Abstract
BACKGROUND
Aminolevulinic acid photodynamic therapy (ALA-PDT) can be effective and well tolerated when applied over a broad area and for short drug incubation times.
OBJECTIVE
To evaluate the effect of short-incubation time and application method on the safety and efficacy of ALA-PDT versus vehicle (VEH-PDT) in the treatment of actinic keratoses (AKs) of the face or scalp.
METHODS
Aminolevulinic acid or VEH was applied to face or scalp as a broad area application for 1, 2, or 3 hours or as a spot application for 2 hours before blue light activation. An identical treatment was repeated at Week 8 if any AK lesions remained.
RESULTS
Median AK clearance rate for ALA-treated subjects ranged from 68% to 79% at Week 12, compared with 7% of the VEH-treated group (p < .0001). Complete clearance rate for ALA-treated subjects ranged from 17% (8/46) to 30% (14/47) at Week 12, compared with 2% (1/46) of the VEH-treated group (p = .0041). The safety profile seen in this study is consistent with previously reported side effects of the therapy.
CONCLUSION
Short-incubation ALA-PDT was found to be superior to VEH-PDT for AK lesion clearance. A second treatment improves efficacy.
Actinic keratoses (AKs) are dysplastic epidermal lesions that occur in fair-skinned individuals who have been chronically exposed to sunlight. Actinic keratoses are usually found on sun-exposed areas, such as the face, bald scalp, forearms, and backs of hands. Individual AKs are identified as scaly, rough, skin color to red, pinpoint to larger patch lesions that may disappear and reappear over months or years. Actinic keratoses are considered to be precancerous lesions with the potential to progress to squamous cell carcinoma (SCC). Results of a previous study suggest that AKs may give rise to nonmelanoma skin cancers (NMSCs) at a greater rate than previously acknowledged.1 Criscione and colleaguesl found that the risk of progression of AK to primary SCC was 0.6% at 1 year and 2.57% at 4 years; in addition, approximately 65% of all primary SCCs diagnosed during the trial arose in lesions that had earlier been clinically diagnosed as AKs. The cellular damage and atypia seen at the histologic level of AKs are similar to those of surrounding nonlesional skin,2,3 suggesting that skin surrounding AKs may also be at increased risk of skin cancer.
Photodynamic therapy (PDT) using a 20% topical solution of aminolevulinic acid HCl (ALA, Levulan Kerastick; DUSA Pharmaceuticals Inc, Wilmington, MA) and activation by 10 J/cm2 blue light (Blue Light Photodynamic Therapy Illuminator, BLU-U; DUSA Pharmaceuticals, Inc, Wilmington, MA) exposure is Food and Drug Administration (FDA) approved for the spot treatment of minimally to moderately thick AKs of the face or scalp after a 14- to 18-hour incubation period, in the United States and Canada.
When ALA is applied to dysplastic lesions, it is absorbed by cells and preferentially metabolized to Protoporphyrin IX (PpIX) relative to normally dividing keratinocytes. When PpIX is activated by light of an appropriate wavelength and energy in the presence of oxygen, it results in a cytotoxic process. There is minimal damage to surrounding unaffected skin, and cutaneous photosensitivity is transient (usually clearing within 24–48 hours) because of metabolic conversion of the remaining PpIX to heme.4
Aminolevulinic acid–PDT, when used on-label for 14- to 18-hour incubation, has been shown to be safe and highly effective in the treatment of minimally to moderately thick AKs of the face and scalp.5,6 However, anecdotal clinical findings from the dermatologic community and also from small investigator–initiated studies indicate that physicians frequently administer ALA over a broad area (BA) rather than as a spot treatment and/or use shorter incubation times.7–9
Fluorescence measurements of PpIX suggest that PpIX builds up gradually over the first hours, reaching peak fluorescence in the range of 6 to 18 hours after application (data on file). A previous study of the kinetics of PpIX accumulation in AK over the first 2 hours after ALA application has shown that PpIX concentration was statistically higher than baseline in 92% of lesions by 1 hour and 100% of lesions by 2 hours and that PpIX levels were also elevated in areas of normal-appearing nonlesional skin.10 Although the study was not designed to assess efficacy, 10 J/cm2 blue light was applied to lesions 2 hours after ALA application, and 1 month after treatment, the vast majority of AKs resolved.10 Data from other studies with short-incubation times also seemed efficacious for AK treatment.7–9 Reducing incubation time might also, by decreasing the amount of PpIX produced in skin, decrease the discomfort of BA PDT while maintaining a high AK clearance rate (AKCR) and potentially treating subclinical lesions that may exist in the presence of clinically typical, visible, or palpable AKs.
This study is designed to evaluate the effect of incubation time and application method on the safety and efficacy of blue light ALA-PDT in the treatment of AKs on the face or scalp.
Materials and Methods
Subjects
Eligible participants were male and nonpregnant females, 18 years or above, with 6 to 20 Grade 1 or 2 AKs (Grade 1: slightly palpable, better felt than seen; Grade 2: moderately thick, easily seen and felt) on the face or scalp, with a history of AK therapy within the treatment area at least twice in the 2 years before study entry. Key exclusion criteria included Grade 3 AKs (very thick and/or hyperkeratotic) within the treatment area, lesions suspicious or proven for skin cancer within the treatment area; a history of cutaneous photosensitization; any condition with associated immunosuppression; keratolytics including >5% urea, alpha hydroxyl acids (e.g., glycolic acid, >5% lactic acid), >2% salicylic acid within 2 days of initiation of treatment; cryotherapy within the previous 2 weeks; topical retinoids within the previous 4 weeks; microdermabrasion, ablative laser, ALA-PDT, chemical peels, 5-fluorouracil, diclofenac, imiquimod within the previous 8 weeks; 2 or more ALA-PDT treatments or systemic retinoids within the previous 6 months.
Procedure
Subjects were randomized to one of 5 treatment groups: BA application of ALA 1, 2, or 3 hours before blue light, spot application of ALA 2 hours before blue light, or vehicle (VEH) before blue light (placebo control), balanced for the 4 active groups (Levulan Kerastick or VEH Kerastick). For spot treatment, 2 applications of ALA or VEH were applied to individual AKs; for BA treatment, the first application was applied to individual AKs, followed by 2 BA applications. Blue light was administered to the treatment area (BLU-U Photodynamic Therapy Illuminator), for 16 minutes, 40 seconds for a total dose of 10 J/cm2. Follow-up was performed at 24 to 48 hours and 2, 4, 8, 12, and 24 weeks after initial treatment. A second treatment was given at Week 8 if treatment areas showed any AKs. Subjects were instructed to prevent exposure of the treated skin to direct sunlight or bright indoor light for 36 hours after each treatment and to apply physical sunblock containing TiO2 and/or ZnO sun protection factor 15+.
The study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki and in compliance with Good Clinical Practice Guidelines. The protocol was approved by an institutional review board for each study center. Written informed consent was obtained from all subjects. The study was registered on ClinicalTrials.gov (Identifier: NCT01475955).
Outcomes
Efficacy endpoints were AKCR compared to baseline, complete (100%) clearance rate, partial (≥75%) clearance rate, and subject satisfaction and acceptability of treatment. Tolerability assessments, including erythema, edema, scaling and dryness, oozing/vesiculation/crusting, and changes in pigmentation, were assessed at each visit using a 5-point scale (0 = none–4 = severe); stinging/burning was assessed using a 4-point scale (0 = none–3 = severe). The evaluating investigator for efficacy was blinded to the treatment assignment. To maintain blinding, personnel who were not involved in efficacy assessments administered the blue light PDT and assessed for tolerability to treatment.
Statistical Analysis
All data tabulations were performed using the SAS System for Windows version 9.3. The primary subject set for analysis of the efficacy data is the intent-to-treat (ITT) population. A last-observation-carried-forward analysis was used to address the effect of dropouts or missing data for the efficacy variables. Subjects receiving VEH were considered a single treatment group. The treatment groups were compared with respect to the AKCR by analysis of variance where the model included treatment effect. The Tukey–Kramer method was used for pairwise comparisons of treatment groups. Treatment groups were also compared with respect to complete and partial clearance rates using a Pearson χ2 test; Fisher exact test was used at Week 8 because of sparse tables. The Cochran–Armitage trend test was used to examine whether complete clearance rate increases as incubation time increases for the BA applications. No adjustment was made for multiple endpoints.
Results
Demographics and Baseline Disease Characteristics
Two hundred thirty-five subjects were enrolled in the study at 13 sites; 234 subjects (211 male and 23 female, mean age 68 years [range, 40–88 years], and Fitzpatrick skin Type I [6%], II [44%], III [43%], IV [6%], and V [0.4%]) are included in the efficacy analysis (ITT population). Ninety-eight percent (231/235) of the subjects completed the study.
Efficacy Results
Table 1 summarizes the absolute AK count at baseline and the median AKCRs at Weeks 8, 12, and 24, which are presented graphically in Figure 1. Most subjects in each treatment group received both PDT treatments. The median AKCR at Week 12 was 71.4 (±34.8)% for ALA-BA1, 73.6 (±31.1)% for ALA-BA2, 78.6 (±30.5)% for ALA-BA3, 68.3 (±39.4)% for ALA-SP2, and 7.1 (±44.3)% for VEH. The median AKCR was significantly greater for all ALA groups versus VEH (p < .001, Tukey–Kramer), at all time points.
TABLE 1.
AK Lesion Clearance Rate at Weeks 8, 12, and 24
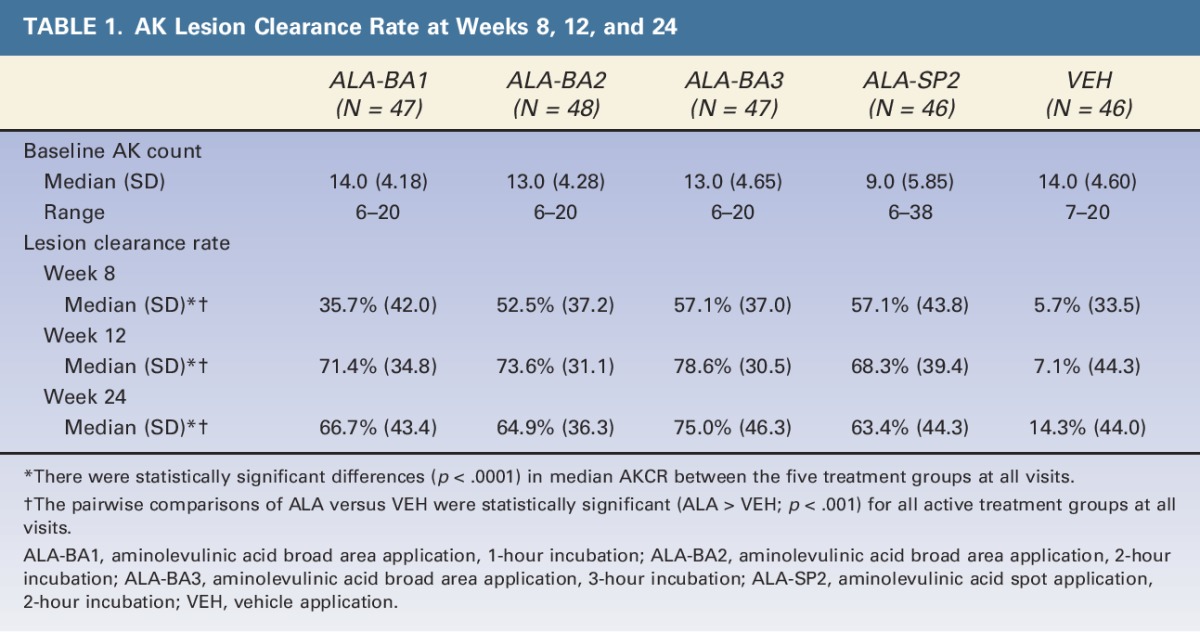
Figure 1.
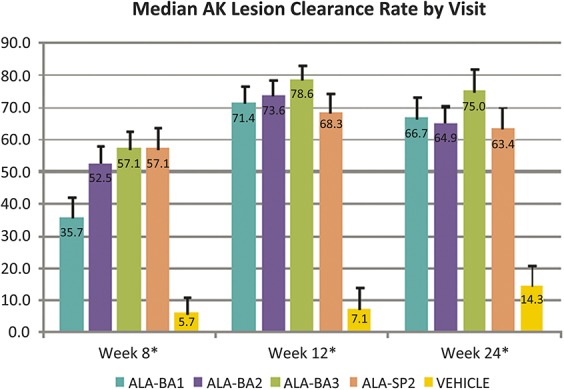
Median AK lesion clearance rate by visit. *There were statistically significant differences (p < .0001) in median AKCR between the 5 treatment groups at all visits; the pairwise comparisons of ALA versus VEH were statistically significant (ALA > VEH; p < .001) for all treatment groups at all visits. In this figure, error bars represent standard error.
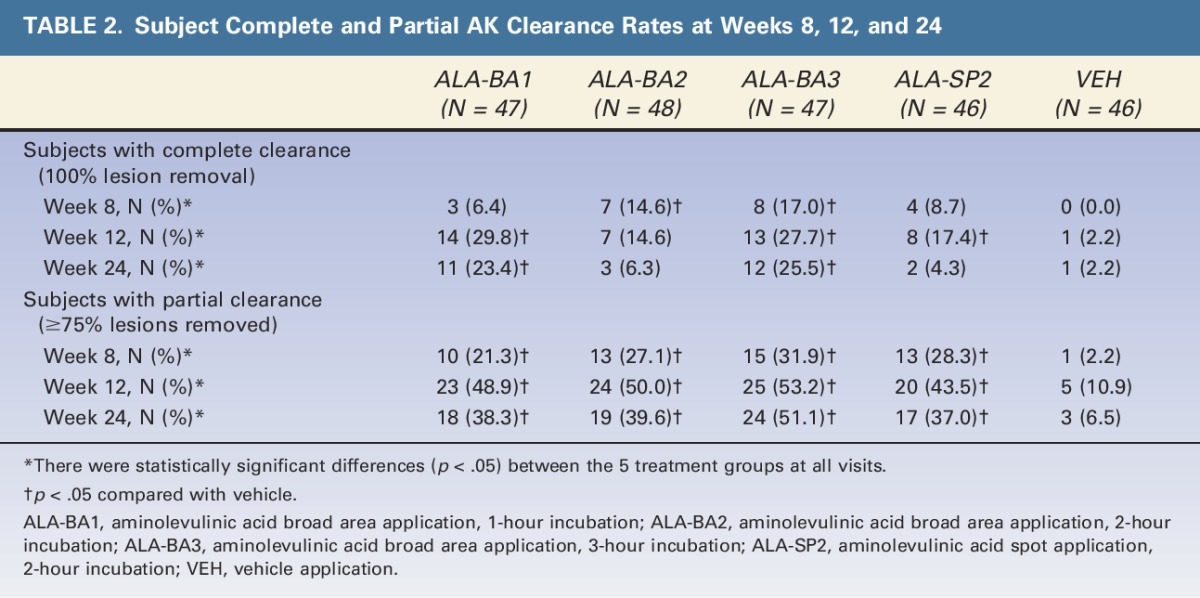
Table 2 shows the number and fraction of subjects with complete AK clearance (no lesions remaining) and partial AK clearance (≥75% of AK lesions cleared) at Weeks 8, 12, and 24. At Week 12, complete clearance for the ALA groups ranged from 15% (7/48, ALA-BA2) to 30% (14/47, ALA-BA1), compared to 2% (1/46) for VEH; partial clearance rates for the ALA groups ranged from 44% (20/46, ALA-SP2) to 53% (25/47, ALA-BA3), compared to 11% (5/46) for VEH.
TABLE 2.
Subject Complete and Partial AK Clearance Rates at Weeks 8, 12, and 24
At the final visit, subjects rated their overall satisfaction with improvement of the appearance of the area treated on a 4-point scale (0 = none/worse, 3 = excellent). For overall satisfaction, 79% (147/185) of subjects treated with ALA-PDT were rated as having a moderate or excellent improvement from baseline, compared to 35% (16/46) of subjects treated with VEH-PDT.
Tolerability/Safety Results
Most subjects tolerated treatments well; 4 ALA-treated subjects did not receive full light dose because of side effects during treatment. Stinging/burning during light treatment for the ALA groups ranged from 93.5% (ALA-SP2) to 100% (ALA-BA3), compared to 24% of subjects treated with VEH (Table 3). For ALA-treated subjects, stinging/burning during light treatment was rated as moderate or severe for 63.8%, 79.2%, 78.7%, and 58.7% of subjects in ALA-BA1, ALA-BA2, ALA-BA3, and ALA-SP2, respectively (Table 4).
TABLE 3.
Percentage of Subjects With Acute Safety Symptoms at Specific Follow-Up Visits by Treatment Group
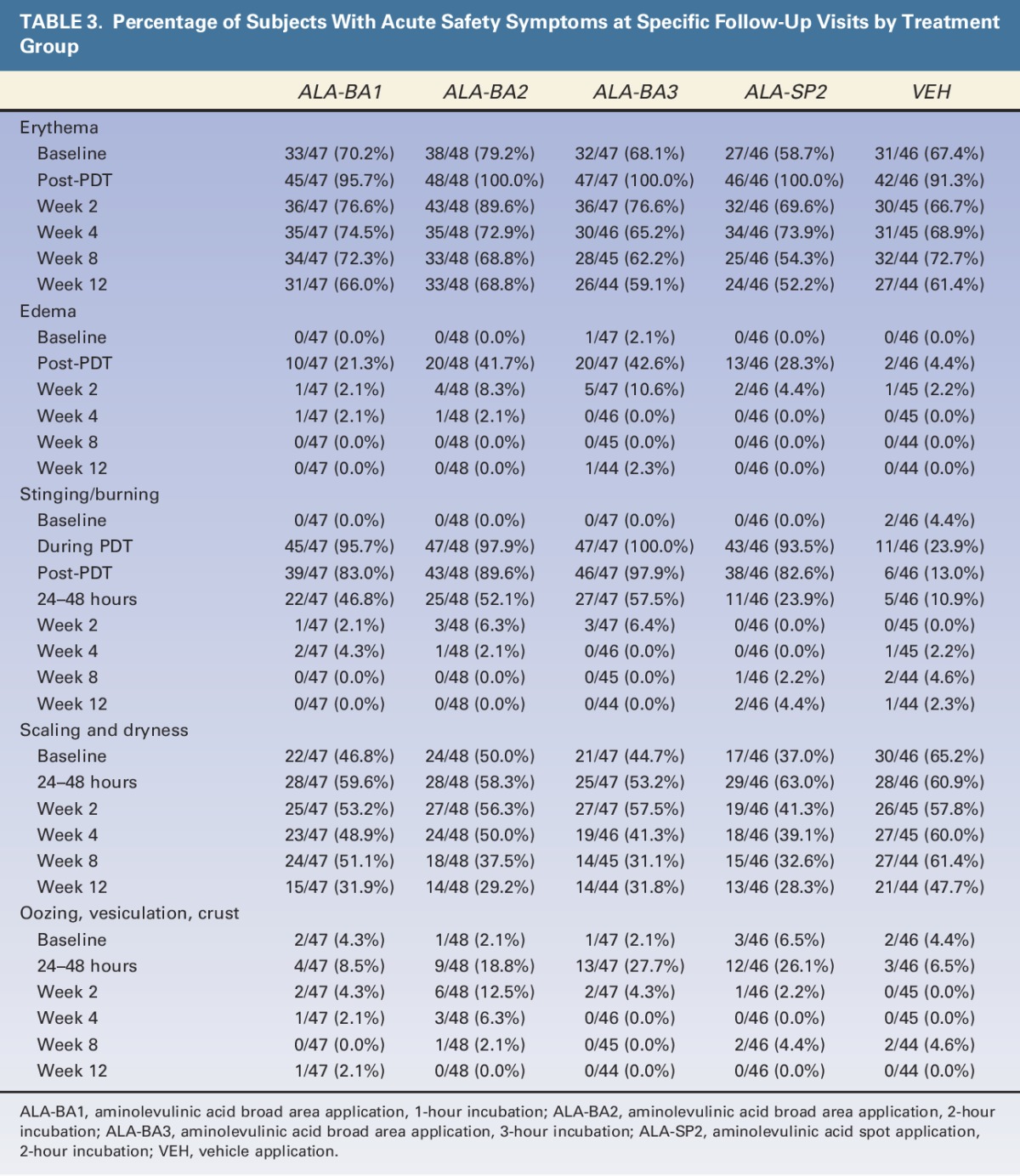
TABLE 4.
Erythema, Stinging/Burning, and Scaling and Dryness by Severity and Treatment Group at Acute Time Points
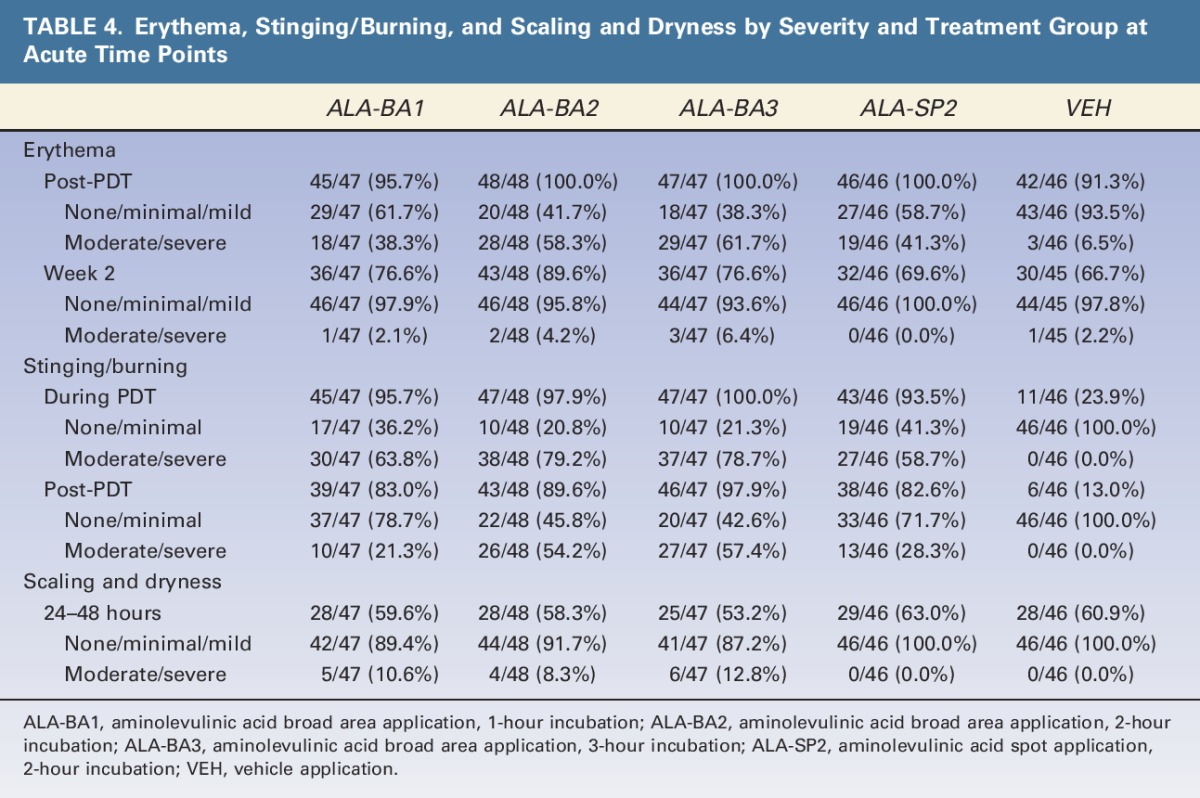
The incidence of erythema increased over baseline levels in all treatment groups immediately after light treatment, but appeared to be more severe in the ALA groups than in the VEH group (Table 3; 38.3%, 58.3%, 61.7%, 41.3%, vs 6.5%). The incidence of erythema declined at Week 2 in all groups. The incidence of edema was greatest for subjects treated with BA ALA for 2 or 3 hours. All ALA groups exhibited an increase in scaling and dryness at the 24- to 48-hour visit, compared to baseline, whereas the VEH group did not (Table 3).
At the final visit, subjects rated their agreement with the acceptability of side effects and duration of adverse events on a 5-point scale (1 = agree totally, 5 = do not agree at all). Ninety-one percent (43/47), 83% (40/48), 68% (30/44), and 83% (38/46) of subjects in ALA-BA1, ALA-BA2, ALA-BA3, and ALA-SP2, respectively, agreed either totally or a little that side effects were acceptable, compared to 76% (35/46) of VEH-treated subjects. Regarding side effect duration, 89% (42/47), 88% (42/48), 82% (36/44), and 83% (38/46) of subjects in ALA-BA1, ALA-BA2, ALA-BA3, and ALA-SP2, respectively, agreed either totally or a little that it was acceptable, compared to 78% (36/46) of VEH-treated subjects.
There were no serious adverse events related to the drug reported during the study. Five adverse events (increased lacrimation, nausea, application site burn, influenza-like illness and rosacea) were judged as related to treatment in ALA-treated subjects, and 2 adverse events (headache and skin tightness) were judged as related to treatment in VEH-treated subjects.
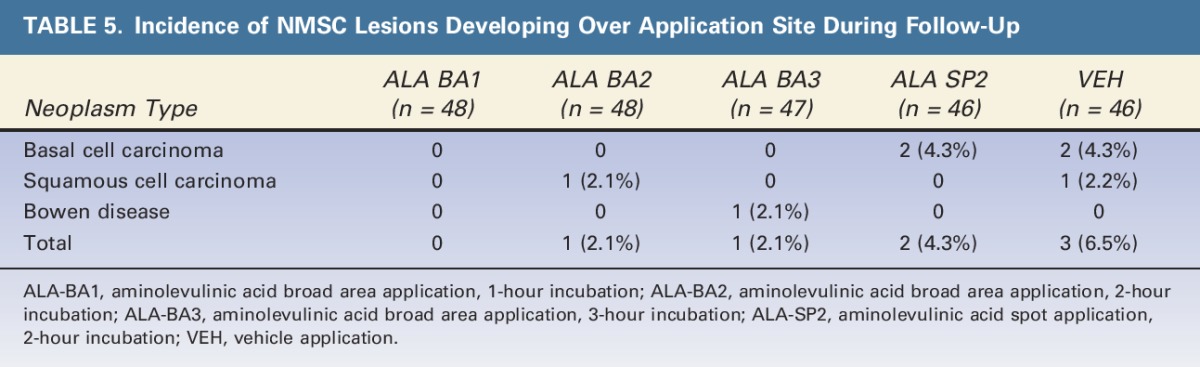
A total of 7 skin cancers were diagnosed within the treated area during the study, including 2 basal cell carcinomas (BCCs), 1 Bowen disease, and 1 SCC in the 188 ALA-treated subjects, and 2 BCCs and 1 SCC in the 46 VEH-treated subjects (Table 5).
TABLE 5.
Incidence of NMSC Lesions Developing Over Application Site During Follow-Up
Limitations
A limitation of this study is the uneven number of men and women, which may be due in part to the fact that males have a higher tendency toward the development of AKs, which is likely the result of higher cumulative lifetime sun exposure and a lower adherence to photoprotection, and is consistent with previous studies of ALA-PDT for AKs on the face and scalp.5,6
Discussion
The results of this study confirm and extend the results of previous smaller studies7,8 showing that blue light ALA-PDT performed using incubation times as short as 1 hour can provide significant clearance of AKs. Although a single short-incubation ALA-PDT treatment in this study produced lesion clearance from 36% to 57%, a second treatment performed at Week 8 increased the AKCR to 68% to 79% (Table 1). The short-incubation AKCRs seen after two treatments also compare favorably with AKCR described after 2 treatments (83.4%) using the FDA-approved 14- to 18-hour incubation time.4 Furthermore, AKCRs appear similar after 2 short-incubation treatments using spot application for 2 hours or BA application for 1 or 2 hours (Table 1).
The complete (100%) subject clearance rates at Week 12 were similar for the 1-hour and 3-hour BA ALA-PDT groups (29.8% and 27.7%, respectively). Both of the 2 hour groups (spot area and BA) had similar 100% clearance rates at Week 12 (17.4% and 14.6%, respectively), which were notably lower than the 1-hour and 3-hour BA rates, for unknown reasons (Table 2). Partial AKCRs (subjects with ≥75% lesions removed) ranged from 44% to 53% and appeared to trend slightly upward with increasing incubation time.
Although subjects in this study showed a high degree of tolerability for the treatment and satisfaction with its outcome, it is clear that increasing incubation time for more than 1 hour, although not increasing efficacy, may increase the incidence and severity of some of the well-known side effects of ALA-PDT (Tables 3 and 4). Notably, the incidence of post-PDT edema and crusting nearly doubled in the 2- and 3-hour BA incubation groups (Table 3). Erythema, although universally observed after PDT and of a similar incidence in all groups, was noted to be of generally greater severity in subjects receiving BA application and incubated for longer than 1 hour. Stinging and burning of a moderate/severe intensity was reported in 21% of subjects after PDT in the BA1 group and increased to 54% and 57% in the BA2 and BA3 groups, respectively (Table 4).
In previous studies of ALA-PDT for AK treatment in which subjects were followed for periods >6 months, NMSCs arose during follow-up, as may be expected in a subject population with heavily sun-damaged skin.6,11 The incidence of NMSCs seen over the course of this study was also determined for each group (Table 5). The incidence of NMSCs within the areas where BA treatment was given ranged from 0/48 (0%) for the BA1 group to 1/48 and 1/47 (2.1% each) for the BA2 and BA3 groups, respectively. This contrasts with the 2/47 (4.4%) incidence in the spot treatment group (SP2) and the highest incidence, 3/46 (6.7%), in the control (VEH) treatment group. The trending toward an indication of some degree of NMSC prevention in areas given BA treatment versus spot or control (sham PDT) treatment is of interest. Studies of long-term ALA-PDT effects in populations at high risk of NMSCs, specifically long-term immunosuppressed solid organ transplant recipients12 and subjects with a previous history of NMSCs in the treatment area,13 have clearly shown that BA application ALA-PDT can significantly reduce the occurrence of NMSCs in these subjects. Future studies will seek to corroborate these results in populations at high risk of NMSC occurrence.
Acknowledgments
The PDT-AK Investigational Group consists of Diane O. McConnehey, DO, Northwest Clinical Trials, Inc., Boise ID; Scott T. Guenthner, MD, The Indiana Clinical Trials Center, Plainville IN; Michael Bukhalo, MD, Altman Dermatology Associates, Arlington Heights, IL; Steven E. Kempers, MD, Minnesota Clinical Study Center, Fridley, MN; Robert T. Matheson, MD, Oregon Medical Research Center, PC, Portland OR; Shang I. Brian Jiang, MD, Mohs Micrographic and Dermatologic Surgery, University of California San Diego, San Diego CA; Pranav B. Sheth, MD, Dermatology Research Center of Cincinnati, Cincinnati OH; Suzanne Bruce, MD, Suzanne Bruce and Associates, P.A., The Center for Skin Research, Houston TX; Howard L. Sofen, MD, Department of Medicine/Dermatology, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA and Dermatology Research Associates, Los Angeles CA; Eugene Y. Huang, MD, PhD, Division of Dermatology, University of California San Diego, San Diego, CA and Therapeutics Clinical Research, San Diego CA; Michael H. Gold, MD, Tennessee Clinical Research Center, Nashville TN; Daniel Piacquadio, MD, Therapeutics, Inc., San Diego CA; and Stuart Marcus, MD, PhD, DUSA Pharmaceuticals, Inc., a Sun Pharma Company, Wilmington MA.
Special thanks to Denise Freeman for supporting manuscript drafting and figure creation and Michael Guttadauro for leading project management activities for this study.
Footnotes
Supported by DUSA Pharmaceuticals, Inc., a Sun Pharma Company. The BLU-U light source was loaned to the authors. Both active and placebo investigational products were provided. Investigator grant funded logistical requirements for the study.
Anna Houlihan and Stuart Marcus of the PDT-AK Investigational Group are both employed by DUSA Pharmaceuticals, Inc.
References
- 1.Criscione VD, Weinstock MA, Naylor MF, Luque C, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer 2009;116:2523–30. [DOI] [PubMed] [Google Scholar]
- 2.Jonason AS, Kunala S, Price GJ, Restifo RJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A 1996;93:14205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl PL, Stranneheim H, Asplund A, Berglund L, et al. Sun-induced nonsynonymous p53 mutations are extensively accumulated and tolerated in normal appearing human skin. J Invest Dermatol 2011;131:504–8. [DOI] [PubMed] [Google Scholar]
- 4.ALA® Kerastick® (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010. [Google Scholar]
- 5.Piacquadio DJ, Chen DM, Farber HF, Fowler JF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004;140:41–6. [DOI] [PubMed] [Google Scholar]
- 6.Tschen EH, Wong DS, Pariser DM, Dunlap F, et al. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow up. Br J Dermatol 2006;155:1262–9. [DOI] [PubMed] [Google Scholar]
- 7.Touma D, Yaar M, Whitehead S, Konnikov N, et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol 2004;140:33–40. [DOI] [PubMed] [Google Scholar]
- 8.Smith S, Piacquadio D, Morhenn V, Atkin D, et al. Short incubation PDT versus 5-FU in treating actinic keratoses. J Drugs Dermatol 2003;2:629–3. [PubMed] [Google Scholar]
- 9.Goldman MP, Atkin D, Kincad S. PDT/ALA in the treatment of actinic damage: real world experience. J Lasers Med Surg 2002;14:S24. [Google Scholar]
- 10.Warren CB, Lohser A, Wene LC, Pogue BW, et al. Noninvasive fluorescence monitoring of protoporphyrin IX production and clinical outcomes in actinic keratoses following short-contact application of 5-aminolevulinate. J Biomed Opt 2010;15:051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmieder G, Huang E, Jarratt M. A multicenter, randomized, vehicle-controlled phase 2 study of blue light photodynamic therapy with aminolevulinic acid HCl 20% topical solution for the treatment of actinic keratoses on the upper extremities: the effect of occlusion during the drug incubation period. J Drugs Dermatol 2012;11:55–62. [PubMed] [Google Scholar]
- 12.Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Derm Surg 2009;35:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Apalla Z, Sotiriou E, Chovarda E, Lefaki I, et al. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol 2010;162:171–5. [DOI] [PubMed] [Google Scholar]


