Abstract
Until recently, paternal effects—the influence of fathers on their offspring due to environmental factors rather than genes—were largely discarded or assumed to be confined to species exhibiting paternal care. It is now recognized that paternal effects can be transmitted through the ejaculate, but unambiguous evidence for them is scarce, because it is difficult to isolate effects operating via changes to the ejaculate from maternal effects driven by female mate assessment. Here, we use artificial insemination to disentangle mate assessment from fertilization in guppies, and show that paternal effects can be transmitted to offspring exclusively via ejaculates. We show that males fed reduced diets produce poor-quality sperm and that offspring sired by such males (via artificial insemination) exhibit reduced body size at birth. These findings may have important implications for the many mating systems in which environmentally induced changes in ejaculate quality have been reported.
Keywords: epigenetic inheritance, condition dependence, Poecilia reticulata
1. Introduction
It is well established that environmentally moderated changes to an individual's phenotype can have fitness implications that transcend generations. Maternal effects, for example, occur when an individual's phenotype is affected by the environment experienced by its mother, which may determine the level of parental care or resources a female invests in her offspring [1]. Similarly, paternal effects can arise when environmental factors experienced (or imposed) by males influence offspring traits, a phenomenon best described in species exhibiting paternal care (e.g. [2]). However, the possibility that paternal environmental effects can be transmitted to offspring in species lacking paternal care (or any other form of direct interaction with their offspring) has received considerably less attention. In particular, the possibility that paternal environmental effects are transmitted via sperm (i.e. cells traditionally assumed to carry exclusively genetic information) or seminal fluid has only recently been considered (e.g. [3,4]).
Recent studies suggest that environmentally induced changes in a male's ejaculate can influence offspring phenotype. For example, diet quality, paternal stress or a change in social conditions may elevate germ-line mutation rates (e.g. [5]) or induce epigenetic effects that ‘reprogramme’ the paternal genome (e.g. [6,7]). Both factors have been implicated as potential sources of paternal effects in a range of species (reviewed in [8]). However, environmental effects on males may also be perceived by females during mate assessment, and females may apportion more resources towards the progeny of certain (e.g. high condition) males (so-called differential allocation (DA); [9]). Thus, experimentally isolating sperm-moderated paternal effects represents a significant challenge [8]. A handful of recent studies have specifically addressed this issue by experimentally controlling mate assessment through the use of artificial (e.g. in vitro) fertilization, which effectively dissociates mating (and thus the potential for DA) from fertilization [10–12].
Here, we test whether environmentally induced paternal effects can be transmitted exclusively via ejaculates in the guppy Poecilia reticulata, a livebearing fish with internal fertilization. Guppies are amenable for addressing this question; methods for artificial insemination are established [13] and used routinely to dissociate pre-copulatory mate assessment from fertilization, thus controlling for DA (e.g. [12]). Moreover, experimental dietary manipulation has consistent phenotypic effects on sperm quality; males fed compositionally impaired or reduced diets suffer reductions in sperm quality (e.g. [14]) and reproductive success [15]. Here, we exploit these attributes and show that a reduction in food intake causes not only a decline in ejaculate quality, but also a reduction in body size in newborn offspring.
2. Material and methods
(a). Study population and dietary treatments
Guppies came from a captive population comprising descendants of wild-caught fish (Townsville, Queensland). Adult males (n = 100) were selected from mixed-sex tanks and assigned haphazardly to either a ‘high’- or ‘low’-quantity diet for one month (see the electronic supplementary material).
(b). Assessment of sperm traits in treated males
After the feeding trials, 20 males were selected haphazardly from each treatment to test the effects of diet on sperm counts, sperm viability (proportion of live sperm in the ejaculate) and sperm velocity (see below). Sperm counts were estimated by counting the total number of sperm in ‘stripped’ ejaculates (electronic supplementary material). Sperm viability was measured using a fluorescence live/dead sperm assay (Invitrogen, Molecular Probes; electronic supplementary material). Sperm velocity was assessed using computer-assisted sperm analysis (CASA; see the electronic supplementary material). Of the measures recorded by CASA, we a priori selected the speed at which sperm swam along their recorded trajectory (curvilinear velocity (VCL)). Previous work has confirmed that these three traits (sperm counts, viability and VCL) are predictors of paternity success in guppies [16,17].
(c). Artificial inseminations
Ejaculates were stripped from the remaining 60 males (n = 30 high and n = 30 low) and used immediately for artificial insemination (AI). We used virgin females (n = 30 in each treatment; mean body size ± s.e. = 20.88 ± 0.22 mm) to ensure that fertilizations could not be attributed to sperm stored from previously mated males. Each female was assigned to one of the two treatments (t-test comparing body size between females paired to high and low males: t57 = 0.83, p = 0.41), anaesthetized and artificially inseminated with 20 spermatozeugmata (sperm bundles) from a single (high or low) male [13]. Females were then revived in freshwater and housed individually in 2 l tanks until they produced their first brood (mean number of offspring from each female: high = 5.57 ± 0.45 s.e.; range = 1–10; low = 5.83 ± 0.67 s.e.; range = 1–11). At this point, brood production time (days to parturition) was recorded (t-test comparing days to parturition between females paired to high and low males: t52 = 0.07, p = 0.95).
(d). Offspring body size and sperm traits
At parturition, offspring were isolated from their mother and kept in separate 2 l tanks until measured for body size at one week of age (juveniles) and at four months (adults). To estimate juvenile body size, offspring were placed in a Petri dish containing ca 5 mm of water and orientated above a piece of graph paper for size calibration. Each fish was photographed and body length (distance in millimetres from snout to the tip of the tail) was estimated using ImageJ (v. 1.48). To estimate adult body size, fish were reared in mixed-sex family cohorts (maximum of four fish per tank) until four months old. At this point, the fish were sexed and photographed for body size measures. For male offspring, we measured sperm counts and sperm velocity (see above). All measurements were performed blind of the treatment.
(e). Statistical analysis
Analyses were performed using R v. 3.3.2 [18]. We used t-tests to determine whether diet treatments influenced sperm traits in adult males. Linear mixed-effects (LME) models were used to compare body size in juvenile and adult offspring. For juveniles, which could not be sexed, we used the ‘lmer’ function in the ‘lme4’ package [19] to construct a model that included offspring body size as the response variable, paternal diet treatment as a fixed factor, female identity (dam) as a random effect (to account for multiple offspring coming from the same dam), and dam body length as a covariate. The same parameters were used to analyse adult body size, but in this model we also included offspring sex and the sex-by-treatment interaction as fixed effects. Finally, we compared sperm traits in adult male offspring using LME models that included sperm counts and VCL as responses, treatment as a fixed effect, and dam as a random effect. The significance of fixed effects in LME models was estimated using the Anova function in the ‘car’ package [20].
3. Results
We found significant effects of diet on sperm traits; males fed the high-quantity diet produced significantly larger ejaculates (t37 = 2.11, p = 0.04) containing faster (t37 = 2.51, p = 0.02) and more viable sperm (t37 = 3.39, p = 0.002) than their low-quantity counterparts.
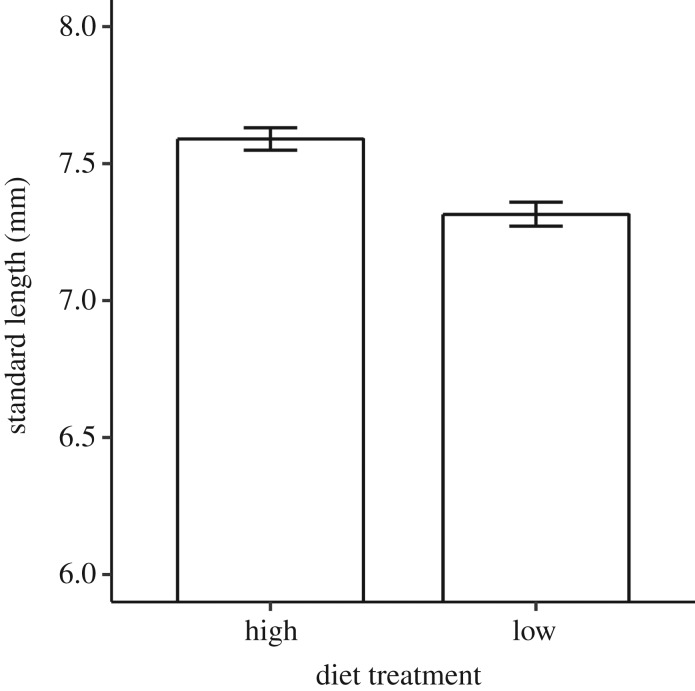
We found a significant effect of the father's diet treatment on the mean body size of juvenile offspring (table 1a). Juveniles sired by males fed the high-quantity diet were significantly larger than those sired by males fed the low diet (figure 1). We found no significant effect of diet treatment (or interactions involving treatment) on either adult body size (table 1b) or male offspring sperm traits (table 1c,d).
Table 1.
Results from LME models analysing the effects of sire diet on body size of juvenile (a) and adult (b) offspring, (c) sperm velocity and (d) sperm counts. SL, standard length.
| (a) juvenile body size | |||
|---|---|---|---|
| random effect | variance | s.d. | |
| dam ID | 0.15 | 0.38 | |
| residual | 0.14 | 0.37 | |
| fixed effects | χ2 | d.f. | p-value |
|---|---|---|---|
| diet treatment | 5.96 | 1 | 0.015 |
| dam SL (covariate) | 0.94 | 1 | 0.331 |
| (b) adult body size | |||
|---|---|---|---|
| random effect | variance | s.d. | |
| dam ID | 0.46 | 0.68 | |
| residual | 1.59 | 1.26 | |
| fixed effects | χ2 | d.f. | p-value |
|---|---|---|---|
| diet treatment | 0.36 | 1 | 0.56 |
| sex | 61.5 | 2 | <0.0001 |
| diet × sex | 0.49 | 2 | 0.78 |
| (c) sperm velocity (VCL) | |||
|---|---|---|---|
| random effect | variance | s.d. | |
| dam ID | 4.10 | 2.02 | |
| residual | 215.48 | 14.68 | |
| fixed effects | χ2 | d.f. | p-value |
|---|---|---|---|
| diet treatment | 1.64 | 1 | 0.20 |
| (d) Sperm count (×103) | |||
|---|---|---|---|
| random effect | variance | s.d. | |
| dam ID | 1 869 018 | 1367 | |
| residual | 8 194 814 | 2863 | |
| fixed effects | χ2 | d.f. | p-value |
|---|---|---|---|
| diet treatment | 0.20 | 1 | 0.65 |
Figure 1.
The effect of sire diet treatment on mean (±s.e.) offspring standard length in guppies following artificial insemination.
4. Discussion
We show that diet intake affects not only sperm traits linked to reproductive fitness, but also offspring traits via sperm-moderated paternal effects. Juveniles fathered by males fed reduced diets were significantly smaller than those sired by males on the high-quantity diet. Interestingly, we found no corresponding effect of paternal diet treatment on adult offspring size or sperm traits, suggesting that this is a transient effect influencing only early fitness.
Our findings for juveniles are likely to have important fitness implications. In guppies, size-dependent mortality means that an individual's survival prospects will depend on its size at birth and growth rate [21]. For example, larger guppy neonates exhibit faster and more effective escape responses to simulated predation threats than their smaller counterparts [22], which follows a general pattern in teleost fishes (e.g. [23]). Furthermore, relatively large juvenile guppies exhibit enhanced fitness in highly competitive environments [24], further attesting to the likely fitness benefits associated with increased body size.
One striking conclusion from our findings is that female preferences for males displaying traits that indicate high foraging efficacy (e.g. [25]) may be under direct selection due to the causal associations between diet quality, sperm traits and ultimately offspring fitness. Until now, such preferences have been attributed to indirect selection, due to a genetic association between male display traits and genes underlying offspring fitness (e.g. [26]). Our findings challenge such conclusions and caution against interpreting associations between sire attractiveness and offspring quality as evidence for female preferences for paternal ‘good genes’.
Finally, our findings complement recent work on guppies showing that environmentally induced changes in sperm phenotype, caused by experimental changes in the duration of sperm storage by males, influence reproductive traits in male offspring [12]. Both studies reveal fitness consequences associated with environmentally induced changes in sperm phenotype, but they also suggest that the nature of the paternal effect depends on the environmental trigger that causes it. In both cases, we require an understanding of the mechanisms that link changes in sperm phenotype to offspring fitness, which may extend to numerous other traits not considered in our study. Such mechanisms may include genetic (e.g. mutation; [27]) and/or epigenetic factors [28] transferred through the ejaculate, including sperm cells and/or components of the seminal fluid. We eagerly await follow-up studies that uncover such mechanisms.
Supplementary Material
Acknowledgements
We thank Cameron Duggin for assistance and three anonymous reviewers for comments on the manuscript.
Ethics
This research was approved by the University of Western Australia's Animal Ethics Committee (RA/3/100/1376).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gr6dq [29].
Authors' contributions
J.P.E. and C.G. designed the study. All authors contributed towards data collection. J.P.E. analysed the data and wrote the first draft of the paper. All authors contributed towards subsequent drafts and analysis, approved the final version of the manuscript and agreed to be held accountable for the content therein.
Competing interests
The authors declare no competing interests.
Funding
J.P.E. and C.G. received funding from the Australian Research Council (grant nos DE150101625 and DP120100773) and the University of Western Australia.
References
- 1.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. 2006. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm. Behav. 50, 699–707. ( 10.1016/j.yhbeh.2006.06.035) [DOI] [PubMed] [Google Scholar]
- 3.Soubry A, Hoyo C, Jirtle RL, Murphy SK. 2014. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays 36, 359–371. ( 10.1002/bies.201300113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggert H, Kurtz J, Diddens-de Buhr MF. 2014. Different effects of paternal transgenerational immune priming on survival and immunity in step and genetic offspring. Proc. R. Soc. B 281, 20142089 ( 10.1098/rspb.2014.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yauk C, et al. 2008. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc. Natl Acad. Sci. USA 105, 605–610. ( 10.1073/pnas.0705896105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgers AB, Morgan CP, Leu NA, Bale TL. 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl Acad. Sci. USA 112, 13 699–13 704. ( 10.1073/pnas.1508347112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terashima M, Barbour S, Ren JK, Yu WS, Han YX, Muegge K. 2015. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics 10, 861–871. ( 10.1080/15592294.2015.1075691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crean AJ, Bonduriansky R. 2014. What is a paternal effect? Trends Ecol. Evol. 29, 554–559. ( 10.1016/j.tree.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 9.Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445. ( 10.1086/284493) [DOI] [Google Scholar]
- 10.Crean AJ, Dwyer JM, Marshall DJ. 2013. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology 94, 2575–2582. ( 10.1890/13-0184.1) [DOI] [PubMed] [Google Scholar]
- 11.Zajitschek S, Hotzy C, Zajitschek F, Immler S. 2014. Short-term variation in sperm competition causes sperm-mediated epigenetic effects on early offspring performance in the zebrafish. Proc. R. Soc. B 281, 20140422 ( 10.1098/rspb.2014.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparini C, Dosselli R, Evans JP. In press. Sperm storage by males causes changes in sperm phenotype and influences the reproductive fitness of males and their sons. Evol. Lett. ( 10.1002/evl3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JP, Zane L, Francescato S, Pilastro A. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363. ( 10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, Turchini GM, Gasparini C, Norambuena F, Evans JP. 2014. The expression of pre- and postcopulatory sexually selected traits reflects levels of dietary stress in guppies. PLoS ONE 9, e105856 ( 10.1371/journal.pone.0105856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman MM, Gasparini C, Turchini GM, Evans JP. 2014. Experimental reduction in dietary omega-3 polyunsaturated fatty acids depresses sperm competitiveness. Biol. Lett. 10, 20140623 ( 10.1098/rsbl.2014.0623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 17.Fitzpatrick JL, Evans JP. 2014. Postcopulatory inbreeding avoidance in guppies. J. Evol. Biol. 27, 2585–2594. ( 10.1111/jeb.12545) [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. 2014. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 19.Bates D, Maechler M, Bolker B, Walker S. 2015. Linear mixed-effects models using Eigen and S4 J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 20.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage; (http://socserv.socsci.mcmaster.ca/jfox/Books/Companion) [Google Scholar]
- 21.Jorgensen C, Auer SK, Reznick DN. 2011. A model for optimal offspring size in fish, including live-bearing and parental effects. Am. Nat. 177, E119–E135. ( 10.1086/659622) [DOI] [PubMed] [Google Scholar]
- 22.Dial TR, Reznick DN, Brainerd EL. 2016. Effects of neonatal size on maturity and escape performance in the Trinidadian guppy. Funct. Ecol. 30, 943–952. ( 10.1111/1365-2435.12565) [DOI] [Google Scholar]
- 23.Gibb AC, Swanson BO, Wesp H, Landels C, Liu C. 2006. Development of the escape response in teleost fishes: do ontogenetic changes enable improved performance? Physiol. Biochem. Zool. 79, 7–19. ( 10.1086/498192) [DOI] [PubMed] [Google Scholar]
- 24.Bashey F. 2008. Competition as a selective mechanism for larger offspring size in guppies. Oikos 117, 104–113. ( 10.1111/j.2007.0030-1299.16094.x) [DOI] [Google Scholar]
- 25.Grether G. 2000. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata). Evolution 54, 1712–1724. ( 10.1111/j.0014-3820.2000.tb00715.x) [DOI] [PubMed] [Google Scholar]
- 26.Reynolds JD, Gross MR. 1992. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc. R. Soc. Lond. B 264, 57–62. ( 10.1098/rspb.1992.0130) [DOI] [Google Scholar]
- 27.Krasovec R, Belavkin RV, Aston JAD, Channon A, Aston E, Rash BM, Kadirvel M, Forbes S, Knight CG. 2014. Mutation rate plasticity in rifampicin resistance depends on Escherichia coli cell-cell interactions. Nat. Comm 5, 3742 ( 10.1038/ncomms4742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–671. ( 10.1038/nn.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans JP, Lymbery RA, Wiid KS, Rahman M, Gasparini C. 2017. Data from: Sperm as moderators of environmentally induced paternal effects in a livebearing fish. Dryad Digital Repository. ( 10.5061/dryad.gr6dq) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Evans JP, Lymbery RA, Wiid KS, Rahman M, Gasparini C. 2017. Data from: Sperm as moderators of environmentally induced paternal effects in a livebearing fish. Dryad Digital Repository. ( 10.5061/dryad.gr6dq) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.gr6dq [29].