Abstract
The neurohormone oxytocin plays a central role in human social behaviour and cognition, and oxytocin dysregulation may contribute to psychiatric disorders. However, genetic factors influencing individual variation in the oxytocinergic system remain poorly understood. We genotyped 169 healthy adults for a functional polymorphism in GTF2I (general transcription factor II-I), a gene associated with high prosociality and reduced social anxiety in Williams syndrome, a condition reported to involve high oxytocin levels and reactivity. Participants’ salivary oxytocin levels were measured before and after watching a validated empathy-inducing video. Oxytocin reactivity, defined as pre- to post-video percentage change in salivary oxytocin, varied substantially and significantly between individuals with different GTF2I genotypes, with, additionally, a trend towards an interaction between genotype and sex. Individuals with more oxytocin-reactive genotypes also reported significantly lower social anxiety. These findings suggest a model whereby GTF2I has a continuum of effects on human sociality, from the extreme social phenotypes and oxytocin dysregulation associated with gene deletion in Williams syndrome, to individual differences in oxytocin reactivity and sociality associated with common polymorphisms in healthy populations.
Keywords: oxytocin, social anxiety, GTF2I gene, Williams syndrome, prosociality
1. Introduction
The neurohormone oxytocin plays central roles in human social cognition, emotionality and behaviour [1–3]. Moreover, dysregulated oxytocin levels have been reported in psychiatric disorders including major depression [4] and autism [5]. Despite intense interest in social effects of oxytocin, the genetic mechanisms regulating individual variation in oxytocin are poorly understood.
Genetic disorders characterized by atypical social behaviour may provide useful insights into genes influencing the human oxytocinergic system. Williams syndrome, a neurodevelopmental disorder caused by hemizygous deletion of approximately 25 genes at chromosomal region 7q.11.23, is characterized by high prosociality and low social anxiety, which may be linked with the increased oxytocin levels and reactivity reported in this condition [6,7]. High prosociality in Williams syndrome, and in mouse models of this syndrome, has been linked with reduced expression of the gene general transcription factor II-I (GTF2I) [8,9], which is located within the 7q11.23 Williams syndrome deletion region. Single nucleotide polymorphisms (SNPs) of this gene are also associated with autism risk [10] and, among healthy populations, with variation in social anxiety, autistic-like traits, threat-related amygdala activity, and extraversion [11–13]. These latter findings provide evidence of roles for GTF2I in sociality and anxiety among healthy humans, which notably resemble the roles of oxytocin itself [7,11,14].
Convergent lines of evidence thus suggest that the associations of GTF2I with social behaviour—in Williams syndrome and in healthy populations—may be mediated by effects of GTF2I genetic variation on oxytocin. We tested this hypothesis using data on salivary oxytocin levels collected before and after experimental empathy induction, data on self-reported social anxiety, and data from genotyping of rs13227433, the GTF2I SNP previously associated with social anxiety, extraversion and amygdala reactivity in healthy populations.
2. Material and methods
(a). Study population
Healthy participants were recruited from a Canadian university (92 females, 77 males). The study population was of mixed ethnicity (42% East Asian, 25% Caucasian, 22% South Asian, 11% other or mixed ethnicity) with an average age of 20.3 ± 2.2 years. No participants reported having children. No female participants reported being pregnant, and mean oxytocin levels and reactivity did not differ with use of hormonal contraception or stage of menstrual cycle (p > 0.24 for all tests, electronic supplementary material, tables S1 and S2).
(b). Experimental design
Saliva samples were collected before and after participants watched a validated empathy-inducing video of a child with terminal cancer [15]. Oxytocin was quantified in both samples, and reactivity was calculated as pre-video to post-video percentage change in salivary oxytocin. As part of the experiment, participants also completed the Schizotypal Personality Questionnaire-Brief Revised [16,17], which includes a four-item excessive social anxiety subscale. Each high-anxiety item endorsed (‘agree’ or ‘strongly agree’) was scored 1; social anxiety scores thus ranged from 0 to 4 with higher scores indicating greater social anxiety.
(c). Salivary oxytocin collection and analysis
Saliva was collected by passive drool into pre-chilled tubes and immediately frozen at −20°C. Consistent with published protocols for measuring salivary oxytocin [18–20], 0.5 ml of saliva was lyophilized overnight to concentrate the sample twofold. Measurement of oxytocin was performed in duplicate using Enzo Life Sciences enzyme-linked immunosorbent assay kit ADI-901-153 (http://www.enzolifesciences.com/ADI-901-153A/oxytocin-elisa-kit/ENZO Oxytocin ELISA). Samples from the same individual were analysed on the same plate. Plates were read at 405 nm and oxytocin concentrations were calculated from standard curves. Intra- and inter-assay coefficients of variability were less than 8% and less than 18%, respectively, for 16 plates, which were consistent with the manufacturer's normative variability ranges (12.6–13.3% and 11.9–20.9%).
Debate exists concerning measurement accuracy of oxytocin by ELISA in unextracted compared with extracted fluids, as well as the relationship between salivary and plasma oxytocin [21]. The assay used in this study has undergone rigorous testing and is highly specific to oxytocin (i.e. it does not detect vasopressin) (http://www.enzolifesciences.com/ADI-901-153A/oxytocin-elisa-kit/ENZO Oxytocin ELISA). Furthermore, salivary oxytocin has been shown to correlate positively with plasma oxytocin [22], and any possible methodological measurement effects are expected to affect all samples, rather than being genotype specific in any way.
(d). GTF2I genotyping
Participants were genotyped for SNP rs13227433, which tags an approximately 73 kb haplotype that includes the promoter region of the GTF2I gene (electronic supplementary material, figure S1). DNA, fluorophore-labelled primers (TaqMan® SNP Genotyping Assays), and TaqMan® Master Mix were combined and run on a Roche LightCycler® 96 Real-Time PCR machine. Fluorescence data were analysed under Endpoint Genotyping with LightCycler® 96 software, v. 1.1.0.1320. Genotype frequencies did not deviate from Hardy–Weinberg equilibrium in our sampled population (χ2 = 2.28, d.f. = 1, p = 0.13).
(e). Statistical analysis
R (v. 3.3.1) was used to analyse all data. Mean differences were tested using t-tests for two-group comparisons and analyses of variance (ANOVA) for comparisons involving more than two groups. Given the rarity of the GG genotype (less than 5%), GG and GT genotypes were combined and compared with TT genotypes. Results were considered significant if p < 0.05.
3. Results
Salivary oxytocin increased, on average across all participants, after viewing the empathy-inducing video (paired t-test: t = −2.95, d.f. = 168, p = 0.004). Variation in oxytocin was analysed using a 2 × 2 × 4 factorial ANOVA (genotype × sex × ethnicity). The analysis indicated a significant main effect of GTF2I rs13227433 genotype on oxytocin reactivity (F = 5.5, p = 0.02, mean difference: 16.6, 95% confidence intervals of difference: 2.8–30.2), with the GG + GT group showing higher reactivity (figure 1), and a trend towards an interaction between genotype and sex (F = 3.0, p = 0.08). Social anxiety was analysed using a 2 × 2 factorial ANOVA (genotype × sex), which also resulted in a significant main effect of genotype (F = 4.4, p = 0.04, mean difference: −0.5, 95% confidence intervals of difference: −0.03 to −0.99) with the GG + GT group self-reporting lower levels of social anxiety. Mean oxytocin reactivity and social anxiety scores for each genotype group and sex are presented in table 1. All other effects and interactions were statistically non-significant (electronic supplementary material, tables S3 and S4), including variation in baseline oxytocin levels between GTF2I genotype groups (p = 0.44, means: 101.0 pg ml−1 GG + GT, 109.9 pg ml−1 TT).
Figure 1.
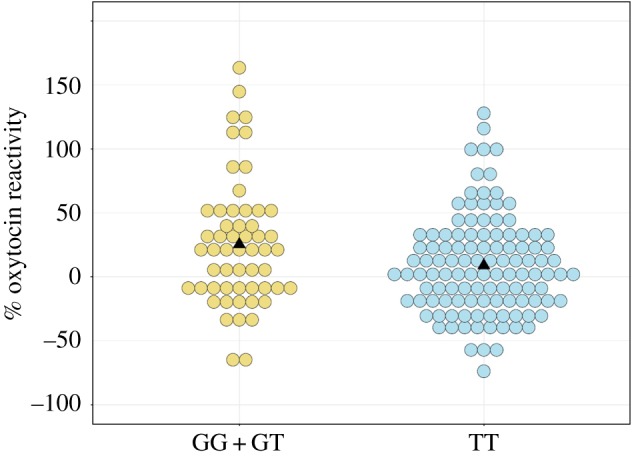
Dot plot indicating percentage change in salivary oxytocin for individuals with GG + GT versus TT genotypes for GTF2I SNP rs13227433. Each dot represents one individual. Triangles indicate the mean for each genotype group (25.4 for GG + GT, 8.8 for TT).
Table 1.
Mean oxytocin reactivity and social anxiety scores for GTF2I SNP rs13227433 genotype groups. Three individuals were excluded from social anxiety analyses due to incomplete questionnaire items. n.a., not applicable.
| All | GG | GT | GG + GT | TT | ||
|---|---|---|---|---|---|---|
| % oxytocin reactivity | females + males | 14.2 (42.9) n = 169 |
32.2 (56.6) n = 9 |
24.0 (48.6) n = 46 |
25.4 (49.5)a n = 55 |
8.8 (38.4)a n = 114 |
| mean (s.d.) | females | 17.3 (47.1) n = 92 |
29.5 (59.8) n = 8 |
36.9 (50.2) n = 24 |
35.1 (51.8) n = 32 |
7.8 (41.8) n = 60 |
| males | 10.5 (37.2) n = 77 |
53.8 (n.a.) n = 1 |
10.0 (43.8) n = 22 |
11.9 (43.7) n = 23 |
10.0 (34.5) n = 54 |
|
| social anxiety | females + males | 1.6 (1.5) n = 166 |
1.1 (1.5) n = 9 |
1.3 (1.4) n = 46 |
1.3 (1.4)a n = 55 |
1.8 (1.5)a n = 111 |
| mean (s.d.) | females | 1.5 (1.4) n = 90 |
1.3 (1.6) n = 8 |
1.0 (1.3) n = 24 |
1.1 (1.3) n = 32 |
1.8 (1.5) n = 58 |
| males | 1.7 (1.6) n = 76 |
0 (n.a.) n = 1 |
1.6 (1.6) n = 22 |
1.6 (1.6) n = 23 |
1.8 (1.5) n = 53 |
aMean oxytocin reactivity and social anxiety differ significantly (p < 0.05) between GG + GT and TT genotype groups for females + males (see the text for ANOVA results).
Given that the more oxytocin-reactive genotype group also reported lower social anxiety, a correlation analysis between oxytocin reactivity and social anxiety was performed. Self-reported social anxiety and oxytocin reactivity to the emotional video were uncorrelated (Pearson product–moment correlation = 0.007, p = 0.93).
4. Discussion
Our results demonstrate a relationship between oxytocin reactivity and genotypes of the SNP rs13227433, a common polymorphism in the Williams-syndrome-associated gene GTF2I. We further show that individuals with more oxytocin-reactive genotypes report lower levels of social anxiety. The lack of correlation between oxytocin reactivity and social anxiety suggests that multiple factors, including some not accounted for in this study, influence these variables, which is not unexpected.
Taken together, the results reported here support a model whereby common genetic variation in GTF2I mediates human sociality and anxiety via effects on oxytocin reactivity. Such a model is consistent with previous studies showing that variation in GTF2I SNPs is associated with social phenotypes in healthy populations, as described above [11–13], and it supports a hormonal basis for the effects. The mechanisms connecting GTF2I with oxytocin remain unknown, but may involve differential methylation of the oxytocin receptor gene OXTR, which has been reported among individuals with 7q11.23 deletions and duplications [23], and alternative splicing of GTF2I mRNA among individuals with different SNP genotypes, including those analysed here [24].
The relationship of GTF2I with oxytocin reactivity is relevant to Williams syndrome as it provides the first evidence that a gene subject to hemizygous deletion in this syndrome, and implicated in its characteristic high empathy and prosociality [8,9], modulates oxytocin reactivity. As such, the reported dysregulation of oxytocin levels and reactivity [7], and the high prosociality, high empathy, and low social anxiety [6] found in Williams syndrome, may arise at least in part from reduced GTF2I expression or activity. Further research will increase our understanding of how polymorphisms in GTF2I, and other oxytocin-associated genes, have contributed to the evolution of human sociality and disorders.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Paul Zak for use of the empathy-inducing video and Justin Jagore for assistance with hormone assays.
Ethics
Participants provided informed consent and protocols were approved by the Office of Research Ethics, Simon Fraser University (study no. 2015s0228).
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
T.L.P., N.V.W. and B.J.C. designed the experiment; T.L.P. conducted the experiment; T.L.P., S.R. and J.S. analysed the data; T.L.P. and B.J.C. wrote the manuscript. All authors revised and approved the manuscript and are accountable for its content.
Competing interests
We have no competing interests.
Funding
This study was supported by Natural Sciences and Engineering Research Council of Canada grants 00194522 to N.V.W. and 2014-06505 to B.J.C.
References
- 1.Crespi BJ. 2015. Oxytocin, testosterone, and human social cognition. Biol. Rev. Camb. Philos. Soc. 91, 390–408. ( 10.1111/brv.12175) [DOI] [PubMed] [Google Scholar]
- 2.Feldman R. 2012. Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391. ( 10.1016/j.yhbeh.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 3.Bartz J, Zaki J, Bolger N, Ochsner K. 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309. ( 10.1016/j.tics.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 4.Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M, Legros JJ. 2007. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 32, 407–410. ( 10.1016/j.psyneuen.2007.01.009) [DOI] [PubMed] [Google Scholar]
- 5.Husarova VM, Lakatosova S, Pivovarciova A, Babinska K, Bakos J, Durdiakova J, Kubranska A, Ondrejka I, Ostatnikova D. 2016. Plasma oxytocin in children with autism and its correlations with behavioral parameters in children and parents. Psychiatry Investig. 13, 174–183. ( 10.4306/pi.2016.13.2.174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Järvinen A, Korenberg JR, Bellugi U. 2013. The social phenotype of Williams syndrome. Curr. Opin. Neurobiol. 23, 414–422. ( 10.1016/j.conb.2012.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai L, Carter CS, Ying J, Bellugi U, Pournajafi-Nazarloo H, Korenberg JR. 2012. Oxytocin and vasopressin are dysregulated in Williams syndrome, a genetic disorder affecting social behavior. PLoS ONE 7, e38513 ( 10.1371/journal.pone.0038513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HH, et al. 2009. Induced chromosome deletions cause hypersociability and other features of Williams–Beuren syndrome in mice. EMBO Mol. Med. 1, 50–65. ( 10.1002/emmm.200900003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelmann L, et al. 2007. An atypical deletion of the Williams–Beuren syndrome interval implicates genes associated with defective visuospatial processing and autism. J. Med. Genet. 44, 136–143. ( 10.1136/jmg.2006.044537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malenfant P, et al. 2012. Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. J. Autism Dev. Disord. 42, 1459–1469. ( 10.1007/s10803-011-1389-4) [DOI] [PubMed] [Google Scholar]
- 11.Crespi BJ, Hurd PL. 2014. Cognitive-behavioral phenotypes of Williams syndrome are associated with genetic variation in the GTF2I gene, in a healthy population. BMC Neurosci. 15, 127 ( 10.1186/s12868-014-0127-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartz JR, Waller R, Bogdan R, Knodt AR, Sabhlok A, Hyde LW, Hariri AR. 2017. A common polymorphism in a Williams Syndrome gene predicts amygdala reactivity and extraversion in healthy adults. Biol. Psychiatry 81, 203–210. ( 10.1016/j.biopsych.2015.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbi M, et al. 2015. Variation in the Williams syndrome GTF2I gene and anxiety proneness interactively affect prefrontal cortical response to aversive stimuli. Transl. Psychiatry 5, e622 ( 10.1038/tp.2015.98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, Korenberg JR. 2008. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Devel. Psychopath. 20, 1–35. ( 10.1017/S0954579408000011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barraza JA, Zak PJ. 2009. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. NY Acad. Sci. 1167, 182–189. ( 10.1111/j.1749-6632.2009.04504.x) [DOI] [PubMed] [Google Scholar]
- 16.Cohen AS, Matthews RA, Najolia GM, Brown LA. 2010. Toward a more psychometrically sound brief measure of schizotypal traits: introducing the SPQ-Brief Revised. J. Pers. Disord. 24, 516–537. ( 10.1521/pedi.2010.24.4.516) [DOI] [PubMed] [Google Scholar]
- 17.Raine A, Benishay D. 1995. The SPQ-B: a brief screening instrument for schizotypal personality disorder. J. Pers. Disord. 9, 346–355. ( 10.1521/pedi.1995.9.4.346) [DOI] [Google Scholar]
- 18.Daughters K, Manstead ASR, Hubble K, Rees A, Thapar A, Van Goozen SHM. 2015. Salivary oxytocin concentrations in males following intranasal administration of oxytocin: a double-blind, cross-over study. PLoS ONE 10, e0145104 ( 10.1371/journal.pone.0145104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisman O, Zagoory-Sharon O, Feldman R. 2012. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 37, 1582–1586. ( 10.1016/j.psyneuen.2012.02.014) [DOI] [PubMed] [Google Scholar]
- 20.Feldman R, Golan O, Hirschler-Guttenberg Y, Ostfeld-Etzion S, Zagoory-Sharon O. 2014. Parent-child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. Br. J. Psychiatry 205, 107–112. ( 10.1192/bjp.bp.113.137513) [DOI] [PubMed] [Google Scholar]
- 21.McCullough ME, Churchland PS, Mendez AJ. 2013. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 37, 1485–1492. ( 10.1016/j.neubiorev.2013.04.018) [DOI] [PubMed] [Google Scholar]
- 22.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. 2010. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology 35, 1133–1141. ( 10.1016/j.psyneuen.2010.01.013) [DOI] [PubMed] [Google Scholar]
- 23.Strong E, Butcher DT, Mervis CB, Morris CA, De Carvalho D, Weksberg R, Osborne LR. 2015. Symmetrical dose-dependent DNA-methylation profiles in children with deletion or duplication of 7q11.23. Am. J. Hum. Genet. 97, 216–227. ( 10.1016/j.ajhg.2015.05.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirai Y, Li W, Suzuki T. 2017. Role of splice variants of Gtf2i, a transcription factor localizing at postsynaptic sites, and its relation to neuropsychiatric diseases. Int. J. Mol. Sci. 18, 411 ( 10.3390/ijms18020411) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material.


