Abstract
Fibrodysplasia ossificans progressiva (FOP) is a genetic disorder characterized by progressive heterotopic ossification in soft tissues, such as the skeletal muscles. FOP has been shown to be caused by gain-of-function mutations in activin receptor-like kinase (ALK)-2, which is a type I receptor for bone morphogenetic proteins (BMPs). In the present study, we examined the molecular mechanisms that underlie the activation of intracellular signaling by mutant ALK2. Mutant ALK2 from FOP patients enhanced the activation of intracellular signaling by type II BMP receptors, such as BMPR-II and activin receptor, type II B, whereas that from heart disease patients did not. This enhancement was dependent on the kinase activity of the type II receptors. Substitution mutations at all nine serine and threonine residues in the ALK2 glycine- and serine-rich domain simultaneously inhibited this enhancement by the type II receptors. Of the nine serine and threonine residues in ALK2, T203 was found to be critical for the enhancement by type II receptors. The T203 residue was conserved in all of the BMP type I receptors, and these residues were essential for intracellular signal transduction in response to ligand stimulation. The phosphorylation levels of the mutant ALK2 related to FOP were higher than those of wild-type ALK2 and were further increased by the presence of type II receptors. The phosphorylation levels of ALK2 were greatly reduced in mutants carrying a mutation at T203, even in the presence of type II receptors. These findings suggest that the mutant ALK2 related to FOP is enhanced by BMP type II receptors via the T203-regulated phosphorylation of ALK2.
Bone morphogenetic proteins (BMPs) are members of the TGF-β family, which contains more than 35 members that control cell proliferation, differentiation, and death in various tissues (1–3). Among the members of this family, several BMPs and growth and differentiation factors (GDFs) have been shown to induce heterotopic bone formation in skeletal muscle tissue (4, 5). Other growth factors, including other members of the TGF-β family and certain hormones, do not show this bone-inducing activity in vivo. Treatment of C2C12 myoblasts with osteogenic BMPs in vitro inhibits their terminal differentiation into mature myocytes and induces the expression of typical osteoblastic phenotypes, such as high levels of alkaline phosphatase (ALP), PTH receptor, and osteocalcin (6). Although nonosteogenic members of the TGF-β family also inhibit myogenesis in C2C12 cells, they do not induce osteoblastic differentiation in these cells (6). Thus, C2C12 cells are used to examine the osteogenic and nonosteogenic activities of TGF-β family members in vitro.
The members of the TGF-β family transduce intracellular signaling via two types of transmembrane serine (Ser)/threonine (Thr) kinase receptors: type I and type II receptors (7). Osteogenic BMPs bind to four types of type I receptors [activin receptor-like kinase (ALK; ALK1, ALK2, ALK3/BMPR-IA, and ALK6/BMPR-IB)] and three types of type II receptors [BMPR-II, activin receptor, type II (ActR-II), and ActR-IIB] (8). The nonosteogenic members of the TGF-β family bind to different type I (ALK4, ALK5, and ALK7) and type II (TβR-II) receptors. ActR-II and ActR-IIB bind both osteogenic and nonosteogenic ligands, indicating that the type I receptors determine ligand-specific biological activities. Type II receptors are constitutively active kinases that directly phosphorylate type I receptors on Ser/Thr residues in their intracellular glycine- and serine-rich (GS) domains (9). The phosphorylated BMP type I receptors, in turn, phosphorylate downstream effectors, such as Smad-1, Smad5, Smad8 (also known as Smad9), and p38 MAPK. The phosphorylated Smad proteins form a heteromeric complex with Smad4 and regulate the transcription of early BMP-responsive genes, such as Id1, Id2, Id3, and BIT-1 (10–12). Thus, a luciferase reporter driven by a BMP-responsive element in the Id1 gene is able to specifically detect BMP signaling in vitro (10).
Fibrodysplasia ossificans progressiva (FOP; online inheritance in man 135100) is a rare hereditary disorder characterized by postnatal progressive heterotopic ossification in the skeletal muscle. The ACVR1 gene on chromosome 2q23–24 encodes ALK2, a BMP type I receptor that has been identified as the gene responsible for both familial and sporadic cases of FOP (13). Typical FOP patients exhibit congenital malformation of the great toes at birth and heterotopic ossification beginning in early childhood (14, 15). Acute heterotopic ossification is induced by muscle injury, such as through accidental trauma, biopsies, or surgical treatment, in typical FOP cases. These patients present a substitution mutation from arginine to histidine at codon 206 (p.R206H) in the GS domain of ALK2 (13). Overexpression of ALK2(R206H) in vitro induces the phosphorylation of Smad1 and Smad5 and activates BMP signaling without ligand stimulation, suggesting that FOP represents the first known case of a natural gain-of-function mutation in the TGF-β family of receptors (16). More than 10 types of mutations in ALK2 have been identified in patients with FOP, and these variations result in different clinical features (17, 18). One of these mutations, a p.G325A mutation in ALK2, was identified in a patient who showed late-onset heterotopic ossification in adulthood without heterotopic ossification after muscle injury (19). Recently several novel mutations in ALK2 have been identified in patients with congenital heart defects (20, 21). In contrast to FOP, these mutant ALK2 showed less activity than wild type in vitro and in vivo, suggesting that they are loss-of-function mutations (20, 21)
In the present study, we examined the molecular mechanisms of signal activation by a typical ALK2 mutant (containing R206H) and a variant ALK2 mutant (containing G325A). Both of these mutant ALK2 proteins were enhanced by BMP type II receptors in a kinase activity-dependent manner. Analysis of a series of substitution mutations at Ser/Thr residues in the GS domain of ALK2 identified the Thr residue at amino acid 203 as the crucial residue for the type II receptor-dependent activation of ALK2. This Thr residue is conserved in all BMP type I receptors and is essential for intracellular signal transduction in response to ligand stimulation.
Materials and Methods
Plasmids
The V5-tagged human wild-type, R206H, G356D, L196P, and Q207D ALK2 constructs used in this study were described previously (16, 22, 23). Mutant ALK2 constructs such as G325A, R202I, PF197–8L, Q207E, R258S, G328R, G328W, G328E, and R375P were generated via a standard PCR technique with Prime Star HS DNA polymerase (TaKaRa) using a plasmid carrying V5-tagged wild-type human ALK2 as a template. Murine ActR-IIA, ActR-IIB, and TβR-II cDNAs were amplified through RT-PCR. Human BMPR-II was kindly provided by Dr K. Miyazono (University of Tokyo, Tokyo, Japan). In accordance with previous studies, we introduced alanine and valine mutations at serine and threonine residues in the GS domain (9, 24): the 9AV, 8AV, 7AV, 2AV, T203V, and T209V mutations were introduced into human ALK2(R206H) and ALK2(G325A) via PCR. Murine ALK1 and ALK3 cDNAs were cloned from C2C12 cells, and murine ALK6 was cloned from cartilage through RT-PCR. The T197V, T229V, and T199V mutations were introduced through PCR. The primer sequences used in these assays are indicated in Supplemental Table 1. Each cDNA was cloned into the pcDEF3 expression vector, which was kindly provided by Dr J. A. Langer (25). All of the final constructs were confirmed via DNA sequencing using an ABI3500 genetic analyzer (Applied Biosystems). The new version of Id1WT4F-luc in pGL4.26 used in this work (Promega), in which firefly luciferase is driven by four copies of a BMP-responsive element in the human Id1 gene (10), was described previously (23).
Cell culture, transfection, ALP assay, and luciferase reporter assay
Murine C2C12 myoblasts were maintained in DMEM containing 15% fetal bovine serum (6). C2C12 cells were plated at a density of 5 × 103 cells/well in a 96-well plate 1 day before each transfection. Human embryonic kidney (HEK) 293A cells maintained in DMEM containing 10% fetal bovine serum were inoculated at a density of 1 × 104 cells/well in a 96-well plate treated with 1.0 ng/mL GDF2/BMP9 (Peprotech), 10 ng/mL BMP7 (Miltenyi Biotec), 10 ng/mL BMP4 (R&D Systems) or 10 ng/mL GDF5 (Peprotech). The cells were transfected with 200 ng of plasmid DNA using 0.5 μL of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, with the additional medium change to fresh growth medium after 2.5 hours. The ALP activity, a marker of osteoblast differentiation, was measured using 1 mg/mL p-nitrophenylphosphate, 100 mM diethanolamine, and 1 mM MgCl2 for 60 minutes at room temperature. The reactions were terminated by adding NaOH to a final concentration of 1 M, and the absorbance was measured at 405 nm (16, 26). The luciferase reporter assay was performed using Id1WT4F-luc (10) and phRL-SV40 (Promega) with the Dual-Glo luciferase assay system (Promega) according to the manufacturer's instructions (10, 27).
Western blot analysis
Whole-cell extracts were prepared using lysis buffer (10 mM Tris-HCl, pH 7.8; 1% Triton X-100; and 1× protease inhibitor cocktail [Roche Diagnostics]). The extracts were then separated via sodium dodecyl sulfate-containing PAGE and transferred to polyvinyl difluoride membranes. Specific proteins were detected by immunoblotting using antibodies targeting the following proteins: phosphorylated Smad1/5 (rabbit monoclonal antibody; Cell Signaling Technology), V5-tag (mouse monoclonal antibody, clone V5005; Nacalai Tesuque), FLAG-tag (mouse monoclonal antibody, clone M2; Sigma), Smad1 (rabbit monoclonal antibody; Cell Signaling Technology), and tubulin (rabbit polyclonal antibody; Cell Signaling Technology). The target proteins were detected using a horseradish peroxidase-conjugated antimouse or antirabbit IgG antibody (Jackson ImmunoResearch Laboratories). Chemiluminescence was detected using the ChemiDoc XRS+ system (Bio-Rad Laboratories). To determine the levels of phosphorylated ALK2, we used a Phos-tag reagent (Wako Chemicals) in SDS-PAGE analysis, according to the manufacturer's instructions (28–31). Briefly, 7.5% polyacrylamide gels containing 75 μM Phos-tag and 100 μM MnCl2 were used to separate phosphorylated and unphosphorylated ALK2 through SDS-PAGE, followed by a Western blot analysis with an antibody against the V5-tag. The amounts of phosphorylated and unphosphorylated ALK2 were determined using ChemiDoc XRS+ and are expressed as the percentage of phosphorylated ALK2 among total (phosphorylated and unphosphorylated) ALK2.
Statistical analysis
Comparisons were performed using unpaired ANOVA with a Tukey-Kramer post hoc test and a Student's t test. The results are expressed as the mean ± SD (n = 3). Statistical significance is indicated (*, P < .05, or **, P < .01).
Results
Overexpression of ALK2(G325A) induces Smad1/5-dependent BMP signaling but more weakly than does ALK2(R206H)
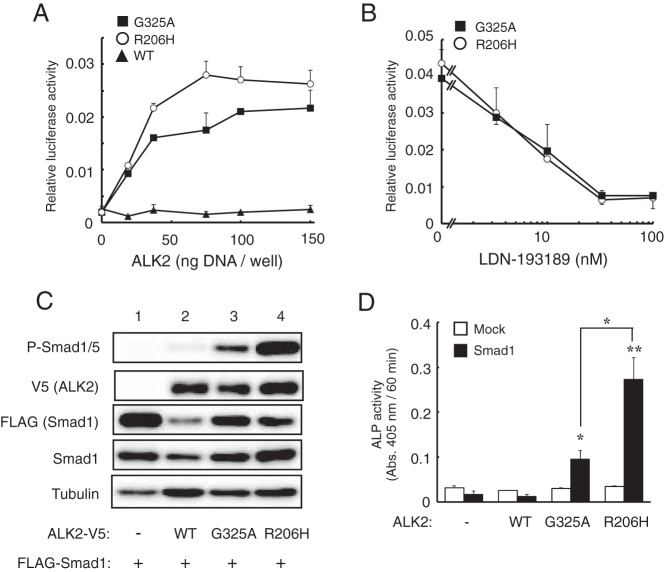
First, we examined the biological activity of ALK2(G325A), which was identified in a FOP patient showing late-onset heterotopic ossification (19). Similar to ALK2(R206H), the overexpression of ALK2(G325A) in C2C12 cells activated a BMP-specific luciferase reporter, Id1WT4F-luc, in a dose-dependent manner (Figure 1A). The luciferase activity induced by ALK2(G325A) was lower than that induced by ALK2(R206H) and was inhibited by the presence of LDN-193189, a specific inhibitor of BMP type I receptor kinases (Figure 1, A and B). Indeed, the levels of phosphorylated Smad1/5 were increased by coexpression of ALK2(G325A) with FLAG-Smad1, but the resultant levels were lower than those induced by ALK2(R206H) (Figure 1C). ALK2(G325A) and ALK2(R206H) induced ALP activity in C2C12 cells in the presence of exogenous Smad1, but the detected ALP activity was lower in ALK2(G325A)-expressing cells than in ALK2(R206H)-expressing cells (Figure 1D). Wild-type ALK2 did not induce BMP activity in these experiments (Figure 1, A, C, and D). These findings indicate that the kinase activity of ALK2(G325A) is activated but is weaker than ALK2(R206H).
Figure 1. Overexpression of ALK2(G325A) in C2C12 cells induces BMP activity via the phosphorylation of Smad1/5.
A, BMP-specific luciferase reporter activity induced by mutant ALK2. C2C12 cells were cotransfected with increasing amounts of wild-type ALK2, ALK2(G325A) or ALK2(R206H) and a BMP-specific reporter plasmid, Id1WT4F-luc. After 24 hours, the activities of firefly and renilla luciferase were determined. The data are expressed as the mean ± SD (n = 3). B, Inhibition of mutant ALK2 activity by a kinase inhibitor of BMP receptors. C2C12 cells transfected with ALK2 and the Id1WT4F-luc reporter were treated overnight with or without increasing amounts of LDN-193189 (0–100 nM). The data are expressed as the mean ± SD (n = 3). C, Western blot analysis of phosphorylated Smad1/5. C2C12 cells were cotransfected with WT or mutant V5-ALK2 and FLAG-Smad1. Whole-cell lysates were analyzed with antibodies against phospho-Smad1/5, V5-tag, FLAG-tag, Smad1, and tubulin. D, ALP activity induced by the cooperative activity of ALK2 and Smad1. C2C12 cells were cotransfected with WT or mutant ALK2 and Smad1. After 3 days, ALP activity was determined by measuring the absorbance at 405 nm. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 vs mock-transfected cells (ANOVA).
BMPR-II and ActR-IIB enhance the kinase activity of mutant ALK2
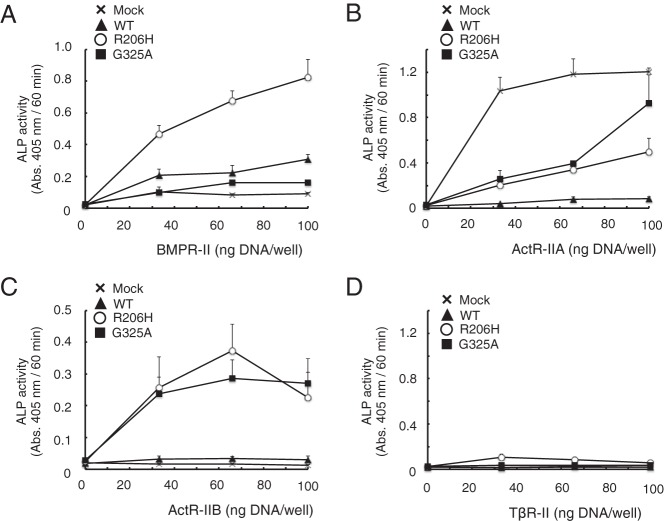
Within the TGF-β family, the kinase activity of type I receptors is activated by type II receptors (15, 32). Therefore, we examined the effect of each type II receptor (such as BMPR-II, ActR-IIA, ActR-IIB, and TβR-II) on the activity of mutant ALK2 by evaluating the induction of ALP activity. Increasing amounts of BMPR-II induced ALP activity in the presence of ALK2(R206H) but not ALK2(WT) or ALK2(G325A) (Figure 2A). Overexpression of ActR-IIA alone induced ALP activity in C2C12 cells, and this activity was decreased by the coexpression of wild-type or mutant ALK2 (Figure 2B). ActR-IIB increased ALP activity in a cooperative manner with either ALK2(R206H) or ALK2(G325A) but not ALK2(WT) (Figure 2C). TβR-II did not induce ALP activity in the absence or presence of any form of ALK2 (Figure 2D). These data suggest that BMPR-II and ActR-IIB are involved in the activation of BMP signaling by ALK2(G325A) and ALK2(R206H), which are ALK2 mutants found in FOP patients.
Figure 2. BMPR-II and ActR-IIB, but not ActR-IIA or TβR-II, enhance ALP activity in C2C12 cells in cooperation with mutant ALK2.
A–D, BMPR-II and ActR-IIB induced ALP activity in cooperation with mutant ALK2. C2C12 cells were cotransfected with 100 ng of WT ALK2 (closed triangles), ALK2(R206H) (open circles), ALK2(G325A) (closed squares), or a mock vector (x) and with increasing amounts (0–100 ng) of BMPR-II (A), ActR-IIA (B), ActR-IIB (C), or TβR-II (D). The total amount of transfected DNA was adjusted to 200 ng/well using an empty vector. ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3).
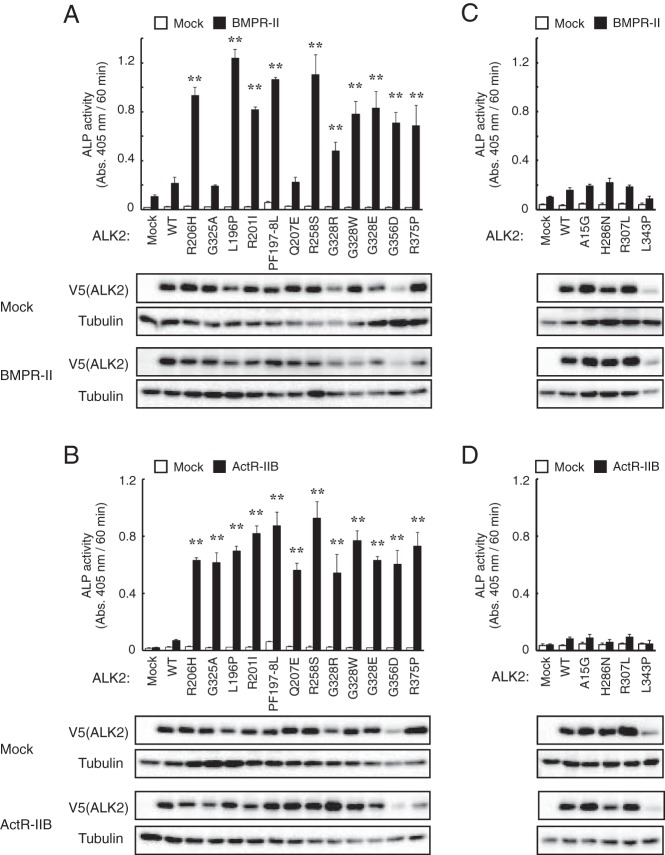
Recently novel mutations in ALK2, including A15G, H286N, R307L, and L343P, were identified in patients with serious congenital heart defects (20, 21). In contrast to the mutant ALK2 in FOP, these ALK2 mutants showed lower activity than that of wild-type (20, 21). We further examined the stimulatory effects of BMPR-II and ActR-IIB on other types of ALK2 mutants identified in FOP or heart disease patients, including L196P, R201I, PF197-8L, Q207E, R258S, G328R, G328W, G328E, G356D, and R375P or A15G, H286N, R307L, and L343P, respectively. ALP activity in C2C12 cells was synergistically increased by the coexpression of BMPR-II and all of the ALK2 mutants identified in FOP patients (more than 4-fold compared with BMPR-II alone), except for G325A and Q207E (less than 3-fold) (Figure 3A). ActR-IIB also increased ALP activity upon the coexpression with each of the ALK2 mutants identified in FOP patients (more than 20-fold compared with ActR-IIB alone) (Figure 3B). However, neither BMPR-II nor ActR-IIB induced ALP activity in C2C12 cells when coexpressed with any of the ALK2 mutants associated with heart disease, and even their protein levels were equivalent to wild-type ALK2 and other ALK2 mutants identified in FOP (Figure 3, C and D). These findings suggest that the pathological phenotypes of FOP are caused by the synergistic activation of BMP signaling by mutant ALK2 and BMPR-II or ActR-IIB, whereas heart disease is caused by a reduction in BMP signaling via ALK2.
Figure 3. BMPR-II and ActR-IIB enhance mutant ALK2 associated with FOP but not mutant ALK2 associated with heart disease.
A–D, The effects of BMPR-II and ActR-IIB on ALK2 mutants identified in FOP and heart disease patients were examined. The C2C12 cells were cotransfected with 100 ng of an ALK2 vector carrying mutations identified in FOP (A and B) or heart disease patients (C and D) and 100 ng of BMPR-II (A and C) or ActR-IIB (B and D). ALP activity was determined on day 3. The expression levels of mutant ALK2 were determined through a Western blot analysis using antibodies against V5-tag and tubulin. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 vs BMPR-II or ActR-IIB transfected cells (ANOVA).
The kinase activity of type II receptors is essential for the activation of ALK2(G325A) and ALK2(R206H) but not ALK2(Q207D)
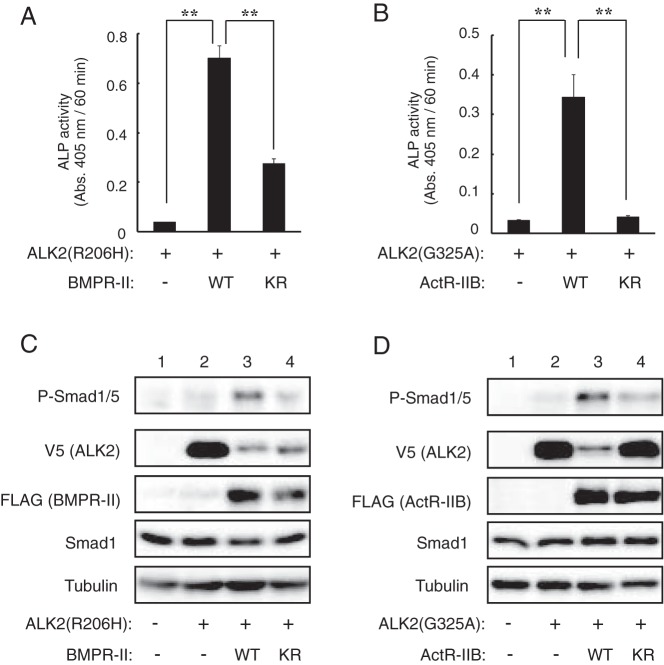
Because type II receptors act as Ser/Thr kinases of type I receptors, we examined the effect of kinase activity-deficient type II receptors (KR) on ALK2 mutants. Coexpression of BMPR-II(KR) with ALK2(R206H) induced ALP activity but at significantly lower levels than that induced by wild-type BMPR-II (Figure 4A). ActR-IIB(KR) failed to induce ALP activity in the presence of ALK2(G325A) (Figure 4B). The levels of phosphorylated Smad1/5 were also lower in cells transfected with BMPR-II(KR) or ActR-IIB(KR) than in cells transfected with wild-type BMPR-II or ActR-IIB (Figure 4, C and D). These findings suggest that the enhancement of mutant ALK2 by type II receptors is a phosphorylation-dependent event. We observed that the protein levels of ALK2(R206H) and ALK2(G325A) detected by V5 were decreased by the coexpression of type II receptors due to an unknown mechanism. The phosphorylation level of Smad1/5 appeared to be independent of the protein levels of ALK2 because both active ALK2 and inactive ALK2 were decreased in the presence of type II receptors (see below).
Figure 4. The kinase activity of BMPR-II and ActR-IIB is essential for the enhancement of mutant ALK2.
A–D, Kinase activity-deficient type II receptors failed to enhance mutant ALK2. ALK2(R206H) (A) and ALK2(G325A) (B) were cotransfected in C2C12 cells with WT or KR BMPR-II (A) and ActR-IIB (B), respectively. ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 (ANOVA). Whole-cell lysates prepared from the transfected cells were analyzed via a Western blot analysis using antibodies against phospho-Smad1/5, V5-tag (ALK2), FLAG-tag (type II receptors), Smad1, and tubulin (C and D).
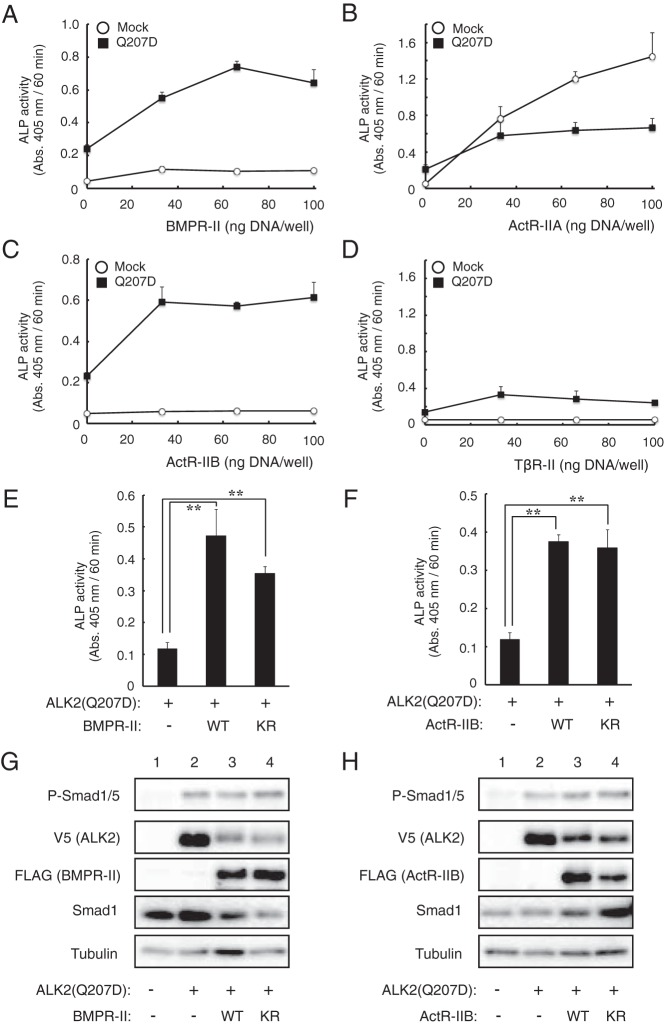
ALK2(Q207D) was created through genetic engineering and is a constitutively activated form of ALK2. However, this mutation has not been identified in FOP patients (33, 34). Because the ALK2 mutants identified in FOP were enhanced by BMPR-II and ActR-IIB, we examined the effect of type II receptors on the constitutively active ALK2(Q207D) mutant in our assay system. Overexpression of ALK2(Q207D) alone induced weak ALP activity in C2C12 cells (Figure 5, A–D, 0 ng DNA/well of type II receptor), and coexpression of ALK2(Q207D) and BMPR-II or ActR-IIB, but not ActR-IIA or TβR-II, further increased the ALP activity (Figure 5, A–D). In contrast to ALK2(R206H) and ALK2(G325A), ALK2(Q207D) still induced high levels of ALP activity cooperatively with the KR mutants of BMPR-II and ActR-IIB (Figure 5, E and F). An increase in phosphorylated Smad1/5 was observed even in cells overexpressing ALK2(Q207D) alone, and that was constant in the presence of wild-type and KR mutant type II receptors (Figure 5, G and H). These findings suggest that the activity of ALK2(Q207D) is also enhanced by BMP type II receptors, but this activity is independent of the kinase activity of type II receptors, in contrast to the findings for ALK2(G325A) and ALK2(R206H).
Figure 5. ALK2(Q207D) is activated by BMPR-II and ActR-IIB in a kinase activity-independent manner.
A–D, The effect of type II receptors on ALK2(Q207D) was examined. ALK2(Q207D) was cotransfected into C2C12 cells with increasing amounts of BMPR-II (A), ActR-IIA (B), ActR-IIB (C), and TβR-II (D). ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). E and F, ALK2(Q207D) was cotransfected in C2C12 cells with WT and KR BMPR-II (E) and ActR-IIB (F), and ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 (ANOVA). G and H, Whole-cell lysates prepared from the transfected cells were analyzed through a Western blot analysis with antibodies against phospho-Smad1/5, V5-tag (ALK2), FLAG-tag (type II receptors), Smad1, and tubulin.
Effects of substitution mutations in the GS domain of ALK2 on the enhancement of signaling by BMP type II receptors
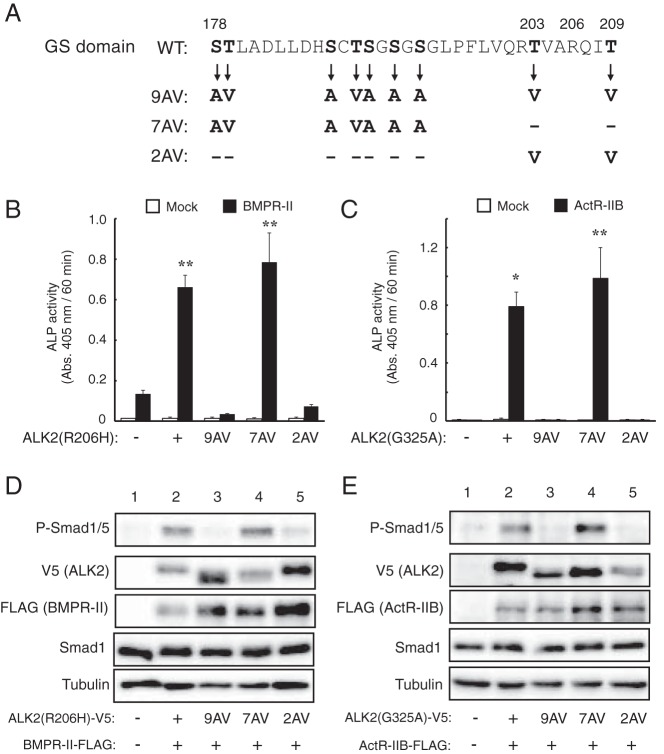
The GS domain of type I receptors has been shown to be phosphorylated by the Ser/Thr kinase activity of type II receptors (9). The nine Ser and Thr residues in the GS domain of ALK2 were substituted with alanine (Ala) and valine (Val) in various combinations in the ALK2(R206H) and ALK2(G325A) mutants (Figure 6A). The activity of each AV mutant ALK2 construct was examined via coexpression with BMPR-II or ActR-IIB in C2C12 cells. The presence of the 9AV mutation in both ALK2(R206H) and ALK2(G325A) almost completely blocked the induction of ALP activity in a cooperative manner with BMPR-II and ActR-IIB (Figure 6, B and C). In contrast, the 7AV mutation, in which both T203 and T209 were left intact, did not block the increase in ALP activity in ALK2(R206H) and ALK2(G325A) in cooperation with the type II receptors (Figure 6, B and C). Moreover, the 2AV mutation, in which only T203 and T209 were mutated (of the nine Ser/Thr residues), blocked the ALP activity of ALK2(R206H) and ALK2(G325A) to a similar extent to the 9AV mutation (Figure 6, B and C). The phosphorylation of Smad1/5 induced by the cooperative action of the mutant ALK2 and type II receptors was also blocked by the 9AV and 2AV mutations but not the 7AV mutation (Figure 6, D and E). The mobility of the mutant ALK2 with the 9AV and 7AV mutations was slightly faster than that of the parental ALK2 and 2AV mutant (the second blots in Figure 6, D and E). These findings suggest that T203 and/or T209 in the ALK2 are crucial for the enhancement of signaling by the type II receptors.
Figure 6. Functional analysis of the role of the ALK2 GS domain in type II receptor-dependent enhancement.
A, Amino acid sequences of the GS domains of WT and mutant ALK2. Of the nine Ser and Thr residues (in bold) present in WT ALK2, nine, seven, and two residues were mutated to Ala and Val residues in the 9AV, 7AV, and 2AV constructs, respectively. These mutations were introduced in ALK2(R206H) and ALK2(G325A), and their activity was analyzed. B and C, Cooperative induction of ALP activity in C2C12 cells by mutant ALK2 carrying mutations in the GS domain plus type II receptors. ALK2(R206H) (B) and ALK2(G325A) (C) carrying the 9AV, 7AV, and 2AV mutations (or empty vector) were cotransfected with BMPR-II (B) and ActR-IIB (C), respectively. ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 vs mock-transfected cells (Student's t test). D and E, Whole-cell lysates from cells transfected with the indicated ALK2 and BMPR-II (D) or ActR-IIB (E) constructs were analyzed via a Western blot analysis with antibodies against phospho-Smad1/5, V5-tag (ALK2), FLAG-tag (type II receptors), Smad1, and tubulin.
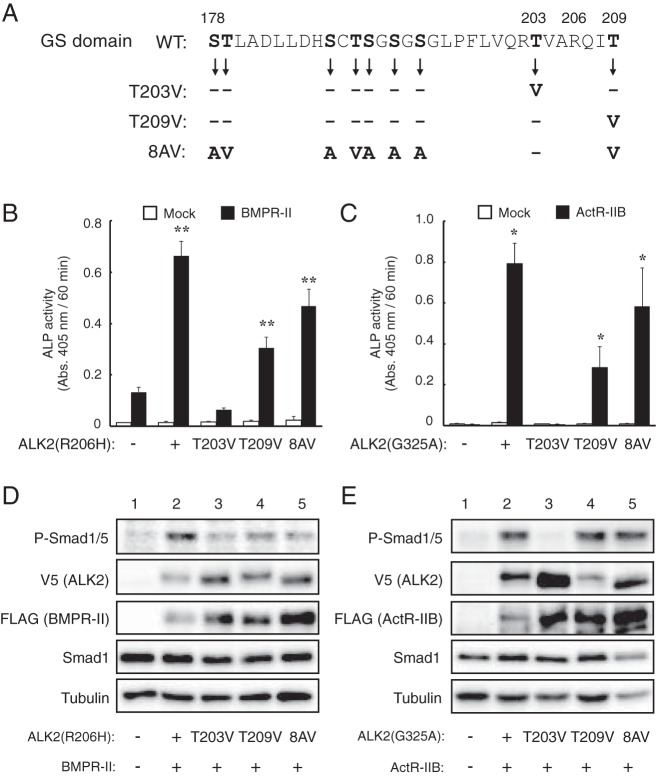
To examine the roles of T203 and T209 in ALK2, we mutated these residues individually in ALK2(R206H) and ALK2(G325A) and examined their activity in C2C12 cells (Figure 7A). As shown in Figure 7, B and C, the T203V mutation almost completely blocked the induction of ALP activity by both ALK2(R206H) and ALK2(G325A) in cooperation with type II receptors. In contrast, significant ALP activity was still induced by ALK2(R206H) and ALK2(G325A) carrying the T209V mutation in the presence of BMPR-II and ActR-IIB (Figure 7, B and C), suggesting that T203, but not T209, is crucial for the observed synergism with type II receptors. To examine this hypothesis, we constructed another ALK2 mutant carrying the 8AV mutation, in which eight Ser/Thr residues (excluding T203) are mutated to the Ala/Val residues (Figure 7A). As expected, both ALK2(R206H) and ALK2(G325A) carrying the 8AV mutation induced ALP activity in the presence of type II receptors (Figure 7, B and C). The T203 mutation inhibited the ALP activity induced by a cooperation of ALK2(Q207D) and type II receptors (data not shown). The levels of phosphorylated Smad1/5 induced by the mutant ALK2 and type II receptors were consistent with the levels of ALP activity, confirming that the Smad-dependent intracellular pathway induces osteoblastic differentiation in C2C12 cells (27) (Figure 7, D and E).
Figure 7. Thr203 of ALK2 is a crucial residue for the type II receptor-dependent enhancement of ALK2.
A, Amino acid sequences of the GS domains of WT and mutant ALK2. Of the nine Ser and Thr residues (in bold) present in WT ALK2, Thr203, Thr209, or the eight residues other than Thr203 were mutated to Ala and Val residues in the T203V, T209V, and 8AV mutants, respectively. These mutations were introduced in ALK2(R206H) and ALK2(G325A), and their activity was analyzed. B and C, Cooperative induction of ALP activity in C2C12 cells by type II receptors and mutant ALK2 constructs bearing mutations in the GS domain. ALK2(R206H) (B) and ALK2(G325A) (C) carrying the T203V, T209V, and 8AV mutations (or empty vector) were cotransfected with BMPR-II (B) and ActR-IIB (C), respectively. ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 vs mock-transfected cells (Student's t test). D and E, Whole-cell lysates from C2C12 cells transfected with the indicated ALK2 and BMPR-II (D) or ActR-IIB (E) constructs were analyzed via a Western blot analysis with antibodies against phospho-Smad1/5, V5-tag (ALK2), FLAG-tag (type II receptors), Smad1, and tubulin.
The conserved Thr residues in the GS domain of BMP type I receptors are crucial for signal transduction in response to ligand stimulation
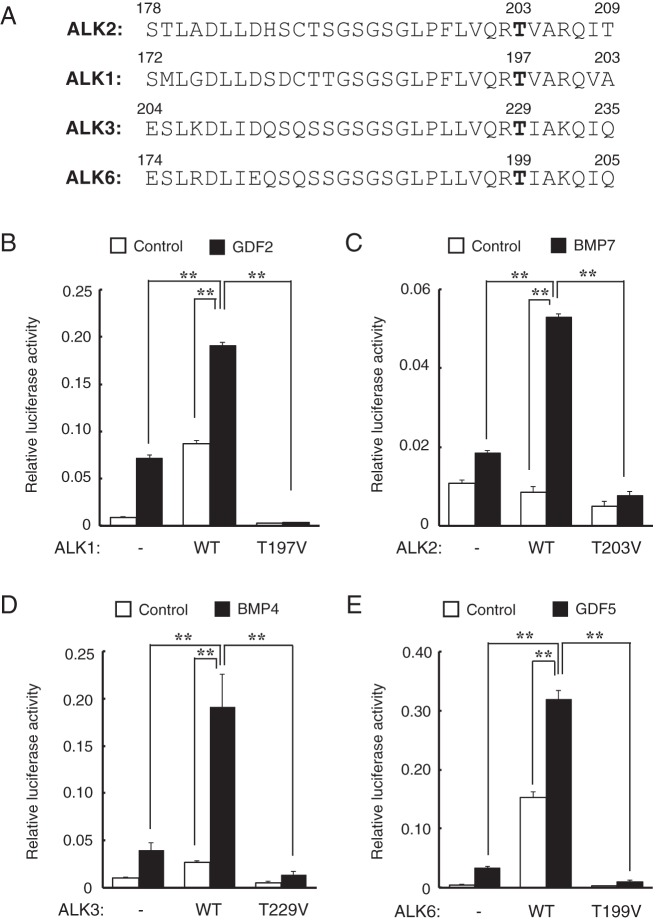
The Thr residue at position 203 in ALK2 is conserved in various species (Homo sapience, Mus musculus, Rattus norvegicus, Canis familiaris, Bos taurus, Gallus gallus, Xenopus laevis, Danio rerio, and Fugu rubripes, data not shown) and among all BMP type I receptors, such as ALK1, ALK3, and ALK6, at positions 197, 229, and 199, respectively (Figure 8A). Hence, these residues were mutated to Val residues, and the resulting constructs were overexpressed in HEK 293A cells to examine their roles in BMP/GDF-induced signal transduction. The cells overexpressing wild-type ALK1, ALK2, ALK3, and ALK6 showed increased BMP-specific Id1WT4F-luc activity in response to corresponding ligands such as GDF2/BMP9, BMP7, BMP4, and GDF5, respectively (Figure 8, B–E). However, cells overexpressing the TV mutant forms of ALK1, ALK2, ALK3, and ALK6 showed decreased luciferase activity in response to the ligand stimulation relative to cells expressing wild-type (WT) receptors and mock vector-transfected cells (Figure 8, B–E). These findings suggest that the conserved Thr residues in the GS domain of all BMP type I receptors are crucial for signal transduction in response to ligand stimulation.
Figure 8. The Thr residue at position 203 in ALK2 is conserved among BMP type I receptors, and these conserved Thr residues are crucial for ligand-induced signal transduction.
A, Alignment of the GS domains of BMP type I receptors human ALK2, murine ALK1, ALK3, and ALK6. The Thr residue at position 203 in ALK2 is conserved in all of the receptors; these residues are mutated to the Val residues in ALK1(T197V), ALK3(T229V), and ALK6(T199V). B–E, Effect of substitution mutations at positions of the conserved BMP type I receptor Thr residues on ligand-induced signaling. HEK 293A cells were cotransfected with WT or mutant type I receptors [T197V, T203V, T229V, and T199V in murine ALK1 (B), human ALK2 (C), murine ALK3 (D), and murine ALK6 (E), respectively] and a BMP-specific Id1WT4F-luc reporter plasmid. The cells were stimulated with 1.0 ng/mL GDF2/BMP9 (B), 10 ng/mL BMP7 (C), 10 ng/mL BMP4 (D) or 10 ng/mL GDF5 (E). Luciferase activity was measured on day 1. The data are expressed as the mean ± SD (n = 3). *, P < .05; **, P < .01 (ANOVA).
The T203 residue in ALK2 regulates the type II receptor-dependent phosphorylation of ALK2
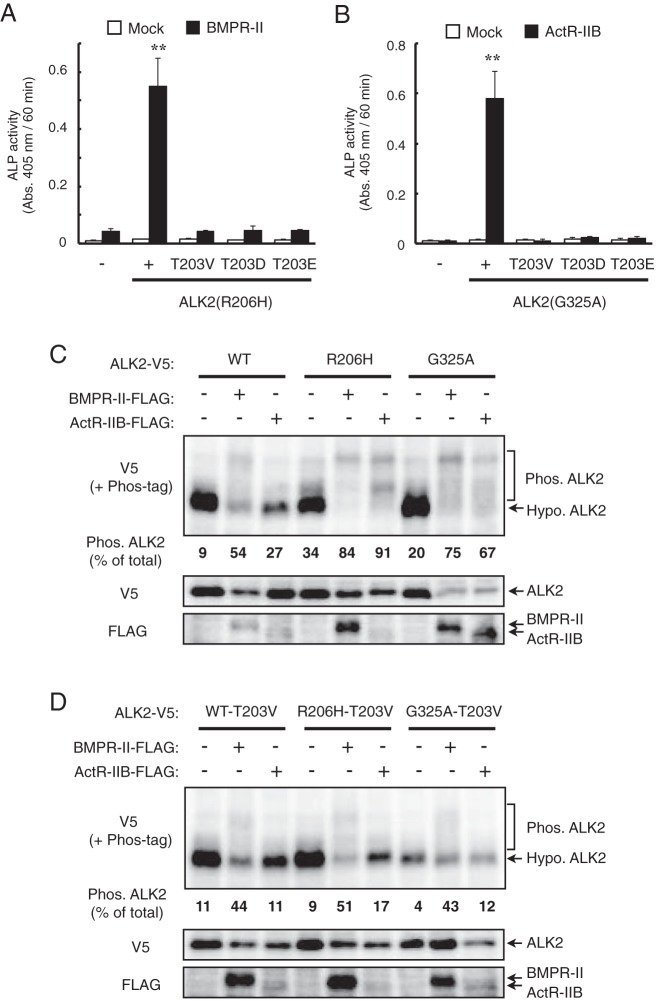
We examined whether the phosphorylation of T203 is involved in the enhancement of type II receptor-dependent BMP signaling by ALK2. To test this possibility, we created two additional mutations at T203, T203D and T203E, in which the threonine residue has been substituted with aspartic acid (D) and glutamic acid (E), respectively. This type of acidic amino acid substitution mutation has been reported to mimic the phosphorylation state of proteins (24, 27, 35). However, in contrast to unmutated T203, neither ALK2(R206H) nor ALK2(G325A) carrying the T203D or T203E mutation induced ALP activity in C2C12 cells, even in the presence of BMPR-II and ActR-IIB (Figure 9, A and B). Thus, both T203D and T203E were inactive, similar to T203V. These results suggest that T203 enhances BMP signaling in an unphosphorylated state, rather than in the presence of a phosphorylated threonine.
Figure 9. T203 in ALK2 regulates the type II receptor-dependent phosphorylation levels of ALK2.
A and B, The T203D and T203E ALK2 mutants did not induce ALP activity, even in the presence of type II receptors. C2C12 cells were cotransfected with ALK2(R206H) (A) or ALK2(G325A) (B), either without or with one of mutations indicated in the figure, and with BMPR-II (A) and ActR-IIB (B), respectively. ALP activity was determined on day 3. The data are expressed as the mean ± SD (n = 3). **, P < .01 vs mock-transfected cells (Student's t test). C and D, Phosphorylation levels of ALK2 without (C) and with (D) the T203V mutation. C2C12 cells were cotransfected with ALK2 wild type, R206H, and G325A with BMPR-II or ActR-IIB. Whole-cell lysates were separated in SDS-PAGE with and without a Phos-tag reagent followed by a Western blot analysis with antibodies against V5-tag (ALK2) and FLAG-tag (type II receptors). The amount of phosphorylated ALK2 was expressed as percentage of total (phosphorylated and hypophosphorylated) ALK2.
To further examine the role of T203 in type II receptor-dependent signal transduction, we analyzed the phosphorylation levels of wild-type ALK2 and the R206H and G325A mutants using a Phos-tag reagent (28, 29). The Phos-tag reagent allows us to distinguish the phosphorylated proteins as more slowly migrating bands in Western blot analysis compared with the unphosphorylated forms (30, 31). Both ALK2(R206H) and ALK2(G325A) showed higher basal phosphorylation levels than the wild-type ALK2 (Figure 9C). The phosphorylation levels of ALK2(R206H) and ALK2(G325A) were further increased by the coexpression with BMPR-II and ActR-IIB (Figure 9C). Moreover, the introduction of the T203V mutation into each form of ALK2 greatly reduced the phosphorylation levels of ALK2, even in the presence of type II receptors (Figure 9D), suggesting that the T203 residue regulates the type II receptor-dependent phosphorylation of ALK2.
Discussion
In the present study, we examined the molecular mechanisms of intracellular signal activation by several mutants of ALK2 identified in patients with FOP. Patients with typical FOP show progressive heterotopic ossification in childhood, and acute heterotopic ossification is induced after muscle trauma or virus infection (14, 15). A recurrent mutation in ALK2, R206H, has been identified in familial and sporadic cases of typical FOP (13). Recently a novel mutation in ALK2, G325A, was found in a patient with atypical FOP, in whom heterotopic ossification had not occurred in childhood or after muscle injury but started at age 47 years (19). This late-onset phenotype led us to examine the molecular mechanisms of signal activation by mutant ALK2. Although both ALK2 mutants activated Smad-dependent BMP signaling without adding ligands in vitro, the kinase activity of ALK2(G325A) was weaker than that of ALK2(R206H). We found that ALK2(R206H) was activated by BMPR-II or ActR-IIB, whereas ALK2(G325A) was activated only by ActR-IIB and not by BMPR-II. In contrast, ALK2 mutants found in heart disease patients were not activated by any type II receptor. Similar enhancement of mutant ALK2 observed in FOP has been reported in an experimental Drosophila model (36). Thus, it was suggested that the differences between ALK2 mutants with respect to sensitivity to BMP type II receptors may affect the clinical features of FOP, especially the onset of heterotopic ossification.
We previously reported that the bovine serum used in cell cultures contains active bovine BMP4, which induces BMP signaling and regulates the differentiation of the cells (26). Moreover, we and others (35, 37–39) have reported that C2C12 cells express the known type I (ALK1, ALK2, and ALK3/BMPR-IA) and type II (BMPR-II, ActR-IIA, and ActR-IIB) receptors. Thus, the endogenous BMP receptors may affect the activity of exogenously expressed receptors, including ALK2 mutants. Indeed, the ALP activity induced by the overexpression of ActR-IIA in C2C12 cells was inhibited by a specific chemical inhibitor of BMP type I receptors, LDN-193189 (data not shown), suggesting that ActR-IIA activates endogenous type I receptor(s) in the cells. ALK3/BMPR-IA is a candidate type I receptor responsible for signal transduction by ActR-IIA in C2C12 cells because it is expressed abundantly and is functional in the cells (37). The dominant-negative effect of ALK2 on the ALP activity induced by ActR-IIA may be caused by a competition between ALK2 and ALK3/BMPR-IA for ActR-IIA. In the present study, we found that BMPR-II and ActR-IIB are potential type II receptors responsible for the activation of the ALK2 mutant related to FOP. Thus, interactions with other types of BMP receptors, ligands, and antagonists in the microenvironment around the cells influence the biological activity of mutant ALK2.
We also observed that the enhancement of mutant ALK2 by type II receptors was dependent on the kinase activity of the receptors. ALK2(Q207D) was created by genetic engineering and is a constitutively active form of ALK2 that activates BMP signaling without the addition of ligands (24). Although the Q207D mutation has not been identified in patients with FOP, transgenic mice carrying this mutation have been used as an in vivo model of FOP (40–42). We found that ALK2(Q207D) was enhanced by the coexpression of BMPR-II or ActR-IIB. However, this effect was independent of the kinase activity of type II receptors; the ALK2(Q207D)-induced phosphorylation levels of Smad1/5 were constant in the presence of both wild-type and KR mutant BMPR-II or ActR-IIB. Our findings were supported by a recent study from Haupt et al (43), in which the activity of FOP-related ALK2(Q207E) was inhibited by adding noggin, an antagonist of BMP ligands, whereas the constitutively active ALK2(Q207D) mutant was not inhibited in the model. These findings suggest that we must be careful when evaluating the phenotypes of ALK2(Q207D) mice because this mutant receptor has different characteristics than the mutant receptors related to FOP. Moreover, it was suggested that a new model based on the ALK2 mutant related to FOP will be needed to establish an in vivo model reflecting the phenotypes of FOP.
We identified T203 in ALK2 as the crucial residue for the activation of intracellular signal transduction in both the mutant ALK2 associated with FOP and wild-type BMP type I receptors in response to ligand stimulation. There are some potential molecular mechanisms that may describe the roles of T203 in signal transduction. First, T203 may act as a site that is directly phosphorylated by the type II receptors because the ALK2 mutant was enhanced in a type II receptor kinase activity-dependent manner. However, the T203D and T203E mutants, which mimic the form of the protein phosphorylated at T203, did not induce ALP activity in C2C12 cells, even in the presence of type II receptors. These mutants were inactive, similar to the T203V mutant. These findings suggested that T203 enhances type II receptor-dependent signal transduction in an unphosphorylated form, rather than as a phosphorylated Thr residue.
Alternatively, T203 could regulate ALK2 phosphorylation at other Thr and/or Ser residues in the GS domain. A similar regulation of phosphorylation levels in the GS domain has been reported for another type I receptor, TβR-I (24). We found that ALK2(R206H) and ALK2(G325A) were phosphorylated at higher basal levels than wild-type ALK2, and these levels were further increased by the presence of type II receptors. However, the introduction of T203V mutation greatly reduced the type II receptor-dependent phosphorylation of ALK2. These findings suggest that the T203 residue is crucial for regulation of the type II receptor-dependent phosphorylation of ALK2.
It is noteworthy that the phosphorylation levels of ALK2(G325A) were increased by the presence of not only ActR-IIB but also BMPR-II, although this increase did not enhance the BMP signaling. It is possible that BMPR-II failed to phosphorylate a critical residue required for such enhancement, although it phosphorylated other residues. Moreover, the 8AV mutant of ALK2, in which all of the Ser/Thr residues except T203 were substituted with Ala/Val residues, was still enhanced by the type II receptors at levels that were equivalent to the wild-type ALK2. Taking these findings together, it was suggested that the critical residue for enhancement by the type II receptors is located outside the GS domain of ALK2. Further studies are required to elucidate the functional role of ALK2 T203 in type II receptor-dependent signal transduction.
In conclusion, the mutant forms of ALK2 found in patients with typical and atypical FOP are enhanced by BMPR-II or ActR-IIB. The sensitivity of the ALK2 mutants to BMP type II receptors may affect the clinical features of individuals with FOP. The T203 residue of ALK2 was shown to be crucial for the enhancement of ALP activity by the type II receptors. The conserved Thr resides were demonstrated to be essential for intracellular signal transduction by all of the BMP type I receptors in response to ligand stimulation. It was suggested that the T203 residue enhances type II receptor-dependent signal transduction by regulating the phosphorylation levels of ALK2.
Acknowledgments
We thank Drs H. Kosako and T. Nikawa of Tokushima University and the members of the Division of Pathophysiology, Research Center for Genomic Medicine, Saitama Medical University, for their valuable discussions.
Author contributions include the following: M.F., S.O., and T.K. designed the experiments. S.O., K.O., S.T., S.K., N.S., and T.K. conducted the experiments. M.F., S.O., A.M., T.M., and T.K. performed the experiments and analyzed the data. M.F., S.O., K.O., A.M., and T.K. wrote the paper.
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grants 25293326, 25861339, and 24592278 and a grant-in-aid from the Support Project for the Formation of a Strategic Center in a private university from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grants 25293326, 25861339, and 24592278 and a grant-in-aid from the Support Project for the Formation of a Strategic Center in a private university from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
- ActR-II
- activin receptor, type II
- Ala
- alanine
- ALK
- activin receptor-like kinase
- ALP
- alkaline phosphatase
- BMP
- bone morphogenetic protein
- D
- aspartic acid
- E
- glutamic acid
- FOP
- fibrodysplasia ossificans progressiva
- GDF
- growth and differentiation factor
- GS
- glycine and serine rich
- KR
- kinase activity-deficient type II receptor
- Ser
- serine
- Thr
- threonine
- TβR-II
- TGF-β type II receptor
- Val
- valine
- WT
- wild type.
References
- 1. Katagiri T, Suda T, Miyazono K. The bone morphogenetic proteins. In: Derynck R, Miyazono K, eds. TGF-β Family. New York: Cold Spring Harbor Press; 2008:121–149. [Google Scholar]
- 2. Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. [DOI] [PubMed] [Google Scholar]
- 3. Nakaoka T, Gonda K, Ogita T, et al. . Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest. 1997;100:2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wozney JM, Rosen V, Celeste AJ, et al. . Novel regulators of bone formation: molecular clones and activities. Science. 1998;242:1528–1534. [DOI] [PubMed] [Google Scholar]
- 5. Kang Q, Sun MH, Cheng H, et al. . Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. [DOI] [PubMed] [Google Scholar]
- 6. Katagiri T, Yamaguchi A, Komaki M, et al. . Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Attisano L, Cárcamo J, Ventura F, Weis FM, Massagué J, Wrana JL. Identification of human activin and TGFβ type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. [DOI] [PubMed] [Google Scholar]
- 8. Muller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586:1846–1859. [DOI] [PubMed] [Google Scholar]
- 9. Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. [DOI] [PubMed] [Google Scholar]
- 10. Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. [DOI] [PubMed] [Google Scholar]
- 11. Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. [DOI] [PubMed] [Google Scholar]
- 12. Shin M, Ohte S, Fukuda T, et al. . Identification of a novel bone morphogenetic protein (BMP)-inducible transcript, BMP-inducible transcript-1, by utilizing the conserved BMP-responsive elements in the Id genes. J Bone Miner Metab. 2013;31:34–43. [DOI] [PubMed] [Google Scholar]
- 13. Shore EM, Xu M, Feldman GJ, et al. . A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan FS, Shen Q, Lounev V, et al. . Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP). J Bone Miner Metab. 2006;26:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katagiri T, Tsukamoto S. The unique activity of bone morphogenetic proteins in bone: a critical role of the Smad signaling pathway. Biol Chem. 2013;394:703–714. [DOI] [PubMed] [Google Scholar]
- 16. Fukuda T, Kohda M, Kanomata K, et al. . Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2008;284:7149–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katagiri T. Heterotopic bone formation induced by bone morphogenetic protein signaling: fibrodysplasia ossificans progressiva. J Oral Biosci. 2010;52:33–41. [Google Scholar]
- 18. Katagiri T. Recent topics in fibrodysplasia ossificans progressiva. J Oral Biosci. 2012;54:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whyte MP, Wenkert D, Demertzis JL, DiCarlo EF, Westenberg E, Mumm S. Fibrodysplasia ossificans progressiva: middle-age onset of heterotopic ossification from a unique missense mutation (c. 974G>C, p.G325A) in ACVR1. J Bone Miner Res. 2012;27:729–737. [DOI] [PubMed] [Google Scholar]
- 20. Smith KA, Joziasse IC, Chocron S, et al. . Dominant-negative ALK2 allele associates with congenital heart defects. Circulation. 2009;119:3062–3069. [DOI] [PubMed] [Google Scholar]
- 21. Joziasse IC, Smith KA, Chocron S, et al. . ALK2 mutation in a patient with Down's syndrome and a congenital heart defect. Eur J Hum Genet. 2011;19:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuda T, Kanomata K, Nojima J, et al. . A unique mutation of ALK2, G356D, found in a patient with fibrodysplasia ossificans progressiva is a moderately activated BMP type I receptor. Biochem Biophys Res Commun. 2008;377:905–909. [DOI] [PubMed] [Google Scholar]
- 23. Ohte S, Shin M, Sasanuma H, et al. . A novel mutation of ALK2, L196P, found in the most benign case of fibrodysplasia ossificans progressiva activates BMP-specific intracellular signaling equivalent to a typical mutation, R206H. Biochem Biophys Res Commun. 2011;407:213–218. [DOI] [PubMed] [Google Scholar]
- 24. Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;10:2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldman LA, Cutrone EC, Kotenko SV, Krause CD, Langer JA. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. Biotechniques. 1996;21:1013–1015. [DOI] [PubMed] [Google Scholar]
- 26. Kodaira K, Imada M, Goto M, et al. . Purification and identification of a BMP-like factor from bovine serum. Biochem Biophys Res Commun. 2006;345:1224–1231. [DOI] [PubMed] [Google Scholar]
- 27. Nojima J, Kanomata K, Takada Y, et al. . Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J Biol Chem. 2010;285:15577–15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;4:749–757. [DOI] [PubMed] [Google Scholar]
- 29. Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc. 2009;10:1513–1521. [DOI] [PubMed] [Google Scholar]
- 30. Iguchi M, Kujuro Y, Okatsu K, et al. . Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J Biol Chem. 2013;30:22019–22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okatsu K, Oka T, Iguchi M, et al. . PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun. 2012;3:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katagiri T, Osawa K, Tsukamoto S, Fujimoto M, Miyamoto A, Mizuta T. Bone morphogenetic protein-induced heterotopic bone formation: what have we learned from the history of a half century? Doi:10.1016/j.jdsr.2014.09.004. [Google Scholar]
- 33. Suzuki A, Kaneko E, Ueno N, Hemmati-Brivanlou A. Regulation of epidermal induction by BMP2 and BMP7 signaling. Dev Biol. 1997;189:112–122. [DOI] [PubMed] [Google Scholar]
- 34. Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O'Keefe RJ. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18:1593–1604. [DOI] [PubMed] [Google Scholar]
- 35. Akiyama S, Katagiri T, Namiki M, et al. . Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp Cell Res. 1997;2:362–369. [DOI] [PubMed] [Google Scholar]
- 36. Le VQ, Wharton KA. Hyperactive BMP signaling induced by ALK2(R206H) requires type II receptor function in a Drosophila model for classic fibrodysplasia ossificans progressiva. Dev Dyn. 2012;241:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Namiki M, Akiyama S, Katagiri T, et al. . A kinase domain-truncated type I receptor blocks bone morphogenetic protein-2-induced signal transduction in C2C12 myoblasts. J Biol Chem. 1997;35:22046–22052. [DOI] [PubMed] [Google Scholar]
- 38. Ebisawa T, Tada K, Kitajima I, et al. . Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;20:3519–3527. [DOI] [PubMed] [Google Scholar]
- 39. Luo J, Tang M, Huang J, et al. . TGFβ/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;38:29588–29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukuda T, Scott G, Komatsu Y, et al. . Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167. [DOI] [PubMed] [Google Scholar]
- 41. Yu PB, Deng DY, Lai CS, et al. . BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagarova J, Vonner AJ, Armstrong KA, et al. . Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol. 2013;33:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haupt J, Deichsel A, Stange K, et al. . ACVR1 p.Q207E causes classic fibrodysplasia ossificans progressiva and is functionally distinct from the engineered constitutively active ACVR1 p.Q207D variant. Hum Mol Genet. 2014;23(20):5364–5377. [DOI] [PMC free article] [PubMed] [Google Scholar]