Abstract
BACKGROUND
Eyebrow loss may have substantial negative functional and social consequences.
OBJECTIVE
Evaluate the safety and efficacy of bimatoprost 0.03% in subjects with eyebrow hypotrichosis.
METHODS
This multicenter, double-masked study randomized adult females or males with eyebrow hypotrichosis to receive bimatoprost 0.03% twice (BID) or once daily (QD) or vehicle BID for 7 months. Primary endpoint was overall eyebrow fullness at Month 7. Secondary endpoints included eyebrow fullness (mm2), darkness (intensity units), and subject satisfaction with treatment. Safety was also assessed.
RESULTS
At Month 7, the proportion of subjects with improvement was significantly higher in bimatoprost groups versus vehicle (both, p < .001). Improvements occurred in both bimatoprost groups versus vehicle after Month 1 and continued through follow-up; eyebrow fullness and darkness improved as early as Months 2 and 1, respectively (both, p < .001). Greater satisfaction was reported with bimatoprost versus vehicle at Month 2 and all subsequent time points. Overall, 38.1%, 42.4%, and 35.5% of subjects in the bimatoprost BID, QD, and vehicle groups, respectively, experienced ≥1 treatment-emergent adverse event (TEAE). Most frequent TEAEs were similar across groups. No skin or iris hyperpigmentation or conjunctival hyperemia occurred.
CONCLUSION
Bimatoprost 0.03% BID and QD is safe, well tolerated, and effective for eyebrow hypotrichosis.
Eyebrows are a key feature of the face and play an important role in determining facial expression and aesthetics.1 Human perception studies have demonstrated that eyebrows may be just as important as the eyes in facial identification.2 Movement of the eyebrows plays a prominent role in an individual's ability to communicate effectively.3,4 Beyond the obvious cosmetic implications, eyebrow loss may have substantial functional and social consequences.2
Despite the negative impact it can have on appearance, eyebrow hypotrichosis has not received the same amount of attention because scalp hair loss or eyelash hypotrichosis and treatment options are more limited.4 Cosmetic approaches include using eyebrow pencils or applying permanent tattoos; however, these approaches can often look unnatural. Complications arising from tattooing have prompted the Food and Drug Administration (FDA) to investigate the safety of cosmetic tattoo ink.5 Furthermore, removal of cosmetic tattoos can be costly and complex.6 Surgical modalities include flaps from the temporal region or from the opposite eyebrow or hair transplantation. Currently approved scalp hair growth products (e.g., minoxidil) are not indicated for eyebrows.7
Bimatoprost ophthalmic solution 0.03% (Latisse; Allergan Inc., Irvine, CA) was approved by the FDA in 2008 for the treatment of hypotrichosis of the eyelashes.8 Early case reports and small exploratory studies of topical application of bimatoprost to the eyebrow indicated improvements in growth and patient satisfaction.9–11 Bimatoprost is a synthetic prostamide F2α analog.12,13 The precise mechanism by which bimatoprost stimulates hair growth is not known; however, it is believed to increase the proportion of follicles in the anagen (growth) phase of the hair cycle by increasing the duration of anagen, and by stimulating transition from telogen (resting phase) to anagen.14–16 Bimatoprost has increased hair darkness as a result of increased melanogenesis and increased hair thickness and fullness by increasing the size of the dermal papilla and hair bulb.15,17,18 The objectives of this study were to assess the safety and efficacy of the application of bimatoprost solution 0.03% to the eyebrows once or twice daily for 7 months to increase overall eyebrow fullness and darkness in adults with eyebrow hypotrichosis.
METHODS
Study Design
This multicenter, double-masked, randomized, vehicle-controlled, 3-arm, parallel-group study was designed to evaluate the safety and efficacy of bimatoprost 0.03% compared with vehicle in subjects with eyebrow hypotrichosis (clinicalTrials.gov identifier: NCT01765764). This study was approved by the Institutional Review Board or Independent Ethics Committee at each site and was conducted in compliance with Good Clinical Practice guidelines. All subjects provided written informed consent.
Subjects were stratified by age group (<45 vs ≥ 45 yrs) and were randomly assigned in a 1:1:1 ratio to receive bimatoprost 0.03% twice daily (BID), bimatoprost 0.03% once daily (QD), or vehicle BID for 7 months of treatment. Randomization was managed through automated interactive voice/web response systems. In all 3 treatment groups, subjects were instructed to apply study medication twice daily, once in the morning (AM dose) and once in the evening (PM dose). In the bimatoprost QD group, the AM dose consisted of vehicle with the PM dose consisting of bimatoprost 0.03%. In the bimatoprost BID group, both the AM and PM doses consisted of bimatoprost 0.03%. In the vehicle group, both the AM and PM doses consisted of vehicle. Treatment group assignment was masked to both the subjects and the investigators throughout the study.
The total duration of study participation from the screening visit was approximately 9 months, with a total of 11 visits: at screening (Day −21 to −7), Day 1 (baseline), Week 1, Months 1, 2, 3, 4, 5, 6, and 7 (or early study discontinuation), and at Month 8 posttreatment follow-up. Eyebrow stencils were used to standardize both the application of the study medication and the area of grooming. Subjects were instructed to groom their eyebrows outside the treatment area, as delineated by the stencil, with scissors or tweezers only; grooming within the eyebrow treatment area was prohibited. The stencil was also used as a guide to determine the area of study medication application.
Subjects
Eligible subjects were female or male, aged 18 years or older with eyebrow hypotrichosis, defined as either a Grade 1 or 2 on the Allergan Global Eyebrow Assessment (GEBA) scale with photonumeric guide. The GEBA instrument is a validated 4-point scale used to grade fullness of the eyebrows (1 = very sparse, 2 = sparse, 3 = full, and 4 = very full).
Subjects were excluded if they had an uncontrolled systemic disease or any known disease, infection, or abnormality in the eyebrow area or hair shaft. Subjects with systemic diseases, including all types of alopecia areata, which could prevent hair growth or produce abnormal growth were excluded, along with subjects with substantial permanent eyebrow loss due to overgrooming; any skin pigmentation/depigmentation, tattoos, moles, scars, or other characteristics within the eyebrow area that could confound evaluation. Subjects who used permanent injectable fillers in and around the eyebrow area within 12 months; or systemic treatment within 12 months of screening with agents that may affect hair growth were excluded, as were subjects who had undergone any facial surgery (e.g., brow lift) or eyebrow extension within 3 months of screening. Subjects who used temporary or semipermanent eyebrow fillers or eyebrow tint, dye, or bleaching within 2 months of screening and female subjects who were pregnant, nursing, or planning for a pregnancy during the study were excluded.
Study Endpoints
Efficacy and safety were assessed posttreatment at Week 1, at Months 1 through 7 (or on early discontinuation), and at the Month 8 follow-up visit. The primary efficacy endpoint was the investigator's assessment of the subject's overall eyebrow fullness at Month 7 using the GEBA scale. The primary efficacy variable was defined as the proportion of subjects with at least a 1-grade increase (improvement) in the GEBA grade from baseline at Month 7. Secondary efficacy endpoints at Month 7 included subjects' eyebrow fullness (mm2) and darkness (intensity units [IU]; negative values are representative of eyebrow darkening) as measured using Digital Monitoring System Image Analysis (DMSIA). Subject satisfaction with eyebrow treatment at Month 7 was measured by item 6 of the 9-item Eyebrow Satisfaction Scale (ESS, Follow-up version). Assessments could range from 1 (very satisfied) to 5 (very dissatisfied). Safety measures included treatment-emergent adverse events (TEAEs), vital sign measurements (i.e., blood pressure, pulse rate, and urine pregnancy test), and laboratory tests (i.e., thyroid-stimulating hormone and ferritin) and hematology values.
Statistical Analysis
To have statistical power to evaluate the primary efficacy variable, a sample size of 348 was proposed, with a 1:1:1 randomization ratio to study groups. Accounting for an attrition rate of 15%, 295 subjects were anticipated to complete the study. Assuming a 25% vehicle response rate based on the primary efficacy endpoint and a 2-sided Type 1 error of 0.05, a sample size of 348 with a 1:1:1 randomization ratio for bimatoprost BID, bimatoprost QD, or vehicle was chosen to detect a difference of 20%, 22%, and 25% in favor of bimatoprost BID, with a statistical power of 86%, 92%, and 97%, respectively. Efficacy analyses reported here were performed on the intent-to-treat population, defined as all randomized subjects. The GEBA and ESS Item 6 grades were analyzed using a pooled point estimate for the treatment difference, based on the Mantel–Haenszel method, stratified by age group. The 95% CIs for the differences were constructed based on the Greenland–Robins formula.19 Treatment-by-age interaction was tested using the Breslow–Day test, with a significance level of 0.1.
The statistical analysis aimed to compare bimatoprost dose groups versus vehicle. Bimatoprost BID or bimatoprost QD was considered to be superior to vehicle if a 2-sided Mantel–Haenszel test showed p ≤ .05. To control the Type 1 error rate for the primary efficacy endpoint, a serial gatekeeping approach was used: the comparison between bimatoprost QD and vehicle was tested only if statistical analysis of bimatoprost BID versus vehicle was p ≤ .05. To control the Type 1 error rate for multiple secondary endpoints, a serial gatekeeping approach was used based on the importance of the secondary variables: eyebrow fullness, eyebrow darkness, and ESS Item 6. In addition, a gatekeeping approach was used for the statistical analysis: bimatoprost BID versus vehicle was tested at the 2-sided α = 0.05 level; if p value was ≤0.05, bimatoprost QD versus vehicle was tested at the 2-sided α = 0.05 level. Changes in eyebrow fullness and darkness were analyzed using DMSIA at Month 7; pairwise comparisons were made using the van Elteren test, stratified by age group. Safety analyses were performed on the safety population, defined as all subjects who received at least 1 dose of study treatment.
RESULTS
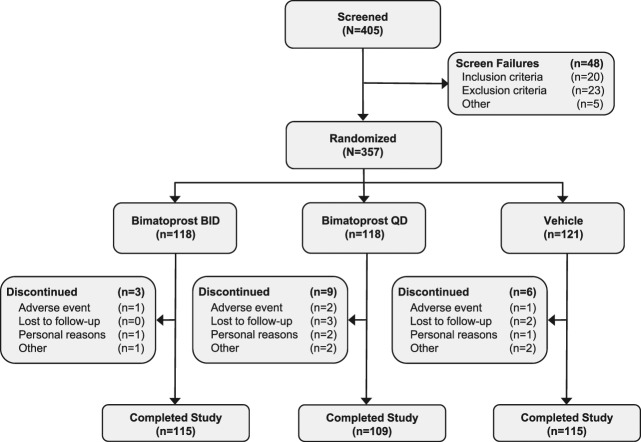
A total of 357 subjects were enrolled in this study, 118 in each of the bimatoprost groups and 121 in the vehicle group (Figure 1). Overall, 339 of 357 (95.0%) subjects completed the study: 115 of 118 (97.5%), 109 of 118 (92.4%), and 115 of 121 (95.0%) in the bimatoprost BID, bimatoprost QD, and vehicle groups, respectively. Eighteen (5.0%) subjects discontinued the study; 4 (1.1%) discontinued because of AEs, 5 (1.4%) were lost to follow-up, 4 (1.1%) discontinued because of personal reasons, and 5 (1.4%) provided reason as “other.”
Figure 1.
Disposition of subjects.
The 3 treatment groups were comparable at baseline (Table 1). The mean age of the subjects was 54.0 years (range, 19–82 years) and most were female (94.7%) and were white (76.8%). Subjects had a similar distribution of GEBA scores across treatment groups at baseline.
TABLE 1.
Demographics and Other Baseline Characteristics (Intent-to-treat Population)

Primary Endpoint: Global Eyebrow Assessment
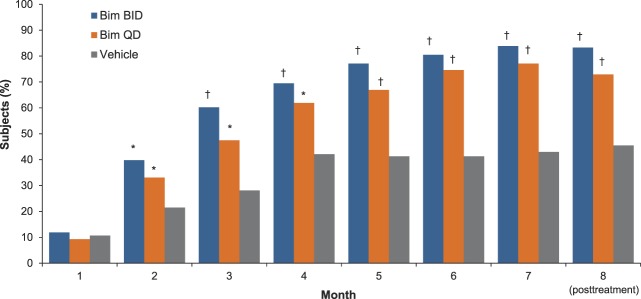
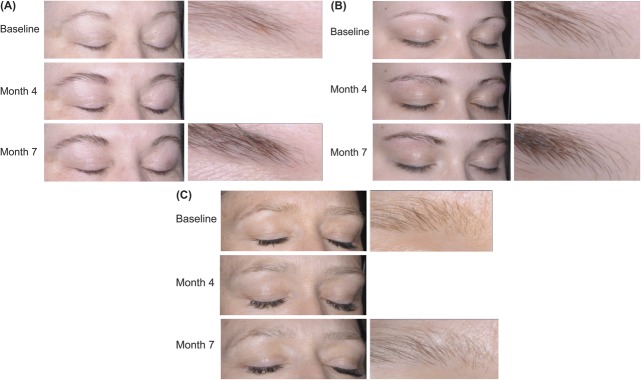
The proportion of subjects with at least a 1-grade increase (improvement) in the GEBA scale from baseline at Month 7 was significantly higher in the bimatoprost BID group (83.9%) and bimatoprost QD group (77.1%) compared with the vehicle group (43.0%) (p < .001, both comparisons) (Figure 2). Note that the bimatoprost BID and QD groups showed similar results. After Month 1, significant improvements were observed in both bimatoprost treatment groups compared with the vehicle group during the treatment period and the posttreatment follow-up period (p ≤ .035, all comparisons; Figure 2). Photographic examples of GEBA changes over time are shown in Figure 3.
Figure 2.
Percentage of subjects with at least a 1-grade improvement from baseline in GEBA by visit (intent-to-treat population). *p < .05 versus vehicle. †p < .001 versus vehicle. Bim, bimatoprost.
Figure 3.
Examples of GEBA changes over time representative of the three treatment groups. One subject receiving bimatoprost BID (A) and one receiving QD (B) had GEBA grades of 2 (sparse) at baseline, 3 (full) at Month 4, and 4 (very full) at Month 7. The subject receiving vehicle (C) had a GEBA grade of 1 (very sparse) at baseline and 2 (sparse) at Months 4 and 7.
Additional Endpoints
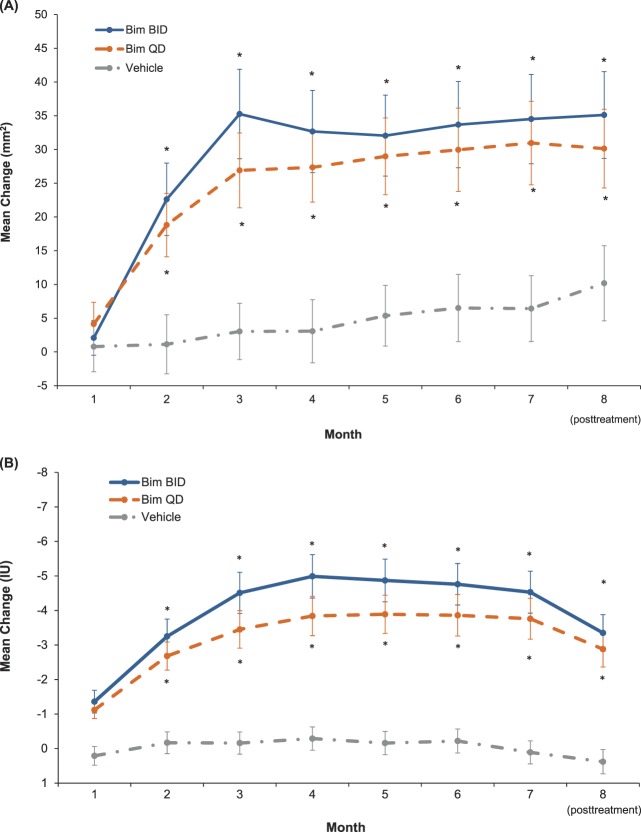
At Month 7, the bimatoprost BID, bimatoprost QD, and vehicle groups experienced mean changes from baseline in eyebrow fullness of 34.51, 30.96, and 6.42 mm2, respectively (p < .001 for bimatoprost BID and bimatoprost QD vs vehicle; Figure 4A). As early as Month 2, significant increases were observed in both bimatoprost treatment groups compared with the vehicle group in eyebrow fullness (p ≤ .001, all comparisons).
Figure 4.
Improvement from baseline in eyebrow fullness (A) and eyebrow darkness (B) by visit (intent-to-treat population). Bim, bimatoprost.
At Month 7, the mean change from baseline in eyebrow darkness as measured by DMSIA was significantly higher in the bimatoprost BID group (−4.53 IU) and bimatoprost QD group (−3.76 IU) compared with the vehicle group (0.11 IU) (p < .001, both comparisons; Figure 4B). Significant improvement was observed in both bimatoprost treatment groups compared with the vehicle group in eyebrow darkness (p ≤ .001, all comparisons) as early as Month 1.
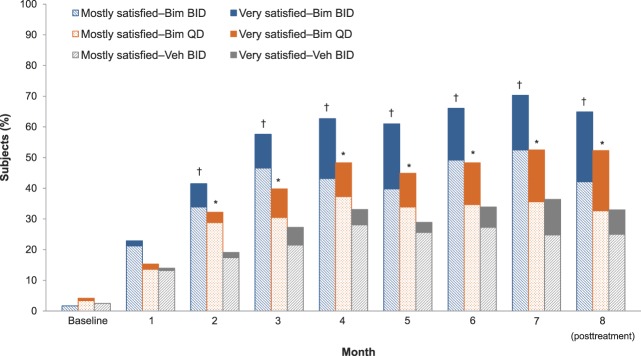
The proportion of subjects who reported being “very satisfied” with how the treatment made their eyebrows look (ESS Item 6) was 17.8% in the bimatoprost BID group, 16.9% in the bimatoprost QD group, and 11.6% in the vehicle group at Month 7. The proportion of subjects who reported being “mostly satisfied” with how the treatment made their eyebrows look was 52.5% in the bimatoprost BID group, 35.6% in the bimatoprost QD group, and 24.8% in the vehicle group. At Month 2 and all subsequent time points, there was a significantly greater proportion of satisfied subjects (very satisfied/mostly satisfied) in both bimatoprost treatment groups compared with the vehicle group (p ≤ .031, all comparisons; Figure 5).
Figure 5.
Percentage of subjects who reported very satisfied/mostly satisfied on ESS Item 6 by visit (intent-to-treat population). *p < .05 versus vehicle for composite “very satisfied” and “mostly satisfied.” †p < .001 versus vehicle for composite very satisfied and mostly satisfied. Bim, bimatoprost.
Safety
Overall, 38.1% of the bimatoprost BID subjects, 42.4% of the bimatoprost QD subjects, and 35.5% of subjects in the vehicle groups reported at least 1 TEAE during the study.
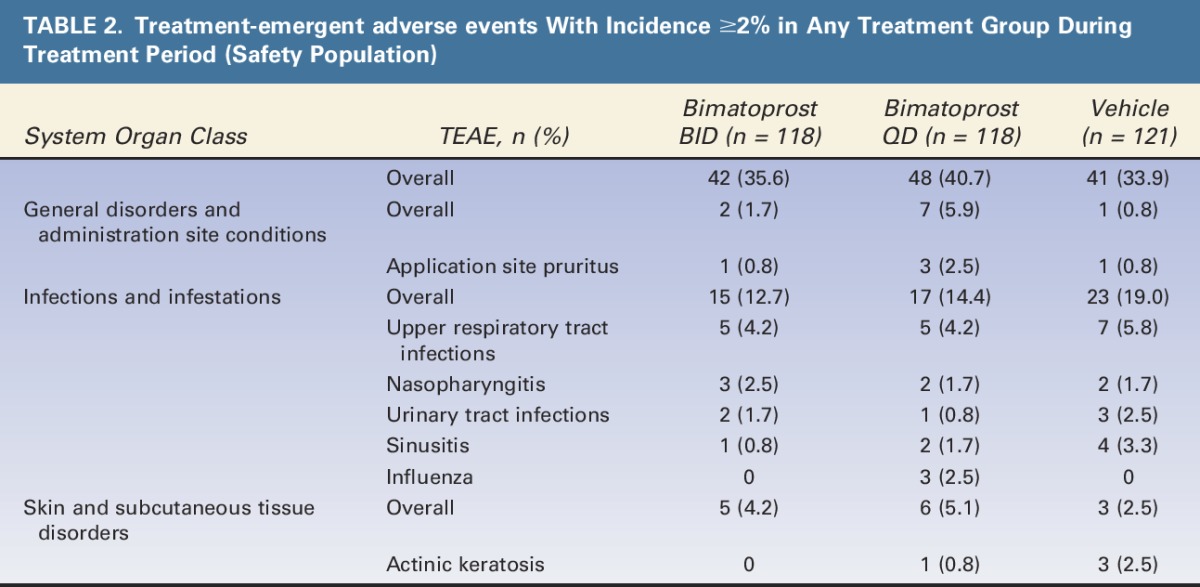
The most frequent TEAE in all groups was upper respiratory tract infection (4.2% bimatoprost BID; 4.2% bimatoprost QD, and 5.8% vehicle). Treatment-emergent adverse events reported in at least 2% of subjects are shown in Table 2. Skin-related TEAEs included application site pruritus, reported by subjects in all groups (0.8% bimatoprost BID, 2.5% bimatoprost QD, and 0.8% vehicle) and actinic keratosis, reported in the bimatoprost QD and vehicle groups (0.8% bimatoprost QD; 2.5% vehicle). Ocular hypertension was noted in 1 subject (0.8%) in the bimatoprost QD treatment group. Overall, for bimatoprost BID, bimatoprost QD, and vehicle, respectively, TEAEs were mild in 18.6%, 22.9%, and 14.9% of subjects; moderate in 12.7%, 16.9%, and 14.0% of subjects, and severe in 4.2%, 0.8%, and 5.0% of subjects. Serious TEAEs were reported in 7 subjects; 2 in the bimatoprost BID group (basal cell carcinoma, breast cancer) and 5 in the vehicle group (sessile lesion of unknown behavior in hepatic flexure, infected toe, 2 cases of basal cell carcinoma, and cervical fusion separation); no serious TEAEs were considered treatment related. No skin or iris hyperpigmentation or conjunctival hyperemia was reported.
TABLE 2.
Treatment-emergent adverse events With Incidence ≥2% in Any Treatment Group During Treatment Period (Safety Population)
Four subjects discontinued because of TEAEs, 1 in the bimatoprost BID group because of breast cancer, 2 in the QD group because of treatment-related moderate dermatitis (n = 1) and treatment-related moderate application site rash (n = 1), and 1 in the vehicle group because of treatment-related moderate application site pruritus. Unintended hair growth in other facial areas (i.e., eyelashes) was not observed, and no clinically meaningful changes in vital signs, laboratory findings, or hematology profiles were noted in any treatment group throughout the study.
Discussion
This first large, Phase 3, randomized, vehicle-controlled study in subjects with eyebrow hypotrichosis demonstrated that bimatoprost 0.03% applied once or twice daily to the eyebrows was effective and safe in subjects with eyebrow hypotrichosis. Although statistical comparisons between once-daily and twice-daily application of bimatoprost 0.03% were not made, differences between the two treatment groups on the GEBA scale and DMSIA do not suggest a substantially greater clinical benefit with twice-daily versus once-daily administration. Improvements in eyebrow fullness in the bimatoprost groups were observed starting at Month 2 on both the GEBA scale and DMSIA, whereas improvements in eyebrow darkness on DMSIA were seen as early as Month 1.
Improvements in eyebrow fullness were noted for subjects in the vehicle group up to Month 4. This may be attributed to natural regrowth of the eyebrows because subjects were instructed not to groom their eyebrows in the treatment area.11 Improvements in the control setting have also been observed with other hair growth products, such as 2% minoxidil. In a randomized, double-masked, split-face study conducted in 39 men and women with eyebrow hypotrichosis receiving minoxidil 2% lotion or placebo, there was a slight increase in eyebrow enhancement in 51% of subjects receiving minoxidil and 23% of subjects receiving placebo.20
The improvements seen with bimatoprost treatment on eyebrow fullness and darkness confirm and add to earlier preliminary findings in case reports and exploratory studies of bimatoprost. Substantial improvement in eyebrow growth was noted for all the reported cases.9,10 A single-center, randomized, double-masked, vehicle-controlled study evaluated bimatoprost 0.03% with daily application to the eyebrows for 9 months in 20 women with mild to moderate hypotrichosis.11 The bimatoprost group demonstrated improvement in eyebrow growth at all time points, with maximum changes seen at Month 7. Subject satisfaction at Month 9 with eyebrow fullness/thickness and darkness/color was higher in the bimatoprost group than that in the vehicle group. No AEs were reported. In another study of 27 subjects with eyebrow hypotrichosis, bimatoprost increased eyebrow growth from baseline, as measured by hair diameter.21 Contact dermatitis occurred in 3 (11.1%) of the 27 subjects.
Although subjects in both bimatoprost groups in this study reported greater satisfaction with the effects of treatment than did subjects in the vehicle group, subjects in the bimatoprost BID group had the highest proportion of satisfied subjects at all time points. This observation could reflect the numerically higher treatment responses on the GEBA scale and DMSIA in the bimatoprost BID group compared with the bimatoprost QD group.
In this study, bimatoprost 0.03% applied to the eyebrows once or twice daily was safe and well tolerated in this population. Overall, no new safety signals were encountered in this study compared with long-term use of bimatoprost for treatment of glaucoma and intraocular hypertension22 or for treatment of eyelash hypotrichosis.23,24 The most commonly reported TEAEs were similar across all treatment groups. A total of 4 subjects were discontinued from the study because of AEs, and the serious AEs that occurred in 7 subjects were considered by the investigator to be not related to study treatment. The overall incidence and severity of TEAEs reported with bimatoprost in this study is comparable with that seen in studies of bimatoprost applied to the eyelashes to treat eyelash hypotrichosis.23,24 In those studies, TEAEs were typically mild to moderate, and the incidence of severe or serious AEs and discontinuations due to TEAEs were relatively low. The most common TEAEs reported after eyelash application were ocular, such as conjunctival hyperemia, eye pruritus, pinguecula, eye irritation, dry eye, and erythema of the eyelid. The incidence of ocular TEAEs in this study was low compared with that in the eyelash studies; this difference may be related to eyebrow application occurring further from the eyes than eyelash application. Treatment-emergent adverse events such as skin hyperpigmentation, iris hyperpigmentation, or conjunctival hyperemia, reported at a low incidence in eyelash hypotrichosis studies, were not seen in this study.24,25
A limitation of this study is the use of stencils. Although this was required to standardize the areas of grooming and treatment across subjects and visits, it does not mirror use in the home environment.
The results of this study demonstrate that bimatoprost 0.03% is a safe, well-tolerated, and effective treatment for hypotrichosis of the eyebrows in this population. Improvements in eyebrow growth and subject satisfaction appeared within 1 to 2 months and were sustained throughout study treatment. The safety profile of bimatoprost in this study is in line with that shown in previous large, controlled studies of bimatoprost for treatment of eyelash hypotrichosis. Based on these results, additional studies on the use of bimatoprost for the treatment of eyebrow hypotrichosis are warranted.
Footnotes
Supported by Allergan plc. Writing and editorial support for this article was provided by Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan plc. J. Carruthers, K. Beer, A. Carruthers, WP Coleman, ZD Draelos, D. Jones, and MP Goldman are consultants for and have received research grants from Allergan plc. M. L. Pucci is an employee of Peloton Advantage, which received funding from Allergan plc. A. VanDenburgh, E. Weng, and S. M. Whitcup are employees of Allergan plc.
The authors have indicated no significant interest with commercial supporters.
References
- 1.Baker SB, Dayan JH, Crane A, Kim S. The influence of brow shape on the perception of facial form and brow aesthetics. Plast Reconstr Surg 2007;119:2240–7. [DOI] [PubMed] [Google Scholar]
- 2.Sadr J, Jarudi I, Sinha P. The role of eyebrows in face recognition. Perception 2003;32:285–93. [DOI] [PubMed] [Google Scholar]
- 3.van der Velden EM, Drost BH, Ijsselmuiden OE, Baruchin AM, et al. Dermatography as a new treatment for alopecia areata of the eyebrows. Int J Dermatol 1998;37:617–21. [DOI] [PubMed] [Google Scholar]
- 4.Velez N, Khera P, English JC., III Eyebrow loss: clinical review. Am J Clin Dermatol 2007;8:337–46. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz AE, Alster TS. Rising concern over cosmetic tattoos. Dermatol Surg 2012;38:424–9. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick RE, Goldman MP, Dierickx C. Laser ablation of facial cosmetic tattoos. Aesthet Plast Surg 1994;18:91–8. [DOI] [PubMed] [Google Scholar]
- 7.Women's Rogaine [package insert]. Skillman, NJ: Johnson & Johnson Healthcare Products; 2014. [Google Scholar]
- 8.Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthet Plast Surg 2011;35:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweiger ES, Pinchover L, Bernstein RM. Topical bimatoprost for the treatment of eyebrow hypotrichosis. J Drugs Dermatol 2012;11:106–8. [PubMed] [Google Scholar]
- 10.Vergilis-Kalner IJ. Application of bimatoprost ophthalmic solution 0.03% for the treatment of eyebrow hypotrichosis: series of ten cases. Dermatol Online J 2014;20. [PubMed] [Google Scholar]
- 11.Beer KR, Julius H, Dunn M, Wilson F. Treatment of eyebrow hypotrichosis using bimatoprost: a randomized, double-blind, vehicle-controlled pilot study. Dermatol Surg 2013;39:1079–87. [DOI] [PubMed] [Google Scholar]
- 12.Smid SD. Role of prostaglandins and specific place in therapy of bimatoprost in the treatment of elevated intraocular pressure and ocular hypertension: a closer look at the agonist properties of bimatoprost and the prostamides. Clin Ophthalmol 2009;3:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol 2008;153:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauchi M, Fuchs TA, Kellenberger AJ, Woodward DF, et al. Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br J Dermatol 2010;162:1186–97. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JL. Enhancing the growth of natural eyelashes: the mechanism of bimatoprost-induced eyelash growth. Dermatol Surg 2010;36:1361–71. [DOI] [PubMed] [Google Scholar]
- 16.Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol 2010;4:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagien S. Management of hypotrichosis of the eyelashes: Focus on bimatoprost. Clin Cosmet Investig Dermatol 2010;3:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur R, Osmanovic S, Toyran S, Edward DP. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol 2005;123:1541–6. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985;41:55–68. [PubMed] [Google Scholar]
- 20.Lee S, Tanglertsampan C, Tanchotikul M, Worapunpong N. Minoxidil 2% lotion for eyebrow enhancement: a randomized, double-blind, placebo-controlled, spilt-face comparative study. J Dermatol 2014;41:149–52. [DOI] [PubMed] [Google Scholar]
- 21.Suwanchatchai W, Tanglertsampan C, Pengsalae N, Makornwattana M. Efficacy and safety of bimatoprost 0.03% versus minoxidil 3% in enhancement of eyebrows: a randomized, double-blind, split-face comparative study. J Dermatol 2012;39:865–6. [DOI] [PubMed] [Google Scholar]
- 22.Williams RD, Cohen JS, Gross RL, Liu CC, et al. Long-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4. Br J Ophthalmol 2008;92:1387–92. [DOI] [PubMed] [Google Scholar]
- 23.Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg 2010;36:638–49. [DOI] [PubMed] [Google Scholar]
- 24.Smith S, Fagien S, Whitcup SM, Ledon F, et al. Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol 2012;66:801–6. [DOI] [PubMed] [Google Scholar]
- 25.Harii K, Arase S, Tsuboi R, Weng E, et al. Bimatoprost for eyelash growth in Japanese subjects: two multicenter controlled studies. Aesthet Plast Surg 2014;38:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]