Abstract
Vertebrate reproduction is controlled by two gonadotropins (FSH and LH) from the pituitary. Despite numerous studies on FSH and LH in fish species, their functions in reproduction still remain poorly defined. This is partly due to the lack of powerful genetic approaches for functional studies in adult fish. This situation is now changing with the emergence of genome-editing technologies, especially Transcription Activator-Like Effector Nuclease (TALEN) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR). In this study, we deleted the hormone-specific β-genes of both FSH and LH in the zebrafish using TALEN. This was followed by a phenotype analysis for key reproductive events, including gonadal differentiation, puberty onset, gametogenesis, final maturation, and fertility. FSH-deficient zebrafish (fshb−/−) were surprisingly fertile in both sexes; however, the development of both the ovary and testis was significantly delayed. In contrast, LH-deficient zebrafish (lhb−/−) showed normal gonadal growth, but the females failed to spawn and were therefore infertile. Using previtellogenic follicles as the marker, we observed a significant delay of puberty onset in the fshb mutant but not the lhb mutant females. Interestingly, FSH seemed to play a role in maintaining the female status because we repeatedly observed sexual reversal in the fshb mutant. Neither the fshb nor lhb mutation alone seemed to affect gonadal differentiation; however, the double mutation of the two genes led to all males, although the development of the testis was significantly delayed. In summary, our data confirmed some well-known functions of FSH and LH in fish while also providing evidence for novel functions, which would be difficult to reveal using traditional biochemical and physiological approaches.
In vertebrates, reproduction is controlled by two master gonadotrophic hormones from the pituitary gland, FSH and LH. Both gonadotropins belong to the glycoprotein hormone family, consisting of one common α-subunit and one hormone-specific β-subunit (1). In mammals, FSH and LH have been extensively studied, and their functions are clearly defined. FSH stimulates follicular growth and estrogen production by binding to its cognate receptor FSH receptor (FSHR) located on the granulosa cells in the ovary and promotes spermatogenesis in the testis by activating FSHR expressed in the Sertoli cells. On the other hand, LH stimulates androgen production by the theca cells in the ovary, providing a substrate for estrogen production in the granulosa cells, and triggers oocyte maturation and ovulation.
In the testis, LH stimulates androgen production by the Leydig cells (2, 3). The clear definition of FSH and LH in mammals has benefited greatly from genetic approaches or gene knockouts in the mouse. In the FSH β-gene knockout (KO) mouse, the mutant females are infertile due to a blockade in folliculogenesis prior to antral follicle formation. However, FSH-deficient males are fertile despite having smaller testes and oligospermia (4). In contrast, the deletion of the LH β-gene causes infertility in both males and females. LH-deficient females have defects in folliculogenesis including the degeneration of antral follicles and absence of corpora lutea in the ovary. In males, the lack of LH leads to a decreased testis size, hypoplasia of Leydig cells, blockade of spermatogenesis at the round spermatid stage, and reduced expression of steroidogenic enzymes and secretion of T (5).
In teleosts, the understanding of gonadotropins has taken a long journey, and our knowledge about their functions still remains rudimentary. Although it has been known for many decades in mammals that the pituitary gland produces two gonadotropins since the first report in 1931 on the follicle-stimulating and luteinizing fractions in the pituitary (6), it was believed for a long time that gonadal development and function in fish were controlled by one single gonadotropin (7–9). The concept of two gonadotropins was proposed later after the mid-1970s based on histochemical and biochemical evidence (10–13), but the chemical nature of the molecules was not defined until 1988 when two chemically distinct gonadotropins, termed GTH-I and GTH-II, were characterized in the chum salmon (Oncorhynchus keta) (14). Ever since then, there have been numerous studies in teleosts demonstrating the duality of gonadotropins, and structural and functional studies have led to the proposal that GTH-I and GTH-II should be renamed to FSH and LH, respectively (15, 16). According to the GenBank record in 2014, gonadotropins (FSH and LH) have been sequenced in more than 70 teleost species. Although the number of fish species whose gonadotropins have been characterized is increasing, one major issue that concerns most in the field is the differential functions of the two hormones.
Numerous studies have been reported in a variety of fish species on the physiological roles of FSH and LH (17) and their regulation (18); however, our knowledge of FSH and LH functions still remains fragmentary. It is now generally believed that similar to their mammalian counterparts, fish FSH is mostly involved in promoting early gonadal development and growth, whereas LH plays an important role in regulating the late stage of gametogenesis, including the final gamete maturation and release (ovulation and spermiation) (19, 20). Most studies on gonadotropin functions have been focused on their expression profiles during the reproductive cycles at the transcript and/or protein levels (21–28), effects on gonadal steroidogenesis in males and females (29–34), vitellogenesis in females (35), and final gamete maturation (36–40).
In the zebrafish, our previous studies have shown that different from the gonadotropins in tetrapods, zebrafish FSH and LH show promiscuous recognition of gonadotropin receptors (Fshr/fshr and Lhcgr/lhcgr). Recombinant zebrafish FSH specifically activates its cognitive receptor Fshr expressed in Chinese hamster ovary cells; however, zebrafish LH can activate both Fshr and Lhcgr although the activation of Fshr requires higher concentrations of zebrafish LH compared with its activation of Lhcgr (41). A similar phenomenon has also been observed in some other species such as the African catfish (Clarias gariepinus) (28) and Senegalese sole (Solea senegalensis) (31). The physiological relevance of activating Fshr by LH remains mysterious. Although the expression profiles of zebrafish FSH and LH β-genes (fshb and lhb) during the reproductive cycle are not fully clear (41), our previous study on their receptors, fshr and lhcgr, has provided strong evidence for their differential roles in controlling folliculogenesis in females (42). Both fshr and lhcgr have a very low expression in the follicles of the primary growth stage (PG). However, the expression of fshr but not lhcgr increases significantly when the follicles are recruited into the secondary growth stage, and its level remains high throughout vitellogenic growth before it drops prior to final oocyte maturation. In contrast, the expression of lhcgr starts to increase in late vitellogenic follicles, and its level surges to the peak at the full-grown (FG) stage before oocyte maturation (42, 43).
Despite all these studies, our understanding of FSH and LH, especially their relative importance in controlling gonadal development and functions, still remains limited as compared with studies in mammals. This is largely due to the lack of pure forms of homologous hormones with biological activity, the international standard of bioassays, and more importantly the genetic or knockout approach available in the mouse model. This situation has changed recently with the emergence of powerful genome-editing technologies, in particular the Transcription Activator-Like Effector Nuclease (TALEN) and Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated system (CRISPR/Cas9) (13).
Both TALEN (44–46) and CRISPR/Cas9 (47–49) have been successfully adopted in the zebrafish model to disrupt functional genes. TALEN was first used in the zebrafish to delete the genes golden, ryr3, tbx6, and ryr1a (44), whereas CRISPR/Cas9 has been shown to be equally efficient to induce gene disruption in the zebrafish (48). However, all studies reported so far in the zebrafish have aimed at demonstrating the utility of the technologies with phenotypes mostly restricted to the embryonic stages. There has been no report studying functions of genes in adults using these technologies in the zebrafish.
To provide genetic evidence for the function and physiological significance of the two gonadotropins in teleosts, we have undertaken the present study by using TALEN approach to disrupt zebrafish FSH and LH β-genes (fshb and lhb). This was followed by a detailed analysis of reproductive phenotypes in both females and males. Our analysis focused on several important events in reproductive development and function, including gonadal differentiation, puberty onset, gametogenesis, final maturation, and fertility. The results from the present study have, for the first time, provided the most comprehensive genetic evidence for gonadotropin functions in a teleost species. Our data not only confirmed some well-established functions of FSH and LH but also revised some views. More importantly, we have provided evidence for novel functions of these hormones that would be difficult to reveal using traditional biochemical and physiological approaches.
Materials and Methods
Zebrafish
The wild type (WT) zebrafish of strain AB was used in the present study to produce mutant lines. Zebrafish were maintained in flow-through aquaria at 28 ± 0.5°C with the photoperiod of 14 hours light and 10 hours dark. All the experiments performed were licensed by the Government of the Hong Kong Special Administrative Region and were approved by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong.
TALEN design and assembly
The specific TALEN target sites were identified using an online tool (TAL Effector-Nucleotide Targeter: https://tale-nt.cac.cornell.edu/). The selected criteria were followed as described (44). Gene-specific TALEN constructs were assembled using the TALEN Golden Gate assembly system as described (50). The two TALEN somatic expression backbones, pCS2TAL3DD and pCS2TAL3RR (44), and the plasmids providing repeat variable diresidue (RVD) repeats for Golden Gate cloning (50) were obtained from Addgene. Briefly, two rounds of Golden Gate cloning assembly were performed to generate a TALEN gene with N RVD repeat modules (N = number of RVDs). First, two arrays were generated, corresponding to repeat modules 1–10 and 11-N-1. The resulting vectors that acquired correct arrays were confirmed first by the size of repeat array inserts amplified by PCR with the primers flanking the insertion site and then sequencing of the plasmids. Second, the two arrays and the sequences encoding the Nth motif were subcloned into the backbone vectors (50). The RVD repeat array sequences were cloned into pCS2TAL3DD to generate a left TALEN arm and pCS2TAL3RR to generate a right TALEN arm. The assemblies were confirmed by sequencing.
Injection of TALEN RNA into zebrafish embryos
The 5′-capped mRNA was generated by in vitro transcription from the customized TALEN plasmid templates using the mMESSAGE and mMACHINE SP6 kit (Invitrogen). The customized TALEN plasmids were linearized with NotI at 37°C for at least 3 hours before in vitro transcription. One microgram of linear template was used in the reaction of in vitro transcription, and the yield of the transcribed mRNA was determined by NanoDrop 2000 (Thermo Scientific). Fifty picograms of left and right TALEN mRNA each were coinjected into the cytoplasm of one- or two-cell stage zebrafish embryos using the Drummond Nanoject system (Drummon Scientific).
Genomic DNA extraction
The genomic DNA (gDNA) was obtained by the NaOH method (51). Briefly, a single embryo or piece of caudal fin was incubated in 50 μL NaOH (50 mM). The caudal fin was sampled after the fish was anesthetized with MS-222 (tricaine methanesulphonate, 250 mg/L; Sigma-Aldrich). The samples were then heated to 95°C until the tissue was noticeably friable, usually 10 minutes for the embryo and 20 minutes for the fin cut. The solution was then cooled to 4°C, and one tenth volume of 1 M Tris-HCL (pH 8.0) was added to neutralize the basic solution. The sample was centrifuged at 13 000 rpm for 2 minutes, and the supernatant was ready for use in PCR.
High-resolution melt analysis (HRMA)
To detect TALEN-induced mutations by HRMA, a 90- to 120-bp amplicon that included the entire genomic target site was generated. Primers flanking the target site were used to amplify the genomic region in a 20 μL PCR containing: 0.5 μl EvaGreen (×20), 2 μL PCR master mix, 2 μL embryonic or fin gDNA, and 400 nM each forward and reverse primers (Table 1). The amplification/duplex formation conditions were as follows: denaturation at 95°C for 3 minutes; 40 cycles (denaturation at 95°C for 15 sec; annealing at 62°C for 15 sec; extension at 72°C for 20 sec); denaturation at 95°C for 15 sec; melt curve 70°C-95°C, with a 0.2°C increment each step. The amplification was performed on the Bio-Rad CFX96 real-time PCR system (Bio-Rad Laboratories), and the HRMA analysis was carried out using the Bio-Rad Precision Melt Analysis software (Bio-Rad Laboratories).
Table 1.
Primers Used for Genotyping, RT-PCR, and Sequencing
| Gene | Primer Name | Primer Sequence (5-′–3-′) | Application |
|---|---|---|---|
| fshb | Fshb_1666 (P1) | AGATGAGGATGCGTGTGCTT | HRMA |
| fshb_1667 (P2) | GATGGAGATGTTTGTGAGTCG | ||
| fshb_1794 (P3) | TTGTTCTGGCGCTGCTGTTGC | RT-PCR | |
| fshb_1795 (P4) | TTCTGGGTGTGCTGTGCCAT | ||
| fshb_1685 | ATTGCTGTATTGGCTGCACT | Sequencing | |
| fshb_1686 | CACAGATGAAAAGGACACACG | ||
| lhb | lhb_1672 (P1) | GGATGTTATTGGCTGGAAATG | HRMA |
| lhb_1717 (P2) | AGCGTGGAAAAACCAAGCTC | ||
| lhb_1797 (P3) | GGTGTCTTCTTTCTCTTCTC | RT-PCR | |
| lhb_1798 (P4) | CGGGCTCTTGTAAACGGGAT | ||
| lhb_1691 | GGATGTTATTGGCTGGAAATG | Sequencing | |
| lhb_1692 | CAGTGGGGGAAATCAGAGCAC | ||
| Lhb_184 | ATGTTATTGGCTGGAAATGG | Real-time qPCR | |
| Lhb_185 | CTAGTATGCGGGGAAATCC | ||
| ef1a | ef1a_728 | GGCTGACTGTGCTGTGCTGATTG | RT-PCR |
| ef1a_729 | CTTGTCGGTGGGACGGCTAGG | Real-time qPCR |
The HRMA profile for each gene can be presented as either temperature-shifted melt curve and/or melt peak. The shifted melt curve is sensitive to distinguish heterozygous (+/−) from homozygous samples (+/+ or −/−), whereas the melt peak is often a better choice to distinguish homozygous mutants (−/−) from homozygous wild type (+/+). When the sequence difference is less than 5 bp (eg, lhb), the melt peaks of HRMA profiles often cannot distinguish homozygous mutants (−/−) from the wild type (+/+). To solve this problem, we spiked the unknown samples with wild-type gDNA to create heteroduplexes, which could be easily detected by either shifted curves or melt peaks.
Heteroduplex motility assay (HMA)
We performed an HMA using PAGE to confirm the mutations detected by HRMA. Briefly, a 90- to 120-bp amplicon that included the entire genomic target site was generated by using the same primers as those for HRMA. A three-step PCR was carried out: 95°C for 3 minutes, 40 cycles of 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 20 seconds. Five microliters of PCR reaction were electrophoresed on 20% polyacrylamide gels at 50 V for at least 10 hours, and the gel was stained with ethidium bromide and visualized on Gel Doc XR+ System (Bio-Rad Laboratories) (52).
Histological examination
All fish sampled were recorded for their body weight, standard body length, and age before processing for histological examination. Briefly, the fish was anesthetized with MS-222 and placed on a petri dish cover to measure body length with a ruler underneath. The body was then gently dried with a piece of tissue for taking body weight on an analytical balance. After the measurements, the fish were either allowed to recover in water or killed by decapitation for histological examination. The decapitated fish was immediately fixed in Bouin's solution for at least 24 hours. The fixed samples were dehydrated and embedded in paraffin and then serially cut into 7-μm sections on a Leica microtome. Slides were stained with hematoxylin and eosin and mounted with Canada balsam (Sigma-Aldrich) for microscopic examination.
RNA isolation and RT-PCR
Total RNA was extracted from individual pituitary glands using Tri-Reagent (Molecular Research Center) according to the manufacturer's protocol and our previous report (42, 53). Reverse transcription (RT) was performed at 37°C for 2 hours in a total volume of a 10-μL reaction solution containing 0.5 μg oligodeoxythymidine, 1× Moloney murine leukemia virus reverse transcription buffer, 0.5 mM each deoxynucleotide triphosphate, 0.1 mM dithiothreitol, and 100 U Moloney murine leukemia virus reverse transcriptase (Invitrogen). Conventional RT-PCR was used to confirm the mutations at mRNA level (15 μL containing 5 μL of 1:15 diluted RT reaction), and real-time quantitative PCR (qPCR) was performed to analyze the lhb expression level in individual pituitary glands (20 μL containing 9.5 μL of 1:10 diluted RT reaction) on the real-time PCR detection system (Bio-Rad Laboratories). The specific primers used are listed in Table 1.
Fertility assay
The fertility of different genotypes was assessed by natural mating with fertile partners. Individuals that failed to spawn or produce fertilized embryos after at least 10 trials were considered infertile.
Data analysis
The expression level of lhb was normalized to that of the internal control ef1a for statistical analysis. All values were expressed as the mean ± SEM, and the data were analyzed by a Student's t test (lhb expression) or a one-way ANOVA followed by a Tukey's multiple comparison test [body weight (BW), standard length (SL), and gonadosomatic index (GSI)] using Prism 6 on Macintosh OS X (GraphPad Software).
Results
High efficiency of TALEN-induced mutagenesis of zebrafish fshb and lhb genes
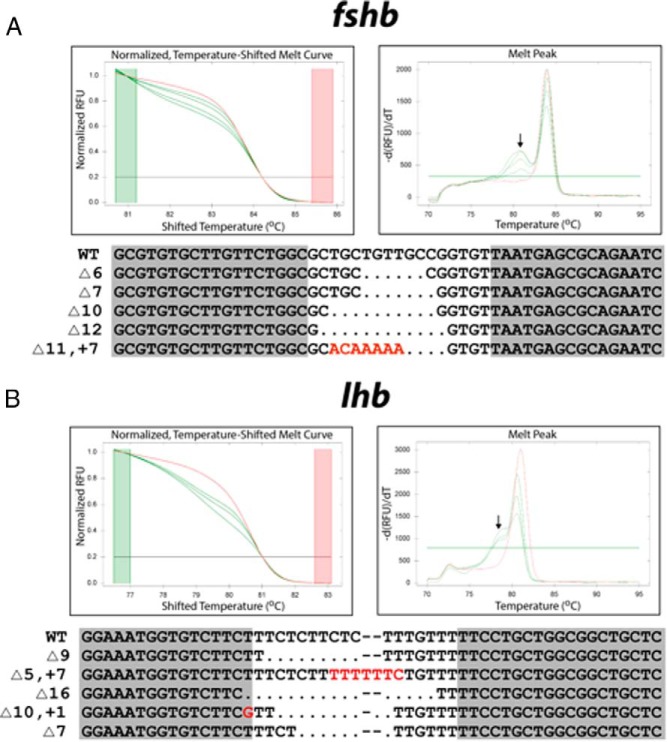
To maximize the chance of inducing loss of function of the targeted gene, the TALEN binding sites were chosen within the second exon immediately downstream of the translation start site for both fshb and lhb (Figure 1). The customized TALEN mRNAs were efficient in inducing somatic mutation at the targeted loci. When analyzed by HRMA at 24 hours after fertilization, the mutation rates were 30% (3 of 10) and 100% (10 of 10) for fshb and lhb, respectively (Table 2). The PCR amplicons were cloned and sequenced to confirm the mutations, and the sequences of some representative mutations are shown in Figure 2. The melt curves of mutation-carrying embryos showed significant shifting as compared with those of the control. Despite the shifting, the curves were generally smooth, indicating mosaicism of the mutations (Figure 2). The remaining F0 founder embryos were raised to adulthood and genotyped by HRMA on gDNA isolated from the fin cuts. Most individuals harbored somatic mutations, with 60% (6 of 10) for fshb and 100% (10 of 10) for lhb. To evaluate the efficiency of germ line transmission of the mutations, individual F0 fish carrying mutations were crossed with wild-type partners to obtain F1 offspring. The gDNA was isolated from individual F1 embryos from one F0 fish and analyzed by HRMA. About 20% (4 of 20) F1 embryos for fshb and 35% (7 of 20) for lhb were heterozygous mutants, demonstrating successful germ line transmission (Table 2).
Figure 1. Schematic representation of the genomic structures of zebrafish fshb (A) and lhb (B) genes and the target sites of TALEN.
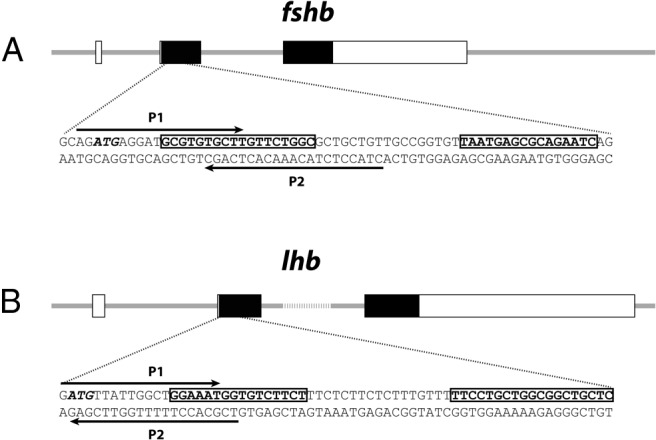
The coding and untranslated exon regions are depicted as solid and open boxes, respectively. The TALEN target sequence for each gene is shown under each illustration, with the left and right TALEN binding sites boxed. Arrows indicate the location of primers used for HRMA and the translation initiation codon (ATG) is italicized.
Table 2.
TALEN-Induced Mutations in F0 Embryos and Adults
| Gene | Postinjection Embryo Survival Rate | Postinjection Abnormal Embryo Rate | Postinjection Mutation Rate at 24 hpf | Somatic Mutation Rate in F0 Adults | Germ Line Transmission Rate |
|---|---|---|---|---|---|
| fshb | 31/60; 52% | 3/31; 10% | 3/10; 30% | 6/10; 60% | 4/20; 20% |
| lhb | 32/60; 53% | 3/32; 10% | 10/10; 100% | 10/10; 100% | 7/20; 35% |
Abbreviation: hpf, hours post fertilization. Germ line mutation rate is the number of F1 fish with mutations among the offspring of one F0 fish.
Figure 2. HRMA detection and sequence analysis of TALEN-induced somatic mutations in fshb (A) and lhb (B) genes in F0 embryos (1–2 dpf).
Each curve represents a single embryo. Both normalized, temperature-shifted melt curve (left panel) and melt peak (right panel) are shown. The red curve represents the uninjected control embryo, and the green ones are from the embryos injected with TANEN mRNAs. The arrow indicates the melt peak generated from the injected embryos with potential somatic mutations. The representative TALEN-induced sequence alterations (indel, insertions and deletions) at targeted locus are shown at the bottom of each panel. Sequences in shadow indicate left and right TALEN binding sites, and red indicate sequence alterations.
Establishment of fshb and lhb KO lines
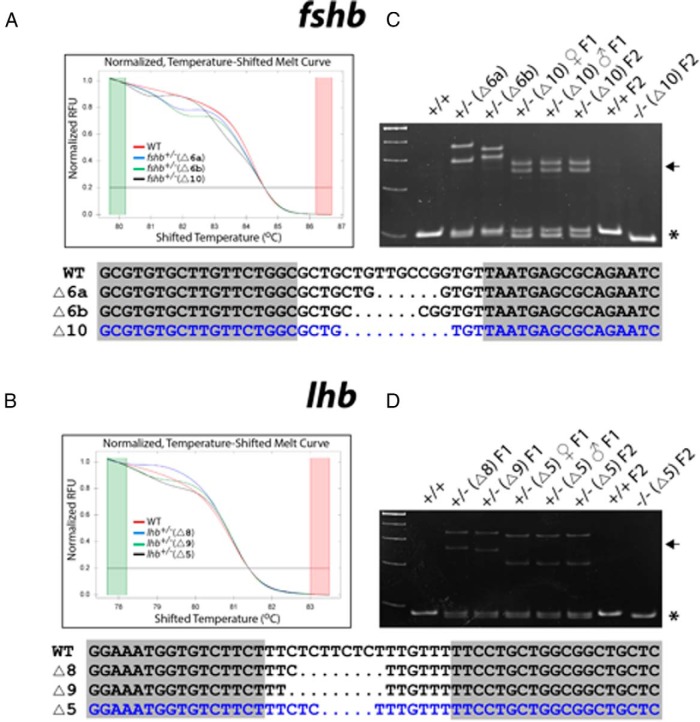
TALEN proteins existed in the embryos for less than 1 day after injection (data not shown). Because there are approximately 25–50 primordial germ cells in the zebrafish at 24 days post fertilization (dpf) (54), the germlines of F0 founders are likely mosaic; however, the level of mosaicism may not be as high as that in the somatic cells. In most of the fshb and lhb F0 founders screened, as many as four mutant sequences could be identified in the F1 embryos from individual F0 fish. With the high fecundity of the zebrafish, it is possible to obtain sibling male and female F1 individuals that carry the same DNA sequence alteration, making it possible to obtain homozygous mutants in F2 generation. HRMA profiles of three different mutated sequences at the targeted loci of fshb and lhb from young adult F1 fish (+/−; 2 mo) are shown in Figure 3, A and B. Different from the smooth HRMA curves from mosaic F0 fish, the curves from the heterozygous F1 individuals showed typical wavy patterns, indicating the existence of three DNA helixes (wild type, mutant, and hybrid). An HMA also confirmed the genotypes of the heterozygous F1 individuals with the presence of both heteroduplex and homoduplex molecules (Figure 3, C and D). For fshb, a 10-bp deletion (Δ10) leading to a frame shifting of the coding sequence was chosen to establish the mutant line for phenotype analysis, and a 5-bp deletion for lhb (Δ5) was selected (Figure 3).
Figure 3. HRMA (A and B) and HMA (C and D) for mutations in F1 and F2 fish.
The F0 founders with mutations detected by HRMA on caudal fin gDNA were crossed with WT partners to generate F1 individuals. The gDNA of 2-month-old F1 progeny was examined by both HRMA and HMA for the presence of mutations. In the HRMA assay (A and B), the smooth red curves are profiles of control WT fish, whereas the heterozygous F1 siblings (+/−) display characteristic wavy HRMA profiles shown in different colors. The genotypes were further confirmed by HMA assay on PCR amplicons (C and D), which shows homoduplexes (asterisk) in WT (+/+) and homozygous F2 mutants (−/−) and both homoduplexes and heteroduplexes (arrow) in heterozygous F1 (+/−). The F1 males and females that carried the same mutation were identified and crossed to generate homozygous mutant F2. The region containing the targeted sites was cloned and sequenced after PCR amplification of F1 gDNA. The representative mutant sequences are listed at the bottom for each gene, and the sequence in blue was chosen for propagation to establish the mutant lines.
Among sibling F1 fish, we successfully identified males and females that carried the same mutant sequences for both fshb▵10/+ and lhb▵5/+. Homozygous fshb−/−(Δ10) and lhb−/−(Δ5) were obtained in F2, and their genotypes were confirmed by HRMA (Figure 4, A and C) and HMA (Figure 3, C and D).
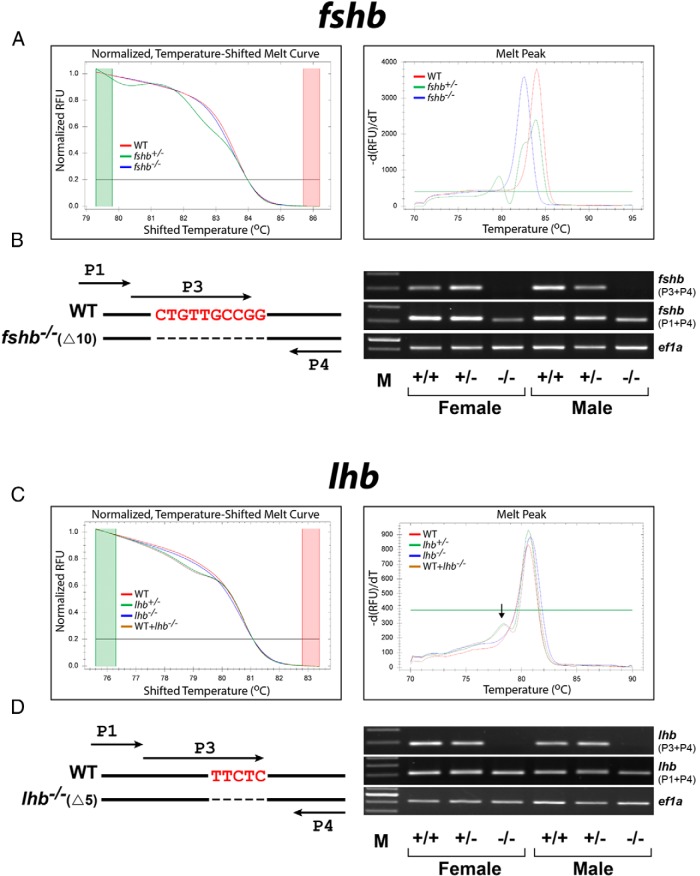
Figure 4. Genotype analysis for fshb (A and B) and lhb (C and D) mutants at gDNA (A and C) and mRNA (B and D) levels.
Male and female F1 fish with the same mutation were crossed to obtain homozygous F2 individuals. The gDNA was isolated from 2-month-old F2 caudal fins and subjected to HRMA (A and C). The WT (+/+), heterozygous (+/−), and homozygous (−/−) F2 fish are labeled in red, green, and blue, respectively. Because the HRMA could not clearly distinguish the homozygous mutant (lhb−/−, blue) from the wild type (lhb+/+, red) well, we spiked the sample with wild-type gDNA to create heteroduplexes (WT+lhb−/−, brown), which could be easily detected by shifted curve and melt peak (arrow). The mutation of fshb and lhb genes at transcription level was further confirmed by RT-PCR (B and D). The primer P3 is specific for the deleted sequences, and ef1a is used as the internal control.
To further confirm the successful deletion of the fshb and lhb genes, we also performed RT-PCR analysis for fshb and lhb mRNAs in the pituitary to ensure no expression of functional transcripts. Specific primers (P3) over the deleted sequences of fshb−/−(Δ10) and lhb−/−(Δ5) were used for the amplification (P3+P4). The result clearly showed the lack of expression of functional transcripts in the mutant pituitary glands. As controls, primers that flank the mutant sites (P1 and P4) could amplify the transcripts as expected with the amplicons from the homozygous mutants slightly smaller than the homozygous and heterozygous individuals (+/+ and +/−) (Figure 4, B and D).
Delayed ovarian growth and follicle development in FSH-deficient but not LH-deficient zebrafish
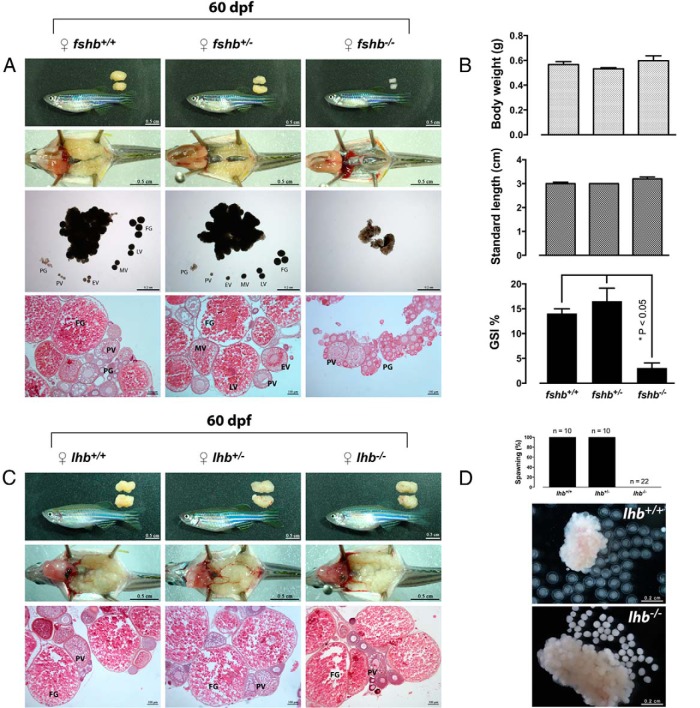
In our aquarium system, the zebrafish starts spawning around 2 months after fertilization, although the fecundity is not high at this young stage. We first analyzed the gross morphology and histology of the ovary in both the controls (fshb+/+ and fshb+/−; lhb+/+ and lhb+/−) and homozygous mutants (fshb−/− and lhb−/−) at 60 dpf. With similar standard length and body weight, the FSH-deficient females (fshb−/−) showed apparently smaller abdomens compared with the control individuals (homozygous fshb+/+ and heterozygous fshb+/−). It was indeed difficult to identify the gender of these fish until after dissection and with the help of microscope. The ovaries of the mutant fish were significantly smaller than those of the control individuals (Figure 5A), as demonstrated by the gonadosomatic index (GSI = gonadal weight/total body weight; P < .05) (Figure 5B). Dissection and histological analysis showed that the ovaries from the control fish (+/+ and +/−) contained a full range of developing follicles from the PG (stage I) to the FG (stage III) stage, in agreement with the fact that these fish had started spawning. In contrast, the ovaries from the mutant FSH-deficient fish (−/−) contained mostly the PG follicles with a few follicles at the PV (previtellogenic; stage II), suggesting a significant block at the PG-PV transition and delayed development into the vitellogenic growth (Figure 5A).
Figure 5. Effects of fshb (A and B) and lhb (C and D) gene disruption on ovarian growth and spawning in female zebrafish at 60 dpf.
A, Anatomical and histological examination of the ovary in fshb-deficient fish (fshb−/−) and controls (fshb+/+ and fshb+/−). B, GSI in controls and fshb-deficient fish (n = 3–4, P < .05). C, Anatomical and histological examination of the ovary in lhb-deficient fish (lhb−/−) and controls (lhb+/+ and lhb+/−). D, Failed oocyte maturation and ovulation in lhb-deficient fish (lhb−/−) at 9:00 am, 1 hour after lights on. LV, late vitellogenic; MV, midvitellogenic.
In contrast, the lack of LH did not seem to have any effect on ovarian growth. The LH-deficient female fish (lhb−/−) at 60 dpf showed no difference from the control of both homozygous (lhb+/+) and heterozygous (lhb+/−) fish at either gross anatomical or histological level. The ovaries in all three genotypes showed full development with all stages of follicles present from PG to FG stage (Figure 5C).
Failed spawning in LH-deficient but not FSH-deficient female zebrafish
Although the LH-deficient females showed normal ovarian growth in contrast to that of FSH-deficient fish, these fish were infertile because they failed to spawn to produce fertilizable eggs. We examined all genotypes (lhb+/+, lhb+/−, and lhb−/−) for spawning. All control individuals of homozygous (lhb+/+) and heterozygous (lhb+/−) genotypes spawned successfully (10 fish each), producing fully vital offspring. In contrast, none of the homozygous mutant individuals (lhb−/−) succeeded in spawning (22 in total). Further examination of the ovaries at 9:00 am when the light turned on showed that the control ovaries normally contained large number of mature and ovulated eggs with the chorion membrane separated from the egg and expanded; in contrast, only FG but immature follicles (no germinal vesicle breakdown) were present in the mutant ovaries, indicating a failure of final oocyte maturation (Figure 5D). A few individual follicles were sometimes found to have undergone germinal vesicle breakdown in the ovary of the lhb KO mutant fish, but they were never ovulated (data not shown).
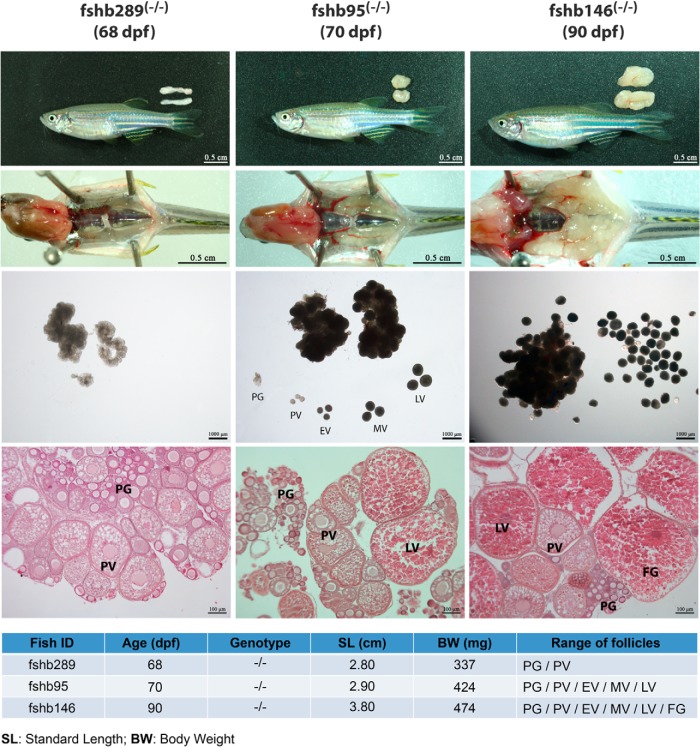
Interestingly, although the FSH-deficient females showed significantly delayed ovarian growth as described above at 60 dpf, the development could quickly catch up in the month afterward. As shown in Figure 6, after a significant delay, the ovaries in FSH-deficient fish (fshb−/−) gradually picked up the growth and became fully grown at 90 dpf (Figure 6). In contrast to the LH-deficient females, the FSH-deficient females could successfully spawn eventually with vital offspring after the ovaries completed the growth (Figure 7).
Figure 6. Catch-up ovarian growth in fshb-deficient mutant (fshb−/−) of different ages (68, 70 and 90 pdf).
PG, primary growth; PV, previtellogenic; EV, early vitellogenic; MV, midvitellogenic; LV, late vitellogenic; and FG, full-grown.
Figure 7. Embryonic offspring from fshb and lhb-deficient zebrafish (fshb−/− and lhb−/−).
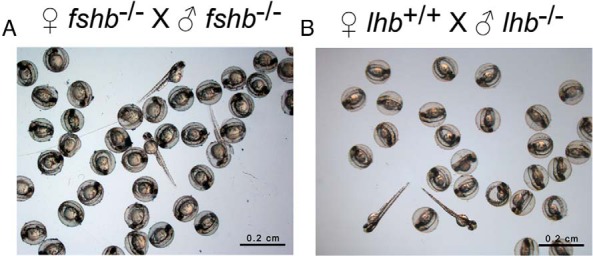
The fertility of homozygous fshb and lhb mutant fish was assessed by natural breeding between mutants or with wild-type fish. Both male and female fshb mutants were fertile with healthy and viable progeny (A), whereas male lhb mutant was fertile but not females (B). Twenty-two lhb-deficient females were examined, but none succeeded in spawning (Figure 5).
Delayed puberty onset in FSH-deficient but not LH-deficient females
Puberty onset is a very important event in reproductive development. The mechanisms underlying puberty onset in vertebrates remain elusive and poorly elucidated, and they are entirely unknown in teleosts, especially with regard to the roles of gonadotropins in the process. After analyzing the impacts of FSH and LH deficiency on ovarian growth at the time of maturity as described above, we shifted our attention to the stage when puberty starts, which is marked by the appearance of the first cohort of PV follicles in the ovary. As we reported recently, the puberty onset in female zebrafish depends largely on body growth rather than age, and we have defined 100 mg body weight and 1.80 cm standard body length to be critical thresholds for the initiation of puberty (55). These criteria were also used in the present experiment to help with the evaluation of puberty onset and the impacts of FSH and LH deficiency.
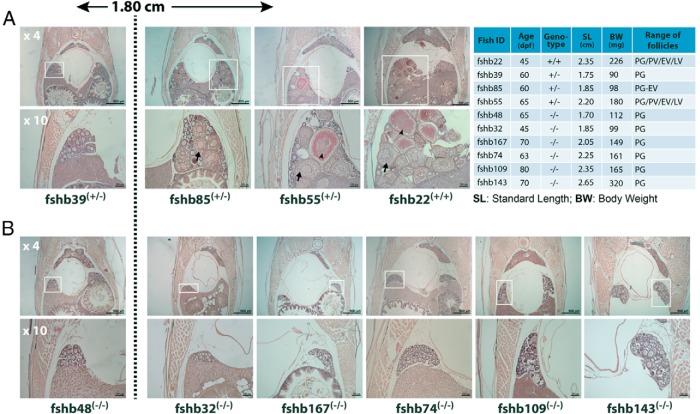
We examined six homozygous mutant individuals (fshb−/−) of different ages (45–80 dpf) with SL and BW, ranging from 1.70 to 2.65 cm and 99 to 320 mg, respectively, covering the point when puberty onset is expected to occur (1.80 cm SL and 100 mg BW). As shown in Figure 8, none of these mutant fish had PV follicles in the ovary, suggesting a significant delay of puberty onset in FSH-deficient females despite the fact that most individuals had crossed the thresholds in terms of either BW or SL or both. Female mutants could not break through the PG-PV transition even at 70–80 dpf with an SL of 2.35 and 2.65 cm, well above the 1.80-cm threshold defined previously (55). Furthermore, a histological examination of whole-body cross-sections showed an overall smaller ovarian size in the mutants as compared with those in the controls. In contrast, all control fish of both homozygous and heterozygous genotypes (+/+ and +/−) showed a normal initiation of puberty with PV and advanced follicles [early vitellogenic (EV) and late vitellogenic] appearing in the ovary once the SL and BW approached or crossed the defined threshold levels (1.80 cm and 100 mg) (Figure 8).
Figure 8. Delayed puberty onset in female fshb−/− mutants of different ages (45–80 dpf).
Juvenile females were sampled at different ages (45–80 pdf) for histological analysis. The upper panel shows low-magnification view of the cross-sections, highlighting the location and relative size of the ovaries (boxed), whereas the lower panel is the close-up of the ovary showing follicle composition. A, The ovaries from control fish (fshb+/+ and fshb+/−) with normal growth rate and advanced follicle stages. B, The ovaries of mutant fish (fshb−/−) with smaller size and PG follicles only. The age, genotype, SL, BW, and stage of follicles in the ovary are shown in the table.
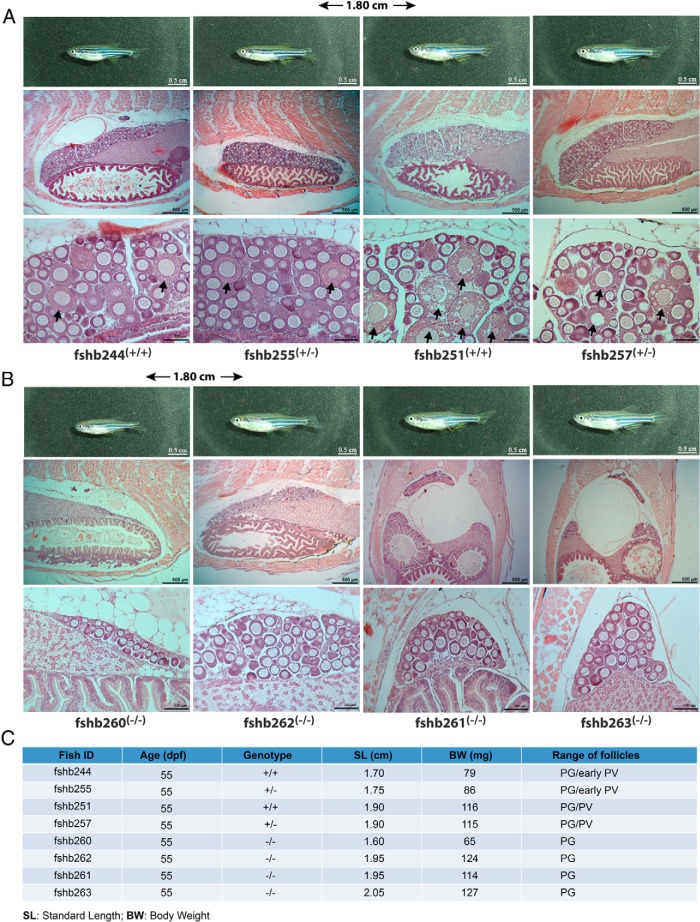
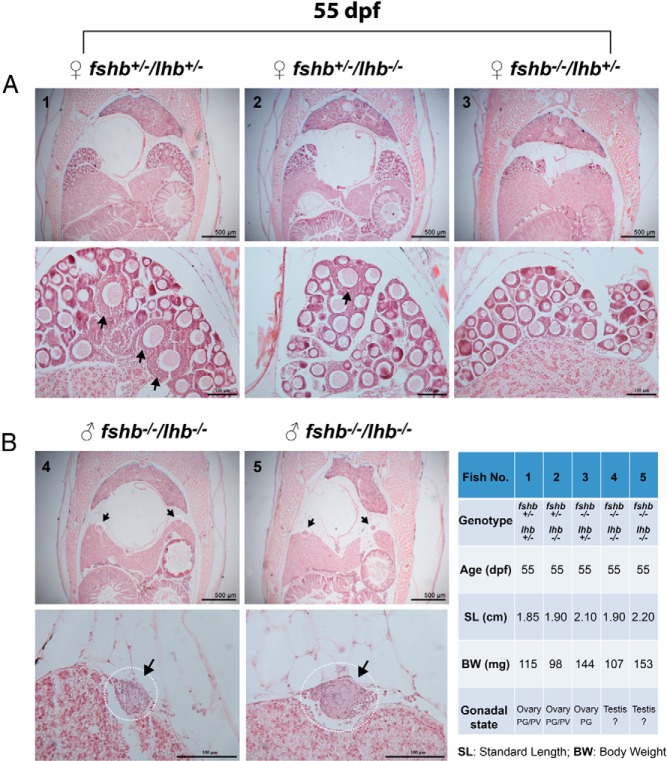
To rule out the influence of age on the result shown above in Figure 8, we carried out one additional experiment by using fish of the same age at 55 dpf, when the puberty onset is expected to have happened according to our recent report (53). The result was consistent with that described in Figure 8. The control fish (+/+ and +/−) with SL and BW above the threshold levels all had PV follicles well formed in the ovary (fshb251 and fshb257). Even those slightly below the thresholds (fshb244 and fshb245) showed signs of early PV follicles. However, none of the fshb KO mutant fish (−/−) showed any sign of PV follicle development, even in those with body sizes much bigger than the controls (Figure 9).
Figure 9. Delayed puberty onset in female fshb−/− mutants of the same age (55 dpf).
Juvenile females of the same age were sampled for morphological and histological analysis. A, The ovaries from control fish (fshb+/+ and fshb+/−) with PV follicles (arrow). B, The ovaries of mutant fish (fshb−/−) with PG follicles only. The age, genotype, SL, BW, and stage of follicles in the ovary are shown in the table (C).
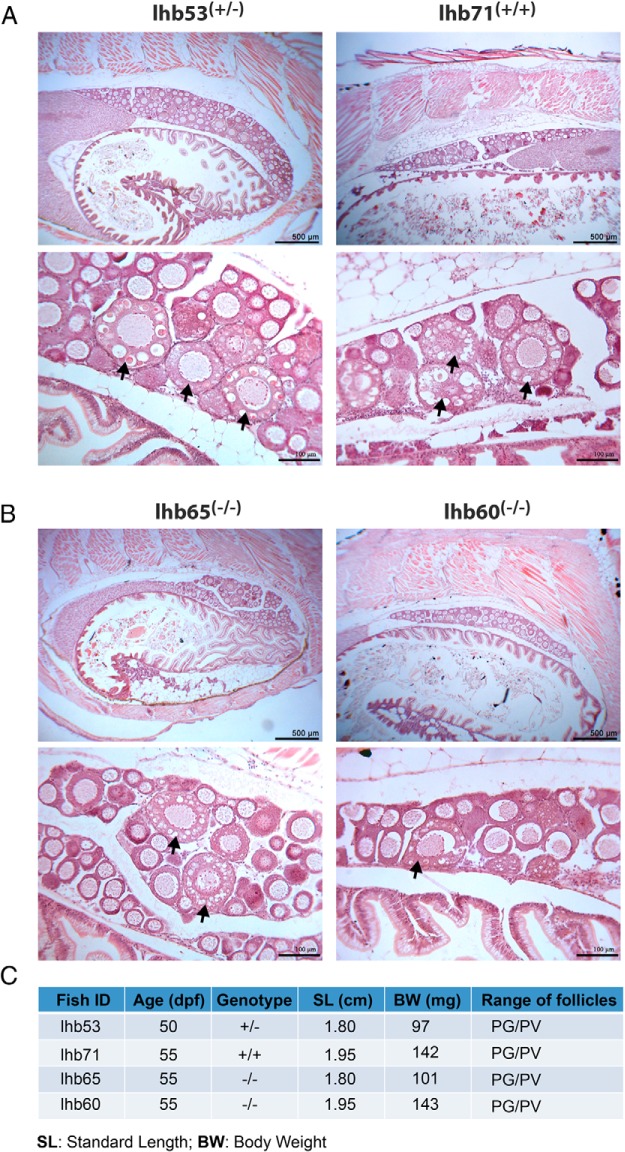
In contrast to FSH-deficient fish, no significant puberty delay was observed in LH-deficient females. We performed the analysis at 50–55 dpf, the time when puberty onset is normally expected to occur (53, 55), on individuals with SL and BW around or above the defined threshold levels (1.80–1.95 cm and 97–143 mg). All individuals had PV follicles well formed in the ovary with no difference among the three genotypes (lhb+/+, lhb+/− and lhb−/−) (Figure 10).
Figure 10. Normal puberty onset in female lhb−/− mutants.
Both control (lhb+/+ and lhb+/−) (A) and mutant fish (lhb−/−) (B) had PV follicles with cortical alveoli (arrow) in their ovaries. The fish had similar age (50–55 dpf) and body size. The age, genotype, SL, BW, and stage of follicles in the ovary are shown in the table (C).
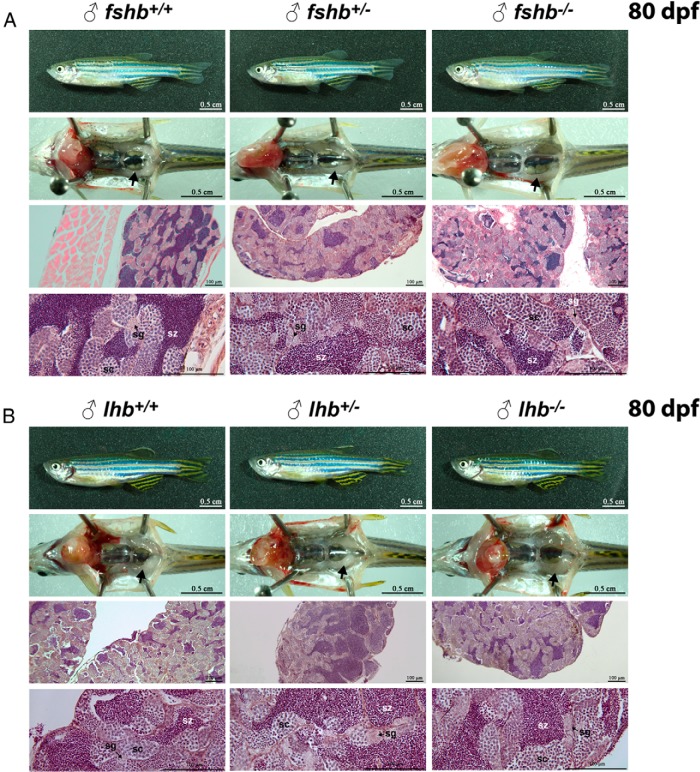
Normal development of testis in FSH and LH-deficient males at maturation stage
We also analyzed the phenotypes of male fshb and lhb KO zebrafish at 80 dpf. Surprisingly, neither fshb nor lhb KO mutants (−/−) showed significant signs of abnormality in their testis development and spermatogenesis at gross anatomical and histological levels as compared with the controls. All stages of sperm cells including spermatogonia (sg), spermatocytes (sc), and spermatozoa (sz) were present in all individuals of the three genotypes (Figure 11). Furthermore, all mutant fshb−/− and lhb−/− males were fertile as demonstrated by their successful spawning with females to produce normal offspring (Figure 7). The lhb−/− males were tested with WT females as the lhb−/− females were unable to spawn and therefore infertile as described above.
Figure 11. No effect of fshb and lhb gene disruption on spermatogenesis in the testis at mature stage (80 dpf).
Phenotypically male individuals were sampled at 80 dpf and their gross morphology and histology were observed. All the individuals contained typical white thread-like testes (arrow), and histological examination showed normal cellular composition of testis in all genotypes for both fshb (A) and lhb (B). The fertility of these male fish had been tested by natural spawning (Figure 7). sc, spermatocytes; sg, spermatogonia; sz, spermatozoa.
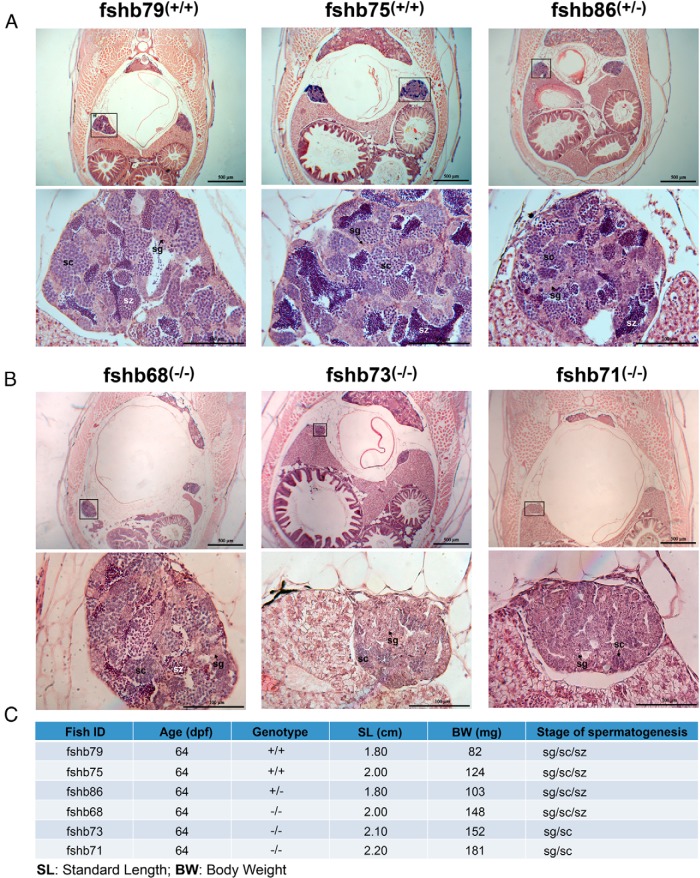
Despite the normal fertility of fshb−/− and lhb−/− males at the mature stage, there was evidence for a role of gonadotropins in promoting early testis development, especially for FSH. As shown in Figure 12, when examined at 64 dpf when all control males (fshb+/+ and fshb+/−) had their testes well developed with all stages of spermatogenesis present, there was an obvious delay of spermatogenesis in the fshb KO mutant fish (−/−), although all mutant fish examined had larger body sizes than those of the controls. The testes of the mutant fish contained germ cells mostly at the early stages of spermatogenesis including spermatogonia and spermatocytes, and the germ cells of advanced stages were seldom present in the mutant testes. Furthermore, the overall sizes of the testes were also much smaller than those in the controls (Figure 12).
Figure 12. Retarded spermatogenesis in FSH-deficient males (fshb−/−) at early developmental stage (64 dpf).
With the help of HRMA, male individuals of different genotypes were sampled at 64 dpf for histological examination. A, The testes of control fish (fshb+/+ and fshb+/−) with normal size and spermatogenesis. B, The testes of mutant fish (fshb−/−) with smaller size and delayed spermatogenesis. The upper panel shows low-magnification view of the cross-sections highlighting the location and relative size of the testes (boxed), whereas the lower panel is the close-up of the testis showing stage of spermatogenesis. C, The age, genotype, SL, and BW of the fish shown.
Increased expression of lhb in the pituitary glands of FSH-deficient fish
The results described above (Figures 6 and 12) showed that although there was a significant retardation of gonadal growth in FSH-deficient females and males, the growth of ovary and testis could both catch up after a period of delay, leading to normal fertility in both sexes. One mechanism for the catch-up growth was likely due to the cross-activation of FSH receptor Fshr by LH as we reported previously (41). To provide supportive evidence for this hypothesis, we examined the expression of lhb in the pituitary glands of FSH-deficient females and males (130 dpf). Interestingly, the expression level of lhb increased significantly in the pituitaries of female fshb−/− fish. The level increased also in males, but it was not statistically significant due to high individual variation (Figure 13). We were unable to measure plasma FSH and LH levels in the zebrafish because of the lack of specific RIAs in this species.
Figure 13. Expression of lhb in the pituitary glands of control (fshb+/−) and FSH-deficient (fshb−/−) female (A) and male (B) fish (130 dpf).
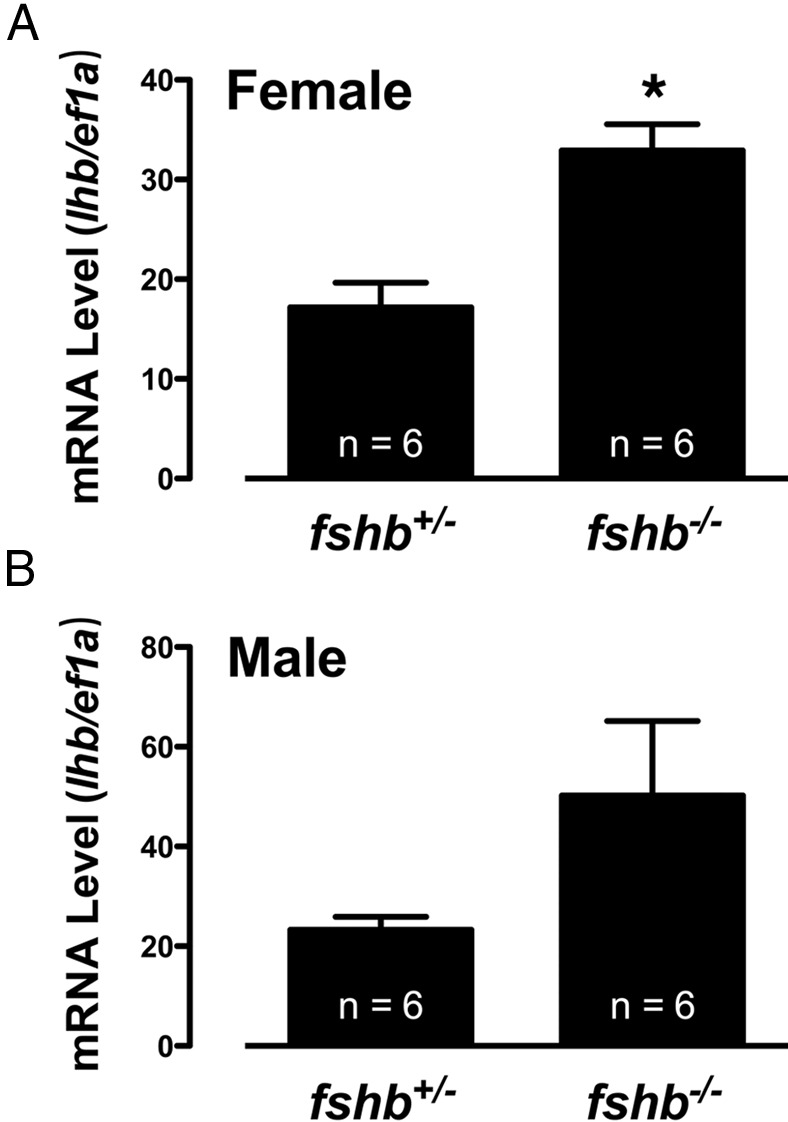
Total RNA from each pituitary gland was reverse transcribed for real-time qPCR quantification of lhb expression, which was normalized to ef1a (mean ± SEM, n = 6). *, P < .05 vs control.
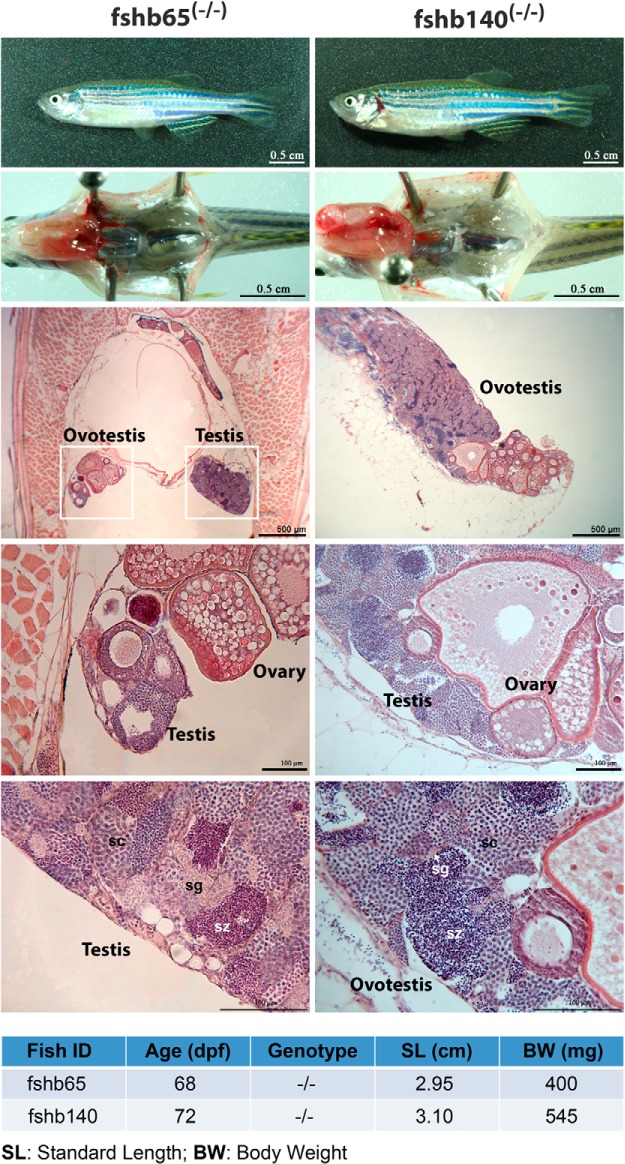
Evidence for a role of FSH in maintaining female status in mature adults
During our large-scale screening by histology, we discovered two fshb−/− mutant fish with ovotestis (fshb65 and fshb140). This was never found in any other genotypes including lhb−/− mutants. Fshb65 (68 dpf) had a testis on one side but ovotestis on the other side with ovarian tissue being dominant, whereas in fshb140 (72 dpf), the testis issue was dominant. The ovarian and testis tissues were well separated without much mingling in both fish, and the ovarian tissues contained well-developed vitellogenic follicles (PV and EV) with some undergoing degeneration or apoptosis (Figure 14). The presence of vitellogenic follicles indicated that the fish were likely genotypically female, and they might possibly be undergoing sex reversal.
Figure 14. Sexual reversal in FSH-deficient adult zebrafish.
Two fish at 68 and 72 dpf were identified with ovotestis. Fshb65(−/−) was fixed and sectioned as a whole with gonadal location clearly shown on both sides, whereas for fshb140(−/−), the gonads were isolated for fixation and sectioning. sc, spermatocytes; sg, spermatogonia; sz, spermatozoa.
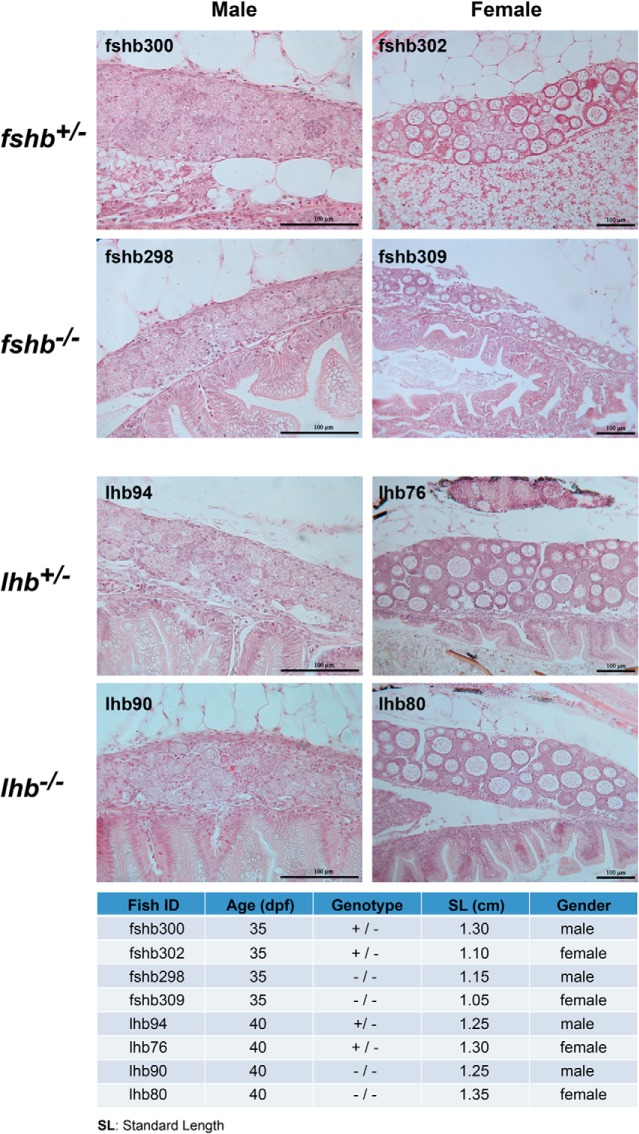
No evidence for the involvement of FSH and LH alone in gonadal differentiation
The discovery of hermaphroditic fshb−/− fish in adult stage prompted us to speculate a possible role for gonadotropins in influencing gonadal differentiation. To address this issue, we did an experiment to analyze the development of gonads at 35–40 dpf (35 dpf for fshb and 40 dpf for lhb). This time window was chosen because our previous study has shown that the zebrafish ovary forms from the undifferentiated so-called ovary-like state around 30 dpf, whereas the formation of testis lagged behind by approximately 2 weeks, ie, approximately 45 dpf (55). Our result showed that all four genotypes analyzed (fshb+/− and fshb−/−; lhb+/− and lhb−/−) had females with well-formed ovaries containing early perinucleolar oocytes (fshb302 and fshb309, lhb76 and lhb80) and undifferentiated gonads, which might likely develop into testis as shown by early signs of spermatogenesis (presence of spermatogonia and spermatocytes) (fshb300 and fshb298, lhb94 and lhb90) (Figure 15). We did not observe any difference between heterozygous fish (fshb+/− and lhb+/−) and homozygous mutants (fshb−/− and lhb−/−).
Figure 15. No effect of fshb and lhb gene disruption on gonadal differentiation.
To investigate whether gonadotropins play any roles in gonadal differentiation, samples were taken at 35 dpf for fshb and 40 dpf for lhb. At 35 dpf, both control (fshb+/−) and mutant (fshb−/−) had already completed gonad differentiation, with no significant difference between the two genotypes. Compared with the ovary, the formation of testis was slower. The same was observed for control and mutant lhb at 40 dpf.
Production of all-male offspring with double mutations of fshb and lhb
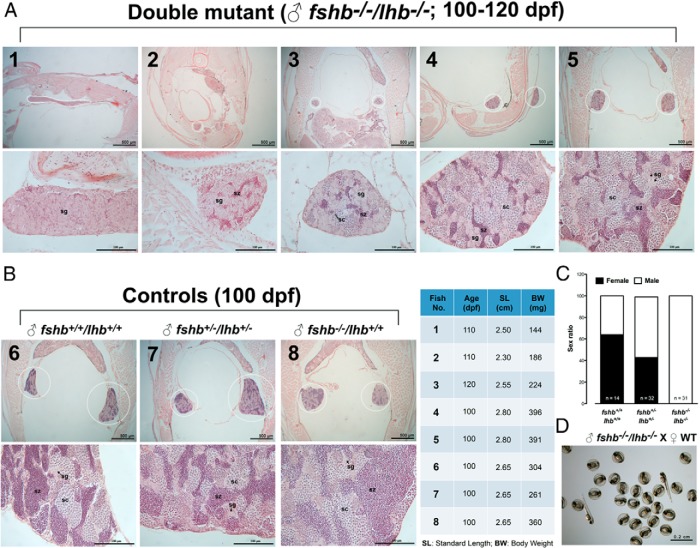
The significant phenotypes of delayed development of ovary and testis but normal fertility in the end in fshb−/− fish led us to hypothesize that LH could play a compensatory role due to its cross-activation of Fshr. This idea was supported by the evidence that the expression of lhb increased in fshb−/− fish (Figure 13). To provide further evidence for this hypothesis, we created a mutant zebrafish with double mutations of fshb and lhb. Interestingly, lack of FSH and LH led to all-male offspring. At 55 dpf, the control fish expressing either fshb and lhb alone or both (fshb+/−/lhb+/−, fshb+/−/lhb−/−, and fshb−/−/lhb+/−) all had normal ovarian development; in contrast, the gonads of double-mutant fish (fshb−/−/lhb−/−) all stayed at very early stage without obvious signs of sexual differentiation (Figure 16). Further analysis of elder fish (100–120 dpf) showed that all double-mutant fish developed into males (Figure 17A) (n = 31), whereas the control fish (fshb+/+/lhb+/+, n = 14; fshb+/−/lhb+/−, n = 32) exhibited normal gonadal differentiation and development (Figure 17B) with an expected sex ratio (∼50% females) (Figure 17C). The all-male phenotype did not seem to be due to sex reversal because no oocytes were observed in any individuals at any stage. The level of spermatogenesis in double-mutant fish varied significantly among individuals. Some individuals showed no development beyond spermatogonial stage at 110 dpf (Fish 1), whereas others exhibited development of spermatocytes and even mature spermatozoa (Fish 2–5, 100–120 dpf). In general, most mutant fish had small testis size compared with those in control fish of similar or even younger age (Figure 17, A and B). Despite the significant delay in spermatogenesis, the double-mutant fish were fertile and they could breed with WT females to produce normal offspring (Figure 17D).
Figure 16. Gonadal development in fshb and lhb double mutants at 55 dpf.
A, Control fish expressing both fshb and lhb (fshb+/−/lhb+/−) or fshb and lhb alone (fshb+/−/lhb−/−, fshb−/−/lhb+/−) had normal ovarian formation. Arrows indicate PV follicles. B, Double mutations of fshb and lhb (fshb−/−/lhb−/−) led to the retarded development of the gonads (testis?) (circled and labeled by arrow).
Figure 17. All-male development in fshb and lhb double mutants at 100–120 dpf.
A, Double mutations of fshb and lhb (fshb−/−/lhb−/−) produced all-male offspring with delayed development of the testis (circled). B, Control fish (fshb+/+/lhb+/+, fshb+/−/lhb+/−, fshb−/−/lhb+/+) had normal testis growth (circled) and spermatogenesis. C, Sex ratio in fshb and lhb double mutants. D, Normal fertility of fshb and lhb double mutants. sc, spermatocytes; sg, spermatogonia; sz, spermatozoa.
Discussion
Using TALEN technology, the present study analyzed the functions of FSH and LH hormones in zebrafish reproduction, which not only confirmed some aspects of FSH and LH functions proposed before but also provided evidence for novel functions of these hormones that would be otherwise difficult to demonstrate with other approaches.
Roles of FSH and LH in folliculogenesis
In the zebrafish model, our previous study on FSH and LH receptors (Fshr and Lhcgr) has provided important clues to the functions of FSH and LH in females, which to some extent agrees with the generally accepted concept that FSH plays a major role in stimulating follicle growth via its cognate receptor Fshr, whereas LH is involved in final maturation and ovulation. However, there has been no direct evidence to verify this concept. By deleting the fshb and lhb genes from the genome, we provide the most comprehensive and conclusive genetic evidence so far in any teleost species for roles of these two important reproductive hormones.
Loss of the fshb gene and therefore the FSH hormone caused a significant retardation of follicle growth in female zebrafish, especially at the PG-PV transition. This is similar to the phenotype observed in the FSH-deficient mice whose follicle development is blocked at the antral stage, resulting in female infertility (4). Interestingly, despite a significant blockade at the PG-PV transition in the absence of FSH, PV follicles could still emerge after a delay in the zebrafish ovary, which differs from the situation in the mouse (4). Once entering the PV stage, the follicles in fshb KO fish could quickly pick up the growth to complete the process of vitellogenesis in about one month, although the growth rate seemed slower than normal, which takes about 2 weeks according to our previous studies (42, 56). This phenomenon raises an interesting question. After a delayed activation, what eventually triggers some follicles to break the blockade at PG-PV transition to enter the vitellogenic growth? The answer remains unknown. We speculate that LH likely plays a compensatory role.
Our previous study has shown that LH can cross-activate Fshr in the zebrafish at high concentrations (41), and this promiscuous activation of Fshr would allow a compensatory role for LH to drive folliculogenesis in the absence of FSH. When the PG follicles grow to their full size prior to entering the PV stage, the expression of Fshr increases (42, 43), therefore increasing the responsiveness of the follicles to FSH and probably, to a lesser extent, LH as well. Without FSH, LH may play a role in initiating the PG-PV transition albeit at slower rate due to its lower potency in stimulating Fshr (Figure 18). This idea is further supported by our evidence that lhb expression in the pituitaries of FSH-deficient fish increased in both females and males. It is conceivable that such an increase in LH biosynthesis may contribute to the compensatory role of LH in the absence of FSH. The mechanism for the increased lhb expression is unknown, and it will be an interesting issue to address in future studies.
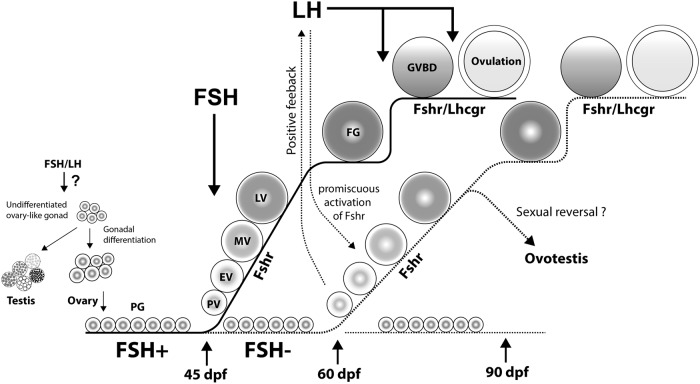
Figure 18. Hypothetical model for roles of FSH and LH in controlling follicle development and maturation in the zebrafish ovary.
FSH rather than LH plays a key role in setting the timing of puberty onset in females, and FSH is a dominant hormone in controlling follicle growth afterward. In the absence of FSH, LH will kick in to play a role in initiating follicle activation (PG-PV transition) and promoting its growth via its cross-reaction with the FSH receptor (Fshr). At the end of folliculogenesis, it is LH but not FSH that plays a critical role in triggering final oocyte maturation and ovulation in females. In addition to promoting follicle growth, FSH may also play a role in maintaining female status in adult, probably through stimulating and maintaining aromatase expression in the ovary.
The promiscuous activation of Fshr by LH has been reported in several species including the zebrafish (41). However, the functional significance of this partial activation of Fshr by LH has remained entirely unknown and mysterious. The genetic approach adopted in this study by deleting the fshb and lhb genes seems to have provided a conceivable answer to the question. Despite this, we still need to be cautious in that the compensatory role of LH was revealed in the absence of FSH, which is a nonphysiological condition. Further studies on the fshr mutant will provide insight into this issue.
In contrast to FSH, the lack of LH had no observable effect on follicle growth in the zebrafish but caused infertility due to failed oocyte maturation and ovulation in vivo. This is in contrast to the infertility in LH-deficient female mice whose ovaries are small with abnormal antral follicles (5). This result also suggests that FSH plays no compensatory role for LH in controlling final oocyte maturation and ovulation in the zebrafish.
Roles of FSH and LH in female puberty onset
In fish, the appearance of the first wave of PV follicles in the ovary is considered a marker for puberty onset in females (55, 57, 58). Little is known about the mechanisms that trigger and control puberty onset in fish species. We previously proposed that FSH could be a potential factor that triggers puberty onset in females because of the significant increase of fshr expression in PV follicles (42). However, our subsequent study on ontogeny of fshb and lhb expression during development led us to a different view that instead of FSH, it was likely LH that played a role in the event because the expression of lhb in the pituitary was very low before puberty but increased dramatically when the first wave of PV follicles appeared in the ovary (53). This view, however, is not supported by the evidence from the present study. In contrast to the loss of fshb, which caused a significant delay in PV follicle development, the disruption of the lhb gene had no effect at all on the timing of the PG-PV transition. Based on this, we can conclude that the previously observed surge in lhb expression at puberty onset is the consequence rather than the cause of puberty onset.
Roles of FSH and LH in spermatogenesis
One of the most striking discoveries in the present study was the role of FSH and LH in testis development and spermatogenesis in males. In the absence of FSH and LH alone or both, the males were all fertile with a normal ability to spawn and produce vital offspring, although fshb−/− mutant and fshb−/−/lhb−/− double-mutant fish had slower testis growth. This result suggests that different from our traditional view on the importance of neuroendocrine regulation of gonadal development and function (59), the classical reproductive axis involving FSH and LH may play a less-than-expected role in the reproduction of male zebrafish. It is likely that after the initial promotion by FSH to trigger male puberty in juveniles, the process of spermatogenesis becomes independent of pituitary gonadotrophic hormones. This agrees with a view in mammals that FSH plays a role in initiating spermatogenesis but not in maintaining the process in adults (4, 60, 61), although this concept still remains controversial (62). In FSH-deficient mice, the males are fertile but oligospermic with smaller testes (4), which agrees well with our finding in the zebrafish. As for the role of LH, our discovery is in sharp contrast to that in mammals. The LH-deficient zebrafish males seemed normal with full capacity to reproduce. In LH-deficient mice, however, both males and females are infertile (5). The major regulators of spermatogenesis in adult male zebrafish may come from other endocrine axes, in particular the somatotrophic axis. This view is supported by in vitro evidence that the spermatogenesis in cultured zebrafish testis could be promoted by IGF-1 alone, which stimulated the development of spermatogonia to spermatocytes and spermatids (63).
Roles of FSH and LH in gonadal differentiation
One interesting discovery in the present study was the hermaphroditic fish in fshb KO mutants at the adult stage. Although we should be cautious in interpreting the result due to the small sample size, it raises a possibility for a role of FSH in maintaining female status after sexual differentiation. It is likely that despite the compensatory role of LH in supporting folliculogenesis, the lack of FSH may lead to a lower expression level of aromatase in the ovary, making the fish susceptible to both internal and external influences that favor a conversion of ovary into testis through sex reversal. This idea is supported by recent studies in the zebrafish that demonstrated sex reversal in mature adult females after treatment with aromatase inhibitor (64, 65).
To test this hypothesis, we went on to generate a mutant zebrafish that carries double mutations of fshb and lhb. Unexpectedly but interestingly, all double mutant fish turned out to be males with normal but retarded testis growth and spermatogenesis. The production of all-male offspring raises an interesting question on the role of gonadotropins in sexual differentiation. Our previous study has demonstrated that during zebrafish life cycle, the expression of lhb appeared first at the time of gonadal differentiation, and its signal could be detected only in the pituitaries of individuals whose gonads contained well-developed oocytes; in contrast, there was no detectable lhb expression in those with either undifferentiated gonads or testis. This has led us to hypothesize that gonadotropins may likely play a role in gonadal differentiation (53). The all-male phenotype of fshb and lhb double mutation provides a strong support to this hypothesis. The effect of LH on ovarian differentiation may be subtle in the presence of FSH; however, it becomes significant in the absence of FSH.
In summary, using the TALEN-mediated genome editing method, the present study has provided the most comprehensive and conclusive genetic evidence in a fish species for functions of pituitary gonadotropins (FSH and LH) in controlling major reproductive events in both females and males. Our results demonstrated both phenotypic similarities and differences of fshb and lhb mutants between the zebrafish and mammals, which are compared in Table 3. The key discoveries of the present study can be summarized as follows (Figure 18): 1) the lack of FSH or LH alone does not seem to influence gonadal differentiation; however, double mutations of fshb and lhb suggest a role for gonadotropins, especially LH in the event; 2) FSH rather than LH plays a key role in setting the timing of puberty onset in both females and males; 3) FSH is a dominant hormone in controlling follicle growth in females, whereas LH does not seem to have any role in this process; 4) LH but not FSH plays a critical role in triggering final oocyte maturation and ovulation in females; 5) FSH may play a role in maintaining the female status in the adult, probably through stimulating and maintaining aromatase expression in the ovary; and 6) the importance of FSH and LH in controlling male reproduction is less than expected in contrast to their roles in females. Alternative mechanisms may exist to regulate spermatogenesis in the zebrafish.
Table 3.
Phenotype Comparison of FSHB/Fshb/fshb and LHB/Lhb/lhb Mutants in Humans, Mice, and Zebrafish
| Gene | Gender | Species | Phenotype | Reference |
|---|---|---|---|---|
| fshb | Female | Human | 1. Hypogonadism | (66–68) |
| 2. Delayed puberty | ||||
| 3. Amenorrhea | ||||
| 4. Infertile | ||||
| Mouse | 1. Arrested folliculogenesis at antral formation | (4) | ||
| 2. Infertile | ||||
| Zebrafish | 1. Delayed puberty | Present study | ||
| 2. Slower folliculogenesis | ||||
| 3. Fertile | ||||
| Male | Human | 1. Hypogonadism | (69) | |
| 2. Azoospermia | ||||
| Mouse | 1. Smaller testis | (4) | ||
| 2. Fertile | ||||
| Zebrafish | 1. Slower progression of initial spermatogenesis | Present study | ||
| 2. Fertile | ||||
| lhb | Female | Mouse | 1. Small ovary | (5) |
| 2. Abnormal folliculogenesis | ||||
| 3. Degenerating antral follicles | ||||
| 4. Absence of corpora lutea | ||||
| 5. Infertile | ||||
| Zebrafish | 1. Normal folliculogenesis | Present study | ||
| 2. Failed oocyte maturation and ovulation | ||||
| 3. Infertile | ||||
| Male | Human | 1. Delayed puberty | (70) | |
| 2. Infertile | ||||
| Mouse | 1. Smaller testis | (5) | ||
| 2. Reduced testosterone level | ||||
| 3. Blocked spermatogenesis at round spermatid stage | ||||
| 4. Infertile | ||||
| Zebrafish | 1. Normal spermatogenesis | Present study | ||
| 2. Fertile |
Acknowledgments
This work was substantially supported by Grants CUHK464308 and CHUK464409 from the Research Grants Council of Hong Kong; grants from the Areas of Excellence (AoE) Scheme of Hong Kong on Marine Environmental Research and Innovative Technology (Project AoE/P-04/04) and Organelle Biogenesis and Function (Grant AoE/M-05/12); Grants SRG2013-00040-FHS, CPG2014-00014-FHS and MYRG2014-00062-FHS from the University of Macau; and The Macau Fund for Development of Science and Technology Grant FDCT114/2013/A3 (to W.G.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was substantially supported by Grants CUHK464308 and CHUK464409 from the Research Grants Council of Hong Kong; grants from the Areas of Excellence (AoE) Scheme of Hong Kong on Marine Environmental Research and Innovative Technology (Project AoE/P-04/04) and Organelle Biogenesis and Function (Grant AoE/M-05/12); Grants SRG2013-00040-FHS, CPG2014-00014-FHS and MYRG2014-00062-FHS from the University of Macau; and The Macau Fund for Development of Science and Technology Grant FDCT114/2013/A3 (to W.G.).
Footnotes
- BW
- body weight
- CRISPR/Cas9
- Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated system
- dpf
- days post fertilization
- EV
- early vitellogenic
- FG
- full grown
- FSHR
- FSH receptor
- gDNA
- genomic DNA
- GSI
- gonadosomatic index
- HMA
- heteroduplex motility assay
- HRMA
- high-resolution melt analysis
- KO
- knockout
- PG
- primary growth stage
- PV
- previtellogenic
- qPCR
- quantitative PCR
- RT
- reverse transcription
- RVD
- repeat variable diresidue
- SL
- standard length
- TALEN
- Transcription Activator-Like Effector Nuclease
- WT
- wild type.
References
- 1. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199. [DOI] [PubMed] [Google Scholar]
- 2. Huhtaniemi I. The Parkes Lecture. Mutations of gonadotrophin and gonadotrophin receptor genes: what do they teach us about reproductive physiology? J Reprod Fertil. 2000;119:173–186. [DOI] [PubMed] [Google Scholar]
- 3. Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. [DOI] [PubMed] [Google Scholar]
- 4. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. [DOI] [PubMed] [Google Scholar]
- 5. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294–17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fevold SL, Hisaw FL, Leonard SL. The gonad stimulating and the luteinizing hormone of the anterior lobe of the hypophysis. Am J Physiol. 1931;97:291–301. [Google Scholar]
- 7. Burzawa-Gerard E. Chemical data on pituitary gonadotropins and their implication to evolution. Can J Fish Aquat Sci. 1982;39:80–91. [Google Scholar]
- 8. Donaldson EM. Reproductive endocrinology of fishes. Am Zool. 1973;73:909–927. [Google Scholar]
- 9. Burzawa-Gerard E, Fontaine YA. The gonadotropins of lower vertebrates. Gen Comp Endocrinol. 1972;3(suppl):715–728. [Google Scholar]
- 10. Idler DR, Ng TB. Teleost gonadotropins: isolation, biochemistry, and function. In: Hoar WS, Randall DJ, Donaldson EM, eds. Fish Physiology. Vol IX (Part A). New York: Academic Press; 1983:187–221. [Google Scholar]
- 11. Idler DR, Bazar LS, Hwang SJ. Fish gonadotropin(s). III. Evidence for more than one gonadotropin in chum salmon pituitary glands. Endocr Res Commun. 1975;2:237–249. [DOI] [PubMed] [Google Scholar]
- 12. Idler DR, Ng TB. Studies on two types of gonadotropins from both salmon and carp pituitaries. Gen Comp Endocrinol. 1979;38:421–440. [DOI] [PubMed] [Google Scholar]
- 13. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki K, Kawauchi H, Nagahama Y. Isolation and characterization of subunits from two distinct salmon gonadotropins. Gen Comp Endocrinol. 1988;71:302–306. [DOI] [PubMed] [Google Scholar]
- 15. Li MD, Ford JJ. A comprehensive evolutionary analysis based on nucleotide and amino acid sequences of the α- and β-subunits of glycoprotein hormone gene family. J Endocrinol. 1998;156:529–542. [DOI] [PubMed] [Google Scholar]
- 16. Querat B, Sellouk A, Salmon C. Phylogenetic analysis of the vertebrate glycoprotein hormone family including new sequences of sturgeon (Acipenser baeri) β subunits of the two gonadotropins and the thyroid-stimulating hormone. Biol Reprod. 2000;63:222–228. [DOI] [PubMed] [Google Scholar]
- 17. Swanson P, Dickey JT, Campbell B. Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem. 2003;28:53–59. [Google Scholar]
- 18. Yaron Z, Gur G, Melamed P, Rosenfeld H, Elizur A, Levavi-Sivan B. Regulation of fish gonadotropins. Int Rev Cytol. 2003;225:131–185. [DOI] [PubMed] [Google Scholar]
- 19. Chauvigne F, Zapater C, Gasol JM, Cerda J. Germ-line activation of the luteinizing hormone receptor directly drives spermiogenesis in a nonmammalian vertebrate. Proc Natl Acad Sci USA. 2014;111:1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogiwara K, Fujimori C, Rajapakse S, Takahashi T. Characterization of luteinizing hormone and luteinizing hormone receptor and their indispensable role in the ovulatory process of the medaka. PLoS One. 2013;8:e54482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomez JM, Weil C, Ollitrault M, Le Bail PY, Breton B, Le Gac F. Growth hormone (GH) and gonadotropin subunit gene expression and pituitary and plasma changes during spermatogenesis and oogenesis in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol. 1999;113:413–428. [DOI] [PubMed] [Google Scholar]
- 22. Davies B, Bromage N, Swanson P. The brain-pituitary-gonadal axis of female rainbow trout Oncorhynchus mykiss: effects of photoperiod manipulation. Gen Comp Endocrinol. 1999;115:155–166. [DOI] [PubMed] [Google Scholar]
- 23. Santos EM, Rand-Weaver M, Tyler CR. Follicle-stimulating hormone and its α and β subunits in rainbow trout (Oncorhynchus mykiss): purification, characterization, development of specific radioimmunoassays, and their seasonal plasma and pituitary concentrations in females. Biol Reprod. 2001;65:288–294. [DOI] [PubMed] [Google Scholar]
- 24. Sohn YC, Yoshiura Y, Kobayashi M, Aida K. Seasonal changes in mRNA levels of gonadotropin and thyrotropin subunits in the goldfish, Carassius auratus. Gen Comp Endocrinol. 1999;113:436–444. [DOI] [PubMed] [Google Scholar]
- 25. Swanson P. Salmon gonadotropins: reconciling old and new ideas. In: Scott AP, Sumpter JP, Kime DE, Rolfe MS, eds. Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish, 1991, Sheffield, UK: FishSymp; 91; 2–7. [Google Scholar]
- 26. Melamed P, Gur G, Rosenfeld H, Elizur A, Yaron Z. The mRNA levels of GtH Ib, GtH IIb and GH in relation to testicular development and testosterone treatment in pituitaries of male tilapia. Fish Physiol Biochem. 1997;17:93–98. [Google Scholar]
- 27. Kajimura S, Yoshiura Y, Suzuki M, Aida K. cDNA cloning of two gonadotropin β subunits (GTH-Iβ and -IIβ) and their expression profiles during gametogenesis in the Japanese flounder (Paralichthys olivaceus). Gen Comp Endocrinol. 2001;122:117–129. [DOI] [PubMed] [Google Scholar]
- 28. Vischer HF, Granneman JC, Linskens MH, Schulz RW, Bogerd J. Both recombinant African catfish LH and FSH are able to activate the African catfish FSH receptor. J Mol Endocrinol. 2003;31:133–140. [DOI] [PubMed] [Google Scholar]
- 29. Van der Kraak G, Suzuki K, Peter RE, Itoh H, Kawauchi H. Properties of common carp gonadotropin I and gonadotropin II. Gen Comp Endocrinol. 1992;85:217–229. [DOI] [PubMed] [Google Scholar]
- 30. Zapater C, Chauvigne F, Scott AP, Gomez A, Katsiadaki I, Cerda J. Piscine follicle-stimulating hormone triggers progestin production in gilthead seabream primary ovarian follicles. Biol Reprod. 2012;87:1–13. [DOI] [PubMed] [Google Scholar]
- 31. Chauvigne F, Verdura S, Mazon MJ, et al. Follicle-stimulating hormone and luteinizing hormone mediate the androgenic pathway in Leydig cells of an evolutionary advanced teleost. Biol Reprod. 2012;87:35. [DOI] [PubMed] [Google Scholar]
- 32. Aizen J, Kobayashi M, Selicharova I, Sohn YC, Yoshizaki G, Levavi-Sivan B. Steroidogenic response of carp ovaries to piscine FSH and LH depends on the reproductive phase. Gen Comp Endocrinol. 2012;178:28–36. [DOI] [PubMed] [Google Scholar]
- 33. Luckenbach JA, Dickey JT, Swanson P. Follicle-stimulating hormone regulation of ovarian transcripts for steroidogenesis-related proteins and cell survival, growth and differentiation factors in vitro during early secondary oocyte growth in Coho salmon. Gen Comp Endocrinol. 2010;171:52–63. [DOI] [PubMed] [Google Scholar]
- 34. Planas JV, Athos J, Goetz FW, Swanson P. Regulation of ovarian steroidogenesis in vitro by follicle-stimulating hormone and luteinizing hormone during sexual maturation in salmonid fish. Biol Reprod. 2000;62:1262–1269. [DOI] [PubMed] [Google Scholar]
- 35. Tyler CR, Sumpter JP, Kawauchi H, Swanson P. Involvement of gonadotropin in the uptake of vitellogenin into vitellogenic oocytes of the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 1991;84:291–299. [DOI] [PubMed] [Google Scholar]
- 36. Sreenivasulu G, Sridevi P, Sahoo PK, et al. Cloning and expression of StAR during gonadal cycle and hCG-induced oocyte maturation of air-breathing catfish, Clarias gariepinus. Comp Biochem Physiol B Biochem Mol Biol. 2009;154:6–11. [DOI] [PubMed] [Google Scholar]
- 37. Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50:S195–S219. [DOI] [PubMed] [Google Scholar]
- 38. Pang Y, Ge W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol Reprod. 1999;61:987–992. [DOI] [PubMed] [Google Scholar]
- 39. Patino R, Thomas P. Effects of gonadotropin on ovarian intrafollicular processes during the development of oocyte maturational competence in a teleost, the Atlantic croaker: evidence for two distinct stages of gonadotropin control of final oocyte maturation. Biol Reprod. 1990;43:818–827. [DOI] [PubMed] [Google Scholar]
- 40. Yoshizaki G, Shusa M, Takeuchi T, Patino R. Gonadotropin-dependent oocyte maturational competence requires activation of the protein kinase A pathway and synthesis of RNA and protein in ovarian follicles of Nibe, Nibea mitsukurii (Teleostei, Sciaenidae). Fish Physiol Biochem. 2001;25:201–208. [Google Scholar]
- 41. So WK, Kwok HF, Ge W. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits—their spatial-temporal expression patterns and receptor specificity. Biol Reprod. 2005;72:1382–1396. [DOI] [PubMed] [Google Scholar]
- 42. Kwok HF, So WK, Wang Y, Ge W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors—evidence for their distinct functions in follicle development. Biol Reprod. 2005;72:1370–1381. [DOI] [PubMed] [Google Scholar]
- 43. Zhou R, Tsang AH, Lau SW, Ge W. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the zebrafish ovary: evidence for potentially dual roles of PACAP in controlling final oocyte maturation. Biol Reprod. 2011;85:615–625. [DOI] [PubMed] [Google Scholar]
- 44. Dahlem TJ, Hoshijima K, Jurynec MJ, et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8:e1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bedell VM, Wang Y, Campbell JM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore FE, Reyon D, Sander JD, et al. Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PLoS One. 2012;7:e37877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang N, Sun C, Gao L, et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 2013;23:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hwang WY, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiao A, Wang Z, Hu Y, et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 2013;41:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cermak T, Doyle EL, Christian M, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meeker N, Hutchinson S, Ho L, Trede N. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. BioTechniques. 2007;43:610–614. [DOI] [PubMed] [Google Scholar]
- 52. Ota S, Hisano Y, Muraki M, et al. Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells. 2013;18:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen W, Ge W. Ontogenic expression profiles of gonadotropins (fshb and lhb) and growth hormone (gh) during sexual differentiation and puberty onset in female zebrafish. Biol Reprod. 2012;86:1–11. [DOI] [PubMed] [Google Scholar]
- 54. Raz E. Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet. 2003;4:690–700. [DOI] [PubMed] [Google Scholar]
- 55. Chen W, Ge W. Gonad differentiation and puberty onset in the zebrafish: evidence for the dependence of puberty onset on body growth but not age in females. Mol Reprod Dev. 2013;80:384–392. [DOI] [PubMed] [Google Scholar]
- 56. Wang Y, Ge W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: evidence for their differential roles during sexual maturation and ovulatory cycle. Biol Reprod. 2004;71:2056–2064. [DOI] [PubMed] [Google Scholar]
- 57. Taranger GL, Carrillo M, Schulz RdW, et al. Control of puberty in farmed fish. Gen Comp Endocrinol. 2010;165:483–515. [DOI] [PubMed] [Google Scholar]
- 58. Okuzawa K. Puberty in teleosts. Fish Physiol Biochem. 2002;26:31–41. [Google Scholar]
- 59. Weltzien FA, Andersson E, Andersen O, Shalchian-Tabrizi K, Norberg B. The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comp Biochem Physiol A Mol Integr Physiol. 2004;137:447–477. [DOI] [PubMed] [Google Scholar]
- 60. Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. [DOI] [PubMed] [Google Scholar]
- 61. Tapanainen JS, Aittomaki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–206. [DOI] [PubMed] [Google Scholar]
- 62. Kumar TR. FSHβ knockout mouse model: a decade ago and into the future. Endocrine. 2009;36:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leal MC, Skaar KS, Franca LR, Schulz RW. The effects of IGF-I on zebrafish testis in tissue culture. Anim Reprod. 2006;3:181. [Google Scholar]
- 64. Takatsu K, Miyaoku K, Roy SR, et al. Induction of female-to-male sex change in adult zebrafish by aromatase inhibitor treatment. Sci Rep. 2013;3:3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A Mol Integr Physiol. 2004;137:11–20. [DOI] [PubMed] [Google Scholar]
- 66. Matthews CH, Borgato S, Beckpeccoz P, et al. Primary amenorrhea and infertility due to a mutation in the β-subunit of follicle-stimulating-hormone. Nat Genet. 1993;5:83–86. [DOI] [PubMed] [Google Scholar]
- 67. Layman LC, Lee EJ, Peak DB, et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N Engl J Med. 1997;337:607–611. [DOI] [PubMed] [Google Scholar]
- 68. Matthews C, Chatterjee VK. Isolated deficiency of follicle-stimulating hormone revisited. New Engl J Med. 1997;337:642–642. [DOI] [PubMed] [Google Scholar]
- 69. Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the β-subunit of follicle-stimulating hormone. N Engl J Med. 1998;338:1729–1732. [DOI] [PubMed] [Google Scholar]
- 70. Weiss J, Axelrod L, Whitcomb RW, Harris PE, Crowley WF, Jameson JL. Hypogonadism caused by a single amino-acid substitution in the β subunit of luteinizing-hormone. New Engl J Med. 1992;326:179–183. [DOI] [PubMed] [Google Scholar]