Abstract
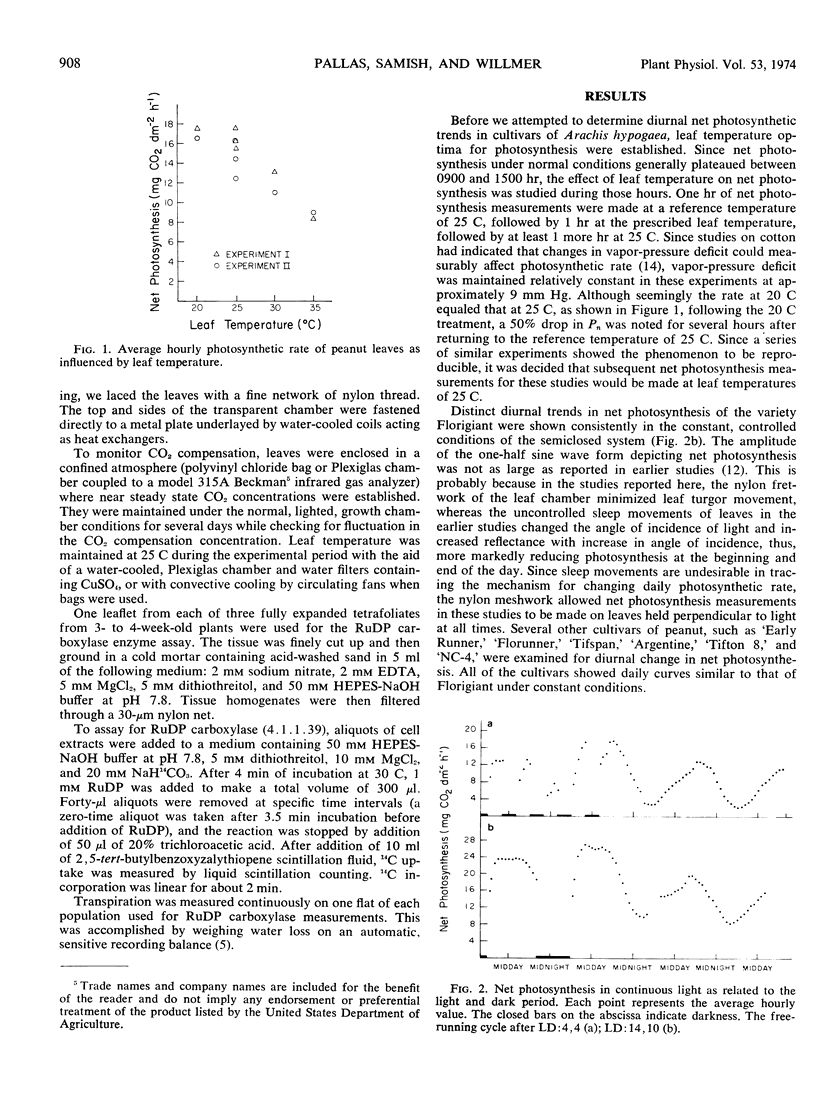
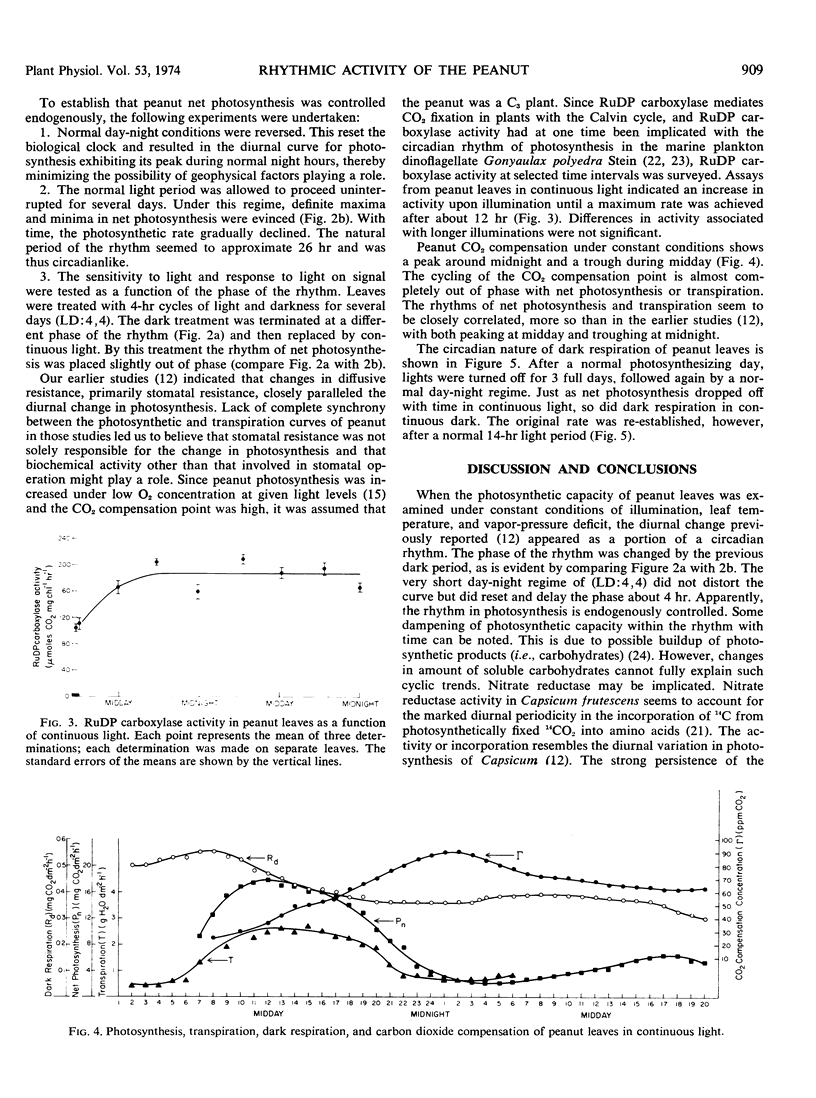
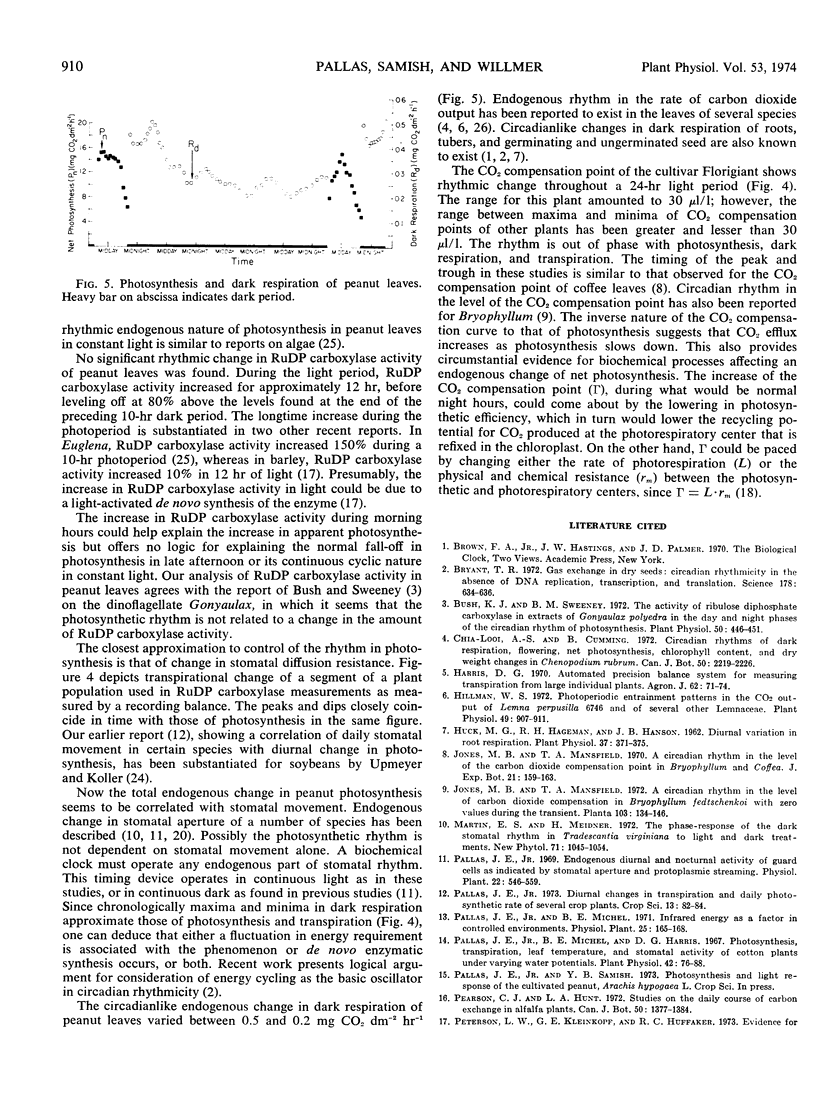
At 14-hour day length, 25 C leaf temperature, 9 mm Hg vapor-pressure deficit, and 1.17 joules cm−2 min−1 irradiance, the diurnal change in daily photosynthesis of the cultivated peanut (Arachis hypogaea L.) is a result of an endogenously controlled circadian rhythm in net photosynthesis which peaks near noon and troughs near midnight. By resetting the day-night light regime, the rhythm rephased in continuous light. The free-running rhythm approximates 26 hours. Both transpiration and dark respiration show similar rhythmicity, with transpiration closely in phase with the rhythm in photosynthesis. The rhythm in carbon dioxide compensation point is approximately 12 hours out of phase, peaking at midnight and troughing at midday. Endogenous changes in stomatal aperture seemed to be the major control of the rhythm in photosynthesis. The activity of ribulose-1,5-diphosphate carboxylase increased during the normal photoperiod, leveling off after 12 hours; however, the activity was not correlated with the rhythmic change in photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant T. R. Gas exchange in dry seeds: circadian rhythmicity in the absence of DNA replication, transcription, and translation. Science. 1972 Nov 10;178(4061):634–636. doi: 10.1126/science.178.4061.634. [DOI] [PubMed] [Google Scholar]
- Bush K. J., Sweeney B. M. The Activity of Ribulose Diphosphate Carboxylase in Extracts of Gonyaulax polyedra in the Day and the Night Phases of the Circadian Rhythm of Photosynthesis. Plant Physiol. 1972 Oct;50(4):446–451. doi: 10.1104/pp.50.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman W. S. Photoperiodic Entrainment Patterns in the CO(2) Output of Lemna perpusilla 6746 and of Several Other Lemnaceae. Plant Physiol. 1972 Jun;49(6):907–911. doi: 10.1104/pp.49.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck M. G., Hageman R. H., Hanson J. B. Diurnal Variation in Root Respiration. Plant Physiol. 1962 May;37(3):371–375. doi: 10.1104/pp.37.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E., Michel B. E., Harris D. G. Photosynthesis, Transpiration, Leaf Temperature, and Stomatal Activity of Cotton Plants under Varying Water Potentials. Plant Physiol. 1967 Jan;42(1):76–88. doi: 10.1104/pp.42.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samish Y., Koller D. Photorespiration in Green Plants During Photosynthesis Estimated by Use of Isotopic CO(2). Plant Physiol. 1968 Jul;43(7):1129–1132. doi: 10.1104/pp.43.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T. Diurnal variations in photosynthetic products and nitrogen metabolism in expanding leaves. Plant Physiol. 1973 Apr;51(4):744–748. doi: 10.1104/pp.51.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upmeyer D. J., Koller H. R. Diurnal trends in net photosynthetic rate and carbohydrate levels of soybean leaves. Plant Physiol. 1973 May;51(5):871–874. doi: 10.1104/pp.51.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther W. G., Edmunds L. N. Studies on the Control of the Rhythm of Photosynthetic Capacity in Synchronized Cultures of Euglena gracilis (Z). Plant Physiol. 1973 Feb;51(2):250–258. doi: 10.1104/pp.51.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]


