Abstract
The mineralocorticoid receptor (MR) plays a central role in salt and water homeostasis via the kidney; however, inappropriate activation of the MR in the heart can lead to heart failure. A selective MR modulator that antagonizes MR signaling in the heart but not the kidney would provide the cardiovascular protection of current MR antagonists but allow for normal electrolyte balance. The development of such a pharmaceutical requires an understanding of coregulators and their tissue-selective interactions with the MR, which is currently limited by the small repertoire of MR coregulators described in the literature. To identify potential novel MR coregulators, we used T7 phage display to screen tissue-selective cDNA libraries for MR-interacting proteins. Thirty MR binding peptides were identified, from which three were chosen for further characterization based on their nuclear localization and their interaction with other MR-interacting proteins or, in the case of x-ray repair cross-complementing protein 6, its known status as an androgen receptor coregulator. Eukaryotic elongation factor 1A1, structure-specific recognition protein 1, and x-ray repair cross-complementing protein 6 modulated MR-mediated transcription in a ligand-, cell- and/or promoter-specific manner and colocalized with the MR upon agonist treatment when imaged using immunofluorescence microscopy. These results highlight the utility of phage display for rapid and sensitive screening of MR binding proteins and suggest that eukaryotic elongation factor 1A1, structure-specific recognition protein 1, and x-ray repair cross-complementing protein 6 may be potential MR coactivators whose activity is dependent on the ligand, cellular context, and target gene promoter.
The mineralocorticoid receptor (MR) belongs to the steroid hormone receptor subfamily of the nuclear receptor (NR) superfamily, which includes the estrogen (ER), glucocorticoid, progesterone (PR), and androgen (AR) receptors (1, 2). It is expressed in a wide range of tissues and binds both the mineralocorticoid, aldosterone, and glucocorticoids, cortisol in humans, and corticosterone in rodents (3). Although aldosterone-bound MR is critical for controlling sodium and potassium transport in epithelial cells (4), inappropriate MR activation in the heart can be mediated by either aldosterone or cortisol in the setting of tissue injury and oxidative stress and leads to cardiac inflammation and fibrosis (5–7). Large clinical trials have confirmed the benefits of MR antagonists in the management of heart failure (8–10), but their widespread use is limited by hyperkalemia (11).
An ideal MR modulator would achieve the cardioprotective benefits of current MR antagonists but avoid the hyperkalemia associated with MR blockade in the kidney. Such tissue selectivity has been achieved for the ER with selective ER modulators such as tamoxifen, which antagonizes ER activity in breast cancer cells, but not in the bone, and thus limit osteoporosis (12). One of the mechanisms underlying the tissue-specific actions of selective NR modulators is the differential recruitment of coregulators, comprised of coactivators and corepressors, by different ligands.
Coactivators function as part of large protein complexes, which are recruited to the target gene by the NR after ligand binding and perform many of the enzymatic reactions needed for the regulation of gene expression (13). Many of the characterized coactivators bind to the ligand-binding domain of the NR via a conserved NR box that contains a leucine-rich LxxLL motif (14, 15). Some coactivators, in particular those that bind the N-terminal domain, do not appear to use this motif (16). In contrast, many corepressors interact with NR via the I/L-xx-I/V-I motif to repress NR activity by inducing histone deacetylase activity and interfering with other aspects of the transcriptional machinery (17–19). Coregulators show structural and function diversity, as well as variable levels of tissue expression, and have been shown to contribute to the ligand- and tissue-specific actions of some NR (20, 21). To date, 11 coregulators have been identified specifically in the context of the MR and none have been definitively shown to have ligand-specific actions (22, 23). The lack of information on MR coregulators thus limits both our understanding of MR signaling and the development of a tissue-selective MR modulator.
Various approaches have been used for the study of NR structure and coregulator discovery, one of which is combinatorial phage display. This rapid and sensitive method has been successfully used to screen for peptides that interact with the ER, AR, peroxisome proliferator-activated receptor and liver receptor homologue-1 (24–27). We have previously used M13 phage display technology to identify ligand-dependent, MR-interacting peptides and demonstrated ligand-dependent structural changes in the MR (28). A limitation of M13 phage display is that only short oligopeptides can be displayed on the M13 phage capsid, and thus, it has limited utility in coregulator discovery. T7 phage allow for expression of larger protein fragments (up to 500 amino acids) on their surface and have been successfully used to identify novel coregulators of the AR and ER (29, 30) but have not been applied to the MR.
In the present study, we have thus used T7 phage display to screen cDNA libraries derived from two tissues critical to MR (patho)physiology (heart, kidney) to identify novel MR-interacting proteins. We screened the recombinant human MR bound to either cortisol or aldosterone to ask whether the nature of the ligand influences MR protein recruitment. Importantly, we used full-length human MR as bait (31), in contrast to previous studies that have focused on isolated MR domains. Of the 30 MR binding proteins identified, three were studied in detail and were shown to modulate MR-mediated transcription in a ligand-, cell-, and/or promoter-specific manner and to colocalize with the ligand-activated MR in live cells. One of these proteins, x-ray repair cross-complementing protein 6 (XRCC6), has already been characterized as a coactivator of the androgen receptor, which supports the biological relevance of data derived from our screen. These data illustrate the utility of the phage display for the identification of potential novel MR coregulators.
Materials and Methods
Plasmids
pBINDa was modified from pBIND (Promega), with an additional alanine inserted seven residues upstream of the BamHI restriction site to allow the T7 peptides to be inserted in frame. A QuikChange site-directed mutagenesis kit (QIAGEN) was used together with the pBINDaF vector sense (5′-ACTGTATCGCCGGAAATTCCCGGGGATCCG) and pBINDaR vector antisense (5′-CGGATCCCCGGGAATTTCCGGCGATACAGT) primers to introduce the amino acids. The pCMV6-XL5 vector and pCMV6-XL5-eukaryotic elongation factor 1A1 (EEF1A1), -structure-specific recognition protein 1 (SSRP1), and -repair cross-complementing protein 6 (XRCC6) were purchased from OriGene. Expression plasmids of MR (PRshMR), glucocorticoid receptor (PRshGR), PR (pSG5-hPR1), AR (pCMV-AR3.1), and ER (pCMV-ERα) and the reporter constructs for MR (MMTV-luc), PR (pA3-PRE2-Luc), AR (pGL4.14-PB3-Luc, also known as ARR3-tk-Luc), and ER (pERE-tk-Luc) were generously provided by Professors R. M. Evans (Salk Insitute for Biological Studies, La Jolla, CA), S. Nordeen (University of Colorado Health Sciences Center, Denver, CO), C. Clarke (Westmead Millenium Institute, Sydney, Australia), B. Katzenellenbogen (University of Illinois, Urbana, IL), and W. Tilley (University of Adelaide, Adelaide, Australia). Alternative MR-responsive promoter constructs (pGL4.23-CNKSR3-Luc and pGL4.23-GILZ-Luc) were kindly provided by Dr Tim Ziera (Bayer Schering Pharma AG, Berlin, Germany) (32).
Production and purification of recombinant full-length human MR and T7 Phage display
Full-length human MR was prepared as previously described (31). The phage display protocol has been described in detail previously (33), although some modifications were introduced for studying the MR. A premade T7Select phage library (heart derived) was purchased from Novagen (catalog number 70640–3), whereas the kidney-derived cDNA-expressing phage library was a kind gift from Professor Donald McDonnell's laboratory. In brief, 2 μg of purified baculovirus-expressed, biotinylated MR was added to each well of a 96-well plate in the presence of 100 mM NaHCO3 to a total volume of 100 μL. One micromole of aldosterone, cortisol, or deoxycorticosterone was added to the wells containing MR purified in the presence of each respective ligand to maintain a ligand-induced receptor conformation and enhance protein stability. The MR was immobilized on plates at 4°C overnight, blocked with 2% milk in PBS for 1 hour, and incubated with 108 T7 phage for 2 hours at room temperature. After three wash steps with PBS + 0.1% Tween 20 (PBST), the bound phage was eluted from the MR using 1% sodium dodecyl sulfate and amplified in Escherichia coli (strain BLT5616) for 5 hours in a shaking incubator at 37°C. Amplified phages were recovered from the bacterial supernatant and subjected to three additional rounds of panning. The enrichment of phage was monitored using an ELISA and the phage in the most enriched pool were plaque purified by dilution and plating on a lawn of E coli. Lytic colonies were extracted using a glass pipette tip and dispensed in phage extraction buffer for phage DNA retrieval. Two microliters of phage containing buffer, containing the DNA template (∼60 ng/μL) were collected for amplification by PCR.
Amplification and sequencing of phage isolated from phage display
PCR amplification of phage was performed using GoTaq Green master mix (Promega; M7122). Two microliters of each DNA template were amplified with 0.5 μL of T7 Select sense (5′-GCGGACCAGATTATCGCTAA) and antisense (5′-AACCCCTCAAGACCCGTTTA) primers at 10 μM and 12.5 μL of GoTaq Green master mix made up to a total volume of 25 μL with nuclease-free water. The PCR conditions were comprised of a 2-minute initial denaturation step at 94°C and then 35 cycles of denaturation (94°C for 50 sec), annealing (50°C for 60 sec), and extension (72°C for 60 sec), followed by a 6-minute extension step at 72°C, using a Veriti 96-well Thermal Cycler (Applied Biosystems).
PCR products were purified using ExoSap-IT according to the manufacturer's protocol (Affymetrix/USB; number 78200). Five microliters of the PCR product and 1 μL of ExoSap-IT was mixed and incubated at 37°C for 30 minutes. ExoSap-IT was then inactivated by heating to 80°C for 15 minutes.
PCR samples were then diluted 1:2 in water and 1 μL used for analysis by sequencing with the T7 Up primer (5′-GTGATGCTCGGGATCCGGA).
Analysis of phage DNA sequences and subcloning
The cardiac and renal phage cDNA libraries contained cDNA inserts between the EcoRI and HindIII restriction enzyme sites within the multiple cloning region of the phage DNA. The sequence at the start of each cDNA was therefore identical and translated into aspartic acid, proline, asparagine, and serine when in frame. All sequences obtained from amplified phage were translated using ExPASY (web.expasy.org/translate/) and the sequence aspartic acid, proline, asparagine, and serine was used to identify the start site of each cDNA insert. cDNAs that could be translated into peptides of greater than 20 amino acids were entered into the Basic Local Alignment Search Tool (National Center for Biotechnology Information) for the sequence alignment within the Homo sapiens protein database. cDNAs that could be translated into an established protein were digested using BamHI and NotI restriction enzymes together with pBINDa. The products of digestion were gel purified and subcloned into pBINDa.
Mammalian cell culture, transfection, and reporter assays
Human embryonic kidney 293 (HEK293) and H9c2 (rat cardiac myoblast) cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 1 mM nonessential amino acid, 1 mM L-glutamate, and 1 mM penicillin (10 U/L) in a humidified 37°C incubator with 5% CO2. HEK293 cells stably transfected with MR (MR+HEK293 cells) were maintained in DMEM supplemented with 0.7 mg/mL G418 (Geneticin selective antibiotic; Invitrogen) and 10% fetal bovine serum.
For transactivation assays, the cells were trypsinized and seeded at a density of 1 × 105 (HEK293) or 5 × 104 (H9c2) cells per well in 24-well plates 1 day before transfection. Transfections were performed following the manufacturer's protocol using Fugene 6 (Roche Molecular Biochemicals). Each well contained 200 ng of PRshMR, 500 ng of mouse mammary tumor virus (MMTV)-luciferase, and 50 ng or 200 ng of empty vector, pBINDa-T7 peptide, or pCMV6-XL5-EEF1A1, -SSRP1, or -XRCC6, with pBluescriptSK as required to normalize the total DNA amount to 900 ng per well. Media were replaced with DMEM with 5% charcoal-stripped fetal bovine serum immediately after transfection, and then 24 hours later vehicle, 10−8 M aldosterone, or 10−8 M cortisol was added to the appropriate wells. Luciferase assays were performed 24 hours after treatment.
For gene expression assays, cells were seeded at a density of 5 × 105 (MR+HEK293) or 1 × 105 (H9c2) cells per well in six-well plates. Transfections were performed using Fugene 6 and each well contained 1000 ng of pCMV6-XL5 vector or pCMV-XL5-EEF1A1, -SSRP1, or -XRCC6. Media were changed to DMEM with 5% charcoal-stripped fetal bovine serum and the appropriate hormones were added 24 hours later. Cells were harvested 3 hours after hormone treatment for RNA extraction and RT-PCR.
Quantitative real-time PCR
Total RNA was extracted from MR+HEK293 and H9c2 cells using an RNeasy minikit (QIAGEN) and subjected to reverse transcription using SuperScript III reverse transcriptase enzyme (Invitrogen). Quantitative real-time RT-PCR analysis was conducted using a 7900HT Fast real-time PCR system (Applied Biosystems). Each 10-μL reaction in a 384-well plate format included 5 μL SYBR Green master mix, 0.25 μL of the forward primer (10 μM), 0.25 μL of the reverse primer (10 μM), 0.5 μL of water, and 4 μL of the diluted cDNA (diluted 1:4). glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as the internal standard to control for reverse transcription efficiency and loading accuracy for HEK293 cells, whereas 18S was the internal control for the H9c2 cells. Two different internal controls were used due to the availability of suitable primers. They did not affect the experimental results when trial runs were performed using both GAPDH and 18S as internal standards. PCR setup in 384-well plates was performed using a robotic QIAGEN CAS1200 liquid handling instrument. Cycling conditions comprised of a 10-minute initial denaturation step at 95°C, 40 cycles of denaturation at 95°C for 15 seconds, followed by annealing at 60°C for 1 minute. Melting curve analysis was performed to assess the homogeneity of PCR products. The resultant mRNA levels were analyzed using SDS Automation Controller software (version 2.3; Applied Biosystems) and normalized to GAPDH or 18S mRNA levels. The data were calculated from the results of three independent experiments, with all reactions performed in triplicate.
Gene-specific primer pairs used for RT-PCR are listed in Table 1.
Table 1.
Gene-Specific Primer Pairs Used for RT-PCR
| Primer Sequence (Sense) (5′) | Primer Sequence (Antisense) (5′) | |
|---|---|---|
| Human | ||
| GAPDH | CCCATCACCATCTTCCAGGAG | GTTGTCATCGATGACCTTGGC |
| 18S | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCCGCT |
| GILZ | TCTGCTTGGAGGGGATGTGG | ACTTGTGGGGATTCGGGAGC |
| SGK | CCCCCTTTTAACCCAAATGT | TCAGAGGAAAGAGTCCGTGG |
| CNKSR | TGGACAGCCTCTTCTCGTTT | TAGCGCGTGTCTTCACATTC |
| MR | GAGAAAAGCCCGTCTGTTTG | AGAGGAGTTCCCTGGGTGAT |
| Rat | ||
| 18S | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCCGCT |
| GILZ | AGTTCCAGCTGGCCAAGGAG | TCCCATCTCTGTCCCGTCT |
| SGK | CCCCCATTTAACCCAAATGT | GAACCATCCTGGGAGCTTTC |
| CNKSR | TCCTTCTTCCTCAGTGGACA | TAGCGCGTGTCTTCACATTC |
| MR | GCAGCGAAACAGATGATCCAG | TCTCCAACTCAAAGCGAACGA |
Fluorescence microscopy
MR+HEK293 cells were cultured on glass coverslips coated with poly-L-lysine. The cells were incubated in DMEM with 5% charcoal-stripped medium for 24 hours and treated with 10−8 M aldosterone for 30 minutes. Samples were rinsed with PBS, fixed at −20°C with ice-cold methanol for 15 minutes and blocked with Image-iT FX Signal Enhancer (I36933; Invitrogen) for 1 hour at room temperature before incubation with primary antibodies at 4°C overnight. Primary antibodies were purchased from Santa Cruz Biotechnologies and include the following: rabbit polyclonal IgG to EF1α1 (H-300, sc-9033), SSRP1 (H-300, sc-25382), and Ku70 (H-308, sc-9033) (34–38). The mouse monoclonal antibody for MR, MR1–18 (1D5), was a gift from Professor C. Gomez-Sanchez (University of Mississippi, Oxford, Mississippi). The cells were washed with PBST three times and incubated with fluorophore-conjugated secondary antibodies for 1 hour at room temperature. Secondary antibodies were purchased from Invitrogen and include AlexaFluor 488 goat antimouse IgG (A11029) and AlexaFluor 546 goat antirabbit IgG (A11035). Cells were washed three times before staining with 4′,6′-diamino-2-phenylindole for 1 minute and washing three times in PBST. Coverslips were mounted using FluroSave reagent (Calbiochem; 345789), and images were acquired at 1024 × 1024 pixels (12 bit) using a Nikon C1 confocal laser-scanning microscope on a Ti-E base. A ×40 0.75NA objective was used for detection with 4′,6′-diamino-2-phenylindole (emission filter 450/35 nm), Alexa488 (emission filter 515/30), and Alexa546 (emission filter 605/75) excited with 405-nm, 488-nm, and 561-nm lasers, respectively.
Statistical analysis
Statistical analysis of each data set was performed using the GraphPad Prism5 software package (GraphPad Software Inc). For transactivation assays, luciferase values obtained with empty vector treated with hormone were normalized to 1, and all other luciferase values were expressed as fold change relative to empty vector plus ligand. Means were compared using a one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. For gene expression assays, relative mRNA expression was calculated using the 2 [-δδ cycle threshold (Ct)] method whereby δ δCt represented the difference between the Ct values of treated compared with untreated samples for each protein investigated. The relative mRNA expression in samples transfected with each protein was then compared with those transfected with empty vector using an unpaired t test. A value of P < .05 was considered statistically significant. The data are presented as mean ± SEM. P values are annotated as follows: *, P < .05; **, P < .01; and ***, P < .001.
Results
Identification of novel MR-interacting peptides using T7 phage display
To identify proteins that interact with ligand-bound MR, we screened T7 phage cDNA libraries derived from either human heart or kidney RNA using full-length human MR as bait and in the presence of aldosterone, deoxycorticosterone, or cortisol. Four rounds of panning were performed to enrich for bound phage, and the degree of enrichment was confirmed by phage ELISA after each round. The cDNA inserts of 918 purified phage particles, including 570 from the heart cDNA library and 348 from the kidney cDNA library, were sequenced and amino acid sequences generated and entered into the protein database of the Basic Local Alignment Search Tool (National Center for Biotechnology Information) for alignment with existing proteins. Any peptide that possessed greater than 90% sequence homology with a protein from the H sapiens protein database was considered a positive hit. Overall, we identified 23 nonredundant peptides from the heart library and seven from the kidney library (Table 2).
Table 2.
MR-Interacting Peptides, Derived From Heart- and Kidney-Specific cDNA Libraries, Identified Using T7 Phage Display
| Clone | Symbol | Gene Name | Fragment | Activity on MR |
||||
|---|---|---|---|---|---|---|---|---|
| HEK293 |
H9c2 |
|||||||
| A | F | A | F | |||||
| Heart | A10 | SFRS18 | Splicing factor, arginine/serine-rich 18 | 474–696 | – | – | – | – |
| A1b | NUCKS1 | Nuclear casein kinase and cyclin-dependent kinase substrate 1 | 53–243 | – | – | – | – | |
| A20 | NOLC1 | Nucleolar and coiled-body phosphoprotein 1 | 105–324 | – | – | – | – | |
| A38 | RBBP6 | Retinoblastoma binding protein 6 | 872–1056 | – | – | – | – | |
| A44 | EIF5B | Eukaryotic translation initiation factor 5B | 160–294 | – | – | ↑ | ↑ | |
| A44b | SSRP1 | Structure-specific recognition protein 1 | 526–709 | ↓ | ↓ | – | – | |
| A53 | NPIPL3 | Same as D15 but longer fragment | 665–854 | n.d. | n.d. | n.d. | n.d. | |
| A78 | ATRX | α-Thalassemia/mental retardation syndrome X linked | 193–327 | ↓ | ↓ | – | – | |
| A9 | SAFB | Scaffold attachment factor B | 4–127 | ↓ | ↓ | – | – | |
| A91 | AHNAK | AHNAK nucleoprotein | 899–1114 | ↓ | ↓ | ↓ | ↓ | |
| D1 | BUB3 | Budding uninhibited by benzimidazoles 3 homolog | 220–328 | ↓ | ↓ | ↓ | ↓ | |
| D15 | NPIPL3 | Nuclear pore complex interacting protein-like 3 | 748–854 | ↓ | ↓ | – | – | |
| D17b | CCDC55 | Coiled-coil domain containing 55 | 296–451 | ↓ | ↓ | ↓ | ↓ | |
| D30 | Hypothetical | Similar to AGAP002076-PA | 299–331 | ↑ | ↑ | – | – | |
| D32b | ATP5I | ATP synthase, H + transporting | 14–66 | – | – | – | – | |
| F18b | RPL4 | Ribosomal protein L4 | 402–462 | ↓ | ↓ | ↓ | ↓ | |
| F19b | MYL2 | Myosin, light chain 2, regulatory, cardiac, slow | 96–153 | ↑ | ↓ | – | – | |
| F20b | XRCC6 | X-ray repair complementing defective repair in CHO cells 6 | 480–609 | ↓ | ↓ | – | – | |
| F45b | RRBP1 | Ribosome binding protein 1 homolog 180 kDa | 79–246 | ↓ | ↓ | ↓ | ↓ | |
| F46b | RPL23A | Ribosomal protein L23a | 42–71 | ↓ | ↓ | ↓ | ↓ | |
| F5b | SERF2 | Small EDRK-rich factor 2 | 02–059 | ↓ | ↓ | – | – | |
| F6 | EEF1A1 | Eukaryotic translation elongation factor 1α1 | 396–462 | – | – | ↓ | ↓ | |
| F81 | ENSA | Endosulfine-α | 17–121 | ↓ | ↓ | ↓ | ↓ | |
| Kidney | KA14a | MUC1 | Mucin 1, cell surface associated | 38–115 | ↓ | ↓ | ↓ | ↓ |
| KA3b | FRMD4B | FERM domain containing 4B | 528–643 | ↑ | – | ↓ | ↓ | |
| KA44b | Chloride intracellular channel 1 variant | 90–133 | ↓ | ↓ | ↓ | ↓ | ||
| KF 22b | GPX3 | Glutathione peroxidase 3 | 62–111 | – | – | ↓ | ↓ | |
| KF18a | RRBP1 | Ribosome binding protein 1 homolog 180 kDa | 102–160 | – | – | ↓ | ↓ | |
| KF24b | PCBP2 | Poly(rC) binding protein 2 | 198–285 | – | – | ↓ | ↓ | |
| KF67b | EEF1A1 | Eukaryotic translation elongation factor 1α1 | 59–92 | n.d. | n.d. | n.d. | n.d. | |
Abbreviations: n.d., not determined; –, no effect; ↑, enhance MR transactivation; ↓, reduce MR transactivation.
Each of the 30 novel peptides was assessed for their ability to modulate MR-mediated transactivation. Peptides or the empty vector (as a control) were transfected together with full-length MR and MMTV-luciferase reporter into two cell lines, the HEK293 cells and H9c2 (rat cardiac myoblast) cells, in the presence of either aldosterone or cortisol. The MMTV-luciferase reporter gene construct contains steroid response elements, which are MR inducible (39, 40), and thus allows for the direct assessment of the effect of these novel peptides on MR-mediated transactivation. The peptides exerted a range of effects, from no effect (<10%) to those with equivalent responses with both ligands in both cell lines to those with differential, ligand-selective effects in the two cells lines (Table 2).
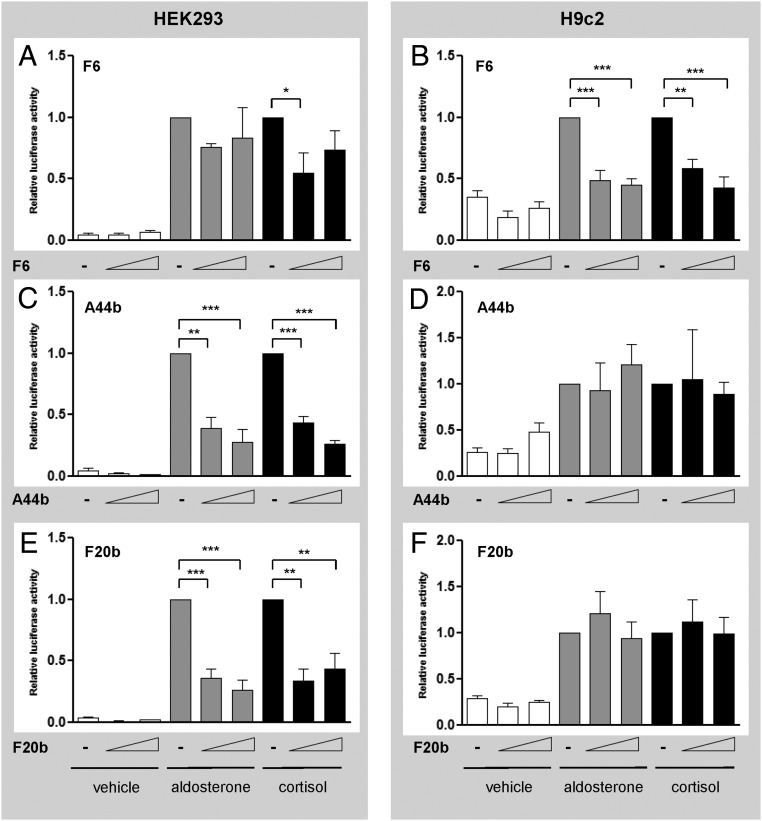
Three peptides (F6, A44b, and F20b, representing fragments of EEFA1, SSRP1, and XRCC6, respectively) were selected for further characterization based on their cell-specific effects on MR activity, known nuclear localization, interaction with other MR-interacting proteins, and/or established status as NR coregulators. Peptide F6 inhibited both aldosterone- and cortisol-induced MR activity in H9c2 cells (Figure 1B) but had little or no effect in HEK293 cells (Figure 1A). Conversely, peptides A44b and F20b potently inhibited MR activity induced by either ligand in HEK293 cells (Figure 1, C and E) but had no effect in H9c2 cells (Figure 1, D and F).
Figure 1.
Peptides F6, A44b, and F20b differentially modulated MR-mediated transactivation of the MMTV-luciferase in HEK293 and H9c2 cells. HEK293 and H9c2 cells transfected with pRshMR, MMTV-luc, and empty vector, F6 (panels A and B), A44b (panels C and D), or F20b (panels E and F) (50 or 200 ng) were treated with vehicle (white), aldosterone 10−8 M (gray) or cortisol 10−8 M (black) for 24 hours. Assays were performed in triplicate in two (for A44b) or three (for F6 and F20b) separate experiments. Luciferase activity is expressed as relative to the hormone treated empty vector. Data are presented as mean ± SEM. Means were compared by ANOVA: *, P < .05; **, P < .01; ***, P < .001.
Ligand- and cell-specific regulation of MR-mediated transactivation by EEF1A1, SSRP1, and XRCC6
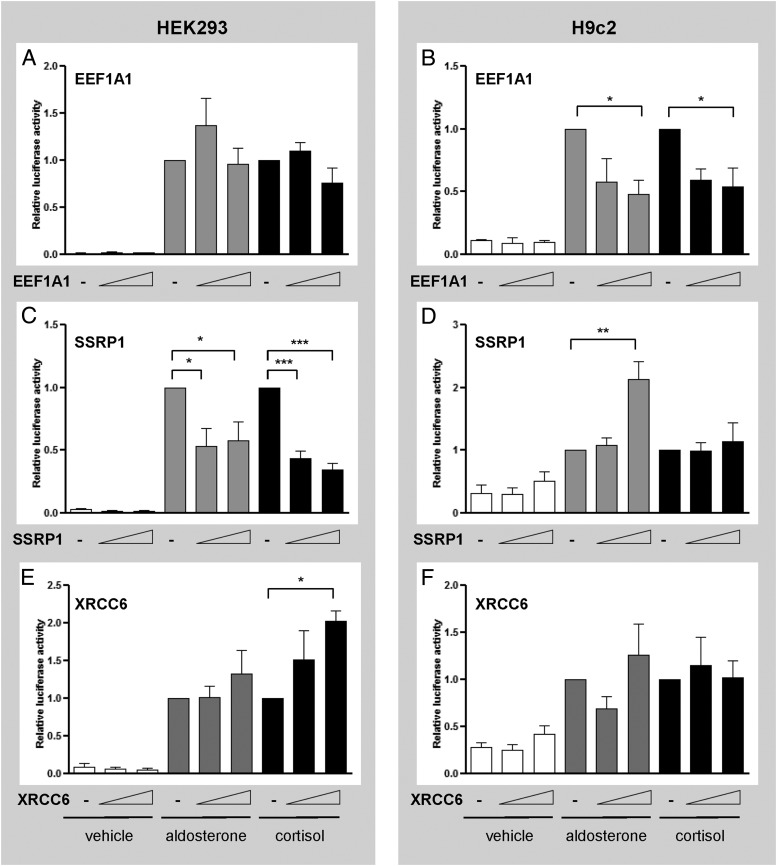
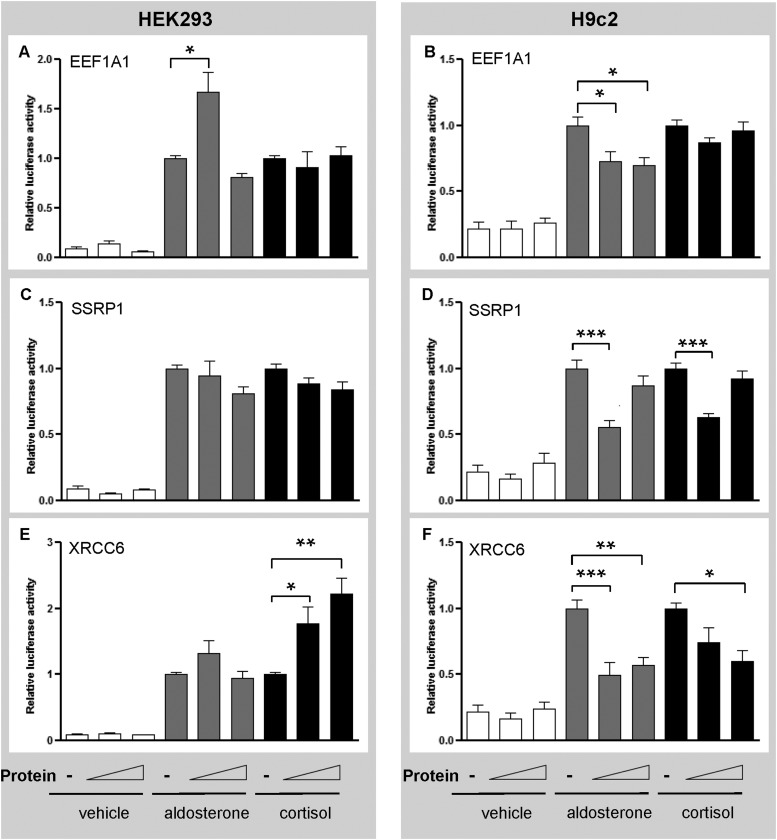
Peptides F6, A44b and F20b could inhibit MR activity either through intrinsic transcription inhibitory effects or alternatively by acting as dominant-negative inhibitors of the corresponding full-length endogenous proteins. We therefore determined the effects of full-length EEF1A1, SSRP1, and XRCC6 on MR-mediated transactivation at the MMTV promoter. Like its fragment F6, EEF1A1 repressed ligand-induced MR transactivation in H9c2 cells (Figure 2B) without affecting HEK293 cells (Figure 2A). Similarly, SSRP1 repressed MR transactivation in HEK293 cells (Figure 2C) in the same manner as its peptide A44b but it augmented aldosterone-induced MR activity in H9c2 cells (Figure 2D). In contrast to fragment F20b, which inhibited MR transactivation, XRCC6 selectively enhanced cortisol-induced MR transactivation in HEK293 cells (Figure 2E). This suggests that F20b acts as a dominant-negative inhibitor of endogenous XRCC6. These results demonstrate that these three proteins can modulate MR-mediated transactivation in a ligand- and cellular context-dependent manner.
Figure 2.
EEF1A1, SSRP1, and XRCC6 modulate aldosterone- or cortisol-induced MR transactivation of the MMTV-luciferase in a ligand- and/or cell-specific manner. HEK293 and H9c2 cells transfected with pRshMR, MMTV-luc, and either empty vector, EEF1A1 (panels A and B), SSRP1 (panels C and D), or XRCC6 (panels E and F) (50 or 200 ng) were treated with vehicle (white), aldosterone 10−8 M (gray), or cortisol 10−8 M (black) for 24 hours. Assays were performed in triplicate in two (for EEF1A1) or three (for SSRP1 and XRCC6) separate experiments. Luciferase activity is expressed as relative to the hormone-treated empty vector. Data are presented as mean ± SEM. Means were compared by ANOVA: *, P < .05; **, P < .01; ***, P < .001.
Cell- and gene-specific regulation of MR-mediated target gene transcription
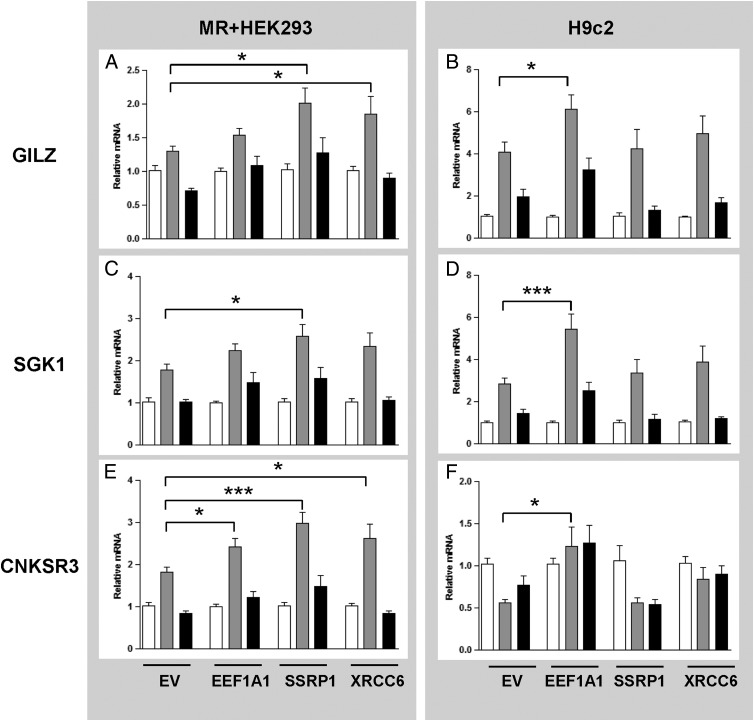
To address the potential effects of EEF1A1, SSRP1, and XRCC6 on endogenous MR target gene expression, MR-expressing HEK293 cells in which the hMR is stably overexpressed (MR+HEK293) (41) were transfected with expression plasmids for full-length EEF1A1, SSRP1, or XRCC6. Expression of three MR target genes, glucocorticoid-induced leucine-zipper protein (GILZ), serum- and glucocorticoid-regulated kinase 1 (SGK1), and connector enhancer of kinase suppressor of Ras 3 (CNKSR3), was evaluated at 3 hours after aldosterone treatment (32, 42, 43).
In the absence of an exogenous coregulator, aldosterone treatment of MR+HEK293 cells (Figure 3, A, C, and E) induced a robust 2-fold increase in endogenous SGK1 and CNKSR3 mRNA (Figure 3, C and E). Transfected EEF1A1 significantly enhanced endogenous CNKSR3 expression (Figure 3E), whereas SSRP1 significantly enhanced the expression of all three MR target genes (Figure 3, A, C, and E), and XRCC6 increased the aldosterone-induced expression of GILZ and CNKSR3 (Figure 3, A and E). In all cases, spironolactone treatment returned gene expression to control levels (Figure 3, black bars). MR mRNA levels did not change after the transfection of any of the plasmids (data not shown).
Figure 3.
EEF1A1, SSRP1, and XRCC6 have varying effects on the transcription of MR target genes in MR+HEK293 and H9c2 cells. MR+HEK293 and H9c2 cells transfected with either empty vector (EV) or each of the three proteins were treated with vehicle (white), aldosterone 10−8 M (gray), or spironolactone 10−6 M and aldosterone 10−8 M (black) for 3 hours. Expression of GILZ (panels A and B), SGK1and (panels C and D) CNKSR3 (panels E and F) were assayed by quantitative real-time PCR. Assays were performed three times in triplicate. mRNA expression was normalized to GAPDH (in MR+HEK293 cells) or 18S (in H9c2 cells). Data are presented as fold change over vehicle treatment and means were compared by ANOVA: *, P < .05; ***, P < .001.
To further characterize the cell-specific differences observed in the transactivation studies, gene expression studies were repeated in H9c2 cells, which contain endogenous MR. In H9c2 cells transfected with an empty vector alone, a strong induction of GILZ and SGK1 expression was observed upon aldosterone treatment, which was blocked by spironolactone (Figure 3, B and D). EEF1A1 was the only protein that significantly enhanced the expression of GILZ and SGK1 in these cells (Figure 3, B and D). In contrast, CNKSR3 expression was repressed in H9c2 cells by aldosterone, a response that was reversed by EEF1A1 (Figure 3F). These results are consistent with earlier transactivation assays that showed EEF1A1-dependent regulation of the MMTV reporter predominantly in H9c2 cells (Figure 2B). SSRP1 and XRCC6 did not have a significant impact on MR endogenous gene expression in H9c2 cells.
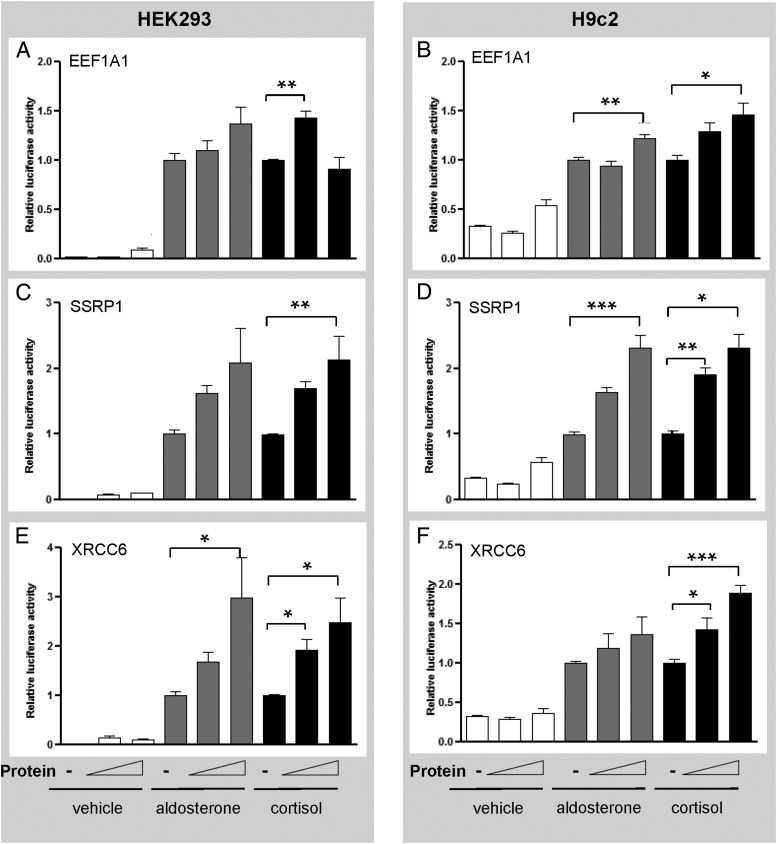
Promoter-specific modulation of MR-mediated transactivation
To further characterize the basis of the gene-specific effects for each protein, MR transactivation assays were performed with CNKSR3 and GILZ promoter constructs in HEK293 and H9c2 cells. All three proteins enhanced MR-mediated transactivation of the CNKSR3 promoter in HEK293 cells (Figure 4, A, C, and E). This is in keeping with their ability to increase endogenous CNKSR3 gene expression in MR+HEK293 cells (Figure 3E). However, the three proteins also increased CNKSR-luciferase activity in the H9c2 cells (Figure 3, B, D, and F), even though only EEF1A1 increased endogenous CNKSR3 expression in H9c2 cells (Figure 3F).
Figure 4.
EEF1A1, SSRP1, and XRCC6 augment MR transactivation at the CNKSR promoter. HEK293 and H9c2 cells transfected with CNKSR-Luc and empty vector or EEF1A1 (panels A and B), SSRP1 (panels C and D), or XRCC6 (panels E and F) (50 or 200 ng) were treated with vehicle (white), aldosterone 10−8 M (gray), or cortisol 10−8 M (black) for 24 hours. Assays were performed twice in triplicate. Luciferase activity is expressed as relative to the hormone treated empty vector. Means were compared by ANOVA: *, P < .05; **, P < .01.
At the GILZ promoter, SSRP1 and XRCC6 did not increase aldosterone-induced MR-mediated transactivation in HEK293 cells (Figure 5, C and E). This is discordant with the endogenous gene expression data, which demonstrated an increase in endogenous GILZ expression in the presence of SSRP1 or XRCC6 (Figure 3A). Similarly, in the H9c2 cells, the MR-mediated transactivation of the GILZ promoter was largely repressed by each protein (Figure 5, B, D, and F), even though a modest enhancement in endogenous GILZ expression was observed with the overexpression of EEF1A1 (Figure 3B). Interestingly, XRCC6 produced a cortisol-selective enhancement of both CNKSR and GILZ promoter activity in HEK293 cells (Figures 4E and 5E), in keeping with its effect at the MMTV promoter (Figure 2E).
Figure 5.
EEF1A1, SSRP1, and XRCC6 exert ligand- and cell-specific effects on MR transactivation at the GILZ promoter. HEK293 and H9c2 cells transfected with GILZ-Luc and empty vector or EEF1A1 (panels A and B), SSRP1 (panels C and D), or XRCC6 (panels E and F) (50 or 200 ng) were treated with vehicle (white), aldosterone 10−8 M (gray) or cortisol 10−8 M (black) for 24 hours. Assays were performed twice in triplicate. Luciferase activity is expressed as relative to the hormone treated empty vector. Means were compared by ANOVA: *, P < .05; **, P < .01; ***, P < .001.
EEF1A1, SSRP1, and XRCC6 colocalize with the MR in MR+HEK293 cells
To determine whether EEF1A1, SSRP1, or XRCC6 colocalizes with the MR at a subcellular level, these endogenous proteins were examined by double-label immunofluorescence microscopy in MR+HEK293 cells. In the absence of aldosterone, MR was primarily localized in the cytoplasm (Supplemental Figure 1, A, C, and E). Treatment with aldosterone resulted in the nuclear localization of MR (Supplemental Figure 1, B, D, and F) as previously described. The subcellular localization of EEF1A1 was similar to that of the MR, as represented by yellow fluorescence in the merged images of MR and EEF1A1 (Supplemental Figure 1, A and B). Both proteins were predominantly cytoplasmic in the absence of aldosterone and nuclear in the presence of aldosterone. There was minimal colocalization of SSRP1 with cytoplasmic MR in the absence of hormone treatment, but colocalization of SSRP1 and MR occurred in the nucleus with the addition of aldosterone (Supplemental Figure 1D). In contrast, XRCC6 is exclusively a nuclear protein in MR+HEK293 cells (Supplemental Figure 1E), and thus, colocalization with the MR occurred only after the aldosterone treatment (Supplemental Figure 1F).
Discussion
In the current study, we identified multiple novel MR-interacting peptides using T7 phage display, of which the majority was able to modulate MR transactivation in HEK293 and/or H9c2 cells in a cell- and/or ligand-specific manner. Three selected peptides and their full-length proteins, EEF1A1, SSRP1, and XRCC6, were further characterized. Their specific regulatory effects on MR-mediated transactivation, evaluated using transiently transfected promoter constructs or endogenous MR target gene expression, were dependent on the target gene promoter, ligand, and cellular context. The predominantly nuclear colocalization of each protein with the MR further supported their potential role as MR coregulators.
Clinical trials have highlighted the potential benefits of MR blockade in heart failure; however, inhibition of renal MR produces hyperkalemia, thus limiting the therapeutic utility of MR antagonists. An ideal selective MR modulator would inhibit MR in the cardiovascular system while sparing its renal effects. Such tissue specificity has been achieved for other steroid hormone receptors, for example, raloxifene that inhibits ERα in breast but acts as an agonist in bone. This specificity arises, at least in part, through the tissue-selective expression and recruitment of coregulators. By analogy, it should therefore be possible to develop tissue-selective MR modulators. However, to date, this goal has not been realized due, in part, to the limited number of coregulators that have been identified for the MR compared with other NRs. A second challenge is that the MR is unique among the steroid hormone receptors in that it responds to at least two classes of steroids: mineralocorticoid and glucocorticoid. Although prereceptor mechanisms confer mineralocorticoid specificity to the MR in epithelial tissues such as the kidney, unprotected MR in nonepithelial tissues likely responds to both classes of hormones. There is increasing evidence that the pathophysiological actions of the MR in the cardiovascular system are partly mediated by glucocorticoids, whereas the beneficial effects on fluid and electrolyte balance are mediated by mineralocorticoids. This presents a clear opportunity for tissue selective drug development, a prerequisite for which is a better understanding of the tissue- and ligand-selective recruitment of coregulators to the MR.
Here we used T7 phage display as a tool for MR coregulator discovery. The phage display approach has a number of features that make it highly suitable for this purpose. First, we used full-length human MR as the bait, thereby exposing the entire protein surface for protein binding. Second, the high throughput nature of the method enabled us to screen cDNA libraries derived from multiple tissues, as well as multiple MR ligands, simultaneously. Finally, the affinity-based nature of the selection process enables low copy number library clones to be readily detected, which is important because coregulators are often expressed at very low level in cells. However, this also means that high-affinity interactions are selected in favor of low affinity binders, a limitation that should be considered. In addition, library expression and selection within a bacterial host means that target proteins may be susceptible to proteolysis or misfolding, and will not undergo posttranslational modifications. Another limitation is the cell-free nature of the phage display system whereby the MR is immobilized on plastic plates rather than DNA response elements, which may not reflect physiological MR protein structure. However, it is a screening process and the isolated peptides are subsequently evaluated in cell-based assays. Of the 30 clones that we obtained in this screening experiment (Table 2), many have known functions in transcription regulation, several have previously been shown to interact with other members of the NR family, and greater than 70% showed significant effects on MR transcriptional activity. This suggests that the phage display process has selected biologically relevant proteins.
Our data show that EEF1A1, SSRP1, and XRCC6 all regulate MR-mediated transcriptional activity in a gene-specific manner, consistent with the literature on gene-specific requirements for coregulators for other NRs (44–46). Interaction of NRs with distinct DNA response elements can induce allosteric changes in receptor conformation and therefore altered binding affinity for different coactivators; similar mechanisms may account for differential MR activity at the four different response elements tested in the present study (47, 48). Indeed, large-scale gene expression profiling and coactivator recruitment assays for novel PR modulators have found that regulation is highly gene specific despite a common coactivator recruitment profile for certain compounds (49).
It is important to note that the effects of the three proteins on MR-mediated transactivation at the CNKSR or GILZ promoters were not always concordant with their effect on endogenous gene expression. For example, EEF1A1 repressed MR-mediated GILZ promoter activity in the H9c2 cells (Figure 5B) but enhanced endogenous gene expression in the same cell line (Figure 3B). This discordance is most probably due to differences between artificial promoter constructs and endogenous promoters, which are additionally regulated by chromatin structure, distant regulatory elements, and other transcription factors (50, 51).
EEF1A1, SSRP1, and XRCC6 also exerted cell-specific effects on MR-mediated transactivation. Differential expression of coregulators between cell types can contribute to cell-specific modulation of NR activity and may account for cell-specific differences in our transactivation assays (52). The gene-specific recruitment of coregulators to the MR together with cell-specific ratios of coregulator expression may ultimately determine the tissue-specific gene transcriptional responses to MR ligands.
The three putative coregulatory molecules chosen for further characterization from the 30 identified in the T7 screen both confirm the validity of the screen and represent proof of principle that the phage system is pulling out meaningful interactions. Although their physiology has not been the focus of this study, each is indeed potentially of physiological relevance to MR signaling. Of the three proteins, XRCC6 is the best characterized in the Nuclear Receptor Signaling Atlas coregulator database.
X-ray repair cross-complementing protein 6
XRCC6, also known as Ku70, is an ATP-dependent helicase (53, 54) that has previously been shown to modulate the transcriptional activity of the AR (55), farnesoid X receptor (56), and thyroid hormone receptor (57). In our study, XRCC6 behaved as a cell-specific coactivator of the MR and showed cortisol- and gene-selective responses for MR transactivation in both cell lines. The cortisol selectivity of XRCC6 in transactivation assays is consistent with the fact that it was isolated from phage that interacted with cortisol-bound MR. It would have been ideal to interrogate the effect of XRCC6 on cortisol-induced MR target gene expression. However, although cortisol was able to enhance MR-mediated transactivation of transfected promoter activity, it did not significantly induce endogenous target gene expression in HEK293 cells despite optimization with increasing doses of cortisol or the addition of an 11β-hydroxysteroid dehydrogenase-2 inhibitor (carbenoxolone) to prevent cortisol inactivation (data not shown). A review of the literature did not reveal examples of cortisol-induced MR target gene expression in HEK293 cells or other cell lines. In one particular study of aldosterone-regulated vascular genes using mouse aortic tissue, it was noted that cortisol did not enhance the expression of several MR-regulated genes including SGK1 (58). The dichotomy between the effect of cortisol on MR-mediated transactivation using promoter constructs and the lack of effect on endogenous MR target genes may be due to differences in the promoter environment and long-range chromatin interactions.
The site of interaction of XRCC6 with the MR has not yet been determined, although XRCC6 contains one LxxLL and one LLxxL motif (Supplemental Figure 2C). Interaction may also occur at the C terminus of XRCC6, which contains the scaffold attachment factor, acinus and protein inhibitor of activated signal transducer and activator of transcription (SAP) domain (residues 559–609; (59, 60), a DNA binding domain found in a range of nuclear proteins including other corepressors of the MR such as protein inhibitor of activated STAT-1 (signal transducer and activator of transcription-1).
Eukaryotic elongation factor 1A1
EEF1A exists as two separately encoded proteins, EEF1A1 and EEF1A2, which share 92% identity (61, 62). EEF1A1 is almost ubiquitously expressed, whereas EEF1A2 has a restricted pattern of expression. We identified EEF1A1 as an MR-interacting protein in both the heart and kidney cDNA libraries. In our study, EEF1A1 behaved as a coactivator of the MR in enhancing endogenous GILZ, SGK1, and CNKSR3 expression, primarily in H9c2 cells. Of potential relevance to the role of MR in cardiovascular pathology is the observation that EEF1A1 is selectively down-regulated after myocardial infarction (63). Furthermore, EEF1A1 has been implicated in mediating the TNFα-induced decrease in endothelial nitric oxide synthase mRNA stability (64). MR activation has similarly been demonstrated to adversely affect endothelial nitric oxide synthase function. Although it is premature to link EEF1A1 and MR in the pathophysiology of cardiac disease at this point in time, the ability of EEF1A1 to enhance MR-mediated target gene expression in a heart-derived cell line warrants further exploration.
Structure-specific recognition protein 1
SSRP1 is a nuclear protein that is essential for transcriptional elongation (38, 65, 66). Our data demonstrate that SSRP1 can increase endogenous MR target gene expression in MR+HEK293 cells but not in H9c2 cells. Colocalization of SSRP1 with the MR, primarily in the nucleus upon aldosterone treatment, is supportive of a functional interaction between these two proteins. SSRP1 contains a C-terminal HMG box (Supplemental Figure 2B) which is required to bind nucleosomal DNA and help reorganize chromatin (65). The high mobility group (HMG) domain is a common motif within the HMG-1 proteins, which are already characterized as NR coactivators for other members of the steroid hormone receptor family but not the MR (67, 68). The HMG box is also found within peptide A44b, the partial cDNA of SSRP1, which was isolated from the T7 phage display.
Conclusions
Taken together, data derived from T7 phage display and presented in the current study identified EEF1A1, SSRP1, and XRCC6 as novel interacting partners of the MR. They demonstrated ligand-, promoter-, and cell-specific modulation of MR-mediated transactivation. Further studies, especially in vivo studies, are required to validate these proteins as physiologically important MR coregulators. Their unique sites of interaction with the MR may represent suitable targets for the development of selective MR modulators and allow them to serve as valuable screening tools for novel MR antagonists.
Acknowledgments
We thank members of the Steroid Receptor Biology Laboratory (Prince Henry's Institute, Clayton, Australia) for their advice on experimental procedures, in particular, Ms Yi Zhou Yao, Ms Maria Alexiadis, Ms Francine Brennan, and Dr Simon Chu; members of the McDonnell laboratory (Duke University Medical Center, Durham, North Carolina), in particular Ms Ching-yi Chang, for her advice on phage display; Ms Sue Panckridge for assistance with figure preparation; and finally, Dr Camden Lo and Monash Micro Imaging for his assistance with confocal microscopy.
This work was supported by Grants 494835 and 1010034 from the National Health and Medical Research Council (NHMRC) of Australia. J.Y. is supported by an NHMRC postgraduate scholarship, and P.J.F. is supported by an NHMRC Principle Senior Research Fellowship (Grant 1002559). Prince Henry's Institute is supported by the Operational Infrastructure Program of the Victorian Government.
Disclosure Summary: J.Y., J.M., H.S., and D.P.M. have nothing to declare. P.J.F., C.D.C., and M.J.Y. have received a grant from the National Health and Medical Research Council of Australia to perform this work.
Funding Statement
This work was supported by Grants 494835 and 1010034 from the National Health and Medical Research Council (NHMRC) of Australia. J.Y. is supported by an NHMRC postgraduate scholarship, and P.J.F. is supported by an NHMRC Principle Senior Research Fellowship (Grant 1002559). Prince Henry's Institute is supported by the Operational Infrastructure Program of the Victorian Government.
Footnotes
- AR
- androgen receptor
- CNKSR3
- connector enhancer of kinase suppressor of Ras 3
- Ct
- cycle threshold
- EEF1A1
- eukaryotic elongation factor 1A1
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GILZ
- glucocorticoid-induced leucine-zipper protein
- HEK293
- human embryonic kidney 293
- HMG
- high mobility group
- MMTV
- mouse mammary tumor virus
- MR
- mineralocorticoid receptor
- NR
- nuclear receptor
- PBST
- PBS + 0.1% Tween 20
- PR
- progesterone receptor
- SAP
- signal transducer and activator of transcription
- SGK1
- serum- and glucocorticoid-regulated kinase 1
- SSRP1
- structure-specific recognition protein 1
- XRCC6
- repair cross-complementing protein 6.
References
- 1. Griekspoor A, Zwart W, Neefjes J, Michalides R. Visualizing the action of steroid hormone receptors in living cells. Nucl Receptor Signaling [Electronic Resource]. 2007;5:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu NZ, Wardell SE, Burnstein KL, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. [DOI] [PubMed] [Google Scholar]
- 3. Sutanto W, de Kloet ER. Mineralocorticoid receptor ligands: biochemical, pharmacological, and clinical aspects. Med Res Rev. 1991;11:617–639. [DOI] [PubMed] [Google Scholar]
- 4. Pearce D, Bhargava A, Cole TJ. Aldosterone: its receptor, target genes, and actions. Vitamins Horm. 2003;66:29–76. [DOI] [PubMed] [Google Scholar]
- 5. Young M, Funder JW. Eplerenone, but not steroid withdrawal, reverses cardiac fibrosis in deoxycorticosterone/ salt-treated rats. Endocrinology. 2004;145:3153–3157. [DOI] [PubMed] [Google Scholar]
- 6. Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 7. Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;109:136242. [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Zannad F, Remme WJ, et al. The Randomized Aldactone Evaluation Study, I. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2010;364:11–21. [DOI] [PubMed] [Google Scholar]
- 11. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 12. McKenna NJ, O'Malley BW. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann NY Acad Sci. 2001;949:3–5. [DOI] [PubMed] [Google Scholar]
- 13. McKenna NJ, O'Malley BW. From ligand to response: generating diversity in nuclear receptor coregulator function. J Steroid Biochem Mol Biol. 2000;74:351–356. [DOI] [PubMed] [Google Scholar]
- 14. Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. [DOI] [PubMed] [Google Scholar]
- 15. Darimont BD, Wagner RL, Apriletti JW, et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12:3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warnmark A, Treuter E, Wright APH, Gustafsson J-A. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol. 2003;17:1901–1909. [DOI] [PubMed] [Google Scholar]
- 17. Goodson M, Jonas BA, Privalsky MA. Corepressors: custom tailoring and alterations while you wait. Nucl Recept Signaling [Electronic Resource]. 2005;3:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merrell KW, Crofts JD, Smith RL, et al. Differential recruitment of nuclear receptor coregulators in ligand-dependent transcriptional repression by estrogen receptor-α. Oncogene. 2011;30(13):1608–1614. [DOI] [PubMed] [Google Scholar]
- 19. Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. [DOI] [PubMed] [Google Scholar]
- 20. Lonard DM, O'Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. [DOI] [PubMed] [Google Scholar]
- 21. Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. [DOI] [PubMed] [Google Scholar]
- 22. Kitagawa H, Yanagisawa J, Fuse H, et al. Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol. 2002;22:3698–3706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Kitagawa H, Yanagisawa J, Fuse H, et al. Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol. 2002;22(11):3698–3706. Retraction in: Mol Cell Biol. 2014;34(5):916. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Chang C-Y, Abdo J, Hartney T, McDonnell DP. Development of peptide antagonists for the androgen receptor using combinatorial peptide phage display. Mol Endocrinol. 2005;19:2478–2490. [DOI] [PubMed] [Google Scholar]
- 25. Hall JM, Chang CY, McDonnell DP. Development of peptide antagonists that target estrogen receptor β-coactivator interactions. Mol Endocrinol. 2000;14:2010–2023. [DOI] [PubMed] [Google Scholar]
- 26. Mettu NB, Stanley TB, Dwyer MA, et al. The nuclear receptor-coactivator interaction surface as a target for peptide antagonists of the peroxisome proliferator-activated receptors. Mol Endocrinol. 2007;21:2361–2377. [DOI] [PubMed] [Google Scholar]
- 27. Safi R, Kovacic A, Gaillard S, et al. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor γ coactivator-1α on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005;65:11762–11770. [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Chang C, Safi R, et al. Identification of ligand-selective peptide antagonists of the mineralocorticoid receptor using phage display. Mol Endocrinol. 2011;25:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norris JD, Joseph JD, Sherk AB, et al. Differential presentation of protein interaction surfaces on the androgen receptor defines the pharmacological actions of bound ligands. Chem Biol. 2009;16:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker MP, Zhang M, Le TP, Wu P, Laine M, Greene GL. RAC3 is a pro-migratory co-activator of ERα. Oncogene. 2011;30(17):1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clyne CD, Chang C-Y, Safi R, Fuller PJ, McDonnell DP, Young MJ. Purification and characterization of recombinant human mineralocorticoid receptor. Mol Cell Endocrinol. 2009;302:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ziera T, Irlbacher H, Fromm A, et al. Cnksr3 is a direct mineralocorticoid receptor target gene and plays a key role in the regulation of the epithelial sodium channel. FASEB J. 2009;23:3936–3946. [DOI] [PubMed] [Google Scholar]
- 33. Chang C, Norris JD, Jansen M, Huang H-J, McDonnell DP. Application of random peptide phage display to the study of nuclear hormone receptors. Methods Enzymol. 2003;364:118–1142. [DOI] [PubMed] [Google Scholar]
- 34. Miura P, Coriati A, Belanger G, et al. The utrophin A 5′-UTR drives cap-independent translation exclusively in skeletal muscles of transgenic mice and interacts with eEF1A2. Hum Mol Genet. 2010;19:1211–1220. [DOI] [PubMed] [Google Scholar]
- 35. Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol. 2003;5:320–329. [DOI] [PubMed] [Google Scholar]
- 36. Lee SW, Cho KJ, Park JH, et al. Expressions of Ku70 and DNA-PKcs as prognostic indicators of local control in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2005;62:1451–1457. [DOI] [PubMed] [Google Scholar]
- 37. Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birch JL, Tan BC, Panov KI, et al. FACT facilitates chromatin transcription by RNA polymerases I and III. EMBO J. 2009;28:854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ham J, Thomson A, Needham M, Webb P, Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988;16:5263–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arriza JL, Weinberger C, Cerelli G, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268. [DOI] [PubMed] [Google Scholar]
- 41. Yokota K, Shibata H, Kurihara I, et al. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem. 2007;282:1998–2010. [DOI] [PubMed] [Google Scholar]
- 42. Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G. SGK Is an aldosterone-induced kinase in the renal collecting duct. J Biol Chem. 1999;274:16973–16978. [DOI] [PubMed] [Google Scholar]
- 43. Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. [DOI] [PubMed] [Google Scholar]
- 44. O'Malley BW. Sequentiality and processivity of nuclear receptor coregulators in regulation of target gene expression. Nucl Recept Signaling [Electronic Resource]. 2003;1:e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Won Jeong K, Chodankar R, Purcell DJ, Bittencourt D, Stallcup MR. Gene-specific patterns of coregulator requirements by estrogen receptor-α in breast cancer cells. Mol Endocrinol. 2012;26:955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karmakar S, Foster EA, Smith CL. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-α transcriptional activity. Endocrinology. 2009;150:1588–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wood JR, Likhite VS, Loven MA, Nardulli AM. Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol Endocrinol. 2001;15:1114–1126. [DOI] [PubMed] [Google Scholar]
- 48. Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol. 2004;33:387–410. [DOI] [PubMed] [Google Scholar]
- 49. Berrodin TJ, Jelinsky SA, Graciani N, et al. Novel progesterone receptor modulators with gene selective and context-dependent partial agonism. Biochem Pharmacol. 2009;77:204–215. [DOI] [PubMed] [Google Scholar]
- 50. Landolin JM, Johnson DS, Trinklein ND, et al. Sequence features that drive human promoter function and tissue specificity. Genome Res. 2010;20:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heintzman ND, Ren B. Finding distal regulatory elements in the human genome. Curr Opin Genet Dev. 2009;19:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bebermeier JH, Brooks JD, DePrimo SE, et al. Cell-line and tissue-specific signatures of androgen receptor-coregulator transcription. J Mol Med (Berl). 2006;84:919–931. [DOI] [PubMed] [Google Scholar]
- 53. Koike M, Miyasaka T, Mimori T, Shiomi T. Subcellular localization and protein-protein interaction regions of Ku proteins. Biochem Biophys Res Commun. 1998;252:679–685. [DOI] [PubMed] [Google Scholar]
- 54. Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. [DOI] [PubMed] [Google Scholar]
- 55. Mayeur GL, Kung W-J, Martinez A, Izumiya C, Chen DJ, Kung H-J. Ku is a novel transcriptional recycling coactivator of the androgen receptor in prostate cancer cells. J Biol Chem. 2005;280:10827–10833. [DOI] [PubMed] [Google Scholar]
- 56. Ohno M, Kunimoto M, Nishizuka M, Osada S, Imagawa M. Ku proteins function as corepressors to regulate farnesoid X receptor-mediated gene expression. Biochem Biophys Res Commun. 2009;390:738–742. [DOI] [PubMed] [Google Scholar]
- 57. Jeyakumar M, Liu X, Erdjument-Bromage H, Tempst P, Bagchi MK. Phosphorylation of thyroid hormone receptor-associated nuclear receptor corepressor holocomplex by the DNA-dependent protein kinase enhances its histone deacetylase activity. J Biol Chem. 2007;282:9312–9322. [DOI] [PubMed] [Google Scholar]
- 58. Newfell BG, Iyer LK, Mohammad NN, et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hu S, Pluth JM, Cucinotta FA. Putative binding modes of Ku70-SAP domain with double strand DNA: a molecular modeling study. J Mol Model. 2012;18:2163–2174. [DOI] [PubMed] [Google Scholar]
- 60. Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. [DOI] [PubMed] [Google Scholar]
- 61. Abbott CM, Newbery HJ, Squires CE, Brownstein D, Griffiths LA, Soares DC. eEF1A2 and neuronal degeneration. Biochem Soc Trans. 2009;37:1293–1297. [DOI] [PubMed] [Google Scholar]
- 62. Mateyak MK, Kinzy TG. eEF1A: thinking outside the ribosome. J Biol Chem. 2010;285:21209–21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Everaert BR, Boulet GA, Timmermans J-P, Vrints CJ. Importance of suitable reference gene selection for quantitative real-time PCR: special reference to mouse myocardial infarction studies. PLoS One. 2011;6:e23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan G, You B, Chen S-P, Liao JK, Sun J. Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α 1. Circ Res. 2008;103:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Winkler DD, Luger K. The histone chaperone FACT: structural insights and mechanisms for nucleosome reorganization. J Biol Chem. 2011;286:18369–18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Orphanides G, Wu W-H, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400:284–288. [DOI] [PubMed] [Google Scholar]
- 67. Boonyaratanakornkit V, Melvin V, Prendergast P, et al. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol. 1998;18:4471–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem. J. 2002;361:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]