Abstract
BACKGROUND
VYC-17.5L (17.5 mg/mL hyaluronic acid, 0.3% lidocaine) is a dermal filler intended for deep dermis injection for the treatment of skin depressions.
OBJECTIVE
To evaluate 12-month effectiveness and safety of VYC-17.5L for the treatment of moderate/severe nasolabial folds (NLFs).
METHODS
Subjects ≥18 years old with moderate/severe NLFs were recruited (N = 70). Injected volume was aimed at achieving optimum correction; top-up treatment was given at 2 weeks if needed. The primary endpoint was investigator-assessed change in NLF severity over 12 months using the validated photonumeric NLF Severity Scale. Secondary endpoints included investigator- and subject-assessed satisfaction and safety. Adverse events judged to be more severe or prolonged than routinely observed were recorded.
RESULTS
Sixty-five subjects completed study requirements. Mean volume injected was 3.0 ± 1.0 mL for both NLFs combined. Significant improvement was maintained in investigator-assessed NLF severity at 12 months, and investigators and subjects reported high satisfaction with VYC-17.5L throughout the study. Two unexpected adverse events were reported: (1) redness, swelling, and decreased sensitivity (resolved after 4 days) and (2) swelling (resolved after 48 hours); neither event was serious or life threatening.
CONCLUSION
VYC-17.5L is effective and well tolerated for the treatment of moderate to severe NLFs for 1 year.
To attain a more youthful appearance, men and women commonly seek treatment for their nasolabial folds (NLFs).1,2 These folds may appear as early as the first decade of life, and by the fourth decade, prevalence has been reported to be as high as 85%.3 Correcting NLFs positively influences an individual's level of attractiveness,1,2 well being,4 quality of life,5 self-esteem,5 and projected first impressions, including improvements in perceptions of social skills and success.6 Individuals frequently request treatment in this area, despite NLF improvement that occurs after mid-face volumizing.
VYC-17.5L (Juvéderm Volift with Lidocaine; Allergan plc, Irvine, CA) is a hyaluronic acid (HA) dermal filler intended for deep dermis injection for the treatment of skin depressions such as NLFs. It is formulated with 17.5 mg/mL of low and high molecular weight HA that is crosslinked using a proprietary manufacturing process (VYCROSS), which gives rise to its performance characteristics, including lift capacity, moldability, and tissue integration. The HA formulation also includes 0.3% lidocaine to enhance the patient's comfort during the procedure and reduce or eliminate the need for additional pain relieving agents.
Biocompatibility tests showed VYC-17.5L to be suitable to be in contact with tissue for sustained periods (Data on file, Allergan plc, 2015). In addition, in vitro experiments comparing VYC-17.5L with products with a similar composition and indication have demonstrated that VYC-17.5L has suitable rheological and extrusion properties for clinical use.7
The purpose of this observational study was to evaluate the safety and effectiveness of VYC-17.5L for the treatment of moderate to severe NLFs in a clinical setting. As this was the first postmarket clinical trial evaluating VYC-17.5L, it was considered exploratory in nature; therefore, no formal clinical hypothesis was tested. A range of subjective endpoints were selected to broadly characterize the effectiveness of VYC-17.5L. In addition, investigator and subject satisfaction with the aesthetic outcome and investigator satisfaction with the ease of use of the filler were assessed.
Methods
This was a prospective, open-label, multicenter, observational, postmarket study evaluating VYC-17.5L for the treatment of moderate to severe NLFs. Assessments were conducted: (1) at baseline, (2) after initial treatment (Day 0), (3) 2 weeks after initial treatment, (4) after top-up (performed at Day 14, as needed), (5) 1 month after last treatment, (6) 9 months after last treatment, and (7) 12 months after last treatment. Repeat treatment was offered to subjects at Month 12 with a follow-up visit at 1 month; however, this article will focus on the data collected from the initial and top-up treatments only. The study was registered at www.clinicaltrials.gov (NCT #01680497) and approved by applicable human subjects institutional review boards. Subjects provided signed informed consent forms before enrollment.
Subjects
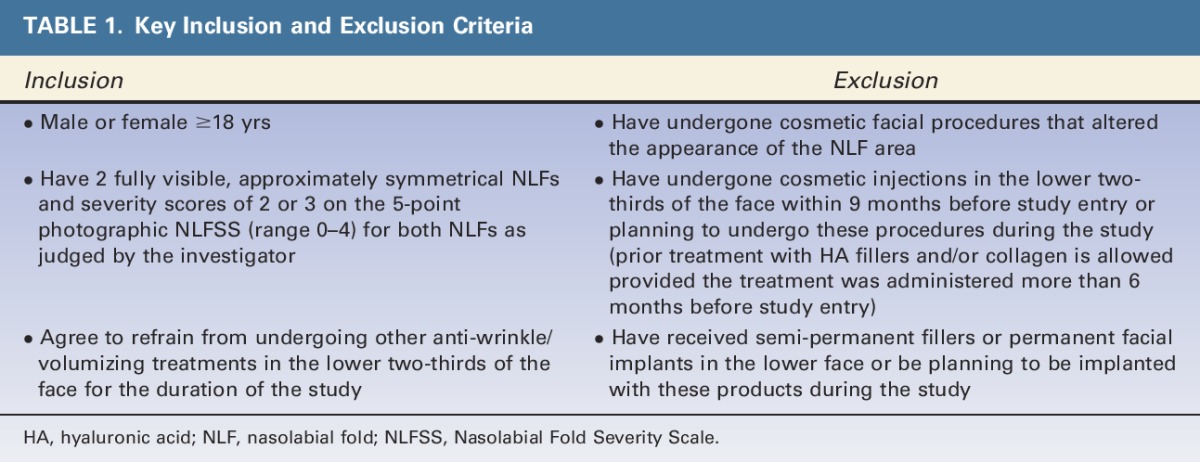
Male and female subjects aged 18 years or older with moderate to severe NLFs were recruited at 3 European centers in Germany and the Netherlands between June 2012 and January 2013. As this study was mainly descriptive in nature, a sample size power calculation was not performed. A conservative 15% dropout rate was assumed; therefore, investigators aimed to enroll 70 subjects to retain at least 60 subjects. Each site aimed to recruit 23 subjects. Key inclusion and exclusion criteria are listed in Table 1.
TABLE 1.
Key Inclusion and Exclusion Criteria
Study Procedures
Subjects were treated with VYC-17.5L in both NLFs. The VYC-17.5L package contained a syringe filled with 1 mL of product and two 30 G ½” needles. To reflect real-world clinical practice, needle/cannula type, injected volume, injection technique, and massage after injection were at the investigators' discretion and guided by the Directions for Use. No ice, topical anesthetics, or nerve block were used. The investigators performed 1 treatment at Day 0. Fourteen days after the initial injection, subjects returned to the clinic for the first follow-up visit. The investigators subjectively assessed if optimum correction had been achieved or if top-up treatment was needed at this visit; top-up treatment followed the same procedures as described for the initial treatment. Follow-up visits occurred 14 days after the initial treatment, and 1, 9, and 12 months after the last treatment. Investigators and subjects completed safety, effectiveness, and satisfaction questionnaires at all follow-up visits. Data collection ended in March 2014.
Measures and Statistics
The primary endpoint was to evaluate investigator-assessed mean change of NLF severity from baseline to Month 12 using the validated 5-point photonumeric NLF Severity Scale (NLFSS) (Data on file, Allergan plc, 2012). The NLFSS scale is defined as 0 = None (No wrinkle), 1 = Mild (Shallow, just perceptible wrinkle), 2 = Moderate (Moderately deep wrinkle), 3 = Severe (Deep wrinkle, well-defined edges but not overlapping), and 4 = Extreme (Very deep wrinkle, redundant fold, and overlapping skin).
Secondary effectiveness endpoints measured by the NLFSS were also assessed. Investigator-assessed mean change of NLF severity from baseline to all other follow-up visits were reported (i.e., after initial/top-up treatment, Month 1, and Month 9). Responder rates at all follow-up visits were also reported. Responders were defined as those who had ≥1 point improvement in NLF severity. In addition, subjects assessed these same NLFSS endpoints.
Investigators and subjects assessed satisfaction with the aesthetic outcome after the initial and top-up (if performed) treatments and at all follow-up visits on an 11-point scale with response options ranging from −5 (Definitely not) to 5 (Definitely). The investigators were asked: (1) Are you satisfied with the result?, (2) Was the result what you expected?, and (3) Do you think that the product gave a smooth finish? Subjects were asked: (1) Do you feel attractive?, (2) Do you feel confident?, and (3) Do you feel comfortable in social situations? Mean change from baseline to Month 1, Month 9, and Month 12 after last treatment was assessed for these subject-assessed satisfaction endpoints. At Month 12, subjects were asked to indicate whether they would like to be injected with the same product again and whether they would recommend the product to others. Additionally, subjects indicated how long it took them to return to normal activities after receiving treatment (<24 hours, 1–3 days, 4–8 days, 9–14 days, or >14 days).
Investigators rated ease of use after the initial and top-up injections. They were asked how they would rate the: (1) ease of injection, (2) extrusion force, and (3) moldability/malleability. Response options ranged from 0 (Easy) to 10 (Hard) on an 11-point scale.
Statistical analyses were performed, and data appendices were created using SAS version 9.2 (Cary, NC). Comparisons from baseline to Month 12 were performed using Wilcoxon Signed-Rank Test. Statistical tests were 2-sided with α = 0.05 for significance.
Safety Endpoints
Safety was monitored throughout the study. All adverse events (AEs) and serious adverse events (SAEs) judged to be more severe or more prolonged than routinely observed were recorded. After initial and top-up treatments, subjects reported pain, swelling, and bruising on an 11-point scale ranging from 0 (None) to 10 (Worst imaginable).
Results
Subjects
Investigators enrolled 70 subjects in the study (94% female, mean age: 49 ± 10, age range: 26–71) (Table 2). Three subjects had major protocol deviations and 2 were lost to follow-up (Figure 1); these subjects were not included in the 12-month effectiveness or satisfaction endpoint analyses. All enrolled subjects were included in the safety analyses (i.e., any adverse event that occurred before loss to follow-up was included in the safety analyses). Eleven subjects did not complete the 12-month questionnaire because of a clerical data collection error.
TABLE 2.
Subject Characteristics (N = 70)
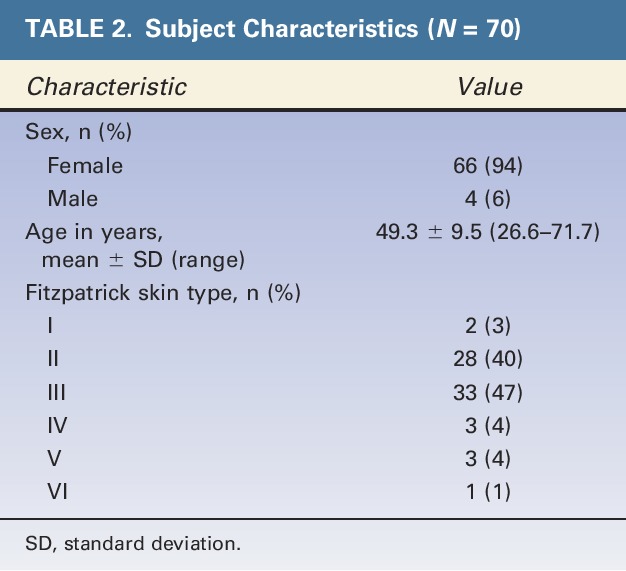
Figure 1.
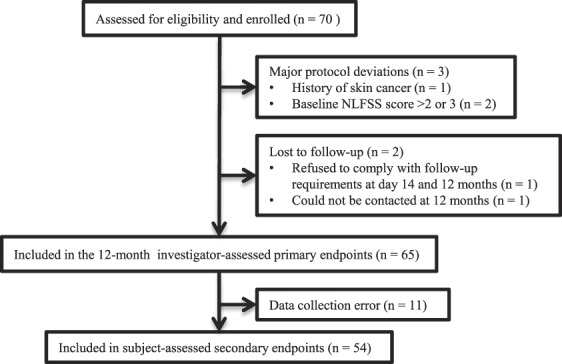
Participant flow chart.
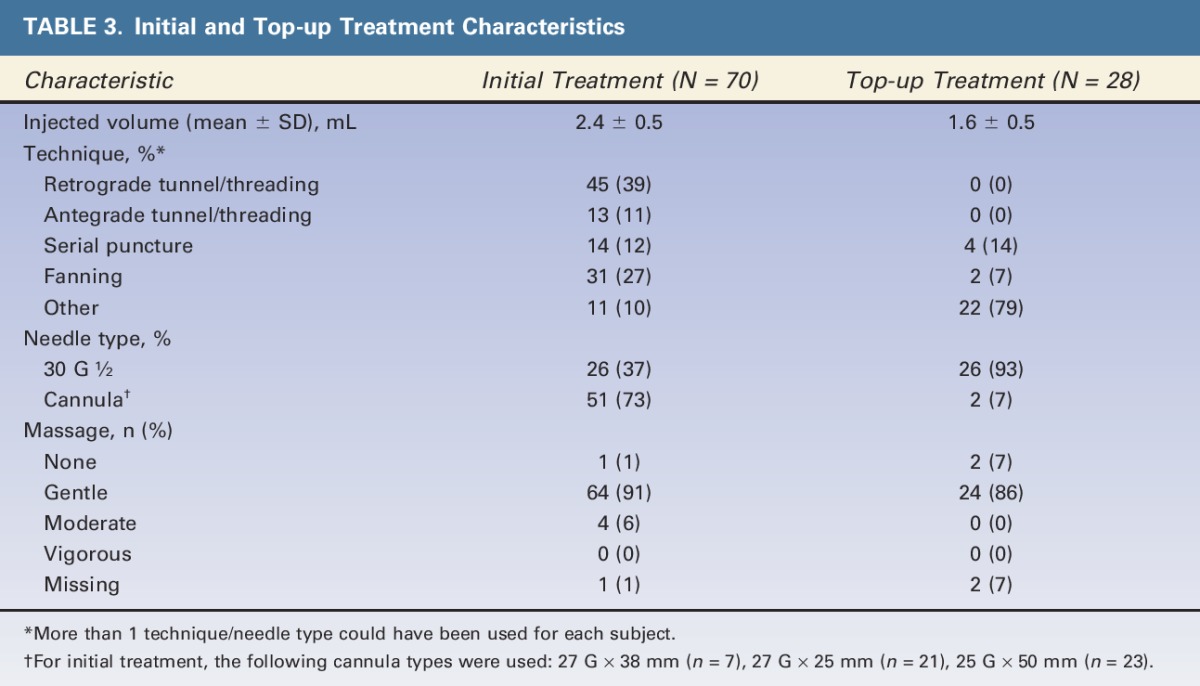
Seventy subjects received initial treatment at baseline, and 40% (n = 28) received top-up treatment at Day 14. Table 3 shows the treatment characteristics for both the right and left NLFs combined. Injected volume to achieve optimum correction, including both initial and top-up treatment, ranged between 2.0 and 6.0 mL (mean = 3.0 ± 1.0 mL) for both NLFs. At initial treatment, Retrograde Tunneling/Threading was the most frequently performed injection technique for both sides. The majority of injection techniques for top-up were reported as Other. Other mainly included the Depot technique (injection into one single plane), the Tower technique (injection through a perpendicular approach to the deep tissue plane with a gradual tapering of product deposition as the needle is withdrawn), and the Vertical Supraperiosteal Depot technique (small aliquots of filler injections deposited on the periosteum for maximum support).8,9 A majority of needles used for initial treatment were cannulas (73%) (27 G × 38 mm [n = 7], 27 G × 25 mm [n = 21], and 25 G × 50 mm [n = 23]); whereas for top-up treatment, standard 30 G ½” needles were mainly used (93%). Gentle massage was performed for most patients at initial and top-up treatments.
TABLE 3.
Initial and Top-up Treatment Characteristics
Primary Endpoint
From baseline to Month 12, there was significant improvement in mean NLF severity for the right (mean change: −0.8 ± 0.7) and left (mean change: −0.8 ± 0.8) NLFs (p < .0001 for both NLFs) as assessed by the investigator (Figure 2).
Figure 2.
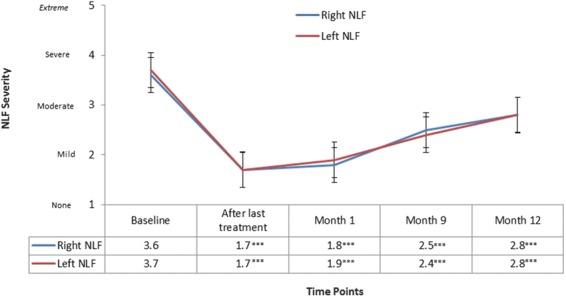
Mean investigator-assessed NLF severity by time point for right and left NLFs. Significant differences between baseline and each time point are shown (p < .001***). NLF, nasolabial fold.
Secondary Effectiveness Endpoints
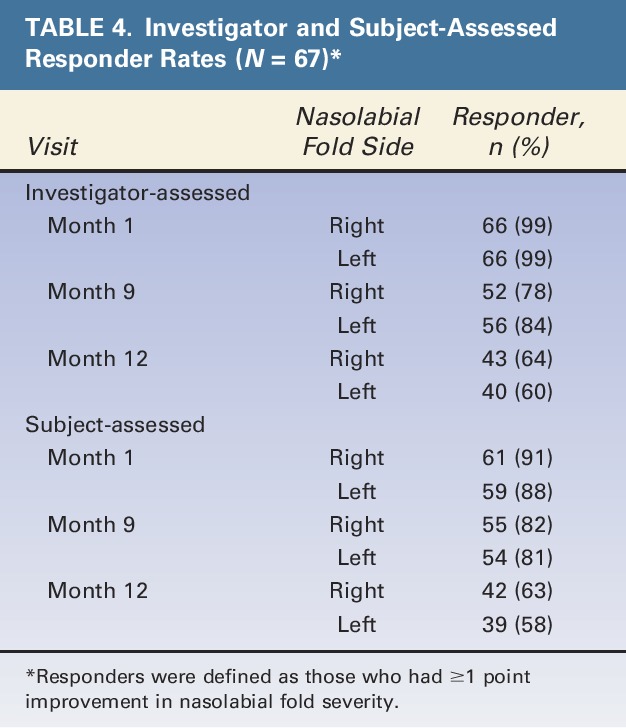
Investigators reported significant improvement in mean NLF severity from baseline to all other time points assessed (i.e., after last treatment, Month 1, and Month 9) (Figure 2). Subject-assessed mean NLFSS scores were similar to the investigator-assessed results at all time points. In addition, investigator and subject-assessed responder rates were high at Month 1 and Month 9 (Table 4). At Month 12, responder rates slightly decreased, but the majority of subjects still had ≥1 point improvement in their NLFs.
TABLE 4.
Investigator and Subject-Assessed Responder Rates (N = 67)*
Satisfaction Endpoints
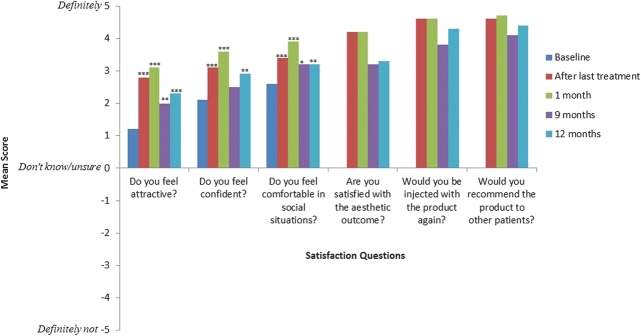
Investigators and subjects indicated high satisfaction with the treatment at all follow-up visits. Mean investigator scores for the question Are you satisfied with the aesthetic outcome? ranged from 4.0 ± 1.0 to 4.6 ± 0.6 (scores could range from −5 = Definitely not to 5 = Definitely). At baseline, most subjects felt somewhat attractive, confident, and comfortable in social situations, and these scores significantly increased from baseline to all follow-up visits (Figure 3). Scores for the question Do you feel confident? were significantly higher at all follow-up visits except at Month 9. Additionally, the majority of subjects (82.1%, N = 67) needed less than 24 hours to return to normal activities after receiving treatment. Only 4 subjects indicated that they needed 4 to 8 days to return to normal activities.
Figure 3.
Subject satisfaction mean scores stratified by time point. Significant differences were assessed between baseline and each time point as appropriate (p < .05*, p < .01**, p < .001***).
Investigator-Rated Ease of Use
Investigators considered the injection treatment Easy after initial and top-up treatments. After initial treatment, the mean scores were as follows: ease of injection = 1.2 ± 1.3, extrusion force = 1.2 ± 1.4, and ease of molding/malleability = 1.1 ± 1.1 (scores could range from 0 = Easy to 10 = Hard). All questions at top-up treatment had a mean score of 0.9 ± 0.5.
Safety Endpoints
Mean scores for subject-reported pain, swelling, and bruising were generally low after both initial and top-up treatments (results could range from 0 = None to 10 = Worst imaginable). Pain was rated as 2.0 ± 1.8 for both sides after initial treatment and decreased to 0.8 ± 1.7 (right) and 0.7 ± 1.5 (left) after Day 14. Pain at top-up was slightly lower: 1.5 ± 1.8 (right) and 1.6 ± 1.8 (left). Swelling was rated as 1.7 ± 1.6 for both sides after initial treatment and decreased to 1.5 ± 2.2 (right) and 1.4 ± 2.2 (left) after Day 14. Swelling at top-up was similar to initial treatment: 1.8 ± 1.4 (right) and 1.9 ± 1.5 (left). Bruising was rated as 0.3 ± 0.9 for both sides after initial treatment and slightly increased to 0.9 ± 1.9 (right) and 0.5 ± 1.3 (left) after Day 14. Bruising at top-up was similar to initial treatment: 0.4 ± 0.8 (right) and 0.5 ± 0.9 (left).
Two AEs were reported; neither event was serious or life threatening. One subject reported redness around the mouth, swelling, and decreased sensitivity 1 day after receiving 1 mL of the product. The subject's symptoms were resolved after using corticosteroid ointment for 4 days. The second AE was described as swelling in the injection area that began 1 hour after receiving 2 mL of the product. Symptoms resolved within 48 hours without additional treatment. This subject had been diagnosed with urticaria in the past and should have been excluded from participation in the study, but he/she had not informed the investigator of this diagnosis.
Discussion
This is the first postmarket study to evaluate VYC-17.5L for the treatment of moderate to severe NLFs. Results indicate that VYC-17.5L is well tolerated and effective for up to 12 months. In addition, investigators and subjects were highly satisfied with the treatment.
The primary endpoint analysis revealed that VYC-17.5L provides a long-lasting filling effect of NLFs up to 1 year after treatment. This sustained correction may be attributed to the crosslinking of low molecular weight HA used in the VYC-17.5L formulation, a manufacturing process that creates a gel with a high level of resistance to degradation.10 In addition, investigators indicated that VYC-17.5L was easy to use (i.e., ease of injection, extrusion force, moldability/malleability). This may be because the VYC-17.5L formulation also contains high molecular weight HA, which contributes to cohesivity and malleability.7,11 The combination of low and high molecular weight HA in VYC-17.5L produces a gel with rheological properties suited to treat facial wrinkles and folds such as NLFs.
Investigators and subjects also were highly satisfied with the treatment at all time points. Subject-assessed mean scores for feeling attractive, confident, and comfortable in social situations significantly increased at all time points after treatment compared with baseline. These results are consistent with previous research indicating that treatment with a dermal filler for correction of NLFs improves psychosocial outcomes, including quality of life and self-esteem.4,5 At all follow-up visits, the majority of subjects indicated that they would like to be injected with the product again and also would recommend the product to others. Most subjects returned to normal activities within 24 hours after treatment, indicating that the treatment had only a minimal impact on their daily routine.
Subjects tolerated VYC-17.5L well. Results indicate that pain, swelling, and bruising after the treatments were generally low. The most likely reason for the low pain scores is that the VYC-17.5L formulation includes lidocaine. Although subject assessment of pain, swelling, and bruising showed that expected injection responses did occur, only 2 events were considered abnormal and reported as AEs. One patient, who had a history of urticaria that was not disclosed during screening, had swelling in the injection area; and the second patient had redness around the mouth with swelling. Neither AE was considered serious.
This study assessed the safety and effectiveness of VYC-17.5L across multiple time points using numerous investigator and patient-assessed outcomes. Although this study enrolled participants across a wide age range, only 6% were men and most were Fitzpatrick skin Type II and III. This study had a high subject retention rate (97%), with only 2.9% lost to follow-up; however, satisfaction data from 11 subjects were lost due to a clerical error. As this was the first postmarket study to assess VYC-17.5L, an open-label design was used, which may introduce the possibility of certain biases. Future studies could strive to include blinding and enroll a more diverse population.
VYC-17.5L is highly effective in treating moderate to severe NLFs with results lasting up to 1 year. In addition, VYC-17.5L was well-tolerated by subjects, investigators rated the product as easy to use, and investigator and subjects were highly satisfied with the aesthetic outcome through 1 year. These results can be attributed to the proprietary formulation and manufacturing process of VYC-17.5L that provides the appropriate rheological properties needed to treat NLFs. As fillers are uniquely suited to treat specific indications and regions of the face, aesthetic clinicians can use the results of this study to help create tailored treatment algorithms and long-term treatment plans to attain optimal treatment outcomes for their patients.
Footnotes
This study was funded by Allergan plc, Dublin, Ireland. G. Sattler is a lecturer, study investigator, and scientific advisory board member for Allergan plc. W.G. Philipp-Dormston and H. Van Den Elzan are lecturers, medical consultants, study investigators, and scientific advisory board members for Allergan plc. C. Van Der Walt, M. Nathan, and B. Dhillon are former Allergan, Ltd. employees. J. Kolodziejczyk, G. Kerson are employees of Allergan plc and may own equity in the company.
References
- 1.Pallua N, Wolter TP. A 5-year assessment of safety and aesthetic results after facial soft-tissue augmentation with polyacrylamide hydrogel (Aquamid): a prospective multicenter study of 251 patients. Plast Reconstr Surg 2010;125:1797–804. [DOI] [PubMed] [Google Scholar]
- 2.Tzikas TL. A 52-month summary of results using calcium hydroxylapatite for facial soft tissue augmentation. Dermatol Surg 2008;34(Suppl 1):S9–S15. [DOI] [PubMed] [Google Scholar]
- 3.Guinot C, Malvy DJM, Ambroisine L, Latreille J, et al. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol 2002;138:1454–60. [DOI] [PubMed] [Google Scholar]
- 4.De Arruda LH, Rocha FT, Rocha A. Studying the satisfaction of patients on the outcome of an aesthetic dermatological filler treatment. J Cosmet Dermatol 2008;7:246–50. [DOI] [PubMed] [Google Scholar]
- 5.De Aquino MS, Haddad A, Ferreira LM. Assessment of quality of life in patients who underwent minimally invasive cosmetic procedures. Aesthet Plast Surg 2013;37:497–503. [DOI] [PubMed] [Google Scholar]
- 6.Dayan SH, Arkins JP, Gal TJ. Blinded evaluation of the effects of hyaluronic acid filler injections on first impressions. Dermatol Surg 2010;36(Suppl 3):1866–73. [DOI] [PubMed] [Google Scholar]
- 7.Bernardin A, Pierre S, Pather S, Martinez A, et al. Vycross: an innovative dermal filler technology. Anti-Aging Medicine European Congress; May 31, 2013; Paris, France.
- 8.Bartus CL, Sattler G, Hanke CW. The tower technique: a novel technique for the injection of hyaluronic acid fillers. J Drugs Dermatol 2011;10:1277–80. [PubMed] [Google Scholar]
- 9.Sattler G, Birgit W. Tower technique of filler injection. In: Carruthers J, Carruthers A, Dover JS, Alam M, editors. Soft Tissue Augmentation: Procedures in Cosmetic Dermatology Series (3rd ed). Elsevier Health Sciences; 2013. [Google Scholar]
- 10.Muhn C, Rosen N, Solish N, Bertucci V, et al. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: a Canadian overview. Clin Cosmet Investig Dermatol 2012;5:147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hee CK, Garrett ST, Narurkar V, Bernardin A, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg 2015;41(Suppl 1):S373–381. [DOI] [PubMed] [Google Scholar]