Abstract
The corpus luteum (CL) is a transient endocrine gland developed from the ovulated follicles, and the most important function is to synthesize and secrete progesterone (P4), a key hormone to maintain normal pregnancy and estrous cycle in most mammals. It is known that estrogen has a vital role in stimulating P4 synthesis in CL, but it still remains unclear about the mechanism of estradiol (E2) regulating P4 production in CL. Our results here first show that all of the CL cells express MAPK 8 (MAP3K8), and the MAP3K8 level is much higher at the midstage than at the early and late stages during CL development. The further functional studies show that the forced inhibition of endogenous MAP3K8 by using MAP3K8 small interfering RNA and MAP3K8 signaling inhibitor (MAP3K8i) in the luteal cells significantly block the P4 synthesis and neutralize the enhancing effect of E2 on P4 production in the CL. In addition, our results here demonstrate that the stimulating effect of E2 on P4 synthesis relies on the estrogen no-classical protein-coupled receptor 30, and MAP3K8 is involved in mediating the protein-coupled receptor 30signaling of E2 affecting P4 synthesis via stimulating ERK phosphorylation. These novel findings are critical for our understanding the ovary physiology and pathological mechanism.
The corpus luteum (CL) is a transient endocrine gland developed from the ovulated follicles through a terminal differentiation process termed luteinization (1, 2), and one of its most important function is to synthesize and secrete progesterone (P4), a key hormone to maintain normal pregnancy in most mammals (3). However, the production of CL P4 is regulated by the numbers of tropic hormones, including prolactin (PRL), luteinizing hormone (LH), and estradiol (E2). The key factors are estrogen and PRL in rodent. PRL and PRL-related proteins prevent the transformation of P4 into an inactive metabolite, elevate luteal cholesterol stores, and enhance cholesterol uptake by luteal cells (4); LH is involved in mediating the delivery of cholesterol to the mitochondrial cholesterol side-chain cleavage system in most mammals (5), but its function is weak in rodents (6). In some species, such as the rodent, rabbit, and pig, E2 is a luteotropic hormone (7, 8). The enhancing effect of E2 on P4 synthesis is accomplished by enhancing the supply of cholesterol substrate and cholesterol uptake, mobilizing cholesterol storage, and transporting to the mitochondria (9, 10). Estradiol also stimulates cholesterol synthesis (11, 12) and luteal cell content of lipoprotein receptors for the uptake of circulating cholesterol (13, 14).
It has been well documented that PRL, PRL-related proteins, and LH bind to the corresponding transmembrane receptors and further act on intracellular cytokine to mediate the synthesis of P4 in the CL. But it is generally thought that estrogen plays its roles by combining with the classical nuclear receptors, including estrogen receptor-(ER)-α and ERβ, which are highly expressed in CL (15–17). The estrogen membrane receptor G protein-coupled receptor 30 (GPR30) has been detected (18), which mediates rapid and transcription-independent estradiol-17β signaling in different tissue and cell types (19, 20). In the ovary, GPR30 expression has been detected in the theca cells, the granulosa cells, and luteal cells (21). However, it still remains unknown that whether E2 influences CL development and P4 synthesis through GPR30.
MAPK 8 (MAP3K8, Tpl2) acts as a MAP3K and is widely expressed in the brain, intestine, kidney, skeletal muscles (22, 23), and the immunological system (24). In addition, MAP3K8 acts as a tumor suppressor, and there is a significant elevation in the cancer tissue or/and cells, such as human gastric/colon adenocarcinomas (25), large granular T cell neoplasias (26), and breast cancer. To identify its physiological functions and pathological significance, the laboratory of Tsichilis and colleagues (27) generated MAP3K8 knockout mouse and the evident abnormalities are not observed. Our recent study demonstrates that MAP3K8 mediates the signaling pathway of corticotropin-releasing factor-regulating proopiomelanocortin in mouse pituitary (28). But up to now, there are no reports about MAP3K8 expression and its function in ovary. The primary aims of the present study were to assay the MAP3K8 expression and related functions. The results demonstrate that MAP3K8 is highly expressed in the mouse ovary and specifically located in the CL. The further functional study shows that MAP3K8 is involved in mediated the stimulating effect of E2 on P4 synthesis through GPR30 in mouse CL. The reported signal molecules of E2 affecting P4 synthesis are potential for our understanding of the related mechanisms of ovary physiology and the pharmacological interventions.
Materials and Methods
Animals and tissue collections
Kunming white mice were purchased from the Animal Institute of the Chinese Medical Academy (Beijing, China) and were raised in a controlled temperature (25°C ± 1°C) and humidity (60%–70%) with a 12-hour light, 12-hour dark cycle. The animal experiments were approved by the Chinese Association for Laboratory Animal Sciences. Adult female mice (6–8 wk) were ip injected with 10 IU pregnant mare serum gonadotropin to stimulate follicle development, which was followed 48 hours later by an injection of 10 IU human chorionic gonadotropin (hCG) to promote ovulation and obtain luteinized ovaries. These mice were then mated with castrated male mice. Day 0 was taken as the day of hCG injection. According to previous studies (29, 30), the ovaries were categorized as early (day 0 to day 5), mid- (day 6 to day 10), and late (day 11 to day 15) luteal phases.
For immunohistochemistry, ovaries of the midluteal stage were separated and fixed in 4% paraformaldehyde in PBS (pH 7.4) for 12 hours at room temperature, embedded in paraffin, and cut into 5-μm sections. The ovaries of early, mid-, and late stages (n ≥ 3 at each stage) were collected and washed in PBS under sterile condition. CL tissues were enucleated from the ovaries under a microscope with the aid of fine forceps. The CL tissues were stored at −80°C until analysis. On day 7 after hCG injection, the mice were ip injected with 2 mg/kg 4-(3-chloro-4-fluorophenylamino)-6-(pyridin-3-yl-methylamino)-3-cyano-(1,7)-naphthyridine (MAP3K8i) (31), and the blood samples were collected from the orbital sinus at 24 hours after MAP3K8i injection (n ≥ 6 for each group). Serum and CL were harvested and frozen at −20°C until further analyzed. On day 7 after hCG injection, the mice were ip injected with 100 ng E2, and the CL and serum were collected at 0, 3, 6, and 12 hours after E2 injection (n ≥ 3 mice/time point) as described above. The mated female mice were pretreated with 2 mg/kg MAP3K8i, given through gavage (32), on day 3 (vaginal plug = d 1 of pregnancy) and day 4. The pregnant mice were killed on day 5 (9:00 am). The serum P4 and E2 levels were analyzed, the implantation sites were visualized by an iv injection of Chicago blue dye solution, and the number of implantation sites was counted.
Granulosa-luteinized cells culture and transient transfections
The granulosa-luteinized cells were cultured as the previous reports (33, 34). Twenty-one-day-old immature female Kunming mice were ip injected with 7.5 IU pregnant mare serum gonadotropin, followed by 7.5 IU of hCG 48 hours later. Seven hours later, the ovaries were obtained and the granulosa-luteinized cells were isolated and cultured in 10% fetal bovine serum-DMEM (Life Technologies, Inc-Invitrogen) supplemented with 100 IU/mL penicillin and 100 IU/mL streptomycin at 37°C with 5% CO2. MAP3K8 small interfering RNA (siRNA) kit was purchased from RiboBio. The procedures for transient transfections were performed as in our previous report (35).
Real-time quantitative PCR (RT-qPCR) and common PCR
According to the protocols provided by the manufacturer, total RNA of the CL tissues and granulosa-luteinized cells were isolated using the TRIzol reagent, purified by deoxyribonuclease I and quantified by spectrophotometry. The purified total RNA (1 μg) was used as a template for cDNA synthesis using moloney murine leukemia virus (Promega) according to the manufacturer's instructions. All reverse transcriptase reactions included no-template controls. RT-qPCR was performed using SYBR Green master mix (DRR420A; TaKaRa) in the ABI PRISM 7500 sequence detection system (Applied Biosystems), and reactions were done in triplicate. RT-qPCR conditions were as follows: 95°C for 2 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Relative RNA quantifications were normalized to endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All primers were designed using Primer 5.0 (Premier), and they are described in Table 1.
Table 1.
Primers Used in the Present Study
| Forward Primer (5′–3′) | Reverse Primer (5′–3′) | |
|---|---|---|
| GAPDH | GGTTGTCTCCTGCGACTTCA | GGGTGGTCCAGGGTTTCTTA |
| StAR | AGTCCTTCGAACACCGATGA | GTCTTGCTATGCCTTCTTTGG |
| MAP3K8 | AGCCCTCACAAGATAGTAACCT | GCTACCATACAATACACACCAGA |
| cyp11a | AGGTGTAGCTCAGGACTTCA | AGGAGGCTATAAAGGACACC |
| GPR30 | AGTCTTTCCGTCACGCCTAC | GCTCGTCTTCTGCTCCACA |
| ERα | TTGACAAGAACCGGAGGA | CCAACAAGGCACTGACCAT |
| ERβ | TGCCCTGGTCTGGGTGAT | TCTGGGAGCCCTCTTTGC |
| 3β-HSD | CAATCTGAAAGGTACCCAGAA | AGATGAAGGCTGGCACACTTG |
GPR30, ERα, and ERβ were detected by common PCR using primers as described in Table 1. Amplifications were carried out on PCR instrument (Bio-Rad Laboratories) using the following protocol: 94°C for 5 minutes (one time); 94°C for 50 seconds, 60°C for 30 seconds, 72°C for 30 seconds (35 times), 72°C for 10 minutes, and 4°C holding.
Western blot (WB)
CL and granulosa-luteinized cells were lysed with radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH7.4; 150 mM NaCl; 1% TritonX-100; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate) containing 1 mM phenylmethanesulfonyl fluoride. The protein concentration of each group was determined by using the bicinchoninic assay reagent (Vigorous Biotechnology) according to the manufacturer's recommendations. Equal amount of proteins (50 μg) were electrophoresed on 11% SDS-PAGE, and the bands were transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories). The membrane was blocked with 5% (wt/vol) nonfat dry milk in 0.05 M Tris-buffered saline (TBS) (pH 7.4) for 3 hours and incubated with MAP3K8 antibody (1:2000; Gene Tex) and internal control GAPDH antibody (1:10 000; Ambion) overnight at 4°C. ERK and p-ERK antibody (1:2000, Cell Signaling Technology) were also incubated overnight at 4°C. The polyvinylidene difluoride membrane was then washed three times for 30 minutes in 0.1% Tween-20 in TBS and incubated for 2 hours with horseradish peroxidase-conjugated goat antirabbit IgG or horseradish peroxidase-conjugated goat antimouse IgG (Zhongshan). After washing for 30 minutes with three changes of 0.1% Tween-20 in TBS, the membrane was treated with the Pierce ECL 2 Western blot substrate (Thermo Scientific). The relative intensity of each blot was assessed and analyzed with the AlphaImager 2200 Software package (Alpha Innotech Corporation). The intensity values pertaining to each group were normalized against the OD of GAPDH corresponding to the same group within a single gel and expressed in terms of the means ± SEM of three independent experiments (Table 2).
Table 2.
Antibody Information
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|
| MAP3K8 | MAP3K8 antibody[N3C3] | GTX102711 | Rabbit polyclonal | 1:50 1:2000 |
| GPR30 | GPR30 antibody (K-19) | Santa Cruz Biotechnology, sc-48524 | Goat polyclonal | 1:50 |
| ERK | p44/42 MAPK (Erk1/2) antibody | Cell Signaling Technology, number 9102 | Rabbit polyclonal | 1:2000 |
| P-ERK | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody | Cell Signaling Technology, number 9101 | Rabbit polyclonal | 1:2000 |
| GAPDH | GAPDH mouse monoclonal antibody (clone 6C5) | Ambion AM4300 | Mouse monoclonal | 1:2000 |
Immunohistochemistry (IHC)
On day 7 after the hCG injection, the ovaries were embedded in paraffin and cut into 5-μm sections. The IHC of ovary sections was performed using the methods described earlier (36). Antibodies against MAP3K8 (1:50) and GPR30 (1:50; Santa Cruz Biotechnology) were incubated at 4°C for 12 hours. Biotinylated goat antirabbit IgG and donkey antigoat IgG (1: 400; Zhongshan) were incubated at room temperature for 3 hours. Subsequently, the avidin biotin complex (Vector Laboratories) was added for 1 hour, and peroxidase activity was detected by using diaminobenzidine (Sigma) staining for 2 minutes. The slides were observed under a microscope (Leica Microsystems) and photographed.
Radioimmunoassays
Transfected granulosa-luteinized cells (105 per well of 24 well plates) were cultured in 10% fetal bovine serum-DMEM for 24 hours. Cell medium was then replaced by DMEM (1 mL/well). Cells were further incubated for 12 hours before harvesting. In the MAP3K8i experiments, cells were incubated in DMEM for 6 hours after MAP3K8i treatment. Then the medium was collected for P4 and E2 determinations. In MAP3K8 expression-interference experiments, granulosa-luteinized cells were subjected to 100 ng/mL LH, 0.10 μM E2, 0.1 μM PRL, or an equal volume DMEM for different times. The media were then collected and determinations were made. CL was lysed in 400 μL lysis buffer for P4 and E2 determinations. Experiments were performed six times. P4 and E2 were analyzed using RIA reagents provided by the Beijing North Institute Biological Technology (Beijing, China). The minimum detectable concentrations were 2 pg/mL for E2 and 0.2 ng/mL for P4. For each RIA, the intra- and interassay coefficients of variation were less than 15% and 10%, respectively.
Statistical analysis
All experiments were independently performed more than three times. All data were analyzed using a one-way ANOVA, followed by a Student's t test. The values were presented as the means ± SEM. Statistical analysis was performed using SPSS 10.0 (SPSS, Inc). A value of P < .05 was considered to be statistically significant.
Results
MAP3K8 expression in the developing mouse CL
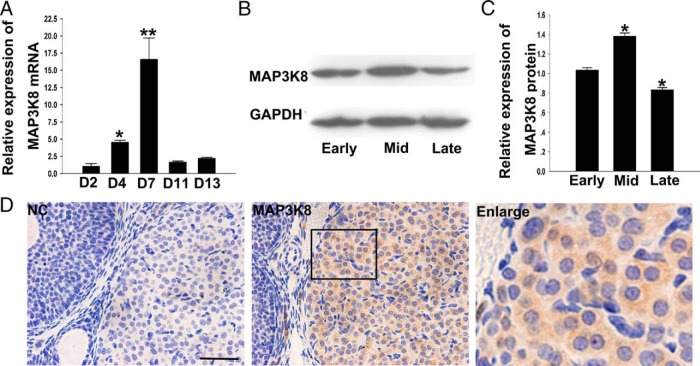
We initially detected MAP3K8 mRNA at different luteal phases of pseudopregnant mice (29, 30) using RT-qPCR. The results showed that the MAP3K8 mRNA level kept increasing from day 2 to day 4, the early luteal stage, which reached the maximum at midluteal stage (d 7) followed by a sharp decline at the late luteal stage (d 11 and d 13) (Figure 1A). In addition, CL MAP3K8 protein levels at different stages were detected by the WB, and the results showed that the MAP3K8 protein level at the midluteal stage was significantly higher than at early and late stages, similar to the change of MAP3K8 mRNA expression (Figure 1, B and C). Furthermore, we located MAP3K8 expression in the ovary by IHC. The results showed that MAP3K8 was stained in all of the luteal cells, and no MAP3K8 signal was observed in the follicular and stromal cells (Figure 1D). The results make us hypothesize that MAP3K8 may involve the regulation of the CL development.
Figure 1. MAP3K8 expression in the developing mouse CL.
A, RT-qPCR analysis of MAP3K8 mRNA level in the staged mouse CL. The experiments were repeated at least three times and normalized to their respective control. Data are shown as means ± SEM (n ≥ 3). *, P < .05; **, P < .01. B, Protein level of MAP3K8 in the staged mouse CL was measured by WB. C, Quantification of MAP3K8 protein levels in the staged mouse CL. The experiments were repeated at least three times and normalized to their respective control. Data are presented as means ± SEM (n ≥ 3). *, P < .05 (ANOVA). D, The expression of MAP3K8 in midstage CL of mouse using IHC. Bar represents 50 μm.
MAP3K8 affects P4 synthesis in mouse granulosa-luteinized cells
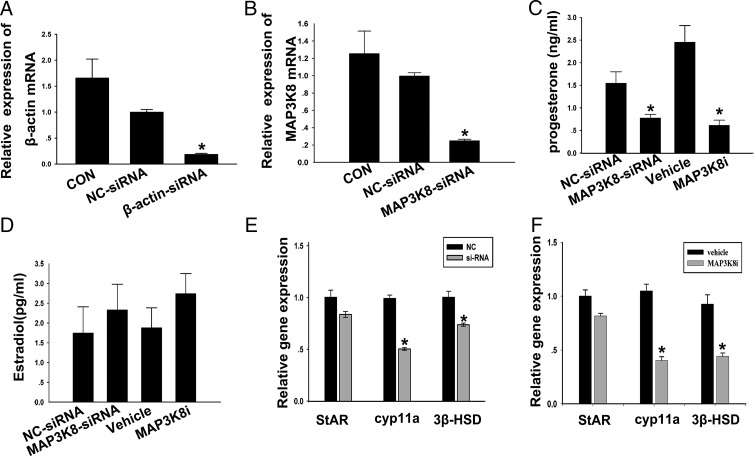
The most important function of the CL is to produce P4; we thus identified the influence of MAP3K8 on the P4 production in the cultured granulosa-luteinized cells by using MAP3K8-siRNA and MAP3K8i. First, MAP3K8 siRNA was carried out to inhibit the MAP3K8 expression, and β-actin siRNA was used as a positive control. The results showed that MAP3K8 siRNA was similar because the positive control could knock down MAP3K8 mRNA level by 75% (Figure 2, A and B) and decreased the P4 level in the medium by 50% compared with negative control (NC) siRNA. In addition, the P4 level in the medium decreased almost 80% after 6 hours treatment with 10 μM MAP3K8i (Figure 2C). In addition, to assay the effect of MAP3K8 on E2 synthesis in cultured cells, the E2 levels in the cultured medium were measured after MAP3K8 was knocked down by, respectively, MAP3K8 siRNA and MAP3K8i treatment as above. The results showed that either MAP3K8 siRNA or MAP3K8i had no obvious effect on the E2 synthesis in cultured cells (Figure 2D). Furthermore, we examined the influence of MAP3K8 siRNA and MAP3K8i on the mRNA levels of cyp11a, 3β-hydroxysteroid dehydrogenase (3β-HSD), and steroidogenic acute regulatory protein (StAR), which are involved in the P4 synthesis. The results demonstrated that no matter in vitro both MAP3K8 siRNA and MAP3K8i significantly decreased cyp11a and 3β-HSD mRNA levels but had no obvious effect on the StAR mRNA (Figure 2, E and F). All of these results showed that MAP3K8 affected the synthesis of P4 in the mouse granulosa-luteinized cells.
Figure 2. MAP3K8 affects P4 synthesis in mouse granulosa-luteinized cells.
A, Quantification of intracellular β-actin mRNA level after the cells were transfected with MAP3K8 siRNA for 24 hours. B, Quantification of intracellular MAP3K8 mRNA level after the cells were transfected with MAP3K8 siRNA for 24 hours. C and D, The cells were transfected with MAP3K8-siRNA for 36 hours or added 10 μM MAP3K8i for 6 hours. The medium was collected. The P4 (C) and E2 (D) levels were measured by RIA, respectively. Data are shown as means ± SEM (n = 5). *, P < .05 vs NC-siRNA or vehicle (t test). E and F, Gene expression levels of the key enzymes was analyzed after the cells were transfected with MAP3K8 siRNA for 24 hours (E) and added 10 μM MAP3K8i for 6 hours (F) by RT-qPCR. Data were shown as means ± SEM (n = 3). *, P < .05 vs NC-siRNA or vehicle (t test). CON, control; StAR, steroidogenic acute regulatory protein.
MAP3K8 affects P4 synthesis in mouse CL and implantation during early pregnancy
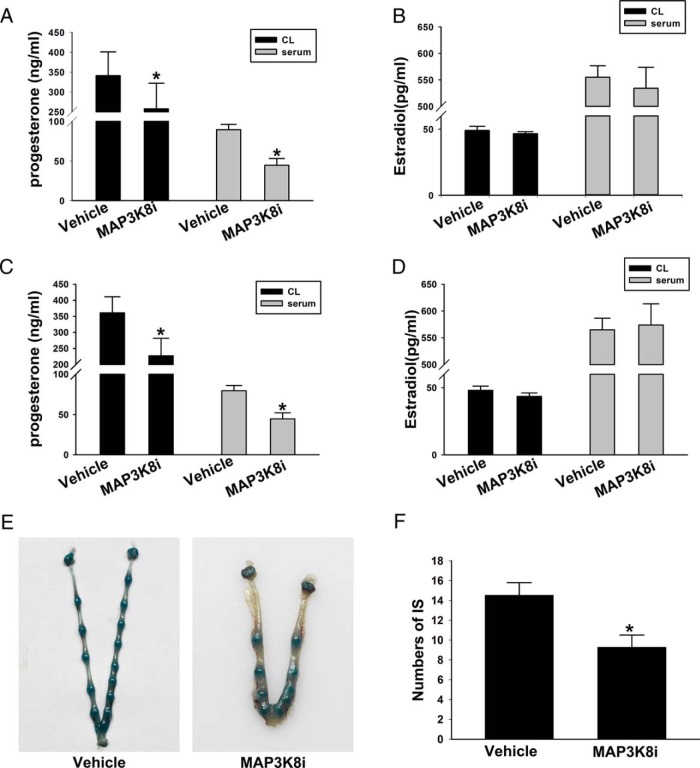
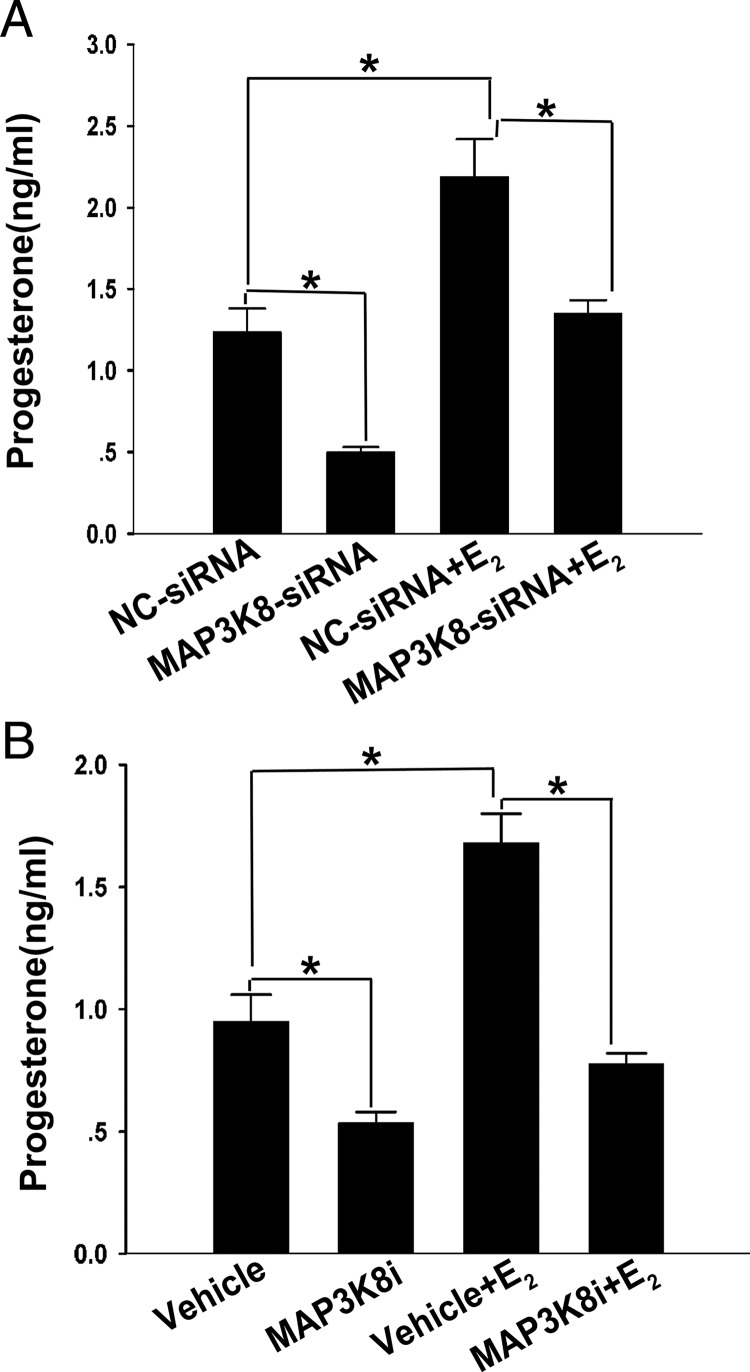
To confirm the effect of MAP3K8 on P4 synthesis, on day 7 after hCG injection, the mice were injected with 2 mg/kg MAP3K8i and vehicle. Then the P4 and E2 in serum and CL were detected. The results showed that after blocking the signal of MAP3K8, the level of P4 in the serum decreased by approximately 26% and approximately 49% in CL (Figure 3A). But E2 had no obvious change (Figure 3B). These results were accordant with the cell experiments. To find the effect of the altered P4 levels after MAP3K8 inhibition, the mated female mice were pretreated with 2 mg/kg MAP3K8i, given through gavage (32), on day 3 (vaginal plug = d 1 of pregnancy) and day 4. The pregnant mice were killed on day 5 (9:00 am). The serum P4 and E2 levels were analyzed, the implantation sites were visualized by an iv injection of Chicago blue dye solution, and the number of implantation sites were counted. The results showed that MAP3K8i treatment decreased the serum P4 level by 29% and the CL P4 level by 40% (Figure 3C), although MAP3K8i treatment had no significant effect on E2 (Figure 3D). In addition, the number of implantation sites decreased approximately 30% (Figure 3, E and F). These data confirm that the altered P4 levels after the MAP3K8 inhibition have significant influence on the related physiological functions of P4.
Figure 3. MAP3K8 affects P4 synthesis in mouse CL and implantation during early pregnancy.
On day 7 after hCG injection, the mice were injected with 2 mg/kg MAP3K8i and vehicle. Then the P4 (A) and E2 (B) in serum and CL were detected by RIA. C, The P4 levels in serum and CL were measured by RIA on day 5 (vaginal plug = d 1 of pregnancy) after treated with MAP3K8i. D, The E2 levels in serum and CL were measured by RIA on day 5 after treated with MAP3K8i. E and F, The number of implantation sites (IS) was analyzed. Data are shown as means ± SEM (n = 5). *, P < .05 vs vehicle (t test).
E2 enhances MAP3K8 expression and P4 synthesis in mouse CL and mouse granulosa-luteinized cells
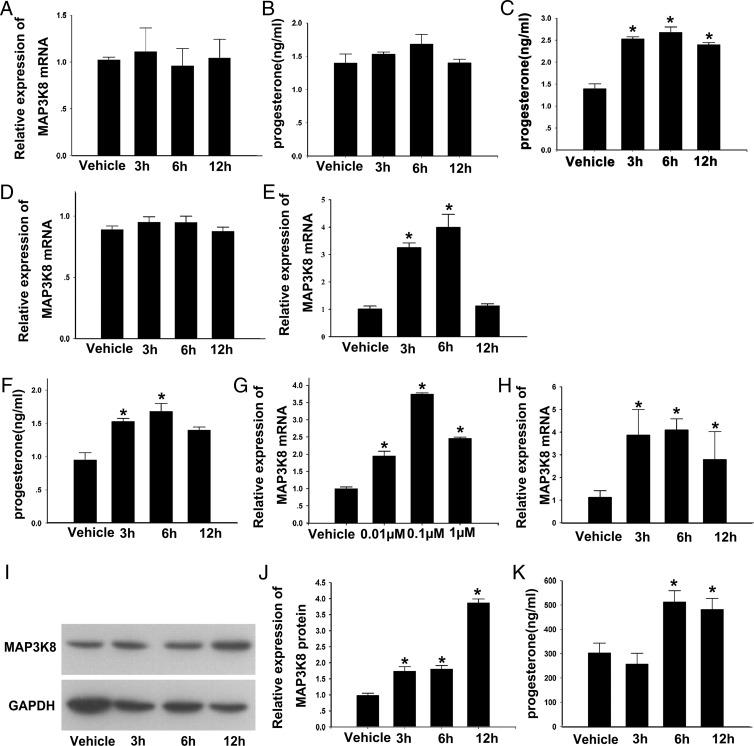
Because PRL, LH, and E2 are key hormones regulating P4 synthesis in the CL, we treated the cultured cells with 100 ng/mL LH (37), 1 μg/mL PRL (38), and 0.10 μM E2 (39, 40) for 0 hours (vehicle), 3 hours, 6 hours, and 12 hours. Then the MAP3K8 mRNA levels and the P4 levels were, respectively, assayed by RT-qPCR and RIA to find the upstream factors of MAP3K8 and determine the relation of MAP3K8 expression to P4 synthesis. The results showed that LH had no effect on the MAP3K8 mRNA level and P4 production at every time point examined (Figure 4, A and B). Although PRL increased the P4 levels (Figure 4C) after 6 and 12 hours of treatments it had no evident influence on MAP3K8 expressions (Figure 4D).
Figure 4. E2 enhances MAP3K8 expression and P4 synthesis in mouse CL and mouse granulosa-luteinized cells.
A, mRNA levels of MAP3K8 and (B) P4 level in the culture medium at vehicle, 3 hours, 6 hours, and 12 hours after incubating the cells with 100 ng/mL LH. P4 level in the culture medium (C) and mRNA levels of MAP3K8 (D) at vehicle (0), 3 hours, 6 hours, and 12 hours after incubating the cells with 1 μg/mL PRL are shown. mRNA levels of MAP3K8 (E) and the P4 level in the culture medium (F) at vehicle (0), 3 hours, 6 hours, and 12 hours after incubating the cells with 0.1 μM E2 are shown. G, The expression of MAP3K8 was measured by RT-qPCR after incubating the cells with vehicle (0) and 0.01, 0.1, and 1 μM E2 for 6 hours. H, mRNA levels of MAP3K8 at the mid-CL at 0, 3, 6, and 12 hours after E2 was injected, as measured by RT-qPCR. I, Protein levels of MAP3K8 in the mid-CL at 0, 3, 6, and 12 hours after E2 was injected, as measured by WB. Results of one representative experiment are shown. J, Quantification of MAP3K8 protein levels at 0, 3, 6, and 12 hours after E2 was injected. K, P4 level in the serum at 0, 3, 6, and 12 hours after E2 was injected. Results are means ± SEM of three independent experiments, each conducted in triplicate and normalized to vehicle group. *, P < .05 (by t test).
It was interesting that 3 hours and 6 hours E2 significantly up-regulated the MAP3K8 mRNA levels by approximately 4 times (Figure 4E) and P4 levels, whereas 12 hours of E2 treatment had no effect either on MAP3K8 expression or P4 secretion. Furthermore, the cultured cells were treated with vehicle (0), 0.01, 0.1, and 1 μM E2 for 6 hours to confirm whether the enhancing effect of E2 on MAP3K8 expression was dose dependent. The results showed that 0.01 and 1 μM E2 increased the MAP3K8 mRNA levels by approximately 2 times, but the 0.1-μM E2 middle dose used increased by 4 times, compared with control (Figure 3G). On this basis, 0.1 μM E2 treatment was used in the following experiments.
In addition, the mice at the midluteal stage were injected with 100 ng E2 (41), the MAP3K8 expression in the CL and serum P4 were detected at vehicle (0 hours), 3 hours, 6 hours, and 12 hours after E2 treatment. The results showed that E2 significantly increased the mRNA level of MAP3K8 at 3 hours and persisted until 12 hours (Figure 4H). The MAP3K8 protein level increased at 3 hours and 6 hours but was followed by a sharp increase at 12 hours (Figure 4, I and J), whereas the serum P4 level obviously increased at 6 and 12 hours (Figure 4K). These collective in vitro and in vivo results demonstrate that E2 up-regulate both the expression of MAP3K8 and P4 synthesis in the CL or luteal cells and suggest that MAP3K8 is potentially involved in P4 production induced by E2.
MAP3K8 mediates the effect of E2 on the P4 synthesis in mouse granulosa-luteinized cells
To determine whether the effect of E2 on P4 synthesis in granulosa-luteinized cells was mediated by MAP3K8, the MAP3K8 in the cultured cells was first knocked down by using MAP3K8 siRNA as above, and then the cells were treated with 0.1 μM E2 for 6 hours. Subsequently, the P4 in the cultured medium was detected by RIA and the results showed that the P4 level was lower approximately 40% than the control (Figure 5A). The MAP3K8i also blocked the enhancing effect of E2 on P4 synthesis by approximately 52% (Figure 5B). These results infer that MAP3K8 mediates the effects of E2 on the P4 synthesis in granulosa-luteinized cells.
Figure 5. MAP3K8 mediates the effect of E2 on the P4 synthesis in mouse granulosa-luteinized cells.
A, Granulosa-luteinized cells were treated 6 hours with or without 0.1 μM E2 after cells were transfected with MAP3K8-siRNA or NC-siRNA for 24 hours. The conditional medium was collected and P4 levels were determined by RIA. B, Granulosa-luteinized cells were treated with or without 0.1 μM E2 for 6 hours in the presence or absence of MAP3K8i. The conditional medium was collected and P4 levels were determined by RIA. Data were shown as means ± SEM from five samples for each group. *, Significant differences (P < .05, ANOVA).
GPR30 expressed in the mouse CL and E2 specifically increases MAP3K8 expression through GPR30
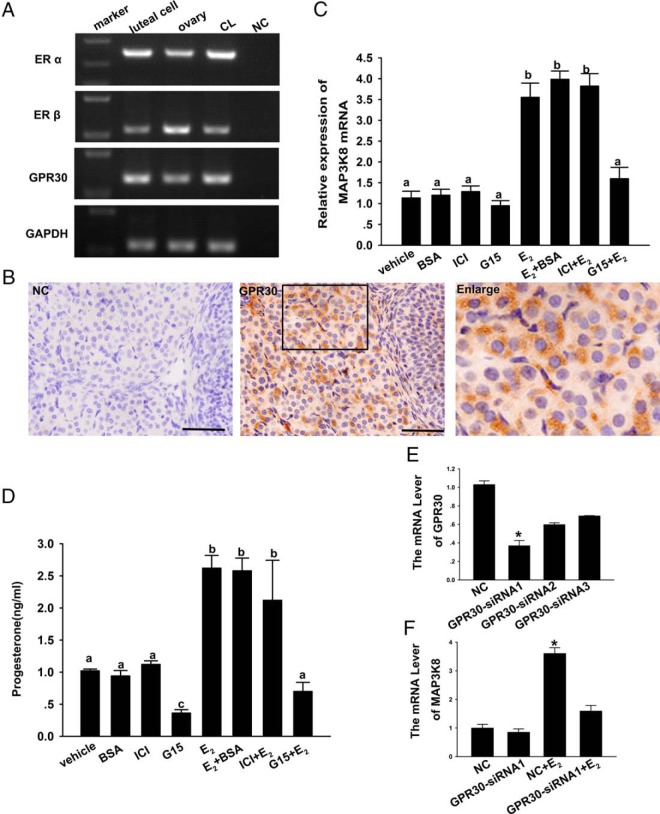
It is generally thought that the actions of E2 are ascribed to the classical nuclear receptors ERα and ERβ. Both ERα and ERβ are expressed and have important roles in CL (15). It has been reported that another receptor (GPR30) of E2 was detected in CL, but they had supplied with no specific evidences. GPR30 has rapid biological responses to E2, and our above results shows that E2 rapidly up-regulated the MAP3K8 expression at 3 hours after treatment. On this basis, we estimate that E2 up-regulated the MAP3K8 expression through GPR30 rather than nuclear receptors. We first detected GPR30 and nuclear receptor mRNA in the CL and luteal cells using the ovary as a positive control by PCR. The result showed that all of the receptors were expressed in CL and granulosa-luteinized cells (Figure 6A). Using IHC, we then located GPR30 in the midluteal-stage ovaries of mice. The results showed that GPR30 was expressed in the mouse CL and follicular and stromal cells and highly existed in the mouse midstage CL (Figure 6B).
Figure 6. GPR30 is expressed in the mouse CL, and E2 specifically increases MAP3K8 expression through GPR30.
A, PCR detected ERα, ERβ, and GPR30 mRNA in the mouse CL and luteal cells. B, The expression of GPR30 in the midluteal-stage ovary of mouse using IHC. Bar represents 50 μm. C, Granulosa-luteinized cells were treated with or without ICI and G15 for 1 hour with 1 μM and then added 0.1 μM E2 or E2-BSA for 3 hours. RT-qPCR analyzed the MAP3K8 mRNA level. D, The conditional medium was collected and P4 levels were determined by RIA. Data are shown as means ± SEM from three repeated experiments. Significance was shown by different letters. E, Quantification of the intracellular GPR30 mRNA level after the cells were transfected with GPR30 siRNA for 24 hours. F, The effect of E2 on the mRNA level of MAP3K8 after GPR30 is blocked by siRNA. Data were shown as means ± SEM from three samples for each group. *, Significant differences were indicated (P < .05, ANOVA).
To study the mechanism of E2 increasing MAP3K8 expression in cultured cells, we used ICI 182780 (ICI) (42, 43) and G15 (44) to inhibit E2 from binding with ERα or ERβ and GPR30. We expected to observe that after blocking E2 binding with its receptors the MAP3K8 mRNA level would not increase. The results showed that ICI binding with ERα or ERβ blocked the nuclear receptors, but MAP3K8 mRNA remained up-regulated by E2 (Figure 5C). On the other hand, when G15 blocked E2 binding with GPR30, the effect of E2 up-regulating MAP3K8 mRNA level disappeared (Figure 6C). Similarly, G15 could inhibit the effect E2 on the P4 synthesis, but ICI had no obvious effects in granulosa-luteinized cells. Furthermore, we treated the cells using E2-BSA and attained the same result as E2 (Figure 6D). In addition, we performed the GPR30 siRNA experiments. The results showed that 24 hours of GPR30 siRNA1 decreased GPR30 mRNA level by 70% (Figure 6E). We then treated the GPR30 siRNA1 knocked-down cells with 0.1 μM E2 for 6 hours, and MAP3K8 mRNA expression was analyzed. The results showed that the up-regulating effect of E2 on the MAP3K8 mRNA expression was lost, similar to the effect of GPR30 inhibitor (Figure 6F). On this basis, we speculate that E2 increases MAP3K8 expression through GPR30 and further affects the P4 synthesis in granulosa-luteinized cells.
E2 increases MAP3K8 expression independently of protein kinase A (PKA) or protein kinase C (PKC) but through ERK1/2
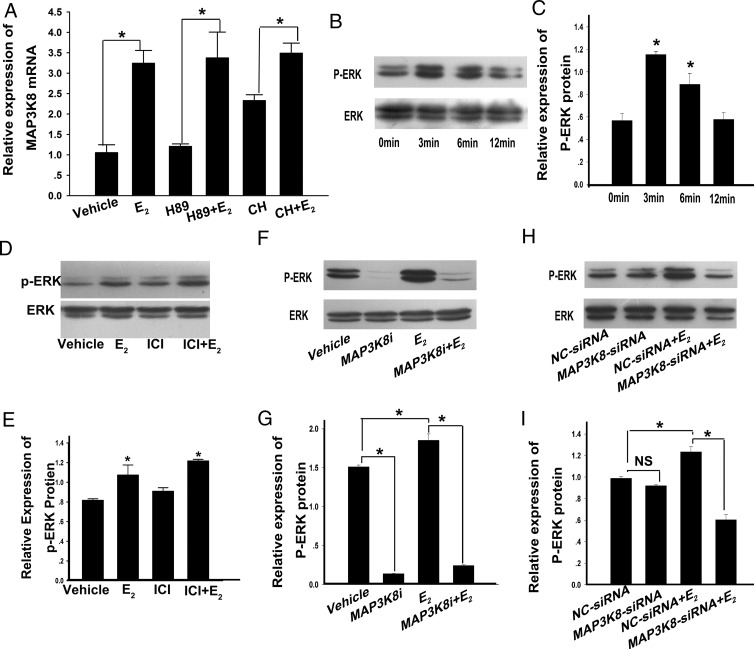
To further study the mechanism of E2 increasing MAP3K8 expression in cultured cells, the cells were separately pretreated with 20 μM of N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89; a PKA inhibitor) (45) and chelerythrine (CH: A PKC inhibitor) (46) for 1 hour, followed by incubation with 0.1 μM E2 for 3 hours. MAP3K8 mRNA levels were detected using RT-qPCR. The results showed that H89 and CH did not modify MAP3K8 mRNA levels induced by E2 (Figure 7A). ERK1/2 was a downstream molecule of MAP3K8 and GPR30 in most pathways, and our results showed that E2 could up-regulate MAP3K8, so we speculated that E2 could stimulate ERK1/2 phosphorylation. To confirm this hypothesis, we added 0.1 μM E2 into the cells for 0, 3, 6, and 12 minutes, and WB results showed that at 3 minutes E2 obviously stimulated ERK1/2 phosphorylation (Figure 7, B and C). In addition, the cells were treated with 0.1 μM E2 for 3 minutes after ICI pretreatments, and ERK1/2 phosphorylation levels were measured. The results showed that E2 enhanced ERK1/2 phosphorylation with or without ICI pretreatment and suggest that E2 stimulated ERK1/2 phosphorylation through GPR30 (Figure 7, D and E).
Figure 7. E2 increases MAP3K8 expression independently of PKA and PKC but through ERK1/2.
A, Inhibitors were added 1 hour before E2 treatment. Both inhibitors had a final concentration of 20 μM. After 3 hours of exposure to E2, MAP3K8 expression in each group was determined by RT-qPCR and then normalized to GAPDH. Data are shown as means ± SEM from three repeated times. *, P < .05. B, Analysis of ERK1/2 and phosphorylated ERK1/2 (P-ERK1/2) proteins. The cells were cultured with 0.1 μM E2 for 0, 3, 6, and 12 minutes, and protein expression levels were analyzed by WB. D, Analysis of ERK1/2 and P-ERK1/2 proteins. The cells were cultured with 0.1 μM E2 3 minutes with or without ICI pretreated. ERK1/2 and P-ERK1/2 protein levels were analyzed by WB. F, Analysis of ERK and P-ERK1/2 proteins. The cells were added with MAP3K8i for 1 hour and 0.1 μM E2 for 3 minutes. H, Analysis of ERK and P-ERK1/2 proteins. The cells were transfected with MAP3K8 siRNA for 24 hours and then treated with 0.1 μM E2 for 3 minutes. C, E, G, and I, Quantification of P-ERK1/2 protein levels. Data are presented as means ± SEM (n = 3). *, P < .05. NS, no significance (P > .05, ANOVA).
To further confirm that in granulosa-luteinized cells ERK1/2 was also the downstream molecule of MAP3K8, we added 10 μM MAP3K8i into the cells. The data showed that MAP3K8i blocked the function of E2 on ERK1/2 phosphorylation (Figure 7, F and G). We then used siRNA against MAP3K8 to confirm its implication in ERK1/2 activation in response to E2. MAP3K8 siRNA was transfected 24 hours later; then the cells were treated with 0.1 μM E2 for 3 minutes, and ERK1/2 phosphorylation levels were measured. The results showed that MAP3K8 siRNA and MAP3K8i had the similar effect on ERK1/2 phosphorylation stimulated by E2 (Figure 7, H and I). These results suggest that MAP3K8 acts as a mediating molecule in the effect of E2 on production of P4 in the granulosa-luteinized cells through ERK1/2.
All these data demonstrate that E2 specifically increases MAP3K8 expression through GPR30. Meanwhile, MAP3K8 acts as a mediating molecule in the effect of E2 on production of P4 in the granulosa-luteinized cells and CL through ERK1/2.
Discussion
The present study first provides the evidence that E2 stimulates P4 synthesis through nonclassical ER GPR30 in mouse CL. In fact, the actions of E2 have traditionally been ascribed to the classical nuclear receptors, ERα and ERβ. Both ERα and ERβ are expressed in mouse ovary (15, 47) which are in agreement with the results presented here. In addition, ERα and ERβ knockout mouse models have indicated that ERα and ERβ are critical for CL formation and maintenance (17). But it was unexpected that the treatment with the nuclear ER antagonist ICI or E2 in combination with ICI did not affect the enhancing of E2 on MAP3K8 expression. These results indicated that ICI did not block E2-induced MAP3K8 expression and made us hypothesize that E2 regulated MAP3K8 expression through GPR30 in mouse CL, and this had been confirmed by the results presented here. These infer that E2 plays different roles in the same target through different mechanisms by its binding to the specific ER molecular type. In support, estrogen is critical for CL formation and maintenance through the classical nuclear ERs in the hamster ovary (21). However, the related intracellular mechanisms need to be elucidated in a further study.
In addition, the present work first shows that MAP3K8 expresses in the CL cells and involves in the functional regulation of the CL by affecting P4 synthesis. The first evidence is that MAP3K8 is expressed in all of the CL cells, and the MAP3K8 expression level is much higher at midstage than at early and late stages during CL development, corresponding to the P4 synthesis in CL (48). In addition, the forced inhibition of endogenous MAP3K8 by using MAP3K8 siRNA and MAP3K8i in granulosa-luteinized cells significantly inhibit the P4 synthesis, corresponding to the recent report that MAP3K8 is involved in steroidogenesis (49). This is supported by in vivo or in vitro experiments that the MAP3K8i administration inhibits the cyp11a and 3β-HSD expression and decreases P4 synthesis.
Furthermore, the results presented here show that MAP3K8 is specifically involved in the signaling pathway of E2-inducing P4 synthesis. It is well known that P4 synthesis in the CL is regulated by PRL, PRL-related proteins (50, 51), LH (6), and E2 (12, 40), among which only E2 significantly increases MAP3K8 expression, but this enhancing effect is blocked by the specific GPR30 inhibitor. Whereas the forced inhibition of endogenous MAP3K8 by using MAP3K8 siRNA and MAP3K8i in the luteal cells significantly blocked the P4 synthesis induced by E2. These suggest that MAP3K8 mediate the signaling pathway of E2-regulating P4 synthesis through GPR30.
It has been reported that MAP3K8 plays its roles by enhancing ERK1/2 phosphorylation (52), and the present study demonstrates that E2 increases MAP3K8 expression, ERK1/2 phosphorylation, and cyp11a and 3β-HSD expressions, which are required for P4 synthesis (Figure 8). The results of the present study have shown that E2 increases ERK1/2 phosphorylation, but MAP3K8i and MAP3K8 siRNA annul ERK1/2 phosphorylation induced by E2. These infer that MAP3K8 mediates the stimulating effects of E2 on P4 synthesis by enhancing ERK1/2 phosphorylation. However, their interactions and the detailed mechanisms need to be elucidated in future studies.
Figure 8. The function of MAP3K8 in the signaling pathway of E2-stimulated P4 synthesis.
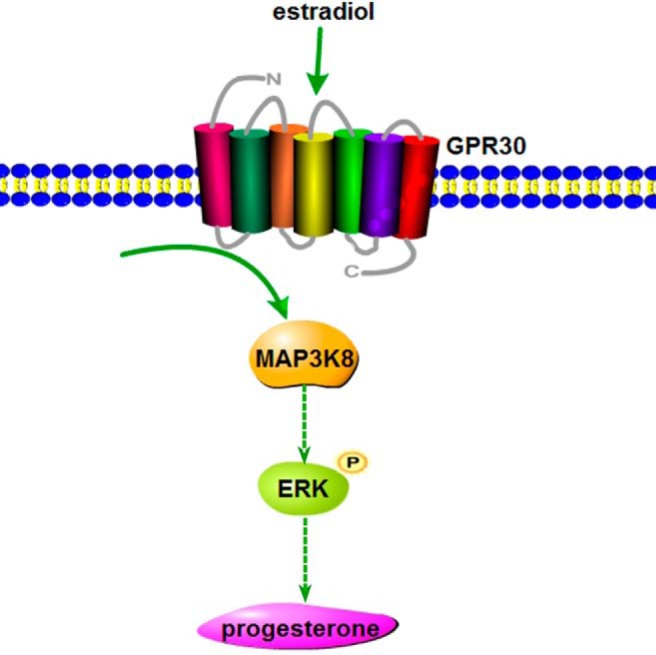
E2 regulates MAP3K8 expression through GPR30, and MAP3K8 subsequently enhances ERK1/2 phosphorylation to stimulate P4 synthesis in mouse granulosa-luteinized cells and CL.
In conclusion, our results show that MAP3K8 is highly expressed in mouse CL and involved in regulating P4 synthesis in mouse CL, and the signaling of E2-stimulating P4 synthesis is through GPR30 and MAP3K8, which subsequently enhances ERK1/2 phosphorylation and the expressions of cyp11a and 3β-HSD. In light of the results of our study, MAP3K8 plays a critical role in regulating CL hormone synthesis, and the reported signal molecules of E2 affecting P4 synthesis are potential for our understanding the related mechanisms of ovary physiology and the pharmacological interventions.
Acknowledgments
S.C. and Y.L. conceived and designed the experiments; Yi.L., Yu.L., and D.Z. performed the experiments; Y.L., J.L., K.G., and S.C. analyzed the data; Yi.Y. and Yu.L. contributed reagents/materials/analysis tools; and S.C. and Yi.L. wrote the manuscript.
This work was supported by the the National Basic Research Program of China (2012CB944703 and 2013CB945503) and the Natural Science Foundation of China (31172288 and 31172287).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the the National Basic Research Program of China (2012CB944703 and 2013CB945503) and the Natural Science Foundation of China (31172288 and 31172287).
Footnotes
- CL
- corpus luteum
- E2
- estradiol
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GPR30
- G protein-coupled receptor 30
- H89
- N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride
- hCG
- human chorionic gonadotropin
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- ICI
- ICI 182780
- IHC
- immunohistochemistry
- MAP3K8
- MAPK 8
- MAP3K8i
- MAP3K8 signaling inhibitor
- P4
- progesterone
- PKA
- protein kinase A
- PKC
- protein kinase C
- PRL
- prolactin
- RT-qPCR
- real-time quantitative PCR
- siRNA
- small interfering RNA
- StAR
- steroidogenic acute regulatory protein
- TBS
- Tris-buffered saline
- WB
- Western blot.
References
- 1. Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. [DOI] [PubMed] [Google Scholar]
- 2. Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. [DOI] [PubMed] [Google Scholar]
- 3. Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DT, Knudsen KA, Miller LS. Effects of prolactin upon cholesterol metabolism and progesterone biosynthesis in corpora lutea of rats hyophysectomized during pseudopregnancy. Endocrinology. 1970;86:634–641. [DOI] [PubMed] [Google Scholar]
- 5. Wiltbank MC, Belfiore CJ, Niswender GD. Steroidogenic enzyme activity after acute activation of protein kinase (PK) A and PKC in ovine small and large luteal cells. Mol Cell Endocrinol. 1993;97:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Menon KM. Expression of high density lipoprotein-binding protein messenger ribonucleic acid in the rat ovary and its regulation by gonadotropin. Endocrinology. 1994;134:2360–2366. [DOI] [PubMed] [Google Scholar]
- 7. Holt JA. Regulation of progesterone production in the rabbit corpus luteum. Biol Reprod. 1989;40:201–208. [DOI] [PubMed] [Google Scholar]
- 8. Gibori G, Khan I, Warshaw ML, et al. Placental-derived regulators and the complex control of luteal cell function. Rec Prog Horm Res. 1988;44:377–429. [DOI] [PubMed] [Google Scholar]
- 9. Khan I, Belanger A, Chen YD, Gibori G. Influence of high-density lipoprotein on estradiol stimulation of luteal steroidogenesis. Biol Reprod. 1985;32:96–104. [DOI] [PubMed] [Google Scholar]
- 10. McLean MP, Puryear TK, Khan I, et al. Estradiol regulation of sterol carrier protein-2 independent of cytochrome P450 side-chain cleavage expression in the rat corpus luteum. Endocrinology. 1989;125:1337–1344. [DOI] [PubMed] [Google Scholar]
- 11. Azhar S, Khan I, Chen YD, Reaven GM, Gibori G. Regulation of luteal cell 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by estradiol. Biol Reprod. 1985;32:333–341. [DOI] [PubMed] [Google Scholar]
- 12. Puryear TK, McLean MP, Khan I, Gibori G. Mechanism for control of hydroxymethylglutaryl-coenzyme A reductase and cytochrome P-450 side chain cleavage message and enzyme in the corpus luteum. Endocrinology. 1990;126:2910–2918. [DOI] [PubMed] [Google Scholar]
- 13. Philipp BW, Shapiro DJ. Estrogen regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and acetyl-CoA carboxylase in Xenopus laevis. J Biol Chem. 1981;256:2922–2927. [PubMed] [Google Scholar]
- 14. Gibori G, Chen YD, Khan I, Azhar S, Reaven GM. Regulation of luteal cell lipoprotein receptors, sterol contents, and steroidogenesis by estradiol in the pregnant rat. Endocrinology. 1984;114:609–617. [DOI] [PubMed] [Google Scholar]
- 15. Telleria CM, Zhong L, Deb S, et al. Differential expression of the estrogen receptors α and β in the rat corpus luteum of pregnancy: regulation by prolactin and placental lactogens. Endocrinology. 1998;139:2432–2442. [DOI] [PubMed] [Google Scholar]
- 16. Frasor J, Barkai U, Zhong L, Fazleabas AT, Gibori G. PRL-induced ERα gene expression is mediated by Janus kinase 2 (Jak2) while signal transducer and activator of transcription 5b (Stat5b) phosphorylation involves Jak2 and a second tyrosine kinase. Mol Endocrinol. 2001;15:1941–1952. [DOI] [PubMed] [Google Scholar]
- 17. Rosenfeld CS, Wagner JS, Roberts RM, Lubahn DB. Intraovarian actions of oestrogen. Reproduction. 2001;122:215–226 [DOI] [PubMed] [Google Scholar]
- 18. Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 19. Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010;204:105–114. [DOI] [PubMed] [Google Scholar]
- 20. Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29:116–123. [DOI] [PubMed] [Google Scholar]
- 21. Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. [DOI] [PubMed] [Google Scholar]
- 22. Kim SO, Irwin P, Katz S, Pelech SL. Expression of mitogen-activated protein kinase pathways during postnatal development of rat heart. J Cell Biochem. 1998;71:286–301. [DOI] [PubMed] [Google Scholar]
- 23. Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol Cell Biol. 2007;85:420–424. [DOI] [PubMed] [Google Scholar]
- 24. Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA. 2006;103:3274–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohara R, Hirota S, Onoue H, Nomura S, Kitamura Y, Toyoshima K. Identification of the cells expressing cot proto-oncogene mRNA. J Cell Sci. 1995;108(Pt 1):97–103. [DOI] [PubMed] [Google Scholar]
- 26. Christoforidou AV, Papadaki HA, Margioris AN, Eliopoulos GD, Tsatsanis C. Expression of the Tpl2/Cot oncogene in human T-cell neoplasias. Mol Cancer. 2004;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. [DOI] [PubMed] [Google Scholar]
- 28. Zhang N, Lin JK, Chen J, et al. MicroRNA 375 mediates the signaling pathway of corticotropin-releasing factor (CRF) regulating pro-opiomelanocortin (POMC) expression by targeting mitogen-activated protein kinase 8. J Biol Chem. 2013;288:10361–10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olofsson J, Selstam G. Changes in corpus luteum content of prostaglandin F2α and E in the adult pseudopregnant rat. Prostaglandins. 1988;35:31–40. [DOI] [PubMed] [Google Scholar]
- 30. Hasumoto K, Sugimoto Y, Yamasaki A, et al. Association of expression of mRNA encoding the PGF2α receptor with luteal cell apoptosis in ovaries of pseudopregnant mice. J Reprod Fertil. 1997;109:45–51. [DOI] [PubMed] [Google Scholar]
- 31. Gavrin LK, Green N, Hu Y, et al. Inhibition of Tpl2 kinase and TNF-α production with 1,7-naphthyridine-3-carbonitriles: synthesis and structure-activity relationships. Bioorg Med Chem Lett. 2005;15:5288–5292. [DOI] [PubMed] [Google Scholar]
- 32. Lee WJ, Lan KH, Chou CT, et al. Tpl2 inhibitors thwart endothelial cell function in angiogenesis and peritoneal dissemination. Neoplasia. 2013;15:1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stocco CO, Zhong L, Sugimoto Y, Ichikawa A, Lau LF, Gibori G. Prostaglandin F2α-induced expression of 20α-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem. 2000;275:37202–37211. [DOI] [PubMed] [Google Scholar]
- 34. Stocco C. In vivo and in vitro inhibition of cyp19 gene expression by prostaglandin F2α in murine luteal cells: implication of GATA-4. Endocrinology. 2004;145:4957–4966. [DOI] [PubMed] [Google Scholar]
- 35. Wu Y, Luo H, Liu J, Kang D, McNeilly AS, Cui S. LIM homeodomain transcription factor Isl-1 enhances follicle stimulating hormone-β and luteinizing hormone-β gene expression and mediates the activation of leptin on gonadotropin synthesis. Endocrinology. 2010;151:4787–4800. [DOI] [PubMed] [Google Scholar]
- 36. Liu J, Cui S. Ontogeny of estrogen receptor (ER) α and its co-localization with pituitary hormones in the pituitary gland of chick embryos. Cell Tissue Res. 2005;320:235–242. [DOI] [PubMed] [Google Scholar]
- 37. Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology. 2009;150:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopez D, Sanchez MD, Shea-Eaton W, McLean MP. Estrogen activates the high-density lipoprotein receptor gene via binding to estrogen response elements and interaction with sterol regulatory element binding protein-1A. Endocrinology. 2002;143:2155–2168. [DOI] [PubMed] [Google Scholar]
- 41. Goyeneche AA, Telleria CM. Exogenous estradiol enhances apoptosis in regressing post-partum rat corpora lutea possibly mediated by prolactin. Reprod Biol Endocrinol. 2005;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. al-Matubsi HY, Fairclough RJ, Jenkin G. Oestrogenic effects of ICI 182,780, a putative anti-oestrogen, on the secretion of oxytocin and prostaglandin F2α during oestrous cycle in the intact ewe. Anim Reprod Sci. 1998;51:81–96. [DOI] [PubMed] [Google Scholar]
- 43. Kurebayashi J, Otsuki T, Yamamoto S, Kurosumi M, Nakata T, Akinaga S, Sonoo H. A pure antiestrogen, ICI 182,780, stimulates the growth of tamoxifen-resistant KPL-1 human breast cancer cells in vivo but not in vitro. Oncology 1998;55(suppl 1):23–34. [DOI] [PubMed] [Google Scholar]
- 44. Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Syin C, Parzy D, Traincard F, et al. The H89 cAMP-dependent protein kinase inhibitor blocks Plasmodium falciparum development in infected erythrocytes. Eur J Biochem. 2001;268:4842–4849. [DOI] [PubMed] [Google Scholar]
- 46. Eckly-Michel AE, Le Bec A, Lugnier C. Chelerythrine, a protein kinase C inhibitor, interacts with cyclic nucleotide phosphodiesterases. Eur J Pharmacol. 1997;324:85–88. [DOI] [PubMed] [Google Scholar]
- 47. Frasor J, Park K, Byers M, et al. Differential roles for signal transducers and activators of transcription 5a and 5b in PRL stimulation of ERα and ERβ transcription. Mol Endocrinol. 2001;15:2172–2181. [DOI] [PubMed] [Google Scholar]
- 48. Redmond AF, Pepe GJ. Uterine progesterone metabolism during early pseudopregnancy in the rat. Biol Reprod. 1986;35:949–955. [DOI] [PubMed] [Google Scholar]
- 49. Manna PR, Stocco DM. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J Signal Trans. 2011;2011:821615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horseman ND, Zhao W, Montecino-Rodriguez E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ormandy CJ, Naylor M, Harris J, et al. Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Rec Prog Horm Res. 2003;58:297–323. [DOI] [PubMed] [Google Scholar]
- 52. Ceci JD, Patriotis CP, Tsatsanis C, et al. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 1997;11:688–700. [DOI] [PubMed] [Google Scholar]