Abstract
Heat-producing beige/brite (brown-in-white) adipocytes in white adipose tissue have the potential to suppress metabolic disease in mice and hold great promise for the treatment of obesity and type 2 diabetes in humans. Here, we demonstrate that human adipose-derived stromal/progenitor cells (hASCs) from subcutaneous white adipose tissue can be efficiently converted into beige adipocytes. Upon pharmacological activation of peroxisome proliferator-activated receptor-γ, hASC-derived adipocytes activated beige fat-selective genes and a brown/beige fat-selective electron transport chain gene program. Importantly, hASC-derived beige fat cells displayed the bioenergetic characteristics of genuine brown fat cells, including a capacity for increased respiratory uncoupling in response to β-adrenergic agonists. Furthermore, knock-down experiments reveal that the thermogenic capacity of human beige fat cells was entirely dependent on the presence of Uncoupling protein 1. In summary, this study reveals that hASCs can be readily differentiated into beige adipocytes that, upon activation, undergo uncoupling protein 1-dependent thermogenesis.
Brown adipose tissue (BAT) is a major site of adaptive thermogenesis in rodents that is activated by cold exposure and certain high calorie diets. Uncoupling protein (UCP)1 mediates thermogenesis in brown fat cells by dissipating the mitochondrial proton gradient that is used to drive ATP synthesis. This results in a high rate of respiration and heat production from the combustion of available substrates (1). Thermogenic adipocytes, called beige or brite (brown-in-white) cells, also develop in rodent white adipose tissue (WAT) in response to various stimuli, including cold, thiazolidinediones (TZDs), and β-adrenergic agonists (2). Importantly, high levels of beige adipocyte activity is commonly associated with reduced obesity and metabolic disease in mice (2). Beige adipocytes in mice originate, in large part, from precursor cells that undergo de novo differentiation in response to certain environmental stimuli such as cold exposure (3–9). It is yet unclear whether human WAT contains an analogous population of beige preadipose cells. Interestingly, immortal cell lines derived from infant fat tissue (pubic region) are able to undergo differentiation into UCP1-expressing adipocytes (10). Multipotent mesenchymal stem cells called human adipose-derived stromal/progenitor cells (hASCs) can be isolated from human WAT depots (11). However, the beige adipogenic potential of primary hASCs from adult tissue has only been partially examined (12, 13). Importantly, it is also unclear whether the expression of UCP1 in human adipocytes is associated with increased thermogenic activity. In fact, other mechanisms of respiratory uncoupling have been identified in ASC-derived adipocytes (14).
In this study, we demonstrate that primary hASCs from adult subcutaneous (sc) depots can be efficiently converted into thermogenic adipocytes. Specifically, treatment of hASCs with rosiglitazone (Rosi) during a 32-day differentiation time course activated the expression of UCP1, a brown fat-selective mitochondrial program as well as a beige fat-specific gene signature. Comprehensive bioenergetic analyses revealed that Rosi-treated but not control adipocytes were competent to increase their levels of respiratory uncoupling in response to β-adrenergic agonists or direct UCP1 activators. Finally, we found that depletion of UCP1 in hASC-derived beige adipocytes blocked β-adrenergic agonist-stimulated respiratory uncoupling. Together, our results suggest that triggering the beige fat differentiation pathway and UCP1 activity in human fat has the potential to increase energy dissipation for the treatment of obesity and associated disease.
Materials and Methods
Patient consent
Samples of adipose tissues were collected from patients undergoing elective surgery at Sahlgrenska University Hospital in Gothenburg, Sweden. All study subjects received written and oral information before giving written informed consent for the use of the tissue. The studies were approved by The Regional Ethical Review Board in Gothenburg, Sweden.
Isolation and culture of hASCs
Human sc WAT was obtained from healthy women undergoing elective fat removal (Table 1). We isolated human adipose-derived stem cells (hASCs) from the stromal vascular fraction. Briefly, biopsies were minced and digested in Hank's Balanced Salt Solution with Mg2+/Ca2+ containing collagenase II (300 U/mL) (4177; Worthington) and fatty acid-free bovine serum albumin (20 mg/mL) (A6003; Sigma) for 45–60 minutes at 37°C with gentle shaking. After digestion, the suspension was mixed 1:1 with DMEM/Ham's F12 containing 10% calf serum (PAA B15–004; GE Healthcare) and filtered through a cell strainer (250 μm in size, 03–250/5 0; Sefar Nitex). The resulting cell suspension was centrifuged 10 minutes at 200g, and cells were resuspended in adipocyte basal medium (ZenBio) containing 10% calf serum, 1% penicillin/streptomycin, and 17-ng/mL basic fibroblast growth factor (F0291; Sigma) and seeded at 19 000 cells/cm2 in tissue culture-treated cell culture flasks. Cells were cultured at 37°C, 5% CO2 in air with 80% humidity. Media were changed the day after isolation, and on day 4 or 5, cells were detached using TrypLE Express (passage 1; Life Technologies). Cells were reseeded at 17 000 cells/cm2 and grown to 90% confluence (2–3 d). At passage 2, cells were cryopreserved in basal medium, 40% fetal bovine serum, and 7% dimethyl sulfoxide at 2.5 × 106 cells/mL. Upon thawing, cells were cultured in a growth medium DMEM/Ham's F12 with 10% fetal bovine serum, 10mM HEPES, 33μM biotin (Sigma), 17μM pantothenate (Sigma), 1nM basic fibroblast growth factor (Sigma), 50-U/mL penicillin, and 50-μg/mL streptomycin at 37°C, 5% CO2 in air with 80% humidity. For adipocyte differentiation, 90% confluent cells were treated with DMEM/F12 with 3% fetal calf serum (Gold; PAA) supplemented with 100nM dexamethasone (Sigma), 500μM 3-isobutyl-1-methyxanthine (Sigma), 0.85μM insulin, and 5nM triiodothyronine (Sigma). To promote beige adipogenesis, 100nM Rosi was included in the differentiation medium. Media were changed every other day during proliferation and differentiation, until fully differentiated (d 32).
Table 1.
Clinical Data of Subjects Used in This Study
| Subject | Gender | Age | Initial weight (kg) | Actual weight (kg) | Height (cm) | BMI | Medications/disease | Figure |
|---|---|---|---|---|---|---|---|---|
| 114 | F | 34 | 105 | 65 | 155 | 27 | Vitamins/none | 1–3 |
| 119 | F | 43 | 126 | 70 | 154 | 30 | Vitamins/none | 1–3 |
| 123 | F | 34 | 105 | 81 | 173 | 27 | Vitamins/none | Supplemental Figure 2 |
| 138 | F | 32 | 138 | 67 | 167 | 24 | Vitamins/none | 1, 3, and 4 and Supplemental Figure 2 |
| 150 | F | 24 | 128 | 63 | 156 | 26 | None/none | 1–3 and Supplemental Figure 2 |
Short hairpin RNA (shRNA) experiments
UCP1 or scramble (Scr) shRNAs were expressed from the pGIPZ-lentiviral shRNAmir vector (Open Biosystems). The useful UCP1 sh sequences were: TACCTAATAACACTGGACG (number 550) and TGACTTTGACAGTTCTCGT (number 711). Lentivirus was produced in HEK-293T cells (Open Biosystems). Culture supernatants were collected 48 hours after transfection and concentrated using polyethylene glycol (System Biosciences).
Immunoblotting
Cells were lysed in modified radioimmunoprecipitation assay A buffer (20mM HEPES [pH 7.9], 5mM MgCl2, 25% glycerol, 1% nonidet P-40, 150mM NaCl, 1× phospho-STOP, and 1× Complete mini [Roche]). Protein concentration was determined using a bicinchoninic acid kit (Pierce). After electrophoresis, proteins were transferred to nitrocellulose membrane for incubation with primary antibody (oxidative phosphorylation [OXPHOS] complexes, MS-604; Mitosciences), Peroxisome proliferator-activated receptor-γ, coactivator 1-α (PGC1) (sc-13067; Santa Cruz Biotechnology, Inc), UCP1 (MAb6158; R&D Systems), Peroxisome Proliferator-Activated Receptor-γ (PPARγ) (sc-7196; Santa Cruz Biotechnology, Inc), and β-ACTIN (A5441; Sigma).
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was extracted using RNAeasy (QIAGEN) and reversed transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). qPCR was performed using TaqMan Gene Expression Assays (Supplemental Table 1). TBP and CYPA served as loading controls.
XF24 O2 consumption analysis
O2 consumption rate (OCR) was measured using the XF24 Analyzer (Seahorse Bioscience). Chemicals used were rotenone (8875; Sigma), antimycin A (8674; Sigma), oligomycin (O4876; Sigma), 2,4DNP (D199303; Sigma), isoproterenol (iso) (I6504; Sigma), all-trans retinoic acid (ATRA) (R2625; Sigma), palmitate (P9767; Sigma), and BSA (A6003; Sigma). After obtaining XF measurements, cells were fixed, stained with a nuclear dye, and counted to allow for normalization of OCRs (per cell number). The apparent mitochondrial coupling efficiency (phosphate/oxygen ratio) during basal respiration as well as the cell respiratory control ratio (cRCR) after β-adrenergic stimuli were determined as outlined previously (15).
Statistical analysis
Statistical analyses were performed by one-way ANOVA with Bonferroni post hoc test to compare all conditions or by paired or unpaired Students't test using Prism GraphPad.
Results
hASCs differentiate into beige adipocytes
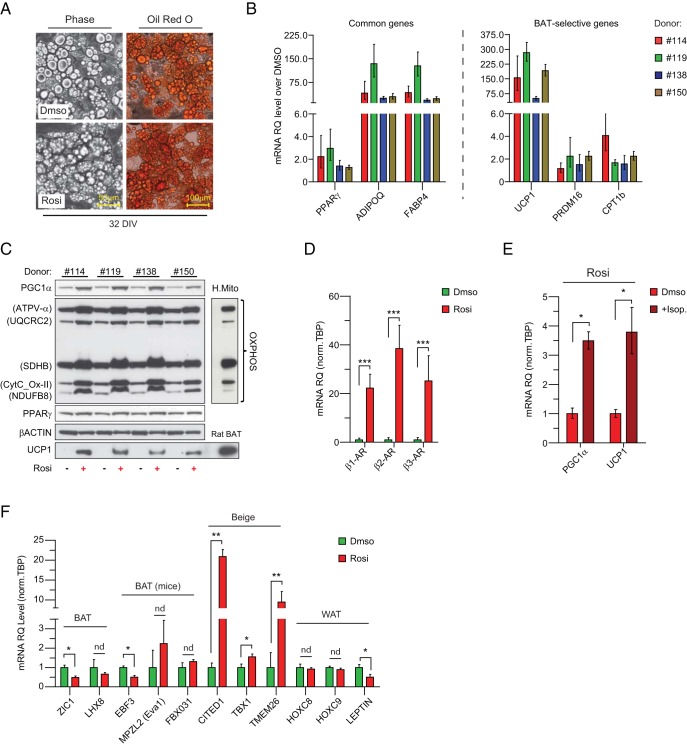
To determine whether hASCs could give rise to beige adipocytes, we isolated sc hASCs from 4 healthy donors and assayed their differentiation potential. hASCs were cultured under adipogenic conditions with or without addition of Rosi. Oil-Red-O staining showed that control and Rosi-treated cultures underwent conversion into lipid droplet-containing adipocytes (Figure 1A). Gene expression studies indicated that Rosi increased the expression levels of PPARγ target genes such as ADIPOQ and FABP4 but had marginal effects on the expression of PPARγ itself (Figure 1, B and C). Remarkably, Rosi treatment led to 50- to 300-fold increases in UCP1 (Figure 1B, right panel), which was paralleled by increases in the expression of other brown fat-selective genes, including PRDM16 and CPT1B (Figure 1B). This Rosi-induced increase in UCP1 mRNA levels was also seen at the protein level (Figure 1C). Interestingly, GW1929, MCC-555, and 15dPG12, other PPARγ agonists, stimulated the expression of general adipose genes like FABP4 and ADIPOQ in hASCs, but among these, only GW1929 activated brown fat-specific genes (UCP1, CPT1B) (Supplemental Figure 1). Finally, Rosi-treated cells expressed much higher levels of subunits in the mitochondrial respiratory complexes (I to V) (Figure 1C).
Figure 1. hASCs undergo beige adipocyte differentiation in culture.
A, Oil-Red-O staining of triglycerides in adipocytes derived from DMSO- and Rosi-treated sc abdominal hASCs 32 days in vitro (32DIV). B, Cell cultures were analyzed by real-time qPCR (RT-qPCR) for their expression levels of differentiation markers common to WAT and BAT (B, left panel, common genes), brown fat cell-selective genes (B, right panel, BAT selective gens). C, Western blot analysis of OXPHOS subunits (CV-α [Atp5B], CIII-core2 [UQCRC2], CII-30 [SdhB], CIV-II [CytC-OxII], CI-20 [NdufB8]), PGC1α, PPARγ, UCP1, and β-ACTIN (loading control) in DMSO- and Rosi- treated cells. Human mitochondria heart and rat BAT extracts served as loading controls for OXPHOS complexes and UCP1. D and E, mRNA levels of β-adrenergic receptors (β1-AR, β2-AR, and β3-AR). mRNA levels of UCP1 and PGC1α ± 4-hour iso treatment (+Iso) of Rosi-differentiated adipocytes. F, mRNA levels of selected BAT, beige, and WAT genes from DMSO- and Rosi-treated sc abdominal hASCs. Data are express as fold changes over DMSO-treated cells (B) and as mean ± SEM (D–F); n = 3 independent experiments; ***, P < .001; **, P < .01; and *, P < .05.
Thermogenesis in brown fat cells is activated through the β-adrenergic signaling cascade (1). We found that Rosi treatment elevated the expression levels of ADRB1, ADBR2, and ADRB3 by 20- to 40-fold in hASC-derived adipocytes (Figure 1D). This was associated with the capacity for Rosi-treated cells to further increase their thermogenic gene levels in response to β-adrenergic agonists. For example, iso treatment increased PGC1α and UCP1 mRNA levels by 3- to 4-fold (Figure 1E). Together, these data show that Rosi treatment robustly activates a broad brown fat/thermogenesis-related gene program in hASC-derived adipocytes.
Recent studies have identified several brown- vs beige fat-specific marker genes (Supplemental Table 2) (2). We found that Rosi-treated hASCs expressed lower/unchanged levels of brown-selective genes relative to control cultures, including ZIC1, EVA1, and EBF3, but expressed higher levels of the beige-specific markers CITED1, TBX1, and TMEM26 (Figure 1F). Additionally, levels of LEPTIN, a white adipose-enriched gene, were decreased by Rosi. These data suggest that Rosi triggers a beige fat differentiation program in hASCs.
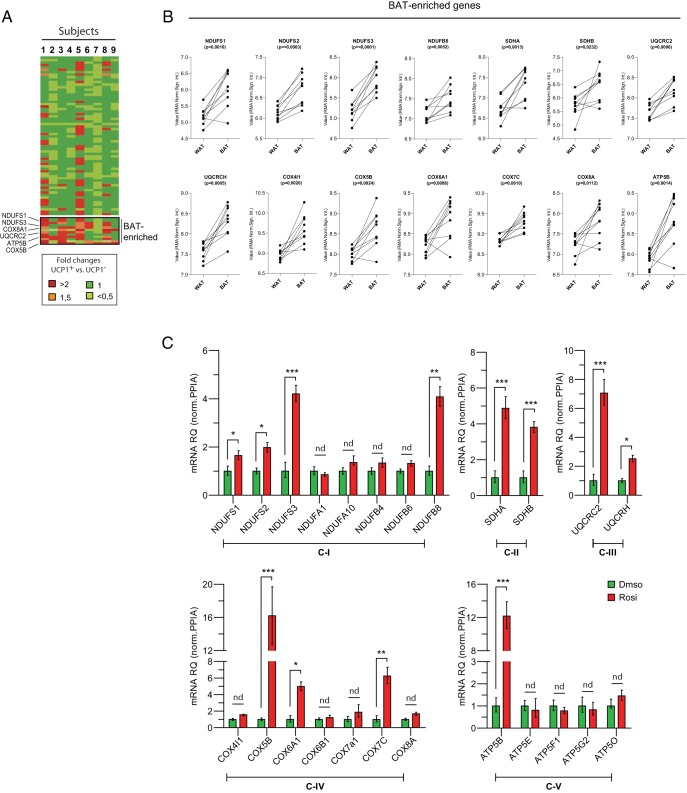
Brown and white fat cell mitochondria have distinctive molecular profiles (16). We sought to determine whether Rosi treatment remodels the mitochondrial profile of hASCs to a “brown-like” state. First, we identified human brown/beige fat-enriched mitochondrial genes by comparing the mRNA levels of all mitochondrial OXPHOS components in human perithyroid BAT and adjacent sc WAT using available microarray datasets (GSE27657) (17). We found 14 OXPHOS genes that were selectively enriched in human BAT (or beige) vs WAT (Figure 2B); this included genes encoding subunits from all 5 complexes: NADH dehydrogenase (NDUFS1, NDUFS2, NDUFS3, and NDUFB8), succinate dehydrogenase (SDHA and SDHB), cytochrome c reductase (UQCRC2, UQCRCH), cytochrome c oxidase (COX4I1, COX5B, COX6A1, COX7C, and COX8A), and ATP synthase (ATP5B). We then measured the expression of these brown-selective mitochondrial genes as well as other nonselective components from each complex in Rosi-treated vs control hASCs. Strikingly, Rosi increased the expression of nearly all brown fat-selective mitochondrial genes with no effect on the expression of the common components (Figure 2C). These data demonstrate that Rosi treatment stimulates a brown/beige fat-specific mitochondrial gene signature in adipocytes that matches the mitochondrial profile of native human BAT/beige fat.
Figure 2. hASC-derived beige adipocytes express a human BAT-specific mitochondrial profile.
A, Heat-map of relative fold changes in expression levels of Oxphos genes in hBAT vs hWAT (paired samples from a total of 9 subjects; GSE27657). Example of up-regulated genes are highlighted as BAT enriched. The color scale shows the fold changes in mRNA expression levels of the genes. B, RMA (Robust Multiarray Average normalization signal; log2) of up-regulated genes are shown individually. C, Correlation of Oxphos gene expression between human BAT biopsies and cultured hASC-derived beige adipocytes. Difference in gene expression between BAT and WAT biopsy was analyzed by paired Student's t test (2-sided) (A); P < .05 was considered statistically significant. Data are mean ± SEM (C); n = 3 independent experiments; ***, P < .001; **, P < .01; and *, P < .05.
hASC-derived beige adipocytes exhibit genuine UCP1-mediated thermogenesis
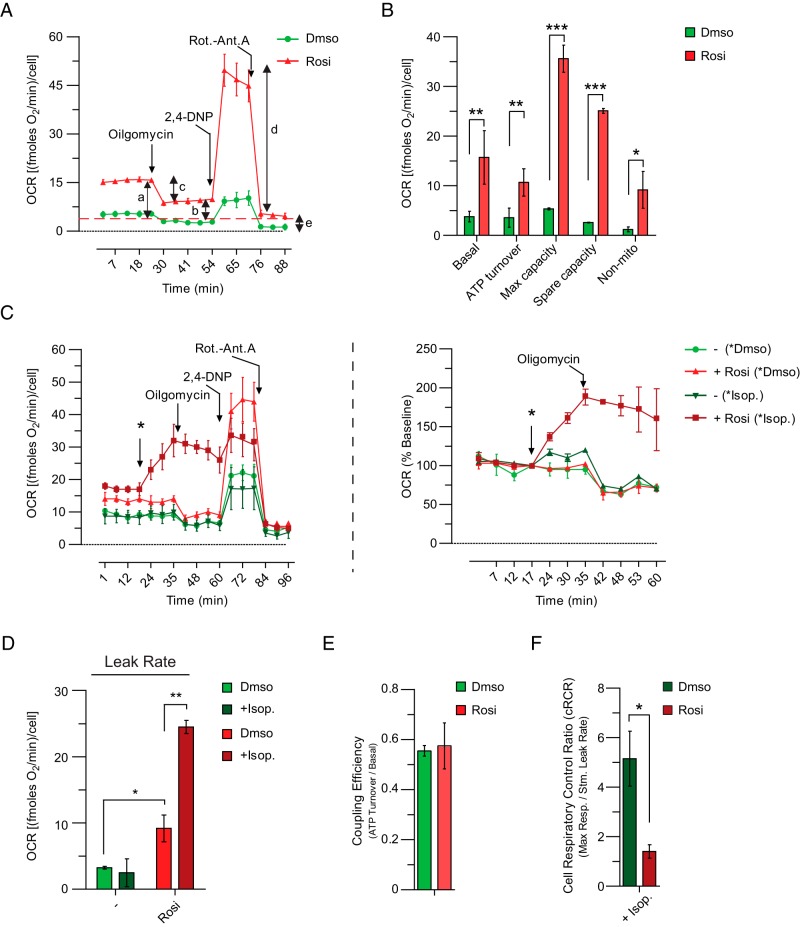
Thermogenesis in brown adipocytes is activated by norepinephrine, which is secreted from nerve terminals in adipose tissue during cold exposure (1). Brown adipocytes respond to norepinephrine by increasing their rate of uncoupled respiration. To examine the potential for uncoupling in hASC-derived beige adipocytes, we measured cellular respiration in control and Rosi-treated cultures using the Seahorse instrument. Under basal conditions, Rosi-treated adipocyte cultures had higher OCRs than control cultures (Figure 3A). More detailed analysis of cellular respiration through the use of various mitochondrial inhibitors showed that Rosi treatment increased ATP turnover, uncoupling, maximum capacity, spare capacity, and nonmitochondrial respiration (Figure 3, A and B).
Figure 3. Thermogenic capacity of human beige adipocytes.
A and B, Representative cell respiratory experiment. A, OCR in differentiated hASCs under basal conditions, after the addition of oligomycin (2μM, ATP turnover), in the presence of 2,4DNP (180μM, Max capacity), or rotenone/antimycin (5μM/5μM, Nonmito). B, Nonmitochondrial respiration after the final addition (e) was subtracted from the other values. Basal respiration (a); oligomycin-insensitive (leak, as an index of uncoupled respiration) respiration (b); oligomycin-sensitive (ATP turnover) respiration (c); maximal respiration in the presence of 2,4DNP (d). Derived parameter: spare respiratory capacity (d–a) (data as average of 3 independent experiments). C, Representative OCR in differentiated hASCs under basal condition, after the addition of DMSO or iso (100nM), oligomycin (2μM), in the presence of 2,4DNP (180μM), or rotenone/antimycin(5μM/5μM). C, left panel, Percentage change in OCRs in differentiated hASCs after injection of DMSO or iso (*) and oligomycin (C, right panel). D, Oligomycin-insensitive OCR (leak rate, as an index of uncoupled respiration) in Rosi-treated hASCs. E and F, Coupling efficiency (ATP turnover [c]/basal respiration [a]; cRCR; Max respiration [d]/leak rate [b]). Data in B and D–F are represented as mean ± SEM; n = 4 independent experiments; ***, P < .001; **, P < .01; and *, P < .05.
To assess thermogenesis in hASC-derived beige adipocytes, we treated adipocyte cultures with the synthetic β-adrenergic activator, iso. As shown in Figure 3C, iso stimulated a nearly 2-fold increase in the OCR of Rosi-treated cells (Figure 3C) but had no impact on control cells. Importantly, oligomycin-insensitive (and hence uncoupled) OCR was increased by iso in Rosi-treated cells but not in control cells (Figure 3D). As in classical brown fat cells, Rosi-treated (UCP1+) hASC-derived adipocytes showed the same coupling efficiency (ATP turnover [c]/basal respiration [a]) as control cells under basal conditions despite the presence of easily catabolized substrate (Na+ pyruvate) in the media (Figure 3E). Conversely, the cRCR (max respiration [d]/leak rate [b]) was significantly decreased in Rosi-treated cells after maximal stimulation (Figure 3F), the only situation in which uncoupled thermogenesis is limited by the availability of endogenous substrate. Palmitate (C16:0), a long chain fatty acid, and ATRA, a fatty acid-like metabolite, can both activate UCP1 (18, 19). Seahorse analysis demonstrated that palmitate and ATRA increased uncoupled respiration in Rosi-treated (UCP1+) cells (Supplemental Figure 2), suggesting that UCP1 can increase O2 consumption in hASCs. Altogether, these data demonstrate that the presence of UCP1 in hASC-derived beige adipose cells correlates with an increased capacity for β-adrenergically stimulated uncoupled respiration.
Thermogenesis in human beige adipocytes is strictly UCP1 dependent
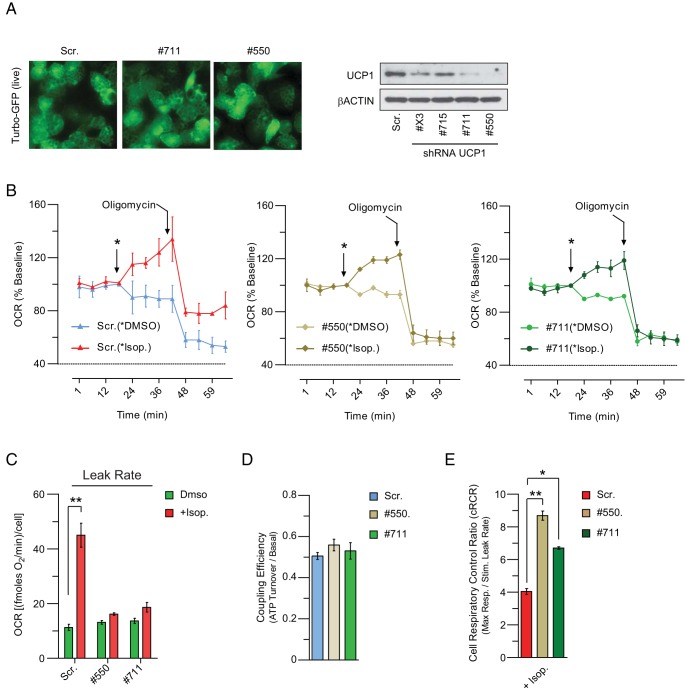
To unambiguously examine the requirement for UCP1 in the respiratory activity of hASC-derived adipocytes, we used shRNAs to suppress UCP1 expression. Specifically, hASCs were induced to differentiate before transducing cells with lentivirus that expressed a UCP1-specific shRNA (4 different clones) or a control Scr sh sequence. Forty-eight hours after transduction, 2 shRNAs (numbers 550 and 711) strongly decreased UCP1 protein (Figure 4A) without affecting adipocyte morphology or lipid accumulation (Figure 4A). The OCR of UCP1-depleted cells and Scr (control) cells was similar, indicating that UCP1 does not affect the basal levels of respiration (Figure 4B). Upon iso treatment, there was a significant increase in the OCR of both Scr cells and UCP1-depleted adipocytes (Figure 4B), which was more pronounced in Scr cells. However, only the Scr cells displayed an increase in oligomycin-insensitive (uncoupled) OCR in response to iso. Indeed, iso-stimulated a 4-fold increase in the uncoupling rate of Scr cells but had no effect in UCP1-depleted cultures (Figure 4C). As anticipated, Scr and UCP1-depleted adipocytes had equal coupling efficiency under basal (unstimulated) conditions (Figure 4D). However, upon maximal stimulation, there was a dramatic decrease in the cRCR of Scr but not of UCP1-depleted adipocytes (Figure 4E). These data demonstrate that β-agonist-stimulated uncoupled respiration in hASC-derived beige adipocytes depends on UCP1.
Figure 4. UCP1 is required for β-adrenergic agonist-stimulated thermogenesis in hASC-derived beige adipocytes.
A, Western blot analysis of UCP1 levels in hASC-derived adipocytes transduced with either sh-UCP1 or sh-Scr. B, OCR in differentiated hASCs after UCP1 depletion under basal condition, after the addiction of DMSO or β-AR agonist iso (100nM), the mitochondrial inhibitor oligomycin (2μM). C, Oligomycin-insensitive OCR (proton leak, as an index of uncoupled respiration) in UCP1-depleted brown-like adipose cells. D and E, Coupling efficiency and cRCR (as calculated in Figure 3). Data are represented as mean ± SEM (C–E); n = 3 independent experiments; **, P < .01; and *, P < .05.
Discussion
A robust model system to study human beige fat function has been lacking. Previous studies have examined human brown or beige fat-like differentiation from a variety of cell sources (10, 20–23), but the thermogenic capacity of these cells and UCP1 functionality, in particular, had been poorly studied.
We reveal that hASCs can be efficiently converted into thermogenic adipocytes that have a molecular signature of beige fat cells. Importantly, UCP1 was absolutely required for the thermogenic activity of these cells in response to β-adrenergic agonists. Thus, other mechanisms of uncoupling, if they exist, do not substitute for the loss of UCP1 under these conditions.
The activation of PPARγ by Rosi and other TZDs powerfully stimulates beige adipocyte differentiation in hASCs. The strongly overlapping gene programs of Rosi-treated hASCs and native human BAT suggests that a “Rosi-like” mechanism functions under physiological conditions to control beige adipose identity. In mouse cells, Rosi mediates beige adipocyte differentiation by increasing the activity of the SIRT1 deacetylase. SIRT1 binds and deacetylates PPARγ to facilitate the recruitment of PRDM16, a powerful transcriptional coactivator of brown fat genes (24, 25). Future studies will be needed to determine whether this mechanism also operates in human cells and whether SIRT1 activators can be used to promote beige fat development.
An outstanding question is whether PPARγ or SIRT1 activators can generate enough beige adipocytes in human WAT to impact systemic metabolism. To do so, the acquired beige fat cells would also need to be efficiently activated to undergo thermogenesis (presumably through the β-adrenergic signaling cascade). The browning capacity of human WAT has not been well studied and is likely to be heterogeneous within and between different depots. In one study, however, TZD treatment of people weakly increased UCP1 levels in a small biopsy of sc adipose (26). One problem is that TZDs also appear to repress the adrenergic activation of adipocytes (27, 28), an effect that would tend to dampen thermogenic gene expression. Thus, it might be important to combine a beige fat cell recruiter, like a TZD with a β3-adrenergic agonist or sensitizer to augment UCP1 function. Unfortunately, many β3-adrenergic activators that were developed to stimulate BAT in man have been unsuccessful (29–32). This could be because there was inadequate local exposure of compounds to BAT, there are different role(s) of the β3-adrenergic receptor in man, or there are insufficient amounts of BAT in humans to affect systemic metabolism. However, it remains unanswered whether activation of an expanded beige or brown fat compartment can significantly raise energy expenditure and/or improve insulin sensitivity. Compounds with an improved capacity to stimulate beige adipogenesis would greatly enhance our understanding of this question.
In summary, our study demonstrates that stem cells in human WAT can be induced to readily undergo a full and functional program of beige adipocyte differentiation. The capacity for these cells to increase their energy consumption suggests that triggering this differentiation pathway in human fat could help reduce obesity and/or metabolic dysfunction. Defining the cell(s) within the perivascular niche of human WAT that can undergo efficient thermogenic programming and examining the pathways that regulate this process will be an important avenue of future research.
Acknowledgments
We thank Cecilia Brännmark, Jeremie Boucher and all the members of the in vitro insulin sensitization team of AstraZeneca (Molndal) for the scientific discussion and helpful comments. We also thank Sahlgrenska University Hospital in Gothenburg, Sweden for the human biopsies.
This work was supported by the AstraZeneca postdoc program (S.B.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the AstraZeneca postdoc program (S.B.).
Footnotes
- ATRA
- all-trans retinoic acid
- BAT
- brown adipose tissue
- cRCR
- cell respiratory control ratio
- dmso
- dimethyl sulfoxide
- hASC
- human adipose-derived stromal/progenitor cell
- iso
- isoproterenol
- max
- maximal rate of respiration
- OCR
- O2 consumption rate
- OXPHOS
- oxidative phosphorylation
- PGC1α
- PPARγ-coactivator 1α
- PPARγ
- peroxisome proliferator-activated receptor-γ
- qPCR
- quantitative real-time PCR
- Rosi
- rosiglitazone
- sc
- subcutaneous
- Scr
- scramble
- shRNA
- short hairpin RNA
- TZD
- thiazolidinedione
- UCP
- uncoupling protein
- WAT
- white adipose tissue.
References
- 1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 2. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. [DOI] [PubMed] [Google Scholar]
- 3. Lee YH, Granneman JG. Seeking the source of adipocytes in adult white adipose tissues. Adipocyte. 2012;1(4):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842(3):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Kissig M, Rajakumari S, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci USA. 2014;111(40):14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elabd C, Chiellini C, Carmona M, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;27(11):2753–2760. [DOI] [PubMed] [Google Scholar]
- 11. Fink T, Zachar V. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol Biol. 2011;698:243–251. [DOI] [PubMed] [Google Scholar]
- 12. Carey AL, Vorlander C, Reddy-Luthmoodoo M, et al. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS One. 2014;9(3):e91997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beranger GE, Karbiener M, Barquissau V, et al. In vitro brown and “brite”/“beige” adipogenesis: human cellular models and molecular aspects. Biochim Biophys Acta. 2013;1831(5):905–914. [DOI] [PubMed] [Google Scholar]
- 14. Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar N, Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes. 2010;59(10):2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 2009;10(4):324–335. [DOI] [PubMed] [Google Scholar]
- 17. Svensson PA, Jernås M, Sjöholm K, et al. Gene expression in human brown adipose tissue. Int J Mol Med. 2011;27(2):227–232. [DOI] [PubMed] [Google Scholar]
- 18. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151(2):400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rial E, González-Barroso M, Fleury C, et al. Retinoids activate proton transport by the uncoupling proteins UCP1 and UCP2. EMBO J. 1999;18(21):5827–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011;152(10):3597–3602. [DOI] [PubMed] [Google Scholar]
- 21. Nishio M, Yoneshiro T, Nakahara M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16(3):394–406. [DOI] [PubMed] [Google Scholar]
- 22. Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798–805. [DOI] [PubMed] [Google Scholar]
- 23. Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504(7478):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150(3):620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54(5):1392–1399. [DOI] [PubMed] [Google Scholar]
- 27. Bakopanos E, Silva JE. Thiazolidinediones inhibit the expression of β3-adrenergic receptors at a transcriptional level. Diabetes. 2000;49(12):2108–2115. [DOI] [PubMed] [Google Scholar]
- 28. Festuccia WT, Oztezcan S, Laplante M, et al. Peroxisome proliferator-activated receptor-γ-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology. 2008;149(5):2121–2130. [DOI] [PubMed] [Google Scholar]
- 29. Buemann B, Toubro S, Astrup A. Effects of the two β3-agonists, ZD7114 and ZD2079 on 24 hour energy expenditure and respiratory quotient in obese subjects. Int J Obes Relat Metab Disord. 2000;24(12):1553–1560. [DOI] [PubMed] [Google Scholar]
- 30. Larsen TM, Toubro S, van Baak MA, et al. Effect of a 28-d treatment with L-796568, a novel β(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76(4):780–788. [DOI] [PubMed] [Google Scholar]
- 31. Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA. 2012;109(25):10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vosselman MJ, van der Lans AA, Brans B, et al. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61(12):3106–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]