Abstract
BACKGROUND
Hyaluronic acid (HA) filler injection is an increasingly popular aesthetic procedure.
OBJECTIVE
To compare the effectiveness and safety of two HA fillers (HAED and HAPER) for the treatment of severe nasolabial folds (NLFs).
MATERIALS AND METHODS
This was an evaluator-blinded and subject-blinded split-face study. At baseline, HAED or HAPER was randomly assigned to the left or right NLF. The follow-up period was 12 months. Effectiveness was assessed using the wrinkle severity rating scale (WSRS) and subject preference. Safety was assessed by adverse events and local tolerability symptoms recorded by subjects during 3 weeks after the treatment.
RESULTS
At 6 months, HAED was noninferior to HAPER (assessed by mean change from baseline in WSRS score). There was a significant difference in mean WSRS score change from baseline in favor of HAED at 3 to 12 months, and a majority of subjects preferred HAED over HAPER at 12 months. However, the overall responder rate was similar between products, and it remained high throughout the study. At 12 months, approximately 80% of subjects were still responders. Both products were well tolerated and associated with a few treatment-related adverse events.
CONCLUSION
To conclude, HAED was at least as effective and well tolerated for the treatment of severe NLFs as HAPER.
Hyaluronic acid (HA) dermal fillers have been used for aesthetic purposes since the 1990s, and Restylane was the first stabilized HA filler of nonanimal origin approved for use in the European Union 1996 and in the United States 2003. Currently, HA fillers are used for different purposes and are available with varying gel characteristics such as firmness and particle size. Emervel (Galderma SA, Lausanne, Switzerland) HA gel dermal fillers (from now on referred to as HAE) have been developed for use in facial tissue augmentation.1 The products in the HAE range are all manufactured using the optimal balance technology and have a HA concentration of 20 mg/mL. However, to allow for adaptation to different injection depths, treatment sites, and tissue quality, the products differ in the degree of cross-linking and calibrated gel particle size. Effectiveness and safety data for the HAE products are available for several facial indications.2–6 Emervel Deep (HAED), which was used in the present study, is intended for injection into the deep dermis to correct moderate to deep facial wrinkles, such as nasolabial folds (NLFs).2
Interim results (6-month data) from this randomized, controlled, split-face study with HAED and Restylane Perlane (HAPER; Q-Med AB, Uppsala, Sweden) for the treatment of severe NLFs have been published previously.7 Here, the final effectiveness and safety outcomes up to 12 months after the baseline treatment were reported, thereby extending the published literature on product duration and safety for HAED in comparison with a well-established HA filler (HAPER) that has a similar intended use. This is also the first long-term study of HAED, and the first long-term comparison of these two products for the treatment of severe NLFs.
Subjects and Methods
Study Design
This was a randomized, controlled, subject-blinded and evaluator-blinded, split-face, multicenter study. To compare the effectiveness and safety of HAED with HAPER for the treatment of severe NLFs, subjects were followed up for 12 months after the treatment. Randomization of treatment to each side of the face was done centrally (one list per study center). Allocation concealment was achieved using randomization envelopes. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by independent ethics committees. The study included an open-label part where the first three subjects per center received HAED in both NLFs. The rationale for this was to give the investigators an opportunity to familiarize themselves with the injection technique because HAED had recently been developed at the time of the study. However, only outcomes from the blinded split-face comparison of HAED and HAPER are presented here.
Subject Selection
Adult subjects (≥18 years) who provided signed informed consent and had severe NLFs on both sides of the face (i.e., Score 4, rated using the 5-point wrinkle severity rating scale [WSRS]8) were eligible for inclusion in the study. Main exclusion criteria were (1) active skin disease or inflammation near or in the NLFs; (2) other relevant medical history (e.g., sensitivity to HA, bleeding disorder, impaired coagulation, severe keloids, or hypertrophic scars); (3) soft tissue augmentation with HA or collagen in the NLFs in the previous 18 months or any other soft tissue augmentation in the NLFs; (4) any aesthetic or dermatologic treatment in the face in the previous 12 months and during the study (except botulinum toxin Type A injections, which were allowed in the upper third of the face up to 1 month prior to baseline and from 3 months after the last injection in the study); (5) and pregnancy, nursing, or planned pregnancy.
Treatments
At baseline, injection treatment with HAED or HAPER (both products without lidocaine) was randomly assigned to either the left or right NLF. The evaluating investigator was not allowed to be present at the injection or assist in the preparations for injection, and subjects were blindfolded prior to and during the injection in order to maintain the blinding. If the blinded evaluator judged it necessary, the unblinded investigator could administer a touch-up injection 3 weeks after the baseline treatment. Local and/or topical anesthetics and/or infraorbital nerve block could be applied at the discretion of the investigator.
Injections were to be placed in the mid dermis using a 27-gauge needle. The aim was to achieve complete correction of the NLFs (without overcorrection). The injection technique and volume was decided by the investigator, provided that no more than 2 mL per product was injected at baseline and no more than 1 mL per product at touch-up.
Assessments
The 5-grade WSRS8 was used separately by blinded evaluators and subjects to evaluate the severity of subjects' NLFs. Mean change in WSRS from baseline was the primary effectiveness parameter. The percentage of subjects with at least one-grade improvement in WSRS from baseline (defined here as responder rate) was a secondary effectiveness parameter. Subjects were offered to receive a final retreatment at the end of the study. Those who wished to receive this retreatment were asked to indicate their preferred treatment side.
Safety assessments included the blinded evaluator's assessment of adverse events (AEs) at each study visit. In addition, subjects assessed local tolerability (bruising, edema/swelling, erythema, nodule formation, pain/tenderness, and pruritus) on a daily basis using a subject diary during 3 weeks after the baseline treatment, and if applicable, touch-up treatment.
Statistical Analyses
Sample size was calculated based on the assumption that HAED and HAPER were equally efficacious at 6 months, with a 90% power to demonstrate noninferiority. The safety and intention-to-treat (ITT) populations included all subjects who received all planned treatments. The per-protocol (PP) population included all randomized subjects with no major protocol deviations.
The primary effectiveness criterion was the mean change in the blinded evaluator's assessment of WSRS from baseline to 6 months after baseline. A Student t-test was used to calculate the 95% confidence interval (CI) of the mean between-treatment difference (HAPER − HAED). If the CI was above the margin of −0.5 in both the ITT and PP populations, noninferiority was to be declared.
Responder rate data are presented descriptively. Imputation for any missing effectiveness data was done for the ITT population (last observation carried forward) but not for the PP population, for which analyses were based on observed cases. A two-sided McNemar test was used to analyze subject preference at the end of the study. Safety data were analyzed descriptively.
Results
Subject Disposition
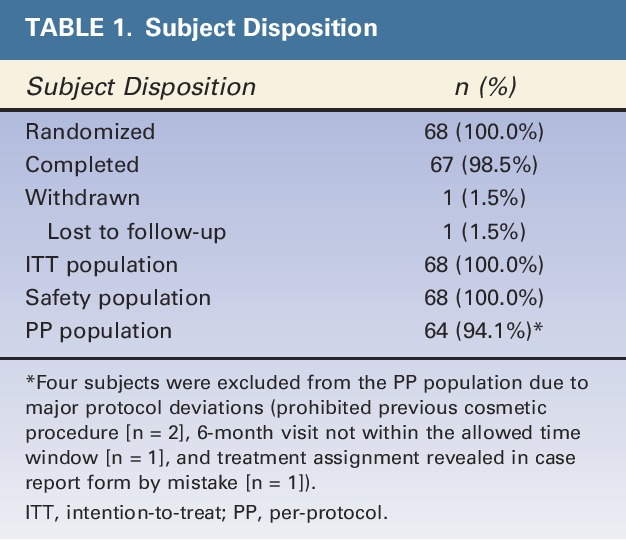
A total of 89 subjects were screened at the 6 study centers. Of these, 68 were randomized to the comparative part of the study, and 67 completed the study (Table 1). The first subject was enrolled March 17, 2009, and the last subject completed the study June 10, 2010.
TABLE 1.
Subject Disposition
Demographics, Baseline Characteristics, and Injection Information
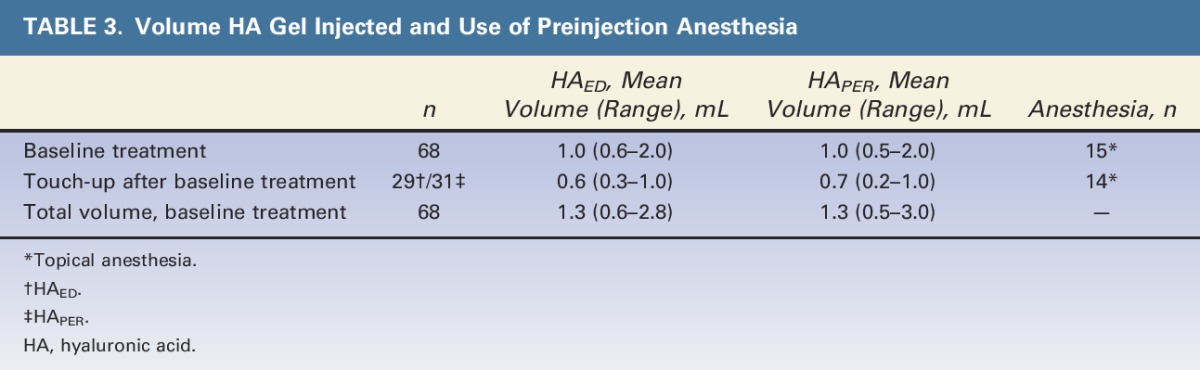
All subjects were white with severe NLFs and had a mean age of 53 years (Table 2). The total mean injection volume (including touch-up) was similar for HAED and HAPER (1.3 mL for both; see Table 3, which also includes details on use of preinjection anesthesia).
TABLE 2.
Demography and Baseline Characteristics
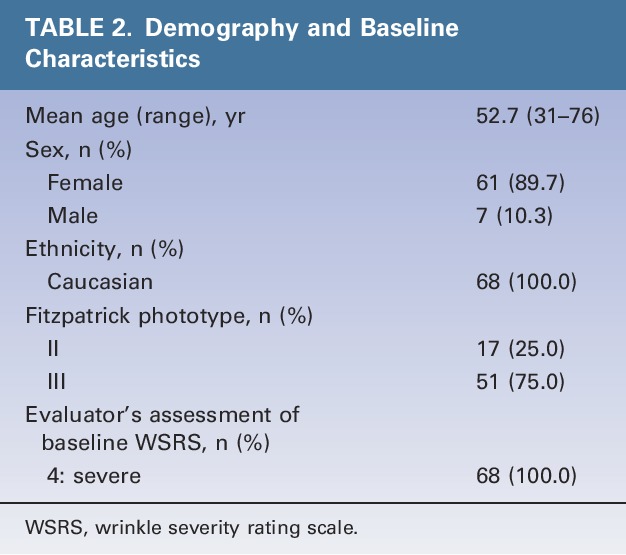
TABLE 3.
Volume HA Gel Injected and Use of Preinjection Anesthesia
Assessment of Effectiveness
Mean Change From Baseline in Wrinkle Severity Rating Scale as Assessed by the Blinded Evaluator (Intention-To-Treat Population)
Similar to previously reported interim data,7 HAED was noninferior to HAPER in terms of mean change from baseline in WSRS score at 6 months after the baseline treatment. At this time point, the mean between-treatment difference (HAPER − HAED) was 0.24, and the 95% CI ranged between 0.10 and 0.37 (i.e., above the predetermined margin [−0.5]).
The statistical comparison of mean change from baseline in WSRS score favored HAED over HAPER from 3 to 12 months after the treatment (Figure 1). However, the difference in mean change from baseline in WSRS score between the 2 products (ΔHAED − HAPER) was small, ranging from 0.17 to 0.24 during this period.
Figure 1.
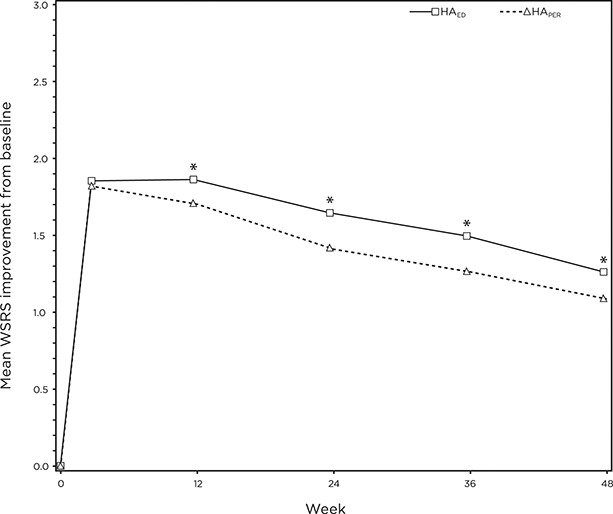
Mean improvement from baseline in WSRS over time as assessed by the blinded evaluator. *HAED > HAPER, p < 0.01. WSRS, wrinkle severity rating scale.
Corresponding comparisons for the PP population (data not shown) were similar to those for the ITT population. Furthermore, the subjects' assessment of WSRS (data not shown) corroborated that of the blinded evaluator.
Responder Rate (Percentage of Subjects With At least One Grade Improvement in Wrinkle Severity Rating Scale) as Assessed by the Blinded Evaluator
The overall responder rate was similar between HAED and HAPER over time, ranging from 79% to 99% during the study (Figure 2).
Figure 2.
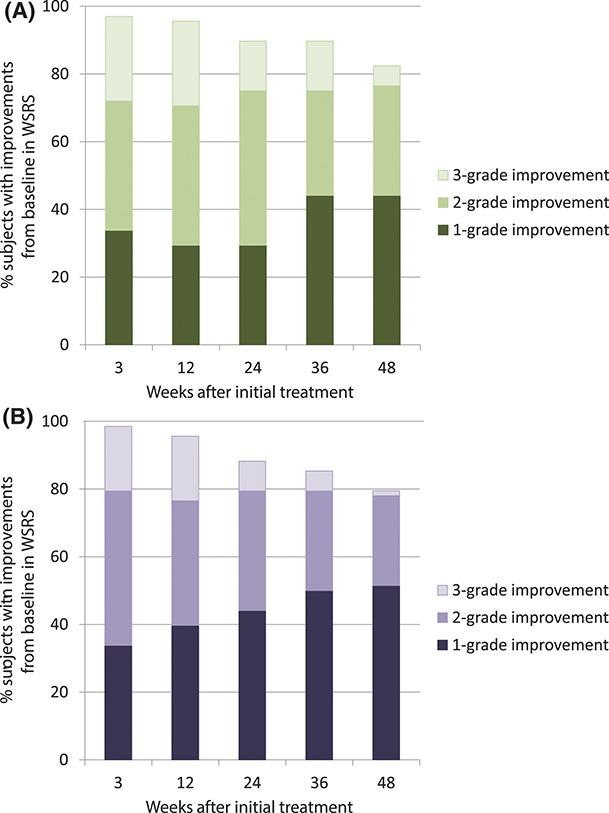
Responder rate over time as assessed by the blinded evaluator. (A) HAED. (B) HAPER.
The responder rate for the PP population (data not shown) was similar to that of the ITT population. Furthermore, the responder rate based on subjects' assessment of WSRS (data not shown) corroborated that of the blinded evaluator.
Subjects' Preference
At the end of the study (12 months after the baseline injection), 58 subjects wished to be retreated. The subject preference assessment favored HAED over HAPER (p < 0.001), with 42 subjects (72%) preferring the side that had been treated with HAED at baseline, whereas the remaining 16 subjects (28%) preferred the side that had been treated with HAPER at baseline. Figure 3 shows photographs of a representative subject.
Figure 3.
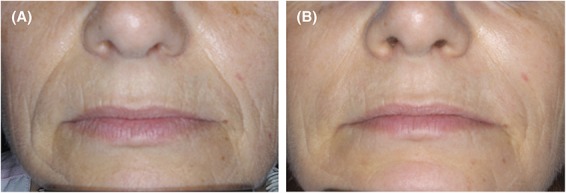
Standardized photographs of a subject before (A) and 12 months after (B) treatment with HAED and HAPER in the right and left nasolabial folds, respectively.
Safety
Adverse Events
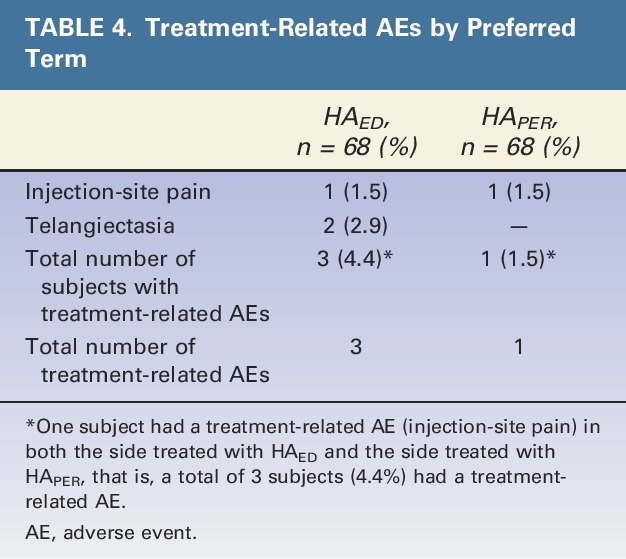
Four treatment-related AEs occurred (Table 4); all were of mild intensity and anticipated reactions after HA filler injection. One subject had bilateral injection site pain, which started the day after the baseline treatment and resolved after 3 days after treatment with paracetamol (acetaminophen). Two subjects had telangiectasia (on the side treated with HAED), but these events did not require intervention. All treatment-related AEs resolved, except one event of telangiectasia that was ongoing at the end of the study.
TABLE 4.
Treatment-Related AEs by Preferred Term
Local Tolerability (Subject Diary Data)
Both HAED and HAPER were well tolerated. The local tolerability reactions (bruising, edema/swelling, erythema, nodule formation, pain/tenderness, and pruritus) recorded in this study were consistent with expected reactions after injection with an HA gel. The frequency of these reactions generally declined within 1 week, and a majority resolved within 2 weeks.
Discussion
This study is the first comparison of HAED and HAPER, with long-term follow-up extending 12 months. The results show that HAED was noninferior to HAPER for the treatment of severe NLFs, assessed by mean change from baseline in WSRS score at 6 months. The effect lasted for at least 12 months in approximately 80% of subjects after 1 treatment. The final effectiveness results reported here are similar to the interim results reported previously.7
Analyses of secondary effectiveness parameters pointed toward that there was a statistically significant difference in the mean change from baseline in WSRS over time, favoring HAED compared with HAPER. However, this difference was seemingly small (ranging between 0.17 and 0.24) and needs to be corroborated by results from further studies. Furthermore, the clinical relevance of this difference needs to be confirmed. However, the preference assessment at the end of the present study indicated that a majority of subjects preferred the side treated with HAED over the side treated with HAPER. These results are in agreement with the comparison of difference in mean WSRS score improvement from baseline.
Carruthers and colleagues9 previously reported that the 6-month responder rate was 75% for HAPER. This is somewhat lower than the corresponding data recorded in the present study (90% for HAED and 88% for HAPER), although similar volumes of HA gel were injected. In fact, the 12-month results from the present study are similar to the 6-month results reported by Carruthers and colleagues.9 It is possible that the difference in responder rates between studies is a reflection of differences in study populations and/or differences due to the subjective components of the outcome criteria.
In the present study, the effectiveness results (both in terms of mean improvement from baseline in WSRS and in terms of responder rate) showed a gradual decline over time after the baseline injection, which is expected as both HAED and HAPER are biodegradable. A majority of subjects (>88%) were responders 6 months after the baseline injection, and approximately 80% of subjects were still responders 1 year after the baseline injection.
The HA fillers used in this study did not contain lidocaine, which was due to the fact the only the nonlidocaine version of HAED was available at the time of the study; HA fillers that do not contain lidocaine have the advantage that they may be used in subjects for whom lidocaine is contraindicated or who are allergic to this class of local anesthetics. However, drawbacks relate to pain and reduced treatment comfort. Therefore, a lidocaine-containing version of HAED is currently available.
Both fillers were well tolerated and associated with few treatment-related AEs. For both HA products, local tolerability reactions generally resolved within a week. The treatment-related AEs that occurred were of mild intensity, and all except one had resolved by the end of the study.
Conclusions
Basically, HAED was noninferior to HAPER as measured by the mean change from baseline in WSRS score at 6 months after the baseline treatment of severe NLFs. There was a statistically significant difference in mean change from baseline in WSRS score in favor of HAED at 3 to 12 months after the treatment. However, this difference was small and needs to be corroborated by results from further studies. Furthermore, the clinical relevance of this difference is unclear. The overall responder rates were similar between HAED and HAPER over time and remained high throughout the 12 months, ranging from 79% to 99%. The preference assessment at the end of the study indicated that a majority of subjects preferred the side treated with HAED over the side treated with HAPER. However, neither product used contained lidocaine, so it is not known if these results would apply to the lidocaine-containing products.
Both HA gel products were well tolerated and were associated with a few treatment-related AEs, all of which were anticipated reactions to HA filler injection.
To summarize, the results of the present study show that HAED was at least as effective and well tolerated for treatment of severe NLFs as HAPER.
Acknowledgments
The authors acknowledge Patrick Brun, MD, Dermatology, Cannes Hospital, Cannes, France, for serving as one of the study investigators and thereby contributing towards the collection of study data.
Footnotes
Galderma provided B. Ascher, C. Bayerl, P. Kestemont, B. Rzany, and M. Podda with a camera for use in the study. Galderma also provided all authors (except C. Edwartz) with the investigational products used in the study, and reimbursed the costs for subject participation in the study. B. Rzany is an advisor for Merz pharma. C. Edwartz is employed by Galderma.
References
- 1.Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol 2012;11(1 Suppl):s5–s8. [PubMed] [Google Scholar]
- 2.Cartier H, Trevidic P, Rzany B, Sattler G, et al. Perioral rejuvenation with a range of customized hyaluronic acid fillers: efficacy and safety over six months with a specific focus on the lips. J Drugs Dermatol 2012;11(1 Suppl):s17–s26. [PubMed] [Google Scholar]
- 3.Kestemont P, Cartier H, Trevidic P, Rzany B, et al. Sustained efficacy and high patient satisfaction after cheek enhancement with a new hyaluronic acid dermal filler. J Drugs Dermatol 2012;11(1 Suppl):s9–16. [PubMed] [Google Scholar]
- 4.Rzany B, Cartier H, Kestermont P, Trevidic P, et al. Correction of tear troughs and periorbital lines with a range of customized hyaluronic acid fillers. J Drugs Dermatol 2012;11(1 Suppl):s27–s34. [PubMed] [Google Scholar]
- 5.Rzany B, Bayerl C, Bodokh I, Boineau D, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Laser Ther 2011;13:107–12. [DOI] [PubMed] [Google Scholar]
- 6.Rzany B, Cartier H, Kestemont P, Trevidic P, et al. Full-face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months. Dermatol Surg 2012;38(7 Pt 2):1153–61. [DOI] [PubMed] [Google Scholar]
- 7.Ascher B, Bayerl C, Brun P, Kestemont P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines—6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol 2011;10:94–8. [DOI] [PubMed] [Google Scholar]
- 8.Day DJ, Littler CM, Swift RW, Gottlieb S. The wrinkle severity rating scale: a validation study. Am J Clin Dermatol 2004;5:49–52. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers A, Carey W, De LC, Remington K, et al. Randomized, double-blind comparison of the efficacy of two hyaluronic acid derivatives, Restylane perlane and hylaform, in the treatment of nasolabial folds. Dermatol Surg 2005;31(11 Pt 2):1591–8. [DOI] [PubMed] [Google Scholar]


