Abstract
Competition for food drives divergence and specialization in feeding morphology. Stomatopod crustaceans have two kinds of highly specialized feeding appendages: either elongate spear-like appendages (spearers) used to ambush soft-bodied evasive prey or hammer-like appendages (smashers) that produce extremely high forces used both to break hard-shelled prey and to capture evasive prey. To evaluate associations between appendage type and feeding ecology, the diet of two small smasher and spearer species (size range: 21–27 mm) that co-occur were compared. Stable isotope analysis and the Bayesian mixing model MixSIAR were used to estimate the proportional contributions of prey types to the diet. Both species had relatively wide diets that included hard-shelled and soft-bodied prey, albeit in different proportions; the smasher consumed a greater proportion of hard-shelled prey, and the spearer consumed mostly soft-bodied prey. Appendage kinematics in stomatopods is known to scale linearly across species. These two small species may produce similar kinematics allowing them both to capture evasive prey and hammer hard-shelled prey, thereby widening their diets. Yet, the spearer species is more highly adept at capturing evasive prey, indicating that small spearers are stronger competitors for soft-bodied prey. These findings suggest that a smasher's ability to access hard prey reduced competition for soft prey, and therefore conferred an important benefit favouring the evolution of the impressive smashing strike.
Keywords: trophic ecology, mantis shrimp, stable isotopes
1. Introduction
Obtaining energy from the environment is necessary for all organisms. Success in foraging drives the evolution of the morphology that many heterotrophs use for feeding. Competition for prey can favour the evolution of specialized feeding morphology that opens access to types of prey for which there are few competitors. This scenario often leads to a specialized diet of those prey [1,2].
Mantis shrimp, or stomatopods, (Crustacea: Stomatopoda) are often touted as having highly specialized feeding morphology [3]. Smashing stomatopods, hereafter ‘smashers’, have stout raptorial appendages that move with speeds of 14–23 m s−1 and accelerations that generate extremely high forces [4] (figure 1). The ability to produce high-impact strikes has been hypothesized to correspond to a specialized diet of hard-shelled molluscs and crustaceans [3,5,6]. However, one Caribbean smasher is known to be a generalist predator that consumes both hard-shelled and soft-bodied prey, primarily fish [5,7]. By contrast, ‘spearer’ stomatopods strike with slower speeds (2–7 m s−1) but with elongate appendages that can reach longer distances to ambush soft-bodied, evasive prey, such as fish and small crustaceans [3,8] (figure 1). Their diets consist primarily of these soft prey but some spearers have been observed consuming crustaceans and bivalve molluscs [3,9].
Figure 1.
Focal stomatopod species and their raptorial appendages. (a) Smasher species, G. childi, and its (c) hammer-like raptorial appendage in lateral view. (b) Spearer species, Raoulserenea n.sp., holding an egg mass, and its (d) elongate, spear-like appendage in lateral view. White lines in (a) and (b) outline raptorial appendages. Scale bars, 5 mm. Images in (a) and (b) courtesy of R. L. Caldwell and (c) and (d) courtesy of T. Claverie. (Online version in colour.)
Competition for prey is thought to contribute to the evolution of these morphologically distinct forms; smashing appendages were derived from spearing and gave smashers access to hard-shelled prey [3,7], while spearers became highly adept at capturing evasive prey [3]. These hypotheses have never been rigorously tested. To begin to determine current associations between morphology and diet, I compared the diets of the smasher, Gonodactylus childi (Gonodactylidea; [10]), and an unnamed species of spearer, Raoulserenea n.sp. (Pseudosquillidae; [11]) (figure 1). These small species are of similar size. They occupy the same coral rubble habitat and therefore are exposed to the same prey. I hypothesized that the diets of these two sympatric species would differ substantially, thereby reducing possible competition between them. Specifically, the spearer would have a narrow diet consisting of mostly soft-bodied prey, while the smasher would have a wide diet that included mostly hard-shelled prey but also some soft-bodied prey.
2. Methods
Stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) were measured to compare interspecific diet breadth [12–14]. Seven individuals of each of the two stomatopod species as well as eight potential prey types: alpheid shrimp, clams, crabs, hermit crabs, fish, planktonic crustaceans, snails and worms (6–15 individuals per prey type; table 1) were collected on the reef flat at the Gump South Pacific Research Station, University of California, Berkeley in Mo'orea, French Polynesia. Animals were measured to the nearest 0.01 mm (mean ± s.d.: G. childi = 25.63 ± 3.61; Raoulserenea n.sp. = 23.37 ± 2.61), and then frozen and stored at −20°C until muscle tissue was dissected and prepared for stable isotope analysis.
Table 1.
Mean ± s.d. δ13C and δ15N values from G. childi (smasher), Raoulserenea n.sp. (spearer), and their potential prey items. Prey values are uncorrected for DFs. n = sample size.
| animal | n | δ13C ± s.d. (‰) | δ15N ± s.d. (‰) |
|---|---|---|---|
| stomatopod | |||
| smasher | 7 | −13.81 ± 1.11 | 8.55 ± 0.58 |
| spearer | 7 | −15.42 ± 1.20 | 12.75 ± 1.62 |
| prey types | |||
| alpheid shrimp | 7 | −13.38 ± 0.96 | 7.34 ± 0.74 |
| clam | 11 | −16.96 ± 1.22 | 7.14 ± 0.57 |
| crab | 9 | −12.58 ± 1.59 | 8.18 ± 1.25 |
| fish | 5 | −15.61 ± 2.68 | 13.50 ± 2.56 |
| hermit crab | 6 | −10.65 ± 1.53 | 4.64 ± 2.30 |
| plankton | 5 | −17.91 ± 0.04 | 9.37 ± 0.20 |
| snail | 15 | −13.31 ± 2.02 | 8.15 ± 1.81 |
| worm | 8 | −13.52 ± 1.12 | 7.01 ± 1.40 |
Bayesian stable isotope mixing models in the program MixSIAR v. 3.1.6 [15] were used to estimate the proportion of different prey in the diet for each stomatopod species (reviewed in [16]). Experimentally determined trophic discrimination factors (Δ, the difference between the predator and prey stable isotope ratios) for stomatopods, hereafter denoted as ‘experimental DFs’, were used in the mixing models (Δ15N = 0.9 ± 0.3‰, Δ13C = 3.0 ± 0.6‰ [17]). To verify the use of the experimental DFs, these results were compared with those generated from models using mean literature values calculated by [18] (Δ15N = 2.75 ± 0.1‰, Δ13C =0.75 ± 0.1‰), hereafter referred to as ‘conventional DFs’. To reduce the number of prey in the model, alpheid shrimp and worms were combined a priori, as were snails and crabs, because their stable isotope values did not differ and the stomatopods handled them similarly [7].
Prey were combined a posteriori into categories of hard-shelled and soft-bodied prey. To quantify diet specialization for each species, a specialization index, ɛ (equation (5) in [19]), was calculated from the estimates of dietary proportions. This index provides a measure of diet specialization that is comparable between species and can range from 0 (ultra-generalist) to 1 (ultra-specialist) [19]. Results are presented as medians (95% credible interval, CI). All analyses were conducted in R v. 3.3.0 [20]. See the electronic supplementary material for additional details of all methods.
3. Results
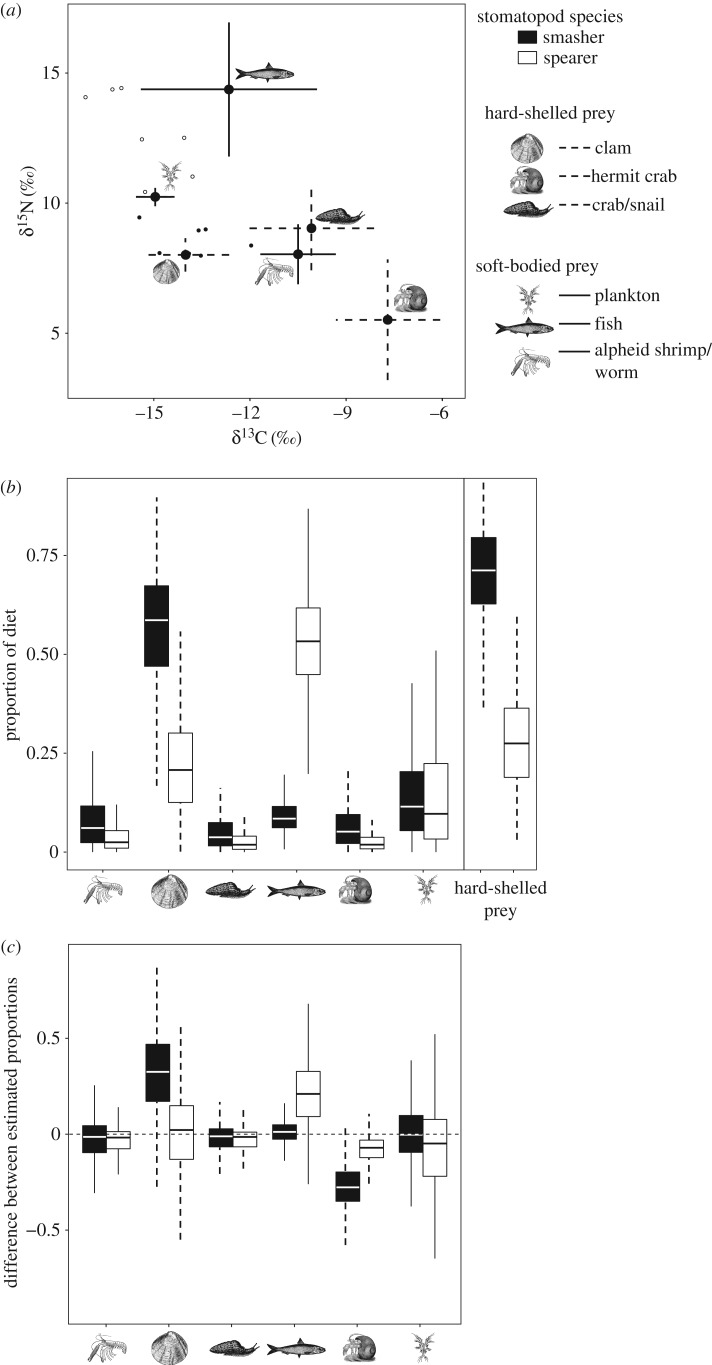
Diet proportion estimates from the mixing model run with experimental DFs indicated that the majority of the smasher diet was clams (59%), whereas the spearer consumed mostly fish (53%) (table 2 and figure 2). Results calculated with conventional DFs were similar (figure 2c) but estimated proportions did not consistently favour one prey type; the majority of the smasher diet was clams and hermit crabs (24% and 34%, respectively), and fish composed a slightly greater proportion of the spearer diet (31%) (table 2; electronic supplementary material, figure S1). However, both models struggled to differentiate between clams and plankton (electronic supplementary material, table S1 and figures S2, S3), suggesting that plankton may have been underestimated in the model results in both species.
Table 2.
Bayesian mixing model median estimates (95% CI) of the proportional contributions of each prey type to the ‘smasher’ and ‘spearer’ diets. Results are given for models run with two DFs: ‘experimental DF’ and ‘conventional DF’. Prey are also aggregated a posteriori into two categories: soft-bodied and hard-shelled prey (italics).
| prey type | experimental DF |
conventional DF |
||
|---|---|---|---|---|
| smasher (%) | spearer (%) | smasher (%) | spearer (%) | |
| soft-bodied | 29.6 (12.2, 65.7) | 72.9 (48.0, 92.1) | 31.9 (10.3, 67.1) | 61.5 (29.8, 87.7) |
| alpheid/worm | 6.1 (0.2, 28.3) | 2.5 (0.1, 14.7) | 8.0 (0.2, 51.5) | 5.1 (0.1, 32.5) |
| fish | 8.5 (2.9, 20.9) | 53.3 (29.1, 79.5) | 7.2 (1.8, 20.5) | 31.4 (10.7, 59.3) |
| plankton | 11.5 (0.5, 43.8) | 9.7 (0.2, 52.7) | 12.3 (0.7, 35.2) | 18.6 (0.3, 54.9) |
| hard-shelled | 70.4 (34.3, 87.8) | 27.1 (7.9, 51.8) | 68.1 (32.9, 89.7) | 38.5 (12.3, 70.2) |
| clam | 58.6 (18.4, 80.5) | 20.8 (3.5, 47.2) | 24.2 (1.2, 55.8) | 18.0 (0.5, 57.6) |
| crab/snail | 3.7 (0.2, 19.8) | 1.9 (0.1, 12.9) | 4.9 (0.2, 33.6) | 3.5 (0.1, 29.4) |
| hermit crab | 5.2 (0.1, 20.0) | 1.9 (0.1, 10.0) | 33.7 (6.2, 53.0) | 9.6 (1.4, 25.3) |
Figure 2.
Analysis of stable isotope data. (a) Position of individual smashers, G. childi, (small filled circles) and spearers, Raoulserenea n.sp., (small open circles) in dual δ13C and δ15N isotope space relative to the mean (±s.d.) isotope values of the potential prey items (large filled circles). The stable isotope values of stomatopods have been corrected using experimental DFs. (b) Median (lines in centres of boxes = median, box boundaries = 50% CI, error bars = 95% CI) proportional contributions of each prey item to the smasher (black boxes) and the spearer (white boxes) diets calculated with experimental DFs. (c) Differences between the estimated proportions calculated with the experimental and conventional DFs; zero is represented by a dashed horizontal line for ease of comparison. Prey types are depicted by line drawings and divided into hard-shelled (error bars = dashed lines) and soft-bodied (error bars = solid lines) categories. For sample sizes of stomatopods and prey sources, see table 1.
When aggregated a posteriori into hard-shelled and soft-bodied prey categories, models run with both DFs consistently indicated that the smasher diet was composed largely of hard-shelled prey (68–70%), while the spearer diet was primarily soft-bodied prey (62–73%) (table 2; electronic supplementary material figures S4 and S5). Regardless, estimates of the specialization index (95% CI) for the smasher (experimental DF: 0.46 (0.03, 0.84), conventional DF: 0.38 (0.02, 0.79)) were indistinguishable from those of the spearer (experimental DF: 0.42 (0.03, 0.76), conventional DF: 0.28 (0.01, 0.75)), as there was high uncertainty in the estimates, indicating that neither species is highly specialized (no values of ɛ are close to 1).
4. Discussion
Given that the smasher, G. childi, and the spearer, Raoulserenea n.sp., occupy the same habitat and have overlapping diets, it is reasonable to assume that they compete for the same food resources. Although the smasher consumed a greater proportion of hard-shelled prey, while the spearer consumed mostly soft-bodied prey, both species included all prey types in their diets, and neither species was highly specialized according to the specialization index estimates. This result was robust to the DF used in the analyses.
How do these species include a range of prey in their diets? Raptorial appendage strikes of smashers are incredibly fast and forceful, allowing smashers to capture and consume a wide diversity of prey [7]. Some spearers are known to both ambush evasive prey with their elongate appendages and actively forage for sedentary hard-shelled prey [9,21]. Additionally, force generation scales linearly across species, and medium-sized smashers (33–82 mm) produce similar forces compared with a slightly larger spearer (87–108 mm), Squilla empusa [22], whose diet consists of crustaceans, molluscs and hydroids [9]. Thus, at the low extreme of their size ranges (10–20 mm), spearers and smashers may generate similar forces allowing them both to break hard-shelled prey. Another possibility is that small, hard-shelled prey items are more weakly calcified [23], making them more vulnerable to both spearing and smashing strikes. Finally, both of these species are in the same superfamily, Gonodactyloidea [11], and thus phylogenetic effects on feeding kinematics and behaviour may also contribute to diet overlap. Testing these hypotheses would require determining the strike kinematics and feeding behaviours used to capture and consume these particular prey, along with measuring the abundances of stomatopods and these prey.
In comparison with the spearer, the high proportion of hard-shelled prey in the diet of G. childi suggests that this smasher is a weaker competitor for soft-bodied prey, even though the raptorial appendages of smashers produce faster, more powerful strikes [8]. Interestingly, G. childi's diet is also narrower than that of the Caribbean smasher, Neogonodactylus bredini [7], whose diet consists primarily of fish and who does not co-occur with many spearers. It is therefore possible that N. bredini would be similarly limited to a diet of mostly hard-shelled prey in the presence of high abundances of spearers. Investigating how indirect competition for soft-bodied prey shapes G. childi's diet and that of other smashers with wide diets, like N. bredini, would lend novel insights into how selection for the ability to consume hard prey may be an evolutionary driver of morphological divergence between fast spearers and powerful smashers.
Regardless, both species consume a diversity of available prey even though they have highly specialized feeding morphologies for consuming either hard or soft prey. This result counters long-standing hypotheses that specialized morphology corresponds to a specialist diet [2]. The diets of these small species are likely a result of both consistent competition for prey and morphological specializations that widened diet breadth, allowing them to consume otherwise inaccessible prey. These findings begin to reveal that competition for prey in stomatopods yields specialized morphology that broadens, as opposed to narrows, diet breadth, and emphasizes the dynamic influence of scaling, behaviour, and competition on the evolution of feeding morphology.
Supplementary Material
Acknowledgements
S. Bush and T. S. Tunstall assisted with sample collections. J. Hassen, C. Liivoja, M. Perez, V. Duong and the Dawson Laboratory helped with sample preparation. T. Dawson and S. Mambelli helped with stable isotope analysis. R. L. Caldwell and T. Claverie provided photographs. I thank R. L. Caldwell for contributing to the intellectual development of this study, S. N. Patek for insightful discussion, B. C. Stock for data analysis advice, and J. H. Christy and J. R. A. Taylor for helpful review of this manuscript.
Ethics
This research complied with animal welfare guidelines of the University of California, Berkeley and the French Polynesian government. Detailed animal welfare information is available in the electronic supplementary material.
Data accessibility
Supplementary methods and results are available in the electronic supplementary material. Datasets and codes are available on Dryad (http://dx.doi.org/10.5061/dryad.5d781) [14].
Competing interests
The author declares no competing financial interests.
Funding
This research was supported by a UC Museum of Paleontology Graduate Student Research Award and the Biology and Geomorphology of Tropical Islands Course at UC Berkeley (all awards to M.S.dV.).
References
- 1.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 2.Ferry-Graham LA, Wainwright PC, Westneat MW, Bellwood DR. 2002. Mechanisms of benthic prey capture in wrasses (Labridae). Mar. Biol. 141, 819–830. ( 10.1007/s00227-002-0882-x) [DOI] [Google Scholar]
- 3.Dingle H, Caldwell RL. 1978. Ecology and morphology of feeding and agonistic behavior in mudflat stomatopods (Squillidae). Biol. Bull. 155, 134–149. ( 10.2307/1540871) [DOI] [Google Scholar]
- 4.Patek SN, Korff WL, Caldwell RL. 2004. Deadly strike mechanism of a mantis shrimp. Nature 428, 819–820. ( 10.1038/428819a) [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RL, Roderick GK, Shuster SM. 1989. Studies of predation by Gonodactylus bredini. In Biology of Stomatopods (ed. Ferrero EA.), pp. 117–131. Modena, Italy: Mucchi. [Google Scholar]
- 6.Caldwell RL, Dingle H. 1976. Stomatopods. Sci. Am. 234, 80–89. ( 10.1038/scientificamerican0176-80)1251183 [DOI] [Google Scholar]
- 7.deVries MS, Stock BC, Christy JH, Goldsmith GR, Dawson TE. 2016. Specialized morphology corresponds to a generalist diet: linking form and function in mantis shrimp crustaceans. Oecologia 182, 429–442. ( 10.1007/s00442-016-3667-5) [DOI] [PubMed] [Google Scholar]
- 8.deVries MS, Murphy EAK, Patek SN. 2012. Strike mechanics of an ambush predator: the spearing mantis shrimp. J. Exp. Biol. 215, 4374–4384. ( 10.1242/jeb.075317) [DOI] [PubMed] [Google Scholar]
- 9.Pihl L, Baden SP, Diaz RJ, Schaffner LC. 1992. Hypoxia-induced structural changes in the diet of bottom-feeding fish and Crustacea. Mar. Biol. 112, 349–361. ( 10.1007/BF00356279) [DOI] [Google Scholar]
- 10.Manning RB. 1971. Two new species of Gonodactylus (Crustacea, Stomatopoda), from Eniwetok Atoll, Pacific Ocean. Proc. Biol. Soc. Washingt. 84, 73–80. [Google Scholar]
- 11.Ahyong ST. 2001. Revision of the Australian stomatopod Crustacea. Rec. Aust. Museum Suppl. 26, 1–326. ( 10.3853/j.0812-7387.26.2001.1333) [DOI] [Google Scholar]
- 12.Sabat P, Maldonado K, Canals M, Martínez del Rio C. 2006. Osmoregulation and adaptive radiation in the ovenbird genus Cinclodes (Passeriformes: Furnariidae). Funct. Ecol. 20, 799–805. ( 10.1111/j.1365-2435.2006.01176.x) [DOI] [Google Scholar]
- 13.Newsome SD, Martínez del Rio C, Bearhop S, Phillips DL. 2007. A niche for isotopic ecology. Front. Ecol. Environ. 5, 429–436. ( 10.1890/060150.01) [DOI] [Google Scholar]
- 14.deVries MS. Data from: The role of feeding morphology and competition in governing the diet breadth of sympatric stomatopod crustaceans. Dryad Digital Respository. ( 10.5061/dryad.5d781) [DOI] [PMC free article] [PubMed]
- 15.Stock BC, Semmens BX.2013. MixSIAR GUI User Manual, version 3.1. https://github.com/brianstock/MixSIAR . (doi:10.5281/zenodo.56159)
- 16.Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. 2014. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 835, 823–835. ( 10.1139/cjz-2014-0127) [DOI] [Google Scholar]
- 17.deVries MS, Martínez del Rio C, Tunstall TS, Dawson TE. 2015. Isotopic incorporation rates and discrimination factors in mantis shrimp crustaceans. PLoS ONE 10, e0122334 ( 10.1371/journal.pone.0122334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caut S, Angulo E, Courchamp F. 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. ( 10.1111/j.1365-2664.2009.01620.x) [DOI] [Google Scholar]
- 19.Newsome SD, Yeakel JD, Wheatley PV, Tinker MT. 2012. Tools for quantifying isotopic niche space and dietary variation at the individual and population level. J. Mammal. 93, 329–341. ( 10.1644/11-MAMM-S-187.1) [DOI] [Google Scholar]
- 20.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 21.Maynou F, Abello P, Sartor P. 2005. A review of the fisheries biology of the mantis shrimp, Squilla mantis (L., 1758) (Stomatopoda, Squillidae) in the Mediterranean. Crustaceana 77, 1081–1099. ( 10.1163/1568540042900295) [DOI] [Google Scholar]
- 22.Patek SN, Rosario MV, Taylor JRA. 2013. Comparative spring mechanics in mantis shrimp. J. Exp. Biol. 216, 1317–1329. ( 10.1242/jeb.078998) [DOI] [PubMed] [Google Scholar]
- 23.Wainwright SA, Biggs WD, Currey JD, Gosline JM. 1976. Mechanical design in organisms. Princeton, NJ: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- deVries MS. Data from: The role of feeding morphology and competition in governing the diet breadth of sympatric stomatopod crustaceans. Dryad Digital Respository. ( 10.5061/dryad.5d781) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supplementary methods and results are available in the electronic supplementary material. Datasets and codes are available on Dryad (http://dx.doi.org/10.5061/dryad.5d781) [14].