Abstract
Progranulin (PGRN) has recently emerged as an important regulator for glucose metabolism and insulin sensitivity. However, the underlying mechanisms of PGRN in the regulation of insulin sensitivity and autophagy remain elusive. In this study, we aimed to address the direct effects of PGRN in vivo and to evaluate the potential interaction of impaired insulin sensitivity and autophagic disorders in hepatic insulin resistance. We found that mice treated with PGRN for 21 days exhibited the impaired glucose tolerance and insulin tolerance and hepatic autophagy imbalance as well as defective insulin signaling. Furthermore, treatment of mice with TNF receptor (TNFR)-1 blocking peptide-Fc, a TNFR1 blocking peptide-Fc fusion protein to competitively block the interaction of PGRN and TNFR1, resulted in the restoration of systemic insulin sensitivity and the recovery of autophagy and insulin signaling in liver. Consistent with these findings in vivo, we also observed that PGRN treatment induced defective autophagy and impaired insulin signaling in hepatocytes, with such effects being drastically nullified by the addition of TNFR1 blocking peptide -Fc or TNFR1-small interference RNA via the TNFR1-nuclear factor-κB-dependent manner, indicating the causative role of PGRN in hepatic insulin resistance. In conclusion, our findings supported the notion that PGRN is a key regulator of hepatic insulin resistance and that PGRN may mediate its effects, at least in part, by inducing defective autophagy via TNFR1/nuclear factor-κB.
Hepatic insulin resistance is highly integrated with chronic inflammation, which contributes to a systemic, low-grade inflammatory state now known as metabolic inflammation and thereby promotes the development of systemic insulin resistance (1). However, the link between inflammation and hepatic insulin resistance is not fully understood. Progranulin (PGRN), a secreted protein that plays an important role in several processes including immune response (2), has recently emerged as an important regulator between inflammatory action and insulin resistance (3).
PGRN, also known as proepithelin, granulin/epithelin precursor or PC cell-derived growth factor, has been shown to be a pluripotent growth factor that mediates cell growth, wound healing, tumor genesis and neurodegenerative disease such as frontotemporal dementia (4–7). However, recent studies supported the novel function of PGRN in regulating the energy metabolism (3, 8–11). For instance, diet-induced-obesity mice with PGRN deficiency exhibited lower body weight and ameliorated insulin sensitivity, whereas administration of recombinant PGRN induced glucose intolerance and insulin resistance in wild-type mice (3). Several clinical investigations also demonstrated that serum PGRN was associated with the parameters of adiposity, glucose tolerance, insulin resistance, and inflammatory factors (12–17). Clinically, circulating PGRN is significantly higher in subjects with type 2 diabetes mellitus and positively associated with high-sensitivity C-reaction protein, IL-6, and macrophage infiltration in omental adipose tissue (12–16). In particular, PGRN is more highly expressed in visceral fat area of the insulin-resistant patients with morbid obesity than in their age-, sex-, and body mass index-matched, insulin-sensitive counterparts (17).
Although the role of PGRN in energy homeostasis has just recently been identified, the functional basis for PGRN-mediated insulin resistance remains elusive. However, the relevance of PGRN to autophagic response involving a variety of adipocytokines including IL-6 and TNF-α has recently drawn considerable attention (18–22). PGRN has been shown to impair insulin signaling in 3T3-L1 adipocytes through IL-6, an inducer of autophagy (3). Consistently, PGRN−/− mice reveal the autophagic disturbance with alterations in lysosomal homeostasis as evidenced by an abnormal accumulation of lipofuscin granules and p62 proteins (23), and inhibition of autophagy with chloroquine exhibit elevated endogenous PGRN levels (24, 25), indicating the causative link between PGRN and autophagic activity in insulin resistance.
Collectively these evidences support a newly regulatory role of PGRN on energy homeostasis and chronic inflammation, which raises the possibility that PGRN may contribute to the progression of hepatic insulin resistance and metabolic dysfunction. Because the PGRN membrane receptor has not yet been identified, it is important to define the early stages of PGRN-mediated signaling from the plasma membrane. In this study, we aimed to address the direct effects of PGRN in vivo and to evaluate the potential interaction of impaired insulin sensitivity and autophagic disturbance during this process. Our results support the therapeutic potential of this new player in the regulation of hepatic insulin resistance and metabolic disorders.
Materials and Methods
Materials
All chemicals used were of analytical grade and were purchased from Sigma-Aldrich unless stated otherwise. The following antibodies were used: anti-Atg7 (1:400), anti-p62 (1:300), and anti-LC3 (1:500) (Cell Signaling Technology Inc); anti-PGRN (1:400), anti-phosphorylated (p) insulin receptor substrate (IRS)-1 (1:300), anti-pY20 (1:300), anti-p-AKT (1:400), anti-TNF receptor (TNFR)-1 (1:400), anti-multisubunit inhibitory-κB kinase (IKK)-β (1:400), anti-nuclear factor-κB (NF-κB) (1:1000), anti-p-inhibitory-κBα (1:500), anti-glyceraldehyde-3-phosphate dehydrogenase (1:5000), and peroxidase goat antirabbit IgG (1:3000) and peroxidase goat antimouse IgG (1:3000) (Santa Cruz Biotechnology Inc).
Preparation of recombinant mouse PGRN and human IgG1 Fc-fused TNFR1 blocking peptide (TNFR1BP)-Fc
The methods were followed by the literature (3) except for the source of Chinese hamster kidney-K1 cells (American Type Culture Collection), and the venders of CD OptiCHO medium (Life Technologies). The pFLAG-CMV1 vector was purchased from Addgene. pCAGIPuro-FLAG was constructed by subcloning the insert encoding the preprotrypsin signal peptide and FLAG epitope of pFLAG-CMV1 into pCAGIPuro. pCAGIPuro-FLAG was constructed by Sangon Biotech. Purified PGRN was made endotoxin free using the Detoxi-Gel endotoxin-removing column (Thermo Scientific) as recommended by the manufacturer. Preparation of human IgG1-Fc fused TNFR1BP-Fc was as described previously (26). The characteristics of TNFR1BP-Fc are as follows: a TNFR1-blocking peptide originated from screening a phage 12-mer peptide library (New England Biolabs) using soluble TNFR1 as bait (26). The DNA sequence encoding the TNFR1 blocking peptide was directly synthesized by Protein technologies Inc.
Animal treatment and cell culture
Four-week-old C57BL/6J female mice were obtained from the Medical Experimental Animal Center (Xi'an Jiaotong University). All studies were performed in accordance with the Institutional Animal Care and Use Committee of Xi'an Jiaotong University. Mice were maintained in a temperature- (22°C), humidity-, and light (12 h light, 12 h darkness, darkness from 7:30 pm to 7:30 am)-controlled environment. Sixty mice fed regular diet and allowed ad libitum access to chow and water were distributed in the following six groups: 1) vehicle (saline solution ip); 2) PGRN (recombinant mouse PGRN, ip 1 μg/g body weight per day, once a day); 3) IgG1Fc (injection via tail vein, 1 μg/g body weight, every 3 d); 4) TNFR1BP-Fc (injection via tail vein, 1 μg/g body weight, every 3 d); 5) PGRN (ip 1 μg/g body weight per day, once a day) + IgG1Fc (injection via tail vein, 1 μg/g body weight, every 3 d); and 6) PGRN (ip 1 μg/g body weight per day, once a day) + TNFR1BP-Fc (injection via tail vein, 1 μg/g body weight, every 3 d). At the end of the 21-day study period, the mice received vehicle or an ip injection of insulin at a dosage of 2 IU/kg for the insulin signaling test; 15 minutes after the injection, all animals were euthanized and their liver tissues and blood samples were obtained and stored at −80°C for subsequent analysis. BNL cl.2 cells were purchased from American Tissue Culture Collection and maintained at 37°C in a humidified atmosphere of 5% CO2 in DMEM (Gibco) with 10% fetal bovine serum (Gibco). In some experiments, the cells were treated with 500 μM of palmitate [free fatty acids (FFA)] (Sigma-Aldrich; catalog number P5585) for 16 hours. For the effect of recombinant mouse PGRN, cells were treated with 100 ng/mL of PGRN for 10 hours. For insulin signaling, cells were stimulated with 10 nM insulin for 10 minutes.
Immunohistochemistry of liver tissues and metabolic tests
The immunohistomorphometry was performed as previously described (27). Glucose tolerance testing (GTT) was performed after the mice were fasted overnight. Insulin tolerance testing (ITT) was performed after the animals were fasted for 4 hours. Metabolic tests were performed using a standard protocol as described previously (28). The in vivo fatty acid oxidation was performed based on a previous report (29, 30). Briefly, [1-14C]oleic acid [specific activity, 56.3 mCi/mmol (2083 MBq/mmol)] was purchased from PerkinElmer Inc. Oleic acid was dissolved in 10 mL chloroform to prepare 50 mg/mL stock solution and stored in a glass container at −20°C. The working solution was prepared by adding 500 μL of cold oleic acid solution (50 mg/mL) into 0.33 mL of fatty acid-free BSA (0.1 mg/mL to prepare the working solution (30 μg/μL). Ten microliters of the working solution was injected ip into the mice with [1-14C]oleic acid [30 μL, 0.1 mCi/mL (1 Ci = 37 GBq)] at 12:00 am. Mice were put in metabolic chambers connected with 1 N NaOH trap to capture died 14CO2. 14C radioactivity from NaOH trap was counted at 30-minute intervals over the next 4 hours, and the slope for the initial 2 hours was plotted because captured radioactivity is saturated after 2 hours. Hepatic triglyceride contents were measured using a triglyceride detection kit (Sigma-Aldrich; TRI19–1KT) according to the manufacturer's instructions.
Autophagy flux analysis
Chloroquine experiment was described as previously (31, 32). Chloroquine (Sigma-Aldrich) dissolved in PBS was injected sc at a dose of 50 mg/kg body weight, and mice were killed 24 hours following the injection. Vehicle-treated mice were injected sc with an equal volume of PBS. The injection dose of chloroquine was determined based on previous reports and our preliminary study, which had no detectable effect on liver function. After the dissection, the liver tissues were immediately homogenized in lysis buffer for western blotting analysis.
Electronic microscopy (EM) analysis
Cell samples were fixed in 4% paraformaldehyde/2% glutaraldehyde/0.1 M sodium cacodylate (pH 7.3), postfixed in 1% osmium tetraoxide, and embedded in epoxy resin (Epon). Ultrathin sections (80 nm) were stained with aqueous uranyl acetate and lead citrate and examined with a JEOL 2000EX transmission EM (JEOL). For quantification of autophagolysosome-like vacuoles, the numbers of autophagolysosomal-like vacuoles were counted in each field and normalized by the surface area.
Extraction of mRNA, real-time PCR, and gene silencing
Total RNA was prepared from isolated liver tissues and cells by Trizol extraction (QIAGEN) according to the manufacturer's protocol. Small interference RNA (siRNA) oligos targeted for mouse PGRN (Santa Cruz Biotechnology; catalog number sc-39262), TNFR1 (Santa Cruz Biotechnology; catalog number sc-36688), and Atg7 (Santa Cruz Biotechnology; catalog number sc-41448) were transfected using Lipofectamine 2000 (Invitrogen). A pool of siRNAs consisting of scrambled sequences of a similar length was transfected similarly as control siRNA.
Western blot analysis
Tissues and cells under various treatments were lysed in lysis buffer containing 25 mM Tris HCl (pH 6.8), 2% sodium dodecyl sulfate, 6% glycerol, 1% 2-mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, 0.2% bromphenol blue, and a protease inhibitor cocktail for 20 minutes. Western blotting was performed using a standard protocol as described previously (33).
Statistical analysis
Statistical analyses were performed using IBM SPSS 20.0 software. The data are expressed as means ± SD. Statistical analysis between the two groups was performed using an unpaired, two-tailed Student t test or ANOVA followed by post hoc tests. A value of P < .05 was considered statistically significant.
Results
Effects of PGRN on glucose metabolism and insulin sensitivity in mice
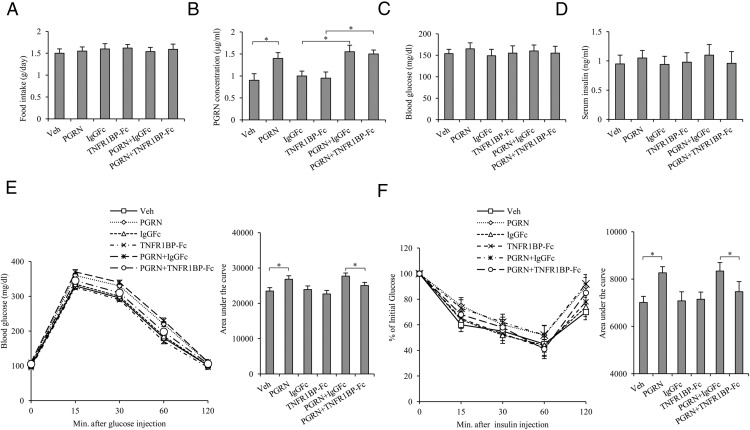
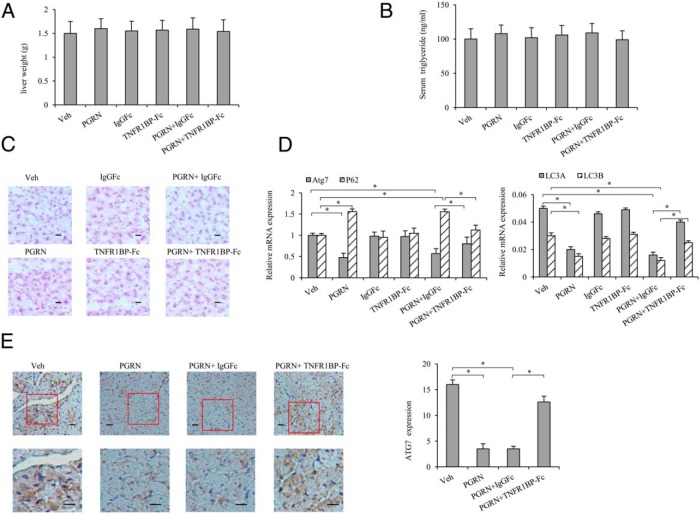
To address the effects of PGRN on glucose metabolism and insulin sensitivity in vivo, mice were administered recombinant PGRN ip daily at the dosage of 1 μg/g body weight per day under a standard diet condition for 3 weeks. We found that serum PGRN levels were elevated to 1.4 μg/mL, about 1.6-fold of control, after treatment with PGRN for 21 days (Figure 1B), whereas no difference was found between control and PGRN-treated mice with respect to other indicators, such as food intake, blood glucose, and serum insulin levels under these conditions (Figure 1, A, C, and D). We also noticed that the TNF-α level had no significant change either in serum or in the liver tissues (Supplemental Figure 1, A and B). Administration of PGRN decreased glucose tolerance and insulin sensitivity as measured by GTT and ITT, with no difference observed in body weight (Figure 1, E and F), indicating the causative role of PGRN involved in insulin resistance in vivo. Nevertheless, there is no significant difference observed either in liver weight or in serum triglyceride among the groups (Figure 2, A and B), and mice injected with recombinant PGRN and/or TNFR1BP-Fc did not accumulate lipids in the liver (Figure 2C). Moreover, the injection of PGRN and TNFR1BP-Fc was associated with improved glucose tolerance and insulin tolerance compared with mice injected with PGRN and IgG1Fc (Figure 1, E and F), indicating that TNFR1BP-Fc, at least partially, blocks the negative effect of PGRN on glucose metabolism in vivo.
Figure 1. Effects of recombinant mouse PGRN on glucose metabolism and insulin sensitivity in vivo.
Mice were injected daily with saline solution as vehicle, 1 μg/g body weight per day recombinant PGRN, 1 μg/g body weight IgG1Fc every 3 days, and 1 μg/g body weight TNFR1BP-Fc every 3 days of for 21 days. A, Food intake. B, Serum PGRN. C, Blood glucose. D, Serum insulin. E, GTT. F, ITT. Data are expressed as means ± SD in each bar graph from 12 to 15 mice per group. *, P < .05. Veh, Vehicle.
Figure 2. Effects of PGRN and TNFR1BP-Fc on liver steatosis and hepatic autophagy.
Mice were injected daily with 0.9% saline solution as vehicle, 1 μg/g body weight/d recombinant PGRN, 1 μg/g body weight IgG1Fc every 3 days, and 1 μg/g body weight TNFR1BP-Fc every 3 days for 21 days. A, Liver weight. B, Serum TGs. C, Oil Red O staining of liver sections (magnification, ×100). Scale bar, 20 μm. D, Relative expression of Atg7, p62, and LC3A/B in the liver normalized to β-actin (real time PCR). E, Immunohistochemical staining (10 times) of the Atg7 protein in tissue sections. The lower panels are high magnification (×40) of the field marked with a red rectangle in the upper panels. Data are expressed as means ± SD in each bar graph from 12 to 15 mice per group. Scale bar, 20 μm. *, P < .05. Veh, Vehicle.
Systemic effects of PGRN on hepatic autophagic response and insulin signaling
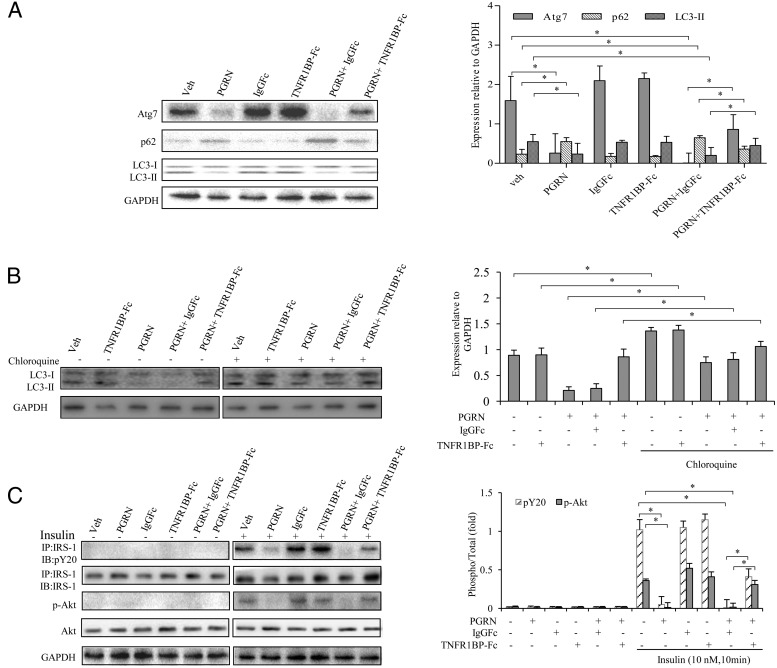
To test whether PGRN is required to affect hepatic insulin signaling and autophagy in vivo, we first examined the expression patterns of several autophagic indicators by real-time PCR in hepatic tissues. Of note, we found that mice treated with PGRN displayed a significant decrease in the mRNA level of Atg7 and a dramatic increase in P62 expression compared with the mice that received vehicle, with such effects being reversed by treatment of TNFR1BP-Fc concomitantly (Figure 2D). Consistent with a decrease in mRNA levels of Atg7, immunohistochemical detection of hepatic tissues revealed that Atg7 expression was decreased in PGRN-treated mice compared with that of mice that received vehicle, supporting the biochemical alterations of Atg7 expression in mice hepatic tissues. The level of Atg7 expression had a significant increase in mice cotreated with PGRN and TNFR1BP-Fc (Figure 2E). Then we detected the protein expression of indicators of autophagy by Western blot. As expected, there was a remarkable inhibition of autophagy as evidenced by the down-regulation of LC3II, in particular, Atg7 protein levels. Meanwhile, p62 was elevated in the liver tissue of mice treated with PGRN compared with that of mice that received vehicle. As a result of PGRN and TNFR1BP-Fc cotreatment, the mice had an increased Atg7 and LC3II expression compared with the mice cotreated by PGRN and IgGFc (Figure 3A). A number of studies have previously indicated that mRNA levels of autophagy genes were correlated with decreased autophagic flux in a variety of experiment systems (34–36). Likewise, significant changes were evident in the expression of Atg7, P62, and LC3 II mRNAs in PGRN and/or TNFR1BP-Fc treatment subjects (Figure 2D).
Figure 3. Effects of PGRN on hepatic autophagy and insulin sensitivity via the TNFR1-dependent manner in vivo.
Mice were injected daily with 0.9% saline solution as vehicle, 1 μg/g body weight per day recombinant PGRN, 1 μg/g body weight IgG1Fc every 3 days, and 1 μg/g body weight TNFR1BP-Fc every 3 days for 21 days. For insulin signaling, they were injected with 2 IU/kg for insulin. A, Atg7, P62, and LC3II in liver samples. B, Determination of autophagic flux in mice receiving PGRN and/or TNFR1BP-Fc in the absence or presence of chloroquine. Liver tissue lysates were analyzed by Western blot using an anti-LC3 antibody. Results are given as fold increase normalized to internal control (GAPDH). C, IRS-1 phosphorylation and Akt phosphorylation in liver samples. Quantification was performed on three different fields per animals. Data are expressed as means ± SD in each bar graph from 12 to 15 mice per group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase IB, immunoblotting; IP, immunoprecipitation; Veh, vehicle. *, P < .05.
We next used the lysosomal protease inhibitor chloroquine to evaluate the autophagic flux. Although an increase of the LC3II level was readily observable with the treatment of chloroquine, the addition of PGRN caused a significant difference in the LC3II expression in hepatic tissue with chloroquine (Figure 3B). Consistently, the insulin sensitivity was also markedly decreased in the hepatic tissues of mice treated with PGRN compared with that of control as assessed by the phosphorylation of IRS-1 and AKT, although the insulin sensitivity was restored in the mice cotreated with PGRN and TNFR1BP-Fc (Figure 3C). These results support a clear tendency toward the relevance of PGRN to hepatic insulin signaling and autophagic actions.
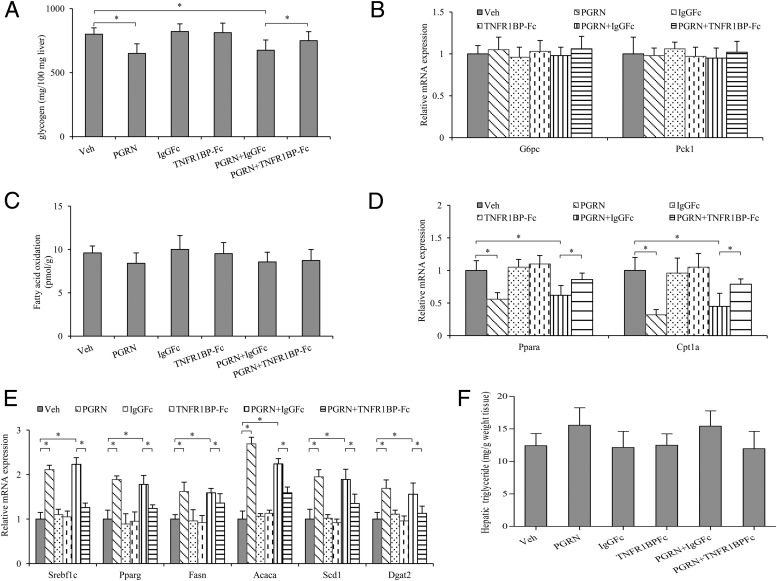
Defective hepatic autophagy in PGRN-treated mice resulted in decreased glycogen and unusual lipolytic and lipogenic genes expression
As the main detoxifying organ of the body, liver is a major regulator of glucose and lipid metabolism in the body and it plays a central role in the synthesis and degradation (oxidation) of fatty acids (1). To address the role of PGRN in hepatic glucose homeostasis, we examined the hepatic glycogen content and lipid metabolism of the mice. As shown in the results, the treatment of PGRN reduced the levels of glycogen in hepatic tissues, whereas the hepatic glycogen content had a significant increase in mice cotreated with PGRN and TNFR1BP-Fc (Figure 4A). Moreover, we found there was no significant change in the expression of gluconeogenic genes such as G6pc and Pck1 among the groups (Figure 4B). We next evaluated β-oxidation by measuring release of 14CO2 after the administration of [1-14C] oleic acid. No significant difference was observed in the β-oxidation rate (Figure 4C). To gain further insights into the unchanged β-oxidation rate, we performed mRNA expression using real-time PCR. We observed a markedly down-regulated expression of the β-oxidation-related genes such as Ppara and Cptla in the hepatic tissues of mice treated with PGRN. Meanwhile, TNFR1BP-Fc treatment partially restored the expression levels of Ppara and Cptla in mice treated with PGRN (Figure 4D). In contrast, lipogenic genes expression in the hepatic tissues was up-regulated in mice injected with PGRN. Mice coinjected with PGRN and TNFR1BP-Fc showed a significant down-regulation of lipogenic genes in hepatic tissues (Figure 4E). An increase trend was also observed in the hepatic triglyceride content in the mice treated with PGRN and/or TNFR1BP-Fc (Figure 4F), although these changes did not seem to be remarkable compared with the controls (P = .064 and P =.057, respectively), implicating that multiple mechanisms may contribute to the PGRN-induced effects.
Figure 4. Effects of PGRN and TNFR1BP-Fc on hepatic glucose and lipid metabolism in vivo.
Mice were injected daily with 0.9% saline solution as vehicle, 1 μg/g body weight per day recombinant PGRN, 1 μg/g body weight IgG1Fc every 3 days and 1 μg/g body weight TNFR1BP-Fc every 3 days for 21 days. A, Glycogen levels. B, Relative mRNA level of gluconeogenesis-associated genes in the liver. C, In vivo β-oxidation of infused [1-14C]oleic acid. D, Relative mRNA levels of genes associated with β-oxidation in hepatic tissues. E, Relative mRNA levels of genes associated with fatty acid and triacylglycerol (TG) synthesis in the liver. F, Hepatic triglyceride content, Results are expressed per gram of liver tissue. Data are expressed as means ± SD in each bar graph from 12 to 15 mice per group. *, P < .05. Veh, vehicle.
Ablation of PGRN increased autophagy and improved insulin signaling on FFA-treated hepatocytes
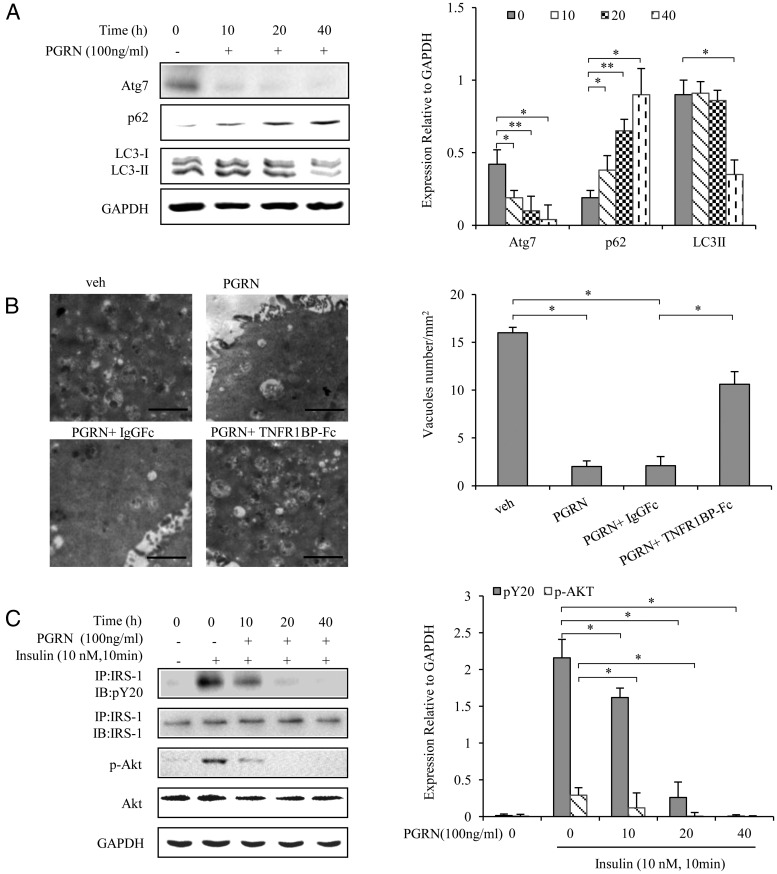
To address the effects of PGRN on autophagy and insulin sensitivity, BNL cl.2 hepatocytes and primary mouse hepatocytes were treated with 100 ng/mL PGRN at different time points, respectively. As shown in Figure 5A and Supplemental Figure 2A, PGRN caused a time-dependent decrease in protein expression of Atg7 and LC3-II as well as an increase in the protein expression of p62. An EM examination in BNL cl.2 hepatocytes demonstrated a significant reduction in autophagosome/autolysosome formation in the cells treated with PGRN compared with control, with this effect of PGRN being reversed by addition of TNFR1BP-Fc (Figure 5B). Because abnormal autophagy has been shown to be implicated in the Akt signaling pathway, we postulated that PGRN might affect insulin signaling in hepatocytes. As expected, decreased insulin sensitivity was observed by the treatment of PGRN both in BNL cl.2 hepatocytes and in primary mouse hepatocytes, as assessed by the phosphorylation of IRS-1 and Akt (Figure 5C and Supplemental Figure 2B). These results indicated that PGRN expression is strongly associated with autophagy dysfunction and insulin resistance at the cell level.
Figure 5. Effects of PGRN in autophagy and insulin receptor signaling in hepatocyte.
BNL cl.2 cells were treated with or without recombination PGRN (100 ng/mL). For insulin signaling, cells were stimulated with 10 nM insulin for 10 minutes. Indicators of autophagy and insulin receptor signaling were measured at protein levels. The relative quantity of proteins was analyzed with Quantity One software. A, Time course for autophagy in hepatocyte after the PGRN treatment. B, Representative electron micrographs (×10 000) of hepatocyte. Quantification of autophagolysosome-like vacuoles per field in the EM images, Scale bars, 1 μm. C, Phosphorylation of IRS-1 and Akt in hepatocyte. Data are expressed as means ± SD in each bar graph and represent the average of three independent experiments. *, P < .05; **, P < .01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation; Veh, vehicle.
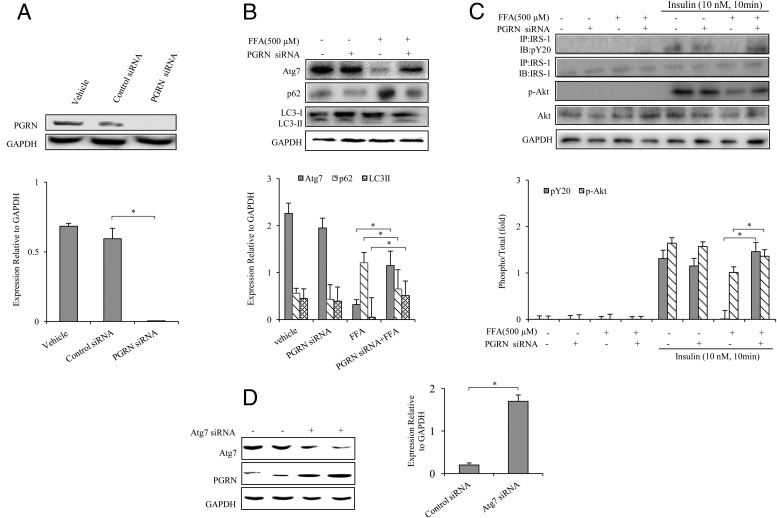
To further confirm the involvement of PGRN in autophagy and insulin signaling of BNL cl.2 hepatocytes and primary mouse hepatocytes, we used siRNA against PGRN to determine whether ablation of PGRN could increase autophagy and improve insulin signaling on different cellular models of insulin resistance induced by palmitate (FFA) or dexamethasone. PGRN siRNA was validated by the measurement of reduced PGRN protein expression in PGRN siRNA-transfected BNL cl.2 hepatocytes (Figure 6A). After transfection with PGRN siRNA, we found that the autophagic imbalance was corrected as evidenced by the enhanced expression of Atg7 and LC3II and the reduced expression of p62 in the presence of FFA (Figure 6B). The insulin-stimulated phosphorylation of both IRS-1 and Akt was increased in the presence of FFA in PGRN-knockdown BNL cl.2 hepatocytes (Figure 6C). In addition, PGRN protein expression was increased in hepatocytes interfered with Atg7 siRNA (Figure 6D). Furthermore, dexamethasone induced autophagy dysfunction and decrement of insulin-stimulated phosphorylation of IRS-1 and Akt were restored in primary mouse hepatocytes (Supplemental Figure 2, C and D) by the transfection of PGRN siRNA. Similarly, insulin signaling and autophagy were also improved in the dexamethasone-treated BNL cl.2 hepatocytes after PGRN ablation (Supplemental Figure 3).
Figure 6. Ablation of PGRN increased autophagy and improved insulin signaling on FFA-treat treated hepatocytes.
Cells were cultured with or without 100 nM PGRN siRNA. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 minutes. Indicators of autophagy and insulin receptor signaling were measured at protein levels. The relative quantity of proteins was analyzed with Quantity One software. A, PGRN siRNA was validated by reduction of PGRN protein level. B, Autophagy indicators in hepatocytes. C, Phosphorylation of IRS-1and Akt in hepatocytes. D, Effect of Atg7 siRNA on PGRN expression in hepatocytes. Data are expressed as means ± SD in each bar graph and represent the average of three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation. *, P < .05.
PGRN-induced autophagy imbalance and impaired insulin signaling via the TNFR/NF-κB-dependent manner
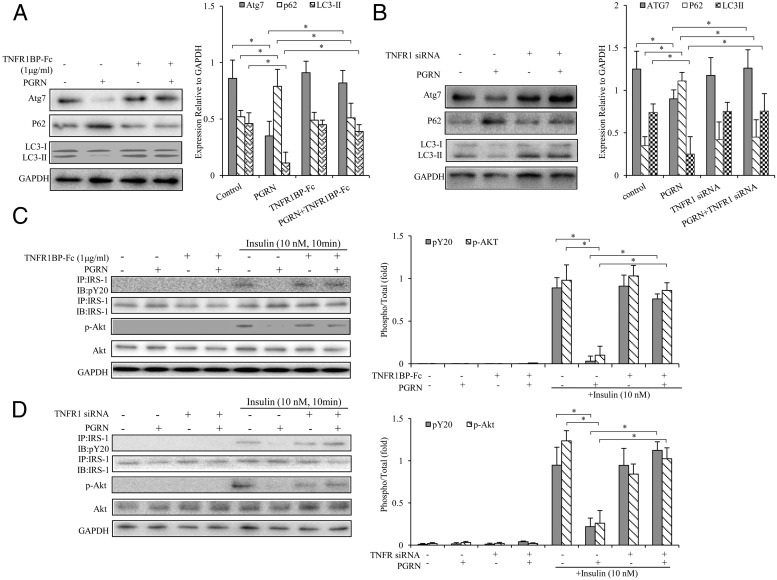
Recent findings suggested that PGRN bind to TNFR1 and mediate its antiinflammatory effects in collagen antibody-induced arthritis and collagen-induced arthritis (37). Therefore, we next sought to explore the molecular mechanism of PGRN/TNFR1 interaction on metabolic regulation in hepatocytes. We used different strategies (TNFR1BP-Fc or TNFR1 siRNA) to interfere with TNFR1 in cultured hepatocytes. We examined autophagic response and insulin action in the presence of 100 ng/mL TNFR1BP-Fc and/or100 ng/mL PGRN in BNL cl.2 hepatocytes. We found the decreased expression of Atg7 and LC3II and elevated p62 expression by PGRN were abolished with the treatment of TNFR1BP-Fc (Figure 7A). Moreover, the blockage of TNFR1 by TNFR1BP-Fc could also enhance the IRS-1 and Akt phosphorylation after PGRN treatment (Figure 7C). As expected, TNFR1 siRNA treatment alone had no effect on autophagic expression, whereas the addition of TNFR1 siRNA could restore the defective autophagy and impaired insulin sensitivity induced by PGRN (Figure 7, B and D). Consistent with these results, there were the expected results that the knockdown of TNFR1 could reverse PGRN-induced autophagy suppression and impaired insulin signaling in primary mouse hepatocytes (Supplemental Figure 4). Taken together, these observations confirmed that PGRN induced autophagy defection and impaired insulin signaling, at least in part, through a TNFR1-dependent manner.
Figure 7. Blockade of TNFR1 or knockdown TNFR1 on autophagy and insulin signaling in hepatocytes.
Cells were cultured with TNFR1BP-Fc or 100 nM TNFR1 siRNA. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 minutes. Indicators of autophagy and insulin receptor signaling were measured at protein levels. The relative quantity of proteins was analyzed with Quantity One software. A and B, Expression of Atg7, P62, and LC3II in hepatocytes. C and D, Phosphorylation of IRS-1 and Akt in hepatocytes. Data are expressed as means ± SD in each bar graph and represent the average of three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation. *, P < .05.
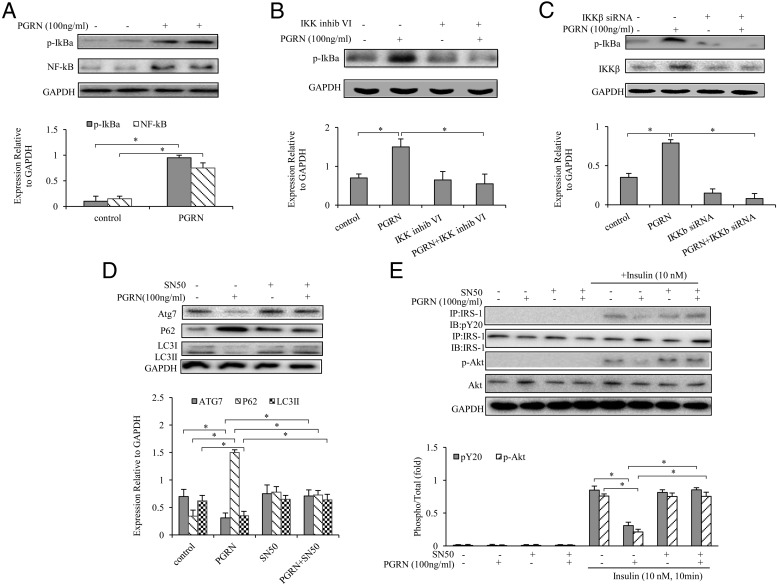
The classical NF-κB pathway is stimulated by a wide variety of cellular stressors and members of the Toll-like and the TNF receptor families particularly recognized by TNFR1 (38). Recent studies suggested that NF-κB activation contributes to hepatic insulin resistance via inhibitory cross talk within the hepatocytes, resulting in the blockade of specific steps in downstream insulin signaling pathways (39, 40). To investigate whether PGRN impaired insulin signaling and induced autophagy defection via the TNFR1/NF-κB-dependent manner, we first observed an induction of inhibitory-κBα phosphorylation after PGRN treatment in hepatocytes, indicating an activation of the canonical NF-κB pathway (Figure 8A). Because the stimulation of the multisubunit IKK complex triggers the phosphorylation-dependent proteasomal degradation of inhibitory-κBα, we then demonstrated that IKKβ inhibitor VI, a selective inhibitor of the canonical pathway-specific IKKβ subunit, blocks inhibitory-κBα phosphorylation, as does the siRNA-mediated knockdown of IKKβ protein (Figure 8, B and C). Treatment of PGRN suppressed autophagy and impaired insulin signaling, whereas the blockade of NF-κB by the addition of SD50, a known NF-κB inhibitor in the culture medium, nullified the effect of PGRN (Figure 8, D and E), suggesting that the effects of PGRN upon autophagy and insulin signal pathway are in IKKβ/NF-κB-dependent mechanisms. Taken together, these results indicated that NF-κB activation is required for PGRN to suppress autophagy and to inhibit insulin signaling and implicated NF-κB as a factor in promoting hepatocellular insulin resistance.
Figure 8. PGRN-dependent NF-κB activation in hepatocytes.
Cells were cultured with or without 100 ng/mL PGRN. For blocking IKKβ, cells were treated with or without inhibitor VI (10 mM) and 100 nM IKKβ siRNA. For blocking NF-κB, cells were treated with or without 10 nM SD50 (an NF-κB inhibitor). For insulin signaling, cells were stimulated with 10 nM of insulin for 10 minutes. Indicators of autophagy and insulin receptor signaling were measured at protein levels. The relative quantity of proteins was analyzed with Quantity One software. A, Phosphorylation of IκBα and NF-κB in liver cells. B, Effect of inhibitor VI (10 mM) on PGRN dependent NF-κB activation. C, Effect of IKKβ siRNA on PGRN -dependent NF-κB activation. D, Expression of Atg7, P62, and LC3II in hepatocytes. E, Phosphorylation of IRS-1 and Akt. Data are expressed as means ± SD in each bar graph and represent the average of three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation. *, P < .05.
Discussion
Recent studies have implicated PGRN in diet-induced obesity and insulin resistance (3, 13–16). However, much remains to be elucidated regarding the mechanism of PGRN action before its putative potential as therapeutic target can be realized. In the present study, mice developed signs of insulin resistance and defective hepatic autophagy upon long-term treatment with PGRN, and these effects were ameliorated when TNFR1 blockade was administered simultaneously. Consistent with these findings in vivo, PGRN also plays a pivotal role in insulin sensitivity involving autophagic mechanism in cultured hepatocytes. Collectively, these findings support the notion that PGRN functions as a potential link between chronic inflammation and hepatic insulin resistance at least partially through TNFR1 via NF-κB signaling.
Clinically, several evidences also indicate that PGRN is an important link between insulin resistance and aggressive inflammatory condition (12, 14). By these data, circulating PGRN levels correlate with body mass index, macrophage infiltration into adipose tissue, and chemotactic activity, suggesting that PGRN reflects the chronic inflammation in obesity, which is in line with those that PGRN may be a novel biomarker of the chronic inflammatory response in insulin resistance and associated disturbances. PGRN is composed of 7.5 repeats of a cysteine-rich motif. Proteolytic cleavage of this precursor protein by extracellular proteases gives rise to smaller peptide fragments termed granulins (GRNs) or epithelins (5, 41, 42). In contrast to the antiinflammatory properties of intact PGRN, GRNs have been shown to promote inflammatory activity (43, 44). It is unclear at this point whether GRNs derived by proteolysis of PGRN is implicated in the inhibition of hepatic insulin sensitivity and autophagy. Because GRNs range in size from 6 to 25 kDa, so far it is difficult to measure serum GRNs or to investigate the function of GRNs in vivo (45). However, our preliminary results showed that elastase-digested PGRN had few effects on insulin signaling and autophagy in cultured hepatocytes, whereas cells exposed to PGRN showed considerable attenuation in insulin signaling and autophagic activity (Supplemental Figure 5).
Although PGRN plays crucial roles in multiple physiological and pathological conditions, efforts to exploit the actions of PGRN and understand the mechanisms involved have been hampered by the inability to identify its binding receptor(s), and it is still hard to clearly define the early stages of PGRN-mediated signaling from the plasma membrane. Some report that PGRN action is not mediated through TNFR, whereas more studies suggest that PGRN is a TNFR antagonist or a cofactor for TNFα action (46). Recently it has been shown that PGRN binds to TNFRs, interfering with the interaction between TNFα and TNFR (37). Further evidence also demonstrated that mice deficient in PGRN are susceptible to collagen-induced arthritis, an experimental model of rheumatoid arthritis (RA) and that the administration of PGRN reversed the arthritic process (37). Several other groups also independently reproduced the binding of PGRN to TNFR1 and TNFR2 and the inhibitory effect of this binding on TNF-α-induced effects (47–50). TNFR1 and TNFR2 do not share homology in the cytoplasmic domains but exhibit a low degree of similarity in the ligand-binding region located in the extracellular domains, which suggests that they are capable of inducing distinct cellular responses (26). Some studies imply PGRN elicits its action more through TNFR2 than TNFR1 because it disturbed the interaction of PGRN with TNFR2 and in turn abolished PGRN-mediated activation of ERK1/2 and Akt signaling and protection against apoptosis in response to endoplasmic reticulum (ER) stress (47). In parallel, our recent study indicated a complimentary effect of both TNFR1 and TNFR2 in mediating PGRN function in cultured adipocytes (51). These discrepancies possibly might be because there is a different distribution between TNFR1 and TNFR2 in different cell types, and the function of PGRN might be diverse in different tissues, which should warrant further investigations.
When compared with TNF-α, PGRN exhibited a higher affinity to both TNFR1 and TNFR2, and PGRN has an approximately 600-fold higher binding affinity than TNF-α (44, 52–54). Similar to PGRN, atsttrin, an engineered protein made of three PGRN fragments, inhibited the interaction between TNF and TNFR and, in turn, the downstream events of TNF/TNFR signaling. In contrast to TNF-α, atsttrin exhibited a higher binding affinity for TNFR2 but a lower affinity for TNFR1 (37). In addition, it was also observed that TNF family ligands bind to the extracellular regions of TNFR1 and TNFR2 in which each receptor subunit contacts two adjacent ligand subunits typically via cysteine-rich repeat domain (CRD)-2 and CRD3 (46, 55). Deletion mutants of TNFR1 and TNFR2 used to map the binding of PGRN revealed that CRD2 and CRD3 of TNFR are essential for the interaction with PGRN, similar to the binding to TNF-α (49, 50, 56, 57).
However, because PGRN is administered systemically in this study, the observed findings cannot exclusively be attributed to PGRN itself, and a systemic state of inflammation could be the reason for the observations because PGRN can induce the release of cytokines such as IL-6 and IL-6 as a key player of hepatic insulin resistance (3). In our supplemental study (Supplemental Figure 6), IL-6 ablation partially ameliorated autophagic defects, although this recovery did not seem to be the most remarkable. In another aspect, recent evidence indicates that PGRN directly binds to TNFRs (37). In our study, the knockdown of TNFR1 resulted in restoration of hepatic insulin signaling and autophagic balance, indicating a potential role of TNFR1 in mediating PGRN function. On the other hand, Several lines of evidences suggested that TNF-α up-regulates mammalian target of rapamycin activity in a NF-κB-dependent manner because this activity is induced in TNF-α-treated, NF-κB-competent cells and is inversely impaired in TNF-α-treated cells lacking NF-κB activity (58). NF-κB activation might be necessary to PGRN-induced hepatic insulin resistance and autophagic imbalance; thus, our findings are reminiscent of previous evidence that NF-κB activation represses TNF-α-induced autophagy (38). These findings supported the conclusion that the NF-κB-dependent inhibition of autophagy may be a general cellular response. Thus, our results provide a more complementary insight into the regulation of PGRN, which suggests that the induction of impaired insulin sensitivity by PGRN is not only secondary to IL-6 but also is more likely to be mediated through TNFR1 via NF-κB signaling during hepatic insulin resistance.
Although defective autophagy induced by PGRN appeared causal to hepatic insulin resistance, a definitive link could be established if the restoration of autophagy could rescue the impaired insulin sensitivity. Our study provided the evidences that reconstitution of Atg7 results in enhanced autophagic activity and improved insulin signaling in cultured hepatocytes (Supplemental Figure 7). Atg7, which encodes an ubiquitin-activating enzyme (E1)-like enzyme, is central for autophagosome formation responsible for both Atg12-Atg5 conjugation and LC3 conversion (59, 60). It has been proposed that the reduction of the insulin level in obese mice fails to recover Atg7 deficiency, but the suppression of Atg7 directly results in hepatic insulin resistance in lean mice (59, 60). It is nevertheless possible that PGRN may contribute to the dampening of the initiation of autophagic machinery, which would further the organelle dysfunction, disrupt metabolic homeostasis, and promote the emergence of metabolic disorders.
The causative role of PGRN was implicated in the pathogenesis of the adipose insulin resistance of obese mice (3); however, the mechanisms of this defect could be diverse. Because obesity is characterized with enhanced intracellular lipid accumulation, the role of PGRN with sustained lipogenesis might be much more complicated than expected. It is plausible that chronic lipid overloading, which impairs insulin signaling and insulin-stimulated glucose uptake, might be one of the triggers in metabolic disturbance. Thus, systematic investigation of the metabolic consequences of PGRN administration should be warranted in the absence of other chronic changes that accompany the obese state. In this study, although we did not investigate lipid metabolism in our model under prolonged conditions, it is possible that severe defects induced by PGRN disrupt major autophagy components and major homeostatic pathways. In fact, the suppression of autophagy also results in alterations of glycogen accumulation in the liver of lean mice (59, 60). Because autophagy is involved in gluconeogenesis and lysosome plays an important role in glycogen breakdown, defective autophagy and insulin resistance are highly integrated in mice and impose a major effect on systemic metabolism. However, the possibility remains that some part of Atg7 action on liver metabolism may still involve yet-unknown and autophagy-independent mechanisms. Future studies are warranted to explore such possibilities to fully understand the metabolic impact of this adaptive response and how insulin action is influenced by different autophagy mediators.
In our study, there was still a clear tendency toward increased hepatic triglyceride (TGs) levels in mice injected with PGRN and/or TNFR1BP-Fc, although these changes did not reach statistical significance. Hepatic TG storage mainly originates from lipolysis of TGs released from white adipose tissue (60%) and the rest derive from dietary fatty acids (15%) and de novo lipogenesis (25%) (29, 61). The fatty acid supply from adipose tissues and diet remains relatively constant in our study; thus, the TG pool derived from these sources accounts for only a minor part of hepatic TG accumulation. Another consideration is from recent observation that PGRN remarkably triggers ER stress in cultured human adipocytes (51). The unfolded protein response by an accumulation of misfolded proteins in the lumen of the ER regulates lipogenesis (62), and the induction of chaperone molecules guiding unfolded proteins through the ER (such as GRP78/BiP) could suppress activation of the unfolded protein response and thus reduce liver lipid content (63). It seems reasonable that the dysfunctional ER might be able to interfere with triacylglycerol synthesis and thereby contribute to liver TG contents during PGRN exposure. Despite ER stress, several other possibilities have also been taken into consideration such as different stage or duration of PGRN intervention and the controversial effects secondary to inflammatory disorders. Thus, the underlying mechanism of hepatic TG deposition is not fully understood, and it remains to be conclusively shown the definite role of ER responses induced by PGRN in parallel with the development of hepatic TG metabolic profile.
In conclusion, our study revealed that administration of PGRN attenuated hepatic insulin sensitivity and suggested a causal link between PGRN, impairment of hepatic insulin signaling, and the attenuation of hepatic autophagy, which may implicate that promising therapeutic approach through the modulation of PGRN secretion/action and consequent amelioration of insulin resistance in subjects with metabolic disorders.
Acknowledgments
We appreciate the technical support and materials from the Animal Experimentation Center of Xi'an Jiaotong University.
This work was supported by the National Natural Science Foundation of China (Programs 81370899, 81170741, 81472038), National Excellent Young Scientist Program 81222026, and the New Century Excellent Talents in University from the Ministry of Education, China.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Programs 81370899, 81170741, 81472038), National Excellent Young Scientist Program 81222026, and the New Century Excellent Talents in University from the Ministry of Education, China.
Footnotes
- CRD
- cysteine-rich repeat domain
- EM
- electron microscopy
- ER
- endoplasmic reticulum
- FFA
- free fatty acid
- GRN
- granulin
- GTT
- glucose tolerance testing
- IKK
- multisubunit inhibitory-κB kinase
- IRS-1
- insulin receptor substrate 1
- ITT
- insulin tolerance testing
- NF-κB
- nuclear factor-κB
- p
- phosphorylated
- PGRN
- progranulin
- siRNA
- small interfering RNA
- TG
- triglyceride
- TNFR
- TNF receptor
- TNFR1BP
- TNFR1 blocking peptide.
References
- 1. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. [DOI] [PubMed] [Google Scholar]
- 2. Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem. 2012;287:32298–32306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsubara T, Mita A, Minami K, et al. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab. 2012;15:38–50. [DOI] [PubMed] [Google Scholar]
- 4. Diaz-Cueto L, Arechavaleta-Velasco F, Diaz-Arizaga A, Dominguez-Lopez P, Robles-Flores M. PKC signaling is involved in the regulation of progranulin (acrogranin/PC-cell-derived growth factor/granulin-epithelin precursor) protein expression in human ovarian cancer cell lines. Int J Gynecol Cancer. 2012;22:945–950. [DOI] [PubMed] [Google Scholar]
- 5. He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. [DOI] [PubMed] [Google Scholar]
- 6. Gijselinck I, van der Zee J, Engelborghs S, et al. Progranulin locus deletion in frontotemporal dementia. Hum Mutat 2008;29:53–58. [DOI] [PubMed] [Google Scholar]
- 7. Nicholson AM, Gass J, Petrucelli L, Rademakers R. Progranulin axis and recent developments in frontotemporal lobar degeneration. Alzheimers Res Ther. 2012;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim HK, Shin MS, Youn BS, et al. Involvement of progranulin in hypothalamic glucose sensing and feeding regulation. Endocrinology. 2011;152:4672–4682. [DOI] [PubMed] [Google Scholar]
- 9. Hossein-Nezhad A, Mirzaei K, Ansar H, Emam-Gholipour S, Tootee A, Keshavarz SA. Obesity, inflammation and resting energy expenditure: possible mechanism of progranulin in this pathway. Minerva Endocrinol. 2012;37:255–266. [PubMed] [Google Scholar]
- 10. Yilmaz Y, Eren F, Yonal O, et al. Serum progranulin as an independent marker of liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Disease Markers. 2011;31:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dupuis L, Petersen A, Weydt P. Progranulin bridges energy homeostasis and fronto-temporal dementia. Cell Metab. 2012;15:269–270; author reply 270. [DOI] [PubMed] [Google Scholar]
- 12. Qu H, Deng H, Hu Z. Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediat Inflamm. 2013;2013:360190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo HJ, Hwang SY, Hong HC, et al. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PloS One. 2013;8:e55744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youn BS, Bang SI, Kloting N, et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes. 2009;58:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tonjes A, Fasshauer M, Kratzsch J, Stumvoll M, Bluher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PloS One. 2010;5:e13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richter J, Focke D, Ebert T, et al. Serum levels of the adipokine progranulin depend on renal function. Diabetes Care. 2013;36:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. [DOI] [PubMed] [Google Scholar]
- 18. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jansen HJ, van Essen P, Koenen T, et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153:5866–5874. [DOI] [PubMed] [Google Scholar]
- 20. Qi Y, Zhang M, Li H, et al. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74:3740–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han HE, Kim TK, Son HJ, Park WJ, Han PL. Activation of autophagy pathway suppresses the expression of iNOS, IL6 and cell death of LPS-stimulated microglia cells. Biomol Ther. 2013;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-α regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. [DOI] [PubMed] [Google Scholar]
- 23. Wils H, Kleinberger G, Pereson S, et al. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol. 2012;228:67–76. [DOI] [PubMed] [Google Scholar]
- 24. Hu SY, Tai CC, Li YH, Wu JL. Progranulin compensates for blocked IGF-1 signaling to promote myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS Lett. 2012;586:3485–3492. [DOI] [PubMed] [Google Scholar]
- 25. Capell A, Liebscher S, Fellerer K, et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J Neurosci. 2011;31:1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang H, Yin B, Zhang H, et al. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-α signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology. 2008;149:2943–2951. [DOI] [PubMed] [Google Scholar]
- 27. Chen P, Zhao SH, Chu YL, et al. Anticancer activity of PDSS2, prenyl diphosphate synthase, subunit 2, in gastric cancer tissue and the SGC7901 cell line. Anticancer Drugs. 2009;20:141–148. [DOI] [PubMed] [Google Scholar]
- 28. Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology. 2013;154:1055–1068. [DOI] [PubMed] [Google Scholar]
- 29. Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. [DOI] [PubMed] [Google Scholar]
- 30. Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:14557–14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zois CE, Giatromanolaki A, Sivridis E, Papaiakovou M, Kainulainen H, Koukourakis MI. “Autophagic flux” in normal mouse tissues: focus on endogenous LC3A processing. Autophagy. 2011;7:1371–1378. [DOI] [PubMed] [Google Scholar]
- 32. Cho HI, Choi JW, Lee SM. Impairment of autophagosome-lysosome fusion contributes to chronic ethanol-induced liver injury. Alcohol. 2014;48:717–725. [DOI] [PubMed] [Google Scholar]
- 33. Sun HZ, Yang TW, Zang WJ, Wu SF. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. J Endocrinol. 2010;204:311–318. [DOI] [PubMed] [Google Scholar]
- 34. Kiyono K, Suzuki HI, Matsuyama H, et al. Autophagy is activated by TGF-β and potentiates TGF-β-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 2009;69:8844–8852. [DOI] [PubMed] [Google Scholar]
- 35. Alirezaei M, Fox HS, Flynn CT, et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy. 2009;5:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rouschop KM, van den Beucken T, Dubois L, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djavaheri-Mergny M, Amelotti M, Mathieu J, et al. NF-κB activation represses tumor necrosis factor-α-induced autophagy. J Biol Chem. 2006;281:30373–30382. [DOI] [PubMed] [Google Scholar]
- 39. Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arkan MC, Hevener AL, Greten FR, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. [DOI] [PubMed] [Google Scholar]
- 41. Daniel R, Daniels E, He Z, Bateman A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev Dyn. 2003;227:593–599. [DOI] [PubMed] [Google Scholar]
- 42. Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990;173:1161–1168. [DOI] [PubMed] [Google Scholar]
- 43. Park B, Buti L, Lee S, et al. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity. 2011;34:505–513. [DOI] [PubMed] [Google Scholar]
- 44. Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. [DOI] [PubMed] [Google Scholar]
- 45. Tanaka A, Tsukamoto H, Mitoma H, et al. Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis Res Ther. 2012;14:R244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. J Ocular Pharmacol Ther. 2012;133:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li M, Liu Y, Xia F, et al. Progranulin is required for proper ER stress response and inhibits ER stress-mediated apoptosis through TNFR2. Cell Signal. 2014;26:1539–1548. [DOI] [PubMed] [Google Scholar]
- 48. Hu Y, Xiao H, Shi T, Oppenheim JJ, Chen X. Progranulin promotes tumour necrosis factor-induced proliferation of suppressive mouse CD4(+) Foxp3(+) regulatory T cells. Immunology. 2014;142:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jian J, Zhao S, Gonzalez-Gugel E, et al. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587:3428–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu C, Li XX, Gao W, Liu W, Liu DS. Progranulin-derived Atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PLoS One. 2014;9:e92743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li H, Zhou B, Xu L, et al. Circulating PGRN is significantly associated with systemic insulin sensitivity and autophagic activity in metabolic syndrome. Endocrinology. 2014;155:3493–3507. [DOI] [PubMed] [Google Scholar]
- 52. Bluml S, Binder NB, Niederreiter B, et al. Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis Rheumatol. 2010;62:1608–1619. [DOI] [PubMed] [Google Scholar]
- 53. Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. [DOI] [PubMed] [Google Scholar]
- 54. Kessenbrock K, Frohlich L, Sixt M, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu H, Siegel RM. Medicine. Progranulin resolves inflammation. Science. 2011;332:427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tian Q, Zhao S, Liu C. A solid-phase assay for studying direct binding of progranulin to TNFR and progranulin antagonism of TNF/TNFR interactions. Methods Mol Biol. 2014;1155:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tian QY, Zhao YP, Liu CJ. Modified yeast-two-hybrid system to identify proteins interacting with the growth factor progranulin. J Vis Exp. 2012;(59)pii:3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kezic A, Becker JU, Thaiss F. The effect of mTOR-inhibition on NF-κB activity in kidney ischemia-reperfusion injury in mice. Transplant Proc. 2013;45:1708–1714. [DOI] [PubMed] [Google Scholar]
- 59. Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kotoulas OB, Kalamidas SA, Kondomerkos DJ. Glycogen autophagy in glucose homeostasis. Pathol Res Pract. 2006;202:631–638. [DOI] [PubMed] [Google Scholar]
- 61. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee JS, Mendez R, Heng HH, Yang ZQ, Zhang K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am J Transl Res. 2012;4:102–113. [PMC free article] [PubMed] [Google Scholar]
- 63. Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]