Abstract
The cannabis withdrawal syndrome (CWS) is a criterion of cannabis use disorders (CUDs) (Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition) and cannabis dependence (International Classification of Diseases [ICD]-10). Several lines of evidence from animal and human studies indicate that cessation from long-term and regular cannabis use precipitates a specific withdrawal syndrome with mainly mood and behavioral symptoms of light to moderate intensity, which can usually be treated in an outpatient setting. Regular cannabis intake is related to a desensitization and downregulation of human brain cannabinoid 1 (CB1) receptors. This starts to reverse within the first 2 days of abstinence and the receptors return to normal functioning within 4 weeks of abstinence, which could constitute a neurobiological time frame for the duration of CWS, not taking into account cellular and synaptic long-term neuroplasticity elicited by long-term cannabis use before cessation, for example, being possibly responsible for cannabis craving. The CWS severity is dependent on the amount of cannabis used pre-cessation, gender, and heritable and several environmental factors. Therefore, naturalistic severity of CWS highly varies. Women reported a stronger CWS than men including physical symptoms, such as nausea and stomach pain. Comorbidity with mental or somatic disorders, severe CUD, and low social functioning may require an inpatient treatment (preferably qualified detox) and post-acute rehabilitation. There are promising results with gabapentin and delta-9-tetrahydrocannabinol analogs in the treatment of CWS. Mirtazapine can be beneficial to treat CWS insomnia. According to small studies, venlafaxine can worsen the CWS, whereas other antidepressants, atomoxetine, lithium, buspirone, and divalproex had no relevant effect. Certainly, further research is required with respect to the impact of the CWS treatment setting on long-term CUD prognosis and with respect to psychopharmacological or behavioral approaches, such as aerobic exercise therapy or psychoeducation, in the treatment of CWS. The up-to-date ICD-11 Beta Draft is recommended to be expanded by physical CWS symptoms, the specification of CWS intensity and duration as well as gender effects.
Keywords: marijuana, humans, neurobiology, treatment, course, detoxification, symptoms
Introduction
Cannabis is a psychotropic substance with widespread recreational use worldwide, surpassed only by nicotine and alcohol.1 Its use continues to be high in West and Central Africa, Western and Central Europe, Australasia, and North America, where recently an increase in the prevalence of past year cannabis use was recorded in the USA (12.6%).1 In Europe, prevalence rates of annual cannabis use rise in Nordic countries (7%–18%) and France (22%). They decline in Spain, UK, and Germany (currently 12%), and there is an increase in the number of treatment demands for cannabis-related problems across Europe2 and the USA.3 Although such prevalence rates are useful to indicate consumption trends, it is doubted whether these rates are relevant to reflect a health risk. Approximately 1% of European adolescents and young adults use cannabis daily or almost daily (defined as use on ≥20 days in the last month),2 a consumption pattern which is more likely to produce cannabis-related disabling disorders.4,5 The prevalence of cannabis dependence (Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition – Text Revision [DSM-IV-TR]) is highest in Australasia (0.68%), followed by North America (0.60%), Western Europe (0.34%), Asia Central (0.28%), and southern Latin America (0.26%).4 In Germany, ~0.5% of the adult population have a cannabis dependence diagnosis.6 Most of the other regions of the world providing data report a prevalence of cannabis dependence of <0.2%.4 There is a significant positive correlation between the region’s economic situation and the prevalence of cannabis dependence.4 A hallmark of cannabis dependence (Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition [DSM-IV] or International Classification of Diseases [ICD]- 10) as well as cannabis use disorder (CUD) (Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition [DSM-5]) is the cannabis withdrawal syndrome (CWS) that characteristically occurs after quitting a regular cannabis use abruptly.
Although there was early evidence from animal experiments7 and despite observations in humans in every decade,8,9 CWS entity was doubted before the 1990s, when a new cannabis wave started to roll in worldwide, particularly in affluent regions.4 This was related with a mounting number of patients seeking treatment due to various cannabis-related disorders, including cognitive deficits, psychosis, and dependence.4,5 Considering these populations and also nontreatment-seeking cannabis-dependent individuals, larger retrospective clinical trials10,11 demonstrated that discontinuation of regular cannabis use is frequently followed by waxing and waning behavioral, mood and physical symptoms such weakness, sweating, restlessness, dysphoria, sleeping problems, anxiety, and craving, which are subsequently positively associated with relapse to cannabis use.11–19 However, other studies did not find this association.20 CWS was further validated by epidemiological,21,22 retrospective,11,19,23 and prospective outpatient12,13,20,24–26 and inpatient laboratory studies27–30 (Table 1). Based on this research, diagnostic criteria of CWS were newly included in DSM-5 (Table 2).31 In ICD-10, CWS is still vaguely defined32 and awaits due definition in ICD-11.33 More recent clinical inpatient detoxification studies arranging controlled abstinence conditions confirmed the entity of CWS.34–36 The CWS was also verified in youths and adolescents (aged 13–19 years), who sought treatment for their disabilitating cannabis dependence.18,37–40
Table 1.
Clinical and laboratory studies on human CWS in the past 20 years
| Authors | Study design | Sample | CWS-measurement and characteristics | CWS type | Subjects reporting CWS (%) | Gender effects | Comorbidity | Other clues |
|---|---|---|---|---|---|---|---|---|
| Wiesbeck et al (USA)10 | Epidemiologic cross-sectional study comparing patients reporting a CWS with those not reporting a CWS Data were generated through the Collaborative Study on the Genetics of Alcoholism (COGA) | N=1,735 frequent cannabis users (using cannabis >21 times in a year) Female <60% Adults >70% Caucasian, >15% African–American |
Face-to-face interview, CWS symptoms (as reviewed by the authors from literature) were retrospectively screened in the COGA population Typical symptoms: nervousness, tense, restlessness, sleep disturbance, appetite change Controlled abstinence period: NA |
NA | 16.5% (all having used the drug almost daily for an average of almost 70 months) | NA | In the CWS group, significantly more psychiatric disorders: alcohol dependence (16.7%), other substance use disorder (28.5%), antisocial personality disorder (36.3%). No psychiatric diagnosis (4%) | Subjects reporting CWS were more likely to have been treated for alcohol or other substance dependence, but were not more likely to have close relatives with substance use disorders or antisocial personality disorder |
| Crowley et al (USA)38 | Cross-sectional study, 13- to 19- year olds referred for substance and conduct problems (usually from social service or criminal justice agencies) | N=180 subjects with cannabis dependence (DSM-III-R) Female 23.1% Youths Caucasian > Hispanic > African–American |
Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM) Typical symptoms: tiredness (61.1% males/42.3% females), anxiety, restlessness, irritability (44.2%/38.5%); trouble concentrating (41.1%/46.2%), yawning (45.3%/26.9%), decreased appetite (37.9%/50.0%), depressed mood (40.0%/34.6%), disturbed sleep (30.5%/34.6%), hallucinations (16.8%/19.2%), tremble, twitch (12.6%/23.1%), running eyes, nose (12.6%/15.4%), sweating, fever (11.6%/11.5%) Controlled abstinence period: NA |
NA | Over two-thirds had withdrawal symptoms | No | Substance dependence (100%), current conduct disorder (82.1%), major depression (17.5%), ADHD (14.8%) | Onset patterns suggest that cannabis was as reinforcing as tobacco or alcohol for the sample, conduct symptoms antedated cannabis use More than one-quarter of the patients reported using cannabis to relief withdrawal symptoms |
| Budney et al (USA)24 | Cross-sectional study of cannabis dependents (DSM-III-R) seeking outpatient treatment | N=54, 82% daily cannabis users seeking treatment Female 15% Adults All Caucasian | 22-item Marijuana Withdrawal Checklist (0–28 points), mean score was 14.4±7.8 Symptom Checklist–90 revised (SCL–90R) Typical symptoms: craving cannabis, irritability, nervousness, depressed mood, sleep difficulties, strange dreams, decreased appetite, headache Controlled abstinence period: NA |
NA | 57% experienced ≥6 symptoms of at least moderate severity and 47% experienced ≥4 symptoms rated as severe | No | Withdrawal severity was greater in those with psychiatric symptomatology and other drug use in the past | Withdrawal severity was greater in those with more frequent marijuana use Most had problems with alcohol or cocaine use or psychiatric symptoms in the past |
| Haney et al (USA)28 | Prospective study in a residential laboratory with frequent cannabis users | N=12 nontreatment-seeking people smoking cannabis 5.8±0.4 days/week All males Adults 7 African–American, 3 Caucasian, 2 Hispanic | 50-item visual analog scale Typical symptoms: anxiousness, irritable, stomach pain, decreased appetite Controlled abstinence period: 4 days |
Type B | All | – | Most participants reported drinking alcohol (2 days/week, 2 drinks per occasion) One person reported occasional cocaine use |
|
| Kouri and Pope (USA)27 | Prospective outpatient laboratory study | N=30 (current chronic users, all cannabis dependent according to DSM-IV, nontreatment-seeking, 4 women) vs N=16 (former users, 6 women) vs N=14 (non-users, 1 woman) >85% Caucasian Adults |
14-item diary Typical symptoms: ability to concentrate, irritability, anxiety, depressed mood, physical tension, decreased appetite, physical symptoms Controlled abstinence period: 28 days. The duration of CWS: symptoms were most pronounced in the first 10 abstinent days, but some symptoms (irritability, physical tension) exist for the entire 28-day study period |
Type A | All | NA | No current comorbidity according to DSM-IV Axis I disorder | Comparison with control groups, relatively long study period (28 days) |
| Swift et al (Australia)41 | Representative epidemiologic study of Australian adults completing a structured diagnostic interview assessing the prevalence of mental and substance use disorders in the last year, National Survey of Mental Health and Wellbeing (NSMHWB) | N=10,641. The 12-month prevalence of DSM-IV (1.5%) and ICD-10 (1.7%) cannabis dependence was similar Abuse (N=572) vs dependence (N=150) Adults | Composite International Diagnostic Interview (CIDI) Controlled abstinence period: NA |
NA | ~90% of the cannabis dependents reported withdrawal symptoms | NA | NA | 12-month prevalence of DSM-IV dependence symptoms of cannabis dependents (N=150): tolerance (72.6±4.8%), withdrawal/withdrawal relief (88.8±3.2%), cannabis used in larger amounts or for longer than intended (62.8±4.5%), persistent desire or unsuccessful efforts to control use (86.9±3.0%), great deal of time spent in obtaining, using and recovering (42.5±6.3%), important activities given up or reduced (9.9±6.1%), continued use despite knowledge of physical or psychological problem (37.0±7.1%) |
| Budney et al (USA)25 | Prospective outpatient study | N=12 daily heavy cannabis smokers, 92% met the DSM-IV criteria for cannabis dependence or abuse Female 41.7% Adults 83.3% Caucasian |
15-item Marijuana Withdrawal Checklist, 10-item Marijuana Craving Questionnaire Typical symptoms: cannabis craving, decreased appetite, weight loss, aggression, anger, irritability, restlessness, strange dreams Controlled abstinence period: 3 days |
NA | NA | NA | No current comorbidity other than nicotine dependence | The symptoms were estimated to be similar in type and magnitude to those observed in studies of nicotine dependence |
| Budney et al (USA)26 | Prospective outpatient study | N=18 frequent, heavy cannabis users seeking treatment (39% female) vs N=12 ex-cannabis users Female 25% Adults 94% Caucasian |
15-item Marijuana Withdrawal Checklist, 10-item Marijuana Craving Questionnaire Typical symptoms: irritability, sleep difficulty, strange dreams, decreased appetite, restlessness, nervousness/anxiety, aggression, anger, sweating, chills, shakiness, stomach pain, cannabis craving, depressed mood Controlled abstinence period: 45 days |
Type A | All | NA | No current comorbidity other than nicotine dependence, 10.9±8.6 days of alcohol use in the past 30 days, 39% tobacco smoker | Significant withdrawal discomfort ~4 weeks of abstinence |
| Vandrey et al (USA)37 | Cross-sectional outpatient study | N=72 treatment seeking cannabis users, 10% female, 57% DSM-IV cannabis dependence Female 90% Adolescents 89% Caucasian |
15-item Marijuana Withdrawal Checklist, Youth Self-Report (YSR) Typical symptoms: craving cannabis, depressed mood, irritability, sleep difficulty, increased anger, decreased appetite, increased aggression, nervousness/anxiety, headache Controlled abstinence period: 4 weeks |
NA | 78% reported two or more symptoms | NA | No current comorbidity other than nicotine dependence. Psychiatric symptoms: YSR – externalizing scale: 59.8±10.4, percent in clinical range (39%), YSR – internalizing scale. 51.5±12.4, percent in clinical range (16%) | Craving, depressed mood, irritability, and sleep difficulty were rated as being moderate or greater severity by at least one-third of the sample, the prevalence and magnitude of withdrawal symptoms were lower than that observed in the similar study with adult treatment seekers24 |
| Arendt et al (Denmark)20 | Prospective cohort study plus follow-up 26±4 months after baseline assessment | N=36 cannabis dependents (ICD-10) seeking treatment, at baseline, 29 and 7 subjects received outpatient and inpatient treatment, respectively Female 19.4% Young adults All Caucasian (putatively) | 22-item Marijuana Withdrawal Checklist according to Budney et al 199924 Clinical Assessment in Neuropsychiatry, computerized version (SCAN) Typical symptoms: significantly elevated after abstinence compared with follow-up: irritability, anger, depression, restlessness, craving, sleep problems, strange dreams, increased appetite, violent outbursts, sweating, hot flashes, chills, and shakiness Controlled abstinence period: median length of time from abstinence to the baseline interview was 6.5±11.3 weeks |
Type A | More than half of the subjects reported symptoms in the moderate to severe range | No | No current comorbidity other than nicotine dependence, lifetime use of other substances was common (mostly amphetamine (91.7%) | Between baseline and follow-up, 24 subjects (67%) had used cannabis at some point. The following substances had also been used: benzodiazepines (5.6%), amphetamines (13.9%), cocaine (27.8%), ecstasy (2.8%), LSD (2.8%), and alcohol (abuse; 13.9%). Average withdrawal scores at baseline did not differ with gender, age, treatment type, extent of cannabis use, or a lifetime history of anxiety or affective disorders. Withdrawal scores at baseline did not predict relapse during follow-up |
| Cornelius et al (USA)17 | Cross-sectional outpatient study | N=104 cannabis dependents (DSM-IV) recruited for the treatment of CUD and depression Female 49.0% Youths and adolescents 65.4% Caucasian, 27.9% African–American | 22-item Marijuana Withdrawal Checklist according to Budney et al 199924 Structured Clinical Interview for DSM-IV (SCID), modified for use with youths and adolescents Typical symptoms: craving cannabis, depressed mood, irritability, restlessness, anxiety, somatic symptoms were reported only rarely Controlled abstinence period: 4 weeks |
NA | 91% reported two or more symptoms | NA | 80% current major depressive disorder (DSM-IV) | CWS was related with rapid relapse of cannabis use |
| Milin et al (Canada)39 | Prospective outpatient study | N=21 cannabis dependents (DSM-IV) voluntarily entering a community youth addiction residential (N=13) or day/outpatient treatment program, female One-third female Youths and adolescents Ethnicity: NA |
16-item Cannabis Withdrawal Scale developed by the authors by reviewing available literature Structured Clinical Interview for DSM-IV Childhood Diagnoses27 (KID-SCID) Typical symptoms: craving cannabis, irritability, depression, twitches and shakes, sleep difficulties, nervousness, perspiring, restlessness, appetite change, tension, body aches, nausea, and malaise Controlled abstinence period: 28 days |
Type B | All | Males began to use cannabis regularly at an earlier age than females. Only few differences in reported withdrawal symptoms and severity between males and females | All self-reported high levels of psychiatric problems. Four of the 21 participants had a lifetime history of another substance dependence. 11 of 13 participants who had attained 2 weeks of abstinence were screened with KID-SCID: 4 were found to have at least one externalizing disorder, 1 had an internalizing disorder only, and 5 had both internalizing and externalizing disorders. One participant did not have any comorbid disorder. | CWS intensity was not related to the quantity of cannabis used, the frequency of exposure, the length of cannabis dependence, and the age at onset of daily cannabis use, or levels of psychiatric problems. 13 patients completed the study |
| Hasin et al (USA)22 | Part of an epidemiologic study, representative for the civilians of the USA 2001–2002, the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC) | N=2,613 frequent cannabis users (≥3 times/week), and a “cannabis-only” subset (N =1,119) never binge-drank or used other drugs (≥3 times/week) Most of the 2,613 individuals used cannabis 5–7 days a week, and 57.2% and 16.2% were diagnosed with DSM-IV cannabis abuse and DSM-IV cannabis dependence, respectively Female 33.1% Adults, aged 18–99 years 75.4% Caucasian, 11.4% African–American, 7.3% Hispanic, 5.9% other ethnicities (N=2,613) | Structured in-person interviews covering substance history, DSM-IV Axis I and II disorders, and withdrawal symptoms after cessation of use Typical symptoms: 2 factors (symptom types) were found, one characterized by weakness, hypersomnia, and psychomotor retardation and the second by anxiety, restlessness, depression, and insomnia |
NA | In the full sample (N=2,613), 57.7%, 44.3%, 34.4% reported ≥1 symptom, ≥2 symptoms, and ≥3 symptoms, respectively. These rates were nearly identical in the “cannabis-only” subset (N=1,119) | Gender was significantly associated with the anxiety/depression symptoms type only | Major depression was significantly associated with anxiety/depression symptoms type, the same was true for Panic Disorder. Generalized Anxiety Disorder was unrelated to CWS symptoms. Personality disorder was associated with both types of withdrawal symptoms | Both the symptom types were significantly associated with significant distress/impairment, substance use to relieve/avoid cannabis withdrawal symptoms, and quantity of cannabis use |
| Agrawal et al (USA)21 | Part of NESARC (Hasin et al)22 | Subsample of the past-12 month cannabis users (N=1,603), 12.2% met criteria for a lifetime history of DSM-IV Cannabis Dependence (not including withdrawal as a criterion) Female 38% Mean age 30.8 years Ethnicity of this subsample: NA |
See Hasin et al22 Typical symptoms: >40% of those with a lifetime history of DSM-IV cannabis dependence reported weakness/tiredness and eating more than usual/weight gain. 20%–40% reported sleeping more than usual, feeling depressed, feeling anxious/nervous, and moving/speaking slowly |
NA | In the full sample (N=1,603), >43% and ~29.4% reported ≥1 symptom and ≥2 symptoms, respectively. Mean number of withdrawal symptoms: 1.37 (range 0–18) | Nausea was more frequently reported in women, goosebumps/dilated pupils were more frequent in men, other symptoms were experienced in men and women with similar prevalence | Co-occurring tobacco use modestly increased the likelihood of reporting certain CWS symptoms (depressed mood, sweating/heart-racing, nausea, frequent yawning, unpleasant dreams, seeing/hearing things, and bad headaches), as did other illicit drug use (feeling weak/tired, depressed mood upon withdrawal, and frequent yawning) | After controlling for intensity of cannabis use, a history of parental alcohol/drug problems was associated with an increased likelihood of experiencing CWS The association between cannabis dependence and CWS was not mediated by a past-12-month diagnosis (DSM-IV) of Major Depressive Disorder, Generalized Anxiety Disorder, or Panic Disorder |
| Budney et al14 | Naturalistic telephone survey study | N=67 daily cannabis users and N=54 daily tobacco cigarette smokers who made quit attempts during the prior 30 days Female 46% (cannabis group) Adults Ethnicity (cannabis group): 66% Caucasian, 30% African–American |
Withdrawal Symptom Checklist,24 which includes common symptoms of both cannabis and tobacco withdrawal Typical symptoms: Withdrawal Discomfort Score (WDS) did not differ significantly between groups. Individual symptom severity ratings were also of similar magnitude, except craving and sweating were slightly higher for tobacco |
NA | NA | No | NA | Both the groups reported that withdrawal contributed substantially to relapse, and the strength of these ratings was similar across groups |
| Vandrey et al (USA)79 | Laboratory outpatient crossover study | Nontreatment-seeking heavy users (N=12) of cannabis and tobacco for 6 months prior to participating Female 50% Adults 100% Caucasian | Withdrawal Symptom Checklist, which includes common symptoms of both cannabis and tobacco withdrawal Typical symptoms: CWS: anxiety/nervousness, decreased appetite, difficulty concentrating, irritability, sleep difficulty, strange dreams, and WDS Tobacco withdrawal: anxiety/nervousness, increased anger, irritability, physical discomfort, restlessness, shakiness, sleep difficulty, tension, and WDS Controlled abstinence period: 5 days |
Type A | All | NA | No current comorbidity other than nicotine dependence | Overall withdrawal severity (WDS) associated with cannabis alone and tobacco alone was of a similar magnitude. Withdrawal during simultaneous cessation of both substances was more severe than for each substance alone, but these differences were of short duration, and substantial individual differences were noted |
| Copersino et al (USA)23 | Retrospective outpatient laboratory study | N=104 regular, nontreatment-seeking cannabis smokers, days used out of past 30 days: 23.9±7.8 Adults 52% Caucasian | 176-item Marijuana Quit Questionnaire addressing 40 withdrawal symptoms, sociodemography characteristics, cannabis use history, and the “most difficult” cannabis quit attempt Typical symptoms: craving cannabis 66%, irritability 48%, and boredom 45%, increased anxiety 33%, difficulty sleeping 33%, increase in appetite 26%, decrease in appetite 24%, physical discomfort 10% |
Type A The onset of physical withdrawal symptoms (means of 1–3 days after last use) was typically sooner than the onset of psychological symptoms (means of 2–10 days after last use) | 98% of subjects reported experiencing at least one cannabis withdrawal symptom, 81% reported experiencing ≥2 symptoms, 49% reported experiencing ≥4 symptoms | No | No current comorbidity other than nicotine dependence | Physical withdrawal symptoms generally had a shorter duration (2–19 days) than psychological symptoms (5 weeks to >1 year) |
| Levin et al (USA)11 | Retrospective outpatient laboratory study | N=469 subjects, 90.6% cannabis dependents (DSM-IV), lifetime, nontreatment seeking. The sample was generally of low socioeconomic status Female 42% Adults 79.5% African–Americans |
176-item Marijuana Quit Questionnaire Typical symptoms: Psychological symptoms: cannabis craving (75.7%), mood changes (33.7%–50.1%), sleep disturbances (21.8%–46.9%), and decreased appetite (38.8%); Physical symptoms: weight gain (23.5%) and headache (23.2%) Controlled abstinence period: duration of withdrawal symptoms was highly variable, ranging from 1.5 weeks to >1 year: Physical symptoms and aggressive behaviors tended to have quicker onset, quicker peak intensity, and shorter duration than sleep disturbances or mood changes |
Type A | 42.4% of subjects had experienced a lifetime withdrawal syndrome, 95.5% of subjects reported ≥1 individual withdrawal symptom (median 9.0); 43.1% reported ≥10 symptoms | Nonsignificant trend for women and African–Americans to be more likely than other subjects to experience CWS | Most subjects used legal psychoactive substances over the 6 months prior to the quit attempt: 69.7% used caffeine (36.3% at least 5 days per week), 75.3% alcohol (15.3%), and 79.3% tobacco (62.0%). There was minimal use of medications or illegal drugs | Number of withdrawal symptoms was significantly associated with greater frequency and amount of cannabis use, symptoms were usually of ≥moderate intensity and often prompted actions to relieve them. Alcohol (41.5%) and tobacco (48.2%) were used more often than cannabis (33.3%) for this purpose. There was little change during withdrawal in use of other legal or illegal substances among subjects reporting at least 2,000 lifetime uses of cannabis |
| Preuss et al (Germany)34 | Prospective inpatient study | N=118 treatment-seeking cannabis dependents (DSM-IV): The educational level was mostly low, almost two-thirds of the patients were unemployed, and more than half reported having been in detention Females 14.4% Age 16–36 years All Caucasian |
Modified version of the Marijuana Withdrawal Checklist (Budney et al)24 Typical symptoms: The most frequently mentioned physical symptoms of strong or very strong intensity on the first day were sleeping problems (20.7%), sweating (28.2%), hot flashes (20.7%), and decreased appetite (15.4%). The most frequent “strong” or “very strong” ratings were given for craving (37.9%). Other often highly rated psychological symptoms: restlessness (19.8%), nervousness (19.7%), and sadness (19.2%). Proportions of the “very strong” response category did not exceed 13.8% on any physical item, 12.2% on any psychological item, or 14.7% for craving Controlled abstinence period: 10 days |
Type B | 68% reported withdrawal symptoms, four withdrawal symptoms of at least moderate intensity were reported by the majority of subjects (69.8%) on the first day | No | No current comorbidity other than nicotine dependence | Most withdrawal symptoms ranged on average between low to moderate intensity |
| Allsop et al (Australia)15 Plus 1 month follow-up: Allsop et al (Australia)16 | Laboratory outpatient prospective study |
DSM-IV-dependent nontreatment-seeking cannabis users (N=49), cannabis use on ≥5 days per week over the previous 3 months Cannabis dependence severity: mild 77%, moderate 23%, severe 0% Female 33% Ethnicity: NA |
26-item Cannabis Withdrawal Scale (adapted from the Marijuana Withdrawal Checklist of Budney et al24 and a literature search of PubMed) Typical symptoms: nightmares and/or strange dreams = most valid item but caused relatively little associated distress. Angry outbursts were considered intense and caused much distress. Trouble getting to sleep is intense withdrawal symptom and caused significant distress Controlled abstinence period: 14 days |
Type A | NA | No | Nicotine dependence and anxiety disorder 14%, mood disorder 14%, psychotic disorder 2%, alcohol/other SUD 4% | |
| Gorelick et al (USA)19 (secondary analyses of the population of Levin et al)11 | Retrospective outpatient laboratory cohort study | N=384 subjects, 92.4% lifetime cannabis dependents (DSM-IV), nontreatment seeking. The sample was generally of low socioeconomic status Female 42% Adults 82.3% African–American |
176-item Marijuana Quit Questionnaire Typical symptoms: craving for cannabis 59.4%, sleep difficulties 50.5%, insomniaa 48.7%, feeling angry and/or aggressive and/or irritable 45.6%, physical symptoms 25.3%, feeling restless 21.9%,increased appetite 20.8%, decreased appetite 17.4% Controlled abstinence period: cf Levin et al11 |
Type A | 40.9% of subjects met the DSM-5 CWS criterion31 (at least 3–7 symptoms), 30.0% met the Budney and Hughes (2006)13 4-symptom criterion (at least 4 of 11 symptoms), and 57.3% met the Budney et al.12 2-symptom criterion (at least 2 of 11 symptoms) | Women were significantly more likely than men to report a DSM-5 physical symptom (30.6% vs 21.4%,) | Among subjects using a drug class at least weekly prior to the quit attempt, only a minority within each class decreased their use during the cannabis quit attempt: 10.1% for caffeine (5.2% of all subjects), 15.4% for alcohol (5.5%), 12.4% for tobacco (8.3%), 44.0% for stimulants (2.9%), 19.1% for opiates (1.0%), 14.3% for sedative/hypnotics (0.5%), 33.3% for hallucinogens (0.3%), and 50% for phencyclidine (0.3%) | Total number of joints smoked in the month prior to the quit attempt was significantly correlated with total number of withdrawal symptoms experienced by a subject. There was no significant association between presence of CWS by any definition and outcome (relapse vs continued abstinence) of the quit attempt |
| Lee et al (USA)35 | Prospective laboratory study at a closed research unit | N=29 nontreatment-seeking, chronic cannabis smokers, 79.3% cannabis dependents (DSM-IV), smoking for at least 1 year and ≥5 days per week for the last 6 months Female: 0% Adults 86.2% African–American |
37-item cannabis withdrawal scale (adapted from Haney et al),28 12-item Marijuana Craving Questionnaire, Symptom Checklist-90 revised (SCL-90R)129 Typical symptoms: craving for marijuana 48.8%, irritable 36.8%, angry/aggressive 36.3%, depressed 31.0%, restless 26.8%, anxious 28.7%, strange/vivid dreams 14.3%, depth of sleep 15.8%, difficulty getting off to sleep 7.29% Residual cannabis symptoms at admission: feel thirsty 35.9%, dry mouth/throat 25.6%, feel hungry 23.8%, increased appetite 18.0%, high appetite 27.0%, stimulated appetite 27.0% Controlled abstinence period: 30 days |
Type A | 38% of the subjects met DSM-5 diagnostic criteria for CWS31 on admission, increasing to 55% (day 1), 38% day 2), 56% (day 3) During days 4–30, 20%–50% of the participants met these criteria31 | No current comorbidity other than nicotine dependence | Severity of symptoms was generally mild to moderate. About 10% had at least moderate severity. Expected residual cannabis effects were positively correlated with plasma THC and 11-OH-THC. Expected withdrawal effects, “difficulty getting off to sleeping” and “anxious,” were negatively correlated with plasma THC | |
| Bonnet et al (Germany)36 | Prospective inpatient study | N=39 treatment-seeking chronic cannabis dependents (ICD-10), mostly lived alone (51.3%), unemployed (69.2%), and had a moderate education level (76.9%); detention history 15.4% Female 20.5% Adults 97.4% Caucasian |
Modified version of the Marijuana Withdrawal Checklist (Budney et al),24 Clinical Global Impression Scale – Severity (CGI-S)80 Typical symptoms: mean intensity of irritability, nervousness, restlessness, and anger increased until day 4 and then decreased. Craving and sleeplessness peaked on day 2. Strange, ie, vivid and lucid dreams reached its maximum not until day 4 The intraindividual CWS peak was first observed on day 1 in 3 patients (7.7%), on day 2 in 12 patients (30.8%), on day 4 in 20 patients (51.3%), and on day 8 in 4 patients (10.3%) Controlled abstinence period: 16 days |
Type A | All | Females had stronger CWS | No current comorbidity other than nicotine dependence | The maximum withdrawal severity according to CGI-S was 4 “moderately ill” in 7 patients (17.9%), 5 “markedly ill” in 16 patients (41%), and 6 “severely ill” in 16 patients (41.1%). On admission, THC and its metabolites did negatively correlate with the severity of CWS. There was no significant correlation afterward, THC-OH in serum declined most rapidly below detection limit, on median at day 4. At abstinence day 16, the THC levels of 28.2% of the patients were still >1 g/mL (range: 1.3–6.4 ng/mL). Concerning the single withdrawal symptoms only for “strange dreams” a significant (negative) correlation with serum THC was found at day 4 |
| Greene and Kelly (USA)42 | Prospective outpatient cohort study plus 1-year follow-up | Modified version of the Customary Drinking and Drug Use Record (CDDR, Brown et al),78 a structured interview that assesses substance involvement, past 90-day withdrawal symptoms, and DSM-IV lifetime cannabis abuse/dependence Typical symptoms: difficulty sleeping 30.56%, headaches 13.89%; feeling irritable 13.89%; stomach upset, nausea, vomiting 11.11%, fatigue, excessive yawning 11.11%, feeling angry, hostile, or acting aggressive 11.11%; loss of appetite 11.1%, feeling depressed 8.33%, feeling anxious or nervous 8.33% Controlled abstinence period: 12 months |
NA | 40% (n=36) reported experiencing cannabis withdrawal. Twenty-four (66.67%) of these subjects reported using drugs to relieve or prevent withdrawal symptoms | No | Most patients had another SUD mostly alcohol use disorder (90%), tobacco (90%),amphetamine <10%, cocaine <10% Anxiety disorders 10%, mood disorders 17.7%, externalizing disorders 42.2% |
Participants reporting withdrawal were more likely 1) to meet criteria for cannabis dependence, 2) to have a mood disorder, 3) have higher levels of substance use severity, 4) report more substance-related consequences. No main effect of withdrawal on percent days abstinent over the 12-month follow-up period. There was no longitudinal relationship between withdrawal and psychiatric symptoms | |
| Herrmann et al (USA)76 | Prospective outpatient study | N=136 treatment-seeking frequent cannabis users Female 26.5% Adults >80% African–American | Marijuana Withdrawal Checklist (Budney et al),24 14-item Withdrawal Discomfort Scale (WDS, Budney et al)26 Controlled abstinence period: NA |
NA | NA | Women had significantly stronger CWS and more withdrawal symptoms than men. Women had significantly higher scores than men on six individual items. These items were in two domains: mood symptoms (irritability, increased anger, restlessness, and violent outbursts) and gastrointestinal symptoms (nausea and stomach pain) | No current comorbidity other than nicotine dependence Reported drinking: <20 standard drinks per week |
|
| Soenksen et al (USA)40 | Cross-sectional study | N=93 pre-adjudicated males between 12 and 18 years of age who were detained at a state juvenile correctional facility: 50.5% of participants reported using cannabis at least once a day during the 3 months prior to detention Female 0% Youths 45.1% Caucasian; 21.5% African–American; 26.88%/5% Hispanic/Latino |
Typical symptoms according to the Marijuana Withdrawal Checklist of Budney et al:24 sleep difficulty, nervousness/anxiety, depressed mood, restlessness, increased anger, decreased appetite, headache, and sweating. Three of these symptoms (sleep difficulty, depressed mood, and nervousness/anxiety) were reported to be of at least moderate severity by 30% of participants Controlled abstinence period: 5.7±12.7 days |
NA | NA | – | 51.6% reported using alcohol at least once a month | Significant main effect for level of marijuana use on the reported severity of two withdrawal symptoms: craving to smoke marijuana; and strange/wild dreams: significant main effect for the level of tobacco use on severity of irritability. African–Americans reporting lower withdrawal discomfort scores and experiencing less severe depressed mood, difficulty sleeping, nervousness/anxiety, and strange/wild dreams |
| Davis et al (USA)109 | Prospective outpatient cohort plus 3-month follow-up | N=110 heavy and recent cannabis users (use ≥45 out of 90 days) seeking community outpatient substance abuse treatment. 28.2% of participants were diagnosed with past year cannabis dependence, and 53.4% reported any lifetime CUD Female 8.2% Young adults (18–25 years old) 34.6% Hispanic, 26.4% African–American, 21.8% Caucasian | 22-item Current Withdrawal Scale. Individuals who reported ≥3 symptoms (eg, scores ≥3) were coded (cannabis withdrawal = 1) as having met criteria for a DSM-5 diagnosis of CWS31 Typical symptoms: feel sad, tense, angry 48.2%, feel nervous 26.4%, have trouble sleeping 40.0%, have trouble sitting still 33.6%, throw up or feel like throwing up (stomach) 10.0%, shaky hands 9.09%; sweat more/heart race/or goose bumps (chills) 11.8%, have a fever 2.73%, have muscle aches (headache) 12.7% Controlled abstinence period: 3 months |
NA | NA | Gender, non-Caucasian, and previous days of substance use treatment were not significant predictors of abstinence in the community at 3 months | No current comorbidity other than nicotine dependence. Drinking alcohol <13 days out of the past 90 days. About 72% of participants reported smoking tobacco in the past week. Depression 3.7%, generalized anxiety disorder 20%, ADHD 7.7% | Of the 110 participants, 28.2% (n=31) reported being abstinent in the community at the 3-month follow-up assessment. Relative to those meeting cannabis withdrawal criteria, those not meeting the cannabis withdrawal criteria have 2.6 times higher odds of being abstinent in the community at 3 months |
| Synthetic cannabinoid receptor agonists | ||||||||
| Macfarlane et al (New Zealand)56 | Retrospective chart review | In the 12-month period, N=47 patients presented to detoxification services reporting problems withdrawing from synthetic cannabinoid receptor agonists. About 21 clients were admitted for medical management within an inpatient setting Female 37.5% 66% Caucasian, 25% Maori, 6% Indian, 3% Pacific Islander |
Cannabis Withdrawal Assessment Scale (CWAS) scores (Allsop et al)15 Typical symptoms: inpatients (N=20): agitation 89%; irritability 83%, anxiety 55%, and mood swings 55%, nausea and vomiting 44%, and loss of appetite 17% Controlled abstinence period: 8 days (mean duration) |
Type A The CWAS scores peaked on day 2 but remained at a similar level throughout the first 5 days |
NA | NA | Coexisting substance dependence apart from nicotine dependence was low. About 30% inpatients had an Axis 1 psychiatric disorder (schizophrenia N=3, depression with psychosis N=1, bipolar disorder N=1, and anxiety disorder N=1) | 87.2% of the clients reported difficulty to stop using due to the development of withdrawal symptoms |
Abbreviations: NA, not applicable; CWS, cannabis withdrawal syndrome; ICD, International Classification of Diseases; CUD, cannabis use disorder; THC, delta-9-tetrahydrocannabinol; ADHD, attention-deficit hyperactivity disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; LSD, lysergic acid diethylamide.
Table 2.
Marijuana Withdrawal Checklist (MWC)
| Symptoms | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Cannabis craving | ||||
| Irritability* | ||||
| Nervousness/anxiety* | ||||
| Increased aggression* | ||||
| Restlessness* | ||||
| Increased anger* | ||||
| Sleep difficulty* | ||||
| Strange/wild dreams* | ||||
| Depressed mood* | ||||
| Decreased appetite* | ||||
| Sweating* | ||||
| Shakiness/tremulousness* | ||||
| Headaches* | ||||
| Stomach pains* | ||||
| Nausea | ||||
| Other |
Notes: A total MWC score is obtained by summing the severity ratings, mild = 1, moderate = 2, severe = 3 points;
symptoms listed in DSM-5. There is no valid definition available for assigning a cannabis withdrawal syndrome to be mild, moderate, or severe. An MWC score of 10 points was found to be comparable with 5 points on the Clinical Global Impression – Severity scale (CGI-S), which is a 7-point scale. Four or more withdrawal symptoms were shown to predict the severity of cannabis-related problems at 1-year follow-up among treated adolescents (N=214, 92% retention). Data from previous studies.18,24,26,31,36,37,80
Abbreviation: DSM-5, Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition.
There is a consistent evidence that CWS occurs in ~90% of the patients being diagnosed with cannabis dependence according to ICD-10 or DSM-IV12,13,38,41,42 (Table 1). Among them, most often, male adolescents and young adults demonstrated a significant loss of quality of life during their cannabis dependence as measured by disability-adjusted life years in the Global Burden of Disease 2010 Study (cf Figures 2 and 3 in http://journals.plos.org/plosone/article?id=info:doi/10.1371/journal.pone.0076635, accessed November 25, 2016).4
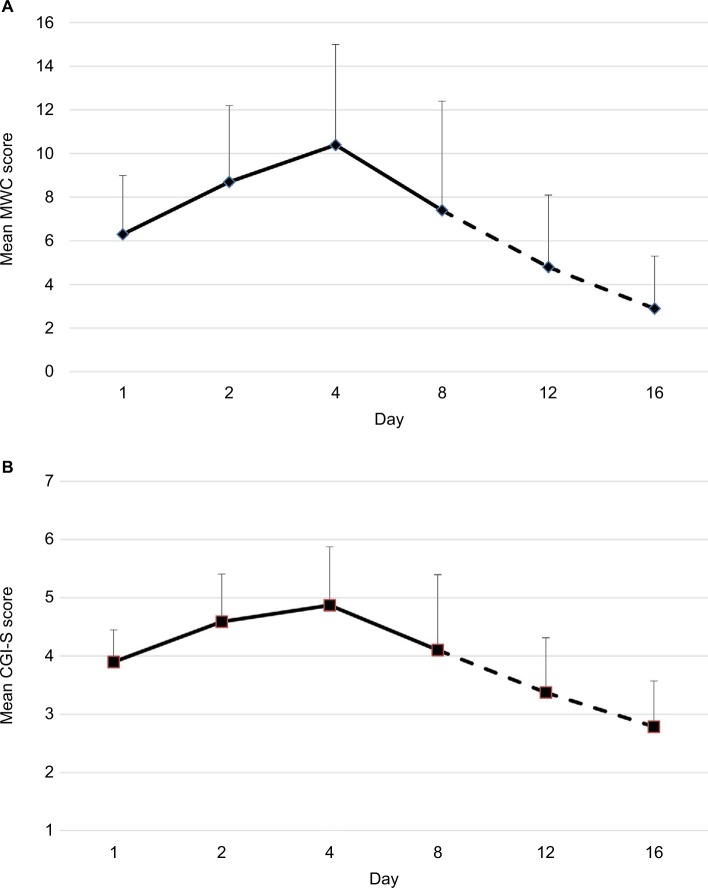
Figure 2.
Mean and standard deviation of the (A) CWS checklist (MWC score according to previous studies24,26,37) and (B) the Clinical Global Impression Scale (CGI-S Score80) during the course of the study. Reduced sample sizes on day 12 (n=35) and day 16 (n=28) due to regular dismissals and missed assessments are indicated by dashed lines. The effect size according to Cohen (Cohen’s d) was 1.1 for the CWS (day 1 to day 16), Cohen’s d ≥0.8 is defined to reflect a strong effect.130 Vertical imaginary Y-axis: severity scores. Horizontal imaginary X-axis: time course.
Note: Reproduced from Drug Alcohol Depend, 143, Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N, Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and -metabolites in serum, 189–197. Copyright (2014), with permission from Elsevier.36
Abbreviations: CWS, cannabis withdrawal syndrome; MWC, Marijuana Withdrawal Checklist.
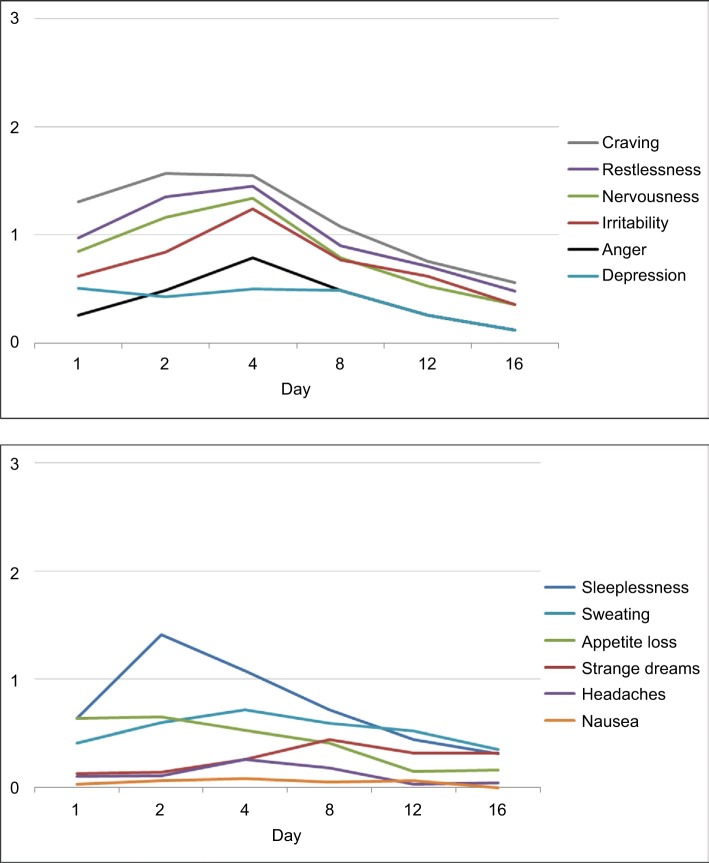
Figure 3.
Mean rating of single symptoms of the MWC (MWC score according to previous studies24,26,37); 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = heavy). Note the delayed occurrence of strange dreams.25 Vertical imaginary Y-axis: severity scores. Horizontal imaginary X-axis: time course.
Note: Reproduced from Drug Alcohol Depend, 143, Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N, Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and -metabolites in serum, 189–197. Copyright (2014), with permission from Elsevier.36
Recent studies revealed that 35%–75% patients seeking outpatient cannabis detoxification developed a CWS post-cessation, which usually seemed to be mild to moderate in severity.11–13,15,16,19 However, most of the cannabis dependents developed a CWS of greater severity.36 Adult cannabis dependents were shown to develop a severe CWS likelier than adolescent frequent users.24,37 A prolonged and heavier cannabis use predicts a stronger CWS.12,13,19 It was confirmed again more recently that the occurrence of CWS is a highly specific indicator of a cannabis dependence, particularly in adolescents and young adults.42
This review intends to provide a synthesis of current evidence on the biology and clinical characteristics of the human CWS and its treatment. In addition, it includes information on the role of CWS in the course of CUD31 or cannabis dependence.22,43
Materials and methods
This study is a review of the current literature on human CWS. The search for articles was performed on the PubMed44 (Med-line) and Scopus,45 using the a combination of the search terms “cannabis withdrawal,” “humans,” “epidemiology,” “disability,” “clinical studies,” “clinical trials,” “case reports,” “cannabis use disorder,” “cannabis dependence,” “treatment,” “psychotherapy,” “psychosocial,” “exercise,” “occupational therapy,” “pharmacotherapy,” and “potency”. In addition, an active search for related literature was carried out in the reference lists of the selected publications. In total, 2,440 documents were screened, and mainly those studies providing information on human CWS and those published in English or German (N=101) were considered. Articles published up to November 25, 2016, were included.
Human biological background
The cannabis plant contains >420 chemical compounds of which 61 being cannabinoids themselves being defined to bind to cannabinoid 1 and 2 (CB1, CB2) receptors.46 Regular cannabis use is associated with neuroanatomic abnormalities within brain regions with a high density of CB1 receptors, particularly the hippocampus and prefrontal cortex.47,48 It is assumed that, the main psychoactive ingredient of cannabis, the partial CB1 receptor agonist delta-9-tetrahydrocannabinol (THC) is involved in the etiology of this damage,47 which certainly awaits further study. For instance, a contribution of receptor-independent mechanisms of cannabinoids49,50 as well as distress due to psychiatric CUD or CWS cannot as yet be excluded. A crucial role of THC in the genesis of CWS in humans is demonstrated by 1) pharmacokinetic studies showing a hysteresis effect between the decrease in plasma THC and onset of CWS,51,52 2) an abstinence syndrome following oral THC12,13 and THC analogs,53 3) alleviation of CWS by oral THC and THC analogs,29,54,55 and 4) the occurrence of CWS-like symptoms after quitting recreational intake of synthetic cannabinoid (SC) receptor agonists, often being full CB1 receptor agonists, differing from THC being a partial agonist.56,57 The withdrawal syndrome of SCs binding closer to CB1 receptors than THC seemed to be stronger than CWS and obviously showed characteristics unknown to CWS, such as seizures.58 Otherwise, single cases of patients with diagnosed epilepsy who quit regular cannabis use are reported to exacerbate,59 which is attributed to an anticonvulsive effect of cannabis.46 The psychoactive potency of bred cannabis products sold for recreational use has been increasing in many markets over the past decade,1,2 which could lead to a stronger withdrawal syndrome than usually known for cannabis. Intriguingly, there is one case report regarding improvement of CWS following the administration of cannabidiol,60 another constituent of cannabis, shown to reverse some adverse effects of THC in the laboratory.61 The cardiovascular functioning seemed to be scarcely altered during CWS.62 Although the endocannabinoid system is involved in the regulation of most of the other peripheral organ systems, the immune system and the gut, too, we are unaware of any such study on the contribution of these organs to human CWS. Notably, applying a CB1 receptor antagonist (rimonabant) to cannabis-dependent patients substituted with THC analogs did not precipitate a relevant CWS.63 This may be due to the low doses of rimonabant applied (20 and 40 mg) or the CWS-generating mechanisms that are at least partly independent upon CB1 receptors.49,50 Cannabis users with opioid dependence are less likely to experience CWS,64 which may indicate the contribution of the endogenous opioid system. In a laboratory study, the µ-opioid receptor antagonist naltrexone was recently shown to reduce self-administration of active cannabis and its related subjective positive effects on heavy cannabis users.65 The authors are unaware of any study having directly examined the effect of naltrexone on the CWS under naturalistic conditions.
Abstinence-induced craving is associated with reduced amygdala volumes in frequent adolescent cannabis users, which was also found in adult alcohol and cocaine users.66 Thus, the specificity of this finding for CWS is doubted and may represent a more general precursor of substance abuse itself;66 that is, early stress in life.67,68 With respect to the three “a”s of CWS (anger, aggression, and anxiety) (Table 1), the threat-related amygdala reactivity was shown to be inversely related to the level of cannabis use in adolescents with comorbid cannabis dependence and major depression.69 This finding may reflect the neurobiological basis of these transient, mostly short-lasting CWS symptoms, thus possibly being even rebound “amygdala-related” symptoms after quitting regular cannabis use. Nevertheless, the CWS symptoms could persist even longer in genetically or epigenetically more susceptible individuals upon withdrawal.
Regular cannabis intake is related to a desensitization and downregulation of human cortical and subcortical CB1 receptors. This starts to reverse within the first 2 days of abstinence and the receptors return to normal functioning after ~4 weeks of abstinence,70 which could constitute a neurobiological time frame for the duration of CWS, not taking into account cellular and synaptic long-term neuroplasticity elicited by long-term cannabis use before cessation, for example, being possibly responsible for craving. In support, cannabis dependents were recently shown to have a robust negative correlation between CB1 receptor availability in almost all brain regions and their withdrawal symptoms after 2 days of cannabis abstinence which in turn resolved in the next 28 days of abstinence.71
If compared with nonusers, long-term cannabis users were demonstrated to have greater brain activity during cannabis cues relative to natural reward cues (ie, fruit itself being superior to neutral cues) in the orbitofrontal cortex, striatum, anterior cingulate gyrus, and ventral tegmental area.72 The users had positive correlations between neural response to cannabis cues in the fronto-striatal-temporal regions and subjective craving, cannabis-related problems, serum levels of THC metabolites, and the intensity of CWS. All of which were not found in non-cannabis users,72 suggesting a sensitization and specificity of the brain response to cannabis cues in long-term cannabis users.72
In the San Francisco Family Study, some symptoms of CWS, craving and cannabis-related paranoia were found to be heritable,73 which could have been confounded by the heritability of age at first-ever use, for instance. It was suggested that genetic factors determine whether an individual may try or use cannabis; however, environmental factors are more crucial in determining whether a person develops dependence or not.73 Recent findings provide evidence that the use of nicotine, alcohol, or cannabis shares genetic and environmental pathways on the way to develop a substance use disorder.74 Regular intake of alcohol, nicotine, cannabis, or other drugs of abuse alters the stress response sustainably75 and, thereby, may precipitate a substance use disorder.
Characteristics of CWS
Considering the cannabis research of the last 20 years,12,13,16,18–20,31 there was no doubt that cessation of heavy or prolonged cannabis use is most likely followed by typical symptoms, such as
irritability
nervousness/anxiety
sleep difficulty
decreased appetite or weight loss
depressed mood
one of the following physical symptoms such as abdominal pain, shakiness/tremors, sweating, fever, chills, or headache.
According to DSM-5,31 CWS (292.0) is diagnosed if three or more of these symptoms (1–6) develop within ~1 week after quitting cannabis use abruptly.31 Withdrawal severity and duration can vary widely between individuals and fluctuate depending on the amount of prior cannabis use, context of cessation (eg, outpatient vs inpatient, voluntary vs involuntary), personality traits, psychiatric and somatic comorbidity, current life stressors, previous experiences, expectations, support, and severity of dependence.12,13 Women seeking treatment for CUD were shown to generate more frequent and more severe withdrawal symptoms than men after quitting their frequent cannabis use.36,76,77 However, older studies did not reveal this gender effect (Table 1).
Additional heavy tobacco use was reported to be associated with stronger irritability during the CWS of adolescents.40 Black adolescents were shown to have lower withdrawal complaints and experience less severe depressed mood, sleep difficulty, and nervousness/anxiety than non-Black adolescents.40 In youths with conduct disorder, this disorder antedated cannabis use.38
Currently, psychometrically validated cannabis withdrawal scales are unavailable. Several versions to measure CWS11–13,16,18,24,78 were developed, some of which compared with each user by Gorelick et al.19 All these versions were based on the Marijuana Withdrawal Checklist (MWC) of Budney et al.24 The MWC was originally designed with 22 items that assessed mood, behavioral, and physical symptoms and was revised to a 15-item version comprising these items that had been most frequently endorsed during cannabis withdrawal12,13,26,37 (Table 2). Later, this version builds the construct of the DSM-5 definition of CWS31 (Table 2), which, however, does not consider cannabis craving and nausea.31
Regarding the course of the overall CWS, there were two different types described in the available literature (Figure 1 and Table 1). One peaked between the second and sixth abstinence day (type A)11,15,16,19,20,23,26,27,35,36,56,79 and the other decreased continuously following cannabis cessation (type B).28,34,39 It is assumed that type-A CWS includes more intoxication symptoms which vanished during the first few days post-cessation, thereby unmasking the “pure” CWS.36 A negative correlation with serum levels of THC at admission, which would support this assumption, was found in type A.35,36 Type-B CWS was not investigated to this subject. Alternative explanations are that the contribution of single items (cf Figures 2 and 3) differed between types A and B or more patients without a measurable CWS were included in the group of patients producing a type-B course.
Figure 1.
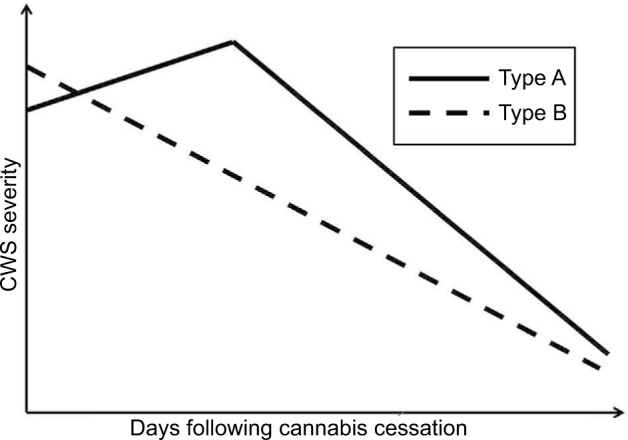
Courses of overall CWS post-cessation. The CWS usually lasts up to 3 weeks and its average peak severity (burden) is comparable to that of a moderate depression or alcohol withdrawal syndrome or in outpatient settings, similar to that of a tobacco withdrawal syndrome. Data from previous studies.14,36,79
Abbreviation: CWS, cannabis withdrawal syndrome.
In the following, the course of a CWS (Figures 2 and 3) is presented, which was recorded during a controlled inpatient detoxification treatment of a sample consisting of long-term cannabis users (N=39, 38 Caucasians, 8 females, median age: 27 years, median daily cannabis use: 2.5 g, median duration of daily cannabis use: 36 months).36 Their cannabis consumption was in the upper range if compared with other clinical studies on CWS.11,12,16,20,34 All patients of this sample developed a considerable CWS.36 Although this study was conducted in a large detoxification ward of a university hospital residing in a metropolitan area (Ruhr Area, Germany), it lasted 5 years (2006–2011) to find 39 appropriate patients seeking treatment due to a current sole cannabis dependence without a competing additional substance use (except for tobacco) or active comorbidity to measure a CWS as pure as possible.36 This study aimed to apply both MWC24 and the Clinical Global Impression Scale (CGI-S)80 to this sample to make the severity of CWS comparable to the severity of other psychiatric disorders.36 The course of the single items is shown in Figure 3. Cronbach’s α coefficients were 0.67, 0.78, and 0.73 on days 4, 8, and 12, respectively.36 The MWC applied to this sample achieved α coefficients comparable to that of the CWS criteria proposed for DSM-5 (0.75)19 and to that of the original MWC of Budney et al (0.77).12,24
CWS severity in comparison with other psychiatric conditions
As outlined earlier, the severity of CWS is positively related to the cumulative amount and potency of cannabis used before cessation,12,13,19 gender,36,76,77 and several environmental12,13,73 as well as heritable73 factors. Therefore, its naturalistic severity varies a lot. There are some evidences that the discomfort due to CWS is similar to that found during tobacco withdrawal14,79 or a moderate alcohol withdrawal syndrome.36 Inpatients detoxifying from heavy cannabis use were rated to be “moderately ill” at the peak of CWS according to CGI-S.36 For a first orientation, inpatients suffering from acute schizophrenic episodes and patients with acute depressive episodes in outpatient settings have been rated in the majority to be “severely ill” and “moderately ill,” respectively.36 Strong CWS can mimic eating disorders associated with gastrointestinal symptoms, food avoidance, and weight loss of adolescents.81
The role of nausea in CWS
There is one case report of severe nausea being associated with CWS.82 In the last years, increasing cases with a cannabis-hyperemesis syndrome were noticed, which characteristically occurred in frequent and long-term cannabis users and vanished in their next 5–20 abstinent days.83–85 In order to differentiate this condition from CWS, we studied the course of the item “nausea” in the “cannabis burdened” sample mentioned earlier and found no correlation (r=−0.14 to 0.19) with the other items of MWC, whose internal consistency did not change, if “nausea” was excluded from the scale.36 Thus, we found no evidence of nausea being a characteristic element in the orchestra of the CWS, which confirms the previous results of others.11,12,19
Nevertheless, nausea seems to be a less common cannabis withdrawal symptom than chills, shaking, sweating, depressed mood, and stomach pain.13 This is supported by the observation that nausea can occur more pronounced in the female CWS than in the male CWS.76,77
A retrospective chart review found preliminary evidence that “nausea and vomiting” might emerge more frequently in the withdrawal syndrome of SC agonists (Table 1)56 often being full agonists at the CB1 receptor, other than the partial agonist THC.57 Is this a clue that nausea breaks through when very potent agonists are removed from CB1 receptors? On the other hand, “severe nausea and vomiting” were key symptoms of the intoxication syndrome following the intravenous application of crude marijuana extracts86 and are typical signs for the overdosing of smoking or swallowing cannabis preparations,46,86 including the first-ever intake experience. However, low to moderate amounts of cannabis preparations or THC analogs have well-known antiemetic properties.46
Treatment of CWS
Cannabis detoxification treatment is usually performed in outpatient settings. However, in the case of a moderate or severe dependence syndrome, low psychosocial functioning or moderate or severe psychiatric comorbidity, an inpatient treatment is required. In Germany, inpatient cannabis detoxification is ideally performed in specialized wards following a “qualified detoxification” protocol. This includes supportive psychosocial interventions, psychoeducation, non-pharmacological symptom management, occupational and exercise therapy, professional care, as well as medical and psychiatric diagnostics and therapy of comorbid conditions. The treatment duration is related to the severity of the comorbidity or the CUD. An inpatient detoxification treatment on grounds of the diagnosis of “cannabis dependence” alone is mostly not accepted by the German health care providers, and they prima vista doubted the existence of a treatment-relevant CWS. This point of view may have a historical background, because many physicians consider cannabis to be a “soft drug,” as probably 20 years ago it contained lower THC contents.87–90 This view may change with a clear definition of CWS in current diagnostic classification systems, such as DSM-5.31 As a rule of thumb, an “acute” inpatient detoxification treatment lasts between a few days and up to 3 weeks. In case of a too high psychiatric comorbidity and too low psychosocial functioning for an outpatient treatment, the patients could be transferred into specialized inpatient rehabilitation wards. Because this post-acute treatment approach is paid by the German Person Fund (DRV), a substantial formal request is required. The rehabilitation treatment normally lasts for several weeks and is a special feature of the German health care system. In Germany, most of the cannabis patients entering outpatient (28,000 individuals in 2014) and inpatient (3,367 individuals in 2014) rehab programs show additional problems with the co-use of alcohol (7%–14%), opioids (30%–55%), cocaine (45%–60%), stimulants (45%–70%), and pathological gambling (6%–18%).91 This pattern of comorbidity is common in other high-income countries.1
The effects of behavioral approaches on the mitigation of CWS were not intentionally studied, even though a beneficial action of aerobic exercise therapy can be assumed.92
Currently, there are no approved medications for the treatment of CUD. Nevertheless, various pharmaceuticals have been studied in small (N<80) controlled, mostly outpatient or laboratory pilot trials: lithium, antidepressants (bupropion, nefazodone, venlafaxine, fluoxetine, escitalopram, and mirtazapine), anticonvulsants (divalproex and gabapentin), norepinephrine reuptake inhibitor (atomoxetine), glutamate modulator and mucolytic agent (N-acetylcysteine), muscle relaxants (baclofen), anxiolytic (buspirone), antipsychotics (quetiapine), and CB receptor agonists (dronabinol and nabiximols).55,93 The antidepres sants, atomoxetine, lithium, buspirone, and divalproex had no relevant effect on the CWS or had worsened it.55,93 For instance, venlafaxine was shown to aggravate CWS and, thus, was accused to uphold cannabis smoking.94 Quetiapine (200 mg/day) improved appetite and sleep quality during the CWS but worsened marijuana craving and drove self-administration of marijuana.95 A more recent open pilot study reported a decrease of cannabis use within 8 weeks of quetiapine treatment (25–600 mg).96
Putatively beneficial agents
There is evidence for an improvement of CWS with gabapentin (1200 mg/day).97 The efficacy of the THC analogs dronabinol and nabiximols (plus cannabidiol) in reducing CWS is demonstrated in three small but well-controlled outpatient studies.98–101 An improvement of the dependence syndrome or craving was not found.98–101 Although innovative compounds, high costs of dronabinol or nabiximols may limit not only the use but also the abuse of these drugs. A significant effect of the THC substitution on the severity of cannabis dependence, craving, or cannabis-related problems was not found yet.98–101 Nabiximols is a drug containing two of the main active cannabinoids, namely, THC and cannabidiol, and has been approved in some countries for the treatment of spasticity of multiple sclerosis (MS).99–101 It is an oral spray formulation, and each puff of 100 µL contains 2.7 mg of THC and 2.5 mg of cannabidiol. A treatment with six puffs over the day revealed >10 times smaller blood THC concentrations than the blood concentrations known to produce psychotropic effects.102 For the nabiximols regimes in the treatment of CWS, cannabis users had been instructed to take eight sprays qid99,100 or a maximum of four sprays every hour (up to 40 sprays/day).101 Oral dronabinol had been administered in 20 mg doses three times a day.98 The effectiveness of N-acetylcysteine (1200 mg/day) on CWS was not assessed directly, but this agent was shown to reduce relapse markers in the urine alongside a large (N=116) well-controlled study.103 A recent laboratory study demonstrated the efficacy of the THC analog nabilon.104
Because sleep difficulty is the withdrawal symptom that is assumed to be most associated with relapse to cannabis use,105 a few sleeping medications were tested in the CWS treatment with first promising results for mirtazapine30 and zolpidem106,107 during the first days of abstinence, necessarily taking care for zolpidem’s potential misuse. Nevertheless, both the drugs had no effects on CWS in general or relapse prevention.30,107
The withdrawal syndrome of SC receptor agonists56 awaits further characterization and may respond to benzodiazepines and quetiapine.108
Impact on CUD or dependence
The CWS is part of a CUD (DSM-5)31 and dependence-syndrome (ID-10, DSM-IV-R)32,43 being characterized by frequent, heavy, or prolonged cannabis use. The importance of the treatment of CWS on the maintenance of cannabis use or substance use trajectories over time is unclear and awaits further study. From previous literature, there is small evidence for both 1) CWS treatment initiated abstinence or dose reduction12,13 and 2) CWS treatment does not influence cannabis use in the following.18,20,42,109 Frequent cannabis users had reported that withdrawal symptoms negatively influence their desire and ability to quit.12,13,18,105 Two actual studies on adolescents and young adults found no association of CWS with abstinence rates being monitored up to 3 months20 and 1 year posttreatment,109 which confirmed the previous finding of Arendt et al.20 Furthermore, patients with CWS relapsed sooner than those without CWS.20 Patients recognizing a problem with CWS were associated with better abstinence rates than patients not recognizing a problem with CWS42 pointing to a potential value of psychoeducation, an approach to be further studied in the management of CUD.
Abstaining from cannabis was reported to be followed by an increase of alcohol and tobacco use, which decreased again after continuation of cannabis use.110 CWS in people with schizophrenia is associated with behavioral change, including relapse with cannabis and increased tobacco use.111 “Religious support” and “prayer” were self-identified by cannabis users to be the most helpful quitting strategies and both were associated with higher 1-month and 1-year abstinence rates in these population.111 Furthermore, the symptom severity of patients with posttraumatic stress disorder was positively associated with the use of cannabis (probably taken as a “self-medication”), cannabis use problems, and severity of CWS.112
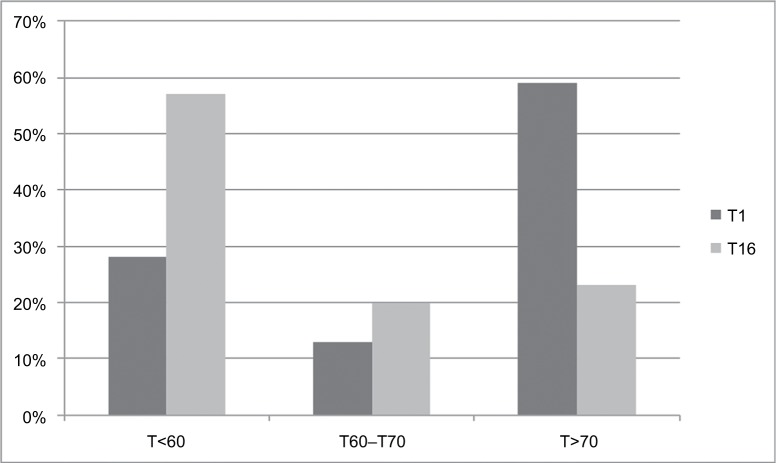
Studies that compared the effectiveness of outpatient versus inpatient treatments with respect to the severity and prognosis of CUD, especially their differential efficacy on long-term relapse prevention, dose reduction, or psychosocial functioning, are missing. At the end of a 16-day lasting inpatient detoxification treatment (qualified detox) of heavy cannabis users, the following effect sizes were found (Cohen’s d): 1.1 (CWS), 1.4 (cognition), psychiatric symptoms (0.8–0.9).113 The bothersome global distress of this sample had improved significantly during the qualified detox (Figure 4).
Figure 4.
Significant improvement (p<0.001) of the subjective global distress of adult heavy cannabis users during inpatient qualified detoxification as measured by the Symptom Checklist 90, revised version (SCL-90-R).129 Y-axis: percent of the sample (N=35); X-axis: global distress according to T-values: T<60: normal global distress; T>70: severe global distress;129 T1 = admission day and T16 = last day (day 16) of the controlled inpatient qualified detoxification treatment.113
Note: Reproduced from Dtsch Med Wochenschr, 141(2), Bonnet U, Specka M, Scherbaum N, Häufiger Konsum von nichtmedizinischem Cannabis, 126–131. Copyright (2016), with permission from Georg Thieme Verlag.113
At present, the effectiveness of different cannabis detoxification treatments on the course of the CUD has not been studied in depth. Outpatient treatment programs improved the psychosocial functioning and dropped the cannabis use for a while.12,13 Currently, only a few long-term follow-ups are available, so far showing no sustaining improvement of CUD subsequent to outpatient detoxification attempts.18,20,42,109
Discussion
With the definition of the DSM-5 criteria,31 the CWS comes of age. Genetic influences on cannabis withdrawal were described to be the same as those affecting cannabis abuse and dependence.114 Beyond that, the existence of the CWS has solid neurobiological underpinnings since it was found that the availability of brain CB1 receptor in cannabis dependents was inversely associated with the occurrence of CWS.70,71 After 4 weeks of abstinence, the anomalies of CB1 receptors binding had been normalized in cannabis dependents, thus giving a rough time frame of the duration of CWS,70,71 which apt to the clinical observations that have been obtained in the past 20 years (Table 1). Two different courses of CWS might result from the different contribution of cannabis residual symptoms assumed to be initially more prominent in the type A than in type B CWS.36 Looking at a key symptom of cannabis use, such as “increase in appetite,”5,7 this was indeed reported more often in the first days of the type A CWS (Table 1).19,20,35
Mood and behavioral symptoms, namely, insomnia, dysphoria, and anxiety, are the key symptoms of the CWS (Tables 1 and 2). Similar symptoms occurred in the obesity treatment with the CB-1 receptor antagonist rimonabant (also known as SR14171) and were the reason why rimonabant was withdrawn from the market in 2008.115 Possibly, a “sustained CWS” had been precipitated when the effects of the physiological tone of endogenous endocannabinoids on brain and peripheral CB1 receptors were impeded by receptor antagonists, even in non-addicted but susceptible individuals. In support, the neurocircuitries involved in the regulation of stress, anxiety, and mood (such as the serotonergic, noradrenergic, and dopaminergic systems) were demonstrated to be sensitive to CB-1 receptor antagonists.115
Influence of alcohol and tobacco
Regular alcohol drinking might influence the clinical expression of the CWS, and this is not through the overlapping alcohol withdrawal symptoms. Continuous exposure to ethanol, in either cell culture or rodent models, led to an increase in endocannabinoid levels that resulted in downregulation of the CB1 receptor and uncoupling of this receptor from downstream G protein signaling pathways.116 A similar downregulation of CB1 receptors was found in multiple brain regions of chronic drinkers.116 Alcoholic drinks were reported to be co-used by 33%–46% of regular cannabis users. The rates of co-use for cocaine, stimulants, and hallucinogens were 37%–43%, 30%–52%, and 36%–42%, respectively1,2,116 – all putatively being able to influence the course and intensity of the CWS. Approximately 90% of cannabis users are also tobacco smokers, possibly reflecting the common route of administration, and even synergistic and compensatory actions of cannabis and tobacco as well as genetic and epigenetic factors assumed to mediate addiction vulnerability.117,118 More specifically, smoking tobacco use was shown to increase the number of cannabis dependence symptoms119 and precipitated cannabis relapse.120 Vice versa, cannabis use decreased the likelihood of abstaining from tobacco.117,118 There is a preliminary evidence that simultaneous tobacco and cannabis abstinence predicts better psychosocial treatment outcomes.117,118 There is still a paucity of clinical studies on this important subject, although alcohol, tobacco, and cannabis were consistently identified to be the substances with earliest onset of use, the highest prevalence of lifetime use, and the highest prevalence of lifetime disorder.1–6,74
Choice of treatment setting
In comparison with outpatient programs, inpatient detoxifications can provide strict abstinence conditions and, thus, can be used to better differentiate CWS from comorbidity, but are much more expensive and usually not the first choice of patients seeking treatment due to CUD. However, 1) the inability to initiate cannabis abstinence due to bothersome CWS, 2) the continuous co-use of other harmful drugs of dependence, or 3) the coexistence of other disabling psychiatric or somatic complaints give reasons for the medical necessity of an inpatient detoxification program, the duration of which depends on the intensity of the withdrawal symptoms and concomitant complaints.5,113 At this juncture, the duration of an inpatient detoxification program of heavy cannabis users is recommended to be not less than 14 days, ideally 21 days, taken into account that their pure CWS itself usually lasted up to 14–21 days (Table 1),12,13,36,113 and the diagnosing of potentially underlying comorbidity is more sensitive from then on. It remains a challenge of future in-depth studies to compare the impact of outpatient and inpatient treatment programs on the long-term course and disability of substance use disorders, which applies to CUD, too.
Influence of high potency cannabis preparations, gender, and so on
Similar to the cannabis addiction syndrome itself,121 one of its hallmark, the CWS, is based upon complex interactions between drug-induced neurobiological changes, environmental factors, genetic and epigenetic factors, comorbidity, personality traits, gender influences, and stress responsivity, all of which contributing to the high inter- and intrapersonal variations in the composition, annoyance, and duration of the CWS (Table 1).12,13,114,121
In addition to an increasing awareness of the existence of the CWS, its increasing emergence in the last 20 years might result from the increasing psychotropic potency of the used marijuana originating from the breeding of strains with high THC (10%–18.5%) and low cannabidiol concentrations (<0.15%) being found especially in high-income countries.1,88–90,122 In the Netherlands, the recent cannabidiol content of imported resin was ~7%.90 According to animal experiments, cannabidiol can counterbalance some adverse effects of cannabis,61 and in patient populations, there is mounting evidence of anticonvulsive and antipsychotic properties of cannabidiol.123 Since the early 1950s, it is known that the chemical composition of the resin itself varies with cannabidiol activities between 0% and 50% depending on the provenance of the drug.87 Whether the users of more potent cannabis strains adjust their intake according to the potency is still unclear.88 However, there is first evidence that the occurrence of first-episode psychosis as well as the intensity of the CUD increased alongside the use of high potency cannabis preparations.124,125 This throws an extremely critical light on emerging modern cannabis ingestion methods (“dabbing” or “cannavaping” of cannabis concentrates with 20%–80% THC) used by individuals seeking a more rapid and even bigger than being possible with smoking flowers that THC contents are usually in the range of 2% and 6%.126,127 Marijuana users who had turned to “dabbing” reported higher tolerance and withdrawal experiences.126
The CWS could have an measurement bias regarding a recent finding that it was endorsed more likely by the US than by Dutch cannabis users, which applies to other CUD criteria, such as tolerance, and gender effects on CWS, too.77 In this context, recent studies revealed a consistent gender impact on CWS, because women experienced a stronger CWS (Table 1)36,76 and were shown to have a greater susceptibility to developing CUD than men.121,128 Women were also found to be more sensitive to the cannabis than men.121,128 Remarkably, women reported physical CWS complaints more likely, such as nausea and stomach pain (Table 1).19,76
CWS in the ICD-11 Beta Draft
In 2018, the 11th revision of the ICD-11 is planned to be published. The so-called Beta-Draft of the chapter about “Mental and Behavioral Disorders” is already available online at http://apps.who.int/classifications/icd11/browse/f/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f637576511 (accessed November 25, 2016).33 The current version of this ICD-11 Beta Draft33 lists the usual mood and behavioral CWS symptoms according to DSM-531 but does not consider physical CWS symptoms.33 We recommend to include at least “nausea” and “stomach pain” into the final version because these symptoms were recently found to be more prominent in the female CWS,76,77,121,128 and yet, it seems likely that the increasing use of high potency cannabis preparations are associated with more physical CWS symptoms. It is also recommended to include a note on the high intra- and interpersonal variability of the CWS intensity and the observation that – if a CWS occurs – it is extra distressing between the first and the third week after quitting a frequent, heavy, or prolonged cannabis use (Table 1).12,13,36 Heavy users were shown to experience a CWS whose average severity is comparable to the burden of a moderate depression or moderate alcohol withdrawal syndrome.36 In outpatient settings, the average discomfort of CWS was similar to that of tobacco withdrawal.14,79
Certainly, it awaits future study whether the inhalation of very potent cannabis concentrates126,127 is indeed associated with a further decrease of psychosocial functioning, higher comorbidity, and a stronger CUD and CWS – eventually with more physical features (eg, hyperalgesia, nausea, sweating, tremor, flu-like symptoms)31 than occurring after the cessation of a heavy or prolonged use of traditional non-concentrated cannabis preparations.
Conclusion
The CWS is a criterion of CUDs (DSM-5) and cannabis dependence (DSM-IV-R, ICD-10). Several lines of evidence from human studies indicate that cessation from long-term and regular cannabis use precipitates a specific withdrawal syndrome with mainly mood and behavioral symptoms of light to moderate intensity, which can usually be treated in an outpatient setting. However, comorbidity with mental or somatic disorders, severe CUD, and low social functioning may require an inpatient treatment (preferably a qualified detox) and post-acute rehabilitation or long-term outpatient care. There are promising results with gabapentin and THC analogs in the treatment of CWS. Mirtazapine could improve insomnia, and venlafaxine was found to worsen the CWS. Certainly, further research is required with respect to the impact of the CWS treatment setting on long-term CUD prognosis and with respect to psychopharmacological or behavioral approaches, such as aerobic exercise therapy or psychoeducation, in the CWS treatment. The preliminary up-to-date content for the ICD-1133 (intended to be finally published in 2018) is recommended to be expanded by physical CWS-symptoms, the specification of CWS severity and duration as well as gender effects.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.UNDOC (United Nations Office on Drugs and Crime) World Drug Report 2015. [Accessed September 28, 2016]. (United Nations publication, Sales No. E.15.XI.6). Available from: https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 2.EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) European Drug Report 2015. Trends and Developments. Lux-embourg Publication Office of the European Union 2015. [Accessed September 28, 2016]. Available from: http://www.emcdda.europa.eu/attachements.cfm/att_239505_EN_TDAT15001ENN.pdf.
- 3.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72(12):1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt L, Ferrari AJ, Calabria B, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8(10):e76635. doi: 10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoch E, Bonnet U, Thomasius R, Ganzer F, Havemann-Reinecke U, Preuss UW. Risks associated with the non-medicinal use of cannabis. Dtsch Arztebl Int. 2015;112(16):271–278. doi: 10.3238/arztebl.2015.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pabst A, Kraus L, Gomes de Matos E, Piontek D. Substanzkonsum und substanzbezogene Störungen in Deutschland im Jahr 2012. Sucht. 2013;59:321–331. [Google Scholar]
- 7.Paton WD. Cannabis and its problems. Proc R Soc Med. 1973;66(7):718–721. [PubMed] [Google Scholar]
- 8.Fraser JD. Withdrawal symptoms in cannabis-indica addicts. Lancet. 1949;2(6582):747. doi: 10.1016/s0140-6736(49)92263-1. [DOI] [PubMed] [Google Scholar]
- 9.Bensuan AD. Marihuana withdrawal symptoms. Br Med J. 1971;3(5766):112. doi: 10.1136/bmj.3.5766.112-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesbeck GA, Schuckit MA, Kalmijn JA, Tipp JE, Bucholz KK, Smith TL. An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction. 1996;91(10):1469–1478. [PubMed] [Google Scholar]
- 11.Levin KH, Copersino ML, Heishman SJ, et al. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 13.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19(3):233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 14.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Allsop DJ, Copeland J, Norberg MM, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33(11):1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103(5):787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. 2012;123:141–147. doi: 10.1016/j.drugalcdep.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendt M, Rosenberg R, Foldager L, Sher L, Munk-Jørgensen P. Withdrawal symptoms do not predict relapse among subjects treated for cannabis dependence. Am J Addict. 2007;16:461–467. doi: 10.1080/10550490701640985. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A, Pergadia ML, Lynskey MT. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? Am J Addict. 2008;17:199–208. doi: 10.1080/10550490802019519. [DOI] [PubMed] [Google Scholar]
- 22.Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copersino ML, Boyd SJ, Tashkin DP, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15(1):8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- 24.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- 25.Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58(10):917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- 26.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 27.Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8(4):483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 28.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141(4):395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 29.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berlin) 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haney M, Hart CL, Vosburg SK, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berlin) 2010;211(2):233–244. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.APA (American Psychiatric Association) Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5TM) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 32.Dilling H, Mombour W, Schmidt MH. Internationale Klassifikation psychischer Störungen. ICD-10 Kapitel V (F). Diagnostische Kriterien für Forschung und Praxis. Bern, Switzerland: Huber; 2004. [Google Scholar]
- 33.ICD-11 Beta Draft (Foundation) Cannabis withdrawal. [Accessed November 25, 2016]. Available from: http://apps.who.int/classifications/icd11/browse/f/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f637576511.
- 34.Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug Alcohol Depend. 2010;106:133–134. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Lee D, Schroeder JR, Karschner EL, et al. Cannabis withdrawal in chronic, frequent smokers during sustained abstinence within a closed residential environment. Am J Addict. 2014;23:234–242. doi: 10.1111/j.1521-0391.2014.12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N. Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and -metabolites in serum. Drug Alcohol Depend. 2014;143:189–197. doi: 10.1016/j.drugalcdep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005;78(2):205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend. 1998;50(1):27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 39.Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. J Am Acad Child Adolesc Psychiatry. 2008;47(2):174–178. doi: 10.1097/chi.0b013e31815cdd73. [DOI] [PubMed] [Google Scholar]
- 40.Soenksen S, Stein LA, Brown JD, Stengel JR, Rossi JS, Lebeau R. Cannabis withdrawal among detained adolescents: exploring the impact of nicotine and race. J Child Adolesc Subst Abuse. 2015;24(2):119–124. doi: 10.1080/1067828X.2013.770379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swift W, Hall W, Teesson M. Characteristics of DSM-IV and ICD-10 cannabis dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Drug Alcohol Depend. 2001;63(2):147–153. doi: 10.1016/s0376-8716(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 42.Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents’ treatment response and outcomes: a 12-month prospective investigation. J Addict Med. 2014;8(5):359–367. doi: 10.1097/ADM.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.APA (American Psychiatric Association) Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 44.PubMed US National Library of Medicine National Institutes of Health Search database. [Accessed November 25, 2016]. Available from: https://www.ncbi.nlm.nih.gov/pubmed.
- 45.Scopus . Document search. Elsevier; [Accessed November 25, 2016]. Available from: https://www.scopus.com/home.uri. [Google Scholar]
- 46.Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, McGuire PK. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: a critical review of neuroimaging studies. Curr Pharmaceut Des. 2012;18:5045–5054. doi: 10.2174/138161212802884636. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzetti V, Solowij N, Yücel M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. 2016;79(7):e17–e31. doi: 10.1016/j.biopsych.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther. 2006;111(1):114–144. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Manjarrez-Marmolejo J, Franco-Pérez J. Gap junction blockers: an overview of their effects on induced seizures in animal models. Curr Neuropharmacol. 2016;14(7):759–771. doi: 10.2174/1570159X14666160603115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cone EJ, Huestis MA. Relating blood concentrations of terahydrocan-nabinoland metabolites to pharmacologic effects and time of marijuana usage. Ther Drug Monit. 1993;15:527–532. doi: 10.1097/00007691-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Laprevote V, Gambier N, Cridlig J, et al. Early withdrawal effects in a heavy cannabis smoker during hemodialysis. Biol Psychiatry. 2015;77(5):e25–e26. doi: 10.1016/j.biopsych.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Muramatsu RS, Silva N, Ahmed I. Suspected dronabinol withdrawal in an elderly cannabis-naïve medically ill patient. Am J Psychiatry. 2013;170(7):804. doi: 10.1176/appi.ajp.2013.13010059. [DOI] [PubMed] [Google Scholar]
- 54.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2015;40(11):2489–2498. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balter RE, Cooper ZD, Haney M. Novel pharmacologic approaches to treating cannabis use disorder. Curr Addict Rep. 2014;1(2):137–143. doi: 10.1007/s40429-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macfarlane V, Christie G. Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 2015;34(2):147–153. doi: 10.1111/dar.12225. [DOI] [PubMed] [Google Scholar]
- 57.Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014;97(1):55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampson CS, Bedy SM, Carlisle T. Withdrawal seizures seen in the setting of synthetic cannabinoid abuse. Am J Emerg Med. 2015;33(11):1712.e3. doi: 10.1016/j.ajem.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 59.Hegde M, Santos-Sanchez C, Hess CP, Kabir AA, Garcia PA. Seizure exacerbation in two patients with focal epilepsy following marijuana cessation. Epilepsy Behav. 2012;25(4):563–566. doi: 10.1016/j.yebeh.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Crippa JA, Hallak JE, Machado-de-Sousa JP, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38(2):162–164. doi: 10.1111/jcpt.12018. [DOI] [PubMed] [Google Scholar]
- 61.Niesink RJ, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnet U. Abrupt quitting of long-term heavy recreational cannabis use is not followed by significant changes in blood pressure and heart rate. Pharmacopsychiatry. 2016;49(1):23–25. doi: 10.1055/s-0035-1565242. [DOI] [PubMed] [Google Scholar]
- 63.Gorelick DA, Goodwin RS, Schwilke E, et al. Antagonist-elicited cannabis withdrawal in humans. Clin Psychopharmacol. 2011;31(5):603–612. doi: 10.1097/JCP.0b013e31822befc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauchard E, Goncharov O, Krupitsky E, Gorelick DA. Cannabis withdrawal in patients with and without opioid dependence. Subst Abuse. 2014;35(3):230–234. doi: 10.1080/08897077.2014.898605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology. 2015;40(11):2489–2498. doi: 10.1038/npp.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padula CB, McQueeny T, Lisdahl KM, Price JS, Tapert SF. Craving is associated with amygdala volumes in adolescent marijuana users during abstinence. Am J Drug Alcohol Abuse. 2015;41(2):127–132. doi: 10.3109/00952990.2014.966198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 68.Aleksić D, Aksić M, Radonjić NV, et al. Long-term effects of maternal deprivation on the volume, number and size of neurons in the amygdala and nucleus accumbens of rats. Psychiatr Danub. 2016;28(3):211–219. [PubMed] [Google Scholar]
- 69.Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35(6):644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1-receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Souza DC, Cortes-Briones JA, Ranganathan M, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(1):60–67. doi: 10.1016/j.bpsc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filbey FM, Dunlop J, Ketcherside A, et al. fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Hum Brain Mapp. 2016;37(10):3431–3443. doi: 10.1002/hbm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102–110. doi: 10.1016/j.addbeh.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richmond-Rakerd LS, Slutske WS, Lynskey MT, et al. Age at first use and later substance use disorder: shared genetic and environmental pathways for nicotine, alcohol, and cannabis. J Abnorm Psychol. 2016;125(7):946–959. doi: 10.1037/abn0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fosnocht AQ, Briand LA. Substance use modulates stress reactivity: behavioral and physiological outcomes. Physiol Behav. 2016;166:32–42. doi: 10.1016/j.physbeh.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23(6):415–421. doi: 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delforterie M, Creemers H, Agrawal A, et al. Functioning of cannabis abuse and dependence criteria across two different countries: the United States and the Netherlands. Subst Use Misuse. 2015;50(2):242–250. doi: 10.3109/10826084.2014.952445. [DOI] [PubMed] [Google Scholar]
- 78.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 79.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Busner J, Targum SD. The clinical global impression scale: applying a research tool in clinical practice. Psychiatry (Edgemont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 81.Chesney T, Matsos L, Couturier J, Johnson N. Cannabis withdrawal syndrome: an important diagnostic consideration in adolescents presenting with disordered eating. Int J Eat Disord. 2014;47(2):219–223. doi: 10.1002/eat.22229. [DOI] [PubMed] [Google Scholar]
- 82.Lam PW, Frost DW. Nabilone therapy for cannabis withdrawal presenting as protracted nausea and vomiting. BMJ Case Rep. 2014:2014. doi: 10.1136/bcr-2014-205287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonnet U, Chang DI, Scherbaum N. Cannabis-Hyperemesis-Syndrom [Cannabis hyperemesis syndrome] Fortschr Neurol Psychiatr. 2012;80(2):98–101. doi: 10.1055/s-0031-1273372. German. [DOI] [PubMed] [Google Scholar]
- 85.Heise L. Cannabinoid hyperemesis syndrome. Adv Emerg Nurs J. 2015;37(2):95–101. doi: 10.1097/TME.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 86.Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol. 1981;18:353–366. doi: 10.3109/15563658108990042. [DOI] [PubMed] [Google Scholar]
- 87.Grlic L. A comparative study on some chemical and biological characteristics of various samples of cannabis resin. UNDOC; 1962. [Accessed November 25, 2016]. Available from: https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1962-01-01_3_page005.html. [Google Scholar]
- 88.Cressey D. The cannabis experiment. Nature. 2015;524(7565):280–283. doi: 10.1038/524280a. [DOI] [PubMed] [Google Scholar]
- 89.Swift W, Wong A, Li KM, Arnold JC, McGregor IS. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLoS One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niesink RJ, Rigter S, Koeter MW, Brunt TM. Potency trends of Δ9-tetrahydrocannabinol, cannabidiol and cannabinol in cannabis in the Netherlands: 2005–15. Addiction. 2015;110(12):1941–1950. doi: 10.1111/add.13082. [DOI] [PubMed] [Google Scholar]
- 91.Braun B, Künzel J, Brand H. Deutsche Hauptstellen für Suchtfragen eV, editor Jahrbuch Sucht 2016. Lengerich, Germany: Pabst Verlag; 2016. Kapitel 31: Jahresstatistik 2014 der professionellen Suchtkrankenhilfe; pp. 173–199. [Google Scholar]
- 92.Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Copeland J, Pokorski I. Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst Abuse Rehab. 2016;7:41–53. doi: 10.2147/SAR.S89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelly MA, Pavlicova M, Glass A, et al. Do withdrawal-like symptoms mediate increased marijuana smoking in individuals treated with venlafaxine-XR? Drug Alcohol Depend. 2014;144:42–46. doi: 10.1016/j.drugalcdep.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2013;18(6):993–1002. doi: 10.1111/j.1369-1600.2012.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mariani JJ, Pavlicova M, Mamczur AK, Bisaga A, Nunes EV, Levin FR. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280–284. doi: 10.3109/00952990.2014.884102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71(3):281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 100.Allsop DJ, Lintzeris N, Copeland J, Dunlop A, McGregor IS. Cannabinoid replacement therapy (CRT): nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Ther. 2015;97(6):571–574. doi: 10.1002/cpt.109. [DOI] [PubMed] [Google Scholar]
- 101.Trigo JM, Lagzdins D, Rehm J, et al. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend. 2016;161:298–306. doi: 10.1016/j.drugalcdep.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Indorato F, Liberto A, Ledda C, Romano G, Barbera N. The therapeutic use of cannabinoids: forensic aspects. Forensic Sci Int. 2016;265:200–203. doi: 10.1016/j.forsciint.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 103.Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2015;40(11):2489–2498. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Budney AJ, Vandrey RG, Stanger C. Pharmacological and psychosocial interventions for cannabis use disorders. Rev Bras Psiquiatr. 2010;32(Suppl 1):S46–S55. [PMC free article] [PubMed] [Google Scholar]
- 106.Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117(1):38–44. doi: 10.1016/j.drugalcdep.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herrmann ES, Cooper ZD, Bedi G, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl) 2016;233(13):2469–2478. doi: 10.1007/s00213-016-4298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooper ZD. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatry Rep. 2016;18(5):52. doi: 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davis JP, Smith DC, Morphew JW, Lei X, Zhang S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: a prospective study. J Drug Issues. 2016;46(1):64–83. doi: 10.1177/0022042615616431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allsop DJ, Dunlop AJ, Saddler C, Rivas GR, McGregor IS, Copeland J. Changes in cigarette and alcohol use during cannabis abstinence. Drug Alcohol Depend. 2014;138:54–60. doi: 10.1016/j.drugalcdep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 111.Koola MM, Boggs DL, Kelly DL, et al. Relief of cannabis withdrawal symptoms and cannabis quitting strategies in people with schizophrenia. Psychiatry Res. 2013;209(3):273–278. doi: 10.1016/j.psychres.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn-Miller MO. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict. 2013;22(3):277–284. doi: 10.1111/j.1521-0391.2012.12018.x. [DOI] [PubMed] [Google Scholar]
- 113.Bonnet U, Specka M, Scherbaum N. Häufiger Konsum von nicht-medizinischem Cannabis [Frequent non-medical cannabis use: health sequelae and effectiveness of detoxification treatment] Dtsch Med Wochenschr. 2016;141(2):126–131. doi: 10.1055/s-0041-106313. German. [DOI] [PubMed] [Google Scholar]
- 114.Verweij KJ, Agrawal A, Nat NO, et al. A genetic perspective on the proposed inclusion of cannabis withdrawal in DSM-5. Psychol Med. 2013;43(8):1713–1722. doi: 10.1017/S0033291712002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gamaleddin IH, Trigo JM, Gueye AB, et al. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front Psychiatry. 2015;6:41. doi: 10.3389/fpsyt.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henderson-Redmond AN, Guindon J, Morgan DJ. Roles for the endocannabinoid system in ethanol-motivated behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:330–339. doi: 10.1016/j.pnpbp.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107(7):1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rabin RA, George TP. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am J Addict. 2015;24(2):105–116. doi: 10.1111/ajad.12186. [DOI] [PubMed] [Google Scholar]
- 119.Ream GL, Benoit E, Johnson BD, et al. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 2008;95:199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haney M, Bedi G, Cooper ZD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry. 2013;73:242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fattore L. Considering gender in cannabinoid research: a step towards personalized treatment of marijuana addicts. Drug Test Anal. 2013;5(1):57–61. doi: 10.1002/dta.1401. [DOI] [PubMed] [Google Scholar]
- 122.Bonnet U. Rauschzustände: Risiken und Nebenwirkungen. Im Focus: nicht-medizinisches Cannabis und synthetische Cannabinoide [States of intoxication: risks and adverse effects: focus on non-medical cannabis and synthetic cannabinoids] Suchttherapie. 2016;17(2):61–70. German. [Google Scholar]
- 123.Leweke FM, Mueller JK, Lange B, Rohleder C. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604–612. doi: 10.1016/j.biopsych.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 124.Di Forti M, Marconi A, Carra E, et al. Proportion of patients in South London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2(3):233–238. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- 125.Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med. 2015;45:3181–3189. doi: 10.1017/S0033291715001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Loflin M, Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict Behav. 2014;39(10):1430–1433. doi: 10.1016/j.addbeh.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 127.Varlet V, Concha-Lozano N, Berthet A, et al. Drug vaping applied to cannabis: is “cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599. doi: 10.1038/srep25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Franke G. SCL-90-R. Die Symptomcheckliste von Derogatis – Deutsche Version – Manual (2.Auflage) Göttingen, Germany: Hogrefe; 2002. [Google Scholar]
- 130.Cohen J. Statistical Power for the Behavioral Sciences. New York: Academic Press; 1988. [Google Scholar]