Abstract
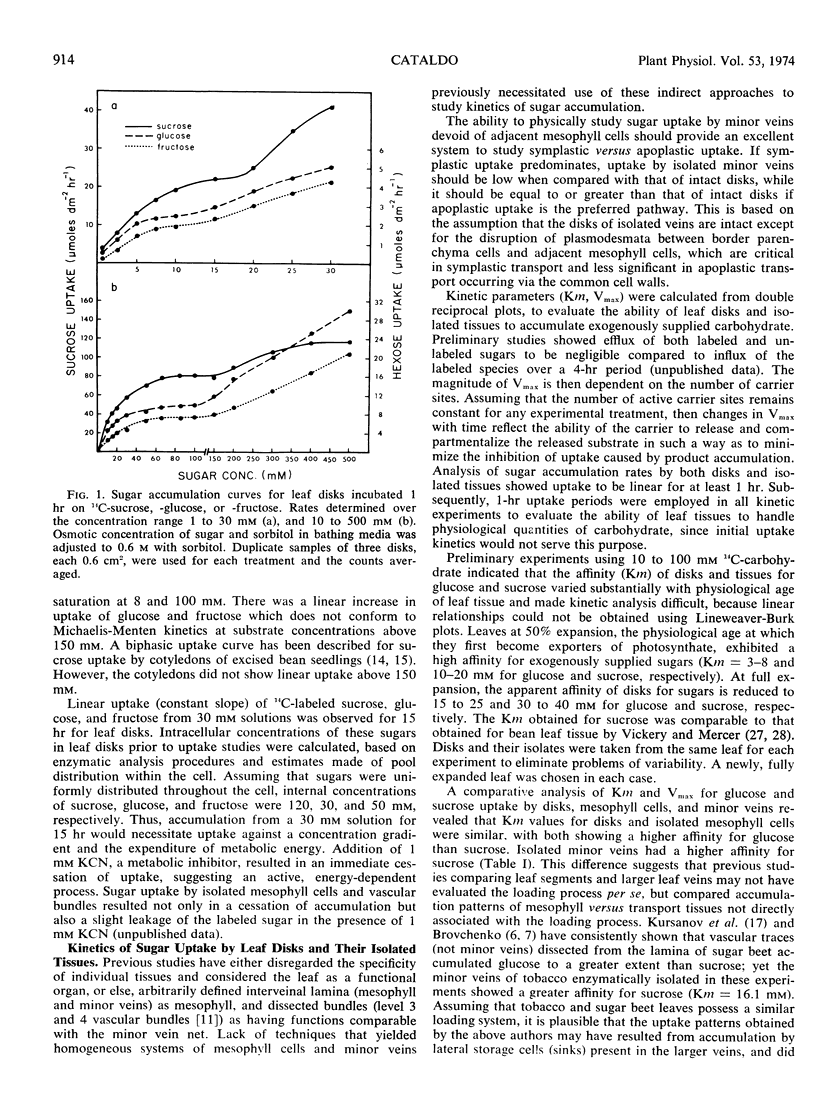
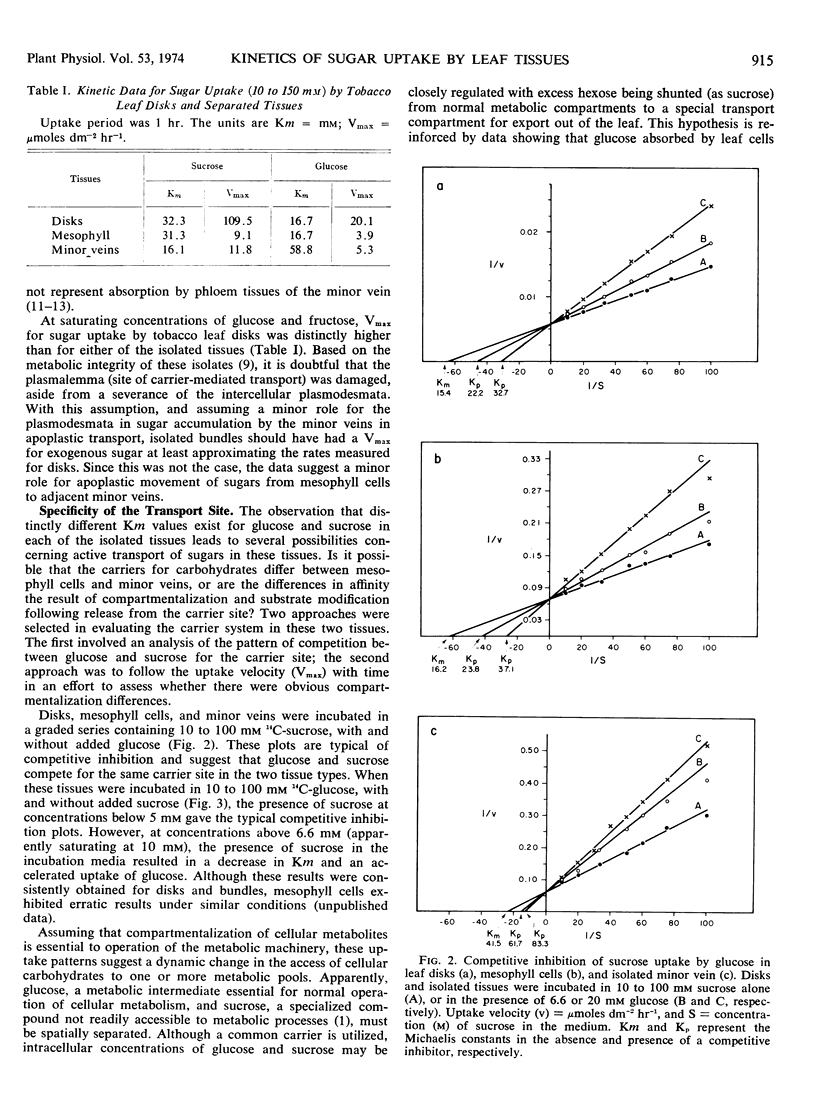
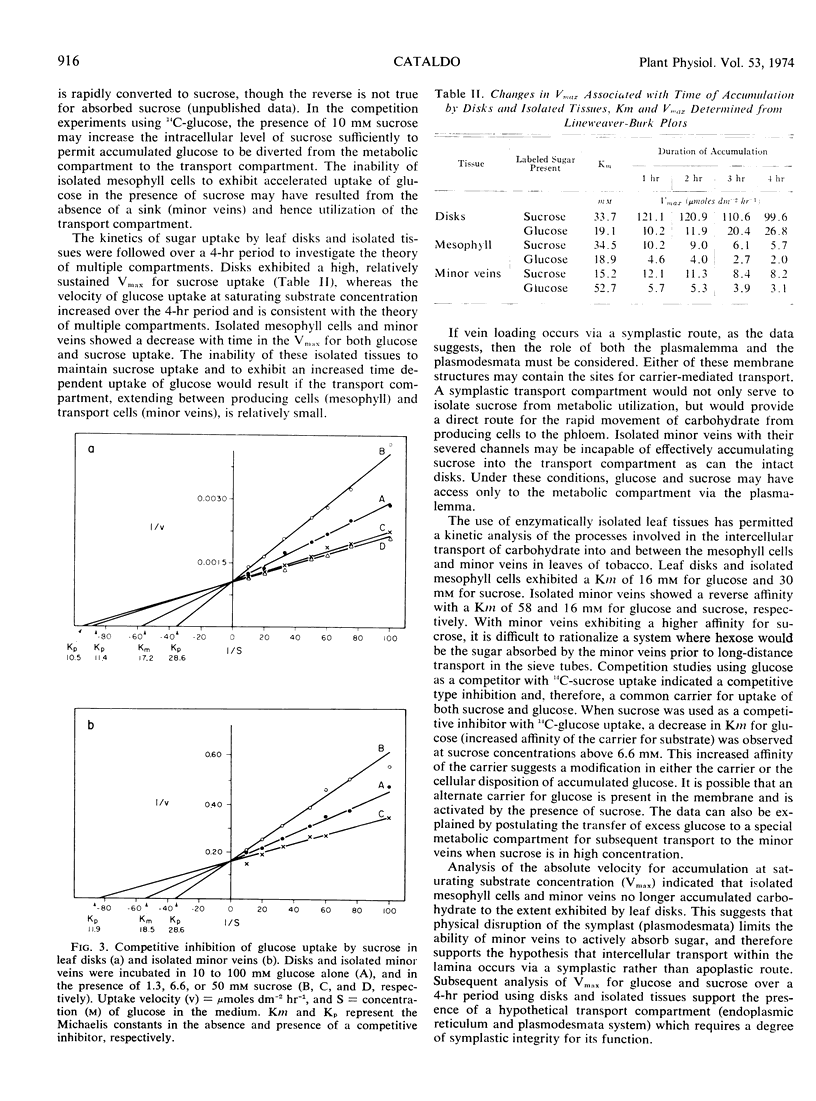
Enzymatically separated leaf tissues of Nicotiana tabacum L., exhibiting good metabolic integrity, were used to evaluate the kinetics of sugar accumulation over the concentration range of 10 to 100 mm. Mesophyll cells exhibited Km values of 16 and 30 mm for glucose and sucrose, respectively; minor veins showed a reverse relationship, with Km values of 58 and 16 mm for glucose and sucrose, respectively. This would suggest that sucrose is preferentially absorbed by the minor vein net. Analysis of Vmax data indicates a reduction in the ability of isolated minor veins to accumulate substrate, implicating a symplastic rather than apoplastic route for intercellular transport. Competition studies demonstrate a common carrier for sucrose and glucose in both tissue types and suggest the presence of a “transport compartment,” entry to which is regulated by a critical intracellular sucrose concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. The selection of sucrose as the translocate of higher plants. J Theor Biol. 1968 Oct;21(1):13–20. doi: 10.1016/0022-5193(68)90056-8. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J. E. Sugar transport in immature internodal tissue of sugarcane: I. Mechanism and kinetics of accumulation. Plant Physiol. 1972 Jan;49(1):82–86. doi: 10.1104/pp.49.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Cataldo D. A. Leaf structure and translocation in sugar beet. Plant Physiol. 1969 Jan;44(1):45–54. doi: 10.1104/pp.44.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar Uptake and Translocation in the Castor Bean Seedling II. Sugar Transformations During Uptake. Plant Physiol. 1967 Feb;42(2):174–180. doi: 10.1104/pp.42.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar uptake and translocation in the castor bean seedling I. Characteristics of transfer in intact and excised seedlings. Plant Physiol. 1967 Feb;42(2):161–173. doi: 10.1104/pp.42.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzki A., Thom M. Membrane transport of sugars in cell suspensions of sugarcane: I. Evidence for sites and specificity. Plant Physiol. 1972 Feb;49(2):177–182. doi: 10.1104/pp.49.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. The regulation of sugar uptake and accumulation in bean pod tissue. Plant Physiol. 1966 Jan;41(1):181–189. doi: 10.1104/pp.41.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T. The symplast concept. A general theory of symplastic transport according to the thermodynamics of irreversible processes. J Theor Biol. 1970 Feb;26(2):181–214. doi: 10.1016/s0022-5193(70)80012-1. [DOI] [PubMed] [Google Scholar]
- Webb J. A., Gorham P. R. Translocation of Photosynthetically Assimilated C in Straight-Necked Squash. Plant Physiol. 1964 Jul;39(4):663–672. doi: 10.1104/pp.39.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]


