Abstract
Pulsatile secretion of GnRH plays a pivotal role in follicular development via stimulating tonic gonadotropin secretion in mammals. Kisspeptin neurons, located in the arcuate nucleus (ARC), are considered to be an intrinsic source of the GnRH pulse generator. The present study aimed to determine ARC-specific enhancer(s) of the Kiss1 gene by an in vivo reporter assay. Three green fluorescent protein (GFP) reporter constructs (long, medium length, and short) were generated by insertion of GFP cDNA at the Kiss1 locus. Transgenic female mice bearing the long and medium-length constructs showed apparent GFP signals in kisspeptin-immunoreactive cells in both the ARC and anteroventral periventricular nucleus, in which another population of kisspeptin neurons are located. On the other hand, transgenic mice bearing 5′-truncated short construct showed few GFP signals in the ARC kisspeptin-immunoreactive cells, whereas they showed colocalization of GFP- and kisspeptin-immunoreactivities in the anteroventral periventricular nucleus. In addition, chromatin immunoprecipitation and chromosome conformation capture assays revealed recruitment of unoccupied estrogen receptor-α in the 5′-upstream region and intricate chromatin loop formation between the 5′-upstream and promoter regions of Kiss1 locus in the ARC. Taken together, the present results indicate that 5′-upstream region of Kiss1 locus plays a critical role in Kiss1 gene expression in an ARC-specific manner and that the recruitment of estrogen receptor-α and formation of a chromatin loop between the Kiss1 promoter and the 5′ enhancer region may be required for the induction of ARC-specific Kiss1 gene expression. These results suggest that the 5′-upstream region of Kiss1 locus functions as an enhancer for ARC Kiss1 gene expression in mice.
A classical concept, the hypothalamo-pituitary-gonadal axis with GnRH placed at the highest position, has been a fundamental dogma in reproductive biology. Pulsatile GnRH release from the hypothalamus drives tonic gonadotropin secretion to regulate gonadal activities such as gametogenesis and steroidogenesis and is observed during the greater part of estrus cycle in females. The mechanism governing the pulsatile GnRH/gonadotropins release, however, has been a mystery in the study of reproductive biology for decades. Kisspeptin [also known as metastin (1)], encoded by Kiss1 gene, may well be the key neuropeptide that unravels the mystery because multiple lines of evidence indicate that that peptide regulates pulsatile GnRH release in mammalian species including primates, ruminants, and rodents (2–5). Loss-of-function mutations in the KISS1 gene in patients also displayed hypogonadotropic hypogonadism (6, 7). Mice with a global disruption of the Kiss1 gene duplicated the phenotype seen in human (8, 9). Of two populations of kisspeptin neurons located in two distinct hypothalamic nuclei in rodents (10–12), kisspeptin neurons in the arcuate nucleus (ARC) are considered to regulate pulsatile GnRH release. Multiple-unit activities recorded near kisspeptin neurons in the ARC were associated with pulsatile LH secretion in rats (13) and goats (3, 4). On the other hand, another population of kisspeptin neurons located in the anteroventral periventricular nucleus (AVPV) may play a role in LH surge induction (11, 12, 14, 15).
Revealing the molecular mechanism regulating ARC-specific Kiss1 gene expression would be a good approach to elucidate the role of kisspeptin in GnRH pulse generation. We previously reported that the epigenetic mechanism regulating ARC Kiss1 gene expression is different from that in the AVPV (16). Estrogen decreases histone acetylation levels of the Kiss1 promoter region in the ARC but increases the levels in the AVPV (16), which would give a good explanation for how estrogen regulates AVPV Kiss1 gene expression and GnRH surge induction. Our previous in vivo reporter assay with transgenic mice suggested that the 3′-downstream region of Kiss1 locus, which is considered as an AVPV-specific enhancer of Kiss1 gene, is most likely not required for ARC Kiss1 gene expression (16). Another region that would function as an ARC-specific enhancer(s) of Kiss1 gene has not been identified yet.
The present study aims to determine enhancer region(s) controlling ARC-specific Kiss1 gene expression in mice with an in vivo reporter assay. We generated several lines of transgenic mice bearing constructs of three different lengths, which sequentially truncated from 5′-upstream region of the Kiss1-green fluorescent protein (GFP) constructs. The estrogen receptor-α (ERα) binding to the 5′-upstream region and chromatin loop formation between the 5′-upstream and Kiss1 promoter regions were analyzed to reveal the recruitment of ERα and ARC-specific conformational change of Kiss1 locus. We also determined putative binding sites for transcriptional factors in the ARC-specific enhancer region of the Kiss1 locus identified in the current study.
Materials and Methods
Generation of Kiss1-GFP mice
Bacterial artificial chromosome (BAC) clone RP24-299J was obtained from BACPAC Resources. Using a counterselection BAC modification kit (Gene Bridge), AcGFP1 (CLONTECH) was substituted for the site between the translational start point and 3′ end of exon 2 of the Kiss1 gene (accession number AB666166). BAC backbone was replaced with a plasmid cassette containing ampicillin resistance gene and ori using a BAC subcloning kit (Gene Bridge). The long, medium-length, and short construct were linearized by digestion with restriction enzymes, MluI, SspI, and XhoI, respectively. Accordingly, 3′ regions were different among three constructs. Linearized DNA fragments were purified by ethanol precipitation.
Female BDF1 mice (3 wk old) were superovulated with an ip injections of equine chronic gonadotropin (ASKA Pharmaceutical) and human chorionic gonadotropin (ASKA Pharmaceutical) with a 47-hour interval. Females were then mated with mature male BDF1 mice. Pronuclear-stage oocytes were collected from the females with a vaginal plug 22 hours after human chorionic gonadotropin injection. The linearized DNA fragments, which were dissolved in injection buffer (Millipore) by 5 ng/μL, were injected into fertilized eggs of the mouse strain BDF1. The manipulated eggs were cultured in KSOM medium for 16 hours in a humidified atmosphere of 5% CO2 in air at 37°C and then transferred into the oviduct ampulla of pseudopregnant foster mothers. Genomic DNA samples were extracted from a founder 0 mouse ear and then screened by PCR to check the presence of the DNA sequence of AcGFP. PCR conditions were 95°C for 10 minutes, 40 cycles at 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 1 minute, and lastly 72°C for 10 minutes to ensure the elongation. A 20-μL aliquot of PCR mixture contained 2 μL of genomic DNA, 0.4 μL with 10 μM of each primer, 2 μL of 10× LA taq PCR buffer, 2 μL of 25 mM MgCl2, 2 μL of 2.5 mM deoxynucleotide triphosphate mix, and 0.2 μL of LA taq DNA polymerase (TAKARA BIO). Primers used were as follows: forward, 5′-AAGTTCATCTGCACCACCG-3′ and reverse, 5′-TCCTCGAAGAAGATGGTGCG-3′. The PCR products were separated by electrophoresis in agarose gels and visualized by ethidium bromide staining under UV irradiation.
Mice
BDF1 mice were purchased from Japan SLC (Hamamatsu) and were maintained at the National Institute of Physiological Sciences under constant temperature (21–23°C) and 12-hour light, 12-hour dark cycle schedule (lights on at 7:00 am). BDF1 and Kiss1-GFP mice were fed CE-2 (Clea Japan) and water ad libitum. Kiss1-GFP mice were transported from the National Institute for Physiological Sciences to Nagoya University.
Kiss1-GFP mice were housed at Nagoya University under constant temperature (21–23°C) and 14-hour light, with 10-hour dark cycle schedule (lights on at 5:00 am). Kiss1-GFP mice were fed CE-2 (Clea Japan) and water ad libitum and were subjected to an analysis of GFP expression. All procedures for animal experimentation were reviewed and approved by the Committees on Animal Experiments of the Nagoya University and the National Institute for Physiological Sciences.
Immunofluorescence
The adult Kiss1-GFP mice (10–18 wk old) were ovariectomized, and some of them were sc implanted with SILASTIC brand tubing (Dow Corning Corp; inner diameter 1.5 mm; outer diameter 3.0 mm; 18 mm in length) filled with estradiol-17β (E2; Sigma) dissolved in peanut oil at 200 μg/mL to produce a preovulatory level of plasma E2 (16). Five days after surgery, animals stereotaxically received a lateral cerebroventricular injection (0.2 mm posterior, 2.0 mm to the bregma, and 1.0 mm lateral from the midline) of colchicine (10 μg per 4 μL saline; Wako). The animals were deeply anesthetized with sodium pentobarbital and perfused with 4% paraformaldehyde in 0.05 M phosphate buffer 2 days after colchicine injection. The brains were immediately removed, postfixed in the same fixative overnight at 4°C, and then immersed in PBS containing 30% sucrose for at least 2 days at 4°C. Frozen frontal sections (50 μm) were prepared with a Cryostat (Leica Microsystems). Free-floating sections were subjected to immunohistochemistry with antikisspeptin rabbit polyclonal antibodies (1:8000, kindly donated by Dr H. Okamura) and anti-GFP chicken polyclonal antibodies (1:2000, Abcam). The specificity of antikisspeptin antibodies was previously confirmed by immunoneutralization with mouse Kp-10 (16). The sections were then incubated with Alexa 594-conjugated antirabbit IgG (1:800; Invitrogen) and Alexa 488-conjugated antichicken IgG (1:800; Molecular Probes).
Kisspeptin and GFP immunoreactivities were examined under a fluorescence microscope with the ApoTome (Carl Zeiss). Signal intensities of GFP immunoreactivity were quantified by ImageJ software (http://imagej.nih.gov/ij/) as previously described (17, 18) and normalized by intensities of kisspeptin immunoreactivity in each individual. According to the instructions, the original color-image files were converted to an eight-bit gray-scale file to quantify the immunoreactivity.
Chromatin conformation capture assay
Chromatin conformation capture (3C) assay was conducted as previously described (19) with some modifications. Cross-linked chromatin was digested with either 500 U of HindIII (Roche) overnight at 37°C and then was ligated in 6 mL of 1× ligation buffer. The 3C products were phenol/chloroform extracted, ethanol-precipitated, and dissolved in Tris/EDTA buffer. The 5′-upstream region of Kiss1 gene contains five HindIII sites, and the primers flanking Hind III sites are listed in Supplemental Table 1. The Kiss1 chromatin loop was detected using each primer P1 in the promoter region in combination with one of the other primers. Each PCR was performed under the following conditions: 95°C for 5 minutes; 35 cycles at 95°C for 30 seconds; 60°C for 1 minute; 72°C for 1 minute; and a final extension at 72°C for 10 minutes. PCR products were analyzed by agarose gel electrophoresis. It should be noted that each primer works at this PCR condition and that specificity of the 3C assay was confirmed with unligated chromatin.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed with punched-out tissue using the ChIP assay kit (Millipore) according to the manufacturer's instructions and the previous study (16). Purified samples were sonicated on ice until chromatin fragments became 200–1000 bp in size and incubated with antibodies at 4°C overnight. Antibody against ERα (Millipore) was used for immunoprecipitation. Mouse nonimmunized IgG (Millipore) was used as a negative control to check the specificity of immunoprecipitation. After immunoprecipitation, a PCR was performed with the primers in Supplemental Table 2. The PCR products were analyzed by agarose gel electrophoresis.
In silico analysis of putative transcription factor-binding sites in the ARC-specific enhancer
In silico analysis for the identification of putative transcription factor-binding sites was conducted. Consensus sequences of binding sites for transcriptional factors were investigated in the 5′-upstream region of Kiss1 locus (from −12 890 to −2165 bp) by TFSERCH (http://www.cbrc.jp/research/db/TFSEARCH.html), with the threshold score set at 85. Transcriptional factors were categorized as the activators (GO:0045893, GO:0045944, and GO:0051091), the repressors (GO:0000122, GO:0010629, GO:0043433, and GO:0045892), and the enhancer sequence-specific factors (GO:0000980, GO:00001158, GO:0001162, GO:0001205, GO:0003705, and GO:0035326) by UniProtKB (http://www.uniprot.org).
Data and statistical analysis
The phenotypes found in transgenic mice bearing long, medium-length, and short constructs are unlikely to have been due to a positional effect of the transgene because the phenotypes were confirmed in at least two founders (total n = 3; long and medium length construct, n = 5; short construct). Values are reported as mean ± SEM, and error bars are SEM. A two-way ANOVA with js-STAR 2.0.6j (http://www.kisnet.or.jp/nappa/software/star/index.htm) was used to determine statistical significance for the relative signal intensity and the number or percentage of immunoreactive cells among Kiss1-GFP transgenic mice bearing the three different lengths of constructs. Differences were considered to be significant when P < .05.
Results
Identification of ARC-specific enhancer by in vivo reporter assay
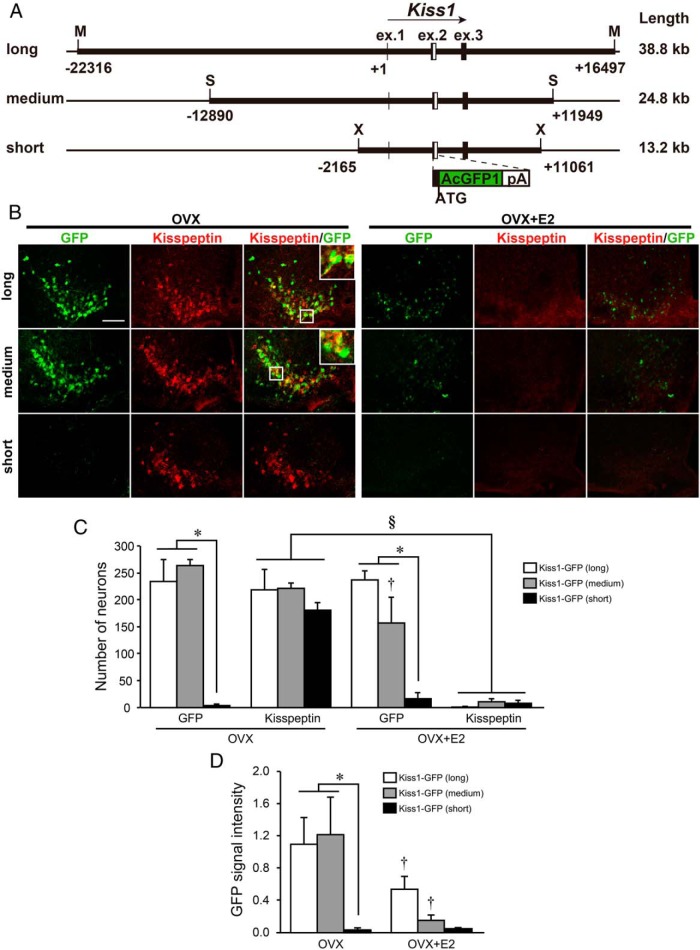
Transgenic mice were generated using three kinds of Kiss1-GFP constructs: long, medium-length, and short, consisting of 38.8, 24.8, and 13.2 kb of Kiss1 gene and its flanking sequences, respectively (Figure 1A). GFP expressions in the ARC and AVPV in these transgenic mice were confirmed in two or three founder mouse lines in each group.
Figure 1. GFP reporter expression in the ARC of transgenic mice carrying three different lengths of GFP reporter constructs.
A, Schematic illustration of three constructs used for in vivo reporter assay. White boxes indicate the inserted AcGFP and polyA tail sequence. Positions and structures of these genes were obtained from the University of California, Santa Cruz, genome browser. Restriction enzyme recognition sites were indicated as follows: X, XhoI; S, SspI; M, MluI. B, Immunofluorescence analysis of kisspeptin and GFP expression in the ARC. Left and center panels show GFP and kisspeptin immunoreactivities in the ARC sections of representative transgenic mice, respectively, and right panels show computer-aided merged images of immunoreactive signals for kisspeptin (red) and GFP (green) in OVX or E2-implanted OVX (OVX+E2) mice. Insets show the sections at higher magnification. Scale bar, 100 μm. C, The number of GFP-expressing and kisspeptin-immunoreactive cells in the ARC of transgenic mice. D, The relative GFP signal intensity in the ARC of transgenic mice. Values are means ± SEM. *, P < .05 vs transgenic mice bearing long and medium-length constructs (two way ANOVA followed by Bonferroni test); †, P < .05 vs OVX mice bearing the same construct; §, P < .05 vs OVX mice as a main effect.
Figure 1B shows representative photomicrographs of GFP and/or kisspeptin-immunoreactive cells in the ARC of ovariectomized (OVX) and E2-implanted OVX (OVX+E2) mice. GFP-expressing cells were abundantly found in the ARC of transgenic OVX mice bearing the long and medium-length constructs. The GFP expressions were localized in most kisspeptin-immunoreactive cells in the ARC of OVX mice. On the other hand, Kiss1-GFP transgenic mice bearing the short construct exhibited few GFP expressions in the ARC of OVX mice (Figure 1B). The number of GFP-expressing cells and relative GFP signal intensity in the ARC of OVX mice were significantly lower in transgenic mice bearing the short construct (n = 5) than in those mice containing long and medium-length constructs (n = 3) (*, P < .05, Figure 1, C and D).
More than 80% of ARC kisspeptin neurons coexpressed GFP in OVX mice bearing the long and medium-length constructs, whereas few kisspeptin neurons coexpressed GFP in OVX mice bearing the short construct (Supplemental Figure 1A). The ARC GFP signals in OVX+E2 mice with the long or medium-length construct were weaker than those in OVX mice (Figure 1B), resulting in the number of GFP-positive cells and relative GFP signal intensity were significantly lower in OVX+E2 mice bearing medium-length construct compared with those in OVX mice (†, P < .05, Figure 1, C and D). The relative GFP signal intensity was significantly lower in OVX+E2 mice bearing the long construct compared to that in OVX mice (†, P < .05, Figure 1D). The number of GFP-positive cells in OVX+E2 mice bearing the short construct (n = 5) was significantly lower than that in mice bearing the long and medium-length constructs (n = 3) (*, P < .05, Figure 1C). Kisspeptin immunoreactivities were hardly detected in the ARC of OVX+E2 mice (Figure 1B), resulting in the number of kisspeptin-immunoreactive cells were significantly lower in OVX+E2 mice compared with that in OVX mice (§, P < .05, Figure 1C).
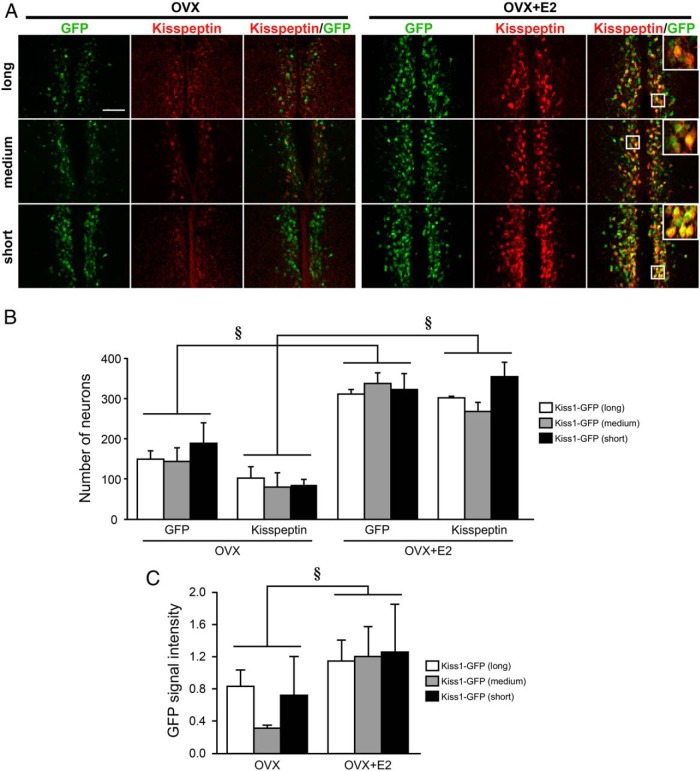
Figure 2A shows representative photomicrographs of GFP and/or kisspeptin-immunoreactive cells in the AVPV of OVX and OVX+E2 mice. All Kiss1-GFP transgenic mice bearing the long, medium-length, or short constructs exhibited GFP expression in the AVPV of OVX+E2 mice (Figure 2A). The apparent GFP expressions were localized in most kisspeptin-immunoreactive cells in the AVPV of OVX+E2 mice. No significant difference was found in the number of AVPV GFP-expressing cells among transgenic OVX+E2 mice bearing long, medium-length (n = 3), and short (n = 5) constructs (Figure 2B). More than 80% of AVPV kisspeptin neurons coexpressed GFP in both OVX and OVX+E2 mice bearing long, medium-length, or short constructs (Supplemental Figure 1B). The AVPV GFP expression and kisspeptin immunoreactivities in OVX+E2 mice with any length of construct were higher than those in OVX mice (Figure 2A), resulting in the number of GFP- and kisspeptin-immunoreactive cells, and the relative GFP signal intensity were significantly higher in OVX+E2 mice than those in OVX mice, regardless of the length of constructs (§, P < .05, Figure 2, B and C). No significant difference was found in the number of GFP- or kisspeptin-expressing cells in the AVPV of OVX mice among groups bearing constructs of different lengths (Figure 2B).
Figure 2. GFP reporter expression in the AVPV of transgenic mice carrying three different lengths of GFP reporter constructs.
A, Immunofluorescence analysis of kisspeptin and GFP expression in the AVPV. Left and center panels show GFP and kisspeptin immunoreactivities in AVPV sections of representative transgenic mice, respectively, and right panels show computer-aided merged images of immunoreactive signals for kisspeptin (red) and GFP (green) in OVX and E2-implanted OVX (OVX+E2) mice. Insets show the sections at higher magnification. Scale bar, 100 μm. B, Number of GFP-expressing and kisspeptin-immunoreactive cells in the AVPV of transgenic mice. C, The relative GFP signal intensity in the AVPV of transgenic mice. Values are means ± SEM. §, P < .05 vs OVX mice as a main effect.
Chromatin loop formation of Kiss1 locus in the ARC
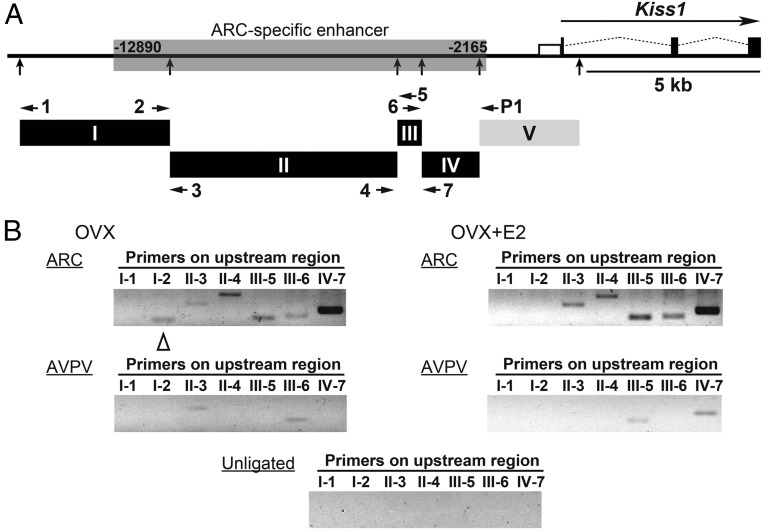
The 3C assay was performed to detect interactions between the Kiss1 promoter region and the 5′-upstream region, the latter of which was identified as an ARC-specific enhancer in the present study (Figure 3A). The 3C assay with ARC tissue showed positive PCR products with a P1 primer in fragment V containing the Kiss1 promoter region, and II-3, II-4, III-5, III-6, or IV-7 primer, respectively, in OVX and OVX+E2 mice (Figure 3B). A PCR product with a P1 primer and I-2 primer was found in the ARC of OVX mice that did not undergo estrogen treatment. The AVPV tissue showed positive PCR products with the P1 primer and II-3 or III-6 primer in OVX mice and those with the P1 primer and III-5 or IV-7 primer in OVX+E2 mice. No band was detected with unligated chromatin showing the specificity of this 3C assay (Figure 3B).
Figure 3. Chromatin conformational change at the Kiss1 locus in the ARC and AVPV.
A, Diagram of Kiss1 locus; filled boxes indicate exons, open box indicates the Kiss1 promoter region, and thin dotted lines indicate introns. HindIII restriction endonuclease sites are indicated by vertical arrows. HindIII restriction fragments (I: −15 585 to −11 263, II: −11 262 to −4713, III: −4712 to −4009, IV: −4008 to −2348, V including the promoter: −2347 to +531) used in the 3C assays are shown. Horizontal arrows within I–V show the position of primers used in the 3C assays. B, 3C assays of Kiss1 locus were performed with the ARC and AVPV tissues of OVX and E2-implanted OVX (OVX+E2) mice. PCR products were generated using the P1 primer in combination with one of the other primers (I-1 to IV-7) as indicated. Open triangle indicates ARC-specific positive band in OVX mice compared with OVX+E2 mice. Note that no band was detected with unligated chromatin showing the specificity of this 3C assay.
ERα recruitment in the putative ARC enhancer region
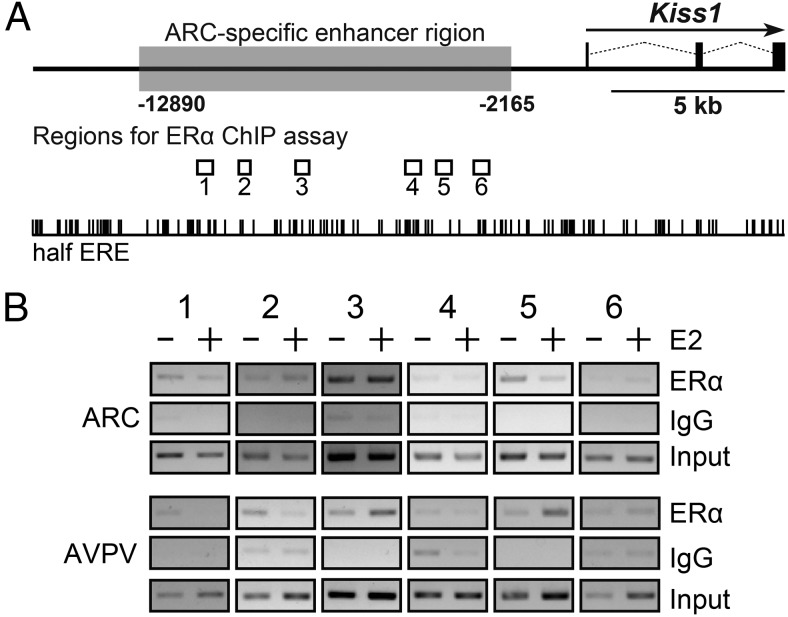
The binding of ERα in the 5′ upstream region (Figure 4) was determined by a ChIP assay with ERα antibody. The ChIP assay showed that ERα bindings were detected in regions 1, 2, 3, and 5, which were obtained from ARC tissue of OVX mice, and that in E2 treatment failed to affect ERα binding in the regions 2 and 3. On the other hand, ERα bindings were detected in regions 1, 2, 3, and 5 in the AVPV tissue of OVX mice, and E2 treatment increased ERα binding in regions 3 and 5.
Figure 4. ChIP analysis of ERα binding with the Kiss1 gene locus.
A, Localization of regions for ERα ChIP assay indicated by open boxes and half estrogen response element sites indicated by vertical lines. B, ERα ChIP assay of Kiss1 locus were performed with the ARC and AVPV tissues of OVX and E2-implanted OVX (OVX+E2) mice. PCR products were generated using primer sets indicated in Supplemental Table 2.
In silico analysis of putative transcription factor-binding sites in ARC-specific enhancer
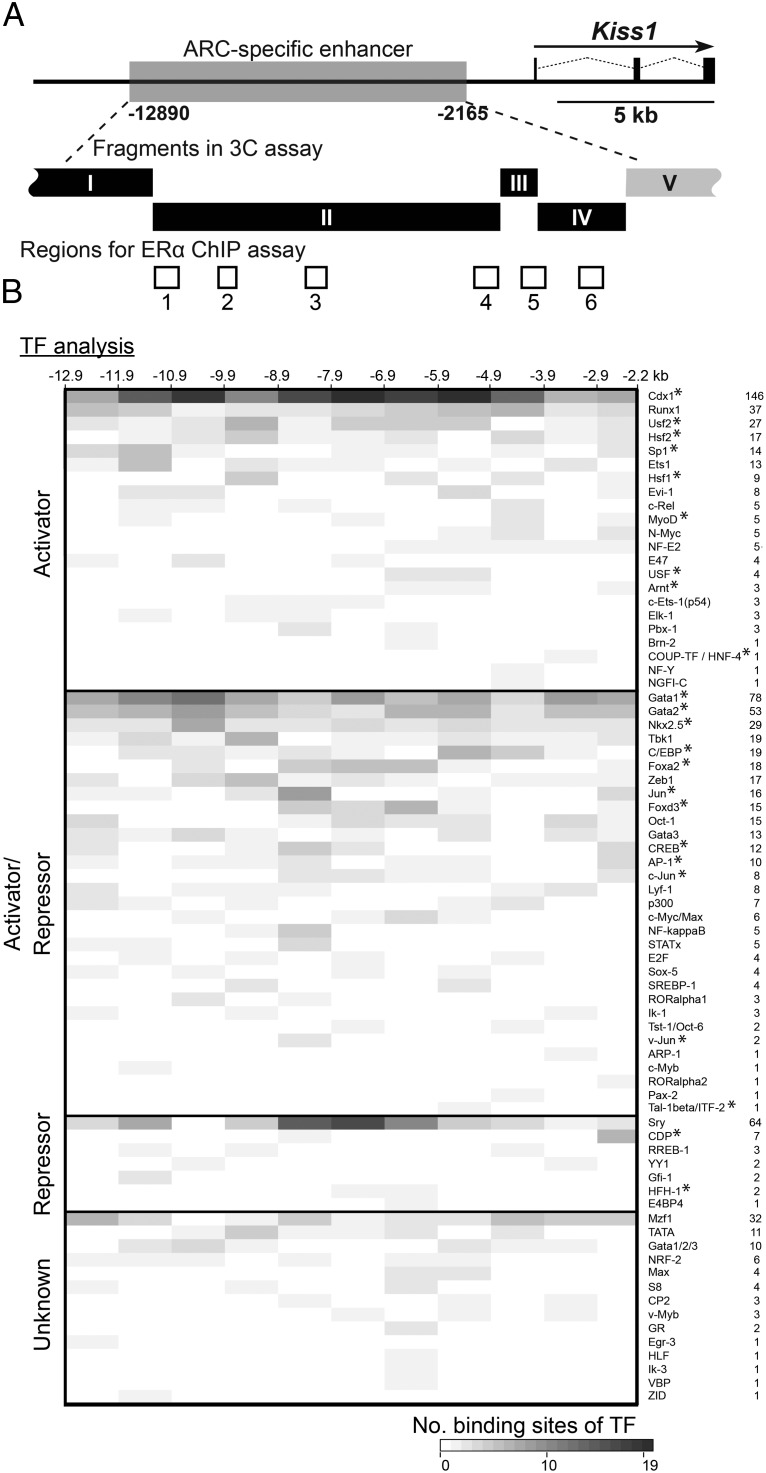
In silico search for putative binding sties of transcriptional factors was used to characterize the 5′-upstream region of the Kiss1 locus. Consensus sequences of 103 binding sites for 75 transcriptional factors were found in the 5′-upstream region of Kiss1 locus (−12 890 to −2165 bp from the Kiss1 translational start point). Some of the factors were previously reported to enhance the transcriptional activity of Kiss1 promoter (20–22). Figure 5 visualized the localization and the number of binding sites for transcriptional factors, which were classified as the activators and/or repressors by UniProtKB. Twenty-two factors were classified as transcriptional activators and 31 factors were reported to play roles as both transcriptional activators and repressors. Twenty-two of 53 activators were also reported to play a role as distal enhancer sequence-specific transcriptional factors, as indicated by asterisks in Figure 5. Seven other factors were classified as transcriptional repressors. The other 14 factors have not been annotated as transcriptional activators or repressors. Binding sites of Cdx1, Sp1, Est1, Gata1, Gata2, and Sry were especially abundant in the 5′ upstream fragment I region.
Figure 5. Distribution of binding sites of transcriptional factors in the ARC-specific enhancer of the Kiss1 locus.
A, Diagram of Kiss1 locus; filled boxes indicate exons and thin dotted lines indicate introns. Localization of HindIII restriction fragments in the 3C assay and regions for ERα ChIP assay are indicated by solid and open boxes, respectively. B, Heat map showing the number of binding sites of each transcriptional factor annotated by UniProtKB. Transcriptional factors are categorized as activator, repressor, activator/repressor, and unknown according to the Gene Ontology terms. Asterisks indicate distal enhancer sequence-specific transcriptional factors (see Materials and Methods for details). The number of consensus sequences for the binding sites of each transcriptional factor are visualized every 1 kb, except for 5′-upstream region of Kiss1 locus.
Discussion
The present study demonstrated that transgenic mice with long and medium-length GFP reporter constructs showed apparent GFP signals in most ARC kisspeptin neurons, whereas GFP signals in ARC kisspeptin neurons disappeared by 5′-truncation from medium-length GFP reporter construct. It should be noted that the transgenic mice with 5′-truncated short GFP reporter construct showed apparent GFP signals in the AVPV kisspeptin neurons. This suggests that the 5′-upstream region (from −12 890 bp to −2165 bp from the Kiss1 translational start point) of Kiss1 locus has a role as an enhancer of ARC-specific Kiss1 gene expression. It is unlikely that the 3′-downstream region (from +11061 bp to +11949 bp) of Kiss1 locus, which was included in medium-length but lacking in short constructs, plays a role as an ARC enhancer, because our previous study showed that transgenic mice bearing another 3′-truncated construct (from −22 316 bp to +5875 bp) showed GFP expression in the ARC kisspeptin neurons (16). Considering the importance of GnRH/LH pulse generation, the determination of the ARC-specific Kiss1 enhancer could provide a powerful tool for manipulating GnRH/LH pulses responsible for follicular development and steroidogenesis. The present results also suggest that the regions corresponding to the medium-length construct may be at least partly involved in estrogen negative feedback for ARC Kiss1 gene expression because GFP signal intensities as well as the number of GFP-expressing cells were significantly reduced by estrogen in mice with medium-length construct.
The current ChIP assay with an ERα antibody suggests that ERα was recruited in several sites of the putative enhancer region located in the 5′-upstream region of Kiss1 locus, regardless of E2 treatment. The 3C assay with ARC tissue indicates that the chromatin loop was formed between the 5′-upstream and Kiss1 promoter regions. These results, together with the results of the current in vivo reporter assay, suggest that recruitment of unoccupied ERα and/or the formation of a chromatin loop between the promoter and the 5′-upstream region of Kiss1 locus might be involved in Kiss1 gene expression in the ARC. The chromatin interaction related with Kiss1 gene expression could be ARC specific because AVPV tissue showed few chromatin loop formations between the promoter region and the 5′-upstream region. This notion is consistent with the apparent GFP expression in the AVPV kisspeptin neurons in transgenic mice bearing the 5′-truncated short construct in the present in vivo reporter assay. Indeed, our previous study suggested that the formation of a chromatin loop between the promoter region and 3′-downstream region of Kiss1 locus is required for Kiss1 gene expression in the AVPV (16). Taken together, the region-specific enhancers (5′-upstream region for ARC; 3′-downstream region for AVPV) of the Kiss1 locus could be responsible for the region-specific expression of Kiss1 gene in the ARC and AVPV. In other words, the 5′-upstream region of the Kiss1 locus in the ARC and the 3′-downstream region in the AVPV may function as a switch, which could turn on the Kiss1 transcription in the absence and presence of estrogen, respectively. It should be noted that the colchicine treatment may have affected Kiss1/kisspeptin and reporter gene expressions because the release of neurokinin B and/or dynorphin, which have a role in regulating GnRH pulse generator activity (3, 23), could be also affected.
The present study showed a number of binding sites for transcriptional factors in the 5′-upstream region. More specifically, binding sites of Cdx1, Sp1, Est1, Gata1, Gata2, and Sry were found in the 5′-upstream fragment I region, a putative ARC Kiss1 enhancer region. Cdx1, Gata1, and Gata2 have already been reported to act on the distal enhancer region of HoxC8, N-myc, and Fli1 genes, respectively (24–26). Our previous in vivo and in vitro study (16) revealed that histone acetylation in the Kiss1 promoter is involved in ARC Kiss1 gene expression. These results suggest that ARC Kiss1 gene up-regulation may be mediated by transcriptional factors, which participate in histone acetylation of the promoter region and chromatin loop formation between the ARC-specific enhancer and the promoter of Kiss1 gene. The complex of Elk-1 and p300, whose binding sites are detected in the 5′-upstream region of Kiss1 locus, reportedly stimulates the histone acetylation of the cIL-8 gene (27). Thus, it is tempting to speculate that those transcriptional factors play a role in up-regulating ARC KIss1 gene expression. Further studies are required to address the issue of transcriptional factors and their binding sites, which regulate ARC Kiss1 gene expression.
In conclusion, the current in vivo reporter assay using transgenic mice bearing three different lengths of GFP reporter constructs suggests that the 5′-upstream region of Kiss1 locus plays a role as an ARC-specific enhancer for Kiss1 gene expression in mice. In addition, the current 3C and ERα ChIP assays suggest that the chromatin loop formation between the ARC-specific enhancer and the promoter region of Kiss1 gene and unoccupied ERα recruitment to the putative enhancer region are involved in Kiss1 gene expression in the ARC.
Acknowledgments
We thank Dr H. Okamura (National Institute of Agrobiological Science, Japan) for kindly providing the antikisspeptin antibody.
This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to K.M.). It was also supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry and Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development Grant REP2002 (to H.T.) and Grant-in Aid 24380157 (to K.M.) and Grants-in Aid 23380163 and 26252046 (to H.T.), Grant-in Aid 23580402 (to Y.U.) from the Japan Society for the Promotion of Science, and the Cooperative Study Program of National Institute for Physiological Sciences, Japan (to M.H.) (National Institute for Physiological Science). This study was also supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science Fellows Grant 26010751 (to T.G.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to K.M.). It was also supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry and Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development Grant REP2002 (to H.T.) and Grant-in Aid 24380157 (to K.M.) and Grants-in Aid 23380163 and 26252046 (to H.T.), Grant-in Aid 23580402 (to Y.U.) from the Japan Society for the Promotion of Science, and the Cooperative Study Program of National Institute for Physiological Sciences, Japan (to M.H.) (National Institute for Physiological Science). This study was also supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science Fellows Grant 26010751 (to T.G.).
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- BAC
- bacterial artificial chromosome
- 3C
- chromatin conformation capture
- ChIP
- chromatin immunoprecipitation
- E2
- estradiol-17β
- ERα
- estrogen receptor-α
- GFP
- green fluorescent protein
- OVX
- ovariectomized.
References
- 1. Ohtaki T, Shintani Y, Honda S, et al. . Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. [DOI] [PubMed] [Google Scholar]
- 2. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wakabayashi Y, Nakada T, Murata K, et al. . Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohkura S, Takase K, Matsuyama S, et al. . Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 5. Roseweir AK, Kauffman AS, Smith JT, et al. . Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan YM, Broder-Fingert S, Paraschos S, et al. . GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab. 2011;96(11):E1771–E1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topaloglu AK, Tello JA, Kotan LD, et al. . Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. [DOI] [PubMed] [Google Scholar]
- 8. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, et al. . Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lapatto R, Pallais JC, Zhang D, et al. . Kiss1−/ − mice exhibit more variable hypogonadism than Gpr54−/ − mice. Endocrinology. 2007;148(10):4927–4936. [DOI] [PubMed] [Google Scholar]
- 10. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 11. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adachi S, Yamada S, Takatsu Y, et al. . Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. [DOI] [PubMed] [Google Scholar]
- 13. Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149(3):1004–1008. [DOI] [PubMed] [Google Scholar]
- 14. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomikawa J, Uenoyama Y, Ozawa M, et al. . Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci USA. 2012;109(20):E1294–E1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada S, Uenoyama Y, Kinoshita M, et al. . Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148(5):2226–2232. [DOI] [PubMed] [Google Scholar]
- 18. Takase K, Uenoyama Y, Inoue N, et al. . Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21(6):527–537. [DOI] [PubMed] [Google Scholar]
- 19. Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306–1311. [DOI] [PubMed] [Google Scholar]
- 20. Li D, Mitchell D, Luo J, et al. . Estrogen regulates KiSS1 gene expression through estrogen receptor α and SP protein complexes. Endocrinology. 2007;148(10):4821–4828. [DOI] [PubMed] [Google Scholar]
- 21. Mitchell DC, Abdelrahim M, Weng J, et al. . Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2α and specificity protein-1. J Biol Chem. 2006;281(1):51–58. [DOI] [PubMed] [Google Scholar]
- 22. Mueller JK, Dietzel A, Lomniczi A, et al. . Transcriptional regulation of the human KiSS1 gene. Mol Cell Endocrinol. 2011;342(1–2):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schyr RB, Shabtai Y, Shashikant CS, Fainsod A. Cdx1 is essential for the initiation of HoxC8 expression during early embryogenesis. FASEB J. 2012;26(6):2674–2684. [DOI] [PubMed] [Google Scholar]
- 25. Potvin E, Beuret L, Cadrin-Girard JF, et al. . Cooperative action of multiple cis-acting elements is required for N-myc expression in branchial arches: specific contribution of GATA3. Mol Cell Biol. 2010;30(22):5348–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pimanda JE, Ottersbach K, Knezevic K, et al. . Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA. 2007;104(45):17692–17697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li QJ, Yang SH, Maeda Y, Sladek FM, Sharrocks AD, Martins-Green M. MAP kinase phosphorylation-dependent activation of Elk-1 leads to activation of the co-activator p300. EMBO J. 2003;22(2):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]