Abstract
SHBG transports and regulates the activities of androgens and estrogens. Several single nucleotide polymorphisms in the human SHBG gene have been linked to sex steroid-dependent diseases, including those associated with the metabolic syndrome. The N-terminal laminin G-like domain of SHBG includes binding sites for calcium, sex steroids, and fibulin family members, as well as a dimerization domain. We have found that 8 of 18 uncharacterized nonsynonymous single nucleotide polymorphisms within this domain alter the production or biochemical properties of SHBG in ways not previously recognized. O-Linked glycosylation at Thr7 is disrupted in SHBG T7N, whereas abnormal glycosylation of SHBG G195E limits its secretion. Three SHBG mutants (R135C, L165M, and E176K) bind estradiol with abnormally high affinity. SHBG R135C also has an increased interaction with fibulin-2. Two different substitutions within the dimer interface at R123 (R123H and R123C) reduce the affinity for 5α-dihydrotestosterone, while increasing the relative binding affinity for estradiol. SHBG T48I is defective in calcium binding, which leads to a defect in dimerization, reduced affinity for sex steroids, and an enhanced interaction with fibulin-2, which can all be restored by calcium supplementation. These naturally occurring mutants provide insight into SHBG structure and function, and defects in SHBG production or function need to be considered in the context of its utility as a biomarker of diseases.
Plasma SHBG is secreted by the liver as a homodimeric glycoprotein with nanomolar affinities for sex steroids (1). With 3 to 4 orders of magnitude greater affinity for androgens and estrogens than albumin, SHBG is the primary determinant of the plasma distribution and access of these sex steroids to their target tissues (2, 3). Human SHBG comprises 373 amino acid residues (4) that constitute a tandem repeat of laminin G-like (LG) domains (1). A crystal structure of the human SHBG N-terminal LG domain in complex with its preferred ligand, 5α-dihydrotestosterone (DHT), revealed the topography of the hydrophobic steroid-binding pocket (5). It also revealed a calcium-binding site previously suspected of being an important determinant of the structural integrity of SHBG (5, 6). Additional crystal structures of this domain together with the biochemical characterization of strategically targeted mutants further demonstrated how androgens and estrogens are preferentially accommodated in opposite orientations within the single steroid-binding site of the SHBG monomer (7). They also provided evidence that human SHBG is a zinc-binding protein (8) and localized amino acids that participate in homodimer formation (9, 10). Further studies have demonstrated that SHBG interacts with other extracellular proteins, including members of the fibulin family of extracellular matrix–associated proteins (11), as well as kallikrein-related peptidase 4 that specifically cleaves human SHBG between its 2 LG domains (12). Less is known about the structural and functional importance of the C-terminal LG domain, but it contains 2 sites for N-glycosylation, the utilization of which affects the plasma clearance of SHBG (13) without influencing its steroid-binding properties (14).
Two nonsynonymous, single nucleotide polymorphisms (SNPs) in the SHBG coding region have been identified and characterized. A common SNP (rs6259) located within exon 8 of the SHBG gene results in the substitution of Asp327 to Asn (D327N) in the mature SHBG polypeptide sequence (15). This substitution introduces an extra N-linked glycosylation site, the use of which retards the plasma clearance of SHBG (16). The resulting higher plasma SHBG levels in individuals who carry the rs6259 SNP have been negatively associated with the risk of developing breast cancer (17, 18) and type 2 diabetes (19). In a genome-wide meta-analysis (20), another nonsynonymous SHBG SNP (rs6258) that results in a P156L substitution was recently found in ∼2% of European men (20). Biochemical analysis of the SHBG P156L mutant indicated that it reduces testosterone-binding affinity, and this explains why its presence is associated with low plasma testosterone levels (20). The rs6258 SNP was originally identified more than a decade ago in a woman who presented with extreme virilization during pregnancy (21). The plasma SHBG levels in this patient were exceptionally low due to a second novel single nucleotide deletion within the exon 8 sequence of her other SHBG allele that results in premature translation termination and a complete loss of SHBG production.
To date there have been no other reports of genetic polymorphisms in the SHBG coding sequence that result in defects in SHBG production or function. We have therefore interrogated recent releases of SNP databases in which more than 49 nonsynonymous SHBG SNPs were identified. Of these, 32 were located within the coding region for the SHBG N-terminal LG domain that contains the steroid-binding site, as well as the dimerization and fibulin-interaction domains. We prioritized SNPs for analysis based on our knowledge of SHBG structures to focus on naturally occurring amino acid substitutions that might be structurally or functionally important and created recombinant SHBG mutants to assess this.
Materials and Methods
Antibodies and reagents
Two mouse monoclonal anti-human SHBG antibodies (7H9 and S1B5) that recognize epitopes within the SHBG N-terminal LG domain were used. The 7H9 antibody was kindly provided by Dr John Lewis (22), and the S1B5 antibody was prepared in-house (23), as was the rabbit anti-human SHBG antibody (24). Peptide-N-glycosidase F (PNGase F) was purchased from New England Biolabs. Kallikrein-related peptidase 4 was kindly provided by Dr Jonathan Harris (Queensland University of Technology). [3H]DHT (specific activity, 110 Ci/mmol) was obtained from PerkinElmer Life Sciences, and [3H]2-methoxyestradiol ([3H]2-MeOE2) (specific activity 60 Ci/mmol) was purchased from American Radiolabeled Chemicals. Unlabeled steroids were obtained from Steraloids and used without further purification.
Cell culture
Cell culture reagents were from Life Technologies. Chinese hamster ovary (CHO) cells were routinely cultured in minimum essential medium α supplemented with 10% fetal bovine serum and antibiotics (100 U of penicillin/mL and 100 μg of streptomycin/mL).
Production of human SHBG mutants
Human SHBG cDNA in the pRC/CMV vector was used for site-directed mutagenesis (25) using a QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies) and site-specific mutagenic oligonucleotide primers (Supplemental Table 1). The mutated plasmids were sequenced to ensure that only the targeted mutations were present. They were then used to transfect CHO cells with Lipofectamine 2000 (Invitrogen). After selection in the presence of 1 mg/mL Geneticin (Life Technologies), stably transfected cells were grown to near confluence and then were washed twice with PBS to remove fetal bovine serum and cultured in minimum essential medium α supplemented with antibiotics (100 U of penicillin/mL and 100 μg of streptomycin/mL) and 100 nM DHT. Culture media containing recombinant human SHBG were filtered with a Millex-GP Filter Unit (Millipore), concentrated 10-fold using an Amicon Ultra-4 Centrifugal Filter with an Ultracel-3 membrane (Millipore), and then equilibrated and stored in Tris-buffered saline, pH 7.5, containing 100 nM DHT and 0.05% sodium azide.
Immunofluorometric assay (IFA)
A modified version of a time-resolved IFA (26) was used to measure SHBG concentrations in culture media. In brief, 96-well plates were coated overnight with 150 μL of rabbit antiserum against SHBG (1:500 diluted in filtered 0.5 M NaHCO3)at 4°C and then were blocked with 300 μL of blocking buffer (1% casein in 20 mM Tris-HCl, pH 8, and 150 mM NaCl) for 2 hours at room temperature. Diluted (1:1000) aliquots (100 μL) of the concentrated culture media samples and 50 μL of europium-labeled S1B5 antibody (2000× diluted in DELFIA assay buffer; PerkinElmer) were then coincubated for 3 hours at room temperature. Wells were washed 6 times with washing buffer (50 mM Tris-HCl, pH 7.5, and 150 mM NaCl), and 150 μL of DELFIA enhancement solution (PerkinElmer) was added to each well. Time-resolved fluorescence was measured using a VICTOR X4 Multimode Plate Reader (PerkinElmer).
Steroid-binding capacity measurements
A standard ligand saturation analysis (27) was used to determine the steroid-binding capacity, affinity, and specificity of wild-type SHBG and SHBG mutants. In brief, concentrated and buffer-exchanged media were preincubated with dextran-coated charcoal (DCC) to remove steroids before incubation with [3H]DHT in the presence or absence of a 100-fold molar excess of unlabeled DHT to monitor nonspecific binding. Unbound steroids were removed by exposure to DCC for 10 minutes at 0°C followed by centrifugation to allow SHBG-bound [3H]DHT to be measured. Affinity constants were determined by Scatchard analysis (27), and the relative binding affinities of steroids were determined using [3H]DHT as the labeled ligand and increasing amounts of DHT, testosterone, or 17β-estradiol as competitors (27).
Steroid-binding kinetics
Equal amounts of wild-type SHBG or SHBG mutants of interest were first incubated with DCC to remove steroids from the binding sites. The stripped SHBG samples and reagents were cooled on ice to ensure that the following reactions occurred at 0°C. The association rate kinetics of DHT to SHBG were determined by incubating ∼1.5 nM SHBG with 10 nM [3H]DHT for 15 seconds to 10 minutes, whereas dissociation rate kinetics were determined by preincubating SHBG with 10 nM [3H]DHT for 1 hour followed by the addition of 3 μM DHT for 0 to 20 minutes. SHBG-bound [3H]DHT was separated and measured, as described above.
The methods described above were modified to examine the effects of S1B5 antibody on steroid-binding kinetics. The association kinetics of steroid binding to wild-type SHBG were determined in the presence or absence of S1B5 antibody by incubating unliganded wild-type SHBG with or without S1B5 antibody for 1 hour at room temperature followed by [3H]DHT or [3H]2-MeOE2 incubation for 30 seconds to 1 hour at 0°C. The dissociation of steroids from wild-type SHBG was also measured by incubating [3H]DHT or [3H]2-MeOE2 saturated wild-type SHBG with or without S1B5 antibody for 1 hour at room temperature followed by DCC treatment for 2.5 to 30 minutes at 10°C.
Western blotting
Recombinant human SHBG from culture media or CHO cell lysates was resolved by 10% PAGE under nondenaturing or denaturing conditions and transferred to 0.45 μm Immobilon-P polyvinylidene difluoride membranes (Millipore) by electroblotting. The membranes were blocked in 5% skim milk for 1 hour, followed by incubation with 1:6000 diluted rabbit antihuman SHBG antiserum or 1:200 diluted 7H9 antibody. Immunoreactive proteins were detected with 1:10 000 diluted horseradish peroxidase-conjugated goat antirabbit or antimouse IgG as a secondary antibody (Sigma-Aldrich).
SHBG dimerization assay
Equal amounts of wild-type or mutated SHBG were first incubated with DCC to remove steroids, and 1 mM EGTA was added to remove calcium. In some samples, 2 mM CaCl2 and/or 500 nM DHT was then added and incubated at room temperature for 3 hours. Nondenaturing PAGE and Western blot analysis were used to detect dimeric vs monomeric SHBG (25).
Glutathione S-transferase (GST) pull-down assays
We used a GST pull-down assay to assess SHBG interactions with fibulin-2 (11). In brief, 10 μg of a GST-fibulin-2 fusion protein, which includes 77 C-terminal residues of fibulin-2 that contain the SHBG-binding site (11), was incubated overnight with 20 nM wild-type or mutated SHBG at 4°C in binding buffer (20 mM Tris-HCl, pH 8.0, 0.02% Nonidet P-40, and 0.2 mg/mL bovine serum albumin). Equilibrated aliquots (50 μL) of glutathione-agarose (Thermo Scientific) were added, and samples were incubated at room temperature for 1 hour. Sedimented glutathione-agarose beads were then washed 3 times in ice-cold buffer (20 mM Tris-HCl, pH 8.0, and 0.02% Nonidet P-40), proteins bound to the washed beads were extracted by boiling in SDS sample-loading buffer for 5 minutes, and SHBG was detected by Western blot analysis.
Statistical analysis
Data are reported as means ± SD of at least 3 independent experiments for all measurements. Differences between mean values were evaluated by the unpaired Student t test using GraphPad Prism (GraphPad Software Inc) software.
Results
Production and measurements of recombinant wild-type and mutant SHBGs in culture media
From entries in the SNP databases, we selected 17 nonsynonymous SNPs within the SHBG N-terminal LG domain and one in the signal polypeptide sequence for analysis (Table 1). These SNPs were studied because the substituted residues are either conserved across several mammalian species or located in regions of the molecule that could potentially alter the biological properties of SHBG according to crystal structure predictions (Figure 1). Recombinant SHBGs were expressed in CHO cells, and culture media were concentrated 10-fold for DHT-binding capacity assays and IFA measurements to assess their levels of production. Wild-type SHBG was analyzed at different dilutions, and this demonstrated a near perfect correlation between these 2 assays, which compare the functional properties of SHBG in terms of steroid-binding capacity with respect to its immunoassay value (Figure 2).
Table 1.
Nonsynonymous SNPs Within Human SHBG Are Identified by Their Corresponding rs Numbers
| SNP Identification No. | Alleles | Amino Acid Position |
|---|---|---|
| rs9282845 | A/G | R (22)H |
| rs373254168 | A/C | T7N |
| rs143521188 | T/C | T48I |
| rs374583574 | C/G | E52Q |
| rs148001698 | A/G | R94Q |
| rs143452836 | A/C | L95M |
| rs147130840 | A/G | D110N |
| rs186960957 | T/G | E119D |
| rs373769356 | T/C | R123C |
| rs143269613 | A/G | R123H |
| rs368589266 | T/C | R135C |
| rs115336700 | C/G | A150P |
| rs143134553 | A/C | N152K |
| rs139379650 | T/C | R154W |
| rs145273466 | A/C | L165M |
| rs372114420 | A/G | E176K |
| rs189578288 | T/C | S192 liter |
| rs146779355 | A/G | G195E |
The nucleotides of SNP/ancestral alleles and the corresponding amino acid substitutions for each SNP identification number are listed. The amino acid positions in the mature human SHBG sequence are indicated. However, R(22)H refers to a substitution at position 22 in the signal peptide of the SHBG precursor.
Figure 1.
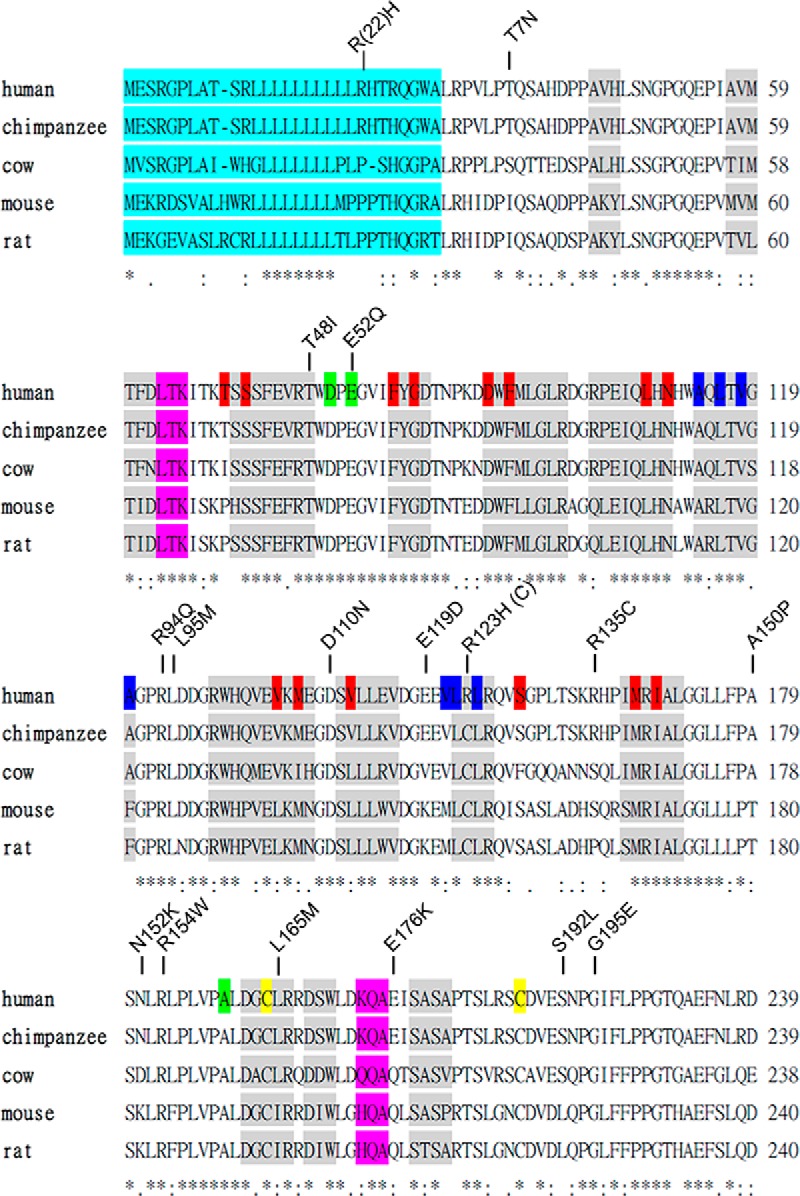
Orthologous sequence alignment of the SHBG N-terminal LG domain and the amino acid substitutions resulting from selected nonsynonymous SNPs. Amino acid sequences between human and other species of SHBG N-terminal LG domain were aligned. Secondary structures of α-helix and β-strands are shown as pink and gray boxes, respectively. Residues known to be involved in steroid binding (red), dimerization (blue), calcium binding (yellow), and disulfide bridge formation (green) are highlighted. The signal peptide is also highlighted in a light-blue color. Amino acid substitutions are presented on top of the sequences. *, conserved residues between species.
Figure 2.
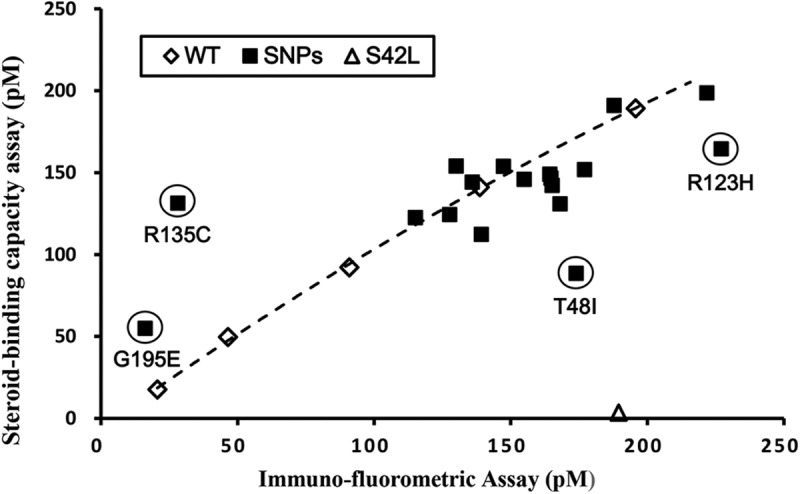
Comparison of DHT-binding capacity assay and IFA values of recombinant wild-type and mutant SHBGs. Values for SHBG mutants in which amino acid substitutions introduced by nonsynonymous SNPs (■) are compared with a reference line generated using the values for wild-type SHBG (WT) determined at 5 different concentrations (♢). For reference, the corresponding values for a known DHT binding–deficient mutant, SHBG S42L (5), are shown (▵). Mutants suspected of exhibiting abnormal DHT-binding affinity or immune recognition in the IFA are circled and are positioned below or above the wild-type reference line, respectively.
Most SHBG mutants, including the mutant with an Arg to His substitution at position 22 in the signal polypeptide sequence, were produced at about the same concentration as that of wild-type SHBG, and their DHT-binding capacity and immunoassay values correlate well. However, one of them (SHBG G195E) was present in the culture media at very low levels, as determined by both assay methodologies, and this implies that SHBG G195E is produced at abnormally low levels (Figure 2). Three other mutants displayed a discrepancy in DHT-binding capacity with respect to the IFA value. One (SHBG R135C) exhibited a low immunoassay value compared with its DHT-binding capacity, which suggests that the recognition of SHBG R135C by the labeled S1B5 antibody in the IFA is impaired, whereas the other 2 (SHBG T48I and SHBG R123H) mutants had lower than expected DHT-binding capacity values compared with their IFA values (Figure 2), suggesting that these mutants have abnormal steroid-binding properties.
Steroid-binding properties of wild-type and mutant SHBGs
To further evaluate the steroid-binding affinities of SHBG T48I and SHBG R123H, as well as those of the other SHBG mutants, Scatchard analysis was performed (Table 2). As anticipated from the above results (Figure 2), SHBG T48I and SHBG R123H have 2.5- and 4-fold lower binding affinities, respectively, for DHT than wild-type SHBG (Table 2). In addition, the SHBG G195E mutant that was produced at very low levels (Figure 2) was also found to have 5-fold lower affinity for DHT than wild-type SHBG (Table 2). All of the other SHBG mutants had affinities for DHT similar to those of wild-type SHBG, apart from another mutant with an amino acid substitution at residue 123 (SHBG R123C), which had a significantly reduced affinity for DHT, although this was less pronounced than that for SHBG R123H (Table 2). To further understand the steroid-binding kinetics responsible for reduced DHT-binding affinity of these SHBG mutants, association rate (Figure 3A) and dissociation rate (Figure 3B) assays were performed using [3H]DHT as the labeled ligand. Compared with that for wild-type SHBG, the association rate of DHT for SHBG R123H is normal (Figure 3A), whereas its dissociation rate is faster (Figure 3B). In contrast, the association rates of DHT for SHBG T48I or SHBG G195E are markedly reduced (Figure 3A), whereas their dissociation rates are similar to those for wild-type SHBG (Figure 3B).
Table 2.
Steroid-Binding Affinities and Specificities of Wild-Type SHBG and SHBG Mutants
| SHBG | Kd, nM | Relative Binding Activity (% of DHT) |
|
|---|---|---|---|
| Testosterone | Estradiol | ||
| Wild type | 0.30 ± 0.08 | 24.02 ± 4.18 | 4.52 ± 0.57 |
| R(22)H | 0.33 ± 0.02 | 23.23 ± 5.34 | 4.64 ± 0.87 |
| T7N | 0.30 ± 0.07 | 21.83 ± 1.72 | 4.75 ± 1.65 |
| T48I | 0.78 ± 0.10a | 23.88 ± 4.69 | 5.32 ± 0.58 |
| E52Q | 0.24 ± 0.03 | 21.43 ± 2.04 | 4.50 ± 0.54 |
| R94Q | 0.35 ± 0.01 | 24.80 ± 4.82 | 4.73 ± 0.88 |
| L95M | 0.30 ± 0.02 | 24.50 ± 4.54 | 4.86 ± 0.52 |
| D110N | 0.39 ± 0.19 | 21.14 ± 0.12 | 4.51 ± 0.16 |
| E119D | 0.31 ± 0.04 | 24.81 ± 3.94 | 4.53 ± 0.89 |
| R123C | 0.49 ± 0.05a | 24.48 ± 3.53 | 5.82 ± 0.36b |
| R123H | 1.29 ± 0.23a | 26.26 ± 4.49 | 5.42 ± 0.34c |
| R135C | 0.27 ± 0.01 | 25.93 ± 3.36 | 7.64 ± 0.94a |
| A150P | 0.40 ± 0.17 | 25.58 ± 4.41 | 5.13 ± 0.97 |
| N152K | 0.28 ± 0.06 | 23.91 ± 1.99 | 4.97 ± 0.70 |
| R154W | 0.33 ± 0.03 | 24.38 ± 4.36 | 3.97 ± 0.71 |
| L165M | 0.24 ± 0.04 | 22.93 ± 1.71 | 7.37 ± 0.32a |
| E176K | 0.35 ± 0.04 | 23.59 ± 0.44 | 8.62 ± 0.47a |
| S192L | 0.27 ± 0.07 | 25.79 ± 1.13 | 4.53 ± 0.61 |
| G195E | 1.52 ± 0.08a | 21.72 ± 3.34 | 3.94 ± 0.62 |
Kd values of wild-type SHBG and SHBG mutants for DHT were determined by Scatchard analysis using [3H]DHT as labeled ligand. The relative binding affinity values of SHBG for testosterone and estradiol were determined as percentages by dividing the IC50 of DHT (ie, the concentration of DHT required to displace 50% of [3H]DHT from SHBG) with the corresponding IC50 values of unlabeled testosterone and estradiol in standard competitive steroid-binding assays (27). The Kd and relative binding affinity values are expressed as means ± SD of 3 independent assays.
P < .001.
P < .01.
P < .05.
Figure 3.
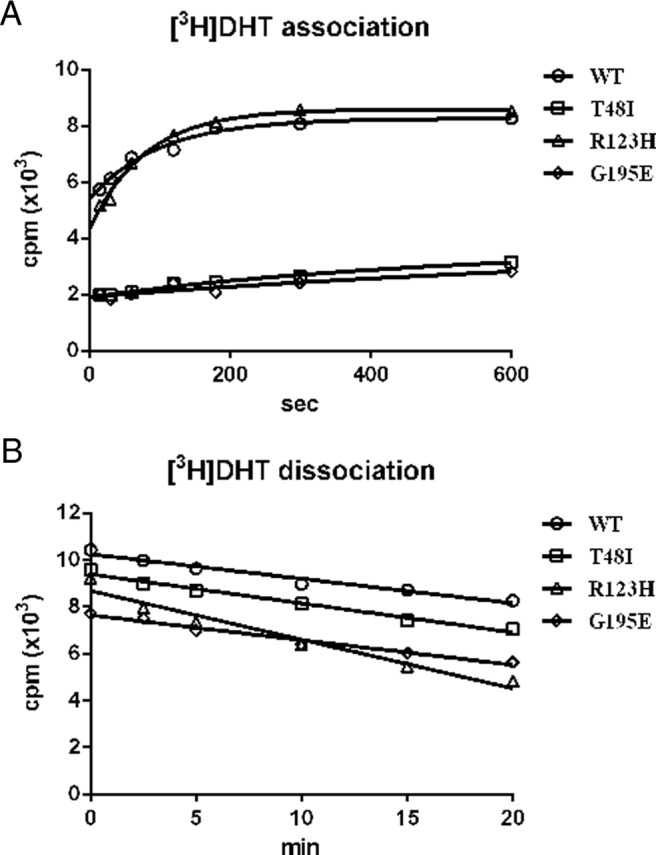
Kinetics of DHT binding to wild-type SHBG (WT) and SHBG mutants with a suspected abnormally low DHT-binding affinity (SHBG T48I, SHBG R123H, and SHBG G195E). A, Association rates were determined by measuring the occupancy of unliganded SHBG samples by [3H]DHT over time at 0°C. B, Dissociation rates were determined in a pulse-chase experiment by preincubating SHBG with 10 nM [3H]DHT for 1 hour followed by adding 3 μM DHT for 0 to 20 minutes at 0°C.
Interestingly, substitutions of R123 by either His or Cys both result in small but significant increases in estradiol-binding affinity relative to those of DHT or testosterone (Table 2). Moreover, 3 other SHBG mutants with essentially normal affinities for DHT (SHBG R135C, SHBG L165M, and SHBG E176K) have approximately 2-fold higher relative binding affinities for estradiol than either DHT or testosterone (Table 2).
Mutations that influence steroid binding through abnormal calcium binding or dimerization
It is known that human SHBG contains 2 calcium-binding sites (6) and that a calcium-binding site within the N-terminal LG domain is important for stabilizing the steroid binding and dimerization of SHBG (25, 28). In a crystal structure of the human SHBG LG domain (5), Thr48 is located close to key residues that interact with calcium, whereas Arg123 is located within the dimerization interface. Although dimerization is not essential for the formation of a functional steroid-binding site (10), we investigated whether the reduced DHT binding affinity that characterizes SHBG T48I is related to a defect in calcium binding. To assess this, we added 1 mM CaCl2 to saturate the SHBG calcium-binding sites of SHBG mutants with lower affinities to DHT (SHBG T48I, SHBG R123H, and SHBG G195E) before steroid-binding capacity assays (Figure 4A), thus demonstrating that calcium supplementation restored the DHT-binding affinity of SHBG T48I to values approaching those observed for wild-type SHBG (Figure 4A). In contrast, the relatively low DHT-binding affinities of SHBG R123H and SHBG G195E were unaltered by calcium supplementation (Figure 4A). Moreover, supplementation of calcium or DHT also reversed a marked defect in SHBG T48I dimer formation, as assessed by a mobility shift in a native PAGE, and this was accentuated when calcium and DHT were added together (Figure 4B). The SHBG R123H mutant exhibited a minor disruption of dimer formation only in the absence of steroid ligands and calcium, but this was resolved by calcium or DHT supplementation (Figure 4B).
Figure 4.
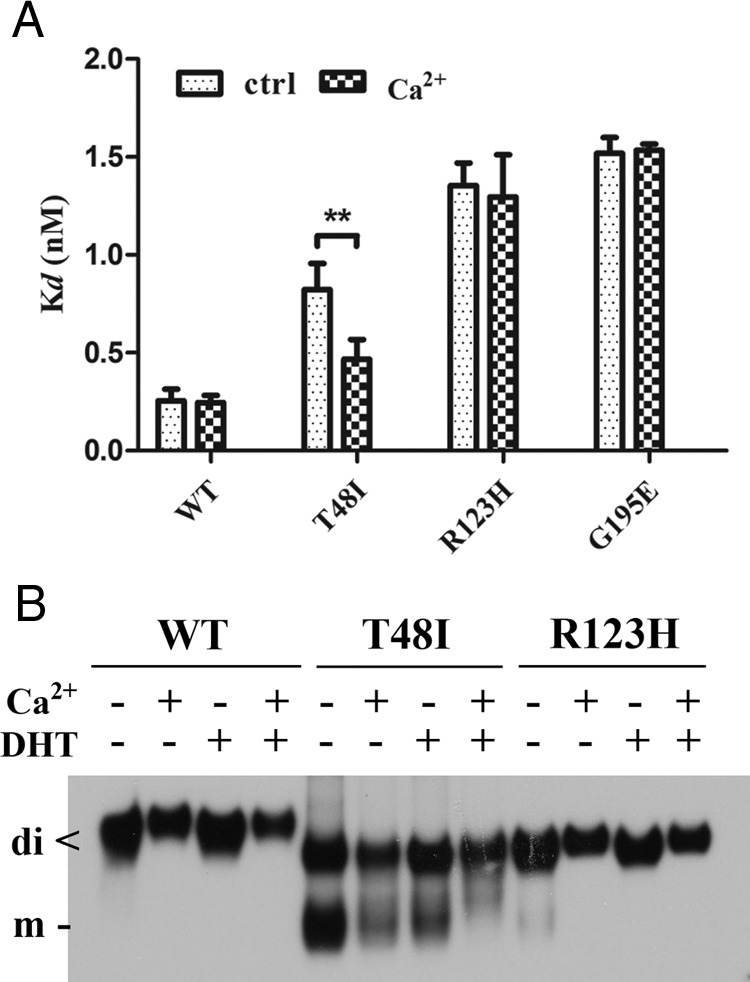
Influence of calcium supplementation on SHBG mutants with abnormally low DHT-binding affinity. A, Calcium partially restores the DHT-binding affinity of SHBG T48I but has no effect on other DHT-binding defective SHBG mutants (SHBG R123H and SHBG G195E). Dissociation constants (Kd) of wild-type SHBG (WT), SHBG T48I, SHBG R123H, and SHBG G195E for [3H]DHT were determined by Scatchard analysis in the presence or absence (control) of 1 mM CaCl2. **, P < .01. B, Calcium and/or DHT stabilizes SHBG T48I and SHBG R123H homodimers. Western blotting analysis under nondenaturing conditions was performed in the presence or absence of DHT or calcium using rabbit anti-human SHBG antiserum as the primary antibody.
SHBG mutations that influence interaction with fibulin-2
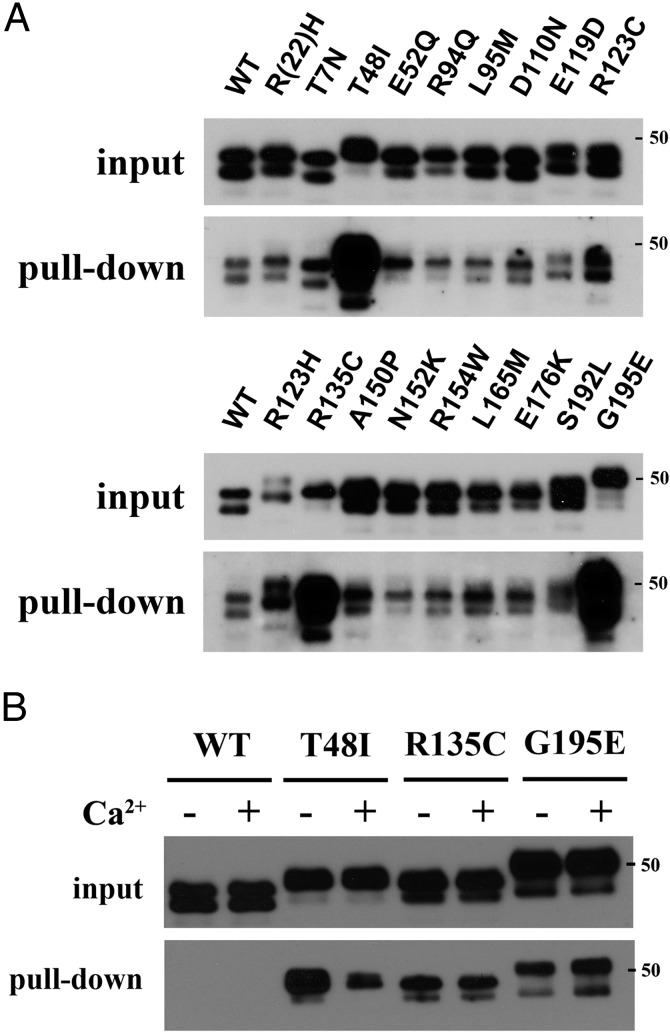
Human SHBG has been shown to interact with the C-terminal domain of fibulin-2 in a steroid ligand–dependent manner (11), and we therefore used a GST-fusion protein comprising the C-terminal domain of fibulin-2 as bait in GST pull-down assays to determine whether the various SHBG mutants interact differently with fibulin-2. This approach revealed that interactions between SHBG T48I, SHBG G195E, or SHBG R135C and fibulin-2 were significantly increased in the presence of DHT, compared with those for wild-type SHBG or the other mutants (Figure 5A). The increased interactions between fibulin-2 and SHBG T48I or SHBG G195E were surprising because they both have abnormally low affinities for DHT (Table 2). Moreover, because calcium restores the DHT-binding affinity and dimerization of SHBG T48I (Figure 4), we examined whether calcium supplementation specifically alters the pronounced interaction between SHBG T48I and fibulin-2. Paradoxically, whereas calcium supplementation increased the DHT-binding affinity of this mutant (Figure 4A), the enhanced interaction with fibulin-2 was lost (Figure 5B). These results suggest that global conformational alterations of SHBG T48I and SHBG G195E may override any effects of steroid binding on the fibulin-2 interaction. The enhanced interaction between fibulin-2 and the SHBG R135C mutant (Figure 5A) is of particular interest because this mutant is secreted normally and is characterized by an increased relative binding affinity for estradiol compared with that for DHT (Table 2).
Figure 5.
Identification of SHBG mutants with abnormal interactions with fibulin-2. A, Interaction of wild-type SHBG or SHBG mutants with the C-terminal domain of fibulin-2. A GST pull-down assay was performed by using 10 μg of a GST-fibulin-2 fusion protein as bait, and the relative amounts of interacting SHBG were determined by SDS-PAGE and Western blotting using rabbit anti-human SHBG antiserum as the primary antibody. Exposure of the Western blots was adjusted to compare the relative amounts of different SHBG mutants that interacted with fibulin-2 in the GST pull-down assay, against wild-type SHBG as a reference. B, Calcium decreases the interaction of T48I SHBG with fibulin-2 when the GST pull-down assay was performed in the presence or absence of 1 mM CaCl2. Note that this Western blot was underexposed to see the effect of calcium supplementation on fibulin-2 interaction with SHBG mutants with enhanced ability to interact with fibulin-2, and at this exposure the pull down of wild-type SHBG (WT) is not detectable. In all of these Western blots, the denatured recombinant SHBG monomers migrate during SDS-PAGE as a doublet, the sizes and intensities of which reflect differential utilization of the 2 N-linked glycosylation sites in the C-terminal LG domain (14). A 50-kDa molecular size marker is shown on the right of the Western blots, which are representative examples of 3 independent experiments.
The electrophoretic microheterogeneity of SHBG subunits during SDS-PAGE (Figure 5) is due to variations in the complexity of carbohydrate structures attached at specific glycosylation sites, whereas the macroheterogeneity is a reflection of the use of individual O-linked and N-linked glycosylation sites (14, 29). For instance, the 2 distinct SHBG subunits of ∼46 and ∼48 kDa typically observed in wild-type SHBG reflect occupancy of one or both of the N-linked glycosylation sites within the C-terminal LG domain of SHBG, respectively (14, 29). In the Western blot of the GST fibulin-2 pull-down assay (Figure 5), SHBG R(22)H, SHBG T7N, SHBG E119D, and SHBG R123H also differed in their microheterogeneity compared with that for wild-type SHBG, whereas SHBG R135C exhibited an obvious difference in macroheterogeneity, indicative of the predominant use of both N-linked glycosylation sites. Moreover, SHBG T48I and SHBG G195E showed differences in both microheterogeneity and macroheterogeneity compared with those for wild-type SHBG.
Flux of steroids into and out of the SHBG steroid-binding site
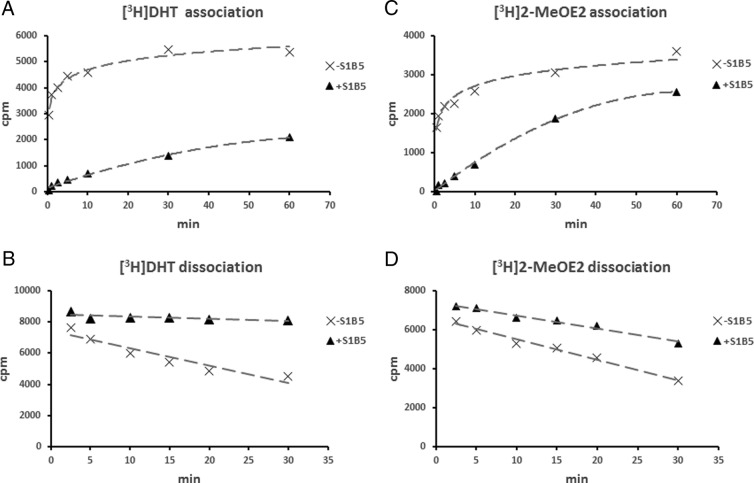
Crystal structure studies have shown that the type of ligand within the steroid-binding site influences the conformation of a flexible loop positioned above the steroid-binding site (7), and the R135C substitution is located within this loop region. It was therefore of interest that the R135C substitution not only changed the SHBG steroid-binding specificity but also interfered with the ability of the S1B5 monoclonal antibody to recognize this mutant in the IFA. The latter observation, together with previous reports that S1B5 recognizes SHBG H136Q poorly (30), indicates that the epitope for S1B5 antibody includes the loop above the steroid-binding site, which prompted us to examine whether the binding of S1B5 to SHBG alters the association or dissociation of steroids from the steroid-binding site. We found that preincubation of S1B5 antibody with unliganded SHBG markedly delayed the association of [3H]DHT with SHBG (Figure 6A), whereas binding of S1B5 antibody after saturation of SHBG with [3H]DHT essentially blocked its dissociation (Figure 6B). Because estrogens occupy the steroid-binding pocket in an opposite orientation compared with that of androgens (7), we also examined whether S1B5 antibody has the same effects on the association and dissociation of estrogens. We used [3H]2-MeOE2 rather than [3H]estradiol for this purpose because 2-MeOE2 has much higher binding affinity to SHBG than estradiol, whereas both estrogens bind in the same orientation (7). Surprisingly, S1B5 also hinders the entrance and exit of [3H]2-MeOE2 into and out of SHBG (Figure 6, C and D), but this effect is less pronounced than that observed using [3H]DHT (Figure 6, A and B).
Figure 6.
Effects of S1B5 antibody binding to SHBG on steroid-binding kinetics. Steroid association rates were determined by incubating unliganded wild-type SHBG with or without S1B5 antibody for 1 hour at room temperature followed by incubation (30 seconds to 1 hour at 0°C) with either [3H]DHT (A) or [3H]2-MeOE2 (C). Dissociation rates were determined by first saturating wild-type SHBG with [3H]DHT (B) or [3H]2-MeOE2 (D) followed by an incubation (1 hour at room temperature) in the presence or absence of S1B5 antibody. The irreversible loss of radiolabeled steroids from SHBG over time was then assessed by DCC exposure for 2.5 to 30 minutes at 10°C.
Characterization of glycosylation variants
In the SHBG T7N mutant, the Thr that is normally O-glycosylated (31) is replaced by an Asn residue that creates a novel N-glycosylation consensus sequence. The observed lower subunit sizes of SHBG T7N than of wild-type SHBG (Figure 5) were therefore surprising because the potential N-glycosylation would be expected to introduce a larger and more complex oligosaccharide. To assess the potential glycosylation at this site in SHBG T7N, we first treated wild-type SHBG and SHBG T7N with PNGase F to remove N-linked glycans and examined their mobilities by SDS-PAGE and Western blotting (Figure 7A). The apparent molecular size of T7N SHBG after treatment with PNGase F is slightly lower than that of wild-type SHBG, most likely due to the lack of the O-linked glycan at residue 7 (Figure 7A, lanes 3 and 4). To further determine whether the O-linked glycan is actually replaced by an N-linked glycan on residue 7, kallikrein-related peptidase 4 was used to specifically cleave the SHBG molecules at a site between their 2 LG domains, as described previously (12). The 7H9 antihuman SHBG monoclonal antibody was then used in a Western blotting experiment to detect the separated N-terminal LG domain (Figure 7A, lanes 5–8). In this experiment, kallikrein-related peptidase 4 cleavage was terminated prematurely to prevent cleavage at nonspecific sites, and there was no difference in the apparent sizes of the separated N-terminal LG domain of SHBG T7N before and after PNGase F treatment (Figure 7A, lanes 6 and 8). Furthermore, the difference in apparent molecular sizes of the N-terminal LG domains of wild-type SHBG and SHBG T7N is consistent with the presence of an O-linked oligosaccharide at this position on wild-type SHBG but not SHBG T7N (Figure 7A, lanes 5 and 6). Taking these results together, we conclude that substitution of Thr7 by an Asn residue eliminates any glycan addition at this position.
Figure 7.
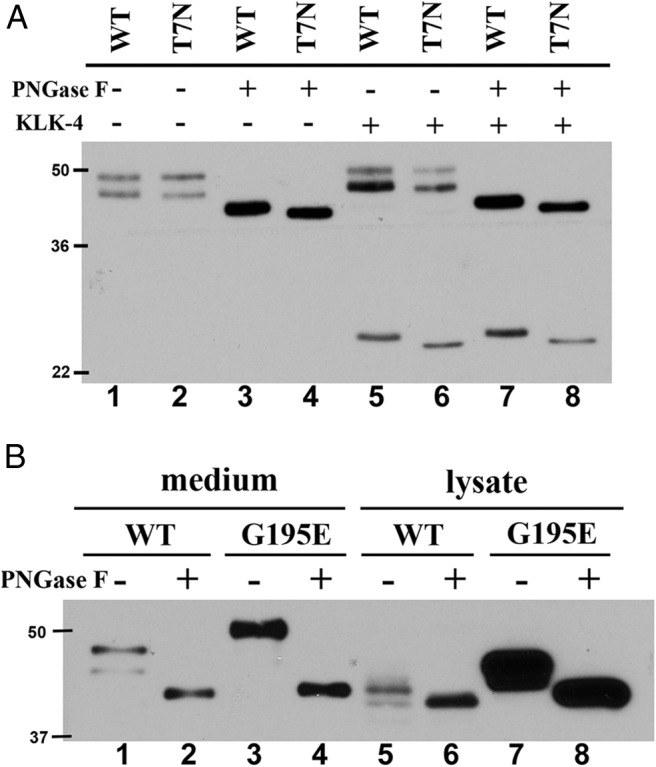
Glycosylation status of SHBG T7N and SHBG G195E. A, Lack of glycosylation at Asn7 of SHBG T7N. Wild-type SHBG (WT) or SHBG T7N was treated with PNGase F and/or kallikrein-related peptidase 4 (KLK-4) for 3 hours at 37°C. B, SHBG G195E mostly accumulated in CHO cells with more complexed N-linked glycans. Equal amounts of wild-type SHBG (WT) and G195E SHBG from media or 20 or 5 μg of total cell lysate from CHO cells expressing SHBG wild-type or SHBG G195E were treated with PNGase F at 37°C for 3 hours. Deglycosylated and/or KLK-4 cleaved SHBGs were detected by SDS-PAGE and Western blotting analysis using mouse 7H9 monoclonal antibody. Molecular size markers (kDa) are shown on the left of each Western blot.
As noted above, it was also apparent that the subunit macroheterogeneity of several SHBG mutants differed from that of wild-type SHBG (Figure 5). In this regard, the SHBG G195E mutant was distinguished by a major subunit size that was greater than that observed for wild-type SHBG or any of the other mutants (Figure 5A). To further characterize this difference, SHBG G195E obtained from cell media or cell lysates was analyzed by Western blotting (Figure 7B). In both types of sample, the SHBG G195E exhibited a greater apparent molecular size than that observed for wild-type SHBG (Figure 7B, lanes 1 and 3 and 5 and 7), but these differences were resolved by removing N-linked glycans (Figure 7B, lanes 2 and 4 and 6 and 8). The greater apparent molecular size of SHBG G195E therefore reflects the presence of more complex N-glycan structures associated with the C-terminal LG domain. Furthermore, a comparison of secreted and intracellular forms of wild-type SHBG and G195E SHBG reveals that more incompletely processed glycoforms of G195E SHBG accumulate within CHO cells (Figure 7B, lanes 5 and 6 and 7 and 8). Taken together, our results indicate that the improper folding of G195E leads to an abnormality in N-glycosylation that restricts its secretion from CHO cells.
Discussion
Despite the widespread use of plasma SHBG measurements for clinical assessments of biologically active androgen levels, only a few genetic differences that result in defects in SHBG production or function have been recorded. Several regulatory sequence variants appear to influence plasma SHBG levels, presumably as a consequence of altered SHBG transcription, including a pentanucleotide (TAAAAn) repeat (32), rs1799941 in the SHBG promoter (33), and rs6257 within intron 1 of SHBG that may additionally influence nascent transcript processing (19). In contrast, only 2 nonsynonymous SNPs in the SHBG coding sequence have been reported to alter SHBG function, including rs6258 that reduces the affinity of SHBG for testosterone by ∼1.8-fold (20) and rs6259 that introduces an additional N-glycosylation site, the use of which increases the half-life and consequently the plasma levels of SHBG (16). In the present study, we demonstrated that 8 uncharacterized nonsynonymous SNPs alter SHBG production or function in previously unrecognized ways (Table 3), which provide insight into the structure and function of this major plasma transport protein for sex steroids.
Table 3.
Summary of the Abnormal Properties Associated With SHBG Mutants
| Mutant | Property |
|---|---|
| T7N | Loss of glycosylation at residue 7 |
| T48I | 2.6-fold lower binding affinity for DHT; increased fibulin-2 interaction, inefficient dimerization, probably due to conformational change caused by defect in calcium binding |
| R123C | 1.6-fold lower binding affinity for DHT; 1.3-fold higher relative binding affinity for estradiol |
| R123H | 4.3-fold lower binding affinity for DHT; 1.2-fold higher relative binding affinity for estradiol; minor defect in dimerization |
| R135C | Reduced S1B5 antibody recognition; 1.7-fold relative higher binding affinity for estradiol; increased fibulin-2 interaction |
| L165M | 1.6-fold higher relative binding affinity for estradiol |
| E176K | 1.9-fold higher relative binding affinity for estradiol |
| G195E | High complexity of N-glycosylation leading to inefficient secretion in CHO cells; 5-fold lower binding affinity for DHT; increased fibulin-2 interaction, probably due to global change of conformation caused by abnormal protein folding |
The most important functional property of human SHBG is its ability to bind both androgens and estrogens with high affinity. Seven of the new SHBG mutants studied displayed an abnormality in steroid-binding affinity or specificity. The G195E substitution caused a general reduction in steroid-binding affinity, and this was surprising because of its location within the linker region between the SHBG LG domains. However, this mutant was also secreted at very low levels, and our results suggest that this is due to a cellular accumulation of incompletely processed glycoforms. Because oligosaccharides do not directly influence the steroid-binding activity of SHBG (14), the G195E mutation may disturb the normal spatial configuration of the 2 LG domains with respect to each other and thereby influence both the steroid-binding activity and the processing of N-linked oligosaccharides attached to sites within the C-terminal LG domain.
Within the N-terminal LG domain, one of the 3 critical residues responsible for calcium binding (Figure 8) is substituted by Gln in SHBG E52Q, but this has no effect on the structure or function of SHBG most likely because the carbonyl oxygens of either Glu or Gln will probably support the coordination of calcium at this site. The Thr48 in human SHBG is also located close to this calcium-binding site (Figure 8). However, although Thr48 does not directly interact with calcium in human SHBG crystal structures (5), its substitution with isoleucine results in a reduction in both steroid-binding activity and dimerization, and these defects can both be restored by calcium supplementation. This finding provides evidence for an allosteric link between steroid binding, dimerization, and calcium binding and helps explain why the steroid-binding activity of SHBG is unstable in EDTA-treated plasma (28, 34).
Figure 8.
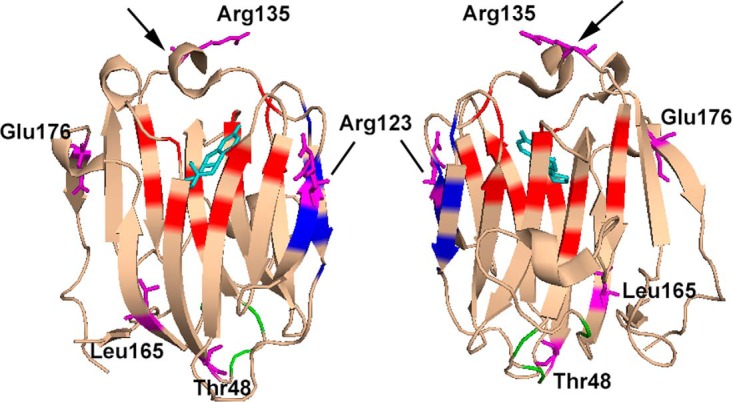
Crystal structure of the N-terminal LG domain of human SHBG in complex with estradiol (Protein Data Bank code: 1LHU; estradiol is shown in cyan) showing the residues where amino acid substitutions were found to alter the functional properties of SHBG. The positions of residues reported previously (5) to be involved in steroid binding (red), dimerization (blue), and calcium binding (green) are highlighted. The loop structure positioned above the steroid-binding pocket is indicted with an arrow. The residues where substitutions result in altered steroid binding (Thr48, Arg123, Arg135, Leu165, and Glu176), dimerization (Arg123 and Thr48), calcium binding (Thr48), or fibulin-2 binding (Thr48 and Arg135) are shown in purple. Note that Gly195 is not included in this crystal structure of the SHBG N-terminal LG domain.
As noted above, the secretion of SHBG G195E is severely compromised, and an abnormal interaction with fibulin-2 may not be functionally very important. Two other SHBG mutants (SHBG T48I and SHBG R135C) are produced normally and exhibited enhanced interactions with fibulin-2. The SHBG T48I mutant is characterized by a generalized 2-fold reduction in steroid-binding affinity, it does not dimerize well, it appears to be more extensively glycosylated than wild-type SHBG, and its enhanced interaction with fibulin-2 is lost after calcium supplementation. We currently have no explanation for the enhanced interaction between SHBG T48I and fibulin-2, but it may reflect a significant structural alteration in the protein, and it is difficult to predict what the physiological implications of this might be. In contrast, SHBG R135C has an increased affinity for estradiol, compared with that of DHT, and this may be physiologically important because SHBG interactions with fibulin-2 are enhanced in a steroid ligand–dependent manner, with occupancy of the SHBG steroid-binding site by estradiol promoting the strongest interaction (11). Moreover, the latter study also demonstrated that the extravascular sequestration and accumulation of plasma SHBG within the endometrial stromal matrix is enhanced by estradiol in both wild-type mice treated with human SHBG and in mice that express a human SHBG transgene and that this accumulation of SHBG occurs in the same location as that of fibulin-2 within the endometrial stroma (11). Furthermore, the R135C substitution occurs within a flexible loop region that covers what appears to be the entrance of the SHBG steroid-binding site (Figure 8). This finding is of interest because this region appears to be more structured when estrogens are located in the steroid-binding site (7), and it is possible that this loop region represents part of the fibulin-2 interaction domain within SHBG.
Two of the naturally occurring SHBG variants we have studied involve substitutions of Arg123, which is located at the dimer interface (Figure 8). In contrast to SHBG T48I, we predict that the SHBG R123H and SHBG R123C mutants probably dimerize normally under physiological conditions because dimerization of SHBG R123H could only be destabilized when calcium and steroids are both removed. Interestingly, the DHT-binding affinities of SHBG R123H and SHBG R123C were both reduced, whereas their affinities for estradiol relative to that of DHT are slightly increased. Although the mechanisms responsible for these effects are not clear, we also found that the rate of DHT association with SHBG R123H is normal, whereas its dissociation rate is abnormally fast. We therefore conclude that amino acid substitutions at the dimer interface exert long-range allosteric effects that influence how specific ligands are accommodated within the steroid-binding pockets of each subunit.
Two of the SHBG mutants that are produced normally, ie, SHBG T48I and SHBG R123H, have general reductions in affinity for androgens (DHT and testosterone) and estradiol. These reductions in affinity (2.6 and 4.3 fold, respectively) are both greater in magnitude than the 1.8 fold reduction in testosterone binding previously observed for SHBG P156L (20). We therefore predict that these SHBG mutants will also be associated with reduced serum sex steroid levels and that these reductions will be even more pronounced than the reductions observed in serum testosterone levels in men with a SHBG P156L mutation (20).
Naturally occurring SHBG variants with abnormal steroid-binding specificity have not been noted previously. It is therefore remarkable that 3 of the SHBG mutants we studied (SHBG R135C, SHBG L165M, and SHBG E176K) bind estradiol with 1.6- to 1.9-fold greater affinities than wild-type SHBG, although their affinities for androgens (DHT and testosterone) are normal. It is difficult to accurately predict how these increases in SHBG affinity for estradiol will affect the concentrations or distribution of estradiol in plasma, but it is reasonable to assume that they will reduce the percentage of free estradiol, as well as its metabolic clearance rate, resulting in higher total plasma estradiol concentrations.
The 3 SHBG mutants with increased affinities for estradiol also provide us with insight into the structural mechanisms that dictate how different classes of steroids are accommodated within the single SHBG steroid-binding site. For instance, we postulate that SHBG R135C may have a different flexible loop conformation that favors estradiol binding, whereas SHBG L165M and SHBG E176K are of interest because Leu165 and Glu176 flank a β-strand (Arg167–Trp170) and a series of residues (ie, Leu171–Lys173) that are specifically displaced when the SHBG steroid-binding site is occupied by estradiol (7). Although L165 and E176 are not located within the steroid-binding site (5), it is possible that they influence the positioning of this β-strand and its C-terminal residues, which appear to specifically influence the accommodation of estradiol within the steroid-binding site.
Our observation that S1B5 differentially hinders the flux of DHT and MeOE2 into and out of the SHBG steroid-binding site is also interesting because it suggests that androgens and estrogens enter and exit the steroid-binding site in different ways. In this context, it is possible that either S1B5 binding physically occludes a common entrance and exit to the steroid-binding pocket in a manner that is less effective in blocking the estrogen flux or that there is an alternative route of entry and/or exit for estrogens compared with that for androgens.
The Thr at position 7 in the mature human SHBG sequence is normally the site of attachment for an O-linked oligosaccharide (31), and we were surprised that the SHBG T7N mutant was not N-glycosylated because an Asn residue in this position introduces a consensus tripeptide (Asn-X-Thr/Ser) site for N-glycosylation (35). One explanation is that the close proximity of this Asn to the N terminus of the nascent SHBG polypeptide limits the enzymatic transfer of the precursor glycan to it during synthesis in endoplasmic reticulum (36, 37). As a result, the SHBG T7N mutant has no oligosaccharide attached at this position at all, but this has no impact on the steroid-binding properties of SHBG, as expected from previous studies (14). Furthermore, based on other previous studies, the loss of an oligosaccharide at this position is not expected to influence the plasma half-life of SHBG appreciably (13). In contrast, use of the 2 N-glycosylation sites in the C-terminal LG domain will have a more significant impact on the half-life of the protein (13). It is therefore of interest that some of the SHBG mutants appear to differ significantly from wild-type SHBG with respect to their subunit macroheterogeneity, and this difference might influence the plasma half-life and consequently the concentrations of these SHBG variants in the blood circulation.
In our initial characterization of recombinant SHBG mutants, a comparison of their DHT-binding capacity and immunoassay values enabled us to identify mutants produced at low levels, as well as mutants that exhibit defects in steroid-binding activity and/or recognition in our immunoassay. A comparison of the values obtained by these 2 different assays also highlights the inherent limitations of the respective methodologies. This comparison is important because SHBG values obtained by these methods are widely used to calculate the free concentrations of sex steroids in clinical samples (38). Moreover, formulas used for this purpose rely on assumptions that immunoassays accurately reflect SHBG concentrations and that SHBG has the same affinity for steroid ligands in all samples. Our results demonstrate that these assumptions are flawed. Although it may be argued that these assay limitations are not a serious concern if SHBG variants occur rarely, the frequency data from current SNP databases reflect healthy individuals in general populations, and it is possible that some of the variants we have identified are more highly represented in specific patient or ethnic groups. For instance, the SHBG P156L variant with a reduced affinity for testosterone occurs in ∼2% of European populations (20), whereas the SHBG D327N that is associated with risk for several different diseases occurs less frequently in African Americans than in individuals of European decent (33).
In an era of increasing emphasis on personalized medicine, our studies highlight the importance of defining how specific nonsynonymous SNPs affect the biochemical properties of widely used biomarkers, such as SHBG. In the case of SHBG SNPs, this will not only facilitate identification of individuals at risk for a variety of hormone-dependent diseases but may shed light on how the plasma levels or functional properties of SHBG are linked to predisposition to diseases including reproductive tissue cancers (33), osteoporosis (39), and type 2 diabetes (40).
Acknowledgments
We thank Caroline Underhill for her excellent technical support and Dr Marc Simard for his critical review of the manuscript.
This work was supported by the Canadian National Institutes of Health Research (Grant MOP 15261 to G.L.H.). G.L.H. is a Canada Research Chair in Reproductive Health and T.-S.W. is the recipient of a 4Y Fellowship from The University of British Columbia.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Canadian National Institutes of Health Research (Grant MOP 15261 to G.L.H.). G.L.H. is a Canada Research Chair in Reproductive Health and T.-S.W. is the recipient of a 4Y Fellowship from The University of British Columbia.
Footnotes
- CHO
- Chinese hamster ovary
- DCC
- dextran-coated charcoal
- DHT
- 5α-dihydrotestosterone
- GST
- glutathione S-transferase
- LG
- laminin G-like
- IFA
- immunofluorometric assay
- 2-MeOE2
- 2-methoxyestradiol
- PNGase F
- peptide-N-glycosidase F
- SNP
- single nucleotide polymorphism.
References
- 1. Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hammond GL. Access of reproductive steroids to target tissues. Obstet Gynecol Clin North Am. 2002;29:411–423. [DOI] [PubMed] [Google Scholar]
- 3. Hammond GL, Wu TS, Simard M. Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr Opin Endocrinol Diabetes Obes. 2012;19:183–189. [DOI] [PubMed] [Google Scholar]
- 4. Hammond GL, Underhill DA, Smith CL, et al. . The cDNA-deduced primary structure of human sex hormone-binding globulin and location of its steroid-binding domain. FEBS Lett. 1987;215:100–104. [DOI] [PubMed] [Google Scholar]
- 5. Grishkovskaya I, Avvakumov GV, Sklenar G, Dales D, Hammond GL, Muller YA. Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J. 2000;19:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross JBA, Contino PB, Lulka MF, Petra PH. Observation and quantitation of metal-binding sites in the sex steroid-binding protein of human and rabbit sera using the luminescent probe terbium. J Protein Chem. 1985;4:299–304. [Google Scholar]
- 7. Grishkovskaya I, Avvakumov GV, Hammond GL, Catalano MG, Muller YA. Steroid ligands bind human sex hormone-binding globulin in specific orientations and produce distinct changes in protein conformation. J Biol Chem. 2002;277:32086–32093. [DOI] [PubMed] [Google Scholar]
- 8. Avvakumov GV, Muller YA, Hammond GL. Steroid-binding specificity of human sex hormone-binding globulin is influenced by occupancy of a zinc-binding site. J Biol Chem. 2000;275:25920–25925. [DOI] [PubMed] [Google Scholar]
- 9. Hammond GL, Robinson PA, Sugino H, Ward DN, Finne J. Physicochemical characteristics of human sex hormone binding globulin: evidence for two identical subunits. J Steroid Biochem. 1986;24:815–824. [DOI] [PubMed] [Google Scholar]
- 10. Avvakumov GV, Grishkovskaya I, Muller YA, Hammond GL. Resolution of the human sex hormone-binding globulin dimer interface and evidence for two steroid-binding sites per homodimer. J Biol Chem. 2001;276:34453–34457. [DOI] [PubMed] [Google Scholar]
- 11. Ng KM, Catalano MG, Pinós T, et al. . Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J Biol Chem. 2006;281:15853–15861. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez WY, de Veer SJ, Swedberg JE, et al. . Selective cleavage of human sex hormone-binding globulin by kallikrein-related peptidases and effects on androgen action in LNCaP prostate cancer cells. Endocrinology. 2012;153:3179–3189. [DOI] [PubMed] [Google Scholar]
- 13. Cousin P, Déchaud H, Grenot C, Lejeune H, Hammond GL, Pugeat M. Influence of glycosylation on the clearance of recombinant human sex hormone-binding globulin from rabbit blood. J Steroid Biochem Mol Biol. 1999;70:115–121. [DOI] [PubMed] [Google Scholar]
- 14. Bocchinfuso WP, Ma KL, Lee WM, Warmels-Rodenhiser S, Hammond GL. Selective removal of glycosylation sites from sex hormone-binding globulin by site-directed mutagenesis. Endocrinology. 1992;131:2331–2336. [DOI] [PubMed] [Google Scholar]
- 15. Power SG, Bocchinfuso WP, Pallesen M, Warmels-Rodenhiser S, Van Baelen H, Hammond GL. Molecular analyses of a human sex hormone-binding globulin variant: evidence for an additional carbohydrate chain. J Clin Endocrinol Metab. 1992;75:1066–1070. [DOI] [PubMed] [Google Scholar]
- 16. Cousin P, Déchaud H, Grenot C, Lejeune H, Pugeat M. Human variant sex hormone-binding globulin (SHBG) with an additional carbohydrate chain has a reduced clearance rate in rabbit. J Clin Endocrinol Metab. 1998;83:235–240. [DOI] [PubMed] [Google Scholar]
- 17. Costantino L, Catalano MG, Frairia R, et al. . Molecular mechanisms of the D327N SHBG protective role on breast cancer development after estrogen exposure. Breast Cancer Res Treat. 2009;114:449–456. [DOI] [PubMed] [Google Scholar]
- 18. Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. [DOI] [PubMed] [Google Scholar]
- 19. Ding EL, Song Y, Manson JE, et al. . Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohlsson C, Wallaschofski H, Lunetta KL, et al. . Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7:e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hogeveen KN, Cousin P, Pugeat M, Dewailly D, Soudan B, Hammond GL. Human sex hormone-binding globulin variants associated with hyperandrogenism and ovarian dysfunction. J Clin Invest. 2002;109:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis JG, Longley NJ, Elder PA. Monoclonal antibodies to human sex hormone-binding globulin (SHBG): characterization and use in a simple enzyme-linked immunosorbent assay (ELISA) of SHBG in plasma. Steroids. 1999;64:259–265. [DOI] [PubMed] [Google Scholar]
- 23. Hammond GL, Robinson PA. Characterization of a monoclonal antibody to human sex hormone binding globulin. FEBS Lett. 1984;168:307–312. [DOI] [PubMed] [Google Scholar]
- 24. Hammond GL, Langley MS, Robinson PA. A liquid-phase immunoradiometric assay (IRMA) for human sex hormone binding globulin (SHBG). J Steroid Biochem. 1985;23:451–460. [DOI] [PubMed] [Google Scholar]
- 25. Bocchinfuso WP, Hammond GL. Steroid-binding and dimerization domains of human sex hormone-binding globulin partially overlap: steroids and Ca2+ stabilize dimer formation. Biochemistry. 1994;33:10622–10629. [DOI] [PubMed] [Google Scholar]
- 26. Niemi S, Mäentausta O, Bolton NJ, Hammond GL. Time-resolved immunofluorometric assay of sex-hormone binding globulin. Clin Chem. 1988;34:63–66. [PubMed] [Google Scholar]
- 27. Hammond GL, Lähteenmäki PL. A versatile method for the determination of serum cortisol binding globulin and sex hormone binding globulin binding capacities. Clin Chim Acta. 1983;132:101–110. [DOI] [PubMed] [Google Scholar]
- 28. Rosner W, Toppel S, Smith RN. Testosterone-estradiol-binding globulin of human plasma: denaturation and protection. Biochim Biophys Acta. 1974;351:92–98. [DOI] [PubMed] [Google Scholar]
- 29. Sumer-Bayraktar Z, Nguyen-Khuong T, Jayo R, et al. . Micro- and macroheterogeneity of N-glycosylation yields size and charge isoforms of human sex hormone binding globulin circulating in serum. Proteomics. 2012;12:3315–3327. [DOI] [PubMed] [Google Scholar]
- 30. Bocchinfuso WP, Warmels-Rodenhiser S, Hammond GL. Structure/function analyses of human sex hormone-binding globulin by site-directed mutagenesis. FEBS Lett. 1992;301:227–230. [DOI] [PubMed] [Google Scholar]
- 31. Avvakumov GV, Matveentseva IV, Akhrem LV, Strel'chyonok OA, Akhrem AA. Study of the carbohydrate moiety of human serum sex hormone-binding globulin. Biochim Biophys Acta. 1983;760:104–110. [DOI] [PubMed] [Google Scholar]
- 32. Hogeveen KN, Talikka M, Hammond GL. Human sex hormone-binding globulin promoter activity is influenced by a (TAAAA)n repeat element within an Alu sequence. J Biol Chem. 2001;276:36383–36390. [DOI] [PubMed] [Google Scholar]
- 33. Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol Cell Endocrinol. 2010;316:60–65. [DOI] [PubMed] [Google Scholar]
- 34. Fillmore CM, Fears TR, Hoover RN, et al. . Methodological note: Biomarkers (sex-hormone binding globulin (SHBG), bioavailable oestradiol, and bioavailable testosterone) and processing of blood samples in epidemiological studies. Biomarkers. 2000;5:395–398. [DOI] [PubMed] [Google Scholar]
- 35. Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. [DOI] [PubMed] [Google Scholar]
- 36. Nilsson IM, von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J Biol Chem. 1993;268:5798–5801. [PubMed] [Google Scholar]
- 37. Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002:310–322. [PubMed] [Google Scholar]
- 38. Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 39. Hoppé E, Bouvard B, Royer M, Audran M, Legrand E. Sex hormone-binding globulin in osteoporosis. Joint Bone Spine. 2010;77:306–312. [DOI] [PubMed] [Google Scholar]
- 40. Le TN, Nestler JE, Strauss JF 3rd, Wickham EP 3rd. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]