Abstract
Myt3 is a prosurvival factor in pancreatic islets; however, its role in islet-cell development is not known. Here, we demonstrate that myelin transcription factor 3 (Myt3) is expressed in migrating islet cells in the developing and neonatal pancreas and thus sought to determine whether Myt3 plays a role in this process. Using an ex vivo model of islet-cell migration, we demonstrate that Myt3 suppression significantly inhibits laminin-V/integrin-β1-dependent α- and β-cell migration onto 804G, and impaired 804G-induced F-actin and E-cadherin redistribution. Exposure of islets to proinflammatory cytokines, which suppress Myt3 expression, had a similar effect, whereas Myt3 overexpression partially rescued the migratory ability of the islet cells. We show that loss of islet-cell migration, due to Myt3 suppression or cytokine exposure, is independent of effects on islet-cell survival or proliferation. Myt3 suppression also had no effect on glucose-induced calcium influx, F-actin remodeling or insulin secretion by β-cells. RNA-sequencing (RNA-seq) analysis of transduced islets showed that Myt3 suppression results in the up-regulation of Tgfbi, a secreted diabetogenic factor thought to impair cellular adhesion. Exposure of islets to exogenous transforming growth factor β-induced (Tgfbi) impaired islet-cell migration similar to Myt3 suppression. Taken together, these data suggest a model by which cytokine-induced Myt3 suppression leads to Tgfbi de-repression and subsequently to impaired islet-cell migration, revealing a novel role for Myt3 in regulating islet-cell migration.
Cell migration is a complex cellular process critical to a wide array of processes, including embryonic development, immune functions, and disease processes (1–4). The underlying mechanisms responsible for controlling the directed migration of cells, both independently and in concert with their neighbors, have been well characterized and are typically well conserved (1–9). In short, the process involves the transmission of extracellular cues, via integrins and cadherins, leading to the activation of Rho-GTPases, such as RAS-related C3 botulinum substrate 1 (Rac1) and cell division cycle 42 (Cdc42), engagement of the actin-related protein-2/3 complex, and actin polymerization, ultimately leading to the extension of the forward edge of the cell (10, 11). In conjunction, the breakdown and formation of cellular adhesions at the leading and trailing edges, and at cell-cell junctions of migrating cells is also required (8, 11, 12). Recently, the involvement of cell migration and adhesion in endocrine cell specification and in β-cell maturation and function has gained increasing interest (10, 13–20).
During pancreas development neurogenin 3 (Ngn3) induces the differentiation of endocrine precursors and initiates an epithelial-to-mesenchymal-like transition, facilitating release of these cells from the tubular epithelium. The newly escaped endocrine precursor cells aggregate into ribbon-like chords in close association with the tubular epithelium, where they are thought to become specified into the various endocrine cell fates. Islets then form from these ribbon-like chords by islet fission and/or by the outgrowth of acinar tissue that acts to separate the endocrine chords from the ducts and breaks them into recognizable islets (21). The newly formed islets proliferate to expand the islet-cell mass and undergo maturation into fully functional islets for up to several weeks postnatally (22–24). Simultaneously, the islets are further separated from the ducts and each other by the continued expansion of the acinar compartment in a process that requires islet-cell migratory machinery (15, 25) to either allow the movement of acinar cells past the islets or to allow the coordinated migration of the islets themselves. Thus, cell migration is critical for both islet formation, and the movement of the islets themselves away from the ducts. Despite this, the mechanisms regulating these processes are unclear; although impairments in wingless-type MMTV integration site family, member 5A epidermal growth factor receptor (Egfr), Cdc42, transducin-like enhancer of split 3, and Rac1 signaling inhibit normal endocrine cell delamination and migration, or the migration of islets away from ducts (10, 15, 25–28).
Myelin transcription factor 3 (Myt3) (also known as suppression of tumorigenicity 18) is a C2HC-type zinc-finger transcription factor that plays roles in regulating cell survival and proinflammatory gene expression (28, 29) and is dysregulated in certain types of cancer, including breast cancer and some types of leukemia (30, 31). We previously demonstrated that Myt3 is highly expressed in all islet-cell types in the adult and that it acts as a prosurvival factor in these cells (29). Although Myt3 has not been implicated in mediating cell migration to date, in our previous work, we noted that its expression in pancreas endocrine cell types during development is initiated in the time frame in which islet-cell migration and morphogenesis is occurring (23, 29). Thus, here, we sought to confirm whether Myt3 is present in migrating islets, and subsequently to determine what role it plays in this process.
Materials and Methods
Mouse maintenance and islet isolations
Mice were maintained according to the guidelines of the Canadian Council on Animal Care. The University of British Columbia Animal Care Committee approved all protocols. Islets were isolated as previously described (32). Briefly, pancreata from both male and female mice were perfused with 1000-U/mL Collagenase XI (Sigma-Aldrich) and incubated for 15 minutes at 37°C. Pancreata were manually disrupted and passed through a 70μM filter (Corning) and islets were handpicked. Isolated islets were allowed to recover for 4–6 hours before use in experiments. Eight- to 12-week-old C57/B6 mice, mice expressing green fluorescent protein (GFP) under the control of the mouse insulin promoter (MIP-GFP) (33) and Bak−/−:Baxfl/fl:Pdx1-CreER mice were used in these studies. Islet-specific ablation of Bax was achieved by injecting 8- to 12-week-old Bak−/−:Baxfl/fl:Pdx1-CreER mice with 0.075 mg/g of tamoxifen (Sigma-Aldrich) on 5 consecutive days (34).
Immunofluorescence and immunohistochemistry
Immunostaining was performed on whole and dispersed paraformaldehyde-fixed islets and paraffin-embedded embryonic day (E) 18.5 and postnatal day (P) 1 pancreas tissues as previously described (29). Primary antibodies included rabbit anti-Myt3 (1:1000; as described in Ref. 29), mouse anti-E-cadherin (1:250; Cell Signaling Technology), guinea pig anti-insulin (1:1000; Abcam), and guinea pig antiglucagon (1:1000; Linco). Fluorescently conjugated secondary antibodies included donkey antirabbit Alexa Fluor 488, goat antiguinea pig Alexa Fluor 594, donkey antimouse Alexa Fluor 546, and donkey antiguinea pig Alexa Fluor 633 (1:2000; Life Technologies). To detect F-actin, islets were labeled with Alexa Fluor 647 conjugated Phalloidin (1:10; Life Technologies). Images within each experiment were taken with the same laser intensity, offset, and gain settings on an SP8 confocal microscope (Leica). The brightness and contrast of the Myt3 localization images were adjusted in Photoshop to better reproduce the data. Staining controls were performed on E18.5 sections to determine cross-reactivity of primary and secondary antibodies (Supplemental Figure 1). Fluorescence intensity for E-cadherin and F-actin was determined using a line segment analysis within the Leica LAS AF confocal software.
Islet plating and transduction
Handpicked islets were plated onto 804G, a complete extracellular matrix (ECM) produced by a rat bladder carcinoma cell line, treated tissue culture dishes. Spreading islets were imaged on day 1, 3, and 7, and area was measured using ImageJ (National Institutes of Health). Islets were incubated with adenoviruses as previously described (29). To visualize F-actin, islets were transduced with 5 × 106 plaque forming units LifeAct TagRFP (ibidi). For cytokine and Tgfbi experiments, islets were incubated with 0.4-ng/mL interferon γ (IFNγ), 0.07-ng/mL interleukin 1, β (IL-1β), and 0.04-ng/mL tumor necrosis factor α (Tnfα) (eBioscience) or 50-μg/mL mouse recombinant Tgfbi (R&D Systems). Images of plated islets were taken at ×20 on an Olympus CKX41 microscope using a Canon EOS60D DSLR camera. Islet area was measured using ImageJ (National Institutes of Health).
Islet-cell migration
To image islet-cell migration in our whole islet model, we plated islets as above and transduced with the control virus at an multiplicity of infection 5 × 10−3. Islets were imaged every 6 hours for 7 days using an SP8 confocal microscope (Leica) with an attached GM-8000 gas mixing stage top incubator (Tokai Hit). For single cell migration analysis islets were dispersed and plated on 804G-treated plates at 200 islets per well and transduced as previously described (23). Cells were imaged as above every 3 hours for 7 days. Cell migration traces were determined using the chemotaxis and migration tool (ibidi).
Quantification of apoptosis and proliferation
Islets were plated on 804G-treated coverslips and transduced or treated with cytokine as above. Islets were cultured for 3 days in the presence of 1μM propidium iodide (Sigma-Aldrich) or 10μM 5-ethynyl-2-deoxyuridine (EdU) (Life Technologies) to label dead and proliferating cells, respectively. Islets were stained as per manufacturer's instructions. All islets were incubated with Topro3 (1:5000; Life Technologies) for 30 minutes before mounting. Cells were imaged with an SP8 confocal microscope (Leica), and quantification was performed with CellProfiler image analysis software (Broad Institute).
Cell adhesion and spreading
For adhesion assays, transduced islets were dispersed and plated at 5000 GFP-positive cells per well. Cells were allowed to adhere for 2 hours, washed, and fresh media added. Wells were imaged at ×4 on an Olympus CKX41 microscope using a Canon EOS60D DSLR camera. Total GFP-positive cells were quantified using CellProfiler. Cell spreading was measured using dispersed islet cells treated as above and then plated in 24-well tissue culture plates. The cells were imaged every hour using an IncuCyte ZOOM (Essen Bioscience) live cell imaging system. Transduced cell area was quantified using the IncuCyte software. For cell morphology, whole islets were transduced as above and cultured for 72 hours. Islets were then dispersed and plated on 804G-treated coverslips and cultured for a further 7 days. Cells were stained for F-actin as described above and imaged with an SP8 confocal microscope (Leica).
Islet functional assays
Calcium imaging was performed on transduced, dispersed islets as previously described (34, 35). In short, islet cells were stained with 5μM fura 2-AM (Life Technologies) for 30 minutes at 37°C in a 5% CO2 humidified incubator in 3mM glucose Ringer's solution (5.5mM KCl, 2mM CaCl2, 1mM MgCl2, 20mM HEPES, and 144mM NaCl; pH 7.4). Before imaging, coverslips were transferred to a 2ml imaging chamber, and cells were perifused with 3mM glucose Ringer's solution at a flow rate of 2.5 mL/min. Baseline intensity was measured for 10 minutes immediately after the wash. Next cells were stimulated by glucose and KCl, as indicated in the figures. Cytosolic calcium changes are represented as the ratio of fura 2-AM emission intensity after excitation at 340 and 380 nm. For glucose-induced F-actin, remodeling cells were incubated in 3mM Krebs-Ringer hepes buffer (115mM NaCl, 5mM KCl, 24mM NaHCO3, 2.5mM CaCl2, 1mM MgCl2, 10mM HEPES, and 2% wt/vol BSA) for 2 hours before treatment with either 3mM or 16.7mM glucose for 5 minutes. Cells were fixed in 2% paraformaldehyde for 10 minutes and mounted. Glucose-stimulated insulin secretion assays were performed as previously described (29) on transduced and untransduced islets with and without culture on 804G.
RNA-seq and qPCR
For RNA-sequencing (RNA-seq), islets were transduced and cultured as above for 7 days. Transduced cells were fluorescence-activated cell sorting (FACS) sorted, and cells were pooled from a minimum of 3 independent experiments. Library construction was performed using the TruSeq Sample Prep kit (Illumina) according to manufacturer's protocol and 100-bp paired-end sequencing was performed at the Canada's Michael Smith Genome Sciences Centre. TopHat and Cufflinks were used to map the reads to known University of California, Santa Cruz transcripts and to identify differentially expressed genes (36). Data are available under gene expression omnibus accession code GSE70699. For quantitative PCR (qPCR), RNA was isolated using TRIzol and the PureLink RNA purification kit. cDNA was generated using Superscript III. TaqMan probes were used to quantify β-actin, and Gapdh, all other primers were designed using Primer3plus and primer sequences are available upon request. A Viia7 real-time PCR system and SYBR Green supermix or TaqMan Fast Advanced Master Mix was used for all reactions. All qPCR reagents were purchased from Life Technologies. Ten nanograms of cDNA were used in each reaction, with all reactions done in triplicate. β-actin and Gapdh were used as internal controls and the change in expression was calculated using 2−ΔΔCt.
Statistical analysis
Statistical analysis was performed using the paired, 2-tailed Student's t test. Data from at least 3 independent experiments are represented as mean ± SEM. Statistical significance was accepted at P ≤ .05.
Results
Myt3 is expressed in developing endocrine cells undergoing migration
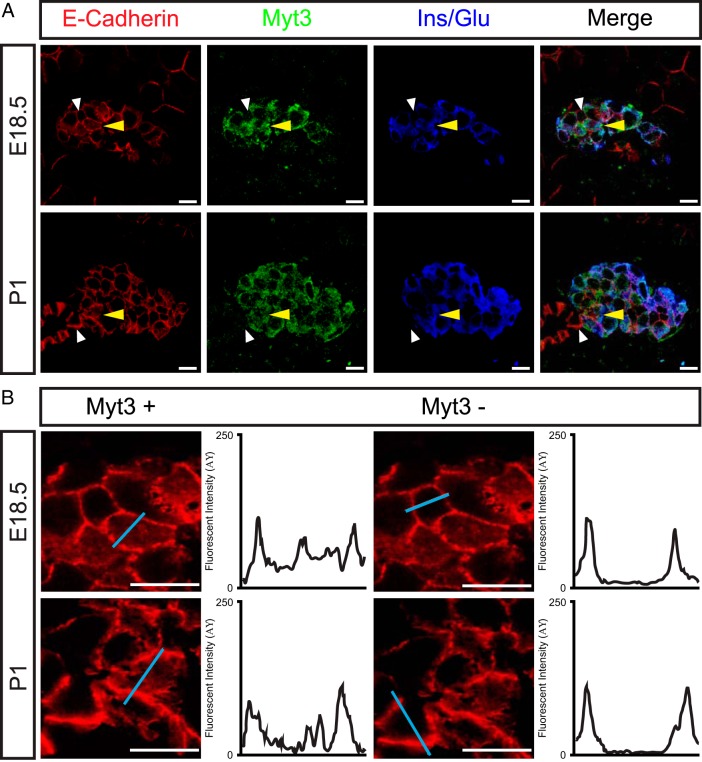
Endocrine precursors undergo an Ngn3-dependent delamination from the pancreas progenitor layer (23, 37). Because overexpression of Ngn3 also induces Myt3 expression (29), we wanted to determine whether Myt3 is expressed in cells undergoing Ngn3-induced delamination. However, at E14.5 and E16.5, time points at which Ngn3 is expressed, we did not observe any Myt3-positive cells within the developing pancreas, and only a few weakly Myt3-positive cells at E17.5 (data not shown), indicating that Myt3 is not expressed in delaminating endocrine precursors. However, at E18.5 and P1, when the islet-like clusters that have formed begin to split and migrate apart, Myt3-positive cells were identified within clusters of insulin or glucagon-positive cells (Figure 1A) in the pancreas. Within these islet-cell clusters, the Myt3-positive cells typically showed E-cadherin both localized at the plasma membrane and redistributed into the cytoplasm, whereas Myt3-negative E-cadherin-positive cells showed E-cadherin staining localized to the plasma membrane (Figure 1B). Given that E-cadherin redistribution is characteristic of migrating cells these data suggest that at E18.5 and during the early postnatal period Myt3 is expressed in endocrine cells within clusters that are actively moving away from the pancreatic ducts.
Figure 1. Myt3 is expressed in migrating islet cells.
A, Immunohistochemical analysis of 5-μm paraffin sections of E18.5 and P1 pancreata for the presence of Myt3 (green), E-cadherin (red), and insulin/glucagon (blue). Yellow arrowheads indicate Myt3-positive cells with redistributed E-cadherin. White arrowheads mark Myt3-negative cells with E-cadherin distributed at their plasma membrane. B, Cells marked by arrowheads are shown at higher magnification, and signal intensity was measured along the blue line and is shown in the histograms beside each image. Scale bars, 10 μm.
Myt3 suppression inhibits islet-cell migration
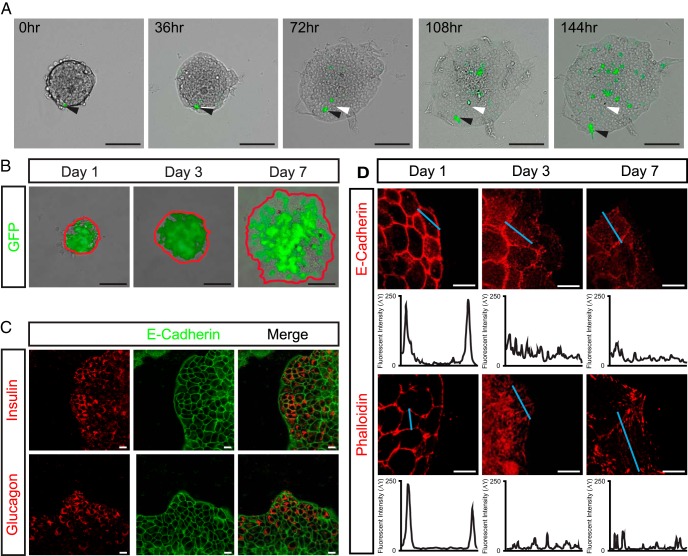
To begin to assess Myt3's role in pancreas endocrine cell migration we used an ex vivo model of islet-cell migration (25). For this, ex vivo pancreatic islets were plated on 804G, a matrix produced by a rat bladder carcinoma cell line that improves islet survival and function in culture (13, 38), and cultured them for 7 days. Tracking individual GFP-positive cells within the islets in real time clearly shows the migration of the islet cells onto the 804G over the 7-day culture period (Figure 2A and Supplemental Movie 1). To confirm the cells migrating onto the 804G included α- and β-cells, we initially used islets from mice that express GFP under the control of the mouse insulin promoter (MIP-GFP) (33). After 3 and 7 days, GFP-positive cells were found at the periphery of the spreading islets, indicating that insulin-expressing β-cells had migrated onto the 804G (Figure 2B). In addition, whole mount staining showed clear insulin and glucagon-positive cells at the periphery of the spreading islets after 7 days (Figure 2C), further confirming the migration of α- and β-cells in this model. Next, to confirm the cells at the periphery of the spreading islets are actively migrating, we assessed their cellular distribution of E-cadherin and F-actin. One day after plating on 804G, the cells at the periphery of the islets displayed plasma membrane localized E-cadherin and F-actin. However, on day 3 and 7 after plating, cells at the periphery largely showed redistributed E-cadherin and F-actin staining, indicating that these cells were in fact migrating (Figure 2D). To confirm that Myt3 expression is not significantly altered over the 7-day culture of the islets, we assessed Myt3 expression by immunofluorescence and qPCR. These data show that Myt3 expression was unaltered by culture on 804G (Supplemental Figure 2, A and B). Further, glucose-stimulated insulin secretion assays indicated that culture time did not significantly impair insulin secretion (Supplemental Figure 2C). Together, these data indicate this is a viable model with which to study the potential regulatory role of Myt3 in pancreas endocrine cell migration.
Figure 2. ECM induces islet-cell migration ex vivo.
A, Time-lapse images of wild-type islets cultured for 7 days on 804G. Transduced cells are marked by GFP expression (green). White arrowheads mark the start position, and black arrowheads mark the current position of the indicated cell. B, Representative images of MIP-GFP islets plated on 804G after 1, 3, and 7 days. The red line indicates the islet boundary, GFP-positive cells are marked in green. The GFP-positive cells at the islet edge indicate insulin-producing β-cells have migrated to the islet periphery. C, Cultured islets were stained for E-cadherin (green) to mark cell-cell boundaries and either insulin or glucagon (red) to identify α- and β-cells. D, E-cadherin (red) and F-actin (red) staining after 1, 3, and 7 days on 804G. Signal intensity was measured along the blue line and is shown in the histograms below each panel. Scale bars, 10 μm (white bar) and 100 μm (black bar).
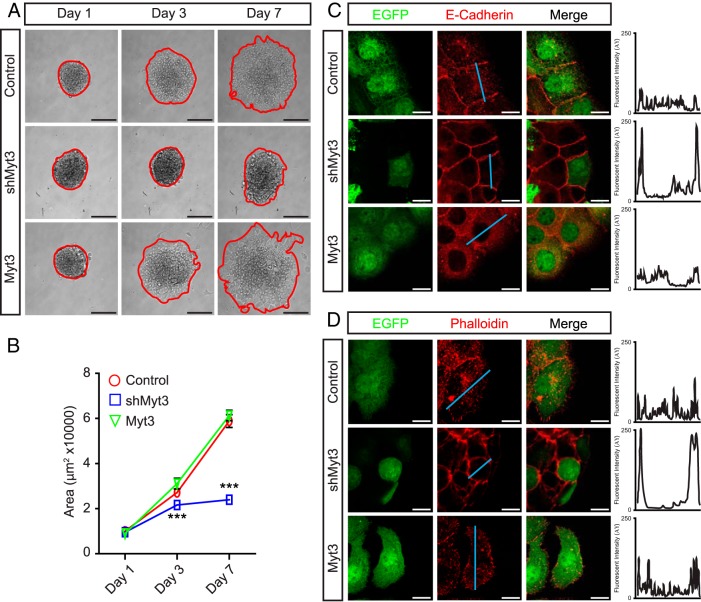
To determine whether Myt3 is involved in regulating islet-cell migration, we transduced the plated islets with adenoviruses expressing a scramble short hairpin RNA (shRNA) (control), an shRNA targeting Myt3 (shMyt3), or full-length Myt3 (Myt3). After 1 day of culture, control-, shMyt3-, and Myt3-transduced islet area was equivalent (Figure 3, A and B). However, 3 days after transduction, shMyt3-transduced islets occupied a significantly smaller area than control- and Myt3-transduced islets (1.3- and 1.4-fold, P < .001), and by 7 days after transduction, shMyt3-transduced islets had a 3-fold smaller area (P < .001) (Figure 3, A and B). In fact, although by 7 days after plating control- and Myt3-transduced islets had an increased islet area of approximately 6-fold, shMyt3-transduced islets had barely reached a 2-fold increase in islet area (Figure 3, A and B). In addition, cells at the periphery of control- and Myt3-transduced islets showed clear signs of E-cadherin and F-actin redistribution, indicating that they are in fact migrating, whereas shMyt3-transduced islets had E-cadherin and F-actin staining only at the plasma membrane, reminiscent of islets that have yet to spread (Figure 3, C and D). These data suggest that in this model Myt3 suppression significantly inhibits islet-cell migration onto 804G.
Figure 3. Myt3 suppression inhibits islet-cell migration.
A, Representative images of control-, shMyt3-, or Myt3-transduced islets cultured for 1, 3, and 7 days on 804G. The red line indicates the islet boundary. B, Quantification of islet area for islets in A. The data represent mean values from at least 3 experiments with 10 islets per experiment. Islets transduced as above were stained after 7 days in culture for E-cadherin (red) (C) or phalloidin (red) (D) to mark F-actin. GFP-positive cells indicate transduced cells (green). Histograms show signal intensity along a line segment (blue line) drawn through the cell. ***, statistically significant difference at P ≤ .001. Scale bars, 10 μm (white bar) and 100 μm (black bar).
Myt3 expression partially rescues cytokine-induced loss of islet-cell migration
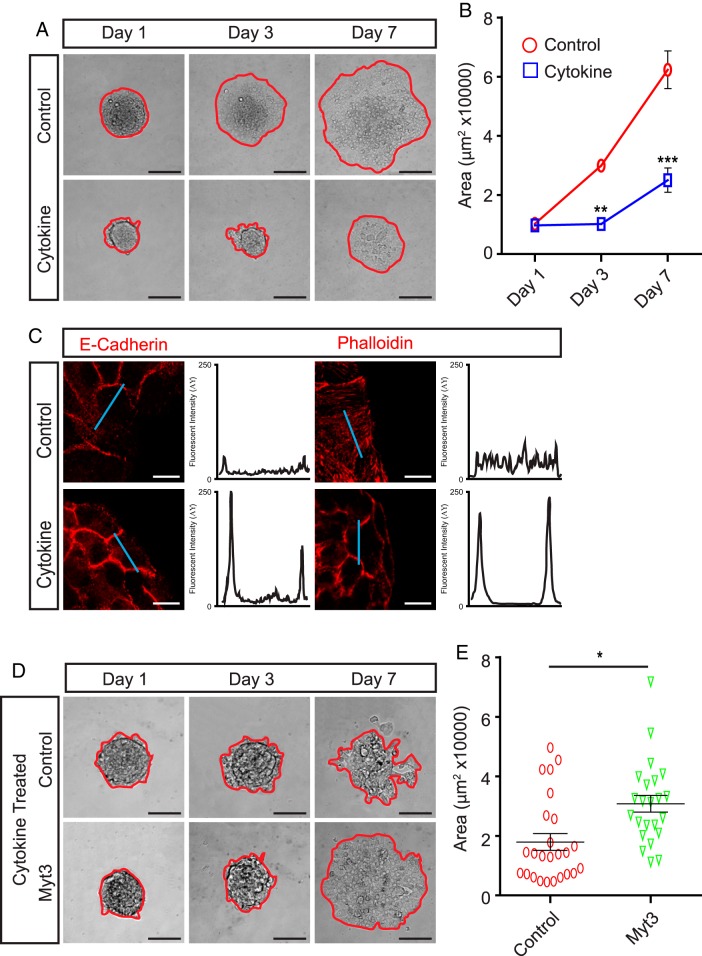
Proinflammatory cytokines, such as IFNγ, TNFα, and IL-1β, are major contributors to the pathogenesis of type 1 diabetes (39–41), and we previously found that exposure of islets to a combination of these cytokines significantly repressed Myt3 expression (29). Thus, we wanted to determine whether exposure of islets to these cytokines impaired islet-cell migration via their suppression of Myt3. For this, we exposed islets cultured on 804G to 0.4-ng/mL IFNγ, 0.07-ng/mL IL-1β, and 0.04-ng/mL TNFα, a dose that is extremely low (roughly 1:256) compared with what is often used to induce β-cell dysfunction and apoptosis (42, 43), yet sufficient to effectively suppresses Myt3 expression (29). Exposure of islets to even this low dose of IFNγ, TNFα, and IL-1β significantly inhibited islet-cell migration onto 804G (2.6-fold, P < .001) (Figure 4, A and B) and prevented the redistribution of E-cadherin and F-actin (Figure 4C). To determine whether this effect was a result of cytokine-induced Myt3 suppression, we transduced cytokine-treated islets with our control or Myt3-overexpressing adenovirus. Myt3-transduced islets exposed to cytokine had significantly improved (1.7-fold, P < .05) ability to spread on 804G as compared with control-transduced islets (Figure 4, D and E). These data suggest that proinflammatory cytokine-induced islet-cell migration impairment, mediated through Myt3 suppression, may play a previously unappreciated role in diabetes pathogenesis.
Figure 4. Cytokine exposure inhibits islet-cell migration.
A, Representative images of control or cytokine-treated islets cultured for 1, 3, and 7 days on 804G. The red line indicates the islet boundary. B, Quantification of islet area for islets in A. The data represent mean values from at least 3 experiments with 10 islets per experiment. C, Control and cytokine-treated islets were stained for E-cadherin (red) and phalloidin (red) to mark F-actin after 3 days in culture. Histograms show signal intensity along a line segment (blue line) drawn through the cell. D, Representative images of cytokine-treated, control-, or Myt3-transduced islets cultured for 1, 3, and 7 days on 804G. The red line indicates the islet boundary. E, Scatter plot of islet area for individual islets from D. Data from 3 independent experiments is represented. Sample means ± SEM are indicated by the black lines. *, statistically significant difference at P ≤ .05; **, P ≤ .01; ***, P ≤ .001. Scale bars, 100 μm.
Myt3 regulates islet-cell migration independently of effects on cell death and proliferation
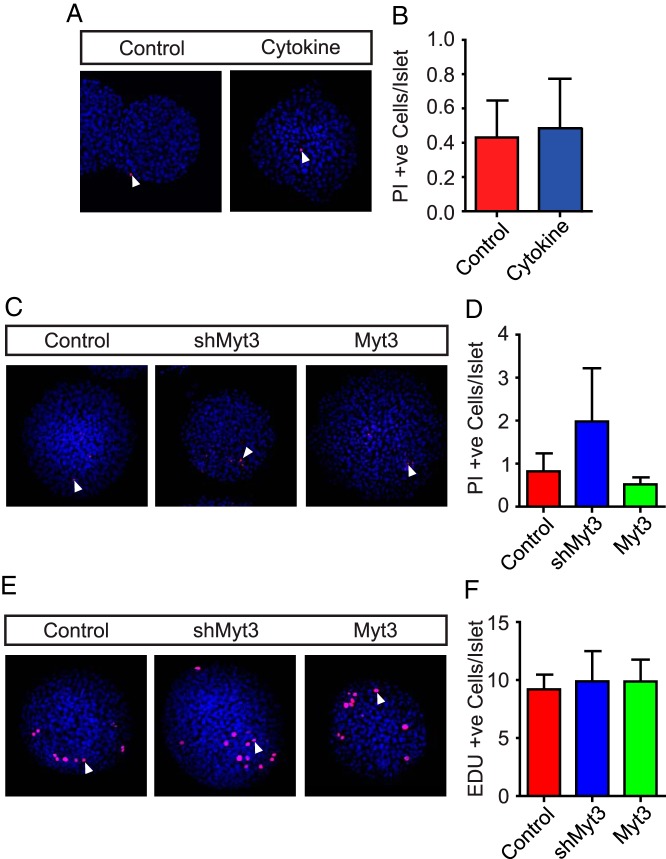
As noted above, Myt3 suppression and IFNγ, TNFα, and IL-1β exposure induce islet-cell death (29, 40, 41, 44, 45). To determine what level of contribution this effect might be having on the failure of shMyt3-transduced and IFNγ, TNFα, and IL-1β-treated islets to spread onto 804G, we assessed the level of cell death in these cultures after 7 days. No significant differences were found in the levels of cell death in IFNγ, TNFα, and IL-1β-treated (Figure 5, A and B), or shMyt3-transduced (Figure 5, C and D) islets as compared with controls. Thus, differences in cell death do not account for the differences in islet area seen. To further confirm this, we performed islet-cell migration assays using islets from Bak−/−:Baxfl/fl:Pdx1-CreER double knockout mice that are highly resistant to undergoing apoptosis (46, 47). Both shMyt3-transduced and IFNγ, TNFα, and IL-1β-treated Bak−/−:Baxfl/fl:Pdx1-CreER double knockout mouse islets showed impaired islet-cell migration, similar to our results using islets from wild-type mice (Supplemental Figure 3, A–D), indicating that the observed phenotype is independent of any effects on apoptosis. Next, to ensure differences in islet area were not a result of changes in cellular proliferation we performed EdU incorporation assays in control-, shMyt3-, and Myt3-transduced islets. No significant differences in EdU incorporation were found in control-, shMyt3-, and Myt3-transduced islets with roughly ten EdU-positive cells found per islet in each (Figure 5, E and F). This level of islet-cell proliferation is clearly insufficient to account for the expansion in islet area seen during our 7-day culture on 804G. Together, these data rule out that the impaired islet-cell migration seen in shMyt3-transduced, or IFNγ, TNFα, and IL-1β-treated islets is a result of increased cell death or impaired proliferation, supporting the assertion that these differences are due to an impairment in shMyt3-transduced islet-cell migratory ability.
Figure 5. Islet-cell migration is independent of apoptosis and proliferation.
A, Representative images of control and IFNγ, TNFα, and IL-1β-treated (cytokine) wild-type islets cultured on 804G for 7 days. Nuclei are stained with Hoescht (blue), and dead cells are stained with propidium iodide (red). B, Quantification of propidium iodide-positive cells in A. The data represent mean values from at least 3 experiments with 50 islets per experiment. C, Representative images of control-, shMyt3-, or Myt3-transduced islets cultured for 7 days on 804G. Nuclei are stained with Hoescht (blue), and dead cells are stained with propidium iodide (red). D, Quantification of propidium iodide-positive cells in C. The data represent mean values from at least 3 experiments with 50 islets per experiment. E, Representative images of control-, shMyt3-, or Myt3-transduced islets cultured for 7 days on 804G. Nuclei are stained with Hoescht (blue), and proliferating cells are stained with EdU (red). F, Quantification of EdU-positive cells in E. The data represent mean values from at least 3 experiments with 50 islets per experiment.
Myt3 regulates integrin mediated cell spreading and migration
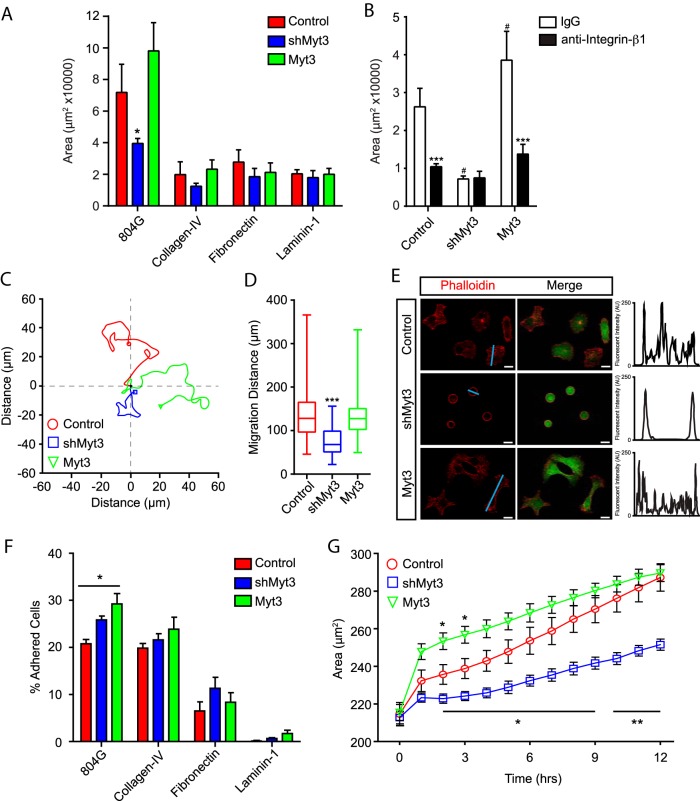
One of the triggering events for the initiation of cell migration is ECM-induced integrin signaling (8–11, 17, 48). Downstream of this initiating signal is the dynamic regulation of cell adhesion to the matrix, of cell-cell contacts, and of the actin cytoskeleton (5, 7). To determine whether Myt3 mediates any of these aspects of cell migration we examined these different processes in whole and dispersed islet cells. First, we compared the ability of transduced islet cells to migrate onto 804G, or the individual matrix components collagen-IV, fibronectin, and laminin-I. As expected, islet cells were able to migrate onto 804G and this was inhibited by suppression of Myt3; however, the islet cells did not migrate to nearly the same extent onto collagen-IV, fibronectin, or laminin-I as they did on 804G (Figure 6A). Laminin-V is the principal component in 804G (20), and laminin-V and its binding partner, integrin-α6β1, are essential for β-cell spreading on 804G, and for 804G's beneficial effects on β-cell function and survival (13, 20, 49, 50). Thus, to determine whether laminin-V is the matrix component in 804G that is responsible for inducing islet-cell migration in our model, we cultured islets in the presence or absence of an antiintegrin-β1 antibody. In fact, antiintegrin-β1 treatment significantly impaired the ability of control and Myt3-expressing islet cells to migrate onto 804G (2.5-fold, P < .001) relative to control IgG-treated islet cells (Figure 6B), indicating that laminin-V is in fact the matrix component in 804G that largely mediates islet-cell migration in our model. Thus, these data suggest that Myt3 suppression impairs islet-cell migration through effects on laminin-V/integrin-β1-induced signaling.
Figure 6. Myt3-mediated islet-cell migration and spreading are dependent on laminin-V and integrin-β1.
A, Islet area was quantified for control-, shMyt3-, or Myt3-transduced islets cultured for 7 days on the indicated ECMs. The data represent mean values from at least 3 experiments with 10 islets per experiment. B, Islet area was quantified for transduced islets treated with control IgG or antiintegrin-β1 blocking antibody for 7 days. The data represent mean values from at least 3 experiments with 10 islets per experiment. C, Representative traces of cell migration paths for transduced, dispersed islets cultured on 804G for 7 days. D, Box-whisker plots representing cumulative migration distance for transduced, dispersed islets cultured on 804G for 7 days. E, Representative images of phalloidin (red)-stained transduced, dispersed islets cultured on 804G for 7 days. GFP (green) indicates transduced cells. Histograms show signal intensity along a line segment (blue line) drawn through the cell. F, Quantification of the number of transduced, dispersed islet cells remaining on the indicated ECM after a 2-hour incubation and subsequent wash. The data represent mean values from 5 independent experiments and are presented as a percentage of starting cells. G, Average cell size over time for transduced, dispersed islet cells plated on 804G. Scale bars, 10 μm.
Next, to specifically confirm that 804G induces islet-cell migration, we performed time-lapse imaging of control-, shMyt3-, and Myt3-transduced dispersed islets over 7 days. Although control- and Myt3-transduced cells migrated on the 804G on average 145 and 134 μm, respectively, over the 7-day culture period, shMyt3-transduced cells migrated on average only 80 μm (P < .001) (Figure 6, C and D). This confirms that Myt3 suppression impairs islet-cell migratory ability on 804G. We noted during these single cell migration experiments that the control- and Myt3-transduced cells showed characteristic elongated or cuboidal morphologies with clear F-actin striations through the cells (Figure 6E), whereas shMyt3-transduced cells remained round in morphology and had F-actin localized at the plasma membrane (Figure 6E). This suggests that shMyt3-transduced cells may also have a reduced ability to adhere or spread on the 804G, because the machinery responsible for cell migration is also required for adhesion and spreading. We, thus, sought to more carefully analyze the adhesion and spreading of these cells. Although there were clear differences in the ability of the islet cells to adhere to the 804G, collagen-IV, fibronectin, or laminin-I, shMyt3-transduced islet cells appeared to adhere equally well as control- or Myt3-transduced cells, although Myt3-transduced cells had a slight increase (1.4-fold, P < .05) in their ability to adhere to 804G (Figure 6F). We next assessed the ability of the control-, shMyt3-, and Myt3-transduced islet cells to spread on 804G over 12 hours. After only 2 hours after plating Myt3-transduced cells were significantly larger (∼250 μm2, P < .05) relative to controls (∼235 μm2), although control and Myt3-transduced cells were similar in size after 12 hours (∼290 μm2). shMyt3-transduced cells, on the other hand, had significantly impaired spreading, with cell area only reaching approximately 220 μm2 2 hours after plating (P < .05). By 12 hours, the average cell size was still significantly lower in shMyt3-transduced cells (∼250 μm2) relative to control- and Myt3-transduced cells (P < .01) (Figure 6G). These results make it clear that shMyt3-transduced cells are unable to spread and migrate in response to signals from the ECM despite being able to adhere to the ECM as well as control- and Myt3-transduced cells.
Myt3 suppression does not affect glucose-induced F-actin remodeling
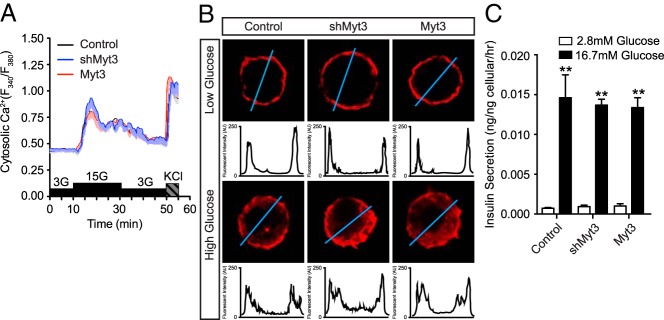
The ability of β-cells to secrete insulin in response to elevated glucose levels is, in part, dependent on their ability to redistribute F-actin to facilitate insulin vesicle trafficking to the membrane (51–53). Because Myt3 suppression affected the ability of the cells to redistribute their F-actin in response to 804G, we next wanted to determine whether Myt3 suppression would also prevent glucose-induced F-actin redistribution and thereby impair glucose-stimulated insulin secretion. First, we confirmed that altered Myt3 expression does not influence signaling upstream of F-actin redistribution by performing calcium imaging on transduced, dispersed islets. Our data show that neither suppression nor overexpression of Myt3 affects the ability of cells to stimulate calcium influx in response to glucose or KCl (Figure 7A). Exposure to high glucose for 5 minutes similarly had no effect on the rapid mobilization of F-actin away from the plasma membrane (Figure 7B). Further, glucose-stimulated insulin secretion assays on transduced islets showed that altered Myt3 expression had no effect on the ability of cells to secrete insulin in response to elevated glucose levels (Figure 7C). These results suggest that loss of Myt3 has no effect on the ability of cells to mobilize F-actin and secrete insulin in response to glucose.
Figure 7. Alterations in Myt3 level do not affect β-cell function.
A, Stimulus coupled calcium influx in control-, shMyt3-, or Myt3-transduced, dispersed islets. B, Glucose-induced F-actin (red) redistribution in control-, shMyt3-, or Myt3-transduced, dispersed islets. Histograms represent signal intensity along a line segment (blue line) drawn through the cell. C, Glucose-stimulated insulin secretion in control-, shMyt3-, or Myt3-transduced whole islets cultured for 7 days.
Myt3 regulates islet-cell migration via Tgfbi
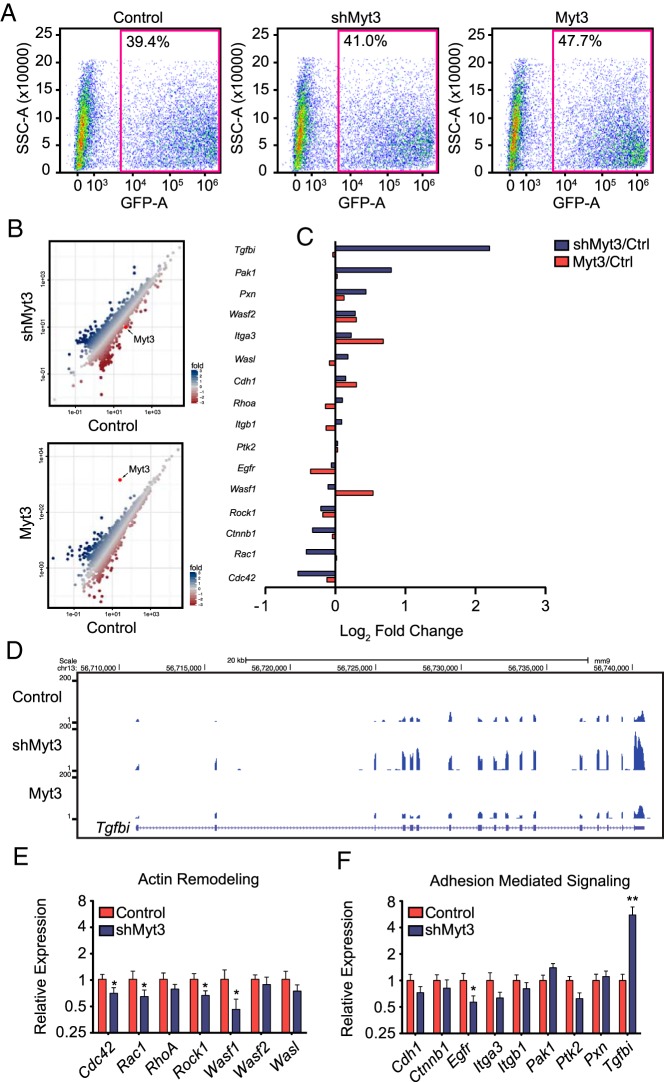
To begin to determine the mechanism by which Myt3 might affect islet-cell migration, we performed RNA-seq on control-, shMyt3-, and Myt3-transduced islets. To ensure only transduced cells were used in the analysis we FACs sorted islets for GFP-positive cells, which accounted for 40%–50% of total cells (Figure 8A). Comparison of gene expression levels between control-transduced islets and either shMyt3- or Myt3-transduced islets resulted in the identification of 51 and 89 significantly altered genes, respectively, including Myt3 in both cases (Figure 8B). Further analysis of 166 genes with known roles in adhesion, migration, and ECM signaling demonstrated that suppression of Myt3 resulted in only minor changes in these genes (Supplemental Figure 4), except for Tgfbi, a known diabetogenic gene that is involved in regulating cellular adhesion (54–57), which showed a significant increase in expression in shMyt3-transduced islets relative to controls (Figure 8C). Examination of read density at the Tgfbi locus confirmed that suppression of Myt3 increases its expression relative to control- and Myt3-transduced islets and that there is only a single splice variant of Tgfbi present in these cells (Figure 8D). qPCR validation of the RNA-seq data showed modest decreases in the expression of Rac1, Cdc42, Wasf1, and Egfr (P < .05) that are likely of little physiological consequence, but a significant increase in the expression of Tgfbi (5.8-fold, P < .01) (Figure 8, E and F). These results suggest that Myt3 likely acts to inhibit Tgfbi expression in islet cells and that Myt3 suppression results in the up-regulation of this gene.
Figure 8. Myt3 suppression regulates Tgfbi expression.
A, FACS plots of control-, shMyt3-, or Myt3-transduced islet cells sorted on the GFP+ population. B, Scatter plots showing gene expression changes for shMyt3- and Myt3-transduced samples relative to control as determined by RNA-seq. C, Bar graph representing the log2 fold changes relative to control islets for adhesion and migration genes. D, Screenshot of UCSC genome browser RNA-seq bigWig tracks for control-, shMyt3-, or Myt3-transduced islet cells at the Tgfbi locus. E, qPCR analysis of genes involved in actin remodeling. F, qPCR analysis of genes involved in adhesion mediated signaling. *, statistically significant difference at P ≤ .05 and **, at P ≤ .01.
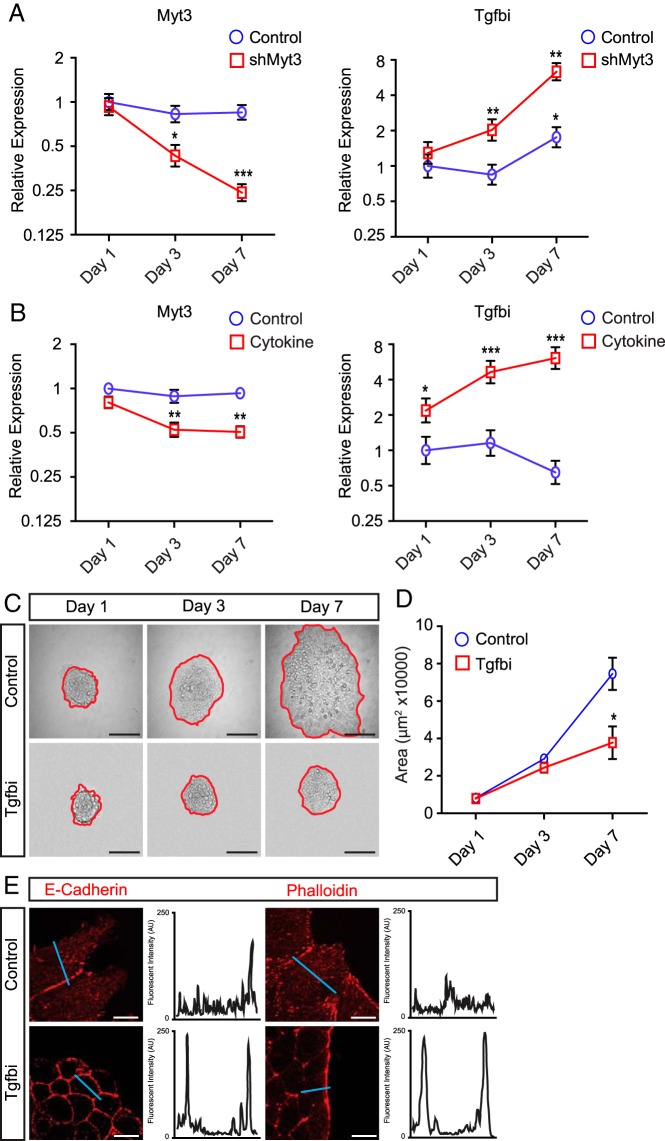
To establish whether Tgfbi up-regulation may be responsible for the effects of Myt3 suppression on islet-cell migration, we first correlated Myt3 and Tgfbi expression with the appearance of the islet-cell migratory defects. qPCR analysis of shMyt3-transduced islets showed that by 3 days, Myt3 is significantly down-regulated (2.3-fold, P < .05), whereas Tgfbi is significantly up-regulated (2-fold, P < .01), and on day 7, these effects are even more pronounced (4-fold, P < .01 and 6.3-fold, P < .01, respectively) (Figure 9A). A similar result was obtained with cytokine exposure, at 3 days, Myt3 was significantly repressed (2-fold, P < .01) with a concomitant increase in Tgfbi (4.6-fold, P < .001) (Figure 9B) at 3 days, whereas at 7 days, Myt3 was suppressed 2-fold (P < .01), and Tgfbi was up-regulated 6-fold (P < .001). Finally, we cultured wild-type islets for 7 days on 804G in the presence of 50-μg/mL Tgfbi. Exogenous Tgfbi inhibited islet-cell migration (2-fold, P < .05) (Figure 9, C and D) and prevented E-cadherin and F-actin redistribution, similar to shMyt3-transduced islets (Figure 9E). Taken together, these results suggest that Tgfbi production may mediate the effects of Myt3 suppression on islet-cell migration in response to signals from the ECM.
Figure 9. Myt3 suppression impairs islet-cell migration via up-regulation of Tgfbi.
A, Time course of Myt3 and Tgfbi expression in control- and shMyt3-transduced islets plated on 804G. B, Time course of Myt3 and Tgfbi expression in cytokine-treated islets plated on 804G. C, Representative images of untreated (control) or Tgfbi-treated (Tgfbi) islets cultured for 1, 3, and 7 days on 804G. The red line indicates the islet boundary. D, Quantification of islet area from C. The data represent mean values from at least 3 experiments with 10 islets per experiment. E, Islets were stained for E-cadherin and phalloidin (red) after 7 days in culture. Histograms show signal intensity along a line segment (blue line) drawn through the cell. *, statistically significant difference at P ≤ .05; **, P ≤ .01; ***, P ≤ .001. Scale bars, 10 μm (white bar) and 100 μm (black bar).
Discussion
After delaminating from the pancreas progenitor layer, pancreas endocrine cells cluster together into islet-like structures adjacent to the pancreatic ducts (23). Subsequently, the fission of these elongated islet-cell clusters is initiated and the newly formed islets are separated from the ducts by acinar tissue (21). Experiments using Rac1 and Egfr knockout mice have also suggested that islet migration participates in the separation of islets from ductal structures implicating cell migration as a necessary component in the final morphology of the developed pancreas (15, 25). We find that Myt3 is expressed widely in these islet-like clusters and is found in all insulin or glucagon-positive cells, with the few Myt3-positive cells that were negative for these hormones likely representing δ-, ϵ-, or PP-cell precursors (29). Although many of these Myt3-positive cells showed signs of E-cadherin redistribution, Myt3-positive cells with E-cadherin largely at the plasma membrane were also frequently found within larger clusters of insulin or glucagon-positive cells, particularly at P1. Taken together, we believe these data suggest that Myt3 is present in pancreas endocrine cells only after they have begun to cluster together into islet-like structures, within islets that are moving away from the pancreatic ducts, and is maintained in mature endocrine cells.
As a model of islet-cell migration, we cultured whole isolated islets for 7 days on 804G, a matrix produced by a rat bladder carcinoma cell line that improves β-cell spreading, function, and survival (13, 20, 49, 50). During this time frame islet area expanded substantially and we show that this is largely due to the migration of endocrine cell types (ie, α- and β-cells) onto the 804G, and not a consequence of islet-cell proliferation or the migration of contaminating endothelial or fibroblast cells. Laminin-V/integrin-β1 interactions are essential for 804G's effects on β-cell adhesion, spreading, and function (13, 20, 49, 50). Likewise, blocking the interaction between laminin-V and integrin-β1 in our model inhibits the ability of islet cells to migrate onto 804G, indicating that islet-cell migration onto 804G is dependent on laminin-V/integrin-β1-induced signaling.
Using this model, we find that Myt3 suppression dramatically reduces the increase in islet area, and our data strongly support that this is largely due to the inability of these cells to migrate onto the 804G. First, we show that altered islet-cell numbers, either via proliferation or apoptosis, caused by Myt3 suppression are not sufficient to account for the differences in islet area seen. Second, we show that Myt3 in fact impairs the ability of dispersed islet cells to migrate on 804G using live-cell imaging. Together, these data strongly suggest that Myt3 suppression in whole islets directly affects the ability of islet cells to migrate onto the 804G. It is, however, still possible that Myt3 also prevents islet cells from breaking down cell-cell junctions, which could also lead to impaired islet spreading.
Interestingly, we saw no impairment in the ability of shMyt3-transduced cells to secrete insulin in response to glucose, which is dependent upon the cell's ability to redistribute their F-actin network at the plasma membrane in order to facilitate access to the membrane for insulin secretory granules (13, 58). This suggests the cells are capable of F-actin, and likely E-cadherin, redistribution, but that they fail to do so specifically in response to laminin-V-induced signaling.
Intriguingly, we find that comparatively low levels of IFNγ, TNFα, and IL-1β cause a similar impairment in islet-cell migration as Myt3 suppression. We have previously shown that exposure of islets to these cytokines significantly inhibits Myt3 gene expression (Figure 9B) (29), and we show here that overexpressing Myt3 in cytokine-treated islets partially rescues their migratory ability. These data suggest that even low levels of proinflammatory cytokines, as may be found early in type 1 diabetes pathogenesis or in type 2 diabetes, may impair islet migratory ability via suppression of Myt3.
Myt3 suppression resulted in a significant increase in the expression of Tgfbi, although whether Myt3 directly binds and represses Tgfbi gene regulatory regions, or whether this effect is indirect, remains to be determined. Tgfbi is a secreted ECM component that has been reported to have diverse effects on cell adhesion and migration in various cancers (59–61) and was previously shown to prevent islet-cell migration, in the absence of any ECM, via dis-regulation of FAK signaling (54). In our model, we show that exogenous Tgfbi inhibited islet-cell migration onto 804G and also resulted in impaired redistribution of E-cadherin and F-actin similar to our results from shMyt3-transduced islets. This result helps explain why in shMyt3-transduced islets both transduced and neighboring untransduced cells seem to be equally affected, because Tgfbi secreted from transduced cells likely inhibits migration of adjacent cells through paracrine mechanisms.
Taken together, these data argue that when whole islets are plated onto 804G, laminin-V/integrin-β1-induced signaling induces islet-cell migration out onto the 804G and that suppression of Myt3 specifically impairs this migration by relieving the repression of the secreted factor Tgfbi. We believe this may be relevant not only to adult islet-cells but also to the migration of islets during pancreas development given the expression of Myt3 in migrating islet clusters. Further, our data imply that exposure to proinflammatory cytokines in the early stages of T1D and in T2D may inhibit islet migratory ability via suppression of Myt3 and subsequent up-regulation of Tgfbi, although the relevance of this to the pathogenesis of these diseases needs to be confirmed. In sum, these data contribute to our understanding of the role of Myt3 in islets and to the mechanisms regulating islet-cell migratory ability.
Acknowledgments
We thank Sarah White for performing the tamoxifen injections. We also thank Dr Bruce Verchere for providing us with the MIP-GFP mice and the staff of the Animal Care Facility at the CFRI for daily maintenance of the mouse colonies.
Author contributions: B.R.T. and B.G.H. conceived of the experiments and wrote the manuscript; B.R.T., J.C., A.Z.L.S., and D.S.L. performed the experiments and analyzed the data; D.S.L. critically reviewed the manuscript; and B.G.H. is the guarantor of this work.
This work was supported by the Child and Family Research Institute and the Canadian Institutes for Health Research Grant MOP-111010 (to B.G.H.) and MOP-119537 (to D.S.L). B.R.T. and B.G.H. are supported by fellowships from the Canadian Diabetes Association.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Child and Family Research Institute and the Canadian Institutes for Health Research Grant MOP-111010 (to B.G.H.) and MOP-119537 (to D.S.L). B.R.T. and B.G.H. are supported by fellowships from the Canadian Diabetes Association.
Footnotes
- Cdc42
- cell division cycle 42
- E
- embryonic day
- ECM
- extracellular matrix
- EdU
- 5-ethynyl-2-deoxyuridine
- Egfr
- epidermal growth factor receptor
- FACS
- fluorescence-activated cell sorting
- GFP
- green fluorescent protein
- IFNγ
- interferon γ
- IL-1β
- interleukin 1, β
- Ngn3
- neurogenin 3
- P
- postnatal day
- qPCR
- quantitative PCR
- Rac1
- RAS-related C3 botulinum substrate 1
- RNA-seq
- RNA-sequencing
- shMyt3
- short hairpin RNA targeting Myt3
- shRNA
- short hairpin RNA
- TNFα
- tumor necrosis factor α.
References
- 1. Ichikawa T, Nakazato K, Keller PJ, et al. . Live imaging of whole mouse embryos during gastrulation: migration analyses of epiblast and mesodermal cells. PLoS One. 2013;8(7):e64506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srinivasan S, Wang F, Glavas S, et al. . Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160(3):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93(5):E44–E52. [PubMed] [Google Scholar]
- 4. Chan AY, Coniglio SJ, Chuang YY, et al. . Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24(53):7821–7829. [DOI] [PubMed] [Google Scholar]
- 5. Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol Biol. 2011;769:1–24. [DOI] [PubMed] [Google Scholar]
- 6. Kuang H-B, Miao C-L, Guo W-X, Peng S, Cao Y-J, Duan E-K. Dickkopf-1 enhances migration of HEK293 cell by β-catenin/E-cadherin degradation. Front Biosci (Landmark Ed). 2009;14:2212–2220. [DOI] [PubMed] [Google Scholar]
- 7. Ridley AJ, Schwartz MA, Burridge K, et al. . Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. [DOI] [PubMed] [Google Scholar]
- 8. Evers EE, Zondag GC, Malliri A, et al. . Rho family proteins in cell adhesion and cell migration. Eur J Cancer. 2000;36(10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 9. Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3(9):a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kesavan G, Lieven O, Mamidi A, et al. . Cdc42/N-WASP signaling links actin dynamics to pancreatic β cell delamination and differentiation. Development. 2014;141(3):685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohatgi R, Ma L, Miki H, et al. . The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97(2):221–231. [DOI] [PubMed] [Google Scholar]
- 12. Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. [DOI] [PubMed] [Google Scholar]
- 13. Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet β-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49(2):233–243. [DOI] [PubMed] [Google Scholar]
- 14. Wang R, Li J, Lyte K, Yashpal NK, Fellows F, Goodyer CG. Role for β1 integrin and its associated α3, α5, and α6 subunits in development of the human fetal pancreas. Diabetes. 2005;54(7):2080–2089. [DOI] [PubMed] [Google Scholar]
- 15. Miettinen PJ, Huotari M, Koivisto T, et al. . Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127(12):2617–2627. [DOI] [PubMed] [Google Scholar]
- 16. Kaido T, Perez B, Yebra M, et al. . αv-integrin utilization in human β-cell adhesion, spreading, and motility. J Biol Chem. 2004;279(17):17731–17737. [DOI] [PubMed] [Google Scholar]
- 17. Cirulli V, Beattie GM, Klier G, et al. . Expression and function of α(v) β(3) and α(v) β(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150(6):1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaido TJ, Yebra M, Kaneto H, Cirulli V, Hayek A, Montgomery AM. Impact of integrin-matrix interaction and signaling on insulin gene expression and the mesenchymal transition of human β-cells. J Cell Physiol. 2010;224(1):101–111. [DOI] [PubMed] [Google Scholar]
- 19. Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human β-cells: effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55(10):2723–2729. [DOI] [PubMed] [Google Scholar]
- 20. Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of β1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat β-cells attached on extracellular matrix. Diabetes. 2006;55(5):1413–1420. [DOI] [PubMed] [Google Scholar]
- 21. Miller K, Kim A, Kilimnik G, et al. . Islet formation during the neonatal development in mice. PLoS One. 2009;4(11):e7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cleaver O, MacDonald RJ. Developmental Molecular Biology of the Pancreas. New York, NY: Springer New York; 2010:71–117. [Google Scholar]
- 23. Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240(3):530–565. [DOI] [PubMed] [Google Scholar]
- 24. Edlund H. Pancreatic organogenesis–developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3(7):524–532. [DOI] [PubMed] [Google Scholar]
- 25. Greiner TU, Kesavan G, Ståhlberg A, Semb H. Rac1 regulates pancreatic islet morphogenesis. BMC Dev Biol. 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim HJ, Schleiffarth JR, Jessurun J, et al. . Wnt5 signaling in vertebrate pancreas development. BMC Biol. 2005;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metzger DE, Gasperowicz M, Otto F, Cross JC, Gradwohl G, Zaret KS. The transcriptional co-repressor Grg3/Tle3 promotes pancreatic endocrine progenitor delamination and β-cell differentiation. Development. 2012;139(8):1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J, Siqueira MF, Behl Y, Alikhani M, Graves DT. The transcription factor ST18 regulates proapoptotic and proinflammatory gene expression in fibroblasts. FASEB J. 2008;22(11):3956–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tennant BR, Islam R, Kramer MM, et al. . The transcription factor Myt3 acts as a pro-survival factor in β-cells. PLoS One. 2012;7(12):e51501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jandrig B, Seitz S, Hinzmann B, et al. . ST18 is a breast cancer tumor suppressor gene at human chromosome 8q11.2. Oncogene. 2004;23(57):9295–9302. [DOI] [PubMed] [Google Scholar]
- 31. Klusmann JH, Li Z, Böhmer K, et al. . miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev. 2010;24(5):478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li DS, Yuan YH, Tu HJ, Liang QL, Dai LJ. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009;4(11):1649–1652. [DOI] [PubMed] [Google Scholar]
- 33. Hara M, Wang X, Kawamura T, et al. . Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab. 2003;284(1):E177–E183. [DOI] [PubMed] [Google Scholar]
- 34. Luciani DS, White SA, Widenmaier SB, et al. . Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes. 2013;62(1):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luciani DS, Ao P, Hu X, Warnock GL, Johnson JD. Voltage-gated Ca(2+) influx and insulin secretion in human and mouse β-cells are impaired by the mitochondrial Na(+)/Ca(2+) exchange inhibitor CGP-37157. Eur J Pharmacol. 2007;576(1–3):18–25. [DOI] [PubMed] [Google Scholar]
- 36. Trapnell C, Roberts A, Goff L, et al. . Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240(3):589–604. [DOI] [PubMed] [Google Scholar]
- 38. Hammar E, Parnaud G, Bosco D, et al. . Extracellular matrix protects pancreatic β-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53(8):2034–2041. [DOI] [PubMed] [Google Scholar]
- 39. Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–226. [DOI] [PubMed] [Google Scholar]
- 40. Cnop M, Welsh N, Jonas J-C, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(suppl 2):S97–S107. [DOI] [PubMed] [Google Scholar]
- 41. Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates β-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cardozo AK, Heimberg H, Heremans Y, et al. . A comprehensive analysis of cytokine-induced and nuclear factor-κ B-dependent genes in primary rat pancreatic β-cells. J Biol Chem. 2001;276(52):48879–48886. [DOI] [PubMed] [Google Scholar]
- 43. Ortis F, Cardozo AK, Crispim D, Störling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-κB activation. Mol Endocrinol. 2006;20(8):1867–1879. [DOI] [PubMed] [Google Scholar]
- 44. Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and β-cell biology: from concept to clinical translation. Endocr Rev. 2008;29(3):334–350. [DOI] [PubMed] [Google Scholar]
- 45. Ehses JA, Böni-Schnetzler M, Faulenbach M, Donath MY. Macrophages, cytokines and β-cell death in type 2 diabetes. Biochem Soc Trans. 2008;36(pt 3):340–342. [DOI] [PubMed] [Google Scholar]
- 46. Wei MC, Zong WX, Cheng EH, et al. . Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rountree AM, Neal AS, Lisowski M, et al. . Control of insulin secretion by cytochrome C and calcium signaling in islets with impaired metabolism. J Biol Chem. 2014;289(27):19110–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen MM, Lee CY, Leland HA, Lin GY, Montgomery AM, Silletti S. Inside-out regulation of L1 conformation, integrin binding, proteolysis, and concomitant cell migration. Mol Biol Cell. 2010;21(10):1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riopel M, Krishnamurthy M, Li J, Liu S, Leask A, Wang R. Conditional β1-integrin-deficient mice display impaired pancreatic β cell function. J Pathol. 2011;224(1):45–55. [DOI] [PubMed] [Google Scholar]
- 50. Saleem S, Li J, Yee SP, Fellows GF, Goodyer CG, Wang R. β1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J Pathol. 2009;219(2):182–192. [DOI] [PubMed] [Google Scholar]
- 51. Cai EP, Casimir M, Schroer SA, et al. . In vivo role of focal adhesion kinase in regulating pancreatic β-cell mass and function through insulin signaling, actin dynamics, and granule trafficking. Diabetes. 2012;61(7):1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalwat MA, Thurmond DC. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp Mol Med. 2013;45(8):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kalwat MA, Yoder SM, Wang Z, Thurmond DC. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic β cells. Biochem Pharmacol. 2013;85(6):808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han B, Qi S, Hu B, Luo H, Wu J. TGF-β i promotes islet β-cell function and regeneration. J Immunol. 2011;186(10):5833–5844. [DOI] [PubMed] [Google Scholar]
- 55. Kim MO, Yun SJ, Kim IS, Sohn S, Lee EH. Transforming growth factor-β-inducible gene-h3 (β(ig)-h3) promotes cell adhesion of human astrocytoma cells in vitro: implication of α6β4 integrin. Neurosci Lett. 2003;336(2):93–96. [DOI] [PubMed] [Google Scholar]
- 56. Ferguson JW, Thoma BS, Mikesh MF, et al. . The extracellular matrix protein βIG-H3 is expressed at myotendinous junctions and supports muscle cell adhesion. Cell Tissue Res. 2003;313(1):93–105. [DOI] [PubMed] [Google Scholar]
- 57. Han B, Luo H, Raelson J, et al. . TGFBI (βIG-H3) is a diabetes-risk gene based on mouse and human genetic studies. Hum Mol Genet. 2014;23(17):4597–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rondas D, Tomas A, Halban PA. Focal adhesion remodeling is crucial for glucose-stimulated insulin secretion and involves activation of focal adhesion kinase and paxillin. Diabetes. 2011;60(4):1146–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shang D, Liu Y, Yang P, Chen Y, Tian Y. TGFBI-promoted adhesion, migration and invasion of human renal cell carcinoma depends on inactivation of von Hippel-Lindau tumor suppressor. Urology. 2012;79(4):966.e1–e7. [DOI] [PubMed] [Google Scholar]
- 60. Nummela P, Lammi J, Soikkeli J, Saksela O, Laakkonen P, Hölttä E. Transforming growth factor β-induced (TGFBI) is an anti-adhesive protein regulating the invasive growth of melanoma cells. Am J Pathol. 2012;180(4):1663–1674. [DOI] [PubMed] [Google Scholar]
- 61. Wen G, Partridge MA, Li B, et al. . TGFBI expression reduces in vitro and in vivo metastatic potential of lung and breast tumor cells. Cancer Lett. 2011;308(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]