Abstract
BACKGROUND
Duration of esthetic treatments may contribute to subject satisfaction.
OBJECTIVE
Describe response duration with onabotulinumtoxinA in crow's feet lines (CFL) and the association of duration with perception of improvement.
METHODS
Subjects from 2 double-blind, placebo-controlled trials received onabotulinumtoxinA 24 U in CFL; Study 2 subjects could also receive 20 U in glabella. At Day 30, responders achieved ≥1-grade improvement in Facial Wrinkle Scale (FWS) scores. Median duration of effect for responders and for responders stratified by Subject's Global Assessment of Change in CFL (SGA-CFL) was determined.
RESULTS
Of 1,362 subjects, 833 received onabotulinumtoxinA. In Study 2, 305 subjects also received 20 U in glabella. In Study 1 (150-day follow-up), per investigator and subject assessments, respectively, median response duration was 125 and 144 days for dynamic lines and 137 and 148 days for static lines. Median response duration for dynamic and static lines in Study 2 (120-day follow-up) was 119 to 121 days per investigator and subject assessments. Subjects reporting greater improvement on the SGA-CFL tended to have a longer duration of response on investigator FWS scores at maximum smile.
CONCLUSION
Response duration with onabotulinumtoxinA in CFL was ≥4 months. Subject perception of CFL improvement may be associated with response duration.
Botulinum toxin Type A was first shown to reduce the appearance of facial lines more than 20 years ago.1–3 Since then, the number of botulinum toxin procedures has increased from almost 790,000 annually in 2000 to more than 6.6 million procedures in 2014.4 OnabotulinumtoxinA (Botox; Allergan plc, Dublin, Ireland) has been extensively evaluated for treatment of glabellar lines (GL) and crow's feet lines (CFL), and has been investigated for treatment of other facial rhytids.5–9 In multicenter, randomized controlled trials, onabotulinumtoxinA produced consistently high responder rates and rapid onset of action in subjects with GL10–12 and, more recently, those with CFL.13,14 OnabotulinumtoxinA is approved for treatment of GL and CFL in the United States, European Union, and countries worldwide.
The duration of treatment response to onabotulinumtoxinA is likely an important factor in subject satisfaction with the treatment experience; it influences retreatment intervals and, in turn, treatment costs and patient convenience.15 In studies of onabotulinumtoxinA for treatment of GL, high subject satisfaction reported at Day 120 coincided with a response duration of at least 4 months.10,11,15 A meta-analysis of data from 4 phase 3 trials found that subject perception of improvement at Day 30 corresponded with a greater duration of effect on dynamic lines.12
Limited information is available on the duration of clinical response in individuals with CFL.16 A multicenter, dose-ranging, placebo-controlled phase 2 study (N = 162) evaluated onabotulinumtoxinA at total doses of 6, 12, 24, or 36 U given through 3 injections per side in the lateral aspect of the orbicularis oculi.16 The median duration of response was 120 days with the 24 U dose, which was longer than the duration with lower doses and, paradoxically, somewhat longer than the duration with the highest dose.16 Based on the Subject's Global Assessment of Change in CFL (SGA-CFL), subjects consistently favored the 24 U dose over the other doses. The clinical efficacy and safety of onabotulinumtoxinA at a 24 U dose were further evaluated in the phase 3 CFL studies.13,14
This report further describes investigator- and subject-assessed duration of response with onabotulinumtoxinA for treatment of CFL. It also examines the association of response duration with subjects' perception of improvement in their CFL.
Methods
Two double-blind, placebo-controlled, multicenter phase 3 trials evaluated the duration of response of onabotulinumtoxinA in subjects with moderate to severe CFL. The study designs and eligibility criteria were reported previously.13,14 Briefly, Study 1 and Study 2 were conducted at 23 and 34 sites, respectively, in the United States, Canada, and the European Union. Eligible subjects were men or women aged ≥18 years with moderate to severe bilaterally symmetrical CFL at maximum smile, measured using the 4-grade Facial Wrinkle Scale with Photonumeric Guide (FWS; 0 = none, 1 = mild, 2 = moderate, 3 = severe). Subjects in Study 2 were also required to have moderate to severe GL at maximum frown as assessed by the investigator using the FWS. In both studies, subjects were excluded if they were currently receiving or had previously received botulinum toxin treatment of any serotype.
Both studies were approved by independent ethics committees or institutional review boards at each site and were conducted in accordance with Good Clinical Practice guidelines and all relevant local and country privacy requirements. All subjects provided written informed consent.
Treatment
Eligible subjects were randomized to receive onabotulinumtoxinA or placebo. Treatment details were previously reported.13,14 In Study 1, subjects were assigned (1:1) to receive onabotulinumtoxinA 24 U or placebo (saline), delivered via 3 injections per side into the CFL. Injections of either onabotulinumtoxinA 4 U or placebo had a volume of 0.1 mL. Follow-up visits occurred at Weeks 1 and 2 and at Days 30, 60, 90, and 120, with the final study visit at Day 150.
In Study 2, subjects were assigned (1:1:1) to receive onabotulinumtoxinA 24 U (24 U to CFL; saline to GL), onabotulinumtoxinA 44 U (24 U to CFL; 20 U to GL), or placebo (to CFL and GL). Six injections were delivered into the CFL, as in Study 1. Five injections of onabotulinumtoxinA 4 U or placebo were delivered to the GL area. Follow-up visits were on the same schedule as in Study 1, except that subjects received a second treatment with onabotulinumtoxinA or placebo on Day 120. Investigators in both studies used protocol-specified injection patterns and injection techniques.13,14
Efficacy Measures
The investigator and subject separately evaluated CFL at each study visit using the FWS. This assessment was performed at maximum smile (i.e., biggest smile) to evaluate dynamic lines and at rest (i.e., relaxed face) to evaluate static lines. Response to treatment was defined by at least a 1-grade improvement from baseline and was evaluated separately for dynamic and static lines. Investigators and subjects were trained in grading the severity of CFL using the FWS.
At each posttreatment visit, subjects completed the 7-point SGA-CFL (1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, and 7 = very much worse).
Duration of response, defined as the number of days from injection until the subject returned to nonresponder status, was determined for subjects who had a treatment response at Day 30. An analysis was performed to assess the median duration of response for Day 30 responders based on SGA-CFL scores at Day 30.
Statistical Analyses
Efficacy analyses were performed on the intent-to-treat (ITT) population, which comprised all randomized subjects. Missing efficacy data at scheduled visits were imputed using a last-observation-carried-forward approach.
The CFL rating at each visit represented the average of both sides of the face.
The proportion of responders in each treatment group was compared using the Cochran-Mantel-Haenszel test, stratified by investigator site. Two-sided 95% CIs for the difference between treatment groups were determined. The median duration of response was estimated for Day 30 responders using Kaplan–Meier methodology. The median duration of response was estimated for Day 30 responders stratified by SGA-CFL score at Day 30 using Kaplan–Meier methodology. SAS version 9.2 or higher (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
In total, 1,362 subjects were enrolled in Studies 1 (N = 445) and 2 (N = 917), of whom 833 subjects (61.2%) received onabotulinumtoxinA and 529 (38.8%) received placebo in the crow's feet region. In Study 2, 305 subjects also received onabotulinumtoxinA 20 U in the glabellar region (total dose, 44 U). Most subjects completed the studies (Study 1, 93.3%; Study 2, 90.4%). Overall, the most common reasons for early discontinuation were personal decision (4.4%) and lost to follow-up (3.0%).13,14
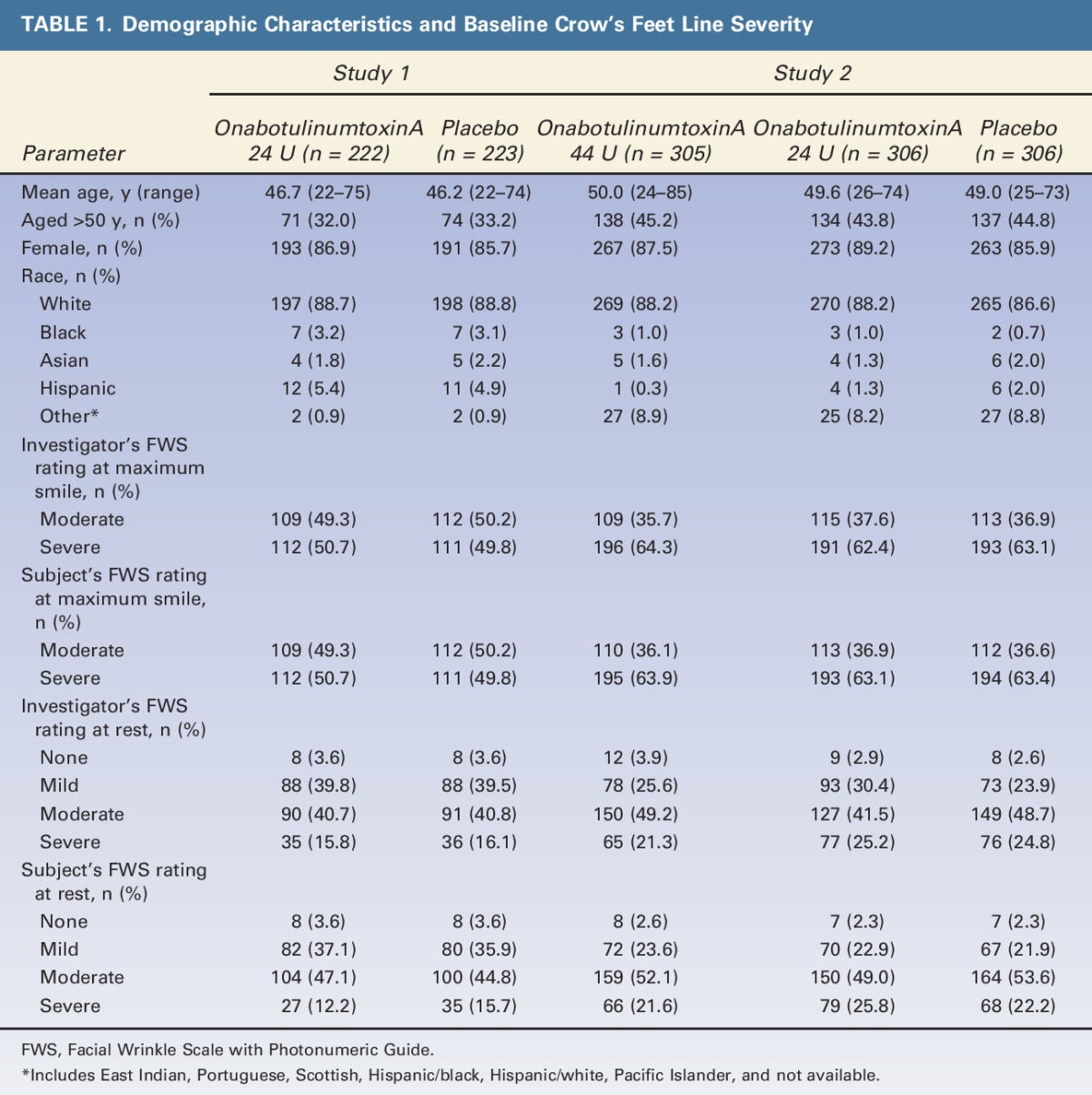
Demographic characteristics were well balanced between onabotulinumtoxinA and placebo treatment groups within each study. However, subjects in Study 2 were, on average, older than those in Study 1 (Table 1). Mean age was 46.4 years in Study 1 and 49.5 years in Study 2, with a greater percentage of subjects in Study 1 versus Study 2 aged ≤50 years (67.4% vs 55.4%, respectively). OnabotulinumtoxinA and placebo treatment groups were also well balanced with respect to CFL severity at baseline within each study. However, compared with Study 1, a greater proportion of subjects in Study 2 had severe dynamic CFL (63.2% vs 50.2%) at baseline; these observations are consistent with the greater mean age in Study 2.
TABLE 1.
Demographic Characteristics and Baseline Crow's Feet Line Severity
Responder Rates
A significantly greater proportion of subjects achieved ≥1-grade improvement from baseline in dynamic and static CFL with onabotulinumtoxinA versus placebo, based on the investigators' and subjects' assessments at Day 30 (p < .001) (Table 2). In Study 1, Day 30 responder rates for dynamic lines with onabotulinumtoxinA versus placebo, respectively, were 87.4% versus 12.1% (p < .001) on the investigator's assessment and 78.8% versus 11.2% (p < .001) on the subject's assessment. Responder rates achieved with onabotulinumtoxinA were somewhat lower for static CFL but were significantly greater than rates in the placebo group (p < .001).13,14
TABLE 2.
Proportion of Subjects Who Achieved ≥1-Grade Improvement from Baseline in Crow's Feet Line Severity at Day 30
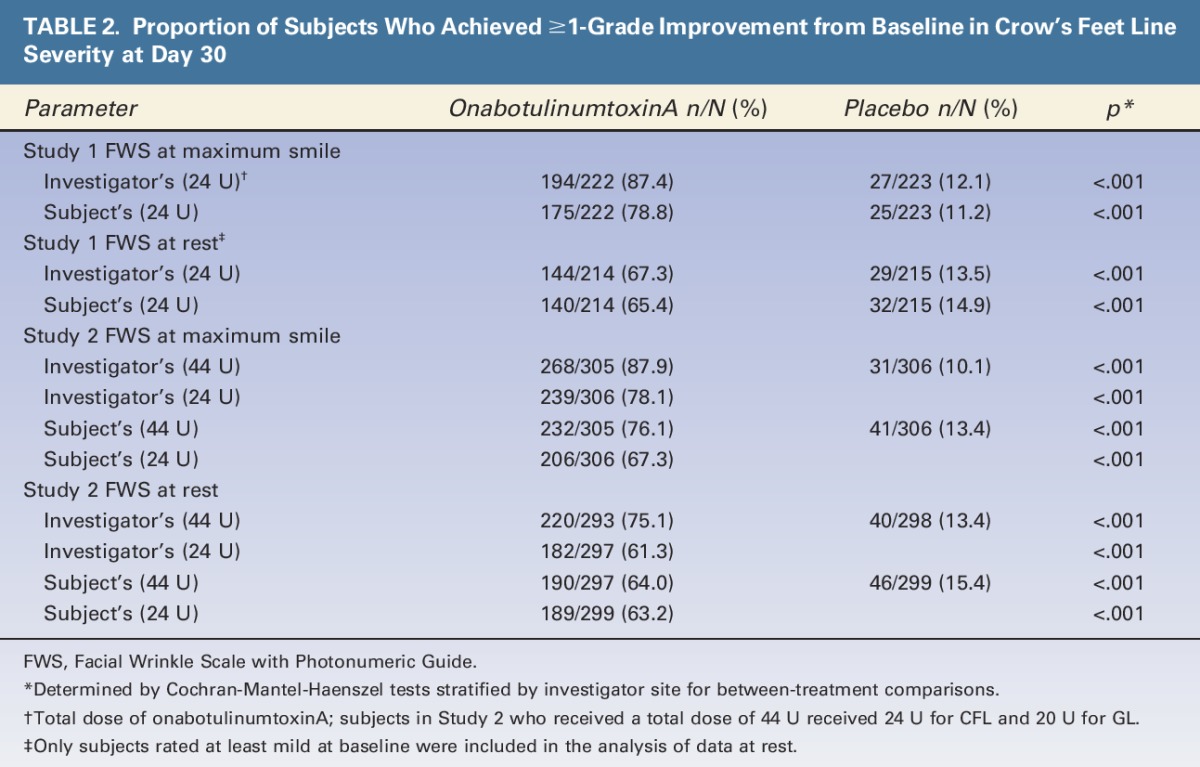
Similar findings were obtained in Study 2, although responder rates tended to be slightly lower, consistent with the greater proportion of subjects with severe CFL at baseline (Table 2). In Study 2, subjects receiving onabotulinumtoxinA for both CFL and GL (44 U) had higher responder rates versus those receiving onabotulinumtoxinA only for CFL (24 U) on the investigator's assessment of dynamic and static CFL (both p < .001) and on the subjects' assessment of dynamic CFL (p = .013).
Duration of Clinical Response
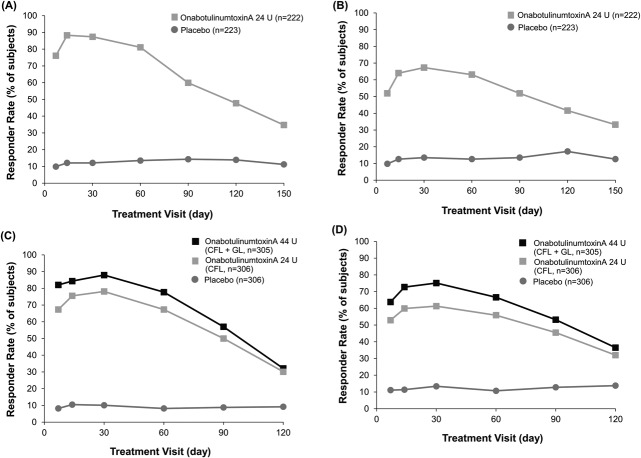
The proportion of subjects who maintained treatment responses at maximum smile and at rest declined slowly after Day 30 in Studies 1 and 2 (first treatment) (Figure 1). Responder rates remained significantly greater with onabotulinumtoxinA versus placebo at all study visits (p < .001).
Figure 1.
Responder rates (≥1-grade improvement) at maximum smile (A) and at rest (B) on the investigator's FWS in Study 1. All between-group differences were statistically significant (p < .001). Responder rates (≥1-grade improvement) at maximum smile (C) and at rest (D) on the investigator's FWS in Study 2 in the first treatment cycle. All differences between the onabotulinumtoxinA groups versus the placebo group were statistically significant (p < .001). Only subjects rated at least mild at baseline were included in the analysis performed at rest in both studies. Day 30 was the primary time point for the efficacy analysis in both studies. FWS, Facial Wrinkle Scale with Photonumeric Guide.
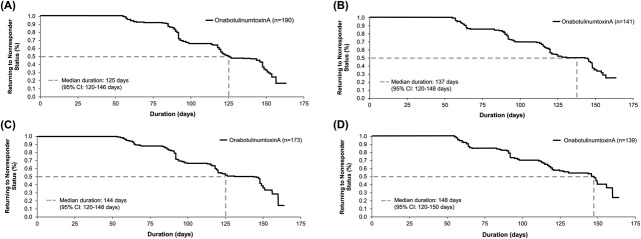
The final visit in Study 1 was scheduled for Day 150. On Kaplan–Meier analysis, the estimated median duration of response on the investigators' and subjects' assessments were, respectively, 125 and 144 days for dynamic CFL and 137 and 148 days for static CFL (Figure 2).
Figure 2.
Duration of treatment response based on Kaplan–Meier analysis in Day 30 responders (≥1-grade improvement) on the investigator's FWS and subject's FWS in Study 1 based on maximum smile (A) and at rest (B) on the investigator's FWS and at maximum smile (C) and at rest (D) on the subject's FWS. FWS, Facial Wrinkle Scale with Photonumeric Guide.
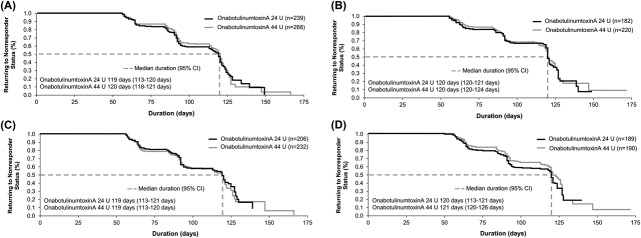
Subjects in Study 2 were to receive a second treatment cycle on Day 120, thus creating an earlier boundary for estimating treatment response duration versus Study 1. On Kaplan–Meier analysis, the estimated median duration of response was 119 to 121 days for both dynamic and static CFL on the investigators' and subjects' assessments (Figure 3). The median response duration was the same for subjects receiving onabotulinumtoxinA for only CFL and for both CFL and GL.
Figure 3.
Duration of treatment response based on Kaplan–Meier analysis in Day 30 responders (≥1-grade improvement) in Study 2 based on maximum smile (A) and at rest (B) on the investigator's FWS and at maximum smile (C) and at rest (D) on the subject's FWS. FWS, Facial Wrinkle Scale with Photonumeric Guide.
Subjects' Perception of Change in CFL
Using the SGA-CFL, a significantly greater proportion of subjects in the onabotulinumtoxinA group versus the placebo group classified themselves as “very much improved” or “much improved” at Day 30 in Studies 1 and 2 (p < .001).17
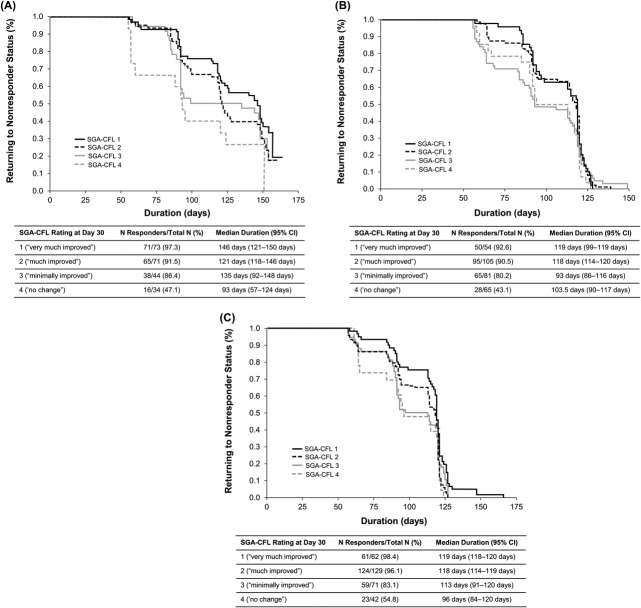
The duration of response with onabotulinumtoxinA (based on FWS responder analysis) stratified by the subjects' SGA-CFL ratings at Day 30 showed a median duration of 146 days in subjects rating themselves as “very much improved” (SGA-CFL score of 1) in Study 1 versus 121 days in subjects rating themselves “much improved” (SGA-CFL score of 2). The median duration of response for subjects with SGA-CFL scores of 3 (“minimally improved”) and 4 (“no change”) was 135 days and 93 days, respectively (Figure 4). In Study 2, the median duration of response for subjects with SGA-CFL scores of 1 or 2 were 119 and 118 days, respectively, and were longer than the median duration of response for subjects with SGA-CFL scores of 3 or 4 in the onabotulinumtoxinA 24 U and 44 U groups (Figure 4). In both studies, the proportion of responders with SGA-CFL scores of 1 and 2 followed parallel and similar courses and were generally different from the proportion of responders with scores of 3 or 4.
Figure 4.
Duration of treatment response based on Kaplan–Meier analysis in Day 30 responders (≥1-grade improvement) on the investigator's FWS at maximum smile according to how they rated their improvement at Day 30 on the SGA-CFL in Study 1 with onabotulinumtoxinA 24 U (A) and for Study 2 with onabotulinumtoxinA 24 U (B) and onabotulinumtoxinA 44 U (C). (A) p = .078. (B) p = .308. (C) p = .009. FWS, Facial Wrinkle Scale with Photonumeric Guide; SGA-CFL in crow's feet lines.
Discussion
Responders in the current analysis maintained treatment response for a median duration of 4 months or longer as assessed by investigators and subjects. In addition, subjects who perceived greater improvement in their CFL at Day 30 tended to have a greater duration of response to onabotulinumtoxinA than did those who reported little or no improvement, suggesting that patients' perceptions of treatment results may be directly associated with duration of effect. These perceptions may determine whether benefits of treatment translate to improvements in patients' well-being and may potentially influence patient behaviors, such as returning for follow-up treatments to maintain long-term effects.18
The estimates of response duration for onabotulinumtoxinA were somewhat longer in Study 1 versus Study 2, particularly on the subjects' assessments. The difference in median duration between studies ranged from 6 days in the investigators' assessments of dynamic CFL to 28 days in the subjects' assessments of static CFL. Multiple factors may account for the observed differences in response duration. First, subjects were followed for 150 days after receiving onabotulinumtoxinA in Study 1, whereas those in Study 2 received a second treatment on Day 120. Thus, duration data for 33% of subjects in Study 2 were censored while they were still responding to onabotulinumtoxinA, leading to a ceiling effect for response duration in Study 2. In addition, the cohort enrolled in Study 2 was generally older and included a greater proportion of subjects with severe CFL at maximum smile at baseline versus Study 1. These differences likely reflect the selection criteria for these studies; specifically, subjects in Study 2 were required to have moderate or severe CFL and GL at maximum smile/frown to qualify for entry, whereas subjects in Study 1 were required to have only moderate or severe CFL. It is also notable that subjects in Study 2 had more severe resting CFL at baseline compared with those in Study 1; greater baseline severity of resting CFL has been associated with a shorter duration of response to onabotulinumtoxinA.19
Subjects perceived that the duration of response with onabotulinumtoxinA was longer than that based on the investigators' assessments in Study 1, particularly for dynamic CFL (median duration, 144 and 125 days based on subject and investigator assessments, respectively). This difference may reflect how subjects and investigators evaluate CFL: namely, subjects generally observe their CFL area from a frontal perspective in the mirror and may be less able to observe a return of activity to that area, whereas physicians can look directly at the lines, from an oblique or lateral view, when making assessments. This explanation may also account for the lower subject-assessed responder rate at Day 30 compared with the investigator-assessed responder rate in Study 1 in that subjects may also be less able to observe a loss of activity in their CFL area. A 1-grade change is considered clinically meaningful in that it is the minimum response for observable benefit of treatment effects and is of relevance to clinicians and subjects. In Study 2, subjects did not perceive a difference in treatment response duration versus investigators. Again, the shorter 120-day follow-up may have limited sensitivity for detecting any differences in duration perceived by subjects and investigators in the second study. The shorter 120-day follow-up may also explain why the higher response rates in the onabotulinumtoxinA 44 U group versus response rates in the onabotulinumtoxinA 24 U group did not translate into a longer median response duration.
The median duration of 4 months or longer with onabotulinumtoxinA in these phase 3 studies is consistent with several smaller studies in subjects treated for CFL. In a multicenter, double-blind, randomized, placebo-controlled, dose-ranging study in 162 subjects with CFL followed for up to 180 days, the median time to return to baseline severity was 120 days for subjects receiving a total dose of onabotulinumtoxinA 24 U.16 Notably, in an open-label study involving 25 women who were followed for 9 months after a single treatment with onabotulinumtoxinA, improvement in static CFL was maintained through 6 months, based on observations by trained observers and 3-dimensional profilometry skin imaging.20
Response duration for onabotulinumtoxinA for CFL is similar to that observed in the treatment of GL. In a meta-analysis of 4 GL registration trials, median duration of response to onabotulinumtoxinA in subjects with moderate to severe GL was 125 days for responders, defined by a 1-grade improvement on the FWS at maximum contraction.12 The corresponding median duration of response for FWS at rest was 131 days. Subject follow-up in these studies was 120 days (with the exception of 1 study for which follow-up was 4 weeks), potentially limiting the response duration. Furthermore, results of a single-center study of 60 women suggest that, after simultaneous injection of onabotulinumtoxinA for treatment of CFL, GL, and forehead lines (total, 16 injections), the median response duration (defined as ≥25% improvement from baseline on a 9-point patient global assessment scale) for subjects receiving 4 U per injection site (as in the present studies) was approximately 12 to 16 weeks (i.e., 84–112 days). Overall, these findings suggest that onabotulinumtoxinA provides a long duration of clinical response when used for treatment of CFL, GL, or multiple upper facial rhytids.
Subject perception of improvement provides guidance regarding retreatment of CFL and, when considered with duration of response, may influence overall satisfaction with treatment.15 Thus, examining the relationship between patient-reported outcomes and clinical outcomes can lead to a more complete understanding of the benefits of treatment. Greater subject-reported improvement on the SGA-CFL at Day 30 in both studies (scores 1 and 2) was associated with a longer response duration on the investigator's FWS at maximum smile. In Study 1, the relatively longer median duration of response for subjects with an SGA-CFL score of 3 compared with subjects with a score of 2 may be due to the small sample size (n = 44) for subjects with a score of 3. As discussed below, additional limitations in study design may have precluded the detection of a significant relationship between SGA-CFL ratings and response duration after treatment of CFL with onabotulinumtoxinA 24 U.
Several factors limit interpretation of the current analyses. As previously discussed, the duration analyses were capped at Day 150 in Study 1 and Day 120 in Study 2, when a significant proportion of subjects were still responding to treatment, thereby potentially underestimating the median response duration. Future studies permitting a return to baseline status of all subjects may yield a longer observed duration of efficacy. In addition, differences in baseline CFL severity between trials may confound comparison of results. Assessments of responder rates and, subsequently, duration of clinical response were based on assessments at maximum smile and at rest: despite the clear instructions on the desired smile, the magnitude of the dynamic expression may have varied across visits based on each subject's effort, and static CFL may vary based on factors such as hydration. Finally, subjects with deep CFL or GL were not included in these studies, and results therefore cannot be extrapolated to those populations.
Dosing and results reported in this study are specific to onabotulinumtoxinA. This formulation is not interchangeable with other botulinum toxin products, and units cannot be converted using a dose ratio. Therefore, the results of this study cannot be extrapolated to other formulations of botulinum toxins.
Conclusions
OnabotulinumtoxinA produced treatment responses in most subjects with CFL, and duration of response was ≥4 months in both studies. The lower responder rate and shorter duration of response in Study 2 compared with Study 1 is most likely explained by the higher baseline CFL wrinkle severity and shorter follow-up period. Patterns were observed suggesting that duration of clinical response to onabotulinumtoxinA may be related to subject perceptions of CFL improvement after treatment. Further comparisons between clinical efficacy measures and patient-reported outcomes are warranted in assessments of esthetic treatments.
Footnotes
This study was funded by Allergan plc. Writing and editorial support for this article was provided by Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan plc.
Presented in part at the annual meeting of the American Society of Plastic Surgeons, October 10 to 14, 2014, Chicago, IL, and at the Anti-aging Medicine European Congress (AMEC), October 11 to 12, 2013, Paris, France.
L. Baumann serves as a consultant for, has received a research grant from, and is a stockholder of Allergan plc. S. Dayan has received research grants from Allergan plc. S. Connolly and N. Silverberg have no relevant financial relationships to disclose. X. Lei and C. J. Gallagher are employees of Allergan plc. The remaining author has indicated no significant interest with commercial supporters.
The opinions expressed in this article are those of the authors. The authors received no honorarium or other form of financial support related to the development of this article.
References
- 1.Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol 1992;18:17–21. [DOI] [PubMed] [Google Scholar]
- 2.Borodic GE. Botulinum A toxin for (expressionistic) ptosis overcorrection after frontalis sling. Ophthal Plast Reconstr Surg 1992;8:137–42. [DOI] [PubMed] [Google Scholar]
- 3.Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg 1993;119:1018–22. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Plastic Surgeons. 2014 Plastic surgery statistics report. Available at: http://www.plasticsurgery.org/news/plastic-surgery-statistics/2014-plastic-surgery-statistics.html. Accessed April 13, 2016. [Google Scholar]
- 5.Lowe NJ, Lask G, Yamauchi P, Moore D. Bilateral, double-blind, randomized comparison of 3 doses of botulinum toxin type A and placebo in patients with crow's feet. J Am Acad Dermatol 2002;47:834–40. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel- group, dose-ranging study of botulinum toxin type A in female subjects with horizontal forehead rhytides. Dermatol Surg 2003;29:461–7. [DOI] [PubMed] [Google Scholar]
- 7.Kaltreider SA, Kennedy RH, Woog JJ, Bradley EA, et al. Cosmetic oculofacial applications of botulinum toxin: a report by the American Academy of Ophthalmology. Ophthalmology 2005;112:1159–67. [DOI] [PubMed] [Google Scholar]
- 8.Dessy LA, Fallico N, Mazzocchi M, Scuderi N. Botulinum toxin for glabellar lines: a review of the efficacy and safety of currently available products. Am J Clin Dermatol 2011;12:377–88. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JL, Dayan SH, Cox SE, Yalamanchili R, et al. OnabotulinumtoxinA dose-ranging study for hyperdynamic perioral lines. Dermatol Surg 2012;38:1497–505. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers JA, Lowe NJ, Menter MA, Gibson J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002;46:840–9. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers JD, Lowe NJ, Menter MA, Gibson J, et al. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg 2003;112:1089–98. [DOI] [PubMed] [Google Scholar]
- 12.Glogau R, Kane M, Beddingfield F, Somogyi C, et al. OnabotulinumtoxinA: a meta-analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg 2012;38:1794–803. [DOI] [PubMed] [Google Scholar]
- 13.Carruthers A, Bruce S, de Coninck A, Connolly S, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crow's feet lines: a multicenter, randomized, controlled trial. Dermatol Surg 2014;40:1181–90. [DOI] [PubMed] [Google Scholar]
- 14.Moers-Carpi M, Carruthers J, Fagien S, Lupo M, et al. Efficacy and safety of onabotulinumtoxinA for treating crow's feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatol Surg 2015;41:102–12. [DOI] [PubMed] [Google Scholar]
- 15.Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol 2010;11:183–99. [DOI] [PubMed] [Google Scholar]
- 16.Lowe NJ, Ascher B, Heckmann M, Kumar C, et al. Double-blind, randomized, placebo-controlled, dose-response study of the safety and efficacy of botulinum toxin type A in subjects with crow's feet. Dermatol Surg 2005;31:257–62. [DOI] [PubMed] [Google Scholar]
- 17.Dayan S, Coleman WP, III, Dover JS, De Boulle K, et al. Effects of onabotulinumtoxinA treatment for crows feet lines on patient-reported outcomes. Dermatol Surg 2015;41(Suppl 1):S67–S74. [DOI] [PubMed] [Google Scholar]
- 18.Stotland MA, Kowalski JW, Ray BB. Patient-reported benefit and satisfaction with botulinum toxin type A treatment of moderate to severe glabellar rhytides: results from a prospective open-label study. Plast Reconstr Surg 2007;120:1386–93. [DOI] [PubMed] [Google Scholar]
- 19.Monheit G, Sattler G, Swift A, et al. Pooled analysis of two double-blind, placebo-controlled clinical trials of onabotulinumtoxinA in crow's feet lines to assess the association of baseline wrinkle severity and age with response and duration of effect [poster]. Paper Presented at: Annual Meeting of the American Society for Dermatologic Surgery; October 3–6, 2013; Chicago, IL.
- 20.Levy JL, Servant JJ, Jouve E. Botulinum toxin A: a 9-month clinical and 3D in vivo profilometric crow's feet wrinkle formation study. J Cosmet Laser Ther 2004;6:16–20. [DOI] [PubMed] [Google Scholar]