Abstract
Estrogens, in particular 17β-estradiol, are well-known regulators of essential cellular functions; however, discrepancies remain over the mechanisms by which they act on mitochondria. Here we propose a novel mechanism for the direct regulation of mitochondrial gene expression by estrogen under metabolic stress. We show that in serum-depleted medium, estrogen stimulates a rapid relocation of estrogen receptor-α to mitochondria, in which it elicits a cellular response, resulting in an increase in mitochondrial RNA abundance. Mitochondrial RNA levels are regulated through the association of estrogen receptor-α with 17β-hydroxysteroid dehydrogenase 10, a multifunctional protein involved in steroid metabolism that is also a core subunit of the mitochondrial ribonuclease P complex responsible for the cleavage of mitochondrial polycistronic transcripts. Processing of mitochondrial transcripts affects mitochondrial gene expression by controlling the levels of mature RNAs available for translation. This work provides the first mechanism linking RNA processing and estrogen activation in mitochondrial gene expression and underscores the coordinated response between the nucleus and mitochondria in response to stress.
Estrogens, in particular 17β-estradiol (E2), are responsible for the regulation of key cellular processes such as proliferation and apoptosis, inducing changes in nuclear gene expression or by activating cell signaling pathways (1, 2). Estrogen receptors (ERs) can function as receptors and transcription factors upon E2 exposure in the nucleus and as signaling molecules in the plasma membrane (3). In the nucleus, ERs regulate the expression of E2-responsive genes through binding to estrogen-responsive elements and inducing changes in transcription (4). In the plasma membrane ERs participate in rapid signal transduction mechanisms and activate G proteins, leading to calcium and cAMP generation and the activation of proximal and distal kinases, which results in the phosphorylation of proteins involved in the modulation of cell migration, survival and proliferation (4, 5).
More recently, research has focused on the effects of estrogen on mitochondrial function because mitochondria are involved in crucial cellular processes in health and in disease. It has been reported that E2 treatment increases the abundance of mitochondrially encoded RNAs that results in elevated respiration (6, 7), indicating that E2 plays a role in the modulation of mitochondrial gene expression. It has been suggested that the E2 effects on mitochondrial function are indirectly mediated by transcription factors acting on nuclear genes that regulate mitochondrial gene expression (7). In particular, it has been suggested that E2 stimulates the nuclear respiratory factor 1, promoting the transcription of mitochondrial transcription factor A (TFAM), which subsequently increases mitochondrial DNA (mtDNA) transcription (7, 8). The localization of ERs to mitochondria in cells has stimulated interest in their specific roles within these organelles. ERs have been localized to mitochondria of several cell lines including the breast cancer-derived MCF-7 and hepatoma-derived HepG2 cells (9–11), in which they have been suggested to act as transcription factors affecting mitochondrial gene expression directly (11–13). Other studies have suggested that the interaction between ERs and putative estrogen-responsive elements in mtDNA is responsible for the induction of mitochondrial transcription by E2 (14, 15). There are different modes suggested by which estrogens exert their effects on the mitochondrial genome; however, a general agreement over the mechanism by which ERs may affect mitochondrial gene expression is lacking.
Most of the genetic information necessary for mitochondrial function is encoded by the nuclear genome, whereas mtDNA encodes 11 mRNAs, 2 rRNAs, and 22 tRNAs that are transcribed as long polycistronic precursor transcripts from both DNA strands (16). Despite their common polycistronic origin, there is wide variation in the abundance of individual mitochondrial tRNAs, mRNAs, and rRNAs in different tissues and cell types (17). This highlights the importance of posttranscriptional processing mechanisms in the regulation of mitochondrial gene expression. RNA processing is a crucial stage in the regulation of mitochondrial RNA metabolism and consequently mitochondrial function (18–20). Two nuclear DNA-encoded protein complexes account for most of the cleavage events involved in mitochondrial RNA processing, the mitochondrial ribonuclease (RNase) P complex that cleaves the 5′ end of tRNAs (18) and the mitochondrial RNase Z, encoded by the ELAC2 gene, which cleaves the 3′ ends of tRNAs (19, 20).
The mitochondrial RNase P complex is composed of three proteins: tRNA methyltransferase 10 C (TRMT10C), 17β-dehydrogenase 10 (HSD17B10), and proteinaceous RNase P (PRORP) that are all necessary for efficient cleavage of the 5′ tRNA ends. TRMT10C and HSD17B10 have additional functions, suggesting that recruitment into the RNase P complex is an additional role for these proteins (20–22). Recently it was shown that TRMT10C, also known as mitochondrial RNase P protein 1, is a methyltransferase responsible for a common methylation modification of tRNAs, and it may be responsible for the RNA recognition for RNase P cleavage (20, 21). HSD17B10 is a member of the short-chain dehydrogenase/reductase family known under a range of names including mitochondrial RNase P protein (MRPP)-2, amyloid β-peptide-binding alcohol dehydrogenase, brain L-3-hydroxyacyl-coenzyme A dehydrogenase, L-3-hydroxyacyl coenzyme A dehydrogenase H2, 2-methyl-3-hydroxybutyryl-CoA dehydrogenase, or short chain L-3-hydroxyacyl-CoA dehydrogenase type 2 (Table 1). It has been described as a multifunctional protein that has an oxidation of nicotinamide adenine dinucleotide (NAD+)-binding domain, although this is not required for RNase P activity (21). In addition, reports suggest that HSD17B10 participates in the degradation of the amino acid isoleucine via its 2-methyl-3-hydroxybutyryl-CoA dehydrogenase activity (23, 24).
Table 1.
Nomenclature and synonyms of nuclear encoded mitochondrial proteins investigated in this study
| UniProtKBa | Geneb | Synonymsc | Descriptiond | |
|---|---|---|---|---|
| ERα | P03372 | ESR1 | ER-α, ESR1 | Estrogen receptor-α |
| TRMT10C | Q7L0Y3 | TRMT10C | MRPP1, RG9MTD1 | tRNA methyltransferase, subunit of the mitochondrial RNase P |
| HSD17B10 | Q99714 | HSD17B10 | MRPP2, ABAD, ERAB, HADH2, MHBD, SCHAD | 17β-hydroxysteroid dehydrogenase 10, subunit of the mitochondrial RNase P |
| PRORP | O15091 | KIAA0391 | MRPP3 | Metalloendonuclease, catalytic subunit of the mitochondrial RNase P |
| ELAC2 | Q9BQ52 | ELAC2 | HPCP2 | Mitochondrial RNase Z |
| PTCD1 | A4D273 | PTCD1 | Pentatricopeptide repeat domain protein 1 |
Abbreviation: ABAD, amyloid β-peptide-binding alcohol dehydrogenase; ERAB, brain L-3-hydroxyacyl-coenzyme A dehydrogenase; HADH2, L-3-hydroxyacyl coenzyme A dehydrogenase H2; MHBD, 2-methyl-3-hydroxybutyryl-CoA dehydrogenase; SCHAD, short chain L-3-hydroxyacyl-CoA dehydrogenase type 2. Nomenclature and synonyms of the studied proteins are listed.
Nuclear encoded proteins used in this work are listed with their UniProtKB identifiers.
Gene symbol according to The human genome organisation (HUGO) nomenclature committee.
Common synonyms according to HUGO Nomenclature Committee.
Their function according to HUGO Nomenclature Committee.
PRORP, also known as MRPP3, belongs to the mammalian pentatricopeptide repeat (PPR) domain protein family (25). PRORP is composed of a PPR domain and a putative metallonuclease domain that is hypothesized to harbor the catalytic site of the RNase P complex. In mammalian mitochondria it was shown that elaC homology 2 (ELAC2) is the mitochondrial RNase Z responsible for processing the 3′ ends of tRNAs and that it associates with PTCD1, another PPR protein that also acts as a negative regulator of leucine tRNAs (20, 26, 27).
Interestingly, HSD17B10 catalyzes the conversion of 17β-estradiol to the less potent estrogen metabolite estrone in mitochondria (28). It has previously been reported that the ERα interacts with HSD17B10 in a bacterial two-hybrid screen (29); however, no further investigation into the relevance of this interaction has been carried out. Therefore, we investigated the biological significance of the interaction between HSD17B10 and ERα in the mitochondria of estrogen-responsive breast adenocarcinoma MCF-7 cells and how this affects mitochondrial gene expression. Here we show that upon exposure to E2, ERα rapidly localizes to mitochondria, in which it interacts with HSD17B10 to modulate the expression of mitochondrial RNA transcripts. We show that changes in the expression of proteins involved in the processing of mitochondrial transcripts can have profound effects on mitochondrial gene expression by affecting the levels of mature species, the final processing of the different RNAs, and overall protein synthesis in the presence of estrogen.
Materials and Methods
Cell culture and estrogen treatment
Estrogen-responsive MCF-7 and ER-negative MDA-468 and MDA-231 human breast adenocarcinoma cells were grown at 37°C under humidified 95% air-5% CO2 in phenol red-free DMEM (Invitrogen) supplemented with 25 mM glucose, 1 mM pyruvate, 2 mM glutamine, uridine (0.05 g/L−1), penicillin (0.1 U/L−1), streptomycin sulfate (0.1 g/L−1), and 10% fetal bovine serum. To evaluate the effects of E2, cells were cultured in serum-depleted medium containing 5% charcoal-stripped fetal bovine serum for 6 days prior to treatment with 100 nM estradiol in ethanol (Sigma-Aldrich).
Mammalian expression plasmids
All expression vectors were based on pcDNA3 (Invitrogen). Full-length human TRMT10C [National Center for Biotechnology Information (NCBI) accession number NP_060289.2], HSD17B10 (NCBI accession number NP_0044841), PRORP (NCBI accession number NP_055487.2), PTCD1 (NCBI accession number NP_056360.2), ELAC2 (NCBI accession number NP_060597.4), or ERα (NCBI accession number NP_000116.2) were expressed with their native termination codon or fused to a tandem affinity purification (TAP) tag (ABO76910) or EGFP (BD Biosciences) at the C terminus. All plasmids were tested for expression by transfection and immunoblotting.
Small interfering RNA (siRNA) and plasmid transfections
MCF-7 cells were plated at 60% confluence in six-well plates or 10-cm dishes and transfected with annealed siRNAs in OptiMEM medium (Invitrogen) according to the manufacturer's instructions. One hundred twenty-five nanomoles (for six well plates) or 145 nM (for 10 cm dishes) of TRMT10C, HSD17B10, PRORP, PTCD1, ELAC2, or nontargeting (NT) control siRNA (Thermo Scientific) were transfected using Lipofectamine 2000 (Invitrogen). Cells were incubated 72 hours after the transfection and then retransfected for an additional 72 hours to enhance knockdown efficiency, followed by a further incubation in the absence or presence of 100 nM E2 for 18 hours and then harvested.
MCF-7 cells were plated at 60% confluence in 10-cm dishes in serum-depleted medium and transfected with mammalian expression plasmids in OptiMEM medium (Invitrogen). One hundred fifty-eight nanograms per square centimeter of plasmid DNA was transfected using Fugene HD (Roche). Cells were incubated for 48 hours after the transfection to allow the integration and expression of the plasmid in serum-depleted medium containing puromycin (1 μg/mL−1) to maintain plasmid expression. This was followed by a further incubation in the absence or presence of 100 nM E2 for 18 hours before the cells were harvested.
Mitochondrial isolation
Mitochondria were prepared from 2 × 107 MCF-7 cells grown in basal, non-serum-depleted or serum-depleted medium or transfected with siRNA and treated with E2 (as described above). Cells in PBS were collected by centrifugation at 150 × g for 5 minutes at 4°C, and the pellet was suspended in 4 mL of ice-cold 10 mM NaCl, 1.5 mM MgCl2, and 10 mM Tris-HCl (pH 7.5). Cells were allowed to swell for 5 minutes on ice and briefly homogenized with a 7-mL glass homogenizer. The nuclei from this suspension were sedimented at 1300 × g for 3 minutes at 4°C, and this centrifugation step was repeated for the supernatant once more. The mitochondria from this suspension were sedimented (10 000 × g for 10 minutes at 4°C) and suspended in 120 mM KCl, 10 mM HEPES, and 1 mM EGTA. Protein concentration was determined by the bicinchoninic acid assay as described previously (30).
Mitochondrial subfractionation
Mitochondria were prepared from MCF-7 cells grown in serum-depleted medium and treated with E2 for 18 hours as described above. Protein concentration was normalized to 100 mg/mL−1 in STE (250 mM sucrose, 5 mM Tris, 1 mM EGTA, pH 7.4) containing 0.1% (wt/vol) fat-free BSA (STE/BSA) and determined by the bicinchoninic acid assay. The mitochondrial outer membrane was solubilized by adding 0.2 mg digitonin (12.5 mg/mL−1 in STE) per milligram protein to the mitochondrial preparation followed by incubation for 15 minutes at 4°C. Three volumes of STE/BSA were added to each sample, mixed, and gently homogenized using micropestles (Ambion). Mitoplasts were sedimented by centrifugation (10 000 × g for 10 minutes at 4°C), and the pellet was suspended in 20 μL STE/BSA, whereas the supernatant was retained for further fractionation. The mitochondrial outer membrane fraction was collected as a pellet after the centrifugation (144 000 × g for 20 min at 4°C) and suspended in 70 μL STE containing 1% lauryl maltoside representing the membrane, insoluble fraction. The supernatant from this centrifugation step contained the soluble fraction.
Immunoblotting
Protein expression was detected by immunoblotting using Sigma-Aldrich antibodies according to the manufacturer's recommendations: TRMT10C (catalog number HPA036671), HSD17B10 (catalog number HPA001432), PRORP (catalog number HPA020459), PTCD1 (catalog number HPA020106), ELAC2 (catalog number HPA019535), and ERα (catalog number HPA000450). Specific mitochondrial proteins were detected using MitoSciences antibodies: cytochrome c oxidase subunit 1 (COXI) (catalog number MS404), COXII (catalog number MS405), and succinate dehydrogenase complex subunit A (SDHA; catalog number MS204). Porin (Abcam; catalog number ab15895) was used as a control to confirm normalized protein loading. The primary antibodies were detected using the Odyssey Infrared IRDye 680RD goat antirabbit or IRDye 800CW goat antimouse secondary antibodies (Li-Cor Biosciences) and visualized using the Odyssey imaging system (Li-Cor Biosciences).
RNA harvesting and northern blotting
RNA (5 μg) was extracted from siRNA-transfected MCF-7, MDA-468, or MDA-231 cells, followed by E2 treatment as described above, using the miRNeasy RNA extraction kit (QIAGEN) with a deoxyribonuclease I on-column digestion step included to remove DNA, according to the manufacturer's instructions. Northern blotting was carried out as described elsewhere (31). The signal was detected using IRDye-labeled streptavidin (Li-Cor Biosciences) diluted 1:10 000 in 3× saline sodium citrate, 5% sodium dodecyl sulfate, and 25 mM Na2HPO4 (pH 7.5) and visualized using the Odyssey imaging system (Li-Cor Biosciences).
Quantitative RT-PCR
The transcript abundance of mRNAs of interest was measured on RNA isolated from MCF-7 cells after siRNA treatment. cDNA was prepared using ThermoScript reverse transcriptase (Invitrogen) and random hexamers and used as a template in the subsequent PCR that was performed using a Corbett Rotorgene 3000 using Platinum UDG SYBR Green master mix (Invitrogen) and normalized to 18S rRNA.
Tandem affinity purification
Tandem affinity purification was carried out from plasmid-transfected MCF-7 cell lysates previously treated with E2 as described previously (27, 31).
Microscopy
MCF-7 cells were plated onto 13-mm diameter glass coverslips and allowed to attach overnight. Before transfection, the cells were placed in serum-depleted medium as described above. Cells were transfected with ERα-EGFP or HSD17B10-EGFP plasmid for 48 hours, followed by incubation with 50 nM MitoTracker Orange (MT Orange, Life Technologies) in PBS for 15 minutes. In experiments examining the effects of E2, the cells were cultured in serum-depleted medium for 6 days prior to E2 treatment. After washing with PBS, the cells were fixed with 4% paraformaldehyde in PBS for 30 minutes and washed again with PBS. Cells were mounted in 1,4-diazabicyclo[2.2.2]octane/polyvinyl alcohol medium, and images were acquired using a Nikon Ellipse Ti fluorescent inverted microscope using a Nikon ×60 or ×100 objective.
Mitochondrial protein synthesis
MCF-7 cells were grown in six-well plates, transfected, and incubated in the presence or absence of E2, and de novo protein synthesis was analyzed as described elsewhere (32).
Mitochondrial respiration
Mitochondrial respiration using 0.5 mM N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride and 2 mM ascorbate was measured in permeabilized cells as described in (20).
Native PAGE
Mitochondria were prepared from MCF-7 cells grown in serum-depleted medium and incubated in the presence or absence of 100 nM E2 for 18 hours. Mitochondrial proteins were solubilized in sample buffer containing 1% dodecyl maltoside for 15 minutes followed by centrifugation (20 000 × g, 30 min at 4°C) and the supernatant resolved by one-dimensional native PAGE in the NativePAGE Novex Bis-Tris gel system (Life Technologies). Electrophoresis was carried out in an XCell SureLock minicell (Life Technologies) and followed by transfer to polyvinyl difluoride membrane (Immobilon FL; Millipore) using a Mini-Protean Trans blot system (Bio-Rad Laboratories). Transfer was followed by incubation in Odyssey blocking buffer (Li-Cor Biosciences) for 1 hour, followed by overnight incubation with primary antibodies at 4°C. The primary antibodies were detected using the Odyssey Infrared IRDye 680RD goat antirabbit or IRDye 800CW goat antimouse secondary antibodies and visualized using the Odyssey imaging system (Li-Cor Biosciences).
Immunoblot densitometry and statistical analysis
Band intensity from the electronic (tiff) images of the immunoblots was calculated by densitometry using Image J freeware (rsb.info.nih.gov/ij). The data are presented as the mean and SD. The statistical significance was examined using a two-tailed Student's t test.
Results
HSD17B10 and ERα expression in MCF-7 cells
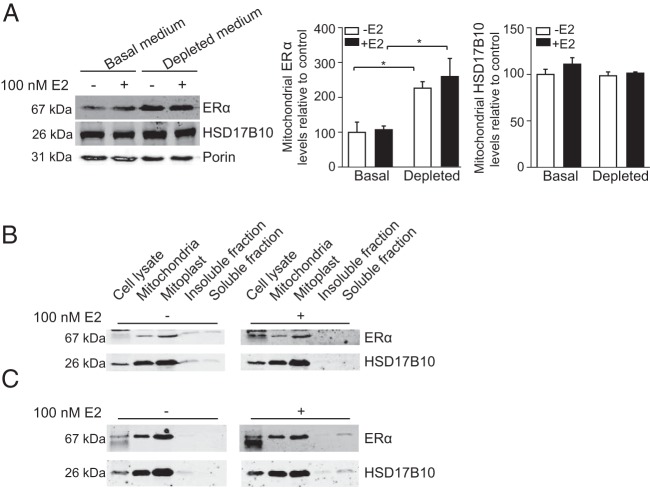
Previous studies have indicated that the E2 effects on mitochondria are mediated by ERα (33) because ERα has been found associated with mitochondria in cells (9–11). To determine whether there is an E2-dependent effect in the abundance of ERα, the endogenous distribution of ERα was assessed by immunoblotting experiments carried out on mitochondria isolated from MCF-7 cells grown in basal, non-serum-depleted medium, compared with mitochondria from cells grown in serum-depleted medium, followed by E2 treatment for 18 hours (Figure 1A). We found ERα associated with mitochondria of MCF-7 cells independent of E2 treatment; however, ERα is significantly enriched in mitochondria from cells grown in serum-depleted medium. This suggests that the serum depletion may enhance the localization of ERα to mitochondria.
Figure 1. HSD17B10 and ERα expression in MCF-7 cells A, ERα was detected by immunoblotting of mitochondrial lysates from MCF-7 cells grown in basal, non-serum-depleted medium and cells grown in serum-depleted medium, followed by incubation with 100 nM E2 for 18 hours.
Porin was used as a control to confirm normalized protein loading. Bars represent mean ± SD of relative mitochondrial ERα levels or HSD17B10 in serum-depleted cells, normalized to porin, and compared with cells grown in basal, nondepleted medium in the presence or absence of E2. Statistical significance was examined using a two-tailed Student's t test. *, P < .05 (n = 3). ERα and HSD17B10 cellular distribution was assessed by immunoblotting from cell lysates and mitochondrial fractions isolated from MCF-7 cells grown in basal medium (B) or serum-depleted medium (C), followed by incubation with 100 nM E2 for 18 hours.
Mitochondria contain the HSD17B10 enzyme that is involved in E2 degradation, and this protein has been suggested to interact with ERα in a bacterial two-hybrid system (29). The levels of this protein are not affected by growth in basal or serum-depleted medium (Figure 1A). However, we wanted to gain further insight into the distribution of HSD17B10 and ERα in mitochondria and in response to E2. We carried out cell and mitochondrial subfractionation experiments to analyze the distribution of ERα and HSD17B10 in mitochondria from MCF-7 cells grown in basal (Figure 1B) and serum-depleted medium (Figure 1C), followed by incubation in the presence or absence of 100 nM E2 for 18 hours. Enriched fractions were obtained containing whole-cell lysates, intact mitochondria, mitoplasts (mitochondria devoid of outer membrane), mitochondrial insoluble fraction (membranes), and mitochondrial soluble fraction. Immunoblotting for ERα and HSD17B10 indicates that both proteins are enriched in mitoplasts under normal and metabolic stress conditions; however, ERα has a significantly stronger association with mitochondria after serum starvation.
HSD17B10 interacts with ERα in MCF-7 cells
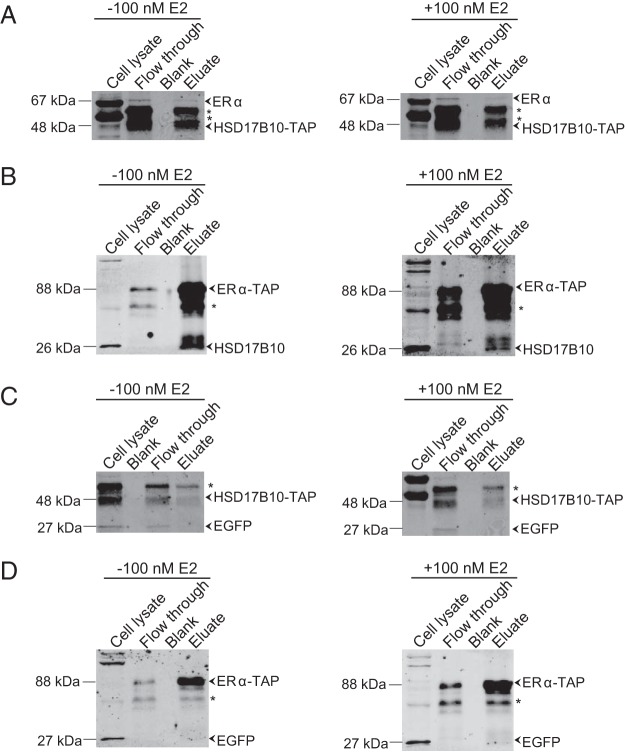
Next, we investigated possible association of ERα with HSD17B10 within the mitochondria of MCF-7 cells. The C terminus of HSD17B10 was fused to a TAP tag to isolate it and determine whether it associates with ERα by immunoblotting (Figure 2). We found that endogenous ERα associated with HSD17B10-TAP, in the presence or absence of E2 (Figure 2A), although in the presence of E2 this association was weaker as suggested previously (29). These interactions were further assessed by reverse TAP, in which the C terminus of ERα was fused to a TAP tag to determine whether it interacts with HSD17B10 (Figure 2B). Endogenous HSD17B10 was detected in association with ERα-TAP, and this association was weaker in the presence of E2. These results indicate that ERα associates with HSD17B10 and that this association can be affected by E2, validating a previous report of this interaction identified in a bacterial two-hybrid screen, hereby establishing the relevance of this interaction in mammalian cells. Enhanced green fluorescent protein (EGFP) was used as a control to show specific antibody detection and that neither HSD17B10 (Figure 2C) nor ERα (Figure 2D) associates with EGFP, indicating the specificity of the interactions between ERα and HSD17B10.
Figure 2. HSD17B10 interacts with ERα.
A, Endogenous ERα was isolated from MCF-7 cells grown in serum-depleted medium, expressing HSD17B10-TAP (48 kDa) in the presence or absence of 100 nM E2 for 18 hours, using IgG agarose beads, followed by TEV protease cleavage. B, TAP analyses show endogenous HSD17B10 (27 kDa) isolated from cells expressing ERα-TAP (88 kDa). IgG heavy (50 kDa) and light chains (23 kDa) can be seen on the immunoblots and are marked with asterisks. C and D, EGFP was used as a control to show the specificity of the TAP procedure.
HSD17B10 and ERα colocalize in mitochondria of MCF-7 cells
The localization of HSD17B10 in mitochondria has been found in rat cardiac myocytes (29), brain vascular smooth muscle cells (34), and monkey kidney fibroblast-like COS-7 cells (35), whereas ERα has been reported to be present in the nucleus and mitochondria of MCF-7 cells (10). Confocal fluorescence microscopy analyses were carried out to determine the specific localization of HSD17B10 and ERα in MCF-7 cells grown in serum-depleted medium, in which we found ERα enriched in our immunoblotting experiments (Figure 1). We transfected plasmids expressing either ERα or HSD17B10 fused to EGFP at the C terminus and incubated the cells with 100 nM E2 for 5 minutes, 3 hours, or 18 hours to investigate possible changes in their intracellular localization relative to control, untreated MCF-7 cells. We used MitoTracker Orange (MT Orange) to specifically stain mitochondria within the cells.
In the absence of E2, ERα is found predominantly in the nuclei of MCF-7 cells, although we also observed it associated with mitochondria (Figure 3A). Next, we observed the distribution of ERα after the addition of 100 nM E2 after 5 minutes and found that ERα is rapidly enriched within mitochondria (Figure 3A, 5 min of E2 treatment). ERα is found localized in the nucleus after prolonged, 3 and 18 hours of treatments with E2 (Figure 3A, 3 and 18 h of E2 treatment). This suggests that E2 has a rapid effect on mitochondrial function via the recruitment of ERα to mitochondria. Next, we investigated the localization of HSD17B10 in MCF-7 cells to determine whether its distribution follows a similar pattern to ERα because these two proteins associate with each other (Figure 2). HSD17B10 was found in the cytoplasm and in specific puncta within the mitochondria of MCF-7 cells; however, its distribution is not changed as a result of E2 treatment (Figure 3B), confirming our immunoblot experiment (Figure 1A). Together with our immunoblotting and TAP experiments, these results show that ERα is rapidly recruited to mitochondria in which it associates with HSD17B10, and this association is affected by E2.
Figure 3. HSD17B10 and ERα colocalize in the mitochondria of MCF-7 cells.
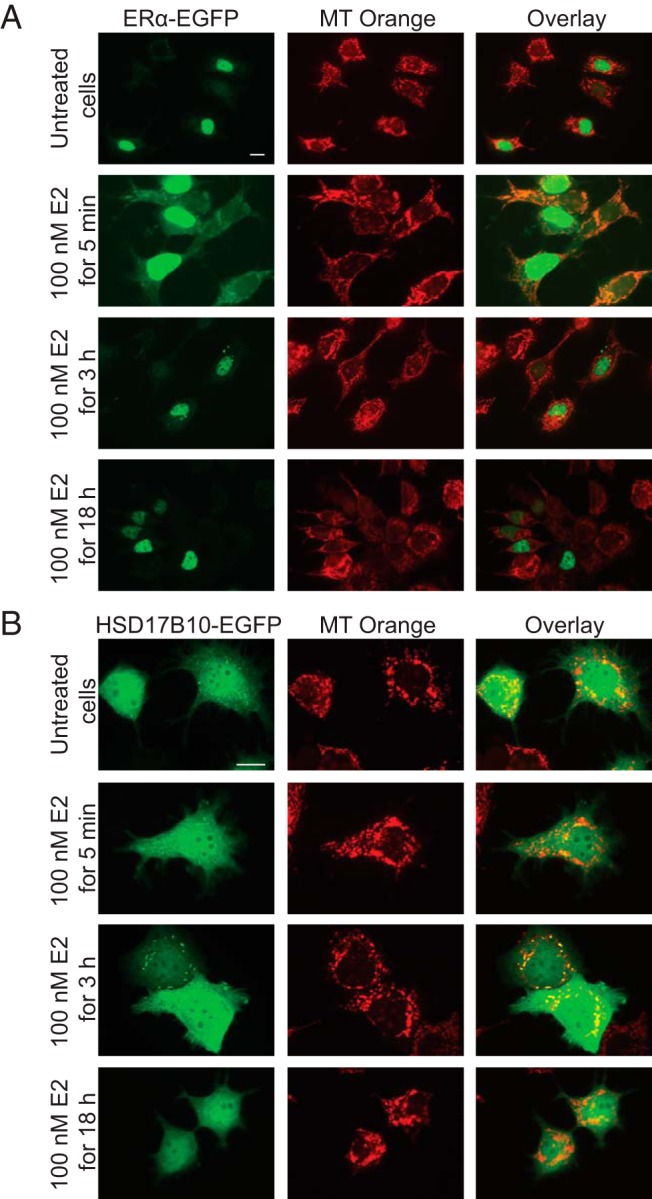
A, ERα is predominantly nuclear but is rapidly recruited to mitochondria of MCF-7 cells upon E2 treatment. Cells grown in serum-depleted medium were transfected with a plasmid expressing ERα fused to EGFP at the C terminus, followed by incubation with 100 nM E2 for 5 minutes, 3 hours, or 18 hours. Cells were then incubated with MitoTracker Orange (MT Orange) to stain mitochondria in live cells and fixed. ERα-EGFP (green) can be seen in the nucleus and also in mitochondria stained with MT Orange (red) in the overlaid images (yellow). Scale bar is 10 μm. B, HSD17B10 is present in the cytoplasm and in puncta within mitochondria of MCF-7 cells. Cells grown in serum-depleted medium were transfected with a plasmid expressing HSD17B10 fused to EGFP at the C terminus, followed by incubation with 100 nM E2 for 5 minutes, 3 hours, or 18 hours. Cells were then incubated with MitoTracker Orange (MT Orange) to stain mitochondria in live cells and fixed. HSD17B10-EGFP (green) can be seen in the cytoplasm and also in mitochondria stained with MT Orange (red) in the overlaid images (yellow). Scale bar is 10 μm.
In addition to its role in estrogen degradation, HSD17B10 is an essential subunit of the mitochondrial RNase P complex that cleaves the 5′ ends of tRNAs. Because ERα translocates to mitochondria in response to E2, in which it interacts with HSD17B10, we investigated whether E2 treatment led to a differential recruitment of HSD17B10 that could affect the formation or stability of the mitochondrial RNase P complex. To maintain mitochondrial proteins in their native complexes, we resolved mitochondrial lysates from MCF-7 cells by native electrophoresis, followed by immunoblotting to detect the three protein subunits of the RNase P complex, TRMT10C, HSD17B10, or PRORP (Supplemental Figure 1). We found that E2 treatment did not affect the stability of the multimeric conformations of the components of the RNase P complex.
Estrogen-dependent regulation of mitochondrial transcripts by RNA processing proteins
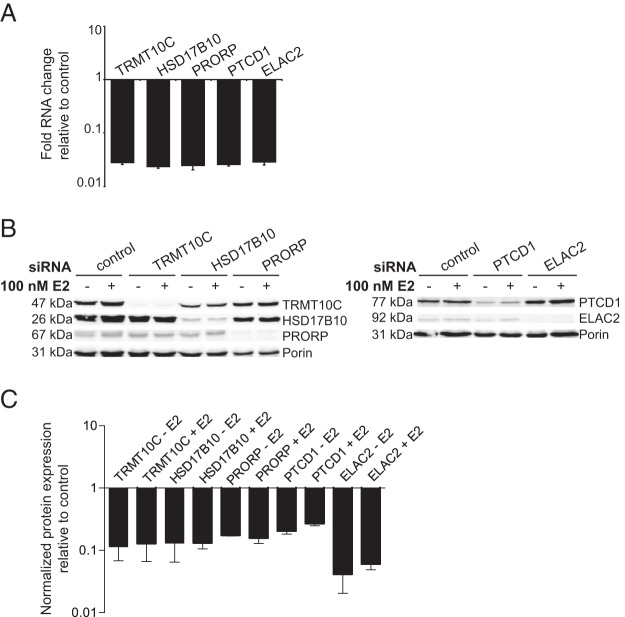
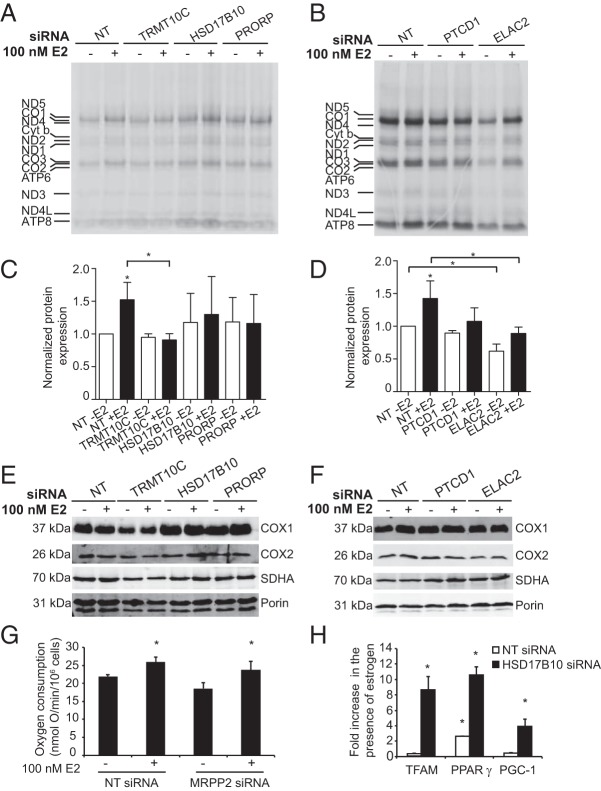
Because HSD17B10 is part of the RNase P complex and RNA processing enzymes regulate mitochondrial gene expression (20, 36), we sought to identify the role of these proteins upon E2 treatment. To examine the role of the RNA processing proteins TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 on RNA metabolism in MCF-7 cells, we assessed the effects of the siRNA-mediated knockdown of these proteins in the presence of E2. We carried out quantitative RT-PCR (qRT-PCR) to measure the efficiency of knockdown and found that the siRNAs significantly decreased the mRNA levels of each RNA processing protein (Figure 4A). In addition, treatment of MCF-7 cells with siRNAs against each of the mitochondrial RNA processing enzymes leads to a significant decrease in their protein abundance compared with cells treated with control siRNAs in the presence and absence of E2 (Figure 4, B and C). Interestingly, the knockdown of HSD17B10 also leads to a decrease in TRMT10C, suggesting that the stability of TRMT10C depends on the presence of HSD17B10. These two proteins have been shown to form a stable subcomplex of the mitochondrial RNase P enzyme previously (18, 21). In addition, the knockdown of HSD17B10 also leads to a subtle decrease of PRORP compared with control, indicating the association of these proteins as part of the RNase P complex. Furthermore, we observed that the knockdown of ELAC2 leads to an increase in PTCD1 abundance in MCF-7 cells (Figure 4B). We have shown that PTCD1 interacts with ELAC2 and affects the 3′ end processing of mitochondrial tRNAs as a negative regulator (20, 27), and this increase may represent a compensatory response.
Figure 4. Knockdown of mitochondrial RNA-processing proteins.
A, The mRNA knockdown levels of the mitochondrial RNA processing proteins in MCF-7 cells were determined by qRT-PCR relative to control NT siRNA-treated cells and normalized to 18S rRNA. Bars represent mean ± SD. A Student's two-tailed t test for paired data was carried out and showed that mRNAs were significantly decreased relative to controls (P < .05; n = 3). B, Immunoblotting of mitochondria isolated from MCF-7 cells transfected with control NT, TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 siRNAs for 72 hours in serum-depleted medium, followed by an additional retransfection for 72 hours to enhance knockdown efficiency and in the presence or absence of 100 nM E2 for 18 hours. Antibodies were used to detect the expression levels of TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 upon siRNA-mediated knockdown. Porin was used as a control to confirm equal protein loading. Blots are representative of four independent biological experiments. C, Densitometry analysis of immunoblotting experiments show that siRNA treatment significantly decreases protein abundance compared with control cells in the presence and absence of E2. The graph shows the relative levels of each siRNA-targeted protein, normalized to porin and compared with NT control. Bars represent relative mean ± SD. A Student's two-tailed t test for paired data was carried out and showed that protein levels were significantly decreased relative to controls (P < .05).
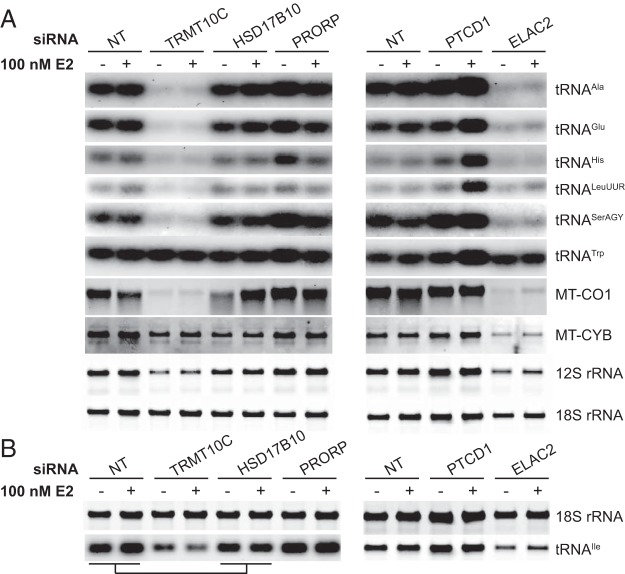
Next, we investigated the effects of knocking down TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2, relative to controls, on the steady-state levels of mitochondrial RNA transcripts by northern blotting in the presence or absence of E2 (Figure 5, A and B). We observed an increase in many mitochondrial RNAs in response to E2 treatment in the control (NT) siRNA-treated cells (Supplemental Figure 2). In contrast, mitochondrially encoded cytochrome c oxidase subunit 1 mRNA abundance was decreased upon E2 treatment, and the levels of mitochondrially encoded cytochrome b (MT-CYB) and the 12S rRNA were not significantly affected (Supplemental Figure 2). Knockdown of TRMT10C or ELAC2 led to a dramatic reduction of most RNA transcripts (Figure 5B and Supplemental Figure 2), and E2 treatment only subtly increased their abundance as in the control samples, providing further evidence that these two enzymes are required for RNA processing in mitochondria. The significant reduction of mature mitochondrial transcripts upon TRMT10C or ELAC2 knockdown is consistent with that observed in HeLa cells (20). In contrast, tRNATrp steady-state levels in MCF-7 cells were not reduced by the knockdown of any of the mitochondrial RNA processing enzymes (Figure 5B and Supplemental Figure 2).
Figure 5. E2-dependent effects of RNA-processing proteins on mitochondrial RNA levels.
A, RNA was isolated from MCF-7 cells transfected with control NT, TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 siRNA in the presence or absence of 100 nM E2 for 18 hours to determine the steady-state levels of mitochondrial transcripts by Northern blotting. Nuclear 18S rRNA abundance was used as an internal control for total RNA loading. Data shown are typical results repeated on three independent RNA preparations. Each blot was probed for the different RNA species by stripping and reprobing. B, Northern blot showing the effects of RNA processing protein knockdown on the steady-state levels of tRNAIle in the presence and absence of 100 nM E2.
Knockdown of HSD17B10 did not result in significant changes in the levels of mitochondrial RNAs (Figure 5A); however, upon addition of E2, we observed a significant increase in the abundance of a particular subset of mitochondrial RNAs (Figure 5B and Supplemental Figure 2). In the absence of E2 treatment, PRORP silencing resulted in the up-regulation of some mitochondrial tRNAs and mRNAs, including tRNAGlu, tRNAHis, and tRNASerAGY, compared with NT siRNA control (Figure 5A). Knockdown of PTCD1 resulted in an overall increase in the steady-state levels of RNA transcripts compared with NT siRNA control upon estrogen treatment (Figure 5A). These findings indicate that the levels of many mitochondrial RNA transcripts are affected by both the hormonal status of the cellular environment and the levels of the RNA processing proteins.
Mutations in the gene encoding HSD17B10 cause 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency, an X-linked neurodegenerative inborn error of isoleucine metabolism, which mimics the symptoms of mitochondrial disease (23, 37). This indicates that HSD17B10 has an additional role to mitochondrial RNA processing, in the regulation of isoleucine metabolism. Therefore, we investigated the steady-state levels of mitochondrial tRNAIle in MCF-7 cells compared with cells treated with TRMT10C, HSD17B10, PRORP, or NT siRNAs in the absence or presence of E2 (Figure 5B). TRMT10C knockdown led to dramatically decreased tRNAIle levels, similar to the changes observed in other RNAs upon knockdown of this protein (Figure 5B). Interestingly, we observed E2-dependent changes in tRNAIle abundance upon knockdown of HSD17B10 that were not observed for any other tRNA investigated in this study. In the absence of E2 treatment, we observed increased levels of tRNAIle upon HSD17B10 knockdown compared with NT siRNA control and PRORP knockdown, whereas E2 treatment led to a significant decrease in the steady-state levels of tRNAIle compared with control and PRORP knockdown (Figure 5B). This pattern was not observed for other mitochondrial RNA transcripts investigated, which indicates that HSD17B10 may play a specific role in the regulation of the tRNAIle transcript in an estrogen-dependent manner.
Next, we investigated the effects of E2 on the processing of mitochondrial RNAs by qRT-PCR (Figure 6). Knockdown of the RNA processing proteins causes an accumulation of preprocessed mitochondrial transcripts (Figure 6, A and B) relative to controls; however, E2 can decrease the abundance of specific preprocessed transcripts by affecting the RNase P complex (Figure 6A) but not the 3′ tRNA processing proteins ELAC2 and PTCD1 (Figure 6B), suggesting that E2 stimulation can partially rescue processing defects, possibly by recruiting HSD17B10 into the RNAse P complex. Taken together, these results show that ERα is rapidly recruited to mitochondria upon E2 activation in which it associates with HSD17B10 and stimulates mitochondrial RNA processing.
Figure 6. E2-dependent effects on mitochondrial RNA processing.
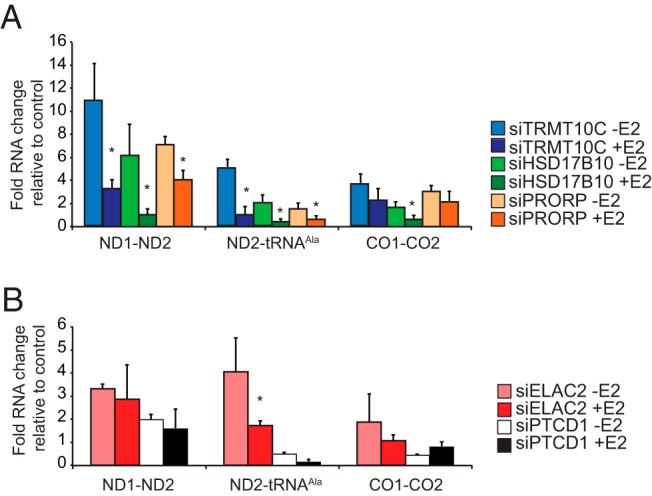
RNA was isolated from MCF-7 cells transfected with control NT, TRMT10C, HSD17B10, PRORP (A) PTCD1, or ELAC2 (B) siRNA in the presence or absence of 100 nM E2 for 18 hours to determine the effects on mitochondrial RNA processing by qRT-PCR relative to control NT siRNA-treated cells and normalized to 18S rRNA. Data shown are typical results repeated on three independent RNA preparations. Data are means ± SD of three separate experiments. P < .05 is marked with an asterisk.
Because we observed E2-stimulated changes in mitochondrial transcripts when PTCD1 was knocked down, we investigated whether this protein associates with ERα by TAP pulldown (Figure 7). We found that endogenous ERα associated with PTCD1-TAP (Figure 7A); however, endogenous PTCD1 could not be detected in association with ERα-TAP (Figure 7B), suggesting that the TAP tag may obscure the site through which it associates with PTCD1. Next, we examined whether PTCD1 and HSD17B10 associated with each other, but we could not detect an association between them by TAP pulldown, independent of E2 treatment (Supplemental Figure 3A). This finding indicates that RNase P and ELAC2/PTCD1 complexes are distinct within mitochondria. Additional TAP pulldown experiments confirmed the association between TRMT10C and HSD17B10 and between HSD17B10 and PRORP (Supplemental Figure 3B) and between PTCD1 and ELAC2 (Supplemental Figure 3C), independent of E2 treatment. These results confirm the coexistence of TRMT10C, HSD17B10, and PRORP in the RNase P complex in cells and confirm the association between PTCD1 and ELAC2, both originally observed in HeLa cells (18, 20).
Figure 7. PTCD1 associates with ERα.
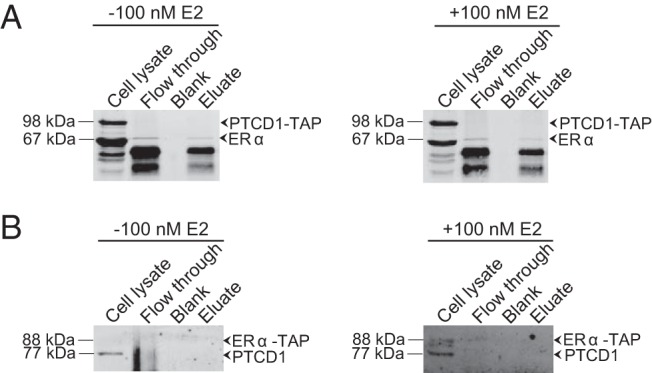
A, Endogenous ERα (67 kDa) was isolated from MCF-7 cells grown in serum-depleted medium, expressing PTCD1-TAP (98 kDa) in the presence or absence of 100 nM E2 for 18 hours, using IgG agarose beads, followed by TEV protease cleavage. B, Reverse TAP analyses were carried out to detect endogenous PTCD1 (77 kDa) isolated from cells expressing ERα-TAP (88 kDa) using a PTCD1-specific monoclonal antibody.
Impaired processing of mitochondrial transcripts leads to decreased protein expression
Changes in mitochondrial tRNA levels as a result of defects in RNA processing can affect the translation of mitochondrially encoded mRNAs (20, 38). To investigate the effects of TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 knockdown on mitochondrial protein synthesis in the presence and absence of E2, we carried out a de novo incorporation assay using 35S methionine and cysteine pulse labeling (Figure 8, A and B). Coomassie staining of pulse-labeling gels showing normalized protein loading is shown in Supplemental Figure 4. As observed for mitochondrial RNA levels (Supplemental Figure 2), we also found a significant increase in mitochondrial protein synthesis in response to E2 treatment in the control cells (Figure 8, C and D). In contrast, we observed decreased mitochondrial protein synthesis when TRMT10C and ELAC2 were knocked down (Figure 8, C and D), consistent with the most significant decrease in mitochondrial RNAs observed by northern blotting (Figure 5A). Knockdown of HSD17B10 or PRORP did not have a significant effect on mitochondrial protein synthesis (Figure 8C). We did not observe changes in mitochondrial protein synthesis upon knockdown of PTCD1, independent of E2 treatment (Figure 8D). PTCD1 knockdown increases the abundance of mature tRNAs that are further stimulated upon E2 treatment (Figure 5E); however, this increase does not affect the rate of protein synthesis, suggesting that these tRNAs are not normally present at rate-limiting amounts for mitochondrial protein synthesis.
Figure 8. Impaired processing of mitochondrial transcripts leads to decreased protein expression.
Mitochondrial protein synthesis was analyzed by the incorporation of 35S-labeled methionine and cysteine in the 13 proteins synthesized by the mitochondrial translation machinery. MCF-7 cells were grown in serum-depleted medium and transfected with siRNAs targeting TRMT10C, HSD17B10, PRORP, or NT control (A) and siRNAs targeting PTCD1, ELAC2, or NT control (B) in serum-depleted medium, followed by treatment with 100 nM E2 for 18 hours. The protein synthesis of cytoplasmic proteins was inhibited with emetine. Coomassie-stained gels showing equal loading of the cell lysates are shown in Supplemental Figure 4A. C, Knockdown of TRMT10C, in the presence of estrogen, decreases mtDNA-encoded protein synthesis. Graph shows relative protein levels normalized to porin. Bars represent relative mean ± SD. Statistical significance was examined using a two-tailed Student's t test, and indicated by an asterisk. *, P < .05 (n = 3). D, Knockdown of ELAC2 decreases mtDNA-encoded protein synthesis. Graph shows relative protein levels normalized to porin. Bars represent relative mean ± SD. Statistical significance was examined using a two-tailed Student's t test, and indicated by an asterisk. *, P < .05 (n = 3). Immunoblotting analyses of mitochondrial proteins isolated from MCF-7 cells grown in serum-depleted medium and transfected with siRNAs targeting TRMT10C, HSD17B10, PRORP, or NT control (E) and siRNAs targeting PTCD1, ELAC2, or NT control (F), followed by treatment with 100 nM E2 for 18 hours. Antibodies were used to detect the expression levels of mtDNA-encoded cytochrome c oxidase subunits COX1 and COX2 and nDNA-encoded complex II SDHA subunit. Porin was used as a control to confirm normalized protein loading. G, Mitochondrial oxygen consumption was measured in MCF-7 cells grown in serum-depleted medium in the presence or absence of 100 nM E2 for 18 hours. Data are means ± SD of three separate experiments (P < .05, n = 3). H, The mRNA levels of TFAM, PGC-1, and PPARγ were measured in MCF-7 cells treated with HSD17B10 or NT control siRNAs in the presence or absence of 100 nM E2 by qRT-PCR. The fold change in expression upon addition of E2 compared with the absence of E2 is shown. Data were normalized to 18S rRNA and bars represent mean ± SD. A Student's two-tailed t test for paired data was carried out and showed that mRNAs were significantly changed relative to controls (P < .05, n = 3).
Next, we investigated the steady-state levels of mitochondrial proteins by immunoblotting from MCF-7 cells grown in serum-depleted medium and transfected with TRMT10C, HSD17B10, PRORP, PTCD1, or ELAC2 siRNAs and control NT siRNAs in the presence and absence of E2. We observed a decrease in the levels of mtDNA-encoded COX1 and COX2 cytochrome c oxidase polypeptides upon TRMT10C and ELAC2 knockdown relative to controls, independent of E2 treatment (Figure 8, E and F), consistent with our northern blots (Figure 5A) and translation measurements (Figure 8, A and B). A decrease was also observed in the expression of nuclear-encoded complex II subunit SDHA upon TRMT10C knockdown, independent of E2 and likely as a response to feedback from the decrease in mitochondrially encoded polypeptides of the electron transport chain. Knockdown of HSD17B10, PRORP, or PTCD1 did not markedly affect the steady state levels of mitochondrial proteins (Supplemental Figure 5). Together the pulse labeling and immunoblotting experiments show a significant decrease of mitochondrial protein expression upon TRMT10C or ELAC2 knockdown. These decreases reflect their crucial role in the processing of mitochondrial transcripts as core subunits of the RNase P and RNase Z complexes and their requirement for mitochondrial gene expression. The dramatic effects of TRMT10C knockdown may be due to the dual role of this protein as a methyltransferase responsible for a tRNA modification and as a component of the RNase P complex (18, 20, 21). The E2-dependent nature of the changes observed upon HSD17B10 or PRORP knockdown may reflect additional roles of these proteins in mitochondrial function, including HSD17B10's enzymatic activity in estrogen metabolism and as a subunit of the RNase P processing complex.
We investigated the effects of E2 on mitochondrial oxygen consumption in MCF7 cells treated with NT siRNAs and upon knockdown of HSD17B10. Mitochondrial respiration is increased upon E2 treatment, which correlates well with increased mitochondrial gene expression (Figure 8G). Knockdown of HSD17B10 led to decreased oxygen consumption, indicating the requirement of this protein for mitochondrial function; however, the addition of E2 in cells in which HSD17B10 was knocked down improved mitochondrial respiration (Figure 8G). We carried out qRT-PCR to show that the increase in mitochondrial gene expression in the control MCF7 cells was not due to increased biogenesis driven by nuclear encoded master regulators such as TFAM, peroxisomal proliferator-activated receptor-γ coactivator 1 (PGC-1), and peroxisomal proliferator-activated receptor (PPAR)γ because their expression is not increased upon E2 treatment (Figure 8H). Instead, we observe up-regulated master regulators in E2-treated cells only when HSD17B10 is knocked down. Taken together, these data suggest a possible mechanism by which HSD17B10 can be recruited into the RNase P complex upon E2 stimulation under stress to increase mitochondrial gene expression that consequently improves energy production. However, if HSD17B10 is decreased or lost, mitochondrial gene expression can be up-regulated by increasing the levels of transcriptional activators of mitochondrial biogenesis in the nucleus.
Discussion
Traditionally, estrogens regulate mitochondrial function by modulating the transcription of nuclear genes involved in mitochondrial gene expression or by acting on receptor-dependent signaling cascades that might affect mitochondrial function. This work explored a new avenue for the regulation of mitochondrial gene expression upon estrogen activation. The results presented here reveal a role for direct action of E2 on mitochondrial RNA metabolism via HSD17B10 in association with ERα. A direct action would allow the hormone to induce rapid changes in mitochondrial gene expression, suggesting that E2 might directly influence energy production by the organelle by regulating the availability of subunits of the respiratory chain. This would be of particular relevance under metabolic stress conditions, during which changes in the energy demand might require a quick, adaptive response by the cell.
The function of HSD17B10 as a hydroxysteroid dehydrogenase involved in the regulation of estrogen levels is well established (39, 40). The association of HSD17B10 with ERα and their colocalization in mitochondria of MCF-7 cells in the presence of estrogen suggests that this hormone affects mitochondrial gene expression via RNA processing. The estrogen response was rapid and stimulated the distribution of ERα to mitochondria, suggesting that the cellular stress response in MCF-7 cells is mediated by ERα through its association with HSD17B10. The interaction of HSD17B10 with ERα was initially found in a bacterial two-hybrid screen (29). In that study, a putative mechanism for the interaction between ERα and HSD17B10 was proposed, in which, under low E2 conditions, ERα's binding to HSD17B10 inhibits its catalytic activity in estradiol degradation. In contrast, in the presence of high concentrations of estradiol, HSD17B10 is proposed to dissociate from the HSD17B10-ERα complex and metabolize estradiol to estrone. Although HSD17B10's binding to ERα could indeed lead to its inactivation in its role in estradiol degradation, this model does not take into account HSD17B10's fundamental role in mitochondrial RNA processing, as a core subunit of the RNase P processing enzyme. It is reasonable to hypothesize that the recruitment of HSD17B10 by the RNase P complex or its association with ERα upon E2 activation may underlie the changes observed in this work on mitochondrial RNA transcript levels. In an E2-responsive cellular context, such as that of MCF-7 cells, HSD17B10's role in estradiol degradation or as a component of the RNase P might result in changes in RNase P activity that in turn lead to variations in mitochondrial transcript abundance.
We have investigated the roles of TRMT10C, HSD17B10, PRORP, PTCD1, and ELAC2 in the regulation of mitochondrial gene expression in MCF-7 cells and showed that mitochondrial RNA processing can be affected by E2 treatment in cells expressing ERα. Some of the changes observed upon knockdown of these proteins on steady-state RNA levels indicate that there are discrete, cell type-specific variations in their action on mitochondrial RNA processing. For instance, in contrast with previous studies in HeLa cells, tRNATrp was not affected by the knockdown of TRMT10C or ELAC2 in MCF-7 cells (20), and it remains to be determined how the levels of tRNATrp are controlled in MCF-7 cells. Knockdown of TRMT10C decreased the levels of most mitochondrial RNAs dramatically, consistent with previous reports that suggest this may be due to its dual role as a tRNA-methyltransferase and as part of the mitochondrial RNase P complex in RNA processing (18, 20, 21).
It has been shown that all three components of the mammalian mitochondrial RNase P complex are required for its activity, and here we observe that knockdown of each component leads to a decrease of mitochondrial RNAs, albeit at different levels. The metallonuclease domain of PRORP provides the catalytic component of the RNase P complex; however, the effects of its knockdown on mitochondrial RNA abundance are not particularly dramatic. With the exception of tRNAIle, the E2 treatment of cells in which HSD17B10 was knocked down did not result in significant changes in mitochondrial RNAs. Control cells showed an increase in many mitochondrial RNAs in response to E2 treatment; interestingly, in the absence of E2 treatment, MCF-7 cells in which PRORP was knocked down increased mitochondrial RNA abundance. The estrogen-dependent changes observed upon PRORP knockdown may reflect the dual role of HSD17B10 as a subunit of the RNase P enzyme and as a binding partner of ERα. In the presence of estrogen, HSD17B10 may be recruited to the steroid metabolism pathway or as a core subunit of the RNase P complex. Hence, in the presence of E2, mitochondrial transcript levels may be recovered because the protein is available to join the RNase P complex and carry out the processing activity that results in increased mature transcript levels. The changes observed at the RNA level were consistent with changes in mitochondrial protein translation, with decreases in mitochondrial protein synthesis upon TRMT10C or ELAC2 knockdown.
The increase in PTCD1 abundance in response to ELAC2 knockdown suggests that the two proteins have opposing roles in tRNA processing, whereby a decreased abundance of PTCD1 and a consequent increase in ELAC2 should result in increased abundance of mature mitochondrial transcripts. Alternatively, it could be that the association of the two proteins is required for 3′ tRNA processing, and the increase in ELAC2 abundance is a compensatory response to decreased PTCD1 levels. The association of ERα with both HSD17B10 and PTCD1 provides the means to affect both 5′ and 3′ mitochondrial RNA processing in response to E2 and enables the coordinated control of mitochondrial gene expression. The effects on mitochondrial gene expression by E2 and ERα under stress growth conditions are specific because no significant changes in mitochondrial gene expression were observed in cells lacking ERα that were grown in basal and serum-depleted medium (Supplemental Figure 6).
Since its identification as a component of the mitochondrial RNase P complex, the role of HSD17B10 in RNA processing has remained obscure. The association between HSD17B10 and TRMT10C into a subcomplex is required for TRMT10C to carry out an important nucleotide modification of mitochondrial tRNAs (18, 20, 21). Initially it was suggested that the NAD+ binding site present in HSD17B10 was necessary for the processing activity of RNase P. However, recent studies showed that both HSD17B10's dehydrogenase activity and NAD+ binding site are not necessary for RNase P activity (21). HSD17B10's role in the conversion of 17β-estradiol to estrone within mitochondria suggests that mitochondria may be able to directly regulate the intracellular levels of the hormone. This is of particular importance, given estrogen's role in cellular proliferation and tumor progression in cancer (41, 42). Furthermore, a number of mutations in the gene encoding HSD17B10 have been associated with disease (43). For instance, patients carrying mutations in HSD17B10 present with neurological abnormalities and neuromotor disorders associated with defects in the metabolism of isoleucine. Accordingly, our work shows a specific role for HSD17B10 in tRNAIle transcript abundance. The role of HSD17B10 on isoleucine availability may explain the effect of HSD17B10 knockdown on tRNAIle abundance. HSD17B10's role in isoleucine degradation represents yet another function of this versatile, multifunctional protein that requires further investigation.
Mitochondria play a range of key roles in the cell, including the integration of extracellular signals, such as hormones, and the execution of intracellular responses. There is increasing evidence suggesting a cross talk between mitochondria and the nucleus, which is directly related to cellular function and reveals the existence of signaling pathways that may provide new therapeutic targets in disease. Estrogen-mediated carcinogenesis caused by spontaneous mutations that lead to enhanced cell proliferation has been associated with increased levels of estrogen and long-term stimulation of estrogen signaling pathways (44). Although the exact mechanisms underlying these signaling pathways are not fully understood, the results presented here indicate that increased estrogen levels affect mitochondrial gene expression by modulating the activities of mitochondrial RNA processing proteins via ERα, further supported by the evidence that the master regulators of mitochondrial biogenesis (TFAM, PGC-1 and PPARγ) are not up-regulated with ERα treatment. Instead, these factors are up-regulated when the levels of HSD17B10 are compromised, suggesting that there is a secondary mechanism that can compensate for decreased mitochondrial function. These findings illustrate the existence of feedback mechanisms between the nucleus and mitochondria directly modulating mitochondrial gene expression. Here HSD17B10 is described as a novel target for the regulation of mitochondrial gene expression by E2 and a clear example of a moonlighting enzyme, a multifunctional protein performing multiple, unrelated functions and involved in different aspects of mitochondrial metabolism.
Acknowledgments
We thank Moira Hibbs for technical assistance and Jacky Bentel for providing the MCF-7 cells and the critical review of the manuscript.
Present address for M.I.G. Lopez Sanchez: Centre for Eye Research Australia, University of Melbourne, Royal Victorian Eye and Ear Hospital, 32 Gisborne Street, East Melbourne, Victoria 3002, Australia.
This work was supported by fellowships and project grants (to A.F and O.R.) from the National Health and Medical Research Council (Grants APP1058442, APP1045677, APP1041582, APP1023460, and APP1005030) and the Australian Research Council (Grants FT0991008, FT0991113, and DP140104111).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by fellowships and project grants (to A.F and O.R.) from the National Health and Medical Research Council (Grants APP1058442, APP1045677, APP1041582, APP1023460, and APP1005030) and the Australian Research Council (Grants FT0991008, FT0991113, and DP140104111).
Footnotes
- COX
- cytochrome c oxidase
- E2
- 17β-estradiol
- EGFP
- enhanced green fluorescent protein
- ERα
- estrogen receptor-α
- HSD17B10
- 17β-dehydrogenase 10
- MRPP
- mitochondrial RNase P protein
- mtDNA
- mitochondrial DNA
- MT Orange
- MitoTracker Orange
- NAD+
- oxidation of nicotinamide adenine dinucleotide
- NCBI
- National Center for Biotechnology Information
- NT
- nontargeting
- PGC-1
- PPAR-γ coactivator 1
- PPAR
- peroxisomal proliferator-activated receptor
- PPR
- pentatricopeptide repeat
- PRORP
- proteinaceous RNase P
- qRT-PCR
- quantitative RT-PCR
- RNase
- ribonuclease
- SDHA
- succinate dehydrogenase complex, subunit A
- siRNA
- small interfering RNA
- TAP
- tandem affinity purification
- TEV
- tobacco etch virus
- TFAM
- mitochondrial transcription factor A
- TRMT10C
- tRNA methyltransferase 10 C.
References
- 1. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biochem. 2001;276(40):36869–36872. [DOI] [PubMed] [Google Scholar]
- 2. Vasconsuelo A, Pronsato L, Ronda AC, Boland R, Milanesi L. Role of 17β-estradiol and testosterone in apoptosis. Steroids. 2011;76(12):1223–1231. [DOI] [PubMed] [Google Scholar]
- 3. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. [DOI] [PubMed] [Google Scholar]
- 4. Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–56. [DOI] [PubMed] [Google Scholar]
- 5. Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20(10):477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68(4):959–965. [DOI] [PubMed] [Google Scholar]
- 7. Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105(6):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattingly KA, Ivanova MM, Riggs KA, et al. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22(3):609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yager JD, Chen JQ. Mitochondrial estrogen receptors—new insights into specific functions. Trends Endocrinol Metab. 2007;18(3):89–91. [DOI] [PubMed] [Google Scholar]
- 10. Chen JQ. Mitochondrial localization of ER and ER in human MCF7 cells. Am J Physiol. 2004;286(6):E1011–E1022. [DOI] [PubMed] [Google Scholar]
- 11. Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17(5):2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Psarra AM, Sekeris CE. Nuclear receptors and other nuclear transcription factors in mitochondria: regulatory molecules in a new environment. Biochim Biophys Acta. 2008;1783(1):1–11. [DOI] [PubMed] [Google Scholar]
- 13. Chen JQ, Yager JD. Estrogen's effects on mitochondrial gene expression: mechanisms and potential contributions to estrogen carcinogenesis. Ann NY Acad Sci. 2004;1028:258–272. [DOI] [PubMed] [Google Scholar]
- 14. Sekeris CE. The mitochondrial genome: a possible primary site of action of steroid hormones. In Vivo. 1990;4(5):317–320. [PubMed] [Google Scholar]
- 15. Chen JQ, Eshete M, Alworth WL, Yager JD. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors α and β to human mitochondrial DNA estrogen response elements. J Cell Biochem. 2004;93(2):358–373. [DOI] [PubMed] [Google Scholar]
- 16. Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290(5806):470–474. [DOI] [PubMed] [Google Scholar]
- 17. Mercer TR, Neph S, Dinger ME, et al. The human mitochondrial transcriptome. Cell. 2011;146(4):645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135(3):462–474. [DOI] [PubMed] [Google Scholar]
- 19. Brzezniak L, Stepien PP, Bijata M, Szczesny RJ. Human ELAC2 gene encodes the tRNAseZ responsible for mitochondrial tRNA 3′processing, which acts on precursors already cleaved by RNAseP. RNA Biol. 2011;8(4):1–11. [DOI] [PubMed] [Google Scholar]
- 20. Lopez Sanchez MI, Mercer TR, Davies SM, et al. RNA processing in human mitochondria. Cell Cycle. 2011;10(17):2904–2916. [DOI] [PubMed] [Google Scholar]
- 21. Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase—extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40(22):11583–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rauschenberger K, Schöler K, Sass JO, et al. A non-enzymatic function of 17β-hydroxysteroid dehydrogenase type 10 is required for mitochondrial integrity and cell survival. EMBO Mol Med. 2010;2(2):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ofman R, Ruiter JP, Feenstra M, et al. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency is caused by mutations in the HADH2 gene. Am J Hum Genet. 2003;72(5):1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korman SH. Inborn errors of isoleucine degradation: a review. Mol Genet Metab. 2006;89(4):289–299. [DOI] [PubMed] [Google Scholar]
- 25. Rackham O, Filipovska A. The role of mammalian PPR domain proteins in the regulation of mitochondrial gene expression. Biochim Biophys Acta. 2011;1819(9–10):1008–1016. [DOI] [PubMed] [Google Scholar]
- 26. Liu G, Mercer TR, Shearwood A-MJ, et al. Mapping of mitochondrial RNA-protein interactions by digital RNase footprinting. Cell Rep. 2013;5(3):839–848. [DOI] [PubMed] [Google Scholar]
- 27. Rackham O, Davies SMK, Shearwood A-MJ, Hamilton KL, Whelan J, Filipovska A. Pentatricopeptide repeat domain protein 1 lowers the levels of mitochondrial leucine tRNAs in cells. Nucleic Acids Res. 2009;37(17):5859–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He XY, Merz G, Yang YZ, Mehta P, Schulz H, Yang SY. Characterization and localization of human type10 17β-hydroxysteroid dehydrogenase. Eur J Biochem. 2001;268(18):4899–4907. [DOI] [PubMed] [Google Scholar]
- 29. Jazbutyte V, Kehl F, Neyses L, Pelzer T. Estrogen receptor α interacts with 17β-hydroxysteroid dehydrogenase type 10 in mitochondria. Biochem Biophys Res Commun. 2009;384(4):450–454. [DOI] [PubMed] [Google Scholar]
- 30. Smith P, Krohn R, Hermanson G, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. [DOI] [PubMed] [Google Scholar]
- 31. Davies SMK, Lopez Sanchez MIG, Narsai R, et al. MRPS27 is a pentatricopeptide repeat domain protein required for the translation of mitochondrially encoded proteins. FEBS Lett. 2012;586(20):3555–3561. [DOI] [PubMed] [Google Scholar]
- 32. Davies SMK, Rackham O, Shearwood A-MJ, et al. Pentatricopeptide repeat domain protein 3 associates with the mitochondrial small ribosomal subunit and regulates translation. FEBS Lett. 2009;583(12):1853–1858. [DOI] [PubMed] [Google Scholar]
- 33. Razmara A, Sunday L, Stirone C, et al. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharm Exp Ther. 2008;325(3):782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frackowiak J, Mazur-Kolecka B, Kaczmarski W, Dickson D. Deposition of Alzheimer's vascular amyloid-β is associated with decreased expression of brain L-3-hydroxyacyl-coenzyme A dehydrogenase (ERAB). Brain Res. 2001;907(1–2):44–53. [DOI] [PubMed] [Google Scholar]
- 35. He XY, Merz G, Mehta P, Schulz H, Yang SY. Human brain short chain L-3-hydroxyacyl coenzyme A dehydrogenase is a single-domain multifunctional enzyme. Characterization of a novel 17β-hydroxysteroid dehydrogenase. J Biochem. 1999;274(21):15014–15019. [DOI] [PubMed] [Google Scholar]
- 36. Brzezniak LK, Bijata M, Szczesny RJ, Stepien PP. Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA Biol. 2011;8(4):616–626. [DOI] [PubMed] [Google Scholar]
- 37. Perez-Cerda C, Garcia-Villoria J, Ofman R, et al. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD) deficiency: an X-linked inborn error of isoleucine metabolism that may mimic a mitochondrial disease. Pediatr Res. 2005;58(3):488–491. [DOI] [PubMed] [Google Scholar]
- 38. Hayashi J, Ohta S, Kagawa Y, et al. Functional and morphological abnormalities of mitochondria in human cells containing mitochondrial DNA with pathogenic point mutations in tRNA genes. J Biol Chem. 1994;269(29):19060–19066. [PubMed] [Google Scholar]
- 39. Yang S, He X, Schulz H. Multiple functions of type 10 17β-hydroxysteroid dehydrogenase. Trends Endocrinol Metab. 2005;16(4):167–175. [DOI] [PubMed] [Google Scholar]
- 40. Moeller G, Adamski J. Multifunctionality of human 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2006;248(1–2):47–55. [DOI] [PubMed] [Google Scholar]
- 41. Nilsson S, Makela S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. [DOI] [PubMed] [Google Scholar]
- 42. Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–170. [DOI] [PubMed] [Google Scholar]
- 43. Zschocke J. HSD10 disease: clinical consequences of mutations in the HSD17B10 gene. J Inherit Metab Dis. 2012;35(1):81–89. [DOI] [PubMed] [Google Scholar]
- 44. Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17(11):2279–2284. [DOI] [PubMed] [Google Scholar]