Abstract
Aim
Anti-osteoporotic therapy requires years of proper compliance to reduce the risk of fractures. This study investigated the effects of 1st-year adherence to anti-osteoporotic treatment on the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures after vertebroplasty.
Patients and methods
This retrospective study included 294 patients (252 females; mean age, 73.93±7.18 years) with osteoporosis and acute vertebral fractures treated with vertebroplasty between January 2001 and December 2007. Sex, age, body mass index, comorbidities, previous hip fracture, number of vertebral fractures, 5-year re-fracture rate, and use of anti-osteoporotic therapy were recorded for each patient. Adherence was determined according to compliance and persistence for 1 year. Compliance was calculated as the medication possession ratio (MPR), and persistence as the time from treatment initiation to discontinuation. Poor adherence was defined as either non-compliance or non-persistence.
Results
The MPR of the patients at 1 year was 55.1%, with a persistence rate of 69.4% and a poor adherence rate of 62.6%. Cox regression analysis revealed that poor adherence to medications was associated with a significantly higher risk of mortality after adjustment for potential confounders (hazard ratio [HR]: 1.75; 95% CI: 1.13–2.71). Poor adherence to medications was significantly associated with an increase in the rate of infection (HR: 4.56; 95% CI: 1.12–18.52), which was the most common cause of death.
Conclusion
Poor adherence to anti-osteoporotic therapy significantly increases the risk of morality, possibly due to an increased risk of infection. Efforts should be made to improve adherence.
Keywords: osteoporosis, vertebral fracture, adherence, mortality
Introduction
Osteoporotic fractures are a serious health problem that can cause severe pain for 2–3 months and have been associated with an increased mortality rate.1 Anti-osteoporotic agents can increase bone mineral density and decrease the incidence of vertebral fractures.2 Several agents are used for the treatment of osteoporosis, including bisphosphonates (zoledronic acid, ibandronate, risedronate, and alendronate), calcitonin, selective estrogen receptor modulators (raloxifene), parathyroid hormone (teriparatide), and nuclear factor-κB ligand (RANK) ligand inhibitors (denosumab).3
Non-adherence to therapy can reduce its beneficial effects4 and subsequently its effectiveness.5 Nevertheless, the non-adherence rate has been estimated to be as high as 50% in chronic diseases.6 Terminology and definitions paper provides a definition that is consistent with the commonly used technique of the medication possession ratio (MPR), noting that it is a ratio of the number of doses dispensed relative to the dispensing period. A previous study reported that the rate of hip fractures increased by 0.4% for every 1% decrease in MPR.7 In studies in the US, poor compliance has been associated with increased health care costs and risk of hospitalization.8–11
The aim of this study, therefore, was to determine the association between adherence to anti-osteoporotic treatment and mortality in patients with vertebral fractures after vertebroplasty.
Patients and methods
This was a retrospective study including patients with osteoporosis and acute vertebral fractures treated with vertebroplasty between January 2001 and December 2007. The institutional review board of Chang Gang Memorial Hospital approved the study protocol (103-3501B), and it was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice Guidelines. According to the institutional review board of Chang Gang Memorial Hospital, no informed consent was required, as patient information was anonymized and de-identified before data analysis.
The inclusion criteria were as follows: 1) osteoporosis with fragile vertebral fractures; 2) acute vertebral fractures defined by magnetic resonance imaging (MRI) with low signal intensity (SI) on T1-, T2-weighted, and fat-suppressed T1-weighted images with enhanced SI of the injured vertebral body;12 and 3) vertebroplasty within 1 week after vertebroplasty. The exclusion criteria were as follows: 1) pyogenic infections or neoplasia and 2) fractures caused by more than minimal trauma.
The patients were followed up from the time of recruitment until December 2014 or the time of death, whichever occurred first. The included patients underwent bone density studies (dual energy X-ray absorptiometry), and data on age; sex; body mass index; comorbidities such as hypertension, diabetes, and liver and renal diseases; the use of anti-osteoporotic agents (ie, raloxifene, alendronate, calcitonin, and teriparatide); and a previous history of fractures were recorded.
Adherence
Cramer et al13 defined adherence using parameters of compliance and persistence. They defined compliance as the MPR and persistence as the time from treatment initiation to discontinuation, with no medication refill gap for a period of 30 days. Poor adherence was defined as either noncompliance or non-persistence.
Statistical analysis
All statistical analyses were performed using SPSS software, version 21.0 (SPSS, Chicago, IL, USA). Patient characteristics were reported as mean ± standard deviation. Kaplan–Meyer analysis with the log-rank test was performed to assess adherence or non-adherence to anti-osteoporotic agents. Comparisons between independent variables were analyzed using the independent t-test, and relationships between categorical variables were evaluated using the chi-square test. Cox regression analysis was used to adjust for confounding factors. Statistical significance was set at P<0.05.
Results
Between January 2001 and December 2007, 294 patients with MRI-proven acute vertebral fractures who received vertebroplasty and anti-osteoporotic therapy were enrolled. Of these patients, 93 (31.6%) took alendronate, 52 (17.6%) took raloxifene, 38 (12.9%) took calcitonin, and 15 (5.1%) took teriparatide. Overall, the MPR at 1 year was 55.1%, with a persistence rate of 69.4% and poor adherence rate of 62.6%.
Of the 294 patients, 85.71% were women. Their mean age was 73.93±7.18 years, with a follow-up period of 7.08±3.67 years. The mean number of vertebral fractures was 1.99±1.32, and 22 patients had a history of previous hip fractures. At the end of the follow-up period, 194 patients were still alive (Table 1). Based on the Kaplan–Meier analysis, poor adherence to anti-osteoporotic therapy had a significant effect on mortality (Figure 1). After adjusting for potential confounding factors such as alcohol consumption, smoking, hypertension, diabetes, and cardiovascular, pulmonary, liver, and neurological diseases, those with poor adherence still had a higher mortality rate than those with good adherence (P=0.012; hazard ratio [HR]: 1.753; 95% CI: 1.133–2.711). Except for a higher mortality rate in those with liver disease (P=0.006; HR: 2.767; 95% CI: 1.337–5.729), smoking; alcohol consumption; hypertension; cardiovascular, pulmonary, and neurological diseases; history of hip fractures; and the number of vertebral fractures were not associated with an increased risk of mortality (P>0.05; Table 2). When subgroup analysis was performed, we found that calcitonin had poor adhesion and higher mortality (Table 3). Poor adherence to medications was significantly associated with an increase in the rate of infection (HR: 4.56; 95% CI: 1.12–18.52), which was the most common cause of death.
Table 1.
Characteristics of the study patients
| Variables | Results |
|---|---|
| Age (years), mean ± SD | 73.93±7.18 |
| BMI (kg/m2), mean ± SD | 23.47±5.12 |
| Spine fractures (number), mean ± SD | 1.99±1.32 |
| Follow-up (years), mean ± SD | 7.08±3.67 |
| Sex (female), n (%) | 252 (85.71) |
| Previous hip fractures, n (%) | 22 (7.5) |
| Smoking, n (%) | 14 (4.8) |
| Alcohol consumption, n (%) | 8 (2.7) |
| Rheumatoid arthritis, n (%) | 36 (8.8) |
| Diabetes mellitus, n (%) | 76 (25.9) |
| Hypertension, n (%) | 150 (51) |
| Neurological disease, n (%) | 8 (2.7) |
| Cardiovascular disease, n (%) | 4 (1.4) |
| Pulmonary disease, n (%) | 10 (3.4) |
| Hepatitis, n (%) | 18 (6.1) |
| Kidney disease, n (%) | 2 (0.7) |
| Glucocorticoid use, n (%) | 44 (15) |
| Poor adherence, n (%) | 184 (62.6) |
Abbreviations: SD, standard deviation; BMI, body mass index.
Figure 1.
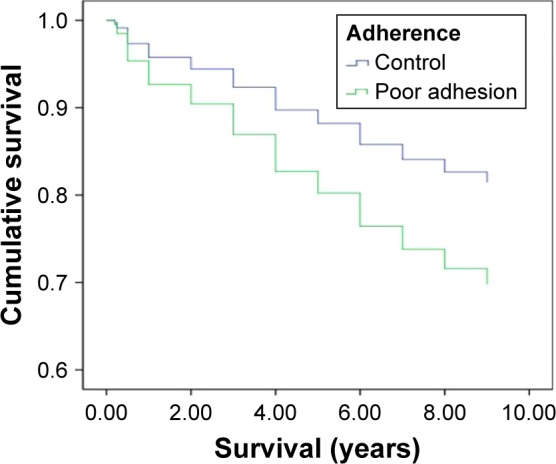
Kaplan–Meier survival curves for the adherence (blue line) and poor adherence (green line).
Note: The poor adhesion group had a lower survival than the adherence group.
Table 2.
Multivariate Cox regression analysis of the HRs for mortality in patients with vertebral fractures
| Variables | Regression coefficient | SE | Wald | P-value | HR (95% CI) |
|---|---|---|---|---|---|
| Sex | 0.115 | 0.328 | 0.12 | 0.727 | 1.121 (0.590–2.133) |
| Age | 0.023 | 0.016 | 2.02 | 0.156 | 1.023 (0.991–1.057) |
| BMI | −0.038 | 0.024 | 2.47 | 0.116 | 0.962 (0.918–1.009) |
| Smoking | −0.33 | 0.579 | 0.33 | 0.569 | 0.718 (0.231–2.237) |
| Alcohol consumption | −12.168 | 239.1 | 0.02 | 0.959 | 1.194 (0.001–1.863) |
| RA | −0.127 | 0.456 | 0.08 | 0.781 | 0.881 (0.361–2.153) |
| DM | 0.274 | 0.241 | 1.29 | 0.256 | 1.314 (0.820–2.109) |
| Hypertension | 0.38 | 0.225 | 2.85 | 0.092 | 1.462 (0.940–2.273) |
| Neurological disease | 0.709 | 0.533 | 1.77 | 0.183 | 2.032 (0.716–5.771) |
| Hepatitis | 1.018 | 0.371 | 7.52 | 0.006 | 2.767 (1.337–5.729) |
| Cardiovascular disease | −12.065 | 303.3 | 0.001 | 0.968 | 5.760 (0.001–8.440) |
| Kidney disease | 1.887 | 0.733 | 5.97 | 0.276 | 1.454 (0.741–2.853) |
| Pulmonary disease | 0.759 | 0.5 | 2.3 | 0.129 | 2.136 (0.801–5.694) |
| Glucocorticoid use | 0.482 | 0.35 | 1.9 | 0.168 | 1.618 (0.816–3.212) |
| Previous hip fracture | −0.368 | 0.478 | 0.59 | 0.441 | 0.692 (0.271–1.765) |
| Poor adherence | 0.561 | 0.223 | 6.36 | 0.012 | 1.753 (1.133–2.711) |
Abbreviations: HR, hazard ratio; SE, standard error; BMI, body mass index; RA, rheumatoid arthritis; DM, diabetes mellitus.
Table 3.
Subgroup analysis of adhesion on mortality rate in different anti-osteoporotic drugs
| Raloxifene | Calcitonin | Teriparatide | Alendronate | |
|---|---|---|---|---|
| Poor adhesion (%) | 67.90 | 84.60 | 88.50 | 59 |
| Mortality rate (%) | 23.70 | 46.20 | 27 | 36 |
Discussion
Patients with established osteoporosis have been reported to have a high mortality rate,14,15 and vertebral deformities are known to predict mortality and fracture rate.16–21 Anti-osteoporotic therapy has been reported to reduce mortality in those at high risk of fractures.2,22,23
In this retrospective analysis, we assessed adherence to anti-osteoporotic therapy in patients with MRI-proven acute osteoporotic vertebral fractures after vertebroplasty. During the first 12 months of anti-osteoporotic therapy, only 37.4% of the patients adhered to treatment. In a review, those who were compliant with the anti-osteoporotic treatment had a 37% reduction in the risk of hip or vertebral fractures,24 whereas those who were not compliant had a higher risk of fractures25 and a higher risk of vertebral and hip fractures.
Adherence is associated with a lower risk of infection, which is possibly through the effects on the immune system such as changes in cytokines and the monocyte–macrophage system.26 It has also been suggested that anti-osteoporotic therapy may modulate the immune process by influencing the production of pro- and anti-inflammatory cytokines (γδ T cells, tumor necrosis factor (TNF)-α, and interferon-γ), so adherence may reduce infection-related deaths. A reduction in pneumonia-related deaths was reported in a zoledronic acid study, supporting this hypothesis.27 Furthermore, patients with hip fractures in a cohort study were reported to have a higher risk of infections, mainly septicemia and pneumonia.28 Patients treated with anti-osteoporotic regimens may be less likely to die from pneumonia.
Liver disease is associated with a higher risk of mortality, with hepatitis being an important factor. In Taiwan, there is a high prevalence of hepatitis B and C.29–31 In previous studies, hepatitis has been shown to increase the risk of osteoporosis in Taiwan.32–34 Thus, patients with hepatitis may be more likely to visit an osteoporosis clinic for low bone mass-related fractures, further strengthening the consideration that hepatitis is an associated medical illness, as noted in this study.
Improving compliance to anti-osteoporotic medication is important, which may be achieved through improved clinical consultation, patient and physician education, and follow-up monitoring.35 Further studies on the causes of non-adherence are also needed.
There are several limitations to this study. First, the sample size is small. Second, because of the retrospective design, incomplete data such as the use of vitamin D and calcium supplements could not be included. The lack of vitamin D levels may impact the results, because it has been demonstrated that low vitamin D levels may affect mortality and also infection risk. Nonetheless, this study also has a number of strengths. First, the mean follow-up period was relatively long at >7 years. In addition, MRI scans were taken for all patients; thus, other causes of vertebral fracture such as pyogenic infection or neoplasia could be excluded.
The results of this study show that poor adherence to pharmacological therapy can lead to higher mortality among patients with osteoporotic vertebral fractures, even after adjusting for comorbidities. Thus, optimal adherence to anti-osteoporosis management may reduce the risk of death, which may be caused by a reduction in the risk of infection.
Conclusion
Poor adhesion to anti-osteoporotic therapy significantly increases the risk of morality, which may be caused by an increased risk of infection. Efforts should be made to improve adherence in these patients.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gordis L. Symposium in honor of Abraham M. Lilienfeld. Chronic disease epidemiology at the threshold of a new decade. Am J Epidemiol. 1980;112(2):169–177. doi: 10.1093/oxfordjournals.aje.a112981. [DOI] [PubMed] [Google Scholar]
- 2.Center JR, Bliuc D, Nguyen ND, Nguyen TV, Eisman JA. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011;96(4):1006–1014. doi: 10.1210/jc.2010-2730. [DOI] [PubMed] [Google Scholar]
- 3.Geusens PP, Roux CH, Reid DM, et al. Drug Insight: choosing a drug treatment strategy for women with osteoporosis-an evidence – based clinical perspective. Nat Clin Prac Rheumatol. 2008;4(5):240–248. doi: 10.1038/ncprheum0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordis L. Assuring the quality of questionnaire data in epidemiologic research. Am J Epidemiol. 1979;109(1):21–24. doi: 10.1093/oxfordjournals.aje.a112654. [DOI] [PubMed] [Google Scholar]
- 5.Haynes RB, Sackett DL, Taylor DW. How to detect and manage low patient compliance in chronic illness. Geriatrics. 1980;35(1):91–93. 96–97. [PubMed] [Google Scholar]
- 6.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19(6):811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 8.Briesacher BA, Andrade SE, Yood RA, Kahler KH. Consequences of poor compliance with bisphosphonates. Bone. 2007;41(5):882–887. doi: 10.1016/j.bone.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38(6):922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Sunyecz JA, Mucha L, Baser O, Barr CE, Amonkar MM. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19(10):1421–1429. doi: 10.1007/s00198-008-0586-2. [DOI] [PubMed] [Google Scholar]
- 11.Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17(1):25–39. doi: 10.18553/jmcp.2011.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin WC, Lu CH, Chen HL, et al. The impact of preoperative magnetic resonance images on outcome of cemented vertebrae. Eur Spine J. 2010;19(11):1899–1906. doi: 10.1007/s00586-010-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 14.Shiraki M, Kuroda T, Tanaka S. Established osteoporosis associated with high mortality after adjustment for age and co-morbidities in postmenopausal Japanese women. Intern Med. 2011;50(5):397–404. doi: 10.2169/internalmedicine.50.4437. [DOI] [PubMed] [Google Scholar]
- 15.Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 1998;8(3):291–297. doi: 10.1007/s001980050067. [DOI] [PubMed] [Google Scholar]
- 16.Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O, European Vertebral Osteoporosis Study Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int. 2003;14(1):61–68. doi: 10.1007/s00198-002-1316-9. [DOI] [PubMed] [Google Scholar]
- 17.Jalava T, Sarna S, Pylkkanen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res. 2003;18(7):1254–1260. doi: 10.1359/jbmr.2003.18.7.1254. [DOI] [PubMed] [Google Scholar]
- 18.Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Rodríguez-García M, Cannata-Andía JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14(6):520–524. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22(6):781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 20.Puisto V, Rissanen H, Heliovaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181–2186. doi: 10.1007/s00586-011-1852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90(7):1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 22.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 23.Grey A, Bolland MJ. The effect of treatments for osteoporosis on mortality. Osteoporos Int. 2013;24(1):1–6. doi: 10.1007/s00198-012-2176-6. [DOI] [PubMed] [Google Scholar]
- 24.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clinic Proc. 2006;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 25.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 26.Tuominen OM, Ylitalo-Heikkala R, Vehmas TI, Mucha I, Ylitalo P, Riutta A. Effects of bisphosphonates on prostaglandin E2 and thromboxane B2 production in human whole blood and monocytes stimulated by lipopolysaccharide and A23187. Methods Find Exp Clin Pharmacol. 2006;28(6):361–367. doi: 10.1358/mf.2006.28.6.1003551. [DOI] [PubMed] [Google Scholar]
- 27.Black DM, Delmas PD, Eastell R, et al. HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 28.Hawkes WG, Wehren L, Orwig D, et al. Gender differences in functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2006;61(5):495–499. doi: 10.1093/gerona/61.5.495. [DOI] [PubMed] [Google Scholar]
- 29.Wu JS, Lu CF, Chou WH, et al. High prevalence of hepatitis C virus infection in aborigines in Taiwan. Jpn J Med Sci Biol. 1992;45(4):165–174. doi: 10.7883/yoken1952.45.165. [DOI] [PubMed] [Google Scholar]
- 30.Lee CM, Chen CH, Lu SN, et al. Prevalence and clinical implications of hepatitis B virus genotypes in southern Taiwan. Scand J Gastroenterol. 2003;38(1):95–101. doi: 10.1080/00365520310000500. [DOI] [PubMed] [Google Scholar]
- 31.Lin CF, Twu SJ, Chen PH, Cheng JS, Wang JD. Prevalence and determinants of hepatitis B antigenemia in 15,007 inmates in Taiwan. J Epidemiol. 2010;20(3):231–236. doi: 10.2188/jea.JE20081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedimo R, Maalouf NM, Lo Re V., 3rd Hepatitis C virus coinfection as a risk factor for osteoporosis and fracture. Curr Opin HIV AIDS. 2016;11(3):285–293. doi: 10.1097/COH.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CH, Lin CL, Kao CH. Association between chronic hepatitis b virus infection and risk of osteoporosis: a nationwide population-based study. Medicine. 2015;94(50):e2276. doi: 10.1097/MD.0000000000002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CH, Lin CL, Kao CH. Relation between hepatitis C virus exposure and risk of osteoporosis: a nationwide population-based study. Medicine. 2015;94(47):e2086. doi: 10.1097/MD.0000000000002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waalen J, Bruning AL, Peters MJ, Blau EM. A telephone-based intervention for increasing the use of osteoporosis medication: a randomized controlled trial. Am J Manag Care. 2009;15(8):e60–e70. [PubMed] [Google Scholar]


