Abstract
Dmrt transcription factors control sex determination or sex-specific differentiation across all invertebrate and vertebrate species, in which they have been studied so far. In addition to important functions in the reproductive system, also nongonadal roles have been assigned to several dmrt family members. One example is dmrt5, which was shown to guide neurogenesis in the forebrain of some vertebrates including fish. Here we show that in zebrafish, dmrt5 is also expressed adjacent to the pituitary anlage and later in the anterior pars distalis in which it organizes differentiation of endocrine cells. We find that pituitary induction, cell survival, proliferation, and early lineage specification in the pituitary is independent of dmrt5. Instead, dmrt5 is required for terminal differentiation of corticotropes and gonadotropes. Gene knockdown and mutant analysis revealed that dmrt5 promotes corticotrope differentiation via tbx19 expression, whereas it prevents gonadotrope differentiation in the anterior pars distalis. In dmrt5 morphants and mutants, reduced corticotrope numbers may result in irregular positioning and reduced maintenance of lactotropes. In conclusion, our study establishes a novel function for dmrt5 for cell differentiation in the anterior pituitary. Intriguingly, its effect on gonadotrope numbers defines a first nongonadal role for a dmrt family member that appears crucial for the activity of the reproductive system.
The pituitary functionally links the nervous system to the endocrine system. It translates regulatory signals originating from the hypothalamus into an endocrine response and regulates multiple body functions including metabolism and reproduction. In zebrafish, similar to all other vertebrates, the pituitary is composed of two functionally distinct domains with different origins. The neurohypophysis originates from neural ectoderm and contains axonal endings of neurons residing in the supraoptic and paraventricular nuclei of the hypothalamus. These zebrafish neurons express oxytocin (oxt) and arginine vasopressin (avp) genes (1). The adenohypophysis (AH), on the other hand, is derived from nonneural ectoderm rostral to the anterior edge of the neural plate, ie, the anterior neural ridge (2–6). The AH can be subdivided into three parts: the anterior pars distalis (aPD), the pars intermedia (PI), and the posterior pars distalis (pPD).
Studies mainly in mice and zebrafish revealed that AH induction, patterning, and cell type specification is spatiotemporally tightly regulated by multiple genes, including pitx3, shh, fgf3, pit1, eya1, and six1b (7–14). Once pituitary precursor cells are specified, distinct precursor populations along the dorsoventral and anterior-posterior pituitary axis differentiate sequentially into various hormone secreting cells: lactotropes and corticotropes differentiate first at approximately 24 hours post fertilization (hpf) in the aPD. In parallel, melanotropes differentiate in the PI. Thyrotropes and somatotropes differentiate at 42 and 48 hpf, respectively (6, 15). Gonadotropes differentiate last and become distinguishable from other pituitary populations from 4 days post fertilization onward (16). Importantly, relatively little is known about the transcriptional networks that regulate how pituitary cells terminally differentiate from specified precursors to differentiated and active endocrine cells. Furthermore, it remains unclear which factors control the maintenance of mature pituitary cells.
Dmrt (doublesex and mab3 related transcription factor) genes encode transcription factors with conserved functions during sex determination and differentiation (17–19). In addition, several nongonadal functions during differentiation of the central and peripheral nervous systems have been described for Doublesex/Mab3 (DM) domain-containing transcription factors (20–26).
In the present study, we analyzed the role of dmrt5 in zebrafish pituitary differentiation. We show that dmrt5 is expressed early in the pituitary anlage and later in a subset of pituitary cells. Using gene knockdown and knockout, we tested whether dmrt5 is required for pituitary induction, initial lineage specification or differentiation, and maintenance of pituitary cell populations. We find that dmrt5 controls differentiation of corticotropes and strikingly also gonadotropes and is required for the maintenance of lactotropes in the aPD. Our data suggest that dmrt5 is permissive for corticotrope development by repressing gonadotrope cell fates during terminal differentiation of pituitary precursor cells. For the first time, this establishes a nongonadal role for a DM-domain transcription factor in the pituitary that is crucial for controlling endocrine aspects of the reproductive system.
Materials and Methods
Zebrafish dmrt5 morphants and mutants
All animal experiments were performed in accordance with approved Institutional Animal Care and Use Committee protocols of the National University of Singapore (protocol numbers 075/07; 082/10; BR19/10). Adult zebrafish of DBS inbred wild-type strain were crossed to obtain embryos that were raised in 30% Danieau's solution at 28°C and staged as described previously (27).
Ethylnitrosourea-induced Dmrt5 mutants (ha2) were kindly provided by the Yutaka Kikuchi laboratory through the Zebrafish National Bioressource Project (22). Heterozygous dmrt5 mutant carriers were genotyped after fin clipping as described before (22) and intercrossed to obtain homozygous mutant embryos. Phenotypes were analyzed after whole-mount in situ hybridization as described below. Embryos were grouped according to phenotypes and genotyped. For this, genomic DNA was isolated from 4 to 10 single embryos and sequenced to confirm presence of the dmrt5 mutation.
To knock down dmrt5 (ENSDARG00000039412), splice site morpholinos (MOs) targeting the boundaries of the single intron (dmrt5 splice up: 5′-AACGTTTCTACTTACCAGAGTTTGA-3′; dmrt5 splice down: 5′-TTTGATTCTCCTGGAATAGATTTGT-3′) were obtained from Gene Tools and diluted to a final concentration of 3.1 mg/mL. A scrambled morpholino with randomized sequence, but the same nucleotide composition as dmrt5 splice down was used as control (5′-GATTCGTCAGCTTTATTGATTTGTA-3′). A total of 0.5–1 nL were injected into one-cell staged wild-type zebrafish embryos. For blocking the p53 mediated apoptosis pathway, a previously described p53 morpholino (p53 Mo: 5′-GCGCCATTGCTTTGCAAGAATTG-3′) (28) was injected at a concentration of 5 mg/mL.
In situ hybridization, immunostaining, and image acquisition
Whole-mount in situ hybridization was performed as described previously (29). Digoxigenin- or fluorescein-labeled riboprobes were used to visualize the expression of the following genes: dmrt5 (21), shh (30), pitx3 (7), lim3 (5), pit1 (12), gh (6), tsh-b (6), gsu-a (31), pomc (6), prl (6), krox-20 (32), eya1 (33), six1b (34), crystalline (35), and tbx19 (36, 37).
For image acquisition, stained embryos were manually deyolked and mounted in 100% glycerol. Single planes or z-stacks were imaged using differential interference contrast (DIC) on a compound microscope (Nikon Eclipse 90i) with the imaging software NIS Basic Elements (Nikon). DIC-contrast imaging at high magnification allowed distinguishing the pituitary from its adjacent tissues based on morphological differences such as cell orientation and cell size. Image analysis and compilation was done with ImageJ (National Institutes of Health, Bethesda, Maryland) and Photoshop (Adobe). Cell counts were performed on high-magnification images using the ImageJ plug-in cell counter, which allows labeling the location of distinct cells within a z-stack. These labels were used as orientation during cell counting in z-stacks of ventral and lateral views of the same embryo and prevented double-counting.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate-biotin nick end labeling (TUNEL) assay
For the TUNEL assay, embryos were fixed in 4% paraformaldehyde for 2 hours at room temperature and subsequently kept in 100% methanol. Apoptotic cells were detected using the ApopTag peroxidase in situ apoptosis detection kit (Millipore).
Data analysis
Expression patterns were analyzed with a stereomicroscope. Numbers of embryos with normal and altered expression patterns were scored (see Supplemental Table 1). Experiments were conducted in triplicates if not stated otherwise. Detailed cell counting was done in 5–15 randomly picked embryos. Average cell numbers were recorded and the SEM was calculated. Two-tailed, unpaired t tests were performed to assess whether average cell numbers differed significantly between controls and dmrt5 morphant embryos.
Results
Zebrafish dmrt5 is expressed in the developing pituitary
The earliest expression of dmrt5 in zebrafish embryos was detected in cells of the anterior neural keel (ANK) at 10 hpf (Figure 1A, arrow). In this region and at this particular stage, it was shown that Shh and Pitx3 play important roles during pituitary precursor cell fate specification (7–10). To test whether dmrt5 is coexpressed with shh or pitx3, single- and two-color in situ hybridization was performed (Figure 1, B and C). This showed that dmrt5 is expressed in the dorsal areas of the anterior neural keel (Figure 1B) away from shh expression that is ventrally restricted (Figure 1B, inset). Pitx3 is expressed in pituitary precursor cells in two antero-lateral domains of the dorsal ANK at the three-somite stage (Figure 1C, blue). In contrast, dmrt5 expression at the same stage is found in a medial position along the dorsal midline (Figure 1C, red). Hence, dmrt5 is not coexpressed with shh or pitx3 in pituitary precursor cells at this early stage but in cells directly adjacent to the pituitary primordium.
Figure 1. Expression of dmrt5 in the zebrafish pituitary.
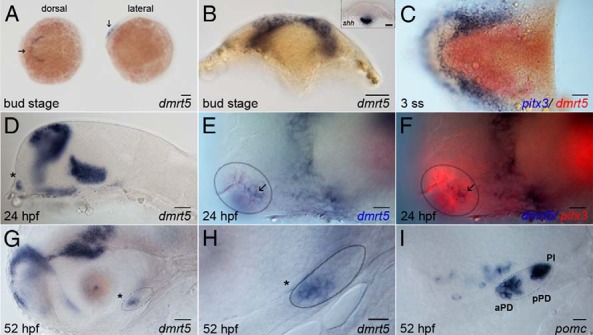
A, Dorsal (left) and lateral views of embryos at bud stage showing dmrt5 expression in the ANK. B, Transverse section showing that dmrt5 expression is restricted to the dorsal ANK. Expression of shh is restricted to the ventral head region (inset). C, Dorsal view of flat-mounted embryo at the three-somite stage (3 ss) showing dmrt5 expression (red) expanding in the telencephalon adjacent to pitx3 (blue). D–F, From 24 hpf onward, dmrt5 is expressed in the most anterior-ventral cells of the head (asterisk, D), partially overlapping with pitx3 (red) in the prospective pituitary (E and F, circled area; arrow marks colocalization). G and H, After 52 hpf, dmrt5 is expressed in the anterior pars distalis (asterisk). Panel H is a high-magnification view of the pituitary shown in panel G. I, pomc expression at 52 hpf demarcates three domains of the adenohypophysis: the aPD, the intermediate, pomc-negative pPD, and the posterior PI. Anterior is to the left in all images except panel B, in which dorsal is to the top. Scale bars, 100 μm (A); 50 μm (B–D, G); 20 μm (E, F, H, and I).
At later stages starting from 24 hpf, dmrt5 expression is found in broad areas of the fore- and ventral midbrain as well as in presumptive pituitary cells at the most anterior and ventral position of the head (Figure 1D, asterisk). To test whether dmrt5-positive cells in this region are pituitary cells, its expression was visualized together with that of the pan-pituitary marker pitx3. This revealed an overlap of dmrt5 expression with pitx3 in several but not all cells (Figure 1, E and F, arrow and circled area), suggesting that dmrt5 is expressed in a distinct subset of presumptive pituitary cells. At more advanced stages, when the pituitary is visible as a distinctive structure (Figure 1, G and I, circled area), dmrt5 expression is limited to its anterior domain (asterisk in Figure 1, G and H), suggesting a possible role for dmrt5 in the aPD.
Knockdown of dmrt5 does not affect early pituitary development or progenitor specification
We next tested whether dmrt5 function is required during early stages of pituitary development. Two dmrt5 splice-blocking morpholinos were coinjected into one-cell stage embryos. This resulted in efficient knockdown of up to 97% (see Supplemental Figure 1).
Although dmrt5 is not expressed in early pituitary precursor cells at the bud stage, it could affect early pituitary formation non-cell autonomously given the proximity of dmrt5 cells and pitx3 pituitary precursor cells (Figure 1C). To analyze whether dmrt5 is required for pituitary induction, expression of the pituitary specification determinants shh and pitx3 was determined in dmrt5 morphants and compared with wild-type and control morpholino injected embryos (further referred to as control embryos). Expression of krox20 was used as landmark to show that head extension was not affected in dmrt5 morphants (Figure 2, A and B). At 10 hpf, neither the length nor width of the shh expression domain (L and W in Figure 2A) were significantly altered in dmrt5 morphants (LMo = 436.5 ± 7.7 μm, WMo = 80.7 ± 1.7 μm) when compared with controls (Lwt = 453.7 ± 5.1 μm, LctrMo = 434.5 ± 4.8 μm; Wwt = 78.6 ± 1.9 μm, WctrMo = 82.1 ± 2.3 μm, P > .05; Figures 2, A and B, and 3A and Supplemental Figure 2A). Furthermore, no transfating of pituitary into lens cells was observed (see Supplemental Figure 3), which was expected if Shh signaling was disrupted (8). Also, the pitx3 expression domain showed no obvious changes in dmrt5 morphants (nMo = 66 of 72; Figure 2D) when compared with control embryos (nwt = 92 of 96, nctrMo = 74 of 77; Figure 2C and Supplemental Figure 2B). This shows that dmrt5 does not affect expression of shh or pitx3 via eg, paracrine signaling or cell-cell interactions between dmrt5 and adjacent pituitary cells.
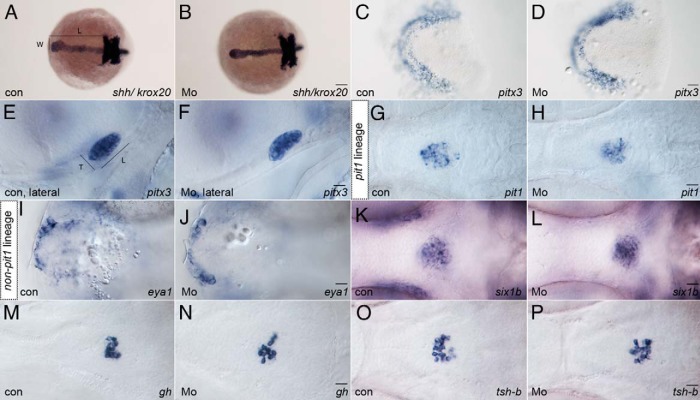
Figure 2. Dmrt5 is dispensable for pituitary induction, lineage specification, and thyrotrope/somatotrope differentiation.
A and B, shh and krox20 expression in uninjected wild type embryos (A; con) and dmrt5 morphants (B; Mo) at bud stage. Neither width (W) nor length (L; from anterior shh to krox20 in r3) of the shh domain is affected in dmrt5 morphants. C and D, Dorsal views of flat-mounted embryos show no change in pitx3 expression in morphants. E and F, Expression of pan-pituitary marker pitx3 was also used to assess pituitary dimensions (L and T) at 52 hpf. Dimensions were not significantly altered in dmrt5 morphants during the first 2 days of pituitary development. G–L, Ventral views of embryos at 52 hpf showing marker expression indicative for specification of pit-1 lineage (pit1, for somatotropes, thyrotropes, and lactotropes) or non-pit-1 lineage (eya1 and six1b for corticotropes, gonadotropes, and melanotropes). Lineage specification was unaffected in dmrt5 morphants. M–P, Expression of growth hormone (gh) in somatotropes and thyroid-stimulating hormone β-subunit (tsh-b) in thyrotropes at 72 hpf. No changes were detected in dmrt5 morphants, indicating normal differentiation. All images are ventral views except E and F, which are lateral views, anterior is to the left. Scale bars, 100 μm (A and B); 50 μm (C and D); 20 μm (E–P).
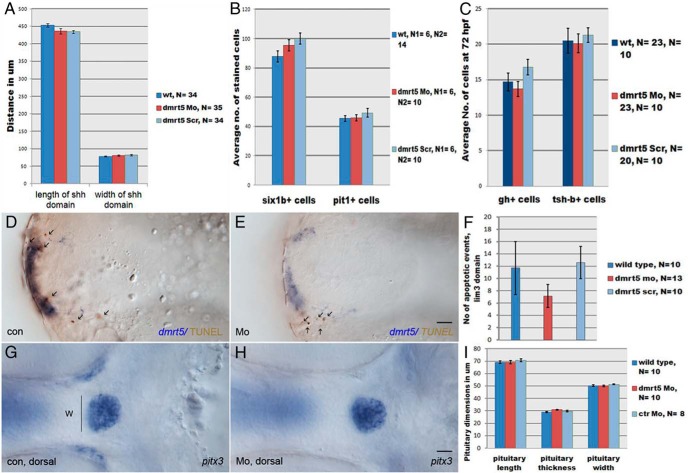
Figure 3. Quantification of early pituitary development in dmrt5 morphants.
A, Analysis of L and W of the anterior shh expression domain in controls (noninjected, wt; scramble MO, Scr) and dmrt5 morphants (Mo) at bud stage did not reveal significant changes (for numbers see text). B, Numbers of six1b (non-pit1-lineage)- and pit-1 (pit-1lineage)-positive cells were counted at 52 hpf. Neither the number of six1b+ nor pit1+ cells differed significantly between dmrt5 morphants and controls. C, Differentiation of somatotropes and thyrotropes was evaluated at 72 hpf based on the expression of GH (gh+) and thyroid-stimulating enzyme (tsh-b+), respectively. No significant changes were observed between morphants and controls. D–F, Determination of apoptotic particles (arrows) in the pituitary lim3 domain (blue) by TUNEL staining (brown). No significant changes were evident in dmrt5 morphants compared with controls. G–I, Analysis of pituitary W, L, and T at 52 hpf after staining with pitx3 revealed no significant changes in dmrt5 morphants. Error bars represent ± SEM, and N is the number of analyzed embryos. Images D and E are ventral views, G and H are dorsal views. Scale bars, 20 μm.
As dmrt5 becomes expressed in pituitary cells after 24 hpf, we next investigated whether general pituitary development was affected upon dmrt5 knockdown at later time points. We first analyzed whether dmrt5 knockdown results in increased apoptosis of specified pituitary cells expressing lim3 (5). For this, TUNEL-positive cell fragments were counted within the lim3 domain (Figure 3, D–F). No significant change was observed in dmrt5 morphants (number of TUNEL positive particles ± SEM: nMo = 7.15 ± 1.84) compared with controls (nwt = 11.7 ± 4.3, nctrMo = 12.6 ± 2.6, P > .05; Figure 3F). This indicates that a dmrt5 knockdown does not result in increased apoptosis of specified pituitary cells at this stage.
Also, the overall size of the pituitary was not affected in dmrt5 morphants, as analyzed by pitx3 expression at 52 hpf. As shown in Figures 2, E and F, and 3, G–I, neither length (L) nor thickness (T) nor width (W) of the pituitary was significantly reduced in dmrt5 morphants (LMo = 69.04 ± 1.35 μm, TMo = 30.95 ± 0.4 μm, WMo = 50.08 ± 0.71 μm) when compared with controls (Lwt = 69.11 ± 1.04 μm, LctrMo = 70.62 ± 1.11 μm; Twt = 28.84 ± 0.47 μm, TctrMo = 29.72 ± 0.66 μm; Wwt = 50.33 ± 0.75 μm, WctrMo = 51.35 ± 0.45 μm, Figure 3I). This suggests that overall cell numbers in the pituitary are normal in dmrt5 morphants and further supports the idea that dmrt5 is not required for early pituitary development.
We next studied early cell fate specification in the pituitary of dmrt5 morphants. Pituitary transcription factor-1 (Pit1) is a POU-homeodomain transcription factor required for the development of lactotropes, thyrotropes, and somatotropes (ie, the pit1 lineage) and prevents their transfating into non-pit1 lineage cell types (12). In dmrt5 morphants at 52 hpf, pit1 expression was comparable (77 of 79) with that of wild-type embryos (103 of 105; Figure 2, G and H), and no significant differences were observed when counting pit1-positive cells in morphants (45.8 ± 2.12) and controls [wild type (wt): 45.43 ± 1.97; ctrMo: 49.2 ± 3.0; P > .05; Figure 3B]. This suggests that dmrt5 does not affect specification of pituitary precursors towards the pit1 lineage.
Cell fate specification toward corticotropes, gonadotropes, and melanotropes (ie, the non-pit1 lineage) depends on the Eya1/Six1b protein complex. It was previously reported that eya1 mutants show a reduced number of non-pit1 lineage cells, which could be enhanced further by a knockdown of six1b (14). To test whether a knockdown of dmrt5 affects pituitary precursor determination toward the non-pit1 lineage, expression of eya1 and six1b was analyzed (Figure 2, I–L). No obvious differences in eya1 expression were detected in dmrt5 morphants at 24 hpf (22 of 22; Figure 2J) when compared with controls (41 of 41; Figure 2I). In addition, expression of six1b was normal in dmrt5 morphants at 52 hpf (151 of 157; Figure 2L) when compared with controls (173 of 177; Figure 2K). Cell counts of six1b cells revealed no significant difference between dmrt5 morphants (95.5 ± 3.84) and controls (wt: 87.83 ± 3.83; ctrMo: 100 ± 3.7; P > .05; Figure 3B). These findings thus strongly suggest that dmrt5 is neither required for early pituitary formation nor for specification of pit1 and non-pit1 lineages.
dmrt5 controls differentiation of corticotropes and gonadotropes
We next tested whether the differentiation of pituitary precursors into specific cell types was altered under dmrt5-deficient conditions. For this, the expression of gh (for somatotropes), tsh-b (for thyrotropes), gsu-a (for thyrotropes and gonadotropes), prl (for lactotropes) and pomc (for corticotropes and melanotropes) was analyzed in dmrt5 morphants at 72 hpf.
In control embryos, the expression of gh (146 of 147) and tsh-b (94 of 107) was restricted to the pPD (Figure 2, M and O). In dmrt5 morphants, no obvious changes were detected for gh (99 of 109; Figure 2N) or tsh-b (69 of 83; Figure 2P). When gh- and tsh-b-expressing cells were counted, comparable numbers were obtained in controls (ghwt: 14.7 ± 1.26; ghctrMo = 16.8 ± 1.1; tshwt 20.5 ± 1.76, tsh-bconMo: 21.1 ± 1.0) and dmrt5 morphants (gh: 13.7 ± 1.07; tsh: 20.1 ± 1.34; Figure 3C). This suggests that dmrt5 is not required for differentiation of somatotropes and thyrotropes.
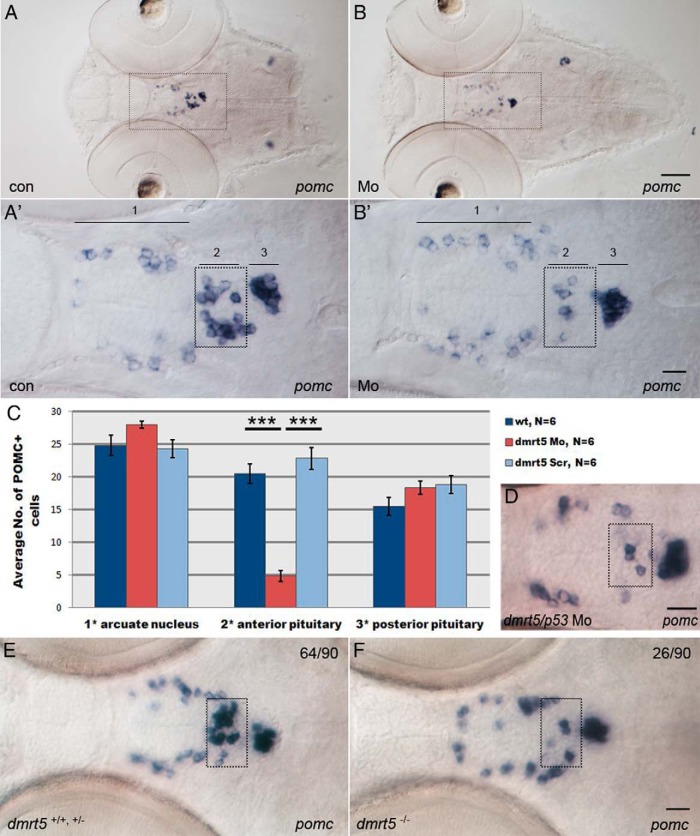
Next, the expression of proopiomelanocortin (pomc) was analyzed at 72 hpf. The pomc-positive cells are located in three distinct regions in the ventral head: the ventral diencephalon (region labeled 1 in Figure 4A′), aPD (2), and PI (3). The pomc-expressing cells in the ventral diencephalon are hypothalamic and belong to the arcuate nucleus, pomc-positive cells in region 2 are corticotropes, whereas the pomc cells in region 3 are predominantly melanotropes (38, 39). In contrast to controls (179 of 181; Figure 4A, A′), almost all dmrt5 morphants (149 of 151) showed strongly reduced numbers of pomc-expressing cells in region 2 (Figure 4B, B′). Cell counting revealed a statistically significant (P < .001) reduction of pomc cell numbers in region 2 of dmrt5 morphants (4.83 ± 0.79; Figure 4C) when compared with controls (20.05 ± 0.56). Importantly, this effect was restricted to the aPD because pomc cell numbers in regions 1 and 3 were unaffected in morphants (region 1: 28 ± 1.53; region 3: 18.33 ± 1.64) when compared with controls (region 1: 24.83 ± 1.54; region 3: 15.5 ± 1.38; P > .05). The observation that development in regions 1 and 3 was unaffected in combination with the finding that initially numbers of lactotropes within the aPD were normal (see below) suggest that a general developmental delay could be excluded. Importantly, the absence of corticotropes from the aPD was not rescuable by coinjection of a p53 morpholino, excluding the possibility that corticotrope reduction was a result of increased apoptosis (Figure 4D) and supporting the idea of defects in corticotrope differentiation.
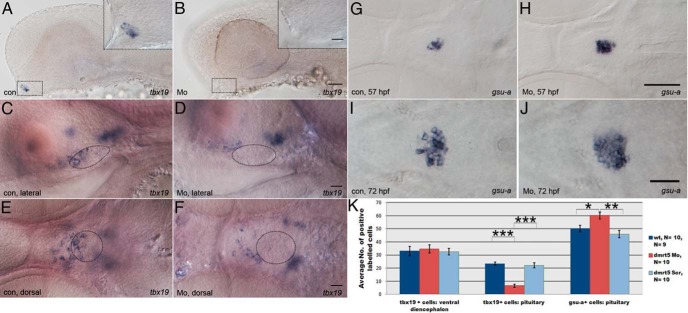
Figure 4. Knockdown of dmrt5 results in reduced corticotrope numbers.
A and B, Expression of pomc in control embryos (A) and dmrt5 morphants (B) at 72 hpf. A′ and B′ are higher-magnification views of areas boxed in panels A and B. pomc expression is shown in ventral diencephalon (arcuate nucleus, 1), corticotropes (anterior pituitary, 2 and boxed), and melanotropes (posterior pituitary, 3). C, Statistical analysis of pomc cell numbers showed that only corticotrope numbers (region 2) were significantly reduced (nMo = 4.8 ± 0.79, nwt = 20.5 ± 0.56, nctrMo = 22.8 ± 1; ***, P < .001). Error bars represent ± SEM and N is the number of analyzed embryos. D, Corticotrope defects could not be rescued by coinjection of p53 morpholino into dmrt5 morphants. E and F, pomc expression in wild-type and dmrt5ha2 mutant embryos at 72 hpf. Wild-type and heterozygous dmrt5 mutants had normal corticotrope numbers (E), whereas homozygous dmrt5 mutants phenocopied dmrt5 morphants and exhibited reduced corticotrope numbers (F). Numbers represent embryos with indicated phenotype in embryo clutch obtained from heterozygous parents. All images are ventral views; anterior is to the left. Corticotropes are boxed. Scale bars, 50 μm (A and B); 20 μm (A′–F).
The pomc expression was also analyzed in dmrt5ha2 mutant embryos at 72 hpf. Approximately 75% of offspring from two heterozygous carriers (64 of 90; Figure 4E) showed normal distribution of pomc-expressing cells, whereas 25% (26 of 90; Figure 4F) fully recapitulated the dmrt5 morpholino phenotype and showed reduced numbers of corticotropes in the aPD. Embryos were genotyped, which revealed that all embryos with normal pomc expression were either wild type (one of four) or heterozygous dmrt5 mutants (three of four), whereas all affected embryos were homozygous dmrt5 mutants (four of four). Importantly, also in dmrt5 mutants as in morphants described above, the pomc expression was reduced only in the aPD. Thus, morphant and mutant data suggest that dmrt5 is required for corticotrope differentiation.
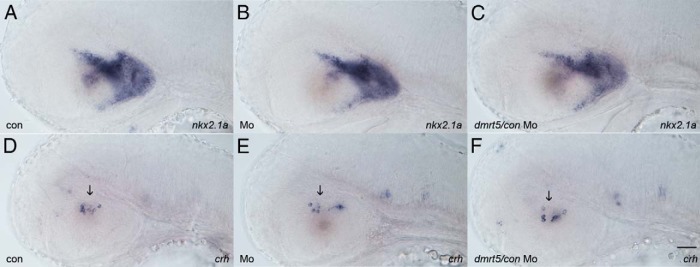
To rule out that the corticotrope phenotype is an indirect consequence of hypothalamic deficiencies, we analyzed the expression of the general hypothalamus marker nkx2.1a (Figure 5, A–C). No difference in the expression pattern was found between controls and morphants, suggesting normal hypothalamus development. Furthermore, to confirm that upstream regulators of pomc are normally expressed in dmrt5 morphants, corticotrophin-releasing hormone (crh) expression was analyzed and found to be normal (Figure 5, D–F, arrow). This indicates that the observed pituitary defects are not due to hypothalamic developmental deficiencies.
Figure 5. Hypothalamus development is not affected in dmrt5 morphants.
A–C, Analysis of hypothalamic development by analysis of nkx2.1 expression in noninjected control embryos (A), dmrt5 morphants (B), and control MO injected embryos at 52 hpf. D–F, Analysis of hypothalamic crh expression at 52 hpf. No difference in expression is evident between controls and morphants indicating normal hypothalamus development. All images are lateral views. Scale bar, 50 μm.
To assess the mechanism of how dmrt5 affects corticotrope differentiation, tbx19 expression was analyzed at 31 and 52 hpf. In mouse, the pituitary restricted, T-box containing transcription factor Tbx19/Tpit is a negative regulator of gonadotrope and a positive regulator of corticotrope precursor cell differentiation (40). Homozygous Tpit mutant mice almost completely lack corticotropes and instead develop more gonadotropes. To address whether tbx19 is reduced upon dmrt5 knockdown, embryos were analyzed at 31 and 52 hpf. At 31 hpf, usually three to five pituitary cells express tbx19 in control embryos (42 of 50; Figure 6A). In stark contrast, most dmrt5 morphants (55 of 61) showed either reduced numbers or complete absence of tbx19-expressing cells (Figure 6B, pituitary is outlined). In control embryos at 52 hpf, more corticotrope precursors have differentiated, and the expression of tbx19 recapitulates the expression of pomc. Accordingly, a subset of cells in the ventral diencephalon as well as corticotropes in the aPD express tbx19 (142 of 164; Figure 6, C and E). dmrt5 morphants showed a down-regulation of tbx19 expression in the ventral head region (Figure 6, D and F). A detailed analysis of 10 morphant embryos at higher magnification revealed that tbx19 expression in the pituitary was almost completely absent, whereas its expression in the ventral diencephalon was normal (Figure 6K). The number of tbx19 cells was unchanged in the ventral diencephalon (tbx19Mo = 34.6 ± 3.11, tbx19wt = 33.1 ± 3.53, tbx19ctrMo = 32.6 ± 2.6; P > .05) but significantly reduced in the pituitary of dmrt5 morphants (tbx19+Mo = 6.6 ± 1.19) when compared with controls (tbx19wt = 23.4 ± 1.13, tbx19ctrMo = 22.2 ± 1.84; P < .001). This shows that dmrt5 is required for tbx19 expression and suggests that Dmrt5 is an important regulator of corticotrope differentiation.
Figure 6. Knockdown of dmrt5 results in reduced tbx19 expression and increased gonadotrope numbers.
A, tbx19 expression in control embryos at 31 hpf. At this stage, 42 of 50 embryos have started to express tbx19 in prospective corticotropes. Inset shows a higher-magnification view of boxed area. B, Most dmrt5 morphants (55 of 61) exhibit absent or strongly reduced tbx19 expression at 31 hpf. C and E, At 52 hpf, tbx19 is expressed in the ventral diencephalon and corticotropes in the aPD (pituitary is circled). D and F, At 52 hpf, tbx19 is expressed in only the ventral diencephalon of dmrt5 morphants but is absent from the aPD (circle). G and I, Expression of gsu-a in gonadotropes and thyrotropes in control embryos at 57 (G) and 72 hpf (I). H and J, gsu-a expression in dmrt5 morphants at 57 (H) and 72 hpf (J). I, Statistical analysis of tbx19 and gsu-a cell numbers in ventral telencephalon and pituitary of control embryos, dmrt5 morphants (Mo), and embryos injected with dmrt5 scrambled control Morpholino (Scr). The number of tbx19 cells is significantly reduced (P < .001) in the pituitary of dmrt5 morphants but not the ventral diencephalon (P > .05). The number of gsu-a cells is significantly increased in dmrt5 morphants. All images are ventral views, except panels A–D, which are lateral; anterior is to the left. Circular outlines in panels C–F delineate the pituitary based on tissue morphology evident in images acquired with DIC contrast (for example, see Supplemental Figure 6). Exact cell numbers were determined by analyzing z-stacks of ventral and lateral views from the same embryo at high magnification. Scale bars, 100 μm (G and H), 50 μm (A and B); 40 μm (I and J), 20 μm (all remaining images and insets in A and B). Error bars represent ± SEM and N is the number of analyzed embryos. *, P < .05; **, P < .01; ***, P < .001.
Because tbx19 is known to promote corticotrope differentiation from a common precursor pool at the expense of gonadotropes in mice (40), we next tested whether gonadotrope development was also affected in dmrt5 zebrafish morphants. Due to the very low expression levels of fsh-b and lh-b at this stage (16), we instead determined the number of gonadotropes by evaluating the expression of gsu-a, which is expressed in gonadotropes and thyrotropes (12). Because we earlier showed that thyrotropes were not affected in dmrt5 morphants (tsh-b in Figure 2, O and P), any changes observed in the number of gsu-a cells would therefore imply that gonadotrope numbers were affected in dmrt5 morphants. Compared with tsh-b (Figure 2O), the expression domain of gsu-a is slightly larger because it contains both thyrotropes and gonadotropes (Figure 6, G and I). The expression domain of gsu-a appeared slightly enlarged in dmrt5 morphants at 57 hpf (14 of 20; Figure 6H) and 72 hpf (32 of 67; Figure 6J). Detailed counting of gsu-α cells revealed a moderate but significant increase in the number of gsu-α cells at 57 hpf (Supplemental Figure 4) and 72 hpf (Figure 6K; 10 embryos counted; gsu-αMo = 60.1 ± 2.78, gsu-αwt = 50 ± 2.53, gsu-αctrMo = 46 ± 2.9; *, P < .05, **, P < .01).
In conclusion, our data suggest that dmrt5 controls formation of corticotropes and restricts the number of gonadotropes. Importantly, the number of additional gonadotropes in morphants correlated with the number of missing corticotropes. This is consistent with the idea that bipotential precursor cells differentiate into gonadotropes instead of corticotropes in the absence of dmrt5 or tbx19.
dmrt5 is required for the maintenance and positioning of lactotropes
Finally, we addressed whether the knockdown of dmrt5 would affect the differentiation of lactotropes in the aPD. In controls, the expression of prl expression was restricted to a compact cell cluster in the most anterior area of the aPD from 48 to 72 hpf (n = 59 of 59; Figure 7, A–C). In dmrt5 morphants, in contrast, we found that the prl domain became increasingly dispersed from 48 to 72 hpf (at 72 hpf, n = 28 of 44; Figure 7, D–F). More importantly, the number of prl cells became gradually reduced in the observed time window. Although the numbers were comparable between controls and morphants at 48 hpf (prlwt = 31, prlMo = 30.8; Figure 7G; P > .05), they were slightly reduced in morphants at 60 hpf (prlwt = 30.8, prl+Mo = 26.8; Figure 7G; P > .05) and significantly reduced at 72 hpf (prlwt = 33.9, prlMo = 25.1; Figure 7G; *, P < .05, **, P < .01). This suggests that dmrt5 is not required for the induction but maintenance of differentiated lactotropes. Interestingly, the progressive loss of lactotropes could be rescued when dmrt5 morphants were coinjected with a p53 morpholino to block p53-mediated apoptosis (nwt = 49 of 50, ndmrt5Mo = 8 of 28, ndmrt5Mo+p53 = 20 of 21; Figure 7, H and I). We therefore performed TUNEL staining in dmrt5Mo-injected embryos at 72 hpf and observed a significant increase in the number of apoptotic fragments in the pituitary (dmrt5Mo = 3.75 ± 0.9, n = 8, P = .0076) when compared with noninjected controls (wt = 0.57 ± 0.3; n = 7; Figure 7, J and K). This further supports the idea that dmrt5 is required for the maintenance of differentiated lactotropes.
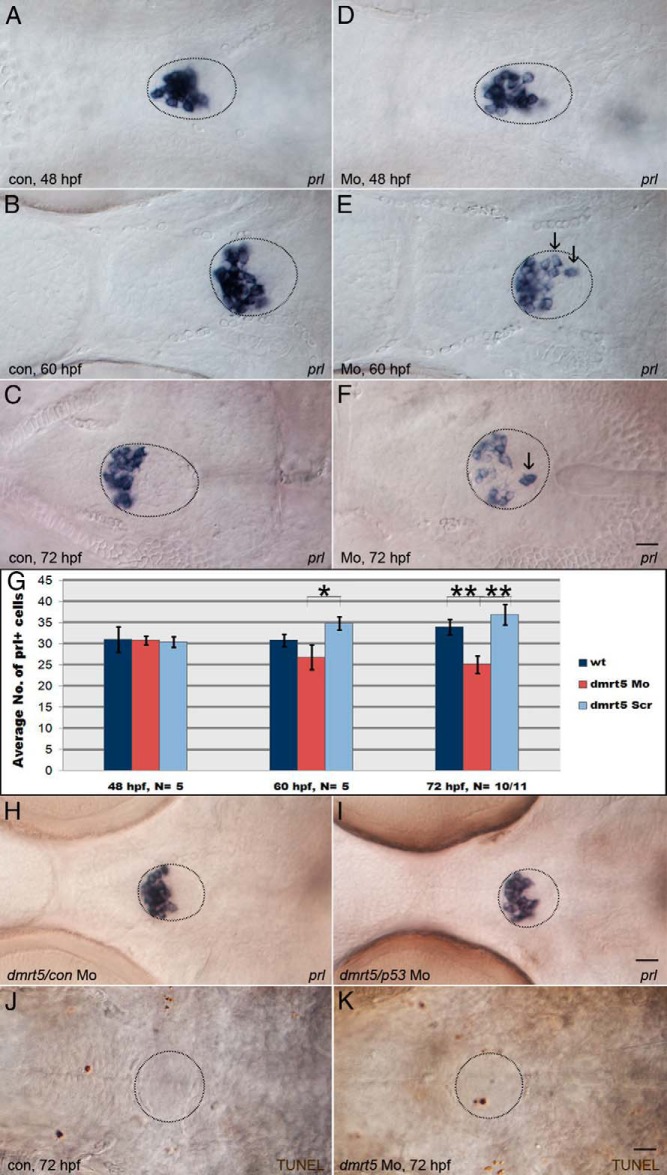
Figure 7. Dmrt5 is required for lactotrope maintenance and positioning.
A–F, prl expression in lactotropes of control (A–C) and dmrt5 morphant (D–F) embryos at 48 (A and D), 60 (B and E), and 72 hpf (C and F). Note loosened arrangement of prl cells in dmrt5 morphants starting at 60 hpf (E) and progressing at 72 hpf (F), with ectopic cells outside the aPD (arrows in E and F). G, Statistical analysis of prl cell numbers in the pituitary of control embryos, dmrt5 morphants, and embryos injected with dmrt5 scrambled control morpholino at 48, 60, and 72 hpf. Error bars represent ± SEM and N is the number of analyzed embryos. Note progressive reduction of prl cell numbers in the pituitary of dmrt5 morphants. Cell counting was performed at different stages in embryos from the same injected batch to minimize possibility of variation. H, Normal prl expression in embryo injected with scrambled control morpholino. I, Rescue of lactotrope phenotype at 72 hpf by coinjection of p53 morpholino into dmrt5 morphants. J and K, Apoptosis assay (TUNEL) in noninjected control embryo (J) and dmrt5 Mo injected embryos (K) at 72 hpf. Circular outlines in panels C, F, and H–K delineate the pituitary based on cell mophology in images acquired with DIC contrast (for example see Supplemental Figure 6). Images are ventral views. Scale bars, 20 μm.
We also analyzed the prl expression in homozygous dmrt5ha2 mutants, with half of the embryos obtained after crossing two heterozygous carriers remaining uninjected, whereas the other half was injected with p53 morpholino. Embryos were analyzed at 96 hpf to exclude developmental delays. Approximately 75% of offspring that remained uninjected showed normal prl expression (109 of 145; Figure 8A). The remaining 25% showed scattered prl cells (36 of 145; Figure 8B), with a significant reduction in cell numbers (nnormal = 20.4, nreduced = 9.8; P < .001) identical to the situation after MO knockdown of dmrt5. Genotyping confirmed that all embryos with reduced prl numbers were homozygous dmrt5 mutants, whereas normal embryos were wild type (three of four) and heterozygous dmrt5 mutants (one of four). When embryos coinjected with p53 morpholino were analyzed, 109 of 147 showed a normal prl phenotype (nnormal = 22.5; Figure 8C). The remaining 38 embryos either showed a strong reduction in the number of prl cells (19 of 147; nreduced = 12.25) that were scattered across the pituitary (data not shown; similar to Figure 8B) or a partial rescue with only mildly reduced numbers of prl cells (19 of 147; nrescued = 17; Figure 8D). Interestingly, these rescued cells were still randomly distributed throughout the pituitary. Genotyping of four normal, four strongly, and nine mildly affected embryos showed that all affected embryos except one were homozygous dmrt5 mutants (12 of 13). This demonstrated that p53 morpholino injection partially rescued the reduction of lactotrope numbers in dmrt5 mutants and confirmed that dmrt5 is required for lactotrope maintenance. In the absence of dmrt5, randomly distributed lactotropes become apoptotic and enter the p53-mediated apoptosis pathway.
Figure 8. Lactotrope loss in dmrt5 mutants is caused by p53-induced apoptosis.
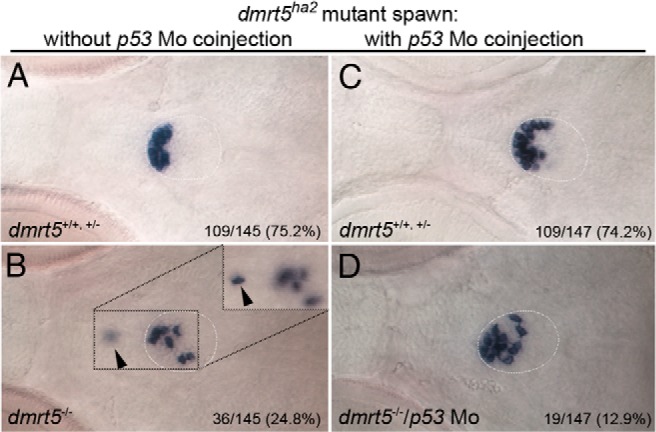
A–D, p53 knockdown rescues lactotrope defects in dmrt5ha2 mutant embryos. Ventral views of prl expression at 96 hpf in embryos obtained from an intercross of heterozygous mutant carriers without (A and B) and after injection of p53 morpholino (C and D). Numbers indicate ratio of embryos with shown phenotype. Note that a subset of mutant embryos injected with p53 morpholino (50%) exhibit a partially rescued prl phenotype (19 of 147, D), while 19 of 147 embryos (or 12.9%) showed no obvious rescue (image not shown, similar to panel B), which is lower than the expected ratio (25%). Inset in B shows different focal plane of pituitary region with prl-positive cell located outside the pituitary (marked by arrowhead).
Discussion
dmrt genes encode transcription factors with crucial functions in several embryonic cell fate decisions. This includes sex determination in fish (41) and sexual differentiation in many animals (42) but also other nongonadal processes such as neurogenesis (23). Due to the close proximity of dmrt5-expressing cells to prospective pituitary progenitors of the medial anterior neural ridge, we tested whether dmrt5 regulates early steps of pituitary formation and analyzed shh and pitx3 expression after dmrt5 knockdown. The pitx3 expression demarcates a progenitor domain, in which Shh-influenced medial cells become pituitary cells, whereas lateral cells that receive lower Shh levels differentiate into lens cells (6, 8). In dmrt5 morphants, the expression of shh and pitx3 was unchanged, indicating that Dmrt5 does not affect either of these genes during pituitary induction. Furthermore, because the survival of pituitary cells and the size of the pituitary before 52 hpf was not affected, we conclude that general pituitary development is normal under dmrt5-deficient conditions during the early stages. Importantly, also early specification of the pit1 and non-pit1 lineages in the early pituitary was normal because the expression of pit1, six1b, and eya1 was not affected.
In contrast, dmrt5 morphants and mutants showed defects in pituitary cell differentiation and exhibited strongly reduced numbers of pomc-expressing cells exclusively in the aPD. A developmental delay was excluded because the pomc expression in the adjacent ventral diencephalon and PI as well as the initial development of lactotropes within the aPD (Figure 7) was normal. The loss of pomc-positive corticotropes in the aPD was not due to apoptosis because p53 morpholino coinjection into dmrt5 morphants or mutants did not rescue the phenotype (Figure 4D and data not shown). In addition, overall pituitary size was normal in the dmrt5-deficient embryos, thus also excluding apoptosis. Instead, we observed a significantly reduced expression of the corticotrope differentiation factor tbx19 in the aPD. In the mouse, Tpit/Tbx19 is a transcription factor expressed in a common precursor pool for gonadotropes and corticotropes in the non-pit1 lineage. Tpit/Tbx19 directs precursor differentiation toward corticotropes by preventing gonadotrope differentiation (40). Consistent with this, a slight but significant increase in the number of presumptive gonadotropes was observed in zebrafish dmrt5 morphants. Notably, the average number of additional gsu-a positive cells (+10.1 cells) nearly matched that of missing corticotropes (−15.7 cells). This therefore is compatible with the idea that also in zebrafish a common progenitor pool gives rise to corticotropes and gonadotropes and that under dmrt5-deficient conditions these progenitors differentiate into gonadotropes instead of corticotropes (Figure 9A). We ruled out that the observed corticotrope defects were secondary to hypothalamic defects. Expression of both the pan-hypothalamic marker nkx2.1 and corticotropin releasing hormone (crh), the upstream regulator of Pomc, was normal (Figure 5). Hence, our findings suggest that Dmrt5 regulates corticotrope differentiation in the pituitary in a cell-autonomous manner and thereby determines gonadotrope numbers.
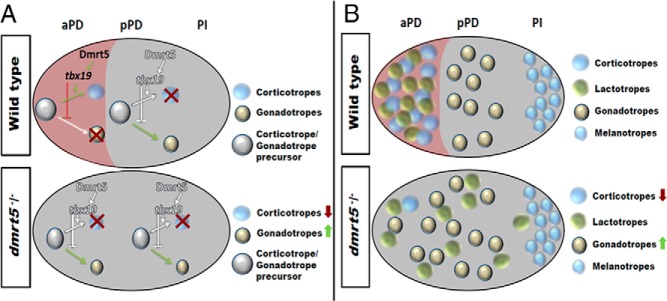
Figure 9. Multiple roles for dmrt5 in pituitary differentiation.
A, Model for dmrt5 function in corticotrope vs gonadotrope differentiation. In wild-type embryos (top panel), dmrt5 expression is restricted to the aPD and regulates corticotrope differentiation from bipotential precursors via regulation of tbx19 expression. The pPD lacks dmrt5 expression and gonadotrope differentiation is not inhibited by Tbx19. In dmrt5 mutants and morphants (bottom panel), absence of Tbx19 mediated repression results in gonadotrope formation also in the aPD. B, Model for dmrt5 role in lactotrope maintenance and positioning. Wild-type embryos (top panel) show tight clustering of corticotropes and lactotropes in aPD, gonadotropes, thyrotropes, and somatotropes in pPD and melanotropes in PI. In dmrt5 morphants and mutants, anterior pituitary precursor cells fail to differentiate into corticotropes. As a result, lactotropes, usually closely linked to corticotropes, become scattered throughout the pituitary. They eventually enter apoptosis after initially normal differentiation. Note that melanotropes in PI as well as somatotropes and thyrotropes (not shown) are not affected.
We found that in dmrt5 morphants both tbx19 and pomc are differentially regulated in the pituitary and ventral diencephalon. This interestingly corresponds to the situation for dmrt5, which is expressed in the pituitary but not the ventral diencephalon (Supplemental Figure 5). In this context, it is interesting to note that different enhancer regions control pomc expression in the arcuate nucleus and corticotropes, respectively (43). It is thus tempting to speculate that Dmrt5 regulates pomc directly, but future studies need to show whether Dmrt5 binds to any of these pituitary-specific enhancers directly.
Numerous studies have shown that dmrt genes are expressed in gonads and control different aspects of sex determination and/or differentiation in a cell-autonomous manner (19, 44). In addition to its gonadal expression at later stages (21), we show here that dmrt5 is expressed in the pituitary and controls gonadotrope numbers. Considering the important roles of dmrt genes in reproductive systems in general, it is tempting to speculate that altered dmrt5 activity could affect gonadal activity and consequently sexual development because of altered gonadotrope numbers and/or activities. A recent study reported that a deficiency in LH and FSH in zebrafish mutants leads to a significant delay of gonad development and onset of puberty as well as infertility and sex reversal (45). It is thus tempting to speculate that an excess of gonadotropes could accelerate gonad maturation and affect sex ratios. This is the first time that a member of the dmrt family is shown to be involved in reproductive control through a mechanism that does not reside in the gonad but instead in the pituitary, in which dmrt5 limits gonadotrope numbers. It will be interesting to test in future studies whether gonadotrope numbers might be controlled by a feedback between gonad and pituitary that involves dmrt5, for example through positive or negative regulation by activin or estrogen and inhibin, respectively.
We also observed that lactotrope development was affected under dmrt5-deficient conditions. The prl-expressing lactotropes are located in the aPD, and its expression becomes gradually reduced in dmrt5 morphants and mutants after 48 hpf. This reduction was partially rescued by p53 knockdown, suggesting that in the absence of dmrt5, lactotropes become apoptotic via p53-mediated apoptosis. Interestingly, in this case lactotropes were no longer restricted to the aPD but instead located in other regions of the pituitary or even outside the pituitary (inset, Figure 8B). It has been shown in mice that the different cell types in the pituitary are not distributed randomly within particular subdomains but instead form intricate cellular networks in which intercellular communication controls differentiation and functionality (46–48). It was shown that cell type-specific ablation of somatotropes resulted in reduced lactotrope numbers (49). This demonstrated the importance of a correct microenvironment for the maintenance of differentiated pituitary cell types. Corticotropes have extensive cytoplasmic extensions that are thought to be needed for direct cell-cell interactions (46). Furthermore, proopiomelanocortin has been shown to have paracrine functions during lactotrope differentiation and growth (50). We therefore propose that the progressive loss of lactotropes in dmrt5 mutants at later stages is a secondary consequence of reduced corticotrope numbers in the aPD that result in changes of the microenvironment (Figure 9B). Accordingly, prl-expressing cells fail to recognize their correct developmental niche and become randomly scattered. We speculate that in the absence of correct cell-cell interactions, lactotropes become apoptotic via the p53 pathway. Interestingly, although apoptosis could be partially rescued by p53 knockdown, this failed to rescue the ectopic location of differentiated lactotropes, suggesting persisting defects in the microenvironment.
Importantly, dmrt5 is expressed only in the aPD, and the mutant phenotypes were restricted to aPD cell populations. The fate choice in bipotential gonadotrope/corticotrope precursors was tipped toward gonadotropes in the absence of dmrt5, whereas the subsequent loss of lactotropes might be attributed to an altered microenvironments within the aPD. Somatotrope, melanotrope, and thyrotrope populations that are located more posteriorly in the zebrafish pituitary were unaffected in dmrt5 morphants. To date, the direct or indirect transcriptional targets of Dmrt5 remain unknown. We cannot exclude that Dmrt5 binds directly to pomc and prl promoters or directly regulates corticotrope and lactotrope differentiation and maintenance. Future studies need to reveal Dmrt5's transcriptional targets that control differentiation and maintenance of diverse pituitary populations.
Acknowledgments
We thank Dr Yutaka Kikuchi (Hiroshima University) as well as the National Bioresource Project Zebrafish in Japan for kindly providing the dmrt5ha2 mutants. We also thank Joey Lim for technical assistance and discussions.
Present address for M.G.: Lee Kong Chian School of Medicine, Proteos, Biopolis, 61 Biopolis Drive, Singapore 138673.
This work was supported by the Singapore Ministry of Education Academic Research Fund (MOE-AcRF; Grants R-154–000-329–133 and R-154–000-478–112, to C.W.). M.G. and F.R. received graduate fellowships from the Faculty of Science, National University of Singapore. M.V.S. receives a SINGA graduate scholarship from the Singapore Agency for Science, Technology and Research. M.G. and F.R. received graduate scholarships from the National University of Singapore, Department of Biological Sciences (NUS-DBS), and M.V.S. holds a Singapore International Graduate Award (SINGA) scholarship.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Singapore Ministry of Education Academic Research Fund (MOE-AcRF; Grants R-154–000-329–133 and R-154–000-478–112, to C.W.). M.G. and F.R. received graduate fellowships from the Faculty of Science, National University of Singapore. M.V.S. receives a SINGA graduate scholarship from the Singapore Agency for Science, Technology and Research. M.G. and F.R. received graduate scholarships from the National University of Singapore, Department of Biological Sciences (NUS-DBS), and M.V.S. holds a Singapore International Graduate Award (SINGA) scholarship.
Footnotes
- AH
- adenohypophysis
- ANK
- anterior neural keel
- aPD
- anterior pars distalis
- DIC
- differential interference contrast
- Dmrt
- doublesex- and mab3-related transcription factor
- hpf
- hours post fertilization
- L
- length
- MO
- morpholino
- PI
- pars intermedia
- Pit1
- pituitary transcription factor-1
- pPD
- posterior pars distalis
- T
- thickness
- TUNEL
- terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate-biotin nick, end labeling
- Tpit
- pituitary-restricted transcription factor
- W
- width.
References
- 1. Pogoda HM, Hammerschmidt M. How to make a teleost adenohypophysis: molecular pathways of pituitary development in zebrafish. Mol Cell Endocrinol. 2009;312(1–2):2–13. [DOI] [PubMed] [Google Scholar]
- 2. Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol. 1990;21(3):427–440. [DOI] [PubMed] [Google Scholar]
- 3. Eagleson GW. Developmental neurobiology of the anterior areas in amphibians: urodele perspectives. Int J Dev Biol. 1996;40(4):735–743. [PubMed] [Google Scholar]
- 4. Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–477. [DOI] [PubMed] [Google Scholar]
- 5. Glasgow E, Karavanov AA, Dawid IB. Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol. 1997;192(2):405–419. [DOI] [PubMed] [Google Scholar]
- 6. Herzog W, Zeng X, Lele Z, et al. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254(1):36–49. [DOI] [PubMed] [Google Scholar]
- 7. Dutta S, Dietrich JE, Aspock G, et al. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132(7):1579–1590. [DOI] [PubMed] [Google Scholar]
- 8. Kondoh H, Uchikawa M, Yoda H, Takeda H, Furutani-Seiki M, Karlstrom RO. Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mech Dev. 2000;96(2):165–174. [DOI] [PubMed] [Google Scholar]
- 9. Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13(4):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toro S, Varga ZM. Equivalent progenitor cells in the zebrafish anterior preplacodal field give rise to adenohypophysis, lens, and olfactory placodes. Semin Cell Dev Biol. 2007;18(4):534–542. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Crenshaw EB 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528–533. [DOI] [PubMed] [Google Scholar]
- 12. Nica G, Herzog W, Sonntag C, Hammerschmidt M. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol. 2004;18(5):1196–1209. [DOI] [PubMed] [Google Scholar]
- 13. Radovick S, Nations M, Du Y, Berg LA, Weintraub BD, Wondisford FE. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992;257(5073):1115–1118. [DOI] [PubMed] [Google Scholar]
- 14. Nica G, Herzog W, Sonntag C, et al. Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis. Dev Biol. 2006;292(1):189–204. [DOI] [PubMed] [Google Scholar]
- 15. Herzog W, Sonntag C, Walderich B, Odenthal J, Maischein HM, Hammerschmidt M. Genetic analysis of adenohypophysis formation in zebrafish. Mol Endocrinol. 2004;18(5):1185–1195. [DOI] [PubMed] [Google Scholar]
- 16. Chen W, Ge W. Ontogenic expression profiles of gonadotropins (fshb and lhb) and growth hormone (gh) during sexual differentiation and puberty onset in female zebrafish. Biol Reprod. 2012;86(3):73. [DOI] [PubMed] [Google Scholar]
- 17. Guo Y, Cheng H, Huang X, Gao S, Yu H, Zhou R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun. 2005;330(3):950–957. [DOI] [PubMed] [Google Scholar]
- 18. Matsuda M, Nagahama Y, Shinomiya A, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–563. [DOI] [PubMed] [Google Scholar]
- 19. Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14(20):2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gennet N, Gale E, Nan X, et al. Doublesex and mab-3-related transcription factor 5 promotes midbrain dopaminergic identity in pluripotent stem cells by enforcing a ventral-medial progenitor fate. Proc Natl Acad Sci USA. 2010;108(22):9131–9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo Y, Li Q, Gao S, et al. Molecular cloning, characterization, and expression in brain and gonad of Dmrt5 of zebrafish. Biochem Biophys Res Commun. 2004;324(2):569–575. [DOI] [PubMed] [Google Scholar]
- 22. Yoshizawa A, Nakahara Y, Izawa T, et al. Zebrafish Dmrta2 regulates neurogenesis in the telencephalon. Genes Cells. 2011;16(11):1097–1109. [DOI] [PubMed] [Google Scholar]
- 23. Huang X, Hong CS, O'Donnell M, Saint-Jeannet JP. The doublesex-related gene, XDmrt4, is required for neurogenesis in the olfactory system. Proc Natl Acad Sci USA. 2005;102(32):11349–11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parlier D, Moers V, Van Campenhout C, et al. The Xenopus doublesex-related gene Dmrt5 is required for olfactory placode neurogenesis. Dev Biol. 2013;373(1):39–52. [DOI] [PubMed] [Google Scholar]
- 25. Konno D, Iwashita M, Satoh Y, et al. The mammalian DM domain transcription factor Dmrta2 is required for early embryonic development of the cerebral cortex. PLoS One. 2012;7(10):e46577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saulnier A, Keruzore M, De Clercq S, et al. The doublesex homolog Dmrt5 is required for the development of the caudomedial cerebral cortex in mammals. Cereb Cortex. 2013;23(11):2552–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. [DOI] [PubMed] [Google Scholar]
- 28. Robu ME, Larson JD, Nasevicius A, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3(5):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schäfer M, Rembold M, Wittbrodt J, Schartl M, Winkler C. Medial floor plate formation in zebrafish consists of two phases and requires trunk-derived Midkine-a. Genes Dev. 2005;19(8):897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113(4):1193–1206. [DOI] [PubMed] [Google Scholar]
- 31. Wong AC, Van Eenennaam AL. Gonadotropin hormone and receptor sequences from model teleost species. Zebrafish. 2004;1(3):203–221. [DOI] [PubMed] [Google Scholar]
- 32. Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21(5):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol. 1999;209(7):399–410. [DOI] [PubMed] [Google Scholar]
- 34. Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn. 2004;230(4):781–786. [DOI] [PubMed] [Google Scholar]
- 35. Runkle S, Hill J, Kantorow M, Horwitz J, Posner M. Sequence and spatial expression of zebrafish (Danio rerio) αA-crystallin. Mol Vis. 2002;8:45–50. [PMC free article] [PubMed] [Google Scholar]
- 36. Camper SA, Saunders TL, Katz RW, Reeves RH. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990;8(3):586–590. [DOI] [PubMed] [Google Scholar]
- 37. Lamolet B, Pulichino AM, Lamonerie T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104(6):849–859. [DOI] [PubMed] [Google Scholar]
- 38. To TT, Hahner S, Nica G, Rohr KB, Hammerschmidt M, Winkler C, Allolio B. Pituitary-interrenal interaction in zebrafish interrenal organ development. Mol Endocrinol. 2007;21(2):472–485. [DOI] [PubMed] [Google Scholar]
- 39. Liu NA, Ren M, Song J, et al. In vivo time-lapse imaging delineates the zebrafish pituitary proopiomelanocortin lineage boundary regulated by FGF3 signal. Dev Biol. 2008;319(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17(6):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nanda I, Kondo M, Hornung U, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA. 2002;99(18):11778–11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zarkower D. DMRT genes in vertebrate gametogenesis. Curr Top Dev Biol. 2013;102:327–356. [DOI] [PubMed] [Google Scholar]
- 43. Langlais D, Couture C, Sylvain-Drolet G, Drouin J. A pituitary-specific enhancer of the POMC gene with preferential activity in corticotrope cells. Mol Endocrinol. 2011;25(2):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307(2):314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Z, Zhu B, Ge W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol Endocrinol. 2014;me20141256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Budry L, Lafont C, El Yandouzi T, et al. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA. 2011;108(30):12515–12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol. 2012;33(3):252–266. [DOI] [PubMed] [Google Scholar]
- 48. Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23(6):261–269. [DOI] [PubMed] [Google Scholar]
- 49. Waite E, Lafont C, Carmignac D, et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology. 2010;151(1):234–243. [DOI] [PubMed] [Google Scholar]
- 50. Denef C. Paracrine control of lactotrope proliferation and differentiation. Trends Endocrinol Metab. 2003;14(4):188–195. [DOI] [PubMed] [Google Scholar]