Abstract
The pivotal role of gonadotropin signaling in regulating gonadal development and functions has attracted much research attention in the past 2 decades. However, the precise physiological role of gonadotropin signaling is still largely unknown in fish. In this study, we have established both LH β-subunit (lhb) and LH receptor (lhr) knockout zebrafish lines by transcription activator-like effector nucleases. Intriguingly, both homozygous lhb and lhr mutant male fish are fertile. The fertilization rate, sperm motility, and histological structure of the testis were not affected in either lhb or lhr mutant males. On the contrary, homozygous lhb mutant females are infertile, whereas homozygous lhr mutant females are fertile. Folliculogenesis was not affected in either lhb or lhr mutants, but oocyte maturation and ovulation were disrupted in lhb mutant, whereas only ovulation was affected in lhr mutant. Differential expression of genes in the ovary involved in steroidogenesis, oocyte maturation, and ovulation was found between the lhb and lhr mutants. These data demonstrate the essential role of LH signaling in oocyte maturation and ovulation, and support the notion that LH acts through the FSH receptor in the absence of LH receptor. Moreover, the defects of lhb mutant could be partially restored by administration of human chorionic gonadotropin. This in vivo evidence in the present study demonstrates, for the first time in any vertebrate species, that LH signaling is indispensable in female reproduction but not in male reproduction. LH signaling is demonstrated to control oocyte maturation and ovulation in the ovary.
Animal reproduction is controlled by the hypothalamus-pituitary-gonad axis. Gonadotropins secreted from the pituitary, ie, FSH and LH, bind to their receptors, ie, FSH receptor (FSHR) and LH receptor (LHR), located in the gonads to orchestrate gonadal growth and development. Gonadotropins and their receptors have attracted much research attention in the past 2 decades because of their pivotal roles in regulating gonadal development and functions. LH is a heterodimer, sharing a common α-subunit with FSH but differing in the β-subunits. Upon binding to LHR, which belongs to the superfamily of G protein–coupled membrane receptors, the cAMP-protein kinase A, B, or C pathway is activated to regulate the synthesis and secretion of steroid hormones and the expression of certain growth factors in the gonad (1, 2).
Most information on gonadotropins and their receptors comes from mammalian studies, especially those using gene knockout mouse models (3–8). These studies have shown that LH exerts its action through LHR, which is expressed in the theca cells of the ovary and Leydig cells of the testis, controlling postnatal sexual development including spermatogenesis at the round spermatid stage in males and folliculogenesis at the antral stage in females. Thus, both males and females of either LH or LHR knockout mice are infertile (6–8). Such information on teleost fish, being the largest and most diverse group of vertebrates, lags far behind that for mammals, particularly regarding the in vivo functions of the gonadotropins and their receptors. Currently, most studies on fish LH and LHR mainly focus on their regulation and expression as well as ligand-binding specificity. The spatial and temporal distribution of LH in the pituitary (9–15) and LHR in the gonads (16–22) was established in several fish species. Although most reports suggest a conserved role of LH signaling from fish to mammals, the physiological functions of LH signaling in controlling fish reproduction still lack in vivo evidence. In addition, marked differences exist between mammals and fish with regard to the specificity of the gonadotropins toward their receptors (1, 23, 24). It has been demonstrated in several fish species including zebrafish that FSH stimulates only FSHR, whereas LH stimulates both FSHR and LHR (11, 25–28). This difference implies functional differentiation of LH signaling from fish to mammals. So far, the most information on the specificity of fish gonadotropins in recognizing their receptors has come from in vitro studies performed in mammalian cell lines. Because of the lack of efficient in vivo gene manipulation methods in fish, the physiological importance of this ligand/receptor differentiation is still unknown.
Recently, a new method has emerged to achieve targeted gene knockout in zebrafish by using the transcription activator-like effector nucleases (TALENs) (29, 30). Transcription activator-like repeats recognize their target sequences in a context-independent modular fashion (31). Therefore, once the target sites are chosen, transcription activator-like effector arrays for these sites can be predicted and assembled. So far, TALENs have been used to edit specific genomic loci in yeast (31), worms (32), plants (33), zebrafish (29, 30, 34, 35), mice (36), rats (37), frogs (38), and pigs (39). Previously, we adapted the original TALENs plasmids and successfully modified the expression vector to make it suitable for gene knockout studies in animals (38, 40). The emergence of this method provides a convenient and robust tool to study in vivo gene functions in fish. In the present study, using zebrafish as the model animal, the TALENs system was used to disrupt lhb and lhr, respectively, and noncanonical functions of LH signaling in zebrafish reproduction were revealed.
Materials and Methods
Zebrafish husbandry
AB zebrafish used in this study were maintained at 28°C in the zebrafish facility of The Chinese University of Hong Kong. Fish were fed twice daily with newly hatched brine shrimp (Brine Shrimp Direct). All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of The Chinese University of Hong Kong.
RNA isolation and real-time PCR
Total RNA samples were isolated from the tissues, ovarian follicles, embryos, and fry of zebrafish using TRIzol reagent (Invitrogen). The amount and purity of the RNA were determined by spectrophotometry. The full names of the genes and the primers used in this study are listed in Supplemental Table 1. The annealing temperature for PCR ranges from 55 to 65°C, depending on the primer set used. Real-time PCR was carried out on an ABI real-time PCR fast system (Applied Biosystems) using an SYBR Green PCR Master Mix Kit (Applied Biosystems).
Gene knockout by TALENs
The pCS2-TALEN-ELD/KKR plasmids were constructed as described previously (38, 41). With use of the modified TALENs vectors, highly effective customized TALENs recognizing 12- to 31-bp half-sites could be assembled in 5 days. The whole procedure involves 2 digestion-ligation steps. The protocol of assembling the TALENs followed our previous study (38, 40). To prepare capped TALENs mRNA, the TALENs expression vectors were linearized by NotI and transcribed using the Sp6 mMESSAGE mMACHINE Kit (Ambion). TALENs mRNAs (100–500 pg) were microinjected into 1-cell stage zebrafish embryos. Two days after injection, genomic DNA (gDNA) was isolated from 8 to 10 pooled larvae with normal morphology. The target genomic region was amplified by limited cycles of PCR and subcloned into pMD18-T (Takara). The mutation was analyzed by PCR or by sequencing. The primers used are listed in Supplemental Table 1. The TALENs-injected embryos were raised to adulthood and outcrossed with wild-type fish. For either mutant, gDNA from 8 pooled F1 embryos of each founder was amplified and subcloned. Thirty-two single clones were analyzed by PCR and sequencing. F1 mutant embryos were raised to adulthood and self-crossed to obtain F2 zebrafish. Part of the fin in each F2 zebrafish was collected for genomic PCR and sequencing for genotyping.
Fertility determination
Egg production assay was performed as described previously (42). At 1 to 2 hours before the end of the light period, the fish were fed and the tanks were cleaned. Adult zebrafish (wild-type, heterozygotes, or homozygotes) were transferred to a breeding aquarium (Tecniplast) at the ratio of one male to one female. After the beginning of the next light cycle, the embryos were collected by siphoning the bottom of the tank. The number of eggs produced by each female fish was recorded for at least 1 month. The egg productions of 10 fish from each group of wild-type, heterozygous, and homozygous mutant females were recorded and compared, respectively. For fertilization rate assessment, the procedure of Brion et al (43) was followed with minor modifications. Each morning, the spawning events were recorded, and eggs were collected and counted. Eggs produced from at least 5 females in each group were collected. The dead embryos were counted and removed at 24 hours postfertilization. The success and survival rate is expressed as a percentage of the fertilization rate. The above experiment was repeated 3 to 5 times.
Sperm motility assessment
Male fish (4 months postfertilization, 4 fish in each group of wild-type, heterozygotes, and homozygotes) were anesthetized using Tris-buffered tricaine (170 mg/mL, pH 7.5). About 1.5 μL of fresh semen was collected using a capillary tube and diluted with 20 μL of zebrafish sperm immobilizing solution. To activate the sperm, 1 μL of semen suspension in zebrafish sperm immobilizing solution was mixed with 20 μL of aged tap water. Approximately 0.5 μL of the activated sperm was quickly applied to a single well of a 12-well multitest slide (MP Biomedicals). The slide and coverslip were coated with 1% (w/v) polyvinyl alcohol to reduce sticking of the sperm. Sperm motility was assessed using computer-assisted sperm analysis by following the method of McAllister and Kime (44).
Morphological and histological analysis
Intact testes and ovaries from adult zebrafish (4 months postfertilization) were carefully dissected after anesthetization and decapitation, and observed in a 100-mm culture dish containing 60% Leibovitz L-15 medium, or fixed in 4% buffered paraformaldehyde (Sigma-Aldrich) or Bouin's fixative buffer (Sigma-Aldrich) overnight at 4°C, dehydrated and embedded in paraffin, and sectioned at 7-μm thickness. Sections were stained with Harris hematoxylin and eosin (Sigma-Aldrich). For testis, periodic acid Schiff/ferric hematoxylin/metanil yellow staining and PCNA immunostaining was also used (Supplemental Methods). The staging system adopted for the ovarian follicles was based on the original definition of Selman et al (45) as modified by Wang and Ge (46) and Pang and Thomas (47). The staging system on spermatogenesis was based on Schulz and colleagues (48). The reproducibility of all the morphological data was verified by similar findings in at least 3 individual fish.
Intraperitoneal injection of adult zebrafish
The procedure of Kinkel et al (49) was followed with minor modifications. In brief, after fasting and anesthetization, adult fish (4 months postfertilization) were quickly placed on an agar gel plate with some water. With use of a microinjection system (Applied Biosystems), 1 to 2 μL human chorionic gonadotropin (hCG) (5 IU/μL; Sigma-Aldrich) were carefully injected into the midline between the pelvic fins. After injection, the fish were immediately transferred back to the water tank for recovery. Four fish in each group of wild-type and homozygous lhb mutant female were used.
Statistical analysis
All data are expressed as mean values ± SEM. A value of P < .05 was considered statistically significant using one-way ANOVA, followed by the Tukey test for multiple comparisons to determine statistical differences between groups using GraphPad Instat software (GraphPad Software). All experiments were performed at least 2 or 3 times to confirm the results.
Results
Gene targeting of lhb and lhr in zebrafish
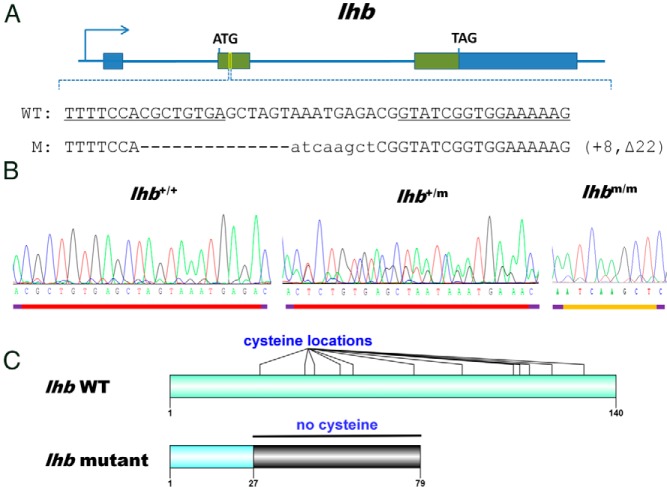
Using our established TALENs platform, we have systematically knocked out the LH β-subunit (lhb) and LHR (lhr) genes in zebrafish. Based on the published sequence of zebrafish lhb, the site for lhb chosen for targeting is located at the middle of exon 2, with 15 bp on the left and 16 bp on the right binding sites, respectively (Figure 1A). Primers were designed to detect deletion of the targeted genomic fragment (Supplemental Figure 1A). PCR amplification of gDNA isolated from the pooled P0 embryos indicated the high frequency of somatic mutation occurring in the spacer region (about 31%) (Supplemental Figure 1B), and sequencing results confirmed that deletions correctly occurred at the target site (Supplemental Figure 1C). The ratio among wild-type (lhb+/+), heterozygous (lhb+/m), and homozygous (lhbm/m) mutants in the F2 zebrafish obeyed Mendelian inheritance. The mutation was confirmed by sequencing gDNA amplified by PCR (Figure 1B). The sequencing results showed that 22 bp were deleted and 8 bp were inserted in targeting sites, causing an open-reading frame shift at amino acid position number 28, which produced a truncated Lhb protein mutant (Figure 1C).
Figure 1. Targeted disruption of the zebrafish lhb gene by TALENs.
A, Location of the engineered TALENs binding site on the lhb gene of zebrafish. The sequences of a pair of TALENs binding sites are underlined. WT, wild-type; M, homozygous mutant line of F2 generation zebrafish. B, Sequencing results of lhb from wild-type (lhb+/+), heterozygous (lhb+/m), and homozygous (lhbm/m) zebrafish. C, Schematic protein structure of lhb from wild-type and mutant zebrafish based on sequencing results.
With use of a similar approach for targeted disruption of lhr in zebrafish, the TALENs binding sites were chosen at exon 1 (Figure 2A). PCR amplification of gDNA isolated from the pooled P0 embryos indicated the high frequency of somatic mutation occurring in the spacer region (about 80%) (Supplemental Figure 1, D and E). The ratio among wild-type (lhr+/+), heterozygous (lhr+/m), and homozygous (lhrm/m) mutants in the F2 also obeyed Mendelian inheritance. For lhrm/m, the mutation leads to the generation of a stop codon at amino acid position 34, thus disrupting the biogenesis of the functional LHR (Figure 2, B and C). These results indicate that we have successfully engineered a null mutation at either the lhb or lhr locus that led to LH or LHR deficiency, respectively, in zebrafish.
Figure 2. Targeted disruption of the zebrafish lhr gene by TALENs.
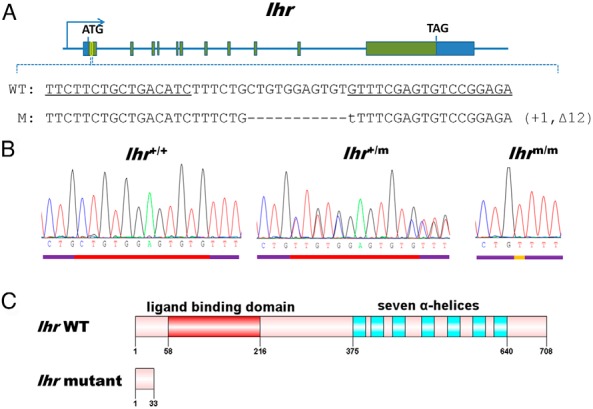
A, Location of the engineered TALENs binding site on the lhr gene of zebrafish. The sequences of a pair of TALENs binding sites are underlined. WT, wild-type; M, homozygous mutant line of F2 generation zebrafish. B, Sequencing results of lhr from wild-type (lhr+/+), heterozygous (lhr+/m), and homozygous (lhrm/m) zebrafish. C, Schematic protein structure of LHR from wild-type and mutant zebrafish based on sequencing results.
Fertility defects in lhb and lhr knockout female but not male zebrafish
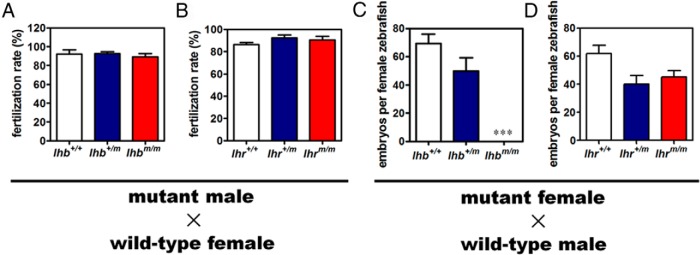
Homozygous lhb or homozygous lhr knockout zebrafish of both sexes were born phenotypically normal, and no distinct differences were noted regarding their survival, embryonic development, and sex differentiation. Mating of lhb or lhr mutant male zebrafish with wild-type females still produced normal numbers of offspring, and no significant difference in the fertilization rate was noticed (Figure 3, A and B). These data indicate that lhb and lhr mutant male zebrafish were fertile. In sharp contrast, mating between homozygous lhb mutant females and wild-type males did not result in any offspring (Figure 3C), indicating that the female lhb-null zebrafish were infertile. Mating between heterozygous or homozygous lhr mutant females and wild-type males resulted in some decrease, although not statistically significant, in the number of embryos (Figure 3D), indicating that ovary development in lhr mutant female was somewhat affected.
Figure 3. Fertility assessment of lhb or lhr mutant male and female zebrafish.
A, Fertilization rate of wild-type (lhb+/+), heterozygous (lhb+/m), and homozygous (lhbm/m) mutant male zebrafish crossing with wild-type females (n = 5 from each group of lhb+/+, lhb+/m, and lhbm/m). B, Fertilization rate of wild-type (lhr+/+), heterozygous (lhr+/m), and homozygous (lhrm/m) mutant male zebrafish crossing with wild-type females (n = 5 from each group of lhr+/+, lhr+/m, and lhrm/m). C, Number of embryos produced in wild-type (lhb+/+), heterozygous (lhb+/m), and homozygous (lhbm/m) mutant female zebrafish crossing with wild-type male. ***, P < .001, compared with lhb+/+ female zebrafish (n = 10 from each group of lhb+/+, lhb+/m, and lhbm/m). D, Number of embryos produced in wild-type (lhr+/+), heterozygous (lhr+/m), and homozygous (lhrm/m) mutant female zebrafish crossing with wild-type males (n = 10 from each group of lhr+/+, lhr+/m, and lhrm/m).
Testicular morphology and histology in lhb and lhr knockout zebrafish
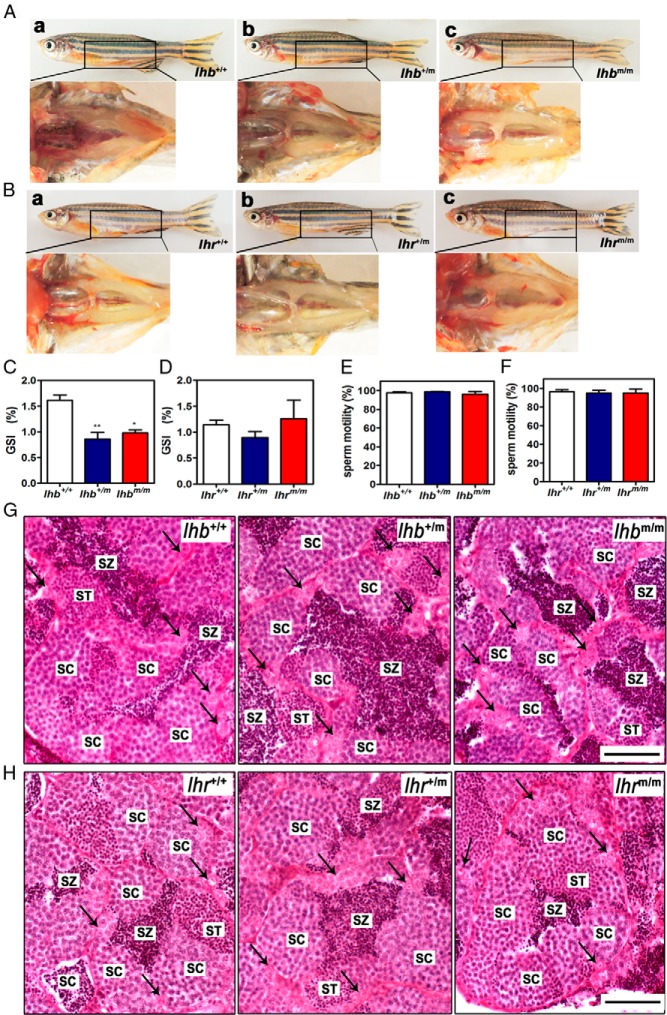
To further assess the fertility of lhb and lhr mutant male zebrafish, the testes from lhb and lhr knockout males were examined morphologically and histologically. In lhb and lhr mutant males, the testis morphology appeared normal (Figure 4, A and B), and a significant decrease in the gonadosomatic index (GSI) was only found in lhb but not in lhr mutants (Figure 4, C and D). Sperm motility of both lhb and lhr mutants is similar to that of the wild type (Figure 4, E and F). Histological analysis revealed that sperm in different stages, including spermatogonia, spermatids, and spermatozoa appeared normal in both lhb and lhr mutants. These results suggest that testis development was not grossly affected in the absence of either LH or LHR in zebrafish as far as the overall histology and ability to produce viable sperm are concerned. Because a decrease in the GSI was observed in lhb mutant male zebrafish, more subtle effects on spermatogenesis were further analyzed using immunostaining of proliferating cell nuclear antigen (Supplemental Figure 2, A–F) and periodic acid Schiff/ferric hematoxylin/metanil yellow staining (Supplemental Figure 2, G–I). Normal Sertoli cells and Leydig cells could be found in mutants (Supplemental Figure 2, G–I). No significant difference in the number of proliferating cell nuclear antigen–labeled nuclei (type B spermatogonia) (Supplemental Figure 2J) was found between the lhb mutants and the wild type, nor was there any statistically significant decrease in the number of spermatids plus spermatozoa between the lhb mutants and the wild type (Supplemental Figure 2K). However, a significant increase in the number of spermatocytes was observed between the homozygous lhb mutant and the wild type (Supplemental Figure 2K). These results suggest that LH signaling might be involved in the regulation of spermatogenesis, especially the process from the meiosis stage to the spermiogenesis stage.
Figure 4. Morphology and histology of testes of wild-type (lhb+/+, lhr+/+), heterozygous (lhb+/m, lhr+/m) and homozygous (lhbm/m, lhrm/m) mutant male zebrafish.
A, Appearance of testes dissected from lhb+/+ (a), lhb+/m (b), and lhbm/m (c) male zebrafish. B, Appearance of testes dissected from lhr+/+ (a), lhr+/m (b), and lhrm/m (c) male zebrafish. C, GSI of lhb+/+, lhb+/m, and lhbm/m male zebrafish.*, P < .05; **, P < .01, compared with lhb+/+ male zebrafish. The data presented are the mean values ± SEM of measurements from 6 individual fish. D, GSI of lhr+/+, lhr+/m, and lhrm/m male zebrafish. E, Sperm motility in lhb+/+, lhb+/m, and lhbm/m male zebrafish. F, Sperm motility in lhr+/+, lhr+/m, and lhrm/m male zebrafish. G, Histology of testes from lhb+/+ (a), lhb+/m (b), and lhbm/m (c) male zebrafish. H, Histology of testes from lhr+/+ (a), lhr+/m (b), and lhrm/m (c) male zebrafish. Bars correspond to 50 μm. SC, spermatocytes; ST, spermatids; SZ, spermatozoa; spermatogonia are marked by arrows.
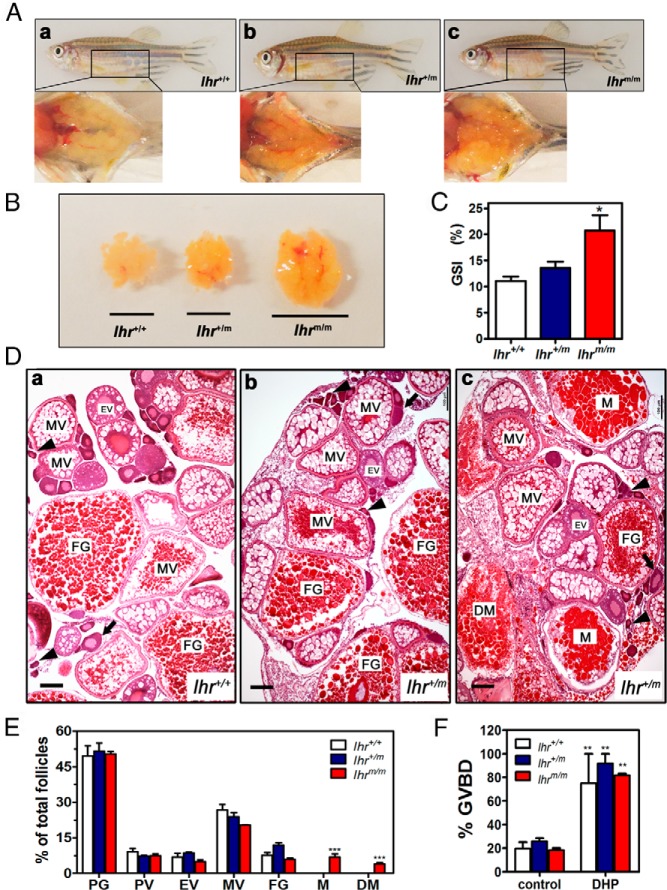
Ovarian morphology and histology in lhb and lhr knockout zebrafish
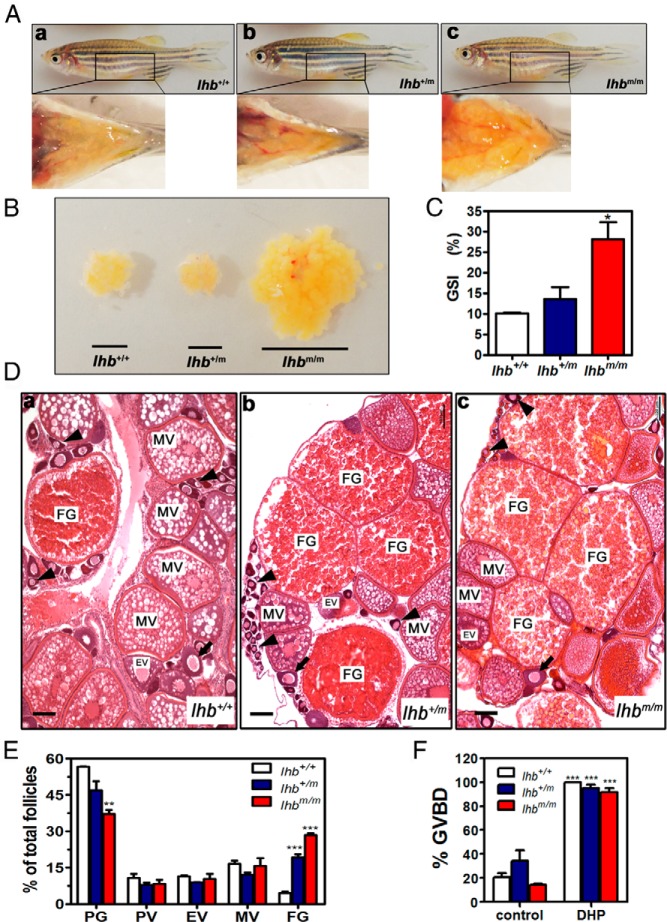
To further delineate the cause of fertility defects observed in lhb and lhr mutant females, we examined their ovaries morphologically and histologically. In lhb mutant females, the ovary size was increased significantly in the homozygotes compared with that in the heterozygotes and wild type at adult stage (Figure 5, A and B). Accordingly, a significant increase in the GSI was found in the homozygous mutants (Figure 5C). Histological analysis revealed that different stages of follicles before maturation could be found in the ovaries of lhb mutant adult fish. Follicles of primary growth (PG), previtellogenic (PV), early vitellogenic (EV), middle vitellogenic (MV), and full grown (FG) stages appeared normal by morphology and histology (Figure 5D). In addition, the ratios of the different stages of follicles between the wild type and mutants were further compared. Because PG stage follicles were too small to be counted directly by morphology, the ratio of the different stages of follicles was assessed by microscopic examination of the sections. The ratio of PG stage follicles decreased, whereas the ratio of FG stage follicles dramatically increased in the ovaries of the mutant fish (Figure 5E). To assess the conditions of the follicles, FG stage follicles were isolated for treatment with 17α,20β-dihydroxy-4-pregnen-3-one (DHP) in vitro. Most of them could enter into the mature stage as indicated by germinal vesicle breakdown (GVBD), and no significant difference was noticed between the mutants and wild type (Figure 5F), indicating that the competency to respond to endogenous hormones was not adversely affected in the follicles of lhb mutant fish.
Figure 5. Morphology and histology of ovaries of wild-type (lhb+/+), heterozygous (lhb+/m), and homozygous (lhbm/m) mutant female zebrafish.
A, Appearance of ovaries dissected from lhb+/+ (a), lhb+/m (b), and lhbm/m (c) female zebrafish. B, Gross appearance of ovaries from lhb+/+, lhb+/m, and lhbm/m female zebrafish. C, GSI of lhb+/+, lhb+/m, and lhbm/m female zebrafish. *, P < .05, compared with lhb+/+ female zebrafish. The data presented are the mean values ± SEM of measurements from six individual fish. D, Histology of ovaries from lhb+/+ (a), lhb+/m (b), and lhbm/m (c) female zebrafish. All stages of follicles including PG, PV, EV, MV, and FG stage follicles but not mature stage follicles were present in the ovaries of lhb+/m (b) and lhbm/m (c) female zebrafish. PV follicles are marked by arrows, and PG follicles are marked by arrowheads. Bars correspond to 100 μm. E, Ratio of different stage follicles in lhb+/+ (a), lhb+/m (b), and lhbm/m (c) female zebrafish. **, P < .01; ***, P < .001, compared with lhb+/+ female zebrafish. The data presented are the mean values ± SEM of measurements from 3 individual zebrafish. F, GVBD ratio after treatment of DHP on FG stage follicles isolated from lhb+/+ (a), lhb+/m (b), and lhbm/m (c) female zebrafish. ***, P < .001, compared with the GVBD ratio of FG stage follicles from wild-type zebrafish. The data presented are the mean values ± SEM of measurements from 3 batches of follicles.
Somewhat similar phenotypes could be observed in the female lhr mutant zebrafish, with the ovary size and GSI dramatically increased in the homozygotes (Figure 6, A–C). All stages of follicles could be readily identified in the ovary. However, unlike lhb mutants, some mature follicles and degenerating mature follicles could be found in the ovaries of homozygous lhr mutant fish (Figure 6D). The ratio of follicles beyond the FG stage including the mature stage and the degenerating mature stage has increased in the mutants (Figure 6E). No significant difference in GVBD after treatment with DHP was found between the mutants and wild type (Figure 6F). Collectively, these data indicate that the absence of LH or LHR does not affect folliculogenesis. Yet both oocyte maturation and ovulation were affected in the lhb mutants, whereas only ovulation but not maturation was affected in lhr mutants.
Figure 6. Morphology and histology of ovaries of wild-type (lhr+/+), heterozygous (lhr+/m), and homozygous (lhrm/m) mutant female zebrafish.
A, Appearance of ovaries dissected from lhr+/+ (a), lhr+/m (b), and lhrm/m (c) female zebrafish. B, Gross appearance of ovaries from lhr+/+, lhr+/m, and lhrm/m female zebrafish. C, GSI of lhr+/+, lhr+/m, and lhrm/m female zebrafish.*, P < .05, compared with lhr+/+ female zebrafish. The data presented are the mean values ± SEM of measurements from 6 individual fish. D, Histology of ovaries from lhr+/+ (a), lhr+/m (b), and lhrm/m (c) female zebrafish. All stages of follicles including PG, PV, EV, MV, FG, mature stage, and degenerating mature stage were present in ovaries of lhrm/m (c) female zebrafish. PV follicles are marked by arrows, and PG follicles are marked by arrowheads. Bars correspond to 100 μm. E, Ratio of different stage follicles in lhr+/+ (a), lhr+/m (b), and lhrm/m (c) female zebrafish. F, GVBD ratio after treatment of DHP on FG stage follicles isolated from lhr+/+ (a), lhr+/m (b), and lhrm/m (c) female zebrafish. **, P < .01, compared with the GVBD ratio of FG stage follicles from wild-type zebrafish. The data presented are the mean values ± SEM of measurements from 3 batches of follicles. M, mature stage; DM, degenerating mature follicles.
Gene expression profiles in the ovary of lhb and lhr knockout zebrafish
To further analyze the molecular mechanism underlining the fertility defects observed in lhb and lhr mutant females as well as the differential phenotype between the 2 mutant lines, a panel of genes involved in ovarian steroidogenesis, oocyte maturation, and ovulation were chosen and their expression profiles were assessed in FG stage follicles from the ovaries of wild-type, heterozygotes, and homozygotes zebrafish by real-time PCR (Table 1 and Supplemental Figure 3). For key enzymes and receptors involved in steroidogenesis, the expression of npr, er2a, star1, p450scc, p450c17a1, and 17β-hsd3 was significantly reduced, whereas only mprb expression was significantly increased in the lhb mutant FG stage follicles. However, the expression of mpra, er2b, cyp19a, star2, 3b-hsd, p450c17a2, 20b-hsd1, 20b-hsd2, and 17b-hsd1 was not significantly changed in the mutants (Supplemental Figure 3). On the other hand, in lhr mutant FG stage follicles, only npr was dramatically decreased. For growth factors reported to be important for ovarian functions, especially oocyte maturation (50–53), the expression of egf, igf3, and inhbb was greatly decreased in the ovaries of lhb but not lhr mutants, whereas inhbab, fst, and tgfb1 expression remained unchanged in both lhb and lhr mutants (Supplemental Figure 3). Moreover, several genes participating in ovulation including plg, plat, plaua, ptgs2a, ptgs2b, and timp2b were up-regulated, whereas only plaub and mmp15b were down-regulated in the lhb mutant ovary. Similar to that in the lhb mutant ovary, the absence of LHR in the ovary leads to up-regulation of many genes related to ovulation including plat, plaua, plaub, ptger4b, ptgs2a, timp2b, mmp2, mmp14b, and mmp15a. These data indicate that genes regulating oocyte maturation and ovulation were affected in the absence of LH or LHR, and differential gene expression between lhb and lhr mutants was evident.
Table 1.
Gene Expression Profile in FG Stage Follicles Isolated From the Ovaries of Wild-Type (lhb+/+ or lhr+/+) and Homozygous (lhbm/m or lhrm/m) Mutant Female Zebrafish
| Gene | lhbm/m | T | lhrm/m | T |
|---|---|---|---|---|
| Steroidogenesis | ||||
| mprb | 1.84 ± 0.22a | ↑ | 1.39 ± 0.22d | |
| npr | 0.37 ± 0.05c | ↓ | 0.12 ± 0.05b | ↓ |
| er2a | 0.74 ± 0.04a | ↓ | 1.21 ± 0.14d | |
| star1 | 0.27 ± 0.03a | ↓ | 1.15 ± 0.18d | |
| p450scc | 0.42 ± 0.03b | ↓ | 1.13 ± 0.10d | |
| p450c17a1 | 0.37 ± 0.02c | ↓ | 1.03 ± 0.05d | |
| 17b-hsd3 | 0.41 ± 0.02a | ↓ | 1.28 ± 0.22d | |
| Growth factors | ||||
| egf | 0.27 ± 0.05a | ↓ | 0.72 ± 0.32d | |
| igf3 | 0.25 ± 0.02a | ↓ | 1.23 ± 0.34d | |
| inhbb | 0.50 ± 0.07a | ↓ | 0.85 ± 0.11d | |
| Ovulation | ||||
| plg | 10.17 ± 2.55a | ↑ | 0.83 ± 0.19d | |
| plat | 2.70 ± 0.36c | ↑ | 1.85 ± 0.12c | ↑ |
| plaua | 3.06 ± 0.46a | ↑ | 3.00 ± 1.03a | ↑ |
| plaub | 0.41 ± 0.06b | ↓ | 2.13 ± 0.20c | ↑ |
| ptger4b | 0.89 ± 0.09d | 2.11 ± 0.17c | ↑ | |
| ptgs2a | 9.74 ± 3.42b | ↑ | 3.16 ± 0.28c | ↑ |
| ptgs2b | 2.06 ± 0.18a | ↑ | 1.34 ± 0.22d | |
| timp2b | 9.75 ± 3.43b | ↑ | 3.22 ± 0.64b | ↑ |
| mmp2 | 1.65 ± 0.33d | 1.80 ± 0.29a | ↑ | |
| mmp14b | 1.06 ± 0.10d | 3.50 ± 0.59c | ↑ | |
| mmp15a | 0.97 ± 0.10d | 1.86 ± 0.34a | ↑ | |
| mmp15b | 0.67 ± 0.04a | ↓ | 1.65 ± 0.39d |
Significant regulation of several genes involved in steroidogenesis, growth factors regulating oocyte maturation, and genes related to ovulation was found between the wild-type and mutants. The expression levels were normalized to that of ef1a. The data are presented as the ratio of mean values by comparing mutant to wild type (n = 6 for lhb and n = 8 for lhr). T indicates gene expression trend: ↑, up-regulation of gene expression; ↓, down-regulation of gene expression. The full names of the genes and the primers used in the real-time PCR are listed in Supplementary Table 1.
Statistical significance: a P < .05; b P < .01; c P < .001; d no statistical significance compared with the wild type.
Defects in oocyte maturation and ovulation partially restored by hCG in lhb knockout zebrafish
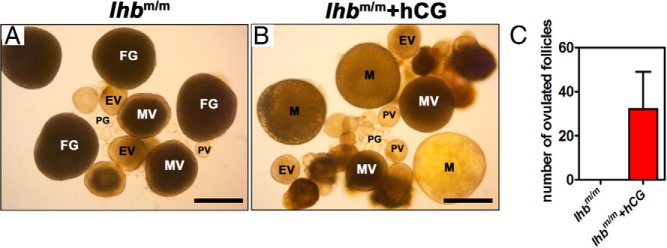
To test whether lhb mutants respond to exogenously given LH, females were intraperitoneally injected with hCG, a hormone commonly used to mimic the action of LH on binding and activating LHR in fish. Mature stage follicles could be observed in the ovary after administration of hCG to homozygous lhb mutant female fish (Figure 7, A and B), and a small number of mature follicles were ovulated (Figure 7C). These results further support the indispensable role of LH in oocyte maturation and ovulation in zebrafish.
Figure 7. Defects of oocyte maturation and ovulation in lhb mutant zebrafish partially rescued by hCG administration.
A, Gross morphology of ovaries dissected from lhb mutant (lhbm/m) zebrafish. B, Gross morphology of ovaries dissected from lhb mutant (lhbm/m) zebrafish after administration of hCG. M, mature stage. Bars correspond to 500 μm. C, Comparison of the number of ovulated follicles from lhbm/m and lhbm/m after administration of hCG (n = 3 from each group).
Discussion
In this study, TALENs were used to knock out lhb and lhr in zebrafish. We have demonstrated that both lhb and lhr mutant male zebrafish are fertile. On the other hand, lhb-null female zebrafish are infertile, and lhr-null female zebrafish are fertile. These results challenge our conventional view of the essential role of LH signaling in both male and female reproduction (1, 2, 24, 54), where direct evidence coming from mammals shows that both LH- and LHR-null male and female mice are infertile (6–8).
Morphological and histological analysis revealed a smaller GSI in lhb mutant males and normal GSI in lhr mutant males compared with that in the wild type, and no differences in sperm motility and testicular structure were observed in lhb and lhr mutants. These results clearly indicate that LH signaling is not crucial for testicular fertility in zebrafish. In mammals, however, both lhb and lhr mutant male mice exhibit testes of reduced size, and spermatogenesis was blocked at the round spermatid stage (6–8). It has been demonstrated in mammals that spermatogenesis is regulated by both FSH and LH. This immense difference in the role of LH signaling in testis between fish and mammals prompted us to speculate that FSH signaling might be the dominant factor in regulating fish spermatogenesis. This view is supported by several recent reports in fish (21, 22, 55–60). Most recently, Schulz and colleagues (22) also proposed that FSH serves as the constitutive driving force for fish spermatogenesis. Thus, in conjunction with the data from the present study, a revision of the present concept on the mode of action of gonadotropins in fish testis should be considered. However, it should be noted that although LH signaling is not crucial for male fertility, its involvement in fish testicular development could not be completely excluded because the GSI decreases in lhb mutant male zebrafish, and a significant increase in the number of spermatocytes was observed in homozygous lhb mutant, suggesting that LH signaling still plays a certain role in the regulation of spermatogenesis, especially the process from meiosis stage to spermiogenesis.
In females, folliculogenesis was normal in both lhb and lhr mutants, but oocyte maturation and ovulation were disrupted in lhb mutant, whereas only ovulation was affected in lhr mutant. These findings in fish ovary are very different from those in mammals in which LH and LHR knockout mice largely phenocopy each other, and ovarian folliculogenesis is blocked at the antral stage in either lhb or lhr knockout female mice (6–8). The differential phenotype of LH and LHR knockout on oocyte maturation and ovulation in zebrafish could be explained by the specificity of ligand binding of the gonadotropins to their receptors. The selectivity of gonadotropin ligand/receptor interaction in mammals is well defined: FSH and LH bind to their respective cognate receptors specifically and show little cross-reactivity (0.01%–0.1%) (61, 62). In contrast, the bioactivity of fish gonadotropins seems to be less well separated as a result of promiscuous ligand-receptor interactions (26, 27, 63–66). Most of the evidence has suggested that the wider but still limited functional selectivity of FSHR for both FSH and LH may depend on the fish species or taxa, whereas fish LHR, like its mammalian counterpart, seems to be more specific for its ligand (1). Previously, So et al suggested (11) in zebrafish that FSH specifically activates FSHR, whereas LH could stimulate both FSHR and LHR. Results from the present study provide the first in vivo evidence that LH probably binds and activates FSHR in the absence of LHR in zebrafish. Furthermore, the presence of abnormal mature follicles in the ovary and a low egg production capacity found in the lhr-null fish indicates the importance of LHR in the ovulation process. It is not known whether LHR is physiologically involved in oocyte maturation of zebrafish, because mature follicles were found in lhr mutant ovary. However, the differential expression profile of LHR and FSHR as well as the higher binding affinity of LH to LHR than FSHR in zebrafish (11) lead us to speculate that LH regulates oocyte maturation through LHR under physiological conditions. Future studies to substantiate this are highly warranted.
To understand the molecular mechanisms of LH signaling in the ovary and the differential phenotype between LH and LHR mutant ovary, the expression of a panel of genes involved in steroidogenesis, oocyte maturation, and ovulation was assessed. First, these results supply direct in vivo evidence on the regulation of downstream genes by LH signaling in fish ovary. For example, the absence of lhb leads to decreased expression of star1, p450scc, and 17b-hsd3 revealed in this study, consistent with the regulation trend from in vitro assays (67). Second, the differential phenotype of lhb and lhr mutant in the ovary could be partially explained by the differential gene expression profiles of the 2 types of mutants. A number of genes related to oocyte maturation such as egf, igf3, and inhbb were down-regulated only in lhb mutant but not in lhr mutant. Finally, several other genes were only changed in the lhb mutant but not in lhr mutant. For example, the expression of mprb, er2a, star1, p450scc, p450c17a1, and 17b-hsd3 was changed in lhb mutant but not in lhr mutant, suggesting that LH might regulate these genes through FSHR in the absence of LHR.
Collectively, we have demonstrated, for the first time in a vertebrate, the noncanonical role of LH signaling in reproduction, being indispensable for gonadal development in females but not in males. Several lines of evidence in the present study support the crucial role of LH signaling in oocyte maturation and ovulation in female zebrafish. This report, representing the first piece of knockout studies on fish LH and LHR, contributes toward a fuller understanding of the role of the gonadotropin system in vertebrate reproduction.
Acknowledgments
We thank Kathy W. Y. Sham for technical assistance. We are also grateful to the Core Laboratories in the School of Biomedical Sciences for provision of equipment and technical support.
This work was supported by the General Research Fund of the Hong Kong Research Grants Council (CUHK 463013), the National Natural Science Foundation of China (Grant 31325026), and the Chinese University of Hong Kong and The State Key Laboratory of Freshwater Ecology and Biotechnology Program (Grant 2014FB10).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the General Research Fund of the Hong Kong Research Grants Council (CUHK 463013), the National Natural Science Foundation of China (Grant 31325026), and the Chinese University of Hong Kong and The State Key Laboratory of Freshwater Ecology and Biotechnology Program (Grant 2014FB10).
Footnotes
- DHP
- 17α,20β-dihydroxy-4-pregnen-3-one
- EV
- early vitellogenic
- FG
- full grown
- FSHR
- FSH receptor
- gDNA
- genomic DNA
- GSI
- gonadosomatic index
- GVBD
- germinal vessel breakdown
- hCG
- human chorionic gonadotropin
- LHR
- LH receptor
- MV
- middle vitellogenic
- PG
- primary growth
- PV
- previtellogenic
- TALE
- transcription activator-like effector
- TALEN
- transcription activator-like effector nuclease.
References
- 1. Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ. Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol. 2010;165:412–437. [DOI] [PubMed] [Google Scholar]
- 2. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. [DOI] [PubMed] [Google Scholar]
- 3. Huhtaniemi I. Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol Cell Endocrinol. 2006;254–255:84–90. [DOI] [PubMed] [Google Scholar]
- 4. Huhtaniemi I, Zhang FP, Kero J, Hämäläinen T, Poutanen M. Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Mol Cell Endocrinol. 2002;187:49–56. [DOI] [PubMed] [Google Scholar]
- 5. Kumar TR. Functional analysis of LHβ knockout mice. Mol Cell Endocrinol. 2007;269:81–84. [DOI] [PubMed] [Google Scholar]
- 6. Lei ZM, Mishra S, Zou W, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. [DOI] [PubMed] [Google Scholar]
- 7. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294–17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. [DOI] [PubMed] [Google Scholar]
- 9. Nozaki M, Naito N, Swanson P, et al. Salmonid pituitary gonadotrophs. I. Distinct cellular distributions of two gonadotropins, GTH I and GTH II. Gen Comp Endocrinol. 1990;77:348–357. [DOI] [PubMed] [Google Scholar]
- 10. Naito N, Suzuki K, Nozaki M, Swanson P, Kawauchi H, Nakai Y. Ultrastructural characteristics of two distinct gonadotropes (GTH I- and GTH II-cells) in the pituitary of rainbow trout Oncorhynchus mykiss. Fish Physiol Biochem. 1993;11:241–246. [DOI] [PubMed] [Google Scholar]
- 11. So WK, Kwok HF, Ge W. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits—their spatial-temporal expression patterns and receptor specificity. Biol Reprod. 2005;72:1382–1396. [DOI] [PubMed] [Google Scholar]
- 12. Chen W, Ge W. Ontogenic expression profiles of gonadotropins (fshb and lhb) and growth hormone (gh) during sexual differentiation and puberty onset in female zebrafish. Biol Reprod. 2012;86:73. [DOI] [PubMed] [Google Scholar]
- 13. Parhar IS, Soga T, Ogawa S, Sakuma Y. FSH and LH-β subunits in the preoptic nucleus: ontogenic expression in teleost. Gen Comp Endocrinol. 2003;132:369–378. [DOI] [PubMed] [Google Scholar]
- 14. Vischer HF, Teves AC, Ackermans JC, van Dijk W, Schulz RW, Bogerd J. Cloning and spatiotemporal expression of the follicle-stimulating hormone β subunit complementary DNA in the African catfish (Clarias gariepinus). Biol Reprod. 2003;68:1324–1332. [DOI] [PubMed] [Google Scholar]
- 15. Kagawa H, Kawazoe I, Tanaka H, Okuzawa K. Immunocytochemical identification of two distinct gonadotropic cells (GTH I and GTH II) in the pituitary of bluefin tuna, Thunnus thynnus. Gen Comp Endocrinol. 1998;110:11–18. [DOI] [PubMed] [Google Scholar]
- 16. Kumar RS, Ijiri S, Trant JM. Molecular biology of channel catfish gonadotropin receptors: 1. Cloning of a functional luteinizing hormone receptor and preovulatory induction of gene expression. Biol Reprod. 2001;64:1010–1018. [DOI] [PubMed] [Google Scholar]
- 17. Guzmán JM, Adam Luckenbach J, Swanson P. Molecular characterization and quantification of sablefish (Anoplopoma fimbria) gonadotropins and their receptors: reproductive dysfunction in female captive broodstock. Gen Comp Endocrinol. 2013;193:37–47. [DOI] [PubMed] [Google Scholar]
- 18. Andersson E, Nijenhuis W, Male R, et al. Pharmacological characterization, localization and quantification of expression of gonadotropin receptors in Atlantic salmon (Salmo salar L.) ovaries. Gen Comp Endocrinol. 2009;163:329–339. [DOI] [PubMed] [Google Scholar]
- 19. Mittelholzer C, Andersson E, Taranger GL, et al. Molecular characterization and quantification of the gonadotropin receptors FSH-R and LH-R from Atlantic cod (Gadus morhua). Gen Comp Endocrinol. 2009;160:47–58. [DOI] [PubMed] [Google Scholar]
- 20. Kwok HF, So WK, Wang Y, Ge W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors–evidence for their distinct functions in follicle development. Biol Reprod. 2005;72:1370–1381. [DOI] [PubMed] [Google Scholar]
- 21. García-López A, Bogerd J, Granneman JC, et al. Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology. 2009;150:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García-López A, de Jonge H, Nóbrega RH, et al. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology. 2010;151:2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bogerd J, Granneman JC, Schulz RW, Vischer HF. Fish FSH receptors bind LH: how to make the human FSH receptor to be more fishy? Gen Comp Endocrinol. 2005;142:34–43. [DOI] [PubMed] [Google Scholar]
- 24. Swanson P, Dickey JT, Campbell B. Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem. 2003;28:7. [Google Scholar]
- 25. Bogerd J, Blomenröhr M, Andersson E, et al. Discrepancy between molecular structure and ligand selectivity of a testicular follicle-stimulating hormone receptor of the African catfish (Clarias gariepinus). Biol Reprod. 2001;64:1633–1643. [DOI] [PubMed] [Google Scholar]
- 26. Miwa S, Yan L, Swanson P. Localization of two gonadotropin receptors in the salmon gonad by in vitro ligand autoradiography. Biol Reprod. 1994;50:629–642. [DOI] [PubMed] [Google Scholar]
- 27. Yan L, Swanson P, Dickhoff WW. A two-receptor model for salmon gonadotropins (GTH I and GTH II). Biol Reprod. 1992;47:418–427. [DOI] [PubMed] [Google Scholar]
- 28. Ogiwara K, Fujimori C, Rajapakse S, Takahashi T. Characterization of luteinizing hormone and luteinizing hormone receptor and their indispensable role in the ovulatory process of the medaka. PloS One. 2013;8:e54482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011;29:699–700. [DOI] [PubMed] [Google Scholar]
- 30. Sander JD, Cade L, Khayter C, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol. 2011;29:697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. [DOI] [PubMed] [Google Scholar]
- 32. Ma S, Zhang S, Wang F, et al. Highly efficient and specific genome editing in silkworm using custom TALENs. PloS One. 2012;7:e45035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li T, Liu B, Spalding MH, Weeks DP, Yang B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol. 2012;30:390–392. [DOI] [PubMed] [Google Scholar]
- 34. Cade L, Reyon D, Hwang WY, et al. Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res. 2012;40:8001–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore FE, Reyon D, Sander JD, et al. Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PloS One. 2012;7:e37877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qiu Z, Liu M, Chen Z, et al. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic Acids Res. 2013;41:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tesson L, Usal C, Ménoret S, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. [DOI] [PubMed] [Google Scholar]
- 38. Lei Y, Guo X, Liu Y, et al. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc Natl Acad Sci USA. 2012;109:17484–17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carlson DF, Tan W, Lillico SG, et al. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci USA. 2012;109:17382–17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Luo D, Zhao H, Zhu Z, Hu W, Cheng CH. Inheritable and precise large genomic deletions of non-coding RNA genes in zebrafish using TALENs. PloS One. 2013;8:e76387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Westerfield M. The Zebrafish Book. 5th ed Eugene, OR: University of Oregon Press; 2007. [Google Scholar]
- 43. Brion F, Tyler CR, Palazzi X, et al. Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat Toxicol. 2004;68:193–217. [DOI] [PubMed] [Google Scholar]
- 44. McAllister BG, Kime DE. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat Toxicol. 2003;65:309–316. [DOI] [PubMed] [Google Scholar]
- 45. Selman K, Wallace R, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:203–224. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Ge W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: evidence for their differential roles during sexual maturation and ovulatory cycle. Biol Reprod. 2004;71:2056–2064. [DOI] [PubMed] [Google Scholar]
- 47. Pang Y, Thomas P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol. 2010;342:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leal MC, Cardoso ER, Nóbrega RH, et al. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod. 2009;81:177–187. [DOI] [PubMed] [Google Scholar]
- 49. Kinkel MD, Eames SC, Philipson LH, Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp. 2010;42:pii:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ge W. Roles of the activin regulatory system in fish reproduction. Can J Physiol Pharmacol. 2000;78:1077–1085. [PubMed] [Google Scholar]
- 51. Wu T, Patel H, Mukai S, et al. Activin, inhibin, and follistatin in zebrafish ovary: expression and role in oocyte maturation. Biol Reprod. 2000;62:1585–1592. [DOI] [PubMed] [Google Scholar]
- 52. Pang Y, Ge W. Epidermal growth factor and TGFα promote zebrafish oocyte maturation in vitro: potential role of the ovarian activin regulatory system. Endocrinology. 2002;143:47–54. [DOI] [PubMed] [Google Scholar]
- 53. Li J, Liu Z, Wang D, Cheng CH. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol Reprod. 2011;84:476–486. [DOI] [PubMed] [Google Scholar]
- 54. Kumar TR. What have we learned about gonadotropin function from gonadotropin subunit and receptor knockout mice? Reproduction. 2005;130:293–302. [DOI] [PubMed] [Google Scholar]
- 55. Planas JV, Athos J, Goetz FW, Swanson P. Regulation of ovarian steroidogenesis in vitro by follicle-stimulating hormone and luteinizing hormone during sexual maturation in salmonid fish. Biol Reprod. 2000;62:1262–1269. [DOI] [PubMed] [Google Scholar]
- 56. Planas JV, Swanson P. Maturation-associated changes in the response of the salmon testis to the steroidogenic actions of gonadotropins (GTH I and GTH II) in vitro. Biol Reprod. 1995;52:697–704. [DOI] [PubMed] [Google Scholar]
- 57. Kazeto Y, Kohara M, Miura T, et al. Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): production of biologically active recombinant Fsh and Lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol Reprod. 2008;79:938–946. [DOI] [PubMed] [Google Scholar]
- 58. Aizen J, Kobayashi M, Selicharova I, Sohn YC, Yoshizaki G, Levavi-Sivan B. Steroidogenic response of carp ovaries to piscine FSH and LH depends on the reproductive phase. Gen Comp Endocrinol. 2012;178:28–36. [DOI] [PubMed] [Google Scholar]
- 59. Aizen J, Kasuto H, Golan M, Zakay H, Levavi-Sivan B. Tilapia follicle-stimulating hormone (FSH): immunochemistry, stimulation by gonadotropin-releasing hormone, and effect of biologically active recombinant FSH on steroid secretion. Biol Reprod. 2007;76:692–700. [DOI] [PubMed] [Google Scholar]
- 60. Weltzien FA, Norberg B, Swanson P. Isolation and characterization of FSH and LH from pituitary glands of Atlantic halibut (Hippoglossus hippoglossus L.). Gen Comp Endocrinol. 2003;131:97–105. [DOI] [PubMed] [Google Scholar]
- 61. Braun T, Schofield PR, Sprengel R. Amino-terminal leucine-rich repeats in gonadotropin receptors determine hormone selectivity. EMBO J. 1991;10:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tilly JL, Aihara T, Nishimori K, et al. Expression of recombinant human follicle-stimulating hormone receptor: species-specific ligand binding, signal transduction, and identification of multiple ovarian messenger ribonucleic acid transcripts. Endocrinology. 1992;131:799–806. [DOI] [PubMed] [Google Scholar]
- 63. Oba Y, Hirai T, Yoshiura Y, Yoshikuni M, Kawauchi H, Nagahama Y. The duality of fish gonadotropin receptors: cloning and functional characterization of a second gonadotropin receptor cDNA expressed in the ovary and testis of amago salmon (Oncorhynchus rhodurus). Biochem Biophys Res Commun. 1999;265:366–371. [DOI] [PubMed] [Google Scholar]
- 64. Vischer HF, Bogerd J. Cloning and functional characterization of a gonadal luteinizing hormone receptor complementary DNA from the African catfish (Clarias gariepinus). Biol Reprod. 2003;68:262–271. [DOI] [PubMed] [Google Scholar]
- 65. Vischer HF, Marques RB, Granneman JC, Linskens MH, Schulz RW, Bogerd J. Receptor-selective determinants in catfish gonadotropin seat-belt loops. Mol Cell Endocrinol. 2004;224:55–63. [DOI] [PubMed] [Google Scholar]
- 66. Sambroni E, Le Gac F, Breton B, Lareyre JJ. Functional specificity of the rainbow trout (Oncorhynchus mykiss) gonadotropin receptors as assayed in a mammalian cell line. J Endocrinol. 2007;195:213–228. [DOI] [PubMed] [Google Scholar]
- 67. Ings JS, Van Der Kraak GJ. Characterization of the mRNA expression of StAR and steroidogenic enzymes in zebrafish ovarian follicles. Mol Reprod Dev. 2006;73:943–954. [DOI] [PubMed] [Google Scholar]