Abstract
Thyroid hormones (THs) are transported across cell membranes by different transmembrane transporter proteins. In previous studies, we showed marked 3,3′-diiodothyronine (3,3′-T2) but moderate T3 uptake by the L-type amino acid transporter 2 (Lat2). We have now studied the structure-function relationships of this transporter and TH-like molecules. Our Lat2 homology model is based on 2 crystal structures of the homologous 12-transmembrane helix transporters arginine/agmatine antiporter and amino acid/polyamine/organocation transporter. Model-driven mutagenesis of residues lining an extracellular recognition site and a TH-traversing channel identified 9 sensitive residues. Using Xenopus laevis oocytes as expression system, we found that side chain shortening (N51S, N133S, N248S, and Y130A) expanded the channel and increased 3,3′-T2 transport. Side chain enlargements (T140F, Y130R, and I137M) decreased 3,3′-T2 uptake, indicating channel obstructions. The opposite results with mutations maintaining (F242W) or impairing (F242V) uptake suggest that F242 may have a gating function. Competitive inhibition studies of 14 TH-like compounds revealed that recognition by Lat2 requires amino and carboxylic acid groups. The size of the adjacent hydrophobic group is restricted. Bulky substituents in positions 3 and 5 of the tyrosine ring are allowed. The phenolic ring may be enlarged, provided that the whole molecule is flexible enough to fit into the distinctly shaped TH-traversing channel of Lat2. Taken together, the next Lat2 features were identified 1) TH recognition site; 2) TH-traversing channel in the center of Lat2; and 3) switch site that potentially facilitates intracellular substrate release. Together with identified substrate features, these data help to elucidate the molecular mechanisms and role of Lat2 in T2 transport.
Transmembrane transporters are essential for substrate exchange between different cell types, including substrate transport from blood stream into target cells. It has been thought that thyroid hormones (THs) can pass the cell membrane via passive diffusion, despite early evidence for carrier-mediated T3 uptake into hepatocytes (1). At the end of the 20th century, the first transporters were characterized that are involved in TH transport (2). Nevertheless, details of the molecular transport mechanisms for TH across cell membranes are still unclear. There are 3 known types of TH transmembrane transporters (THTTs) at the blood-brain barrier (3, 4). The first identified TH transporters are organic anion transporting polypeptide (OATP)2 und OATP3 (2). Transport of TH was subsequently demonstrated for monocarboxylate transporter (MCT)8 (5) and MCT10 (6) and also for L-type amino acid transporter (LAT)1 and LAT2 (7, 8). For MCT8, TH specificity for T3 and T4 has been shown (5). Pathogenic mutations of MCT8 are known to cause Allan-Herndon-Dudley syndrome, leading to severe psychomotor retardation (9). Moreover, studies with Mct8-knockout (KO) mice showed its importance for TH transport (10, 11), and Lat2 has been suggested to compensate for this defect in TH transport (12). In contrast to single KO mice (MCT8), double KO mice (MCT8/LAT2) did not show increased T3 levels and expression of T3 target genes in the cerebral cortex, so that it was assumed that Lat2 is important for the uptake of T3 in the cerebral cortex (11).
It has been predicted that all 3 types of THTT contain 12 transmembrane helices (TMHs) (13–16). Despite their similar topology, the initial studies indicate that the different types of THTT employ different molecular transport mechanisms. Experimental crystal structures at atomic resolution are currently not available for the TH transporting proteins. We employed the structure of a solute carrier (SLC)1 homolog, the glycerol-3-phosphate transporter (GlpT) (PDB, 1PW4) from Escherichia coli (17), as a structural template to generate a homology model for MCT8 (15, 18).
Although OATPs exhibit different substrate interaction sites from MCT8, the sequence similarity to the GlpT structure has also been used by others to create a homology model for Oatp1c1 (19). Oatp1c1 exhibits preferential T4 and rT3 transport (20). Because LAT2 differs in sequence from MCT8 and OATP1C1, other structural templates are necessary to estimate its structural properties. Thus homology modeling for different transporters with differing transport mechanisms requires different templates for the crystal structures (reviewed in Ref. 13). The structure of the leucine transporter LeuT from Aquifex aeolicus (SLC6 homolog) also comprises 12 TMHs but is dissimilar to the structure of the GlpT (21). LeuT has been extensively studied and 4 different conformational states have been identified (22); it exhibits less sequence similarity to LAT2. The arginine/agmatine antiporter (AdiC) (23, 24) and the amino acid/polyamine/organocation (APC) transporter (ApcT) are also members of the SLC7 class; their crystal structures do exhibit a fold similar to LeuT, but the sequences are more similar to LAT2 (25). Little is yet known about the heterodimeric transmembrane transporter protein made up of the light chain LAT2 (SLC7A8) and the glycosylated single-pass heavy chain CD98 (SLC3A2). The latter protein associates with the 12-TMH spanning polypeptide LAT2. CD98 makes up most of the extracellular part of the transporter and thus acts as an escort protein for the transport of LAT2 into the cell membrane (26, 27). LAT2 functions as an ion independent exchanger and, in comparison with LAT1, it has a broader substrate specificity for neutral and aromatic amino acids, including small and large amino acids (28, 29). The transport of these amino acids through LAT transporters can be inhibited by the low molecular weight compound 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) (30). For LAT1 and LAT2, no crystal structure is available. It was also unclear how LAT2 transports TH and interacts with TH and other substrates. Both LAT1 and LAT2 are mainly expressed in brain, spleen, and placenta (28, 31, 32), and high levels of LAT2 are found in the kidney, with lower levels in the liver (28, 29). This expression suggests that LAT2 has a role in the renal reabsorption of neutral amino acids (28), and murine Lat2 is thought to have a role in T3 delivery to neurons in mice during the perinatal period (11). It has been reported that LAT1 transports 3,3′-diiodothyronine (3,3′-T2), T3, and rT3 (7, 33–35). We have recently shown that Lat2 transports 3,3′-T2; transport of T3 is less effective and rT3 and T4 are not transported at all (36).
Our objective in the present study is to gain insight into the mechanisms of substrate binding, recognition and transport of LAT2. We therefore investigated the structure-function relationships of both murine Lat2, by identification of amino acids involved in TH uptake, and of the substrates, by characterizing the molecule features needed for translocation of TH and derivatives.
By combining molecular interaction models, site-directed mutagenesis and functional characterization in Xenopus laevis oocytes, we explored determinants for extracellular substrate recognition, a potential traversing channel of Lat2 and important substrate properties that enable uptake by Lat2.
Materials and Methods
Materials
3,3′-T2, L-amino acids (Arg, Phe, and Tyr), and BCH were purchased from Sigma. 3-Iodo-L-thyronine (3-T1) and 3-iodo-thyroacetic acid were kindly provided by Henning Berlin GmbH. 3-Iodo-thyronamine was synthesized by Dr R. Smits, ABX Advanced Biochemical Compounds, and purified by HPLC by Dr R. Thoma (Formula GmbH). Iodo-L-thyronines were dissolved in dimethyl sulfoxide. Hartmann Analytic GmbH supplied us with 3,3′-[T2-125I]-diiodo-L-thyronine. Compounds for competitive inhibition studies were purchased as indicated in Table 1. All other reagents were purchased from Sigma.
Table 1.
Compounds Used for Competitive Inhibition Studies
| Compound | Chemical Name | Supplier |
|---|---|---|
| S1 | 3,4-dihydroxy-L-phenylalanine | Sigma-Aldrich |
| S3 | 3,5-bis(trifluoromethyl)-DL-phenylalanine | Apollo Scientific |
| S4 | 3-amino-L-tyrosine dihydrochloride monohydrate | InterBioScreen |
| S5 | 2-amino-3- (3,5-dibromo-4-hydroxyphenyl)propanoic acid | Key Organics |
| S6 | 2-amino-2-cyclopropylpropanoic acid | Key Organics |
| S7 | 3,5-diiodo-L (-)-tyrosine | Labotest KG |
| S9 | 1-amino-3-methylcyclohexane-1-carboxylic acid | Uorsy |
| S10 | 1-amino-5-methyl-2- (propan-2-yl)cyclohexane-1-carboxylic acid | Uorsy |
| S11 | 1-amino-2-methylcyclohexane-1-carboxylic acid | Uorsy |
| S12 | 2-[1-(aminomethyl)cyclohexyl]acetic acid | Uorsy |
| S13 | 3-fluoro-4- (2-hydroxyphenoxy)benzamide | Uorsy |
| S14 | 4- (4-hydroxyphenoxy)-3-methylbenzamide | Uorsy |
Site-directed mutagenesis
To replace amino acids, we followed 2 strategies; either 1) slight alterations to maintain as far as possible the properties of the side chains of Lat2 WT (eg, Asn to Ser; both are still hydrophilic); or 2) chimeric mutations introducing the respective LAT1 residue (N133S and Y130R). Lat2 mutants were cloned into the BamHI and XbaI site of the pTLB expression vector, which was kindly provided by T. J. Jentsch Leibniz-Institut für Molekulare Pharmakologie. Mutations were obtained by site-directed mutagenesis (Stratagene) with oligonucleotides listed in Table 2 and verified by sequencing.
Table 2.
Lat2 Oligonucleotides for Site-Directed Mutagenesis
| Mutation | Oligonucleotides (5′ to 3′) Wild Type | Oligonucleotides (5′ to 3′) Mutation |
|---|---|---|
| N51A | CATTGTAGGGAACATCATTGGC | CATTGTAGGGGCAATCATTGGC |
| N51S | CATTGTAGGGAACATCATTGGC | CATTGTAGGGTCAATCATTGGC |
| Y130R | GCTGGTGATCTACCCCACCAAG | GCTGGTGATCCGCCCCACCAAG |
| Y130A | CTGGTGATCTACCCCACCAAG | CTGGTGATCGCCCCCACCAAG |
| T132A | GTGATCTACCCCACCAACCAAGCTG | GTGATCTACCCCGCAAACCAAGCTG |
| N133A | GATCTACCCCACCAACCAAGCTGTCATC | GATCTACCCCACCAGTCAAGCTGTCATC |
| Q134T | CCCACCAACCAAGCTGTCATCG | CCCACCAACACAGCTGTCATCG |
| I137M | CAAGCTGTCATCGCCCTCACC | CAAGCTGTCATGGCCCTCACC |
| T140F | CGCCCTCACCTTCTCCAAC | CGCCCTCTTCTTCTCCAAC |
| Y144A | CTTCTCCAACTACGTGCTGCAGCC | CTTCTCCAACGCAGTGCTGCAGCC |
| K193A | CTTCACAGCTGGGAAGCTCCTGGC | CTTCACAGCTGGGGCACTCCTGGC |
| F242V | CAGGGCTCCTTTGCCTATGGTG | CAGGGCTCCGTTGCCTATGGTG |
| F242W | CAGGGCTCCTTTGCCTATGGTG | CAGGGCTCCTGGGCCTATGGTG |
| N248S | GGCTGGAACTTCCTTAATT | GGCTGGAGCTTCCTTAATT |
| F249A | GTGGCTGGAATTCCCTTAAT | GTGGCTGGAACGCCCTTAAT |
cRNA preparation and injection
Plasmid DNA (pTLB-Lat2-FLAG or pTLB-Lat2-FLAG variants and pTLB-CD98-His) was linearized by MluI (New England Biolabs) and transcribed into cRNA using the mMESSAGE mMACHINE kit (Life Technologies) according to the manufacturer's protocol. X. laevis oocytes were isolated by the group of T. J. Jentsch Leibniz-Institut für Molekulare Pharmakologie; 2-mg/mL collagenase A (Roche) in ORII buffer (100mM NaCl, 2mM KCl, 1mM MgCl2 hexahydrate, and 5mM HEPES; pH 7.5) was used to separate oocytes over 1.5 hours, including 6 buffer changes and washes in ND96 buffer (96mM NaCl, 2mM KCl, 1.8mM CaCl2 dihydrate, 1mM MgCl2 hexahydrate, and 5mM HEPES; pH 7.5). Stage V–VI oocytes were selected for microinjection of 9- to 46-ng cRNA per oocyte (4.5 or 23 ng each of Lat2 and CD98). Oocytes were incubated in ND96 buffer with 100-μg/mL amikacin (Alfa Aesar) at 17°C.
Uptake measurements
After 2 days of expression, groups of 10–20 oocytes were incubated 2 minutes in uptake buffer (100mM ChCl, 2mM KCl, 1mM MgCl2 hexahydrate, 1mM CaCl2 dihydrate, 10mM HEPES, 10mM Tris, and 0.1% BSA; pH 7.5) at room temperature, followed by incubation for 60 minutes in 600-μL tracer (uptake buffer with 100nM 3,3′-T2 and 0.1nM [125I]3,3′-T2). Oocytes were washed 6 times in ice-cold uptake buffer and uptake of [125I]3,3′-T2 was measured by a γ-counter (Wizard 1470; PerkinElmer) for individual oocytes. For competitive inhibition studies, 1mM test compounds or 100μM THs were dissolved in the tracer, and [125I]3,3′-T2 uptake was measured in absence and presence of test compounds. Experiments were performed at least twice.
Western blotting
Expression of Lat2 in oocytes of X. laevis was analyzed 2 days after coinjection (Lat2/CD98 or Lat2 mutants/CD98). Cells were lysed in homogenization buffer (250mM saccharose, 20mM HEPES, 1mM EDTA, and 1mM dithiothreitol; pH 7.4), treated with ultrasound and subsequently centrifuged (4000g, 20 min, 4°C). For Western blottings, equal amounts of the different cell lysates were loaded on an sodium dodecyl sulfate gel and further transferred onto a nitrocellulose membrane and incubated with the antibodies for Lat2 (immunoGlobe) or α-tubulin (Sigma-Aldrich GmbH). Immune reaction proteins were visualized using Enhanced Chemiluminescence (GE Healthcare), and their intensity was analyzed with ImageJ software (NIH). To quantify expression, the areas of the Lat2 bands were determined, and bands of α-tubulin were used as loading control; these were similar for each user. Experiments were performed at least twice. The level of protein expression in the whole cell extracts used for the Western blotting is not influenced by mutations, but an increase or decrease in transporter membrane insertion cannot be excluded.
Amino acid sequence alignment
A multiple sequence alignment was carried out with the help of BioEdit in 2 stages. The ClustalW algorithm was used to automatically align the sequences. This was followed by manual fine tuning to move multiple gaps in the aligned sequence. The amino acid sequence alignment of arginine/ agmatine antiporter AdiC (PDB, 3L1L), a member of the amino acid/ polyamine/ organocation (APC) superfamily ApcT (PDB, 3GI8), Lat2 (mouse) and Lat1 (mouse), was calculated with the BLOSOM62 similarity matrix using the software Geneious Basic 5.4.6 (Biomatters Ltd). Selection of the template is based on sequence similarity between Lat2 and AdiC of about 27%, whereas the overlap between Lat2 and ApcT is about 32%. The sequence identity is even lower. The match between Lat2 and AdiC is only 13% but slightly higher for Lat2 and ApcT with 18%. Nevertheless, analysis shows a high degree of structural similarity, even with low sequence identity of transmembrane proteins (37).
Homology modeling and refinement
An Lat2 comparative model was generated from 2 crystal structures. The construction was with reference to the homologous crystal structures of AdiC and ApcT with respect to their amino acid sequences and 3 dimensional structures. These proteins, as well as Lat1 and Lat2, are all members of the APC superfamily and share the 12 TMH structure. Additionally, they have a reentrant loop between TMH2 and TMH3 (38). The manual refinement of the chimeric model was performed according to sequence similarity and finger print features. Structure motifs or TMH were transferred from the outward facing protein ApcT into the structure of the substrate-bound occluded conformation of AdiC. A slight deviation of the α-helix dimension was observed for Lat2. Each of the 12-TM domains encompasses the next residues: THM1, Gly40–Leu66; TMH2, Gly72–Val97; TMH3, Leu116–Val145; THM4, Ser158–Trp173; TMH5, Arg180–Ile205; TMH6, Gly231–Val253; THM7, Lys262–Thr288; TMH8, Leu310–Ala344; TMH9, Pro363–Val379; THM10, Met383–Leu408; TMH11, Ser422–Trp442; and TMH12, Gly451–His470.
Fitting the loops was implemented with the Sybyl-X2 Protein Loop conformational search (Certara, Inc). This tool provides an ab initio loop method which uses the PRODAT database of known protein fragments. That implies closing gaps, adding residues or finding structures for loops which are totally different. Side chains and loops were repaired and optimized using the AMBER7 FF99 force fields and the Gasteiger-Marsili charges. Finally, the gradient was minimized until a terminal gradient of 0.1 kcal/(mol × Å) was attained. Images were created using Sybyl X2 and PyMOL v1.7.2 software.
Computational compound docking
The compounds in Table 1 were docked into the Lat2 putative recognition site. All dockings and optimizations were performed with Sybyl-X2 and energetically minimized using the AMBER7 FF99 force field.
Results
In order to combine in silico and in vitro studies to elucidate structure-function relationships of TH transport by Lat2, we considered variations in both the transporter protein Lat2 and the TH scaffold.
Generation of the Lat2 homology model indicates a substrate recognition site similar to AdiC
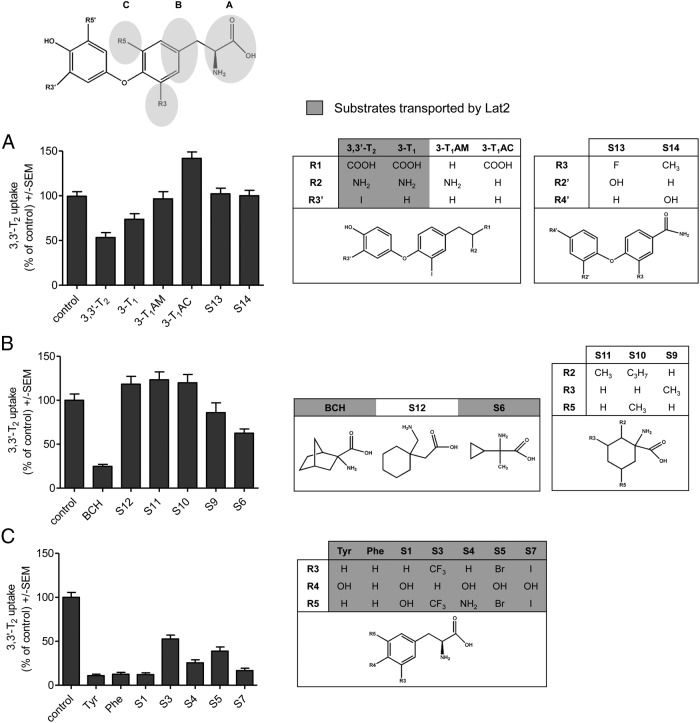
The Lat2 sequence shows sequence similarity of about 27% to the AdiC (PDB, 3L1L) (24) and of about 32% to ApcT, a member of the amino APC superfamily (PDB, 3GI8) (25) (sequence alignment in Supplemental Figure 1). For both antiporters, crystal structures exhibiting 12 TMHs are available. Therefore, it is proposed that Lat2 contains a membrane spanning light chain with 12 TMHs. However, unfortunately not all TMHs of Lat2 show sufficient sequence similarity to either of the 2 transporters. Because the 2 transporters exhibit an identical fold, the Lat2 model was created on the basis of these 2 crystal structures. The structural templates for helices 1–4 and 6–7 of Lat2 were chosen from the AdiC crystal structure and for helices 5 and 8–12 the corresponding structural fragments of the ApcT crystal structure were used. Highly conserved residues and fingerprint features, like 53IxGSGxF58 in the TMH1 and 242FSFxG246 in the TMH6, strongly suggest that the substrate recognition site is similar in AdiC and Lat2. Recognition of substrate (3,3′-T2) by Lat2 is therefore very probably similar to the recognition of arginine by AdiC, where the amino acid moiety is bound to the backbone via hydrogen bonds between TMH1 and TMH6 (Figure 1A). Consequently, an Lat2 model was generated where the amino acid moiety of 3,3′-T2 is equally bound to TMH1 and TMH6 (Figure 1B). The Lat2 model revealed amino acids for potential extracellular recognition and which formed a cavity/channel in the center of the Lat2 model, where the substrate traverses the cell membrane (Figures 2A and 3). Mutants of these residues have been characterized by uptake assays in X. laevis oocytes, to determine the substrate transport (29, 40, 41), and by expression analysis by Western blotting. The 3,3′-T2 uptake results, normalized to the respective total expression (Supplemental Figure 2), clearly show that residues Asn51, Tyr130, Thr132, Asn133, Ile137, Thr140, Lys193, Asn248, and Phe242 are involved in TH translocation through Lat2 (Figure 2). The results showed that shortening the side chains from asparagine to serine (N51S, N133S, and N248S) or Tyr130 to alanine, whereas retaining their hydrophilic or hydrophobic properties, enlarges the traversing channel. Consequently, increased uptake of 3,3′-T2 is observed (Figure 2B). In contrast, enlarging the side chain from Thr140 to phenylalanine, or from Tyr130 to arginine (Figure 3A) or from Ile137 to methionine, decreases 3,3′-T2 uptake (Figure 2B), indicating obstruction of a traversing channel in the center of Lat2. Mutation of Lys193 to alanine disturbs the potential stabilizing interaction between TMH1 and TMH8 (Figure 2A), and disrupts 3,3′-T2 transport. The traversing channel of Lat2 is not simply cylinder-like, but has 2 asymmetric hollows in our model. These are large enough for the passage of aromatic ring structures with bulky substituents, such as iodo-substituents in the 3,3′-position (Figure 3B), in 3,5-position (Figure 3 C), or in 3′,5′-position (Figure 3D) of T2-isomers or S3.
Figure 1. A, Crystal structure of the 12-TMH transporter AdiC, 3L1L (magenta), in the conformation of openward occluded state bound arginine (close up view, yellow). B, Homology model of the 12-TMH Lat2 light chain (green) and analogous binding site bound 3,3′-T2 (orange). The amino acid moiety of arginine and accordingly of 3,3′-T2 is bound to their recognition site, due to common fingerprint motifs (see Supplemental Figure 1), and is located between TMH1 and TMH6 via hydrogen bonds to the backbone.
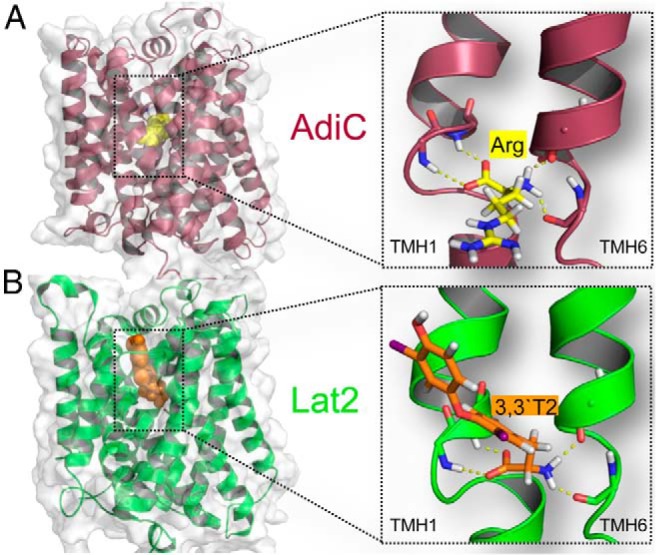
Figure 2. Mutation results of residues lining the potential TH-membrane-traversing channel in Lat2.
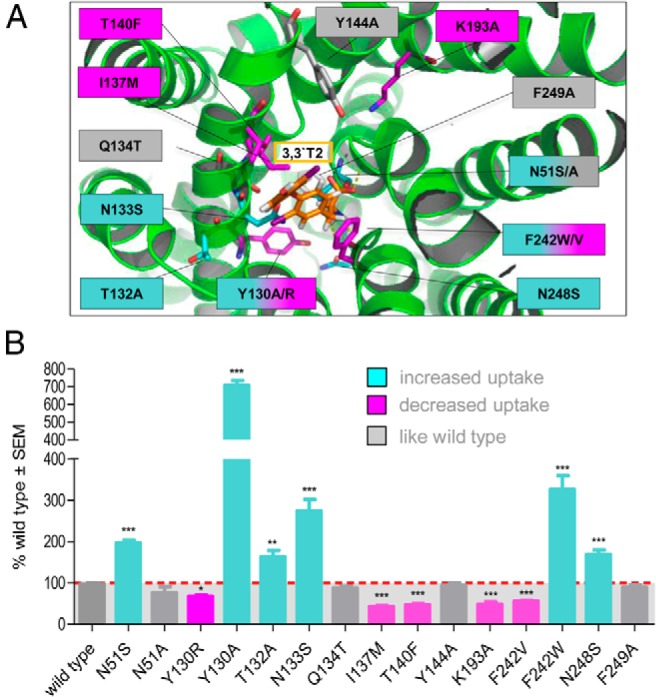
A, The Lat2 model indicates the putative locations of residues potentially involved in translocation of 3,3′-T2 (orange) covering the potential channel in the center of Lat2. The color of the selected residues and mutations corresponds to the color of the uptake bar diagram. B, 3,3′-T2 uptake (100nM) as percentage of wild type and normalized to the corresponding total expression of the different Lat2 variants that were coinjected with CD98 in X. laevis oocytes. Three different types of results are indicated; like wild type (gray), decreased (magenta) and increased 3,3′-T2 uptake (cyan). Data are representative of at least 3 independent transport experiments. Significance level is indicated with *, P < .01; **, P < .001; ***, P < .0001.
Figure 3. Lat2 homology model (green) bound 3,3′-T2 (orange) within the traversing channel (gray).
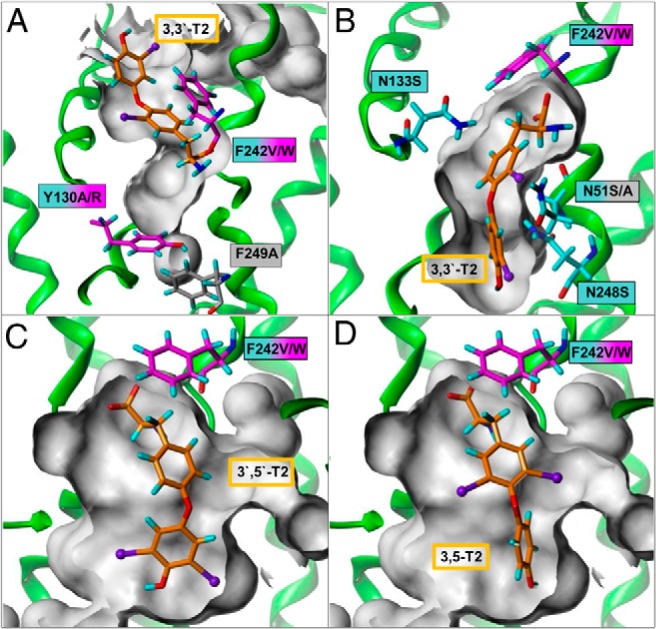
A, Asparagines lining the TH-traversing channel, side chain shortening led to channel enlargement and increased the TH uptake. Potential gate function of Phe242, here forming a lid covering 3,3′-T2 in the bound openward-occluded state. B, Side chain enlargement of Tyr130 to arginine obstructs the channel and results in a decrease in 3,3′-T2 uptake. Phe242 is interacts with the aromatic rings of 3,3′-T2 via π-system stacking of the extracellular open state. Asymmetric excavations in the traversing channel allow bulky substituents either (C) at the tyrosine ring 3′,5′-T2 or (D) at the phenyl ring for 3,5-T2.
Gating function of Phe242
Changing the aromatic properties of Phe242 to nonaromatic valine led to decreased 3,3′-T2 uptake, whereas mutation to tryptophan not only rescues but even increases 3,3′-T2 transport. Our model revealed, on the one hand, that Phe242 might support π-stacking interactions with the aromatic moiety of 3,3′-T2 for extracellular recognition. On the other hand, when 3,3′-T2 is bound into the traversing channel, the aromatic ring of Phe242 might act as a lid covering the channel (Figure 3, B–D). Thus Phe242 is probably involved in the gating of the substrate transport.
Distinct substrates competitively inhibit 3,3′-T2 uptake
A substructure search within a database of commercially available chemical compounds for TH-like scaffolds resulted in 512 compounds, from which the most promising 12 derivatives were manually chosen and ordered from the respective company.
Three types of substrate variants (Figure 4, A–C) have been used to inhibit competitively the uptake of 3,3′-T2 by Lat2. THs contain an amino and a carboxyl group. To reveal the impact of the amino acid group of TH-like substrates, they have been compared with 2 amides S13 and S14 and 2 molecules lacking 1 of the functional groups (3-iodo-thyronamine and 3-iodo-thyroacetic acid). Only when both groups of the amino acid moiety are available, is competitive inhibition of the T2 uptake by Lat2 observed (3-T1 and 3,3′-T2), whereas no other substrates exhibit competitive inhibition of T2 transport (Figure 4A).
Figure 4. Competitive inhibition of the transport by Lat2/CD98 using various substrates.
Amino acid-functional groups (A), proximate hydrophobic extension (B), and substituents in the 3 and 5 positions of the tyrosine ring (C) are studied. Transported substrates are highlighted in gray. Uptake of 3,3′-T2 (100nM) in X. laevis oocytes coinjected with cRNA of CD98 and Lat2. Experiments have been performed in the absence (control) and presence of indicated substrates (A, 10μM; B and C, 1mM). Mean ± SEM of 10–15 oocytes per group of 2–3 independent experiments normalized to the uptake of 3,3′-T2 (after subtraction of noninjected oocytes) are shown.
BCH and similar substrates have been used in competitive inhibition studies. The substrates S12, S11, and S10 contain additional hydrophobic extensions and are larger than BCH, whereas in comparison, S6 possess a small cyclopropane ring. Uptake of 3,3′-T2 by Lat2 cannot be prevented by S12, S11 and S10. Reduction of the uptake by 15% can be achieved with S9 and even more, about 40%, with S6 (Figure 4B).
Further investigation of substituents at tyrosine ring positions 3 and 5 revealed that hydroxyl, trifluoromethyl, or amino groups as well as an iodine atom competitively inhibit the transport of 3,3′-T2. Only bromine- and trifluoromethyl-substituted substrates S3 and S5 resulted in decreased 3,3′-T2 uptake, of about 50% (Figure 4C).
Discussion
Among the TH transporter proteins (MCT, OATP, and LAT), least has been known about structure-function-relationships of Lat2. In recent studies we showed that Lat2 is involved in 3,3′-T2 and to lower extent in T3 uptake (36).
Because details of the LAT2/CD98 interaction have been previously described (26), we now focus on the identification of determinants for TH transport at Lat2.
Advantage of 2 structural templates used for the Lat2 model
Our comparative model of Lat2 was generated on the basis of crystal structures of the transporters AdiC and ApcT. The advantage of using the AdiC structure lies in the bound substrate arginine between TMH1 and TMH6. Due to the sequence similarity between Lat1 and Lat2 of 63%, it is not surprising that the AdiC structure has been used by others as single template for a LAT1 model (42). However, we realized that AdiC is only an adequate template for Lat2 for particular TMHs (1–4, 6, and 7), whereas for the remaining TMHs (5 and 7–12), some sequence fingerprints of ApcT match the Lat2 sequence better (alignment Supplemental Figure 1). Using features originating from AdiC for substrate recognition and from ApcT to form the traversing channel in the center of Lat2 allowed us to develop a more accurate Lat2 model for both steps (Figure 5).
Figure 5. Scheme of substrate translocation from the extracellular side routed through the center of Lat2.
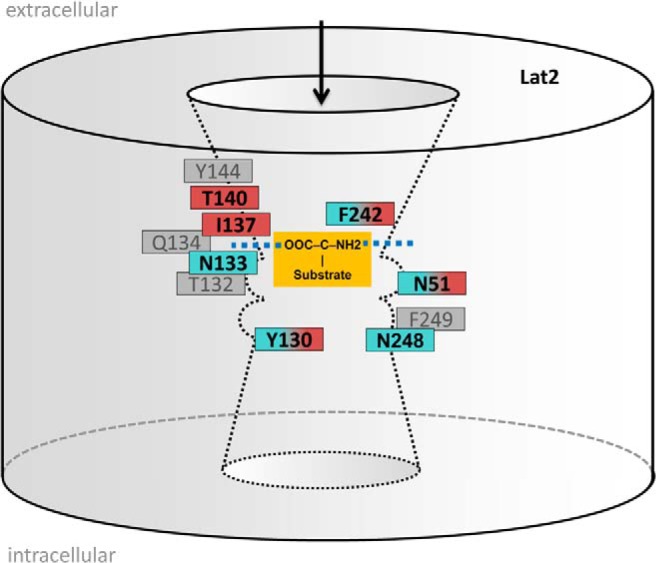
Localizations are depicted of the recognition site (blue dashed) for carboxylic acid and amino group of the substrate (orange) and of a channel for substrate passage with asymmetric hollows. Arrangements of transport-sensitive residues are given and colored according to their mutational effects, eg, wild-type (gray), decreased (magenta), and increased 3,3′-T2 uptake (cyan). F242 is suggested to be a switch site that potentially facilitates intracellular substrate release.
To test our chimeric Lat2 model, we first mutated residues that are identical in corresponding positions of the template-crystal structures and for which experimental mutation data exist. The concordant effects of the 2 mutations in both the template and Lat2 strengthened our chimeric Lat2 model.
Mutations support a potential traversing channel in the center of Lat2
The Lat2 model guides mutagenesis of further residues, which line a channel for TH to traverse the membrane. Shortening and enlargements of selected side chains led to an enlarged or to an obstructed channel, respectively. Consequently, significantly increased or decreased 3,3′-T2 transport is observed (Figures 2 and 3). For example, the mutation Y130 to alanine enlarges the channel and increases transport; the mutation Y130 to arginine obstructs the channel and decreases transport.
Side chain shortening, while maintaining (N51S) or altering (N51A) hydrophilicity, gives significantly increased or unaltered uptake, respectively. This indicates the necessity of small residues within the interior of the central channel, preferably with polar properties, for passage of TH.
Gate function of Phe242 in Lat2 similar to Trp202 in AdiC
Trp202 exhibits a key function in the template structures of AdiC (43), because in the outward-open, substrate-free state, the orientation of Trp202 allows accessibility to the recognition site (44), whereas when arginine is bound, Trp202 moves as a lid over the binding site (43, 45, 46). Aromatic residues acting as a moving lid have also been reported for other transporters such as LeuT and neurotransmitter/Na+ symporters (47, 48).
The mutagenesis results with Phe242 are exactly the opposite of those with the residues characterizing the traversing channel of Lat2 (Figure 2B). This indicates a distinct functionality for Phe242. The fact that Phe242 of Lat2 corresponds to the identical location of Trp202 in AdiC strongly supports the hypothesis that Phe242 is involved in the influx mechanism of Lat2. This is probably connected to a gating function, which may trigger an inversion of the transporter. However, further studies at the intracellular side are necessary to clarify this hypothesis.
TH and TH-like substrates need distinct features to traverse the Lat2 ligand channel
Remarkably, different substrate features for the transport by Lat2/CD98 have been identified in our competitive inhibition studies of substrates, which comprise fragments of TH-like scaffold and which were confirmed by in silico studies. Apart from a complete amino acid moiety, a distinct spatial dimension of the substrate is needed to fit into the traversing channel of Lat2. For example, this is shown for gabapentin (S12) (Figure 6A) compared with the transported BCH, which is in agreement with previous transport studies (34). 3,4-dihydroxy-L-phenylalanine (S1) competitively inhibits 3,3′-T2 transport by Lat2, which is consistent with reports from other groups (34, 49). Compound S7, which is a precursor of T3 and T4, exhibits inhibition of 3,3′-T2 transport by Lat2, because it fits into the binding pocket (Figure 6B). Further studies of the allowed conformational space for the respective TH derivative indicated (for example, with 3,3′-T2) that flexibility of both aromatic rings is necessary for the whole molecule to be translocated. Moreover 3′,5′-T2, with 2 bulky iodines at the phenyl ring (Figure 7), exhibits inhibition of 3,3′-T2 and may pass through the 2 asymmetrically shaped hollows of the transport channel. TH molecules possessing 3 or 4 bulky substituents (iodines) distributed over the tyrosine and phenyl rings exhibit limited (T3) or no flexibility (rT3 and T4) due to steric hindrance (Figure 7). This explains why uptake of T3 by Lat2 is somewhat restricted and of T4 and rT3 is impeded. In contrast, THs possessing only 2 iodines (or fewer) in the 3(3′) and/or 5(5′) position, irrespective of the 2 rings on which they are located, are flexible enough and thus transported by Lat2 (Figures 3, C and D, and 7).
Figure 6. Overlay of (A) BCH (yellow) with S12 (blue) and (B) 3,3′-T2 (orange) with S7 (cyan) (see also Figure 4) docket into the binding pocket (gray surface) of Lat2, showing the allowed spatial dimensions of substrates to be transported.
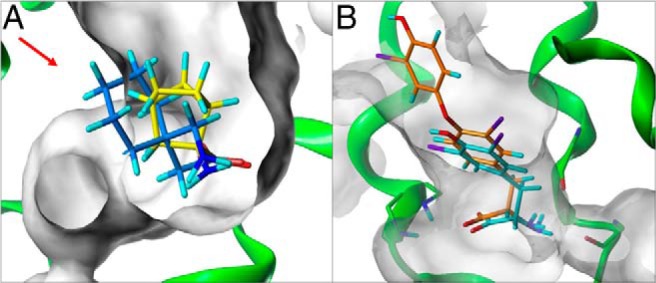
Overlayed S12 (blue in A) is not transported, probably due to its large size (red arrow) that does not fit the binding pocket.
Figure 7.
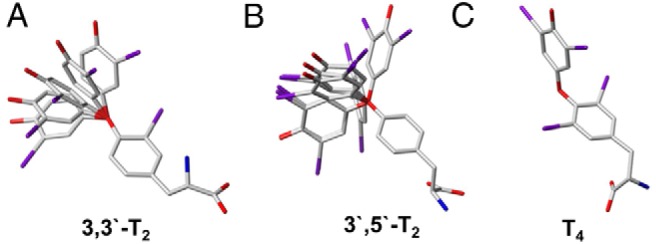
Conformational flexibility search between the 2 aromatic rings of TH yielded the result that (A) 3,3′-T2 and (B) 3′,5′T2 are flexible enough for uptake, whereas in C, T4 steric hindrance of the bulky iodines restricts flexibility and prevents its uptake by Lat2.
There is considerable and increasing evidence that diiodo-L-thyronines are important for triggering cellular responses, eg, affecting cell metabolism, rather than being mere degradation products as they have most often been regarded (50). For example, 3,3′-T2 binding sites have been discovered in rat mitochondria, suggesting a role in metabolic regulation of the cell (51). 3,5-T2 circulates in human serum (52) and may rapidly and directly act on mitochondrial function and, at higher concentrations, also mimics T3 effects via modulation of TH receptor-dependent transcription in many target cells (50, 53–56). Moreover, 3,5-T2 influences the volume of adipocytes by inducing lipolysis and increases mitochondrial content in brown adipocytes and thermogenesis (8). Epidemiological cohort studies indicate that, apart from its role in glucose metabolism, 3,5-T2 is also involved in TH homeostasis (52). Recent findings in fish even suggest an activating role for 3,5-T2 by binding to the ß1 isoform of the TH receptor. This indicates that T2 has a distinct role in the mode of action of TH (39, 57).
Compared with other TH transporters (MCT8, MCT10, and OATP1C1), our data indicate that Lat2 prefers 3,3′-T2 and, very probably, also other T2 and T1 derivatives (36) as substrates, at least for uptake. Thus, Lat2 might contribute to termination of TH action by importing 3,3′-T2, which is generated either by T3 inactivation or by rapid deiodinase 1-mediated rT3 degradation.
In summary, we have provided an initial insight into the molecular details of the uptake mechanisms of TH and their derivatives across the cell membrane by Lat2. Because intracellular and extracellular molecular arrangements seems to be different, further studies of intracellular conformations and efflux of variant substrates are necessary to understand the complete transport mechanism of Lat2.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Project SPP Thyroid Trans Act 1629, Ki1751/1-1 (to A.K.), Ko-922/17-1 (to J.K.), and Kr1273/5-1 (G.K.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft, Project SPP Thyroid Trans Act 1629, Ki1751/1-1 (to A.K.), Ko-922/17-1 (to J.K.), and Kr1273/5-1 (G.K.).
Footnotes
- AdiC
- arginine/agmatine antiporter
- APC
- amino acid/polyamine/organocation
- ApcT
- APC transporter
- BCH
- 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid
- CD98
- glycosylated 4F2 heavy chain
- GlpT
- glycerol-3-phosphate transporter
- KO
- knockout
- LAT
- L-type amino acid transporter
- LeuT
- Leucine transporter
- MCT
- monocarboxylate transporter
- OATP
- organic anion transporting polypeptide
- SLC
- solute carrier
- 3-T1
- 3-iodo-L-thyronine
- 3,3′-T2
- 3,3′-diiodothyronine
- TH
- thyroid hormone
- THTT
- TH transmembrane transporter
- TMH
- transmembrane helix.
References
- 1. Rao GS, Eckel J, Rao ML, Breuer H. Uptake of thyroid hormone by isolated rat liver cells. Biochem Bioph Res Commun. 1976;73:98–104. [DOI] [PubMed] [Google Scholar]
- 2. Abe T, Kakyo M, Sakagami H, et al. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. [DOI] [PubMed] [Google Scholar]
- 3. Wirth EK, Schweizer U, Kohrle J. Transport of thyroid hormone in brain. Front Endocrinol (Lausanne). 2014;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. [DOI] [PubMed] [Google Scholar]
- 6. Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol. 2008;22:1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friesema EC, Docter R, Moerings EP, et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001;142:4339–4348. [DOI] [PubMed] [Google Scholar]
- 8. Smit J, Visser T. European Thyroid Association 37th Annual Meeting, Leiden, September 2013: Programme and Abstracts. Eur Thyroid J. 2013;2(suppl 1):75–194. [Google Scholar]
- 9. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. [DOI] [PubMed] [Google Scholar]
- 10. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Núñez B, de Mena RM, Obregon MJ, et al. Cerebral cortex hyperthyroidism of newborn Mct8-deficient mice transiently suppressed by Lat2 inactivation. PLoS One. 2014;9:e96915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wirth EK, Roth S, Blechschmidt C, et al. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009;29:9439–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlessinger A, Khuri N, Giacomini KM, Sali A. Molecular modeling and ligand docking for solute carrier (SLC) transporters. Curr Top Med Chem. 2013;13:843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlessinger A, Yee SW, Sali A, Giacomini KM. SLC classification: an update. Clin Pharmacol Ther. 2013;94:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinne A, Kleinau G, Hoefig C, et al. Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem. 2010;285:28054–28063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med. 2013;34:396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. [DOI] [PubMed] [Google Scholar]
- 18. Braun D, Lelios I, Krause G, Schweizer U. Histidines in potential substrate recognition sites affect thyroid hormone transport by monocarboxylate transporter 8 (MCT8). Endocrinology. 2013;154:2553–2561. [DOI] [PubMed] [Google Scholar]
- 19. Westholm DE, Marold JD, Viken KJ, Duerst AH, Anderson GW, Rumbley JN. Evidence of evolutionary conservation of function between the thyroxine transporter Oatp1c1 and major facilitator superfamily members. Endocrinology. 2010;151:5941–5951. [DOI] [PubMed] [Google Scholar]
- 20. Tohyama K, Kusuhara H, Sugiyama Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004;145:4384–4391. [DOI] [PubMed] [Google Scholar]
- 21. Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature. 2005;437:215–223. [DOI] [PubMed] [Google Scholar]
- 22. Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology. 2009;24:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao X, Lu F, Zhou L, et al. Structure and mechanism of an amino acid antiporter. Science. 2009;324:1565–1568. [DOI] [PubMed] [Google Scholar]
- 24. Gao X, Zhou L, Jiao X, et al. Mechanism of substrate recognition and transport by an amino acid antiporter. Nature. 2010;463:828–832. [DOI] [PubMed] [Google Scholar]
- 25. Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosell A, Meury M, Álvarez-Marimon E, et al. Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc. Proc Natl Acad Sci USA. 2014;111:2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meury M, Costa M, Harder D, et al. Detergent-induced stabilization and improved 3D map of the human heteromeric amino acid transporter 4F2hc-LAT2. PLoS One. 2014;9:e109882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pineda M, Fernández E, Torrents D, et al. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. [DOI] [PubMed] [Google Scholar]
- 29. Rossier G, Meier C, Bauch C, et al. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948–34954. [DOI] [PubMed] [Google Scholar]
- 30. Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. [DOI] [PubMed] [Google Scholar]
- 31. Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629–23632. [DOI] [PubMed] [Google Scholar]
- 32. Nakada N, Mikami T, Hana K, et al. Unique and selective expression of L-amino acid transporter 1 in human tissue as well as being an aspect of oncofetal protein. Histol Histopathol. 2014;29:217–227. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell FE, Roy LA, Taylor PM. Iodothyronine interactions with the system L1 amino acid exchanger in 3T3-L1 adipocytes. J Thyroid Res. 2010;2010:726098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morimoto E, Kanai Y, Kim do K, et al. Establishment and characterization of mammalian cell lines stably expressing human L-type amino acid transporters. J Pharmacol Sci. 2008;108:505–516. [DOI] [PubMed] [Google Scholar]
- 35. Ritchie JW, Taylor PM. Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J. 2001;356:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinne A, Winttner M, Wirth EK, Hinz KM, Schülein R, Köhrle J, Krause G. Involvement of the L-type amino acid transporter Lat2 in the transport of 3,3′-diiodothyronine across the plasma membrane. Eur Thyroid J. 2015. doi: 10.1159/000381542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olivella M, Gonzalez A, Pardo L, Deupi X. Relation between sequence and structure in membrane proteins. Bioinformatics. 2013;29:1589–1592. [DOI] [PubMed] [Google Scholar]
- 38. Gasol E, Jiménez-Vidal M, Chillarón J, Zorzano A, Palacín M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility. J Biol Chem. 2004;279:31228–31236. [DOI] [PubMed] [Google Scholar]
- 39. Navarrete-Ramírez P, Luna M, Valverde-R C, Orozco A. 3,5-di-iodothyronine stimulates tilapia growth through an alternate isoform of thyroid hormone receptor β1. J Mol Endocrinol. 2014;52:1–9. [DOI] [PubMed] [Google Scholar]
- 40. Ritchie JW, Peter GJ, Shi YB, Taylor PM. Thyroid hormone transport by 4F2hc-IU12 heterodimers expressed in Xenopus oocytes. J Endocrinol. 1999;163:R5–R9. [DOI] [PubMed] [Google Scholar]
- 41. Jansen J, Friesema EC, Milici C, Visser TJ. Thyroid hormone transporters in health and disease. Thyroid. 2005;15:757–768. [DOI] [PubMed] [Google Scholar]
- 42. Geier EG, Schlessinger A, Fan H, et al. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc Natl Acad Sci USA. 2013;110:5480–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsai MF, Fang Y, Miller C. Sided functions of an arginine-agmatine antiporter oriented in liposomes. Biochemistry. 2012;51:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fang Y, Jayaram H, Shane T, et al. Structure of a prokaryotic virtual proton pump at 3.2 A resolution. Nature. 2009;460:1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kowalczyk L, Ratera M, Paladino A, et al. Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc Natl Acad Sci USA. 2011;108:3935–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Claxton DP, Quick M, Shi L, et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. [DOI] [PubMed] [Google Scholar]
- 48. Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter–inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khunweeraphong N, Nagamori S, Wiriyasermkul P, et al. Establishment of stable cell lines with high expression of heterodimers of human 4F2hc and human amino acid transporter LAT1 or LAT2 and delineation of their differential interaction with α-alkyl moieties. J Pharmacol Sci. 2012;119:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pietzner M, Lehmphul I, Friedrich N, et al. Translating pharmacological findings from hypothyroid rodents to euthyroid humans: is there a functional role of endogenous 3,5-T2? Thyroid. 2014; February;25(2):188–197. doi: 10.1089/thy.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lanni A, Moreno M, Horst C, Lombardi A, Goglia F. Specific binding sites for 3,3′-diiodo-L-thyronine (3,3′-T2) in rat liver mitochondria. FEBS Lett. 1994;351:237–240. [DOI] [PubMed] [Google Scholar]
- 52. Lehmphul I, Brabant G, Wallaschofski H, et al. Detection of 3,5-diiodothyronine in sera of patients with altered thyroid status using a new monoclonal antibody-based chemiluminescence immunoassay. Thyroid. 2014;24:1350–1360. [DOI] [PubMed] [Google Scholar]
- 53. Jonas W, Lietzow J, Wohlgemuth F, et al. 3,5-Diiodo-L-thyronine (3,5-T2) exerts thyromimetic effects on hypothalamus-pituitary-thyroid axis, body composition, and energy metabolism in male dietinduced obese mice. Endocrinology 2014:en.2014-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Padron AS, Neto RA, Pantaleão TU, et al. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J Endocrinol. 2014;221:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. [DOI] [PubMed] [Google Scholar]
- 56. Ball SG, Sokolov J, Chin WW. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J Mol Endocrinol. 1997;19:137–147. [DOI] [PubMed] [Google Scholar]
- 57. Mendoza A, Navarrete-Ramírez P, Hernández-Puga G, et al. 3,5-T2 is an alternative ligand for the thyroid hormone receptor β1. Endocrinology. 2013;154:2948–2958. [DOI] [PubMed] [Google Scholar]