Supplemental Digital Content is Available in the Text.
Abstract
BACKGROUND
OnabotulinumtoxinA has demonstrated the ability to eliminate mild glabellar lines at rest; however, less is known regarding the effect of repeat treatment on more severe lines at rest.
OBJECTIVE
To assess the effect of repeated onabotulinumtoxinA treatment for reduction of glabellar lines at rest.
METHODS
Subjects 18 to 75 years old with at least mild glabellar lines at rest, as assessed by the validated Facial Wrinkle Scale (FWS) with photonumeric guide (score ≥ 1), received 3 treatments of 20 U onabotulinumtoxinA 4 months apart (N = 225). “Response” was defined as elimination of glabellar lines at rest (FWS score = 0) at any time point (Days 7, 30, 60, 90, and 120). Effect of treatment cycle on response was analyzed using repeated measures logistic regressions (p < .05).
RESULTS
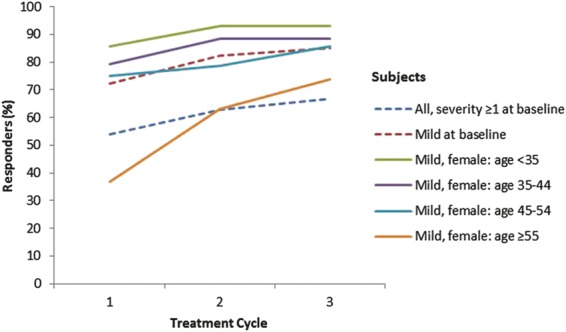
Most subjects were female (85%) and white (88%) (age range: 35–54 years). The likelihood of significant response was as follows: for all subjects combined (odds ratio [OR]: 1.31), for subjects with mild resting lines at baseline (OR: 1.49), and for older women (≥55 years) with mild resting lines at baseline (OR: 2.22). Of all subjects, 76% responded after 1 treatment, and 45% responded in all 3 cycles.
CONCLUSION
Subjects repeatedly treated with onabotulinumtoxinA showed progressive improvement in glabellar lines at rest.
OnabotulinumtoxinA (BOTOX Cosmetic; Allergan plc, Irvine, CA) is a safe and effective neuromodulator that imparts the positive aesthetic effect of reducing dynamic facial lines.1,2 The most commonly treated area is the glabellar region where onabotulinumtoxinA limits the ability to contract the corrugator supercilii, orbicularis oculi, procerus, and frontalis muscles.3 More recently, intramuscular onabotulinumtoxinA treatment has demonstrated the additional benefit of improving mild lines at rest and overall skin quality.4–8
The authors previously reported the efficacy of onabotulinumtoxinA to eliminate mild glabellar lines when individuals are not actively animating (i.e., lines at rest), an important albeit more subtle effect for those seeking a smoother and more youthful facial appearance.4 The authors hypothesized that this is due to local relaxation of transverse muscle cells, tissue remodeling in response to reduced muscle activity, or both. This effect also has been demonstrated quantitatively by Dessy and colleagues9 (2008) using electron microscope image analysis of silicon replicas after onabotulinumtoxinA treatment in the glabellar region. They reported a significant reduction in skin surface topographic parameters, including average skin roughness, and concluded these reductions were associated with the observed “smoother and lighter” appearance of the skin.
Clinical experience has suggested that there may be increased efficacy for lines at rest with repeated treatments of onabotulinumtoxinA. As such, this article reports on the effect of repeated treatments of glabellar lines at rest in a subset of the population the authors studied in their initial report4; namely, those who had 3 consecutive cycles of onabotulinumtoxinA treatment. The authors extended the analysis from their initial report that was limited to subjects with “mild” lines at rest to include subjects with “moderate” and “severe” resting lines at baseline. In addition, the authors evaluated differences in lines at rest by age group among women with mild resting lines at baseline.
Methods
Data were extracted from a 1-year repeat-treatment evaluation consisting of two 4-month randomized, double-blind, placebo-controlled clinical studies (Period 1) followed by an 8-month open-label follow-up study (Period 2). The 2 studies in Period 1 followed identical protocols and enrolled similar populations; therefore, these data were pooled. Methods and safety data for these studies have been published previously.10–12 Both study periods complied with the Declaration of Helsinki guidelines on human biomedical research including written informed consent and institutional review board approval before study initiation.
Male and female subjects were eligible for Period 1 if they were between 18 and 75 years of age and had glabellar lines of at least moderate severity at maximum frown. Lines were measured by the validated Facial Wrinkle Scale (FWS) with photonumeric guide of which 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Subjects were eligible for Period 2 if they completed Period 1 and had glabellar lines with an FWS score of ≥1 at maximum frown at the end of Period 1. Subjects had to continue to meet this requirement for subsequent treatments in Period 2. There were no severity requirements for glabellar lines at rest during Period 1 or 2; however, these data were recorded and are the focus of this report.
In Period 1, subjects received 1 treatment, and in Period 2, they could have received up to 2 treatments 4 months apart. Each treatment cycle consisted of 20 U onabotulinumtoxinA. Treatment was administered as five 0.1-mL injections of 4 U each, 2 each in the corrugators and orbicularis oculi, and 1 in the procerus.
At Days 7 (Period 1 only), 30, 60, 90, and 120 after treatment, physicians assessed the appearance of glabellar lines using the FWS. For this report, only onabotulinumtoxinA treatments were included, and only glabellar lines at rest were analyzed. Furthermore, only subjects with baseline FWS ≥1 at rest who had 3 treatment cycles were included. For the analysis presented here, response during a treatment cycle was defined as elimination of glabellar lines at rest (i.e., FWS = 0 at any visit during the treatment cycle).
Repeated measures logistic regression using PROC GENMOD in SAS v9.1 (SAS Institute, Inc., Cary, NC) was used to analyze the effect of treatment cycle number (1, 2, or 3) on response. For analysis of the age effect in women, age was categorized in 10-year cohorts (i.e., <35, 35–44, 45–55, and >55). Cochran–Armitage tests were used to assess trends across age groups. Statistical tests were 2-sided with a .05 significance level.
Results
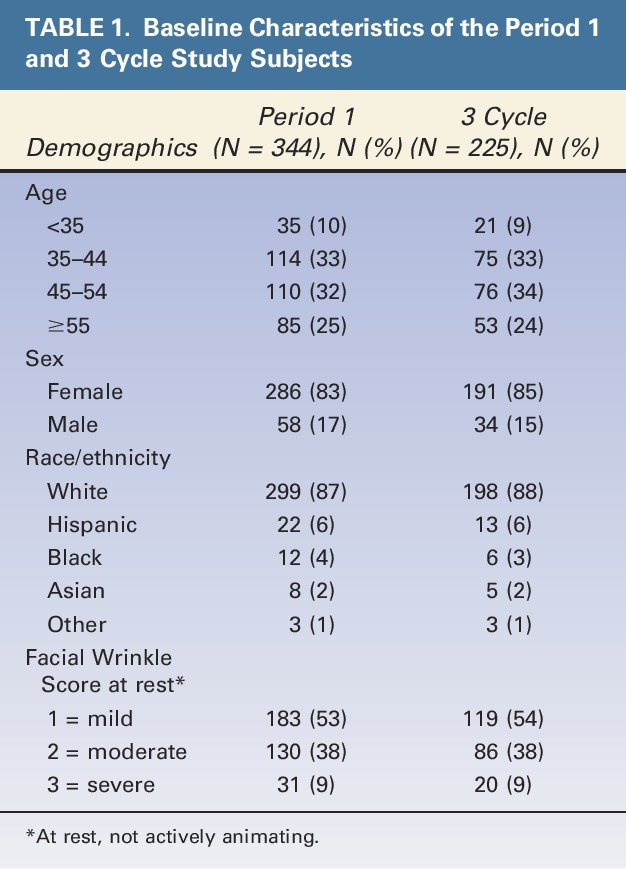
As shown in Figure 1, there were 344 subjects who received onabotulinumtoxinA in Period 1 and who had a baseline FWS score of ≥1 (Mild) at rest (“Period 1 population”). Of these subjects, 183 (53%) had a baseline FWS score of 1 at rest. These 183 subjects constituted the study population of the initial report on the effects of onabotulinumtoxinA on mild lines at rest. A total of 258 subjects had 3 onabotulinumtoxinA treatment cycles. Of these subjects, 225 (87%) had a baseline FWS score of ≥1 at rest and thus constituted the study population of this report (“3-cycle population”). As expected, demographic characteristics and baseline FWS scores were similar for the Period 1 and 3-cycle populations (Table 1). Subjects (age range: 35–54 years) were mainly female and white and had a baseline FWS score of 1 at rest. It is relevant to note that a total of 47 subjects from Period 1 had onabotulinumtoxinA treatment before study enrollment (mean time since last treatment was approximately 9 months); however, the percentage of responders who had previous treatment (55.3%, 26/47) did not differ from those who did not have this history (55.3%, 163/295) (2 subjects had missing data).
Figure 1.
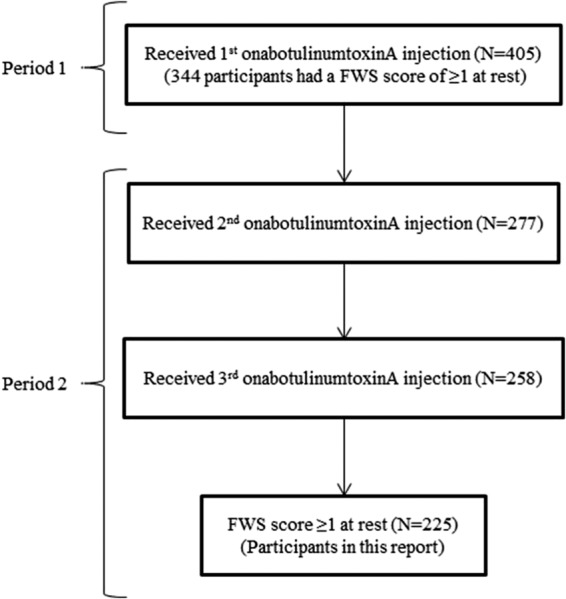
Subject flowchart.
TABLE 1.
Baseline Characteristics of the Period 1 and 3 Cycle Study Subjects
The subpopulations significantly more likely to achieve elimination of glabellar lines at rest (i.e., FWS = 0) with repeat treatment compared with single treatment were1 all subjects combined,2 all subjects with mild resting lines at baseline,3 and women with mild resting lines at baseline who were in the oldest age category (≥55 years) (Table 2). Women in this oldest age category had the highest odds ratio (OR), meaning they were most likely to respond to treatment (OR: 2.22, p < .01). Figure 2 depicts the responder rates by subpopulation, showing that the percentage of responders increased with repeat treatment, especially for women in the oldest age category. Figure 2 also depicts that the response rate decreased with age; Cochran–Armitage tests showed that this decrease was statistically significant (p = .02).
TABLE 2.
Odds Ratio of Repeat-Treatment Effect Among Subjects With 3 OnabotulinumtoxinA Treatment Cycles by Subpopulation
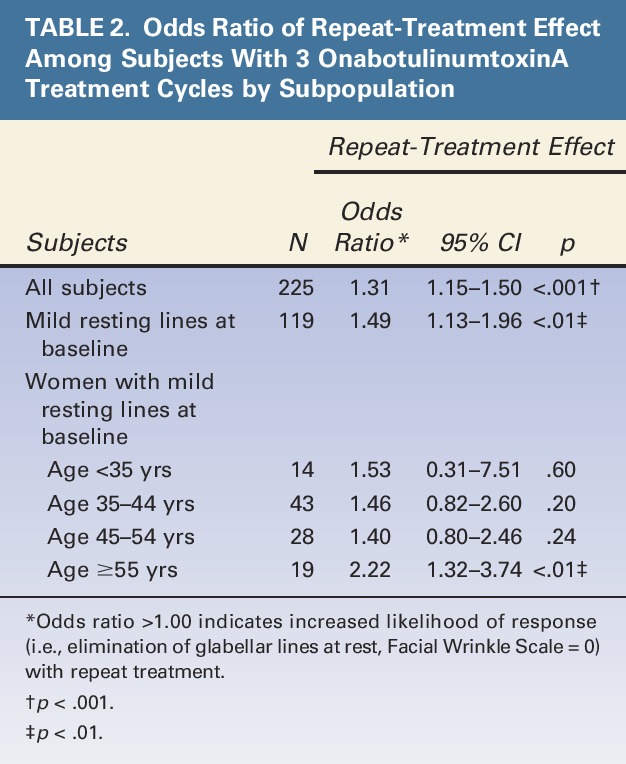
Figure 2.
Percentage of women by baseline and demographic characteristics who responded to repeat treatment of onabotulinumtoxinA. “Response” during a treatment cycle was defined as elimination of glabellar lines at rest.
Summaries of response patterns by subpopulation are shown in Table 3. Of those who did not respond after the initial treatment (i.e., achieve FWS = 0), 25% to 52% (depending on baseline severity of the FWS score) responded to the second or third treatment. Among all subjects, response in more than 1 cycle was likely with 45% to 65% of subjects responding in all 3 cycles. The pattern of response across cycles is shown in detail for all subjects combined and for subjects with mild resting lines at baseline (Figures 3 and 4, respectively). Supplemental Digital Content, Figures E1–E4, (http://links.lww.com/DSS/A46, http://links.lww.com/DSS/A47, http://links.lww.com/DSS/A48, http://links.lww.com/DSS/A49), show the pattern of response across the cycles for women with mild resting lines at baseline by age group (Figure E1 = <35 years, Figure E2 = 35–44 years, Figure E3 = 45–54 years, and Figure E4 = ≥55 years).
TABLE 3.
Percentage of Responders to 3 OnabotulinumtoxinA Treatment Cycles by Subpopulation
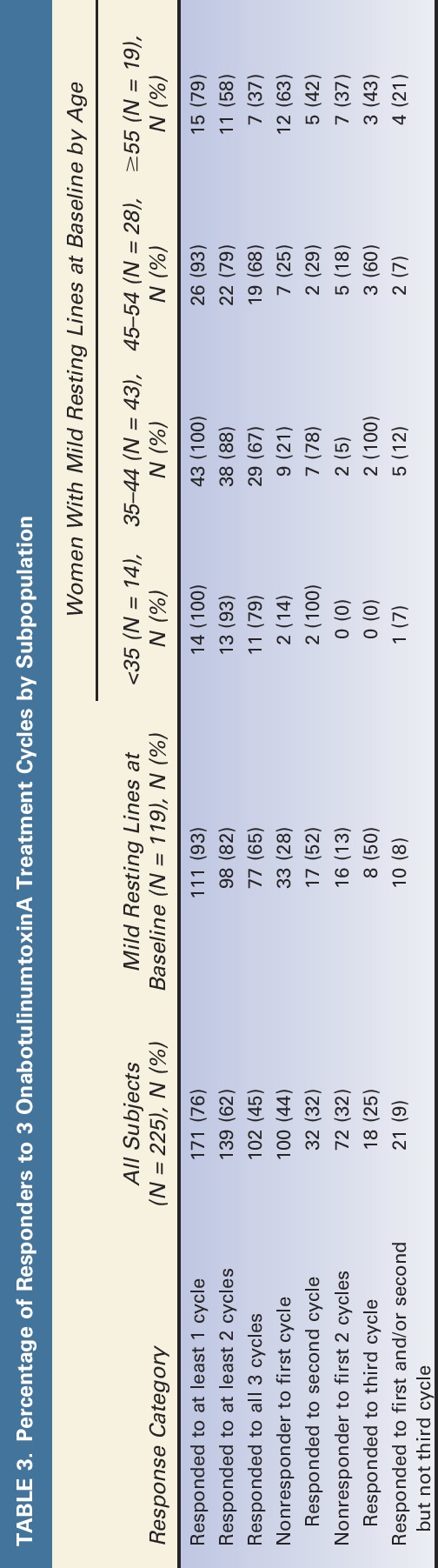
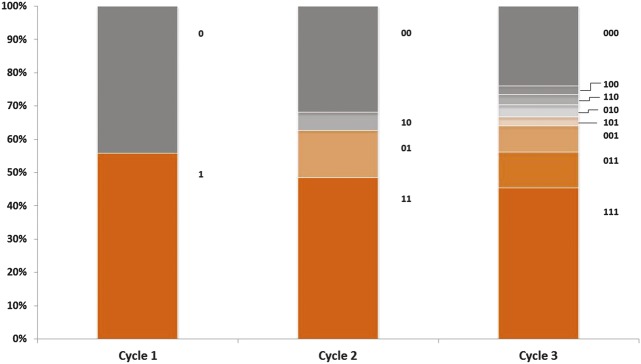
Figure 3.
Response status by treatment cycle for the repeat-treatment subjects with resting line severity ≥1 at baseline who received 3 treatment cycles (N = 225). This graph displays responders and nonresponders for each cycle: “1” = response during the cycle and “0” = no response during the cycle. Digit position indicates cycle number (e.g., in Cycle 3, “011” = no response in Cycle 1 and response in cycles 2 and 3). “Response” during a treatment cycle was defined as elimination of glabellar lines at rest.
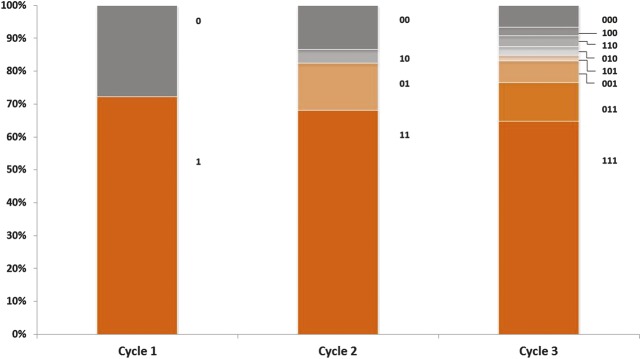
Figure 4.
Response status by treatment cycle for the repeat-treatment subjects with mild resting lines (Facial Wrinkle Scale = 1) at baseline who received 3 treatment cycles (N = 119). This graph displays responders and nonresponders for each cycle: “1” = response during the cycle and “0” = no response during the cycle. Digit position indicates cycle number (e.g., in Cycle 3, “011” = no response in Cycle 1 and response in Cycles 2 and 3). “Response” during a treatment cycle was defined as elimination of glabellar lines at rest.
Discussion
This work extends the analysis the authors presented previously in which they showed that 68% of subjects with mild glabellar lines at rest who were treated once with onabotulinumtoxinA achieved elimination of their resting lines compared with only 5% in the placebo group.4 In the current analysis, one-quarter to one-half of subjects who did not respond after the initial treatment of onabotulinumtoxinA responded after the second or third treatments, with most subjects responding to all 3 treatments. The subpopulation that had the highest likelihood of response from repeated treatments for glabellar lines at rest was those with mild resting lines at baseline and, in particular, women age ≥55 years.
Results from this study are consistent with previous research demonstrating that intramuscular treatment with onabotulinumtoxinA improves appearance of lines at rest and overall skin quality.5–8 For instance, intramuscular injection of onabotulinumtoxinA into the lateral orbital, forehead, and glabellar regions resulted in increased skin pliability and elastic recoil.5 One possible explanation for this effect is that muscle weakening eliminates repetitive skin folding and alleviates chronic stress applied to the overlying skin, which causes elastin and collagen to strengthen at these sites over time.5,6 It seems plausible that this process would not create an immediate effect but would be more observable with repeat treatments. In addition, recent work has established that the C-terminal binding domain region of onabotulinumtoxinA, HC/A, is homologous to fibroblast growth factors, making HC/A a possible ligand for fibroblast growth factor receptor 3.13
Moreover, onabotulinumtoxinA injections into the glabellar region may improve the appearance of lines at rest because weakening the facial muscles in this area results in unopposed elevation and a lifting effect from the noninjected brow elevators.14,15 More specifically, in addition to the corrugator and procerus muscles lowering eyebrows, the medial inferior fibers of the frontalis may contribute to the downward positioning and may be partially inactivated with treatment.14 Alleviation of the inferior pull releases the skin to the corresponding upwardly directed frontalis muscle.14
The smoothing effect on lines at rest from repeated onabotulinumtoxinA treatment helps achieve and sustain patient satisfaction. Not only are patients more satisfied with improvement in both lines at rest and dynamic lines versus improvement in one type of line16 but also the effect on lines at rest may last longer than the effect on dynamic lines.8 This longer-lasting improvement in lines at rest may explain why research has shown that patient satisfaction levels remain high even after dynamic lines return to baseline levels.17
Some limitations should be noted. For ease of understanding, response was considered at any time point after treatment, which limits any conclusions regarding onset of resting line improvement. Exclusion criteria did not include previous onabotulinumtoxinA treatment in the glabellar region; however, the percentage of responders who had previous treatment did not differ from those without this history, and as such, those who had previous treatment should not bias the results.
OnabotulinumtoxinA has proven efficacy in treating dynamic lines,1,2 and more recently, it also has been shown to treat lines at rest.4–7 This study extends previous research by demonstrating that repeat treatment improves the likelihood of eliminating lines at rest. The smoothing effect of eliminating both types of lines is a highly desirable result as it is associated with a more youthful, attractive, and harmonious facial appearance.18,19 Results from this study can help aesthetic clinicians provide more effective guidance to patients regarding potential outcomes and help create tailored treatment algorithms and long-term treatment plans to attain optimal outcomes for facial lines. Dosing and results reported in this study are specific to the formulation of onabotulinumtoxinA manufactured by Allergan plc. This formulation is not interchangeable with other botulinum toxin products, and dosing cannot be converted to that of any other products by use of a dose ratio.20,21
Supplementary Material
Acknowledgments
The authors thank Janice Pagoda, PhD (Allergan plc), for her statistical support.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
This study was sponsored by Allergan plc. Neither honoraria nor other form of payments were made for authorship. A. Carruthers is a consultant for and has received research grants from Allergan plc, Merz Pharmaceuticals GmbH, and Revance, Inc. J. Carruthers is a consultant and investigator for Allergan plc, Merz Pharmaceuticals GmbH, Solstice Neurosciences, and Revance, Inc. S. Fagien is a consultant and a clinical investigator for Allergan plc. X. Lei, J. Kolodziejczyk, and M. F. Brin are employees of Allergan plc and may own equity in the company.
References
- 1.Beer K, Cohen JL. Upper face rejuvenation. Cosmet Dermatol 2006;19:567–70. [Google Scholar]
- 2.Lorenc ZP, Kenkel JM, Fagien S, Hirmand H, et al. A review of onabotulinumtoxinA (Botox). Aesthet Surg J 2013;33(1 Suppl):9S–12S. [DOI] [PubMed] [Google Scholar]
- 3.Dorizas A, Krueger N, Sadick NS. Aesthetic uses of the botulinum toxin. Dermatol Clin 2014;32:23–36. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers A, Carruthers J, Lei X, Pogoda JM, et al. OnabotulinumtoxinA treatment of mild glabellar lines in repose. Dermatol Surg 2010;36(Suppl 4):2168–71. [DOI] [PubMed] [Google Scholar]
- 5.Bonaparte JP, Ellis DA. A prospective assessment of biomechanical changes in skin after the injection of onabotulinum toxin. Otolaryngol Head Neck Surg 2014;149(2 suppl):41. [DOI] [PubMed] [Google Scholar]
- 6.Small R. Botulinum toxin injection for facial wrinkles. Am Fam Physician 2014;90:168–75. [PubMed] [Google Scholar]
- 7.Levy JL, Servant JJ, Jouve E. Botulinum toxin A: a 9-month clinical and 3D in vivo profilometric crow's feet wrinkle formation study. J Cosmet Laser Ther 2004;6:16–20. [DOI] [PubMed] [Google Scholar]
- 8.Glogau R, Kane M, Beddingfield F, Somogyi C, et al. OnabotulinumtoxinA: a meta-analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg 2012;38:1794–803. [DOI] [PubMed] [Google Scholar]
- 9.Dessy LA, Mazzocchi M, Rubino C, Mazzarello V, et al. An objective assessment of botulinum toxin A effect on superficial skin texture. Ann Plast Surg 2007;58:469–73. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers JA, Lowe NJ, Menter MA, Gibson J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002;46:840–9. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers JD, Lowe NJ, Menter MA, Gibson J, et al. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg 2003;112:1089–98. [DOI] [PubMed] [Google Scholar]
- 12.Carruthers A, Carruthers J, Lowe NJ, Menter A, et al. One-year, randomised, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. J Clin Res 2004;7:1–20. [Google Scholar]
- 13.Jacky BPS, Garay PE, Dupuy J, Nelson JB, et al. Identification of fibroblast growth factor receptor 3 (FGFR3) as a protein receptor for botulinum neurotoxin serotype A (BoNT/A). PLoS Pathog 2013;9:e1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carruthers A, Carruthers J. Eyebrow height after botulinum toxin type A to the glabella. Dermatol Surg 2007;33(Suppl 1):S26–S31. [DOI] [PubMed] [Google Scholar]
- 15.Petchngaovilai C. Midface lifting with botulinum toxin: intradermal technique. J Cosmet Dermatol 2009;8:312–6. [DOI] [PubMed] [Google Scholar]
- 16.Patel MP, Talmor M, Nolan WB, Hurwitz DJ, et al. Botox and collagen for glabellar furrows: advantages of combination therapy. Ann Plast Surg 2004;52:442–7. [DOI] [PubMed] [Google Scholar]
- 17.Rivers J, Bertucci V, Muhn C, Rosen N, et al. Patient satisfaction with onabotulinumtoxinA treatment of glabellar and lateral canthal lines evaluated using the FLSQ: a new patient-reported outcome measure. Plast Reconstr Surg 2014;134:162. [DOI] [PubMed] [Google Scholar]
- 18.Carruthers J, Carruthers A. Botulinum toxin type A treatment of multiple upper facial sites: patient-reported outcomes. Dermatol Surg 2007;33(Suppl 1):S10–S17. [DOI] [PubMed] [Google Scholar]
- 19.Raspaldo H, Baspeyras M, Bellity P, Dallara JM, et al. Upper- and mid-face anti-aging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus—part 1. J Cosmet Dermatol 2011;10:36–50. [DOI] [PubMed] [Google Scholar]
- 20.Chen JJ, Dashtipour K. Abo-, inco-, ona-, and rima-botulinum toxins in clinical therapy: a primer. Pharmacotherapy 2013;33:304–18. [DOI] [PubMed] [Google Scholar]
- 21.Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.