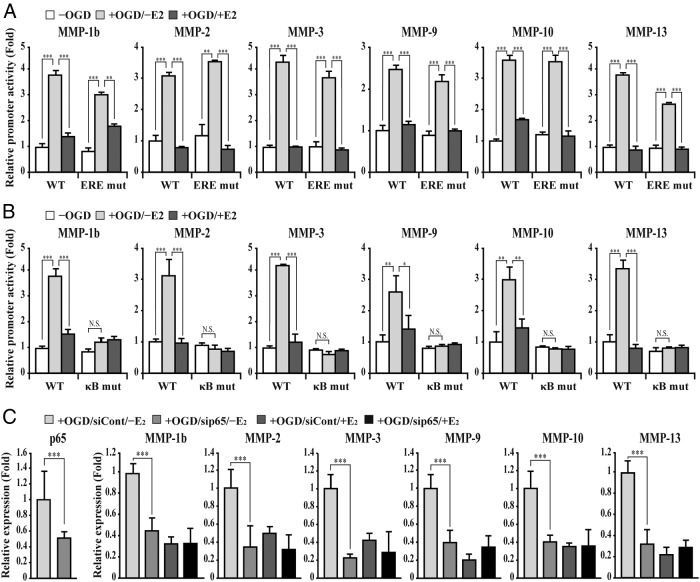
We next performed a reporter assay using the MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene promoter luciferase reporters, respectively. As expected, each of the MMPs' gene promoter activity elevated significantly in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 3A). In addition, E2 treatment suppressed the up-regulated promoter activities of MMP genes in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 3A). To test whether the ERE on the MMP gene promoter is required for MMP gene repression by E2 treatment, we identified as putative ERE in the MMP gene promoter using in silico analysis (Supplemental Figure 2). Unexpectedly, mutations of putative ERE have marginal or no effects on E2-induced suppression of up-regulated promoter activities of MMP genes by OGD/reperfusion injury (Figure 3A). Consistent with our results, ERα is not enriched at the MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene promoters in E2-treated MCF-7 cells and mouse uterus when we reanalyzed genome-wide ChIP data, which have been published previously (30–33).
Figure 3. NF-κB p65 is required for both OGD/reperfusion injury-induced MMP gene activation and E2-induced suppression of MMP gene expression in bEnd.3 cells.
A, Cells were transiently transfected with the wild or mutant type of mouse MMP gene promoter-driven firefly luciferase reporter vector in conjunction with a control renilla luciferase expression vector. Reporter activity is represented as fold activation relative to renilla luciferase activity. Activities of reporters containing MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene promoter are elevated in bEnd.3 cells subjected to OGD/reperfusion injury (−OGD vs +OGD/−E2). In addition, E2 suppresses up-regulated activities of promoter reporters in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/−E2 vs +OGD/+E2). However, ERE mutations do not affect activities of MMP gene promoter in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2. B, Mutations of NF-κB binding site significantly attenuates up-regulated MMP gene promoter activities (−OGD vs +OGD/−E2). However, marginal or no change of the mutated promoter activities was observed in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2 treatment (+OGD/−E2 vs +OGD/+E2). C, After control or NF-κB p65 siRNA was transfected, transcripts of p65, MMPs, and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. NF-κB p65 transcript was depleted efficiently by siRNA. Depletion of NF-κB p65 attenuates MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/siCont/−E2 vs +OGD/sip65/−E2). In addition, the depletion of p65 does not alter the MMP gene expression in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2 treatment (+OGD/sip65/−E2 vs +OGD/sip65/+E2). All data represent mean ± SEM for three independent experiments. *, P ≤ .05; **, P ≤ .01; ***, P ≤ .005.
Given that NF-κB activation is involved in MMP gene activation and ERs negatively regulate target genes of NF-κB through diverse mechanisms (34–45), we first examined whether an intact NF-κB binding site is required for MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury. When NF-κB binding sites of MMP genes were mutated, none of the promoter activities of the MMP genes elevated significantly by OGD/reperfusion injury (Figure 3B). Furthermore, we found marginal or no change of the mutated promoter activities in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2 treatment, indicating that NF-κB is required for both OGD/reperfusion injury-induced MMP gene activation and E2-induced suppression of MMP gene expression (Figure 3B). To confirm the NF-κB p65-dependent MMP gene regulation, a p65 transcript was depleted by siRNA. As shown in Figure 3C, p65 depletion significantly abolished OGD/reperfusion-induced MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene activation. Consistently, we found marginal or no change of MMP gene expression in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2 treatment.
E2 induces repressive activity of NF-κB by forming ERα/N-CoR/NF-κB association on the promoter of MMPs gene in bEnd.3 cells subjected to OGD/reperfusion injury
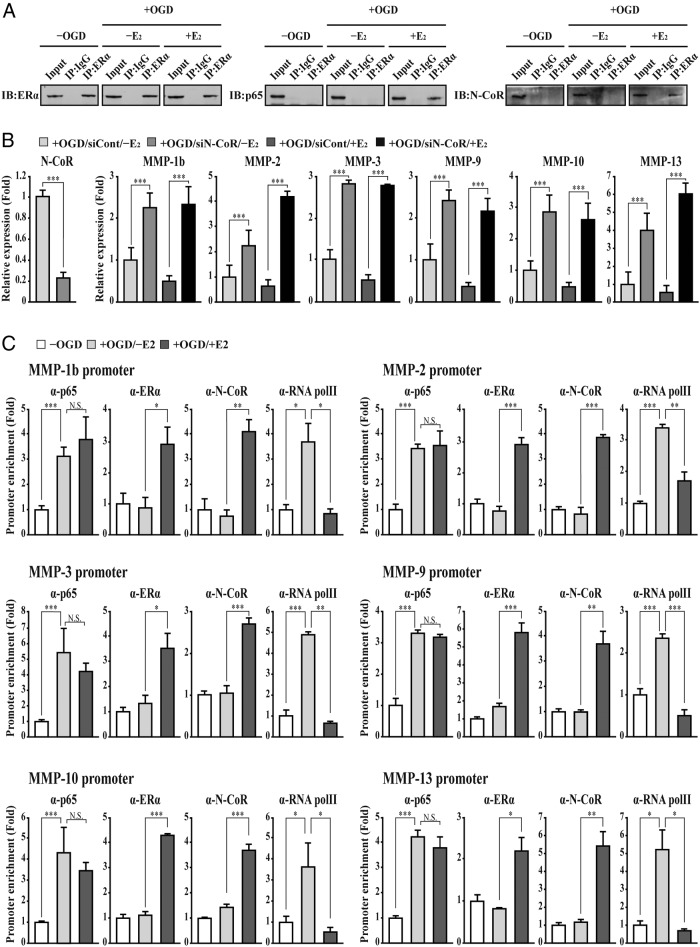
We next investigated E2-induced ERα interaction with NF-κB p65 in bEnd.3 cells subjected to OGD/reperfusion injury. Although ERα did not interact with p65 in untreated and OGD/reperfusion-treated bEnd.3 cells, E2 treatment induced ERα interaction with p65 by OGD/reperfusion injury (Figure 4A). We also tested whether ERα interacts with the N-CoR corepressor, which is involved in E2-induced gene repression, in bEnd.3 cells subjected to OGD/reperfusion injury. Similar to previous studies (46–49), E2 treatment induced ERα interaction with N-CoR by OGD/reperfusion injury (Figure 4A). To confirm the requirement of N-CoR in E2-induced suppression of MMP gene activation, N-CoR transcripts were depleted by siRNA and quantitative PCR was performed. Similar to ERα depletion, N-CoR depletion significantly abolished E2-induced suppression of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 4B). Interestingly, we also found that depletion of N-CoR elevates MMP gene expression in bEnd.3 cells subjected to OGD/reperfusion injury without E2 treatment. Among several possibilities, we tested whether NF-κB p65 gene expression is negatively regulated by N-CoR. In fact, depletion of N-CoR increases NF-κB p65 gene expression, resulting in the up-regulation of MMP gene expression in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 4B and Supplemental Figure 3). Using a ChIP assay, we examined the recruitment of ERα, NF-κB p65, N-CoR, and phosphorylated RNA polymerase II to the MMP gene promoters. Chromatin was immunoprecipitated with the anti-ERα, anti-p65, N-CoR, and anti-RNA polymerase II antibody, respectively. Occupancy of each protein at the MMP gene promoter was determined using oligonucleotide primers to amplify the region of the NF-κB binding sites by quantitative PCR. Upon OGD/reperfusion injury, p65 and RNA polymerase II, but not ERα, significantly bound to the NF-κB binding site of the MMP gene promoters. In addition, E2 treatment induced recruitment of ERα and N-CoR in OGD/reperfusion-treated bEnd.3 cells (Figure 4C). The occupancy of p65 did not change in OGD/reperfusion-treated cells with or without E2 treatment. Collectively our findings suggest that E2 induces ERα to interact with p65 and N-CoR, resulting in the suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury.
Figure 4. E2 induces repressive transcription activity of NF-κB by forming an ERα/N-CoR/NF-κB association on the promoter of MMP genes in bEnd.3 cells subjected to OGD/reperfusion injury.
A, E2 induces ERα interaction with NF-κB p65 and N-CoR in bEnd.3 cells subjected to OGD/reperfusion injury. Lysates were immunoprecipitated with anti-ERα antibody and immunoblotted with anti-ERα, p65, and N-CoR antibodies, respectively (n = 3). Immunoprecipitation with normal IgG was used for a negative control. B, After control or N-CoR siRNA was transfected, transcripts of N-CoR, MMPs, and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. N-CoR transcript was depleted efficiently by siRNA. The depletion of N-CoR attenuates E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/siCont/+E2 vs +OGD/siN-CoR/+E2). In addition, depletion of N-CoR elevates MMP gene expression in bEnd.3 cells subjected to OGD/reperfusion injury without E2 treatment (+OGD/siCont/−E2 vs +OGD/siN-CoR/−E2). C, A ChIP assay was performed using the anti-ERα, p65, N-CoR, and phosphorylated RNA polymerase II antibodies, respectively. The occupancy of each protein was quantified with real-time PCR at the gene promoter region encompassing the NF-κB binding site. ChIP using normal IgG was performed as a negative control. Recruitment of NF-κB p65 and RNA pol II increases to the promoter of MMP genes in bEnd.3 cells subjected to OGD/reperfusion injury (−OGD vs +OGD/−E2). E2 induces the recruitment of ERα and N-CoR to the promoter of MMP genes in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/−E2 vs +OGD/+E2). Simultaneously, RNA polymerase II occupancy is decreased by E2 treatment (+OGD/−E2 vs +OGD/+E2). However, NF-κB p65 occupancy is not changed in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2 treatment (+OGD/−E2 vs +OGD/+E2). All data represent mean ± SEM for three independent experiments. *, P ≤ .05; **, P ≤ .01; ***, P ≤ .005.
Effects of ER antagonist on MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury
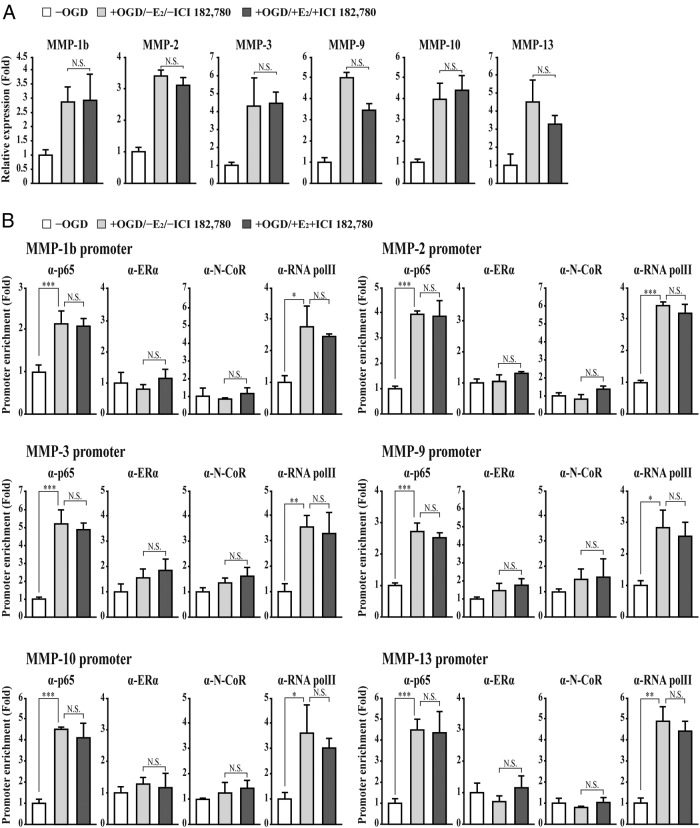
To further confirm our results, we used the ER antagonist ICI 182.780, which causes a conformational change and accelerated degradation of the ERα (50). ICI 182.780 abolished the E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 5A). Consistently, ICI 182.780 treatment abolished the E2-mediated recruitment of ERα and N-CoR to the MMP gene promoter in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 5B).
Figure 5. ER antagonist ICI 182.780 attenuates the E2-injuced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury.
A, The expression of MMPs was determined in bEnd.3 cells subjected to OGD/reperfusion with or without E2+ICI 182.780 treatment. Transcripts of MMPs and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. Inhibition of ERα by ICI 182.780 attenuates E2-induced suppression of MMP gene activation in bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/−E2/−ICI 182.780 vs +OGD/+E2/+ICI 182.780). B, Recruitment of NF-κB p65 and RNA polymerase II increases to the promoter of MMP genes in bEnd.3 cells subjected to OGD/reperfusion injury (−OGD vs +OGD/−E2). However, ICI 182.780 inhibits the recruitment of ERα and N-CoR on the promoter of MMP genes in E2-treated bEnd.3 cells subjected to OGD/reperfusion injury (+OGD/−E2/−ICI 182.780 vs +OGD/+E2/+ICI 182.780). The occupancies of RNA polymerase II and NF-κB p65 are not changed in bEnd.3 cells subjected to OGD/reperfusion injury with or without E2+ICI 182.780 treatment. (+OGD/−E2/−ICI 182.780 vs +OGD/+E2/+ICI 182.780). A ChIP assay was performed using the anti-ERα, p65, N-CoR, and phosphorylated RNA polymerase II antibodies, respectively. The occupancy of each protein was quantified with real-time PCR at the gene promoter region encompassing the NF-κB binding site. A ChIP using normal IgG was performed as a negative control. All data represent mean ± SEM for three independent experiments. ***, P ≤ .005.
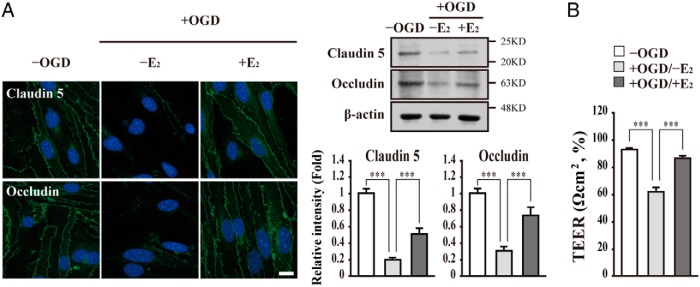
E2 enhances the integrity of tight junction proteins in bEnd.3 cells subjected to OGD/reperfusion injury
MMPs play an important role in BBB/BSCB integrity of via regulation of tight junctional proteins' stability (14, 51–53). Therefore, we investigated whether E2 treatment affects the stability of tight junctional proteins such as Claudin 5 and Occludin in bEnd.3 cells subjected to OGD/reperfusion injury. We first examined the protein level of tight junction proteins by Western blot analysis and immunocytochemistry. The levels of Claudin 5 and Occludin were decreased in bEnd.3 cells subjected to OGD/reperfusion injury (Figure 6A). However, E2 treatment attenuated the decreased levels of Claudin 5 and Occludin by OGD/reperfusion injury (Figure 6A). Consistently, OGD/reperfusion injury induced a decrease in TEER as compared with the untreated control. Parallel with the above results, E2 treatment resulted in no significantly decreased TEER in bEnd.3 cell subjected to OGD/reperfusion injury (Figure 6B).
Figure 6. E2 enhances tight junction integrity in bEnd.3 cells subjected to OGD/reperfusion injury.
A, Representative photomicrographs of Claudin 5 and Occludin in bEnd.3 cells. E2 attenuates the degradation of Claudin 5 and Occludin in bEnd.3 cells subjected to OGD/reperfusion injury. Cells were immunostained with anti-Claudin 5 and Occludin antibodies, respectively (n = 3). Nuclei were identified using DAPI staining. Scale bar, 20 μm. Lysates were immunoblotted with anti-Claudin 5 and Occludin antibodies, respectively. Western blots were analyzed quantitatively. B, E2 increases TEER in bEnd.3 cells subjected to OGD/reperfusion injury. TEER was determined using a voltohmmeter (Millipore) in bEnd.3 cells subjected to OGD/reperfusion with or without E2. All data represent mean ± SEM for three independent experiments. ***, P ≤ .005.
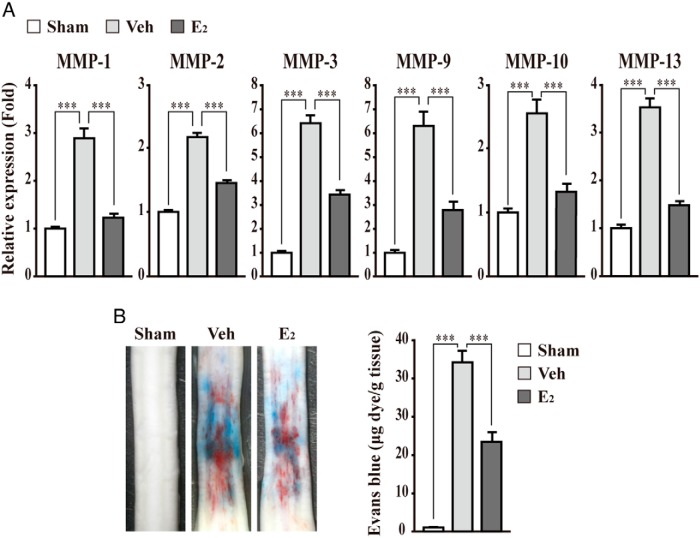
E2 suppresses up-regulation of MMP gene expression and BSCB disruption in injured spinal cord
We next wanted to recapitulate the protective role of E2 on BBB/BSCB disruption using an in vivo animal model. A male rat was injected with E2 after a spinal cord injury (28). Consistent with the in vitro cell model, the expression of the MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene is up-regulated significantly in an injured spinal cord. In addition, E2 treatment suppresses up-regulated MMPs gene expression (Figure 7A). Given that up-regulated MMP expression contributes the disruption of BBB/BSCB, resulting in hemorrhage after a spinal cord injury, we performed a hemorrhage assay using Evans blue dye. As expected, E2 treatment significantly reduced hemorrhage in the injured spinal cord as compared with the control groups (Figure 7B).
Figure 7. E2 suppresses the up-regulation of MMP gene expression and BSCB disruption in an injured spinal cord.
A, The expression of MMPs was determined in the injured spinal cord of a male rat injected with E2 (300 μg/kg). E2 treatment suppresses the up-regulated MMP gene expression in injured spinal cord. Transcripts of MMPs and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR. As controls, rats were sham operated (Sham) or injected with β-cyclodextrin (Veh) as described in Materials and Methods. B, Representative photographs of leakage of Evans blue dye after the spinal cord injury. E2 treatment suppresses hemorrhage, showing decreased leakage of Evans blue in the injured spinal cord. Hemorrhage was measured at 1 day after the spinal cord injury using Evans blue dye. The quantification of Evans blue extravasation was performed using a fluorometer (excitation at 620 nm and emission at 680 nm). All data represent mean ± SEM for three independent experiments. ***, P ≤ .005.
In conclusion, our results suggest that estrogen may maintain the integrity of the BBB/BSCB through the down-regulation of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene expression, which is directly related to tight junction degradation in microvessel endothelial cells subjected to OGD/reperfusion injury. Consistently, estrogen attenuated the disruption of BSCB though the suppression of MMP expression in the injured spinal cord.
Discussion
Disruption of BBB/BSCB by activated MMPs has deleterious effects on neurological damage after a CNS injury. Estrogen has been shown to attenuate BBB/BSCB disruption in in vitro and in vivo models. However, the molecular mechanism underlying estrogen-mediated attenuation of BBB/BSCB disruption has not been elucidated fully. In this study, we report for the first time that as far as we know, E2 attenuates up-regulated MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene expression by binding of ERα/NF-κB/N-CoR to the NF-κB binding site on the MMP gene promoters, further resulting in decreased BBB/BSCB disruption.
It has been previously shown that MMPs contribute to BBB/BSCB disruption in many pathological situations via the degradation of the tight junction and basal lamina proteins (14, 51–53). In this study, we found that several MMP genes such as MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 are up-regulated in bEnd.3 cells subjected to OGD/reperfusion injury. Consistently, MMP-1 is up-regulated in the infarcted tissues after ischemic stroke or spinal cord injury (54–56). Moreover, activation of MMP-1 is related to BBB disruption in some infectious diseases (57, 58). MMP-2 seems to be associated with the degradation of tight junction proteins and components of the basal lamina around the cerebral blood vessels, leading to BBB disruption in the early stages after cerebral ischemia (59, 60). After a spinal cord injury, expression of MMP-2 is also up-regulated (61, 62). Significant reduction of hemorrhage has been observed in MMP-2 knockout (KO) mice after ischemic injury (63). MMP-3 is up-regulated in infiltrated neutrophils and blood vessels in injured spinal cords as well as brain vascular endothelial cells subjected to OGD injury (45, 51). MMP-3 KO mice further exhibit less degradation of the tight junction proteins of the BBB and reduced neutrophil infiltration as compared with wild-type mice by intracerebral lipopolysaccharide (LPS) injection or spinal cord injury (51, 64). In addition, MMP-3 seems to activate MMP-9 (51, 65). Expression of MMP-9 is also up-regulated in brain vascular endothelial cells and infiltrated neutrophils in the early phase after cerebral ischemia or spinal cord injury (14, 61, 62, 66–68).
It has been well reported that MMP-9 degrades tight junction proteins and extracellular matrix (ECM), leading to BBB/BSCB disruption. In fact, MMP-9 KO mice demonstrated reduced infarct size with reduced degradation of the ZO-1 tight junction protein after cerebral ischemia (69). In addition, the pharmacological blockade of MMP-9 inhibits BBB disruption after a traumatic brain injury (70). Although the physiological function of MMP-10 has not been characterized well in BBB regulation, it is up-regulated in the human brain after ischemic stroke and brain microvascular endothelial cells treated with HIV tat protein (55, 71). Because MMP-10 can activate several MMPs including MMP-1 and MMP-10, it may regulate BBB integrity (72). Expression of MMP-13 is increased in damaged hippocampal vessels and the brain after cerebral ischemic stroke (55, 73). MMP-13 leads to the disorganization of ZO-1 and hyperpermeability of the BBB in response to hypoxia (74).
Our study also demonstrates that E2 attenuates the up-regulation of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene expression in bEnd.3 cells subjected to OGD/reperfusion injury. Similarly, previous studies demonstrated that E2 treatment attenuates MMP-1, MMP-2, MMP-9, and MMP-13 gene repression (13, 19–21). Although two forms of ER (ERα, ERβ) are expressed in the CNS endothelium (75, 76), we found that ERα mainly mediated the suppression of OGD/reperfusion-induced up-regulation of MMP gene expression, given that depletion of ERβ did not affect MMPs gene expression significantly compared with ERα depletion. In fact, ERα rather than ERβ is involved in estrogen-mediated protection after brain injury (77). In addition, the ERα-specific agonist (propylpyrazoletriol), but not the ERβ-specific agonist (diarylpropionitrile), protects bEnd.3 endothelial cells against OGD/reperfusion-induced cell death (25). Similarly, E2 treatment induces MMP-1, MMP-2, and MMP-13 gene repression in an ERα-dependent manner (19–21). However, we cannot exclude the possibility that ERβ or both ERs play important roles in BBB regulation. ERβ has been shown to be required for vascular structural integrity by tight junction Claudin 5 gene activation in cerebral endothelial cells and ERβ−/− mice with no stress treatment (78). The ERβ agonist also attenuates BBB disruption in the cortex through the inhibition of vascular endothelial growth factor and hypoxia-inducible factor-1α expression, but not Claudin 5, after transient focal ischemia (16). This discrepancy may be caused by differences in the types of endothelial cells, stimuli, and animal models as well as different target genes used in the studies. Recently it has been reported that both ERs are recruited to the Claudin 5 gene promoter in response to E2-treated cEND cells (22). Similarly, MMP-1b gene expression seems to be regulated by both ERs because the depletion of ERα or ERβ attenuated significantly MMP-1b gene activation by OGD/reperfusion injury (Figure 2D).
Several different mechanisms are involved in the E2-mediated suppression of NF-κB target gene activation. For instance, E2 inhibits binding activity of NF-κB to the IL-6 gene promoter (34, 43). Ligand-bound ER interacts with NF-κB and suppresses MCP-1 and IL-8 gene activation by displacing the cAMP response element-binding protein coactivator (40). In addition, E2 inhibits LPS-induced inflammatory gene activation through the prevention of NF-κB p65 translocation into the nucleus (35). Although N-CoR-independent repression of TNFα gene expression by E2 treatment has been reported (36, 79), our results from ChIP and siRNA experiments clearly indicate that E2 induces ERα/NF-κB/N-CoR association on the MMP gene promoters for MMP gene repression. Similarly, E2 inhibits the up-regulation of FRα, vascular endothelial growth factor receptor-2, p21, and PROS1 gene expression through ERα interaction with N-CoR (46–49). Depletion of N-CoR further results in the up-regulation of MMPs in bEnd.3 cells subjected to OGD/reperfusion via the up-regulation of NF-κB gene expression (Figure 4B and Supplemental Figure 3). Similarly, it has been reported that the zinc finger and BTB domain containing 2/N-CoR/histone deacetylase complex binds to p65 proximal promoter and represses p65 gene expression (80).
As we demonstrate in this study, NF-κB has a critical function in the regulation of BBB integrity in response to diverse injury stimuli (81). NF-κB activation by LPS or TNFα induces the tight junction disruption of vascular endothelial cells, leading to BBB disruption (29, 82, 83). Previous studies have demonstrated the requirement of NF-κB for the up-regulation of mouse MMP-1, MMP-2, MMP-9, and MMP-13 genes (37, 39, 42, 44). Although there has been no report on the existence of a functional NF-κB binding site within mouse MMP-3 and MMP-10 gene promoters, we found a putative NF-κB binding site using promoter reporter and ChIP assays. In addition, NF-κB-dependent MMP-3 and MMP-10 gene activation in various mouse cells was reported previously (38, 41, 45).
Our study further demonstrates that E2 attenuates tight junction disruption through the down-regulation of MMP-1b, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 gene expression in bEnd.3 cells subjected to OGD/reperfusion injury, showing no or marginal decreased level of tight junction protein and TEER. Previous studies support the protective roles of estrogen in the BBB/BSCB integrity in diverse CNS diseases and injuries. For example, estrogen or ER agonists reduce BBB/BSCB disruption in rodents after an ischemic or traumatic CNS injury (12–16). E2 treatment also reduces the expression and activity of MMP-2 and MMP-9 in ovariectomized rats in response to cerebral ischemia-reperfusion injury (13). Similarly, our study demonstrated that E2 attenuates BSCB disruption through the suppression of up-regulated MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-13 expression in the injured spinal cord. Interestingly, we also found that the magnitude of MMP gene expression such as MMP-2 is relatively low as compared with the result from the bEnd.3 endothelial cell line model. This discrepancy may be a result of the fact that the spinal cord consists of diverse cell types such as neurons, astrocytes, endothelial cells, pericytes, and immune cells.
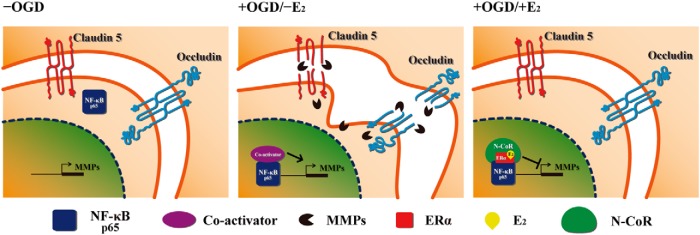
In summary, we found that E2 suppresses the NF-κB-dependent up-regulation of MMP genes by recruitment of ERα and N-CoR in microvessel endothelial bEnd.3 cells subjected to OGD/reperfusion injury (Figure 8). Given that there is no current therapeutic intervention to prevent BBB/BSCB disruption after CNS injuries, our findings suggest that estrogen or estrogen-like molecules may provide a new strategy to protect against BBB/BSCB disruption.
Figure 8. A proposed model.
Upon OGD/reperfusion injury (+OGD), NF-κB p65 translocates into the nucleus (Supplemental Figure 4). In turn, activated NF-κB p65 binds to MMP gene promoters thereby stimulates MMP gene expression in bEnd.3 cells. This event results in the degradation of tight junction proteins and BBB disruption (31, 49, 50). However, E2 treatment (+OGD/+E2) induces nuclear translocation of ERα and association of ERα with NF-κB p65 and N-CoR corepressor, which leads to the down-regulation of MMP gene expression. Therefore, E2 has a protective effect on the BBB disruption via preservation of tight junction proteins.
Acknowledgments
We thank the other members of the laboratory for technical help.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea supported by Ministry of Education Grants 2009-0093822 and 2013R1A1A2008384 (to B.-G.J.). This work also was supported by a grant from the Korea Health Technology research and development project through the Korea Health Industry Development Institute, supported by Ministry of Health and Welfare Grant HI13C14600000 (to T.Y.Y.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea supported by Ministry of Education Grants 2009-0093822 and 2013R1A1A2008384 (to B.-G.J.). This work also was supported by a grant from the Korea Health Technology research and development project through the Korea Health Industry Development Institute, supported by Ministry of Health and Welfare Grant HI13C14600000 (to T.Y.Y.).
Footnotes
- BBB
- blood-brain barrier
- BSCB
- blood-spinal cord barrier
- ChIP
- chromatin immunoprecipitation
- CNS
- central nervous system
- DAPI
- 4′,6′-diamino-2-phenylindole
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- KO
- knockout
- LPS
- lipopolysaccharide
- MMP
- matrix metalloproteinase
- N-CoR
- nuclear receptor corepressor
- NF-κB
- nuclear factor-κB
- OGD
- oxygen-glucose deprivation
- siRNA
- small interfering RNA
- TEER
- transendothelial electrical resistance
- ZO
- zonula occludens.
References
- 1. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. [DOI] [PubMed] [Google Scholar]
- 2. Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206. [DOI] [PubMed] [Google Scholar]
- 3. Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin Cell Dev Biol. 2014;38:16–25. [DOI] [PubMed] [Google Scholar]
- 4. Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drewes LR. Molecular architecture of the brain microvasculature: perspective on blood-brain transport. J Mol Neurosci. 2001;16:93–98. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero IA, Radewicz K, Jubin E, et al. . Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. [DOI] [PubMed] [Google Scholar]
- 8. Förster C, Silwedel C, Golenhofen N, et al. . Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol. 2005;565:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paul C, Bolton C. Inhibition of blood-brain barrier disruption in experimental allergic encephalomyelitis by short-term therapy with dexamethasone or cyclosporin A. Int J Immunopharmacol. 1995;17:497–503. [DOI] [PubMed] [Google Scholar]
- 10. Kleinschnitz C, Blecharz K, Kahles T, et al. . Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke. 2011;42:1081–1089. [DOI] [PubMed] [Google Scholar]
- 11. Pascual JL, Murcy MA, Li S, et al. . Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013;206:840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chi OZ, Liu X, Weiss HR. Effects of 17β-estradiol on blood-brain barrier disruption during focal ischemia in rats. Horm Metab Res. 2002;34:530–534. [DOI] [PubMed] [Google Scholar]
- 13. Liu R, Wen Y, Perez E, et al. . 17β-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. [DOI] [PubMed] [Google Scholar]
- 14. Lee JY, Choi HY, Na WH, Ju BG, Yune TY. 17β-Estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology. 2015;156:1838–1850. [DOI] [PubMed] [Google Scholar]
- 15. Asl SZ, Khaksari M, Khachki AS, Shahrokhi N, Nourizade S. Contribution of estrogen receptors α and β in the brain response to traumatic brain injury. J Neurosurg. 2013;119:353–361. [DOI] [PubMed] [Google Scholar]
- 16. Shin JA, Yang SJ, Jeong SI, et al. . Activation of estrogen receptor β reduces blood-brain barrier breakdown following ischemic injury. Neuroscience. 2013;235:165–173. [DOI] [PubMed] [Google Scholar]
- 17. Sun X, Ji C, Hu T, Wang Z, Chen G. Tamoxifen as an effective neuroprotectant against early brain injury and learning deficits induced by subarachnoid hemorrhage: possible involvement of inflammatory signaling. J Neuroinflammation. 2013;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Zhang Z, Sun W, Koehler RC, Huang J. 17β-Estradiol attenuates breakdown of blood-brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu T, Achari Y, Sciore P, Hart DA. Estrogen receptor α regulates matrix metalloproteinase-13 promoter activity primarily through the AP-1 transcriptional regulatory site. Biochim Biophys Acta. 2006;1762:719–731. [DOI] [PubMed] [Google Scholar]
- 20. Scafonas A, Reszka AA, Kimmel DB, et al. . Agonist-like SERM effects on ERα-mediated repression of MMP1 promoter activity predict in vivo effects on bone and uterus. J Steroid Biochem Mol Biol. 2008;110:197–206. [DOI] [PubMed] [Google Scholar]
- 21. Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17β-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burek M, Steinberg K, Förster CY. Mechanisms of transcriptional activation of the mouse claudin-5 promoter by estrogen receptor α and β. Mol Cell Endocrinol. 2014;392:144–151. [DOI] [PubMed] [Google Scholar]
- 23. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. [DOI] [PubMed] [Google Scholar]
- 24. Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF Stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo J, Krause DN, Horne J, et al. . Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J Cereb Blood Flow Metab. 2010;30:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee K, Na W, Lee JY, et al. . Molecular mechanism of Jmjd3-mediated interleukin-6 gene regulation in endothelial cells underlying spinal cord injury. J Neurochem. 2012;122:272–282. [DOI] [PubMed] [Google Scholar]
- 27. Yune TY, Lee JY, Jung GY, et al. . Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JY, Choi SY, OH TH, Yune TY. 17β-Estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting RhoA-JNK3 activation after spinal cord injury. Endocrinology. 2012;153:3815–3827. [DOI] [PubMed] [Google Scholar]
- 29. He F, Peng J, Deng XL, et al. . RhoA and NF-κB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience. 2011;188:35–47. [DOI] [PubMed] [Google Scholar]
- 30. Welboren WJ, van Driel MA, Janssen-Megens EM, et al. . ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hewitt SC, Li L, Grimm SA, et al. . Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol. 2012;26:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll JS, Meyer CA, Song J, et al. . Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. [DOI] [PubMed] [Google Scholar]
- 33. Handel AE, Sandve GK, Disanto G, et al. . Integrating multiple oestrogen receptor α ChIP studies: overlap with disease susceptibility regions, DNase I hypersensitivity peaks and gene expression. BMC Med Genomics. 2013;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res. 1997;25:2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghisletti S, Meda C, Maggi A, Vegeto E. 17β-Estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cvoro A, Tzagarakis-Foster C, Tatomer D, et al. . Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol Cell. 2006;21:555–564. [DOI] [PubMed] [Google Scholar]
- 37. Elsharkawy AM, Oakley F, Lin F, et al. . The NF-κB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J Hepatol. 2010;53:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kobayashi K, Arimura Y, Goto A, et al. . Therapeutic implications of the specific inhibition of causative matrix metalloproteinases in experimental colitis induced by dextran sulphate sodium. J Pathol. 2006;209:376–383. [DOI] [PubMed] [Google Scholar]
- 39. Lee SJ, Seo KW, Yun MR, et al. . 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-κB signaling pathways. Free Radic Biol Med. 2008;45:1487–1492. [DOI] [PubMed] [Google Scholar]
- 40. Nettles KW, Gil G, Nowak J, et al. . CBP Is a dosage-dependent regulator of nuclear factor-κB suppression by the estrogen receptor. Mol Endocrinol. 2008;22:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murray MY, Birkland TP, Howe JD, et al. . Macrophage migration and invasion is regulated by MMP10 expression. PLoS One. 2013;8:e63555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Kane CM, Elkington PT, Jones MD, et al. . STAT3, p38 MAPK, and NF-κB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2010;43:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-κB by the estrogen receptor. FEBS Lett. 1997;409:79–85. [DOI] [PubMed] [Google Scholar]
- 44. Rhee JW, Lee KW, Kim D, et al. . NF-κB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J Biochem Mol Biol. 2007;40:88–94. [DOI] [PubMed] [Google Scholar]
- 45. Suzuki Y, Nagai N, Yamakawa K, et al. . Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. [DOI] [PubMed] [Google Scholar]
- 46. Hao H, d'Alincourt-Salazar M, Kelley KM, et al. . Estrogen-induced and TAFII30-mediated gene repression by direct recruitment of the estrogen receptor and co-repressors to the core promoter and its reversal by tamoxifen. Oncogene. 2007;26:7872–7884. [DOI] [PubMed] [Google Scholar]
- 47. Higgins KJ, Liu S, Abdelrahim M, et al. . Vascular endothelial growth factor receptor-2 expression is down-regulated by 17β-estradiol in MCF-7 breast cancer cells by estrogen receptor α/Sp proteins. Mol Endocrinol. 2008;22:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Konduri SD, Medisetty R, Liu W, et al. . Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci USA. 2010;107:15081–15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suzuki A, Sanda N, Miyawaki Y, et al. . Down-regulation of PROS1 gene expression by 17β-estradiol via estrogen receptor α (ERα)-Sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-HDAC3 complex. J Biol Chem. 2010;285:13444–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicholson RI, Gee JM, Manning DL, et al. . Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann NY Acad Sci. 1995;761:148–163. [DOI] [PubMed] [Google Scholar]
- 51. Lee JY, Choi HY, Ahn HJ, Ju BG, Yune TY. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol. 2014;184:2985–3000. [DOI] [PubMed] [Google Scholar]
- 52. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. [DOI] [PubMed] [Google Scholar]
- 53. Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci. 2014;124:707–716. [DOI] [PubMed] [Google Scholar]
- 54. Cuadrado E, Rosell A, Penalba A, et al. . Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. [DOI] [PubMed] [Google Scholar]
- 55. Lenglet S, Montecucco F, Mach F, et al. . Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost. 2014;112:363–378. [DOI] [PubMed] [Google Scholar]
- 56. Zhou Y, Cui Z, Xia X, et al. . Matrix metalloproteinase-1 (MMP-1) expression in rat spinal cord injury model. Cell Mol Neurobiol. 2014;34:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grab DJ, Perides G, Dumler JS, et al. . Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. Infect Immun. 2005;73:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roe K, Kumar M, Lum S, et al. . West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J Gen Virol. 2012;93:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. [DOI] [PubMed] [Google Scholar]
- 60. Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang J, Wang G, Gao C, Shao G, Kang N. Effects of hyperbaric oxygen on MMP-2 and MMP-9 expressions and spinal cord edema after spinal cord injury. Life Sci. 2013;93:1033–1038. [PubMed] [Google Scholar]
- 62. Lee JY, Kim HS, Choi HY, OH TH, Yune TY. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain. 2012;135:2375–2389. [DOI] [PubMed] [Google Scholar]
- 63. Suofu Y, Clark JF, Broderick JP, et al. . Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. [DOI] [PubMed] [Google Scholar]
- 65. Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol. 2011;31:e35–e44. [DOI] [PubMed] [Google Scholar]
- 66. Justicia C, Panés J, Solé S, et al. . Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. [DOI] [PubMed] [Google Scholar]
- 67. McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Asahi M, Wang X, Mori T, et al. . Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Higashida T, Kreipke CW, Rafols JA, et al. . The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114:92–101. [DOI] [PubMed] [Google Scholar]
- 71. Woollard SM, Bhargavan B, Yu F, Kanmogne GD. Differential effects of Tat proteins derived from HIV-1 subtypes B and recombinant CRF02_AG on human brain microvascular endothelial cells: implications for blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 2014;34:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. [DOI] [PubMed] [Google Scholar]
- 73. Ueno M, Wu B, Nishiyama A, et al. . The expression of matrix metalloproteinase-13 is increased in vessels with blood-brain barrier impairment in a stroke-prone hypertensive model. Hypertens Res. 2009;32:332–338. [DOI] [PubMed] [Google Scholar]
- 74. Lu DY, Yu WH, Yeh WL, et al. . Hypoxia-induced matrix metalloproteinase-13 expression in astrocytes enhances permeability of brain endothelial cells. J Cell Physiol. 2009;220:163–173. [DOI] [PubMed] [Google Scholar]
- 75. Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-α in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–E192. [DOI] [PubMed] [Google Scholar]
- 76. Tu J, Jufri NF. Estrogen signaling through estrogen receptor β and G-protein-coupled estrogen receptor 1 in human cerebral vascular endothelial cells: implications for cerebral aneurysms. Biomed Res Int. 2013;2013:524324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dubal DB, Zhu H, Yu J, et al. . Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burek M, Arias-Loza PA, Roewer N, Förster CY. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler Thromb Vasc Biol. 2010;30:298–304. [DOI] [PubMed] [Google Scholar]
- 79. An J, Ribeiro RC, Webb P, et al. . Estradiol repression of tumor necrosis factor-α transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci USA. 1999;96:15161–15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim MY, Koh DI, Choi WI, et al. . ZBTB2 increases PDK4 expression by transcriptional repression of RelA/p65. Nucleic Acids Res. 2015;43:1609–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kielland A, Camassa LM, Døhlen G, et al. . NF-κB activity in perinatal brain during infectious and hypoxic-ischemic insults revealed by a reporter mouse. Brain Pathol. 2012;22:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trickler WJ, Mayhan WG, Miller DW. Brain microvessel endothelial cell responses to tumor necrosis factor-α involve a nuclear factor κB (NF-κB) signal transduction pathway. Brain Res. 2005;1048:24–31. [DOI] [PubMed] [Google Scholar]
- 83. Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-α induced NFκB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2012;57:269–275. [DOI] [PubMed] [Google Scholar]