Abstract
Deimatic or ‘startle’ displays cause a receiver to recoil reflexively in response to a sudden change in sensory input. Deimatism is sometimes implicitly treated as a form of aposematism (unprofitability associated with a signal). However, the fundamental difference is, in order to provide protection, deimatism does not require a predator to have any learned or innate aversion. Instead, deimatism can confer a survival advantage by exploiting existing neural mechanisms in a way that releases a reflexive response in the predator. We discuss the differences among deimatism, aposematism, and forms of mimicry, and their ecological and evolutionary implications. We highlight outstanding questions critical to progress in understanding deimatism.
Keywords: aposematism, startle reflex, warning colours, camouflage, predator–prey, Acripeza
1. Introduction
Predation drives the evolution of diverse antipredator defences in prey. Some defences are static (‘unswitchable’), while others are performed in response to external cues. Studies of antipredator defences have often focused on the protective value of conspicuous, static signals, and their co-occurrence with traits that render their bearers unprofitable, such as high speed or manoeuvrability, physical weapons, toxins or venom [1–7]. Studies of aposematism—unprofitability associated with a signal—have led to major advances in evolutionary theory and some iconic examples of natural selection (e.g. [8–11]), but a strong focus on static signals neglects the other antipredator strategies. By undergoing sudden transitions when under attack, deimatic prey species can cause their predators to recoil reflexively [12–14]. For example, reflexive responses can be invoked by a sudden transition between camouflage and aposematism, by a change from small to large apparent body size, or by emitting a loud sound. Crucially, unlike aposematism, reflexive responses to transitory, i.e. deimatic, elements do not require learned or innate aversion.
Until now the idea that deimatic or ‘startle’ displays are distinct from aposematism, in that they do not require learned or innate avoidance, has been implied to greater or lesser degree across the field's sparse literature [4,12–19]. However, so far, it has not been made explicit. Here, we wish to bring the idea into sharp relief. We discuss the consequences of this idea in the context of the distinctive way in which deimatism confers a survival advantage and how it may evolve, and we suggest outstanding questions for empirical and theoretical scrutiny.
2. How do deimatism and aposematism differ?
Aposematic organisms advertise their unprofitability through signals (both static and/or switchable, ‘facultative aposematism’ [19]) [4,20]. Survival by aposematism is paradoxical in the sense that prey bearing conspicuous signals are more easily detected by naive predators than camouflaged prey [21]. Predator populations often contain a fraction of naive predators, and aposematic prey populations must sustain the loss of a proportion of individuals before predators learn to associate the signal with unprofitability. This process occurs in every predator breeding season [22]. Naive predators can be innately averse to conspicuous prey (e.g. wariness [23,24]). However, available evidence also suggests that conspicuous prey are on average more likely to be attacked by naive predators than their camouflaged counterparts [22,24,25]. Aposematic prey can benefit from ‘safety in numbers’ as well as positive frequency dependence where the higher the density and frequency of individuals bearing the signal, the better they are protected [8,26]. The protection afforded to aposematic species is shared by Müllerian (‘honest’) mimics, via positive frequency dependence, and exploited by Batesian (‘dishonest’) mimics via negative frequency dependence [8]. While Batesian mimics do not have to be learned anew, and cannot be, else the species’ profitability is discovered, both types of mimics benefit from predators learning to avoid unprofitable prey, and generalizing their learned avoidance to mimics.
Deimatism involves a behaviour by a sender that gives rise to a sudden transition in sensory input, causing the receiver to recoil reflexively. Such behaviours can take the form of sudden, possibly momentary, changes in appearance in any modality, but visual and auditory signals are most commonly studied [12–14,16,27–31]. For example, there is a significant body of work on the interaction between auditory startle and emotional state in humans [32–34] and on auditory arms races between moths and bats [35]. There has been a long-standing focus on displays involving sudden transitions in visual appearance, for example between camouflage and aposematism (e.g. newts [35]) or between camouflage and mimicry (e.g. lepidopteran and amphibian eyespots [36]). These transitions often include movement, though colour change may give rise to deimatism without movement, as occurs in cephalopods [37,38]. Deimatic transitions can reveal an aposematic signal but, crucially, the aposematic component conveys information to a predator about prey unprofitability, whereas the deimatic component (sudden transition) does not necessarily convey any information. Deimatism exploits purely reflexive responses in the predator, sidestepping the requirement for learned or innate aversion.
Animal defences are well defined by the proximate mechanisms through which they protect their bearers [20,29,36]. There have been many partly-overlapping suggestions for the mechanism by which deimatism is thought to confer a survival advantage, including the following, non-exclusive, processes: (i) releasing the ‘startle reflex’ in the predator [28], (ii) overwhelming the predator's senses [14,37], (iii) exploiting the predator's fear responses [5,13,16], and (iv) causing misclassification by predator of potential prey as a threat [13]. Each mechanism works without the need for predator learning; it merely takes advantage of existing neural machinery.
3. Deimatism and predator experience
The differences and similarities between deimatism and other defensive strategies are evident when they are viewed in light of predator experience (table 1). All defences that exploit innate aversion can protect prey against naive predators. However, unlike aposematic prey and their mimics (Müllerian and Batesian), deimatic prey may be protected against completely naive predators without requiring learned or innate aversion. While predator learning is not required for deimatism to provide protection, it is of course possible that predators can learn that a species’ defence includes a deimatic component (unless the displays inhibit learning). As predators gain experience with unprofitable deimatic prey, they may learn the association between the prey's resting state, its deimatic component and its unprofitability [31], potentially reinforcing or improving the initial protection. However, released from the requirement of learned avoidance, deimatic prey do not necessarily require any further defence, such as a chemical defence, in addition to the deimatic component; the sudden transition itself may provide protection for otherwise profitable prey. For example, when prey are scarce or predator condition is poor, predators may be forced to attack suboptimal prey and hence test the profitability of deimatic prey. In deimatic prey with no further defence, predators may learn to ignore the transitory component that previously provided protection [17]. Thus, like Batesian mimics (profitable mimics), the evolution of profitable deimatic prey may be negatively frequency dependent. All else being equal, we suggest that the probability of surviving an encounter with a naive predator is greater for prey with a deimatic component to their defence than prey without.
Table 1.
Comparison of characteristics of defensive strategies with reference to predator learning. (‘yes’ or ‘no’ refers to the requirement in the first column.)
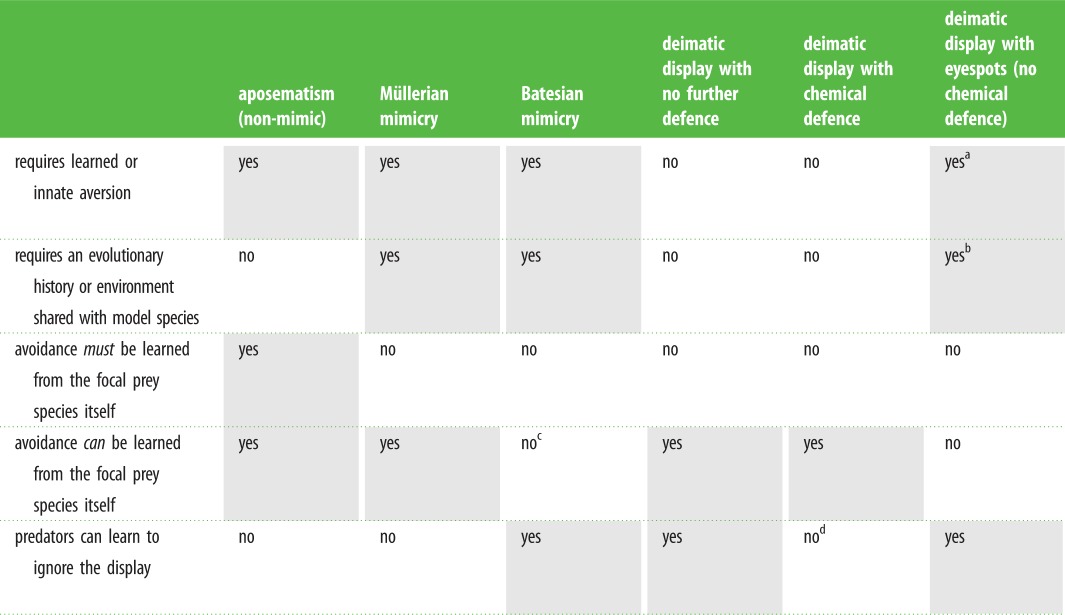 |
aNot for movement and conspicuous colour pattern.
bPredator must have at least innate aversion of its own predator's eyes.
cNot without its model.
dPredators can learn to ignore the deimatic component, but not the chemical defence.
4. Evolutionary pathways to deimatism
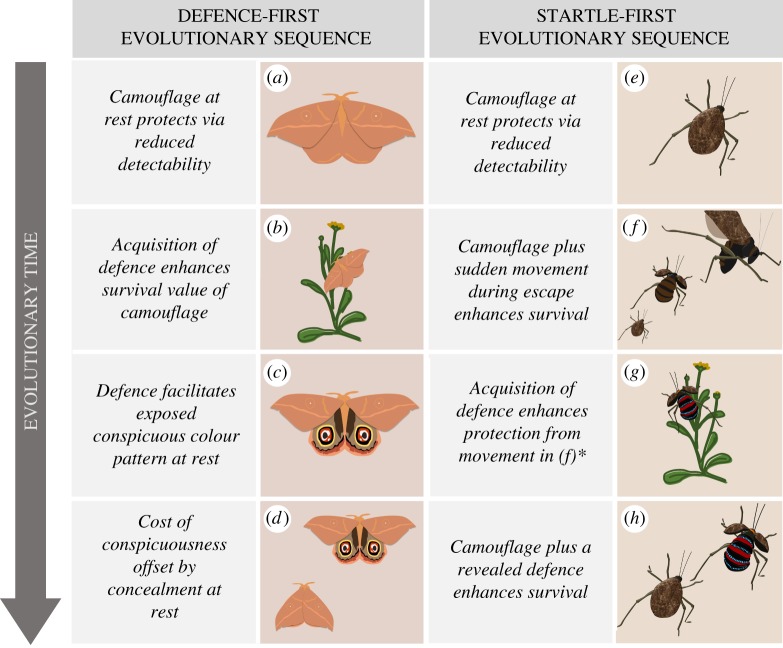
There are two important potential pathways to deimatism: (i) the defence-first hypothesis and (ii) the startle-first hypothesis. The defence-first hypothesis proposes that deimatism evolves as a ‘next step’ along a trajectory to aposematism (figure 1a–d). When aposematism evolves, prey may be more likely to survive attacks by naive or indiscriminate predators by concealing their conspicuousness to avoid the costs of enhanced detectability. Essentially, prey revert to camouflage, but retain the ability to make both a sudden deimatic transition that can grant additional protection by causing reflexive recoil, and a revealable, potentially learnable, aposematic signal. This shifts the defence to a later stage in the predation sequence and the deimatic transition eliminates the requirement for learning [29].
Figure 1.
Evolutionary routes to deimatism. In the defence-first hypothesis (a–d) initially camouflaged prey gain defences followed by conspicuous signals (a–c; as in aposematism), followed by concealment of the signal (d). In the startle-first hypothesis (e–h), initially camouflaged prey benefit from incidentally exploiting a reflexive response in predators (e.g. when fleeing an encounter; a,b). The effective behaviour is evolutionarily co-opted and enhanced through a conspicuous signal (g,h). *In the startle-first hypothesis it is possible that further defences are acquired or not.
The startle-first hypothesis suggests that deimatism evolves in camouflaged ancestors that move suddenly when avoiding attack, thereby startling their predator (figure 1e–h). As alluded to in Edmunds [16] and Cott [4], in the camouflaged ancestor, movement alone could release the predator's startle reflex often or effectively enough to confer a survival advantage, e.g. protean defences or flash coloration [16]. If the protective value of the movement is enhanced by a conspicuous and unexpected colour pattern revealed during escape, such coloration would be favoured by selection. Phylogenetically controlled studies are required to test these hypotheses, as are experiments that disentangle the salient components in the display. Interestingly, the startle-first trajectory seems to circumvent the aposematism paradox [6,38] because conspicuous surfaces are only exposed to selection after the predator has detected and begun approaching the prey [29].
5. Outstanding questions
Experimental, comparative and theoretical studies are required to address many outstanding questions, four of which we see as focal. (i) What is the effect of learning on deimatism and vice versa? Understanding the efficacy of deimatism against naive and experienced predators will test the hypothesis that deimatic displays exploit reflexive responses, and whether they enhance or inhibit learning. (ii) Under what conditions do profitable and unprofitable deimatic prey evolve? Comparing the mode of frequency dependence in profitable and unprofitable deimatic prey will allow us to understand how unprofitable deimatic prey can persist. (iii) By what mechanism(s) does deimatism deter predators? We urgently need knowledge of the mechanism(s) by which deimatism deters predators to guide work in this field. (iv) What are the evolutionary pathways to deimatism? Phylogenetic approaches involving ancestral state reconstruction will provide insight into the evolutionary path(s) to deimatism.
Acknowledgements
We thank Hannah Rowland, Indrikis Krams, Innes Cuthill and Piotr Jablonski for insightful and engaging feedback on this manuscript.
Authors' contributions
K.D.L.U., S.D.B., T.E.W., J.M., J.L. and J.A.E. conceived the ideas and wrote the paper.
Competing interests
The authors declare they have no competing interests.
Funding
This study was supported by the Hermon Slade Foundation (HSF14/3) and Western Sydney University.
References
- 1.Wallace AR. 1877. The colors of animals and plants. Am. Nat. 11, 641–662. ( 10.1086/271979) [DOI] [Google Scholar]
- 2.Bates HW. 1863. The naturalist on the River Amazons: a record of adventures, habits of animals, sketches of Brazilian and Indian life and aspects of nature under the Equator during eleven years of travel. London, UK: John Murray. [Google Scholar]
- 3.Poulton EB. 1890. The colours of animals: their meaning and use, especially considered in the case of insects. London, UK: Trench & Trübner. See; http://books.google.com/books?id=-sVRAAAAMAAJ. [Google Scholar]
- 4.Cott HB. 1940. Adaptive coloration in animals. London, UK: Methuen. [Google Scholar]
- 5.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Mappes J, Marples N, Endler JA. 2005. The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603. ( 10.1016/j.tree.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 7.Rowe C, Guilford T. 1999. The evolution of multimodal warning displays. Evol. Ecol. 13, 655–671. ( 10.1023/A:1011021630244) [DOI] [Google Scholar]
- 8.Joron M, Mallet JLB. 1998. Diversity in mimicry: paradox or paradigm? Trends Ecol. Evol. 13, 461–466. ( 10.1016/S0169-5347(98)01483-9) [DOI] [PubMed] [Google Scholar]
- 9.Brodie E. 1993. Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa-Rica. Evolution 47, 227–235. ( 10.2307/2410131) [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485. ( 10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 11.Mallet J, Joron M. 1999. Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation. Annu. Rev. Ecol. Syst. 30, 201–233. ( 10.1146/annurev.ecolsys.30.1.201) [DOI] [Google Scholar]
- 12.Umbers KDL, Lehtonen J, Mappes J. 2015. Deimatic displays. Curr. Biol. 25, R58–R59. ( 10.1016/j.cub.2014.11.011) [DOI] [PubMed] [Google Scholar]
- 13.Skelhorn J, Holmes GG, Rowe C. 2016. Deimatic or aposematic? Anim. Behav. 113, E1–E3. ( 10.1016/j.anbehav.2015.07.021) [DOI] [Google Scholar]
- 14.Umbers KDL, Mappes J. 2016. Towards a tractable working hypothesis for deimatic displays. Anim. Behav. 113, e5–e7. ( 10.1016/j.anbehav.2016.01.002) [DOI] [Google Scholar]
- 15.Sivinski J. 1981. The nature and possible functions of luminescence in Coleoptera larvae. Coleopt. Bull. 35, 167–179. [Google Scholar]
- 16.Edmunds M. 1974. Defence in animals: a survey of anti-predator defences New York, NY: Longman. [Google Scholar]
- 17.Sargent TD. 1990. Startle as an anti-predator mechanism, with special reference to the undenting moths, (Catocala). In Insect defenses: adaptive mechanisms and strategies of prey and predators (eds DL Evans, JO Schmidt), p. 229. Albany, NY: State University of New York Press. [Google Scholar]
- 18.Umbers KDL, Mappes J. 2015. Post-attack deimatic display in the mountain katydid (Acripeza reticulata). Anim. Behav. 100, 68–73. ( 10.1016/j.anbehav.2014.11.009) [DOI] [Google Scholar]
- 19.Kang C-K, Lee S-I, Jablonski PG. 2011. Effect of sex and bright coloration on survival and predator-induced wing damage in an aposematic lantern fly with startle display. Ecol. Entomol. 36, 709–716. ( 10.1111/j.1365-2311.2011.01319.x) [DOI] [Google Scholar]
- 20.Guilford T, Dawkins MS. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14. ( 10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 21.Endler JA, Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547. ( 10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 22.Mappes J, Kokko H, Ojala K, Lindström L. 2014. Seasonal changes in predator community switch the direction of selection for prey defences. Nat. Commun. 5, 5016 ( 10.1038/ncomms6016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuler W, Hesse E. 1985. On the function of warning coloration: a black and yellow pattern inhibits prey-attack by naïve domestic chicks. Behav. Ecol. Sociobiol. 16, 249–255. ( 10.1007/BF00310988) [DOI] [Google Scholar]
- 24.Lindstrom L, Alatalo RV, Mappes J. 1999. Reactions of hand-reared and wild-caught predators toward warningly colored, gregarious, and conspicuous prey. Behav. Ecol. 10, 317–322. ( 10.1093/beheco/10.3.317) [DOI] [Google Scholar]
- 25.Gittleman JL, Harvey PH. 1980. Why are distasteful prey not cryptic? Nature 286, 149–150. ( 10.1038/286149a0) [DOI] [Google Scholar]
- 26.Endler J. 1988. Frequency-dependent predation, crypsis and aposematic coloration. Phil. Trans. R. Soc. Lond. B 319, 505–523. ( 10.1098/rstb.1988.0062) [DOI] [PubMed] [Google Scholar]
- 27.Maldonado H. 1970. The deimatic reaction in the praying mantis Stagmatoptera biocellata. Zeit. Verg. Physiol. 68, 60–71. ( 10.1007/BF00297812) [DOI] [Google Scholar]
- 28.Crane J. 1952. A comparative study of innate defensive behavior in Trinidad mantids (Orthoptera, Mantoidea). Zoologica 37, 259–293. [Google Scholar]
- 29.Endler JA. 1991. Interactions between predators and prey. In Behavioural ecology: an evolutionary approach, 3rd edn (eds JR Krebs, NB Davies), pp. 169–196. Oxford: Blackwell Publishing. [Google Scholar]
- 30.Kang C-K, Moon H, Sherratt TN, Lee S-I, Jablonski PG. 2017. Multiple lines of anti-predator defence in the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Biol. J. Linn. Soc. 120, 115–124. ( 10.1111/bij.12847) [DOI] [Google Scholar]
- 31.Kang C, Cho H-J, Lee S-I, Jablonski PG. 2016. Post-attack aposematic display in prey facilitates predator avoidance learning. Front. Ecol. Evol. 4, 35 ( 10.3389/fevo.2016.00035) [DOI] [Google Scholar]
- 32.Lang PJ, Bradley MM, Cuthbert BN. 1990. Emotion, attention, and the startle reflex. Psychol. Rev. 97, 377–395. ( 10.1037/0033-295X.97.3.377) [DOI] [PubMed] [Google Scholar]
- 33.Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. 1991. New observations on the normal auditory startle reflex in man. Brain 114, 1891–1902. ( 10.1093/brain/114.4.1891) [DOI] [PubMed] [Google Scholar]
- 34.Öhman A, Flykt A, Esteves F. 2001. Emotion drives attention: detecting the snake in the grass. J. Exp. Psychol. Gen. 130, 466–478. ( 10.1037/0096-3445.130.3.466) [DOI] [PubMed] [Google Scholar]
- 35.Conner WE, Corcoran AJ. 2011. Sound strategies: the 65-million-year-old battle between bats and insects. Annu. Rev. Entomol. 57, 21–39. ( 10.1146/annurev-ento-121510-133537) [DOI] [PubMed] [Google Scholar]
- 36.Endler JA. 1986. Defense against predation. In Predator-prey relationships, perspectives and approaches from the study of lower vertebrates (eds Feder ME, Lauder GV), pp. 109–134. Chicago, IL: University of Chicago Press. [Google Scholar]
- 37.Barber JR, Razak KA, Fuzessery ZM. 2003. Can two streams of auditory information be processed simultaneously? Evidence from the gleaning bat Antrozous pallidus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 189, 843–855. ( 10.1007/s00359-003-0463-6) [DOI] [PubMed] [Google Scholar]
- 38.Marples NM, Kelly DJ, Thomas RJ. 2005. Perspective: the evolution of warning coloration is not paradoxical. Evolution 59, 933–940. ( 10.1111/j.0014-3820.2005.tb01032.x) [DOI] [PubMed] [Google Scholar]