Abstract
Osteocytes are derived from osteoblast lineage cells that become progressively embedded in mineralized bone. Development of the osteocytogenic cell line IDG-SW3 has enabled a temporal and mechanistic investigation of this process. Through RNA-sequencing analyses, we show that although substantial changes in gene expression occur during the osteoblast to osteocyte transition, the majority of the transcriptome remains qualitatively osteoblast like. Genes either up-regulated or expressed uniquely in the osteocyte include local and systemic factors such as Sost and Fgf23 as well as genes implicated in neuronal, muscle, vascular, or regulatory function. As assessed by chromatin immunoprecipitation coupled to high-throughput sequencing, numerous changes in epigenetic histone modifications also occur during osteocytogenesis; these are largely qualitative rather than quantitative. Specific epigenetic changes correlate with altered gene expression patterns that are observed during the transition. These genomic changes likely influence the highly restricted transcriptomic response to 1,25(OH)2D3 that occurs during differentiation. VDR binding in osteocytes revealed an extensive cistrome co-occupied by retinoid X receptor and located predominantly at sites distal to regulated genes. Although sites of VDR binding were apparent near many 1,25(OH)2D3-regulated genes, the expression of others adjacent to VDR-binding sites were unaffected; lack of VDR binding was particularly prevalent at down-regulated genes. Interestingly, 1,25(OH)2D3 was found to induce the Boc and Cdon coreceptors that are active in hedgehog signaling in osteocytes. We conclude that osteocytogenesis is accompanied by changes in gene expression that may be driven by both genetic and epigenetic components. These changes are likely responsible for the osteocyte phenotype and may contribute to reduced sensitivity to 1,25(OH)2D3.
Osteocytes represent one of the most abundant of the primary cell types that make up the metabolically active tissue component of the vertebrate skeleton and are derived from an osteoblast subpopulation that becomes fully embedded in matrix (1–4). These cells are morphologically, functionally, and genetically unique, due in part to the underlying expression of selective gene subsets that characterize the osteocyte phenotype. Gene products include dentin matrix protein 1 (DMP1) podoplanin (E11), matrix extracellular phosphoglycoprotein (MEPE), and phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) (5, 6), as well as sclerostin and fibroblast growth factor 23 (FGF23) that represent key markers of differentiated osteocytes (6–8). Although additional osteocyte-specific gene products have been identified, the vast majority of the genes that contribute to the unique function of the osteocyte remain to be identified (5, 9, 10).
Despite emerging insight into osteocyte function and identification of many of the genes that are associated with this cell's activities, little is known of the cell-autonomous genetic and epigenetic events that underlie the genomic transition from the osteoblast to the terminally differentiated osteocyte or of the internal and external signals that control these processes. For example, although runt-related transcription factor 2 (RUNX2) (11, 12), osterix (OSX) (10), and activating transcription factor 4 (13) are central to osteoblast differentiation, their roles, as well as those of many other transcription factors expressed in osteocytes during differentiation, remain unclear. Similarly, the contribution of many of the signal transduction pathways that are active in the osteoblast are poorly defined in the osteocyte (2). Exceptions include the Wnt/β-catenin pathway and its role in mechanotransduction (2), the unique actions of the Notch pathway in promoting bone formation (14), and the role of PTH in the regulation of bone remodeling (15). In addition, although chromatin-regulatory proteins such as histone deacetylase (HDAC)7 (16) and NO66 (17) are involved in osteoblast differentiation, virtually nothing is known of their role(s) or that of other epigenetic modifiers in osteocyte differentiation and/or function.
Like osteoblasts, osteocyte activity is regulated by a number of systemic factors that include not only PTH, but also 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (18). The role of PTH in osteocytes has emerged as a result of recent cellular and genetic-based analyses (19–22), which suggest that this hormone acts largely to regulate bone remodeling. 1,25(OH)2D3 is also involved in bone remodeling, although its actions include the global regulation of mineral homeostatic activities at the level of the intestine (23) and kidney (24–26), as well as in osteoblasts (27) and osteocytes (28). In these latter bone cell types, 1,25(OH)2D3 induces expression of receptor activator of NF-κB ligand (RANKL), a local factor that acts in a paracrine fashion to stimulate the formation and activity of bone-resorbing osteoclasts (29–31). 1,25(OH)2D3 also induces the expression of mineralization inhibitors such as osteopontin (SPP1/OPN) (32), ankyrin (ANK), ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) and ENPP3 during periods of dietary mineral deficiency to maintain extracellular levels of both calcium and phosphorus (28). Finally, 1,25(OH)2D3 also facilitates the expression of cystathionine β synthase, which functions to curb the deleterious effects of homocysteine on skeletal integrity and strength (33). At each of these genes, the molecular actions of 1,25(OH)2D3 are mediated through its capacity to stimulate the nuclear vitamin D receptor (VDR), which interacts at multiple sites in the Tnfsf11 (the gene that encodes the RANKL protein) locus (29, 34–37) and at novel enhancers near the Spp1, Ank, Enpp1, Enpp3 (28), and Cbs (33) genes. In the case of Tnfsf11, the function of at least one of these enhancers has been confirmed in vivo (38). Finally, 1,25(OH)2D3 is also a primary regulator of Fgf23 expression, although the cis-regulatory mechanism involved in this modulation has not yet been identified (39). Selective deletion of the VDR in osteocytes of mice results in loss of these regulatory capabilities, thereby proving that the osteocyte is a unique target of 1,25(OH)2D3 action in vivo (28).
In the present study, we explore both genetic and epigenetic components of osteocyte differentiation using the mouse IDG-SW3 osteocyte cell line that, upon osteogenic stimulation, undergoes a temporal dependent process that replicates the osteoblast to osteocyte transition (6). We show using RNA-sequencing (RNA-seq) analysis that a striking change in gene expression occurs during osteocyte differentiation period and that these changes are accompanied by significant epigenetic modifications to histones H3 and H4. These changes alter transcriptomic response to 1,25(OH)2D3, likely through modification to the VDR cistrome.
Materials and Methods
Reagents
1,25(OH)2D3 was obtained from SAFC Global. Antibodies to VDR (C-20, sc-1008) and RXR (ΔN-197, sc-774) were purchased from Santa Cruz Biotechnology, Inc. Antibodies against the following histone modifications were purchased from Abcam: H3K4me1 (ab8895), H3K4me3 (ab1012), H3K9me3 (ab8898), H3K36me3 (ab9050), H4K20me1 (ab9051), and H3K27ac (ab4729). Antibodies against the following histone modifications were purchased from Millipore Corp: H4K5ac (07–327), H3K9ac (06–942), and H3K4me2 (07–030). All quantitative real-time PCR reagents (Fast Start SYBR Green Master Mix [with Rox]) were obtained from Roche and TaqMan gene expression assays from Life Technologies (Applied Biosystems [ABI]). All quantitative real-time PCR was conducted on a StepOnePlus from ABI. Primers for chromatin immunoprecipitation (ChIP) assays and recombineering were obtained from Integrated DNA Technologies, Inc and TaqMan primers for gene expression were obtained from Life Technologies (ABI). Sequencing reagents for ChIP-seq (no 11257047 RevA) were obtained from Illumina. Recombinant human/mouse Indian Hedgehog/Ihh (C28II) N-term was purchased from R&D Systems.
IDG-SW3 cell culture
Custom αMEM-powdered tissue culture medium was purchased from Cellgro and supplemented with 2.20g/L NaHCO3 (S233–3) (Fisher Scientific). Fetal Bovine Serum (FBS) (SH30396) was purchased from HyClone and heat inactivated by incubation at 55°C for 30 minutes. Penicillin-streptomycin solution was purchased from Fisher Scientific, and recombinant mouse interferon-γ (IFN-γ) from Gibco/Invitrogen. Collagen I-coated plates (10 cm and 6 well) were purchased from BD Biosciences. IDG-SW3 cells (6) were expanded in 10-cm collagen-coated plates at 33°C in αMEM with 10% heat-inactivated FBS, 100 U/mL penicillin/streptomycin, and 50 U/mL IFNγ. Cells were harvested with 0.05% Trypsin/0.53 mM EDTA (Fisher Scientific) and plated in 6-well collagen-coated plates at 40 000 cells/cm2. To induce osteocytogenesis, 2 days after plating, cells were transferred to 37°C and media was changed to osteogenic media (αMEM with 10% heat-inactivated FBS, 100 U/mL penicillin/streptomycin, 50 μg/mL ascorbic acid [A4034, Sigma] and 4 mM β-glycerophosphate [G9422, Sigma]) without IFNγ. Osteogenic media was changed 3 times weekly.
Visualization of differentiation
Alizarin red staining was performed as previously described (40). Briefly, cells were washed twice with 1× PBS and fixed with 10% formaldehyde (BP531, Fisher Scientific) in 1× PBS for 20 minutes. Cells were washed twice with ddH2O and then stained with Alizarin red solution (pH 4.1) (A5533, Sigma) for 20 minutes. After staining, cells were immediately rinsed with tap water and air dried. All images for green fluorescent protein fluorescence and bright-field microscopy were taken using an ECLIPSE Ti-S microscope (Nikon) and a QICAM 12-bit Mono Fast 1394 Cooled camera (QImaging).
Bacterial artificial chromosome (BAC) clone reporters
Large-scale mouse Cdon (mCdon) or Boc (mBoc) luciferase reporter constructs were generated via recombinogenic targeting (41, 42). BAC clones RP24-151E17 (Cdon) and RP11–116G20 (Boc) were obtained from BACPAC Resource Center and introduced into Escherichia coli strain SW106 by electroporation. The integrity of the DNA was verified by restriction enzyme digestion and pulse-field gel electrophoresis. Prior to reporter insertion, an internal NotI site was removed from the BAC using galactokinase (galk) positive/negative selection (41). The targeting constructs used to prepare the reporter BAC were generated by PCR amplification of a 2A peptide (43) (P2A)-luciferase-Tk-neomycin cassette (P2ALTN) from an in-house engineered plasmid (pP2ALTN) using primers that contain 50 bp of homology to the 3′-untranslated region and 20 bp of homology to the end of the cassette. The amplified DNA was introduced into SW106 cells harboring the unmodified BAC clone. Enhancer deletion BACs were generated sequentially using galk recombineering as done for NotI removal, targeting 500–1500 bp around each ChIP peak as indicated in the text. For the mCdon BAC clone, the +15kb deletion removed 1168 bp of DNA corresponding to chromosome (chr) 9: 35 274 753–35 275 920 and the −85kb region deletion removed 616 bp of DNA corresponding to chr9: 35 173 207–35 173 822. For the mBoc BAC clone the +3kb deletion removed 1368 bp of DNA corresponding to chr16: 44 555 229–44 556 596, the +20kb region deletion removed 1313 bp of DNA corresponding to chr16: 44 538 458–4 539 770, and the +63kb region deletion removed 895 bp of DNA corresponding to chr16: 44 494 367–44 495 261. Primer sequences are available upon request.
Stable cell line preparation
MC3T3-E1 cells were seeded into 6-well plates at a concentrations of 150 000 cells per well in αMEM containing 10% FBS. Cells were transfected 24 hours later with 1–4 μg of NotI linearized BAC luciferase reporter vector in serum and antibiotic-free medium using Lipofectamine LTX (Invitrogen) (44). After transfection, the cells were cultured in medium supplemented with 10% heat inactivated FBS for 24 hours. Cells were then collected by trypsinization, replated into two 10-cm dishes, and subjected to positive selection 24 hours later using G418 (200 μg/mL). Colonies emerging after approximately 14 days were harvested, pooled, and examined. MC3T3-E1 mCdon and mBoc BAC stable cell lines were cultured in αMEM with 10% heat-inactivated FBS and 100 U/mL penicillin/streptomycin at 37°C.
Gibson assembly of luciferase reporter plasmids
Gibson Isothermal Assembly was performed as described by Gibson et al (45). The cloned fragment/region of interest and pTK vector were amplified by PFU UltraII enzyme (Stratagene). PCR fragments were digested with DpnI (New England Biolabs) and subsequently purified with the DNA Clean and Concentrator Kit (Zymo Research) according to manufacturer's instructions with a 12 μL water final elution volume. For the Gibson reaction, 7.5 μL of Gibson master mix (45), 1.5 μL purified insert, and 1 μL of purified vector were combined and incubated at 50°C for 1 hour. Gibson reaction (3 μL) was transformed into chemically competent DH5α cells by heat shock. Resulting clones were sequenced for correct assembly. Mutagenesis was also performed by Gibson assembly using primers to introduce mutated sequences. Primer sequences are available upon request.
Transient transfection luciferase reporter assay
MC3T3-E1 cells were seeded into 24-well plates in α-MEM containing 10% FBS at a concentration of 5.0 × 104 cells per well and transfected the next day with Lipofectamine PLUS (Invitrogen) in serum and antibiotic-free medium. Individual wells were cotransfected with 250 ng of a luciferase reporter vector and 50 ng of pCH110-βgal (46). After transfection, the cells were cultured in medium supplemented with 20% FBS with or without 100 nM 1,25(OH)2D3, administered in ethanol (0.1% final volume). Cells were harvested 24 hours after treatment and the lysates assayed for luciferase and β-galactosidase activities as previously described (46). Luciferase activity was normalized to β-galactosidase activity in all cases.
Indian Hedgehog (IHH) Treatments
IDG-SW3 cells were differentiated for 3 or 35 days and treated 24 hours prior with 3 μg/mL IHH (R&D Systems) in 1× PBS in combination with 100 nM 1,25(OH)2D3 or ethanol vehicle (0.1% final volume) before RNA was isolated using the TRI-Reagent protocol (Molecular Research Center). RNA (1 μg) was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Life Technologies, ABI) and analyzed with TaqMan Real Time PCR, as described above. TaqMan Probes used are available in Supplemental Table 1.
Western blot analyses
Cells were differentiated for 3 or 35 days as described above. Prior to collection, the cells were treated with vehicle or 100 nM 1,25(OH)2D3 for 24 hours. Cells were washed with 1× PBS, total protein lysates were extracted with radioimmune precipitation assay buffer supplemented with proteinase inhibitor cocktail (Roche), and cell lysate was scraped, collected, and stored at −80°C. The differentiated samples were subjected to 3 × 15 second pulses with a Polytron Homogenizer (Power Gen 125; Fisher Scientific). Protein concentrations were measured using Protein Assay (Bio-Rad Laboratories). Sample (20 μg) was heated to 95°C for 5 minutes and loaded on Tris-Glycine 4%–20% gels (Invitrogen). Blots were transferred to polyvinylidene difluoride membrane and blocked with 5% milk in PBS with 1% Tween 20 (PBST) overnight at 4°C. Primary antibodies for sclerostin (AF1589; R&D Systems; 1:100), VDR (9A7; 1:2000), or β-tubulin (H-235; Santa Cruz Biotechnology; 1:5000) were applied in 1% milk PBST at room temperature (RT) for 1 hour. Blots were washed 3 × 15 minutes with PBST. Secondary antibodies were goat α-rabbit IgG-HRP (Calbiochem; 1:2000), goat α-rat IgG-HRP (Santa Cruz; 1:2000), and goat α-rabbit IgG-horseradish peroxidase (HRP) (Santa Cruz; 1:5000) for sclerostin, VDR, and β-tubulin, respectively, in 1% milk PBST at RT for 1 hour. Polyvinylidene difluoride membranes were stripped 3 times (50 mM glycine, pH 2.5; 1% SDS) at RT and washed 3 times with PBST prior to β-tubulin western analysis. Detection was completed with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) on an ImageQuant LAS-4000 (GE HealthCare).
Animal studies
All animal studies were reviewed and approved by Research Animal Care and Use Committee of University of Wisconsin-Madison. Mice were exposed to a 12-hour light, 12-hour dark cycle. C57BL/6 mice, obtained from Harlan, were fed a standard rodent chow diet (catalog no. 5008). Treatments with 1,25(OH)2D3 (10 ng/g body weight) were performed by ip injection using propylene glycol as a delivery vehicle. Tissues for RNA preparation were collected 6 hours after 1,25(OH)2D3 injections.
RNA-seq library preparation for differentiation experiment
IDG-SW3 cells were differentiated for 3, 7, 14, 21, 28, or 35 days, and RNA was isolated using the TRI-Regent protocol (MRC) with an additional LiCl extraction. Total RNA (8 μg) was DNase1 treated (Invitrogen) after which rRNA was depleted using the RiboMinus Eukaryote Kit (Invitrogen) according to manufacturer's instructions. Directional RNA-seq libraries were prepared with Illumina Small RNA Sample Prep Kit and TruSeq RNA Sample Preparation Kits v2 according to the directional mRNA-seq Sample Preparation protocol (Illumina) with the following modification: the library was purified using 2 rounds of purification with AMPure Beads in 1.5-mL tubes. rRNA depletion (RNA 6000; Nano Kit) and final library quality (High Sensitivity DNA kit) were analyzed with the Agilent Bioanalyzer (Agilent Technologies). For TaqMan gene expression analysis, 1 μg of RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Life Technologies) and analyzed with TaqMan Real Time PCR, as described above. TaqMan probes used are listed in Supplemental Table 1.
RNA-seq library preparation for vitamin D hormone experiment
IDG-SW3 cells were differentiated for 3 or 35 days and treated 24 hours prior with 100 nM 1,25(OH)2D3 or ethanol vehicle before RNA was isolated using the TRI-Reagent protocol (MRC) with an additional LiCl extraction. Maximum response to 1,25(OH)2D3 was achieved at this time point. Total RNA (8 μg) was DNase1 treated (Invitrogen) and then ribosomally depleted using the RiboMinus Eukaryote Kit (Invitrogen) according to manufacturer's instructions. Directional RNA-seq libraries were prepared with the Epicenter ScriptSeq v2 RNA-seq Library Preparation Kit as per manufacturer's instructions. rRNA depletion (RNA 6000 Nano Kit) and final library quality (High Sensitivity DNA kit) were analyzed with the Agilent Bioanalyzer (Agilent Technologies).
Bioinformatic and statistical analyses for RNA-seq
Directional RNA-seq libraries were sequenced for 75- or 100-bp single-read and performed on an Illumina HiSeq2000 using the Illumina Sequencing kit (ver. 3) by the University of Wisconsin-Madison DNA Sequencing Facility in the University of Wisconsin-Madison Biotechnology Center. Fluorescent images were analyzed using the CASAVA 1.8.2 (Illumina) to obtain FASTQ formatted sequence data. Resulting FASTQ data files were processed with TopHat (47) using default settings and with the sample type of “fr-secondstrand” for directional libraries. Gene Expression data were further analyzed with ArrayStar (DNASTAR) Software. DAVID Bioinformatics Resources 6.7 (NIAID, NIH) was used for Gene Ontology (GO) analyses (48, 49). Clustering analyses were carried out in R (R Statistical Software) and data were normalized using the approach developed by Ander and Huber (50). The empirical Bayes hierarchical modeling approach EBSeq-HMM was used to identify genes differentially expressed over time. EBSeq-HMM extends EBSeq (51), which was developed to identify differentially expressed genes across 2 or more conditions. In EBSeq-HMM, a hidden Markov model is implemented to accommodate dependence in gene expression over time. EBSeq-HMM provides posterior probabilities for each gene and each possible expression path. The approach model counts at a given transition (t, t+1) as arising from negative binomial distribution with latent mean levels of expression. At each transition, 2 patterns of expression are possible: up-regulated and down-regulated. For each transition, the marginal distribution is modeled as a mixture over these 2 expression patterns. The full set of RNA-Seq data is used to estimate the model's parameters, including the estimated mixing proportion of each pattern. The fitted model is then used to derive a probability distribution for every gene. Specifically, each gene-specific distribution gives the posterior probability of that gene's pattern. When applied to the time course data, a total of 32 possible patterns were considered along 5 transitions. The target fold change for up- or down-regulated transitions was set to 3, which assumes that the up (down)-regulated transitions are expected to be with fold change around or greater than 3 (around or less than 1/3). To study patterns with less than 2 transitions, we considered genes whose posterior probability of being a certain pattern was greater than 0.5. To study the “off” related patterns, we considered genes whose posterior probabilities of being certain pattern at non-off transitions was greater than 0.5. We further filtered the lists and took genes whose mean expression at off/non-off time points was less/greater than 1.
ChIP-seq analyses
Chromatin immunoprecipitation was performed as described previously (46). Briefly, samples were subjected to immunoprecipitation using either a control IgG antibody or the indicated experimental antibody (VDR, RXR, H3K4me1, H3K4me2, H3K4me3, H3K27ac, H3K5ac, H3K9ac, H4K20me1, H3K36me3, or H3K9me3). To remove the calcified matrix from the differentiated cells, the cells were subjected to three 15-minute 300 mM EDTA washes following fixation. The remainder of ChIP and chromatin immunoprecipitation coupled to high-throughput sequencing (ChIP-seq) methodology, including statistical information and data processing, was performed as recently reported (40).
Data access
All data are deposited in the Gene Expression Omnibus (GSE54784).
Results
The osteoblast to osteocyte transition in IDG-SW3 cells is accompanied by global changes in the transcriptome
IDG-SW3 cells recapitulate the osteoblast to mature osteocyte differentiation transition over a 35 day period in vitro, as previously reported (6) and confirmed in the experiments documented in Supplemental Figure 1. This progression can be tracked morphologically and through fluorescence generated by green fluorescent protein, a reporter expressed under the temporal control of the osteocyte-specific Dmp1 enhancer region. It can also be tracked through the temporally selective expression of several endogenous genes expressed by osteocytes including Dmp1, E11, Mepe, Phex, Sost, and Fgf23. Supported by these findings, we hypothesized that a more broad-based set of changes in gene expression were likely to accompany the osteoblast to osteocyte transition as well. To explore this concept, we isolated RNA from differentiating IDG-SW3 cells periodically beginning on day 3 (when T-antigen protein levels have declined to undetectable levels) and ending on day 35 and subjected these samples to global RNA sequencing analysis. The RNA sequencing profiles for several specific gene examples can be found in Supplemental Figure 2. The expressed genes were clustered using the Euclidean distance metric and visualized with the heat map documented in Figure 1A; a clustering analysis is illustrated in Figure 1B with complete gene lists documented in Supplemental Table 1. Whereas multiple patterns of gene expression were observed in this analysis, a number of clusters containing the targeted genes described in Supplemental Figure 1 were identified, suggesting that these clusters, and the genes within, likely reflect patterns selective for osteoblasts, early osteocytes, and mature osteocytes. Sost, for example, is associated with genes in cluster 3 expressed specifically in mature osteocytes.
Figure 1.
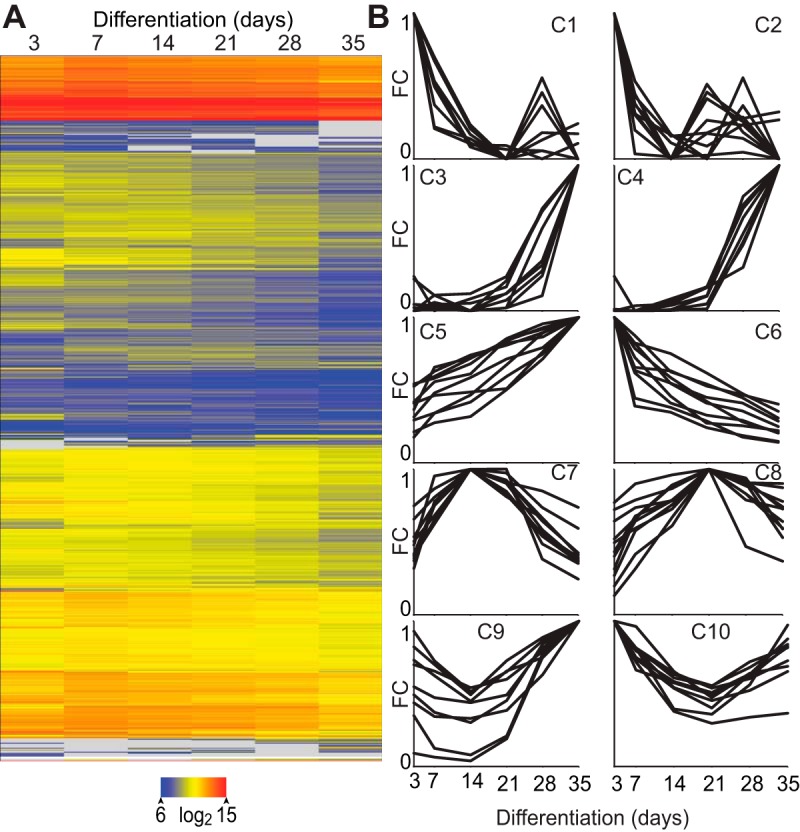
The osteoblast to osteocyte transition is accompanied by global changes in the transcriptome. A, Heat map of gene expression during differentiation. B, Example profiles for 10 clusters of genes with similar gene expression patterns over differentiation plotted as fold change (FC) against indicated time point (FC = 1).
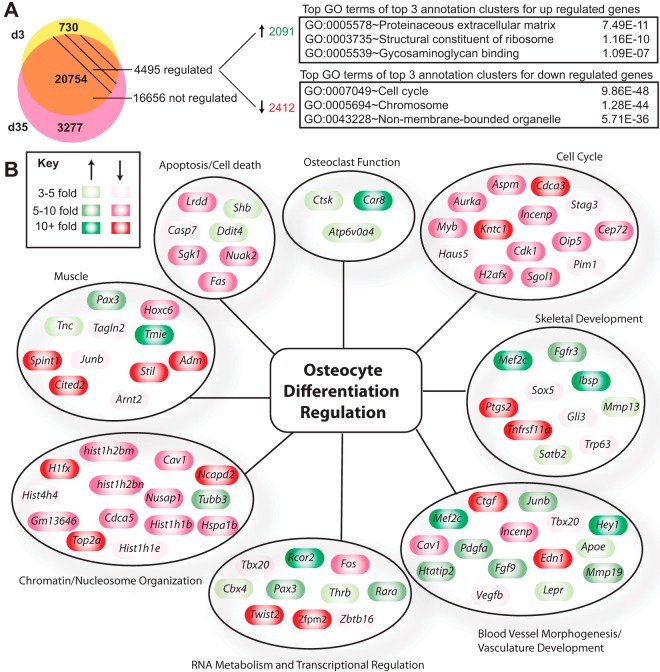
To explore differences in gene expression between osteoblasts and osteocytes in more detail, we contrasted in a separate RNA-seq analysis IDG-SW3 transcriptomes identified on day 3 and day 35 in the form of a Venn diagram (Figure 2A). Whereas 730 and 3277 genes were found to be unique to day 3 and day 35, respectively, 20 754 genes were found to be common to cells of both stages of differentiation (Figure 2A). Of these, however, the expression of 16 656 were unchanged while 4495 were either significantly up (2091) or down-regulated (2412) more than 2-fold (95% confidence; moderated t test). These data suggest a strong lineage relationship between osteoblasts and osteocytes coincident with the emergence of a phenotype unique to the osteocyte, due both to an up-regulation of genes specific to the osteocyte and also to a suppression of genes inherent to the osteoblast precursor. GO term analysis of genes up- and down-regulated during differentiation revealed a number of functional categories, many associated with skeletal biology and function (see Figure 2A and Supplemental Table 1). A further evaluation of functional categories and identification of subsets of genes within these categories is documented in Figure 2B. These genes include candidates associated with cellular function such as cell cycle, apoptosis, RNA metabolism and transcriptional regulation, chromatin/nucleosome organization, cell adhesion, and skeletal development and ossification. An up-regulation of genes involved in osteolytic function and reminiscent of the osteoclast can also be seen following differentiation, including carbonic anhydrase (Ca) genes such as Ca8 as well as Ctsk (cathepsin K), Mmp13 (matrix metalloprotein 13), and Atp6v0a4 (component of the hydrogen ATPase pump) (Figure 2B). Osteoclastic factors are known to be up-regulated in bone during lactation in mice (53). Interestingly, gene categories involved in muscle function, blood vessel development, and vasculature function are also observed. We also note diverse regulation of gene networks encoding DNA-binding proteins such as Mef2c, Creb3l3, Hey 1, and Egr2 (Supplemental Table 2); chromatin regulators such as Rcor2, and Hdac11 (Supplemental Table 1); and components of signaling pathways for Wnt/β-catenin (Wif1, Mdfi, Dkk1, Cthrc1, Sost); Indian hedgehog (Ihh); and Notch (Jag2, Dtx4) pathways (Supplemental Table 3). The results of this global analysis suggest that although osteocytes clearly retain bulk features of their osteoblast-lineage precursors, they are also functionally distinct as identified through the lens of gene expression.
Figure 2.
The osteoblast to osteocyte transition is accompanied by global changes in the transcriptome. A, Venn diagram of genes expressed in the osteoblast (d3) only (yellow) and osteocyte (d35) only (pink) stage. Genes expressed at both time points (orange) are classified as regulated if their gene expression levels are changed by 2-fold or more during differentiation (d35 vs d3) with 95% confidence by a moderated t test. The top GO term from the top 3 annotation clusters from DAVID are shown with associated P values. B, Regulated genes from panel A were manually grouped by category and classified according to their degree of regulation (green, up-regulated; red, down-regulated). d3, day 3; d35, day 35.
Osteoblasts are epigenetically engaged to become osteocytes
Although the selective and combinatorial actions of multiple transcription factor classes are central to transcriptomic changes that occur during cellular differentiation, changes in some of these classes of genes suggest potential modulation of epigenetic modifications associated with the genome as well. This highly dynamic, changeable, and frequently inheritable layer of chemical changes that occur either directly to DNA or to histones provides, on a genome-wide scale, key structural, functional, and regulatory information at individual genetic loci. To explore the role of these epigenetic features on osteocytogenesis, we conducted an extensive ChIP-seq analysis of the distribution of several key histone marks across the genome in IDG-SW3 cells at day 3 and day 35. These include histone 3 lysine (H3K) 36 trimethylation (H3K36me3), histone 4 lysine (H4K) 20 monomethylation (H4K20me1), H3K4 mono-, di-, and trimethylation (H3K4me1, H3K4me2, and H3K4me3), H3K9 acetylation (H3K9ac), H3K27 acetylation (H3K27ac), H4K5 acetylation (H4K5ac), and H3K9 trimethylation (H3K9me3). The gene body is defined by H3K4me3, which marks active transcriptional start sites, and H4K20me1 and H3K36me3, which mark elongation and peak at the 5′- or 3′-end of the gene, respectively (54–56). H3K4me1, in particular, is associated with enhancers whereas H4K5ac, H3K9ac, H3K27ac, and H3K4me2 are more generally associated with open chromatin (55–57). H3K9me3, in contrast, is associated with regions of less active, condensed chromatin (57). The latter 2 categories are of special interest, because the identification of sites containing these marks are particularly informative with regard to regulatory mechanism. GREAT analysis (58) assigned the histone peaks to genes they are proximal to and associated with. Full data sets for all histones, as well as the subsets up- and down-regulated during differentiation (Figure 2), can be found in Supplemental Table 4. As documented in Supplemental Figure 3, analysis of several individual histone marks via the cis-regulatory element annotation system (CEAS) confirmed enrichment at conceptual sites across annotated gene loci as was previously described (56). The CEAS analysis, as depicted in Supplemental Figure 4, also confirmed that the density of several of these epigenetic marks was correlated with the overall level of expression of individual genes. Based upon these findings, we examined 1) whether epigenetic marks were changed quantitatively at individual sites near cohorts of genes (4495 genes) the expression levels of which were altered during the osteoblast to osteocyte transition (but not unique to either cell state); and 2) whether novel histone marks were qualitatively created or dismissed. In the first instance, we clustered sets of genes that were common to both osteoblasts and osteocytes but either up- or down-regulated more than 2-fold (95% confidence limits; moderated t test) following differentiation, respectively. As seen in Figure 3A, basal histone marks associated with elongation (H4K20me1 and H3K36me3), gene enhancer activity (H3K4me1 and H34K27ac), and chromatin condensation (H3K9ac and H4K5ac) were elevated in the gene subset that was up-regulated and decreased in the gene subset that was down-regulated during differentiation, suggesting broad quantitative changes. A search for newly created sites of histone modification or for sites where existing modifications were removed, however this revealed only a limited number of examples, 2 of which (Sp7 and Pth1r gene loci) are depicted in the data tracks seen in Figure 3, B and C. These analyses suggest that the vast majority of epigenetic changes that are measured here and occur during the osteoblast to osteocyte transition are quantitative (increased or decreased) rather than qualitative (gained or lost) and that they are likely established early in the osteoblast lineage.
Figure 3.
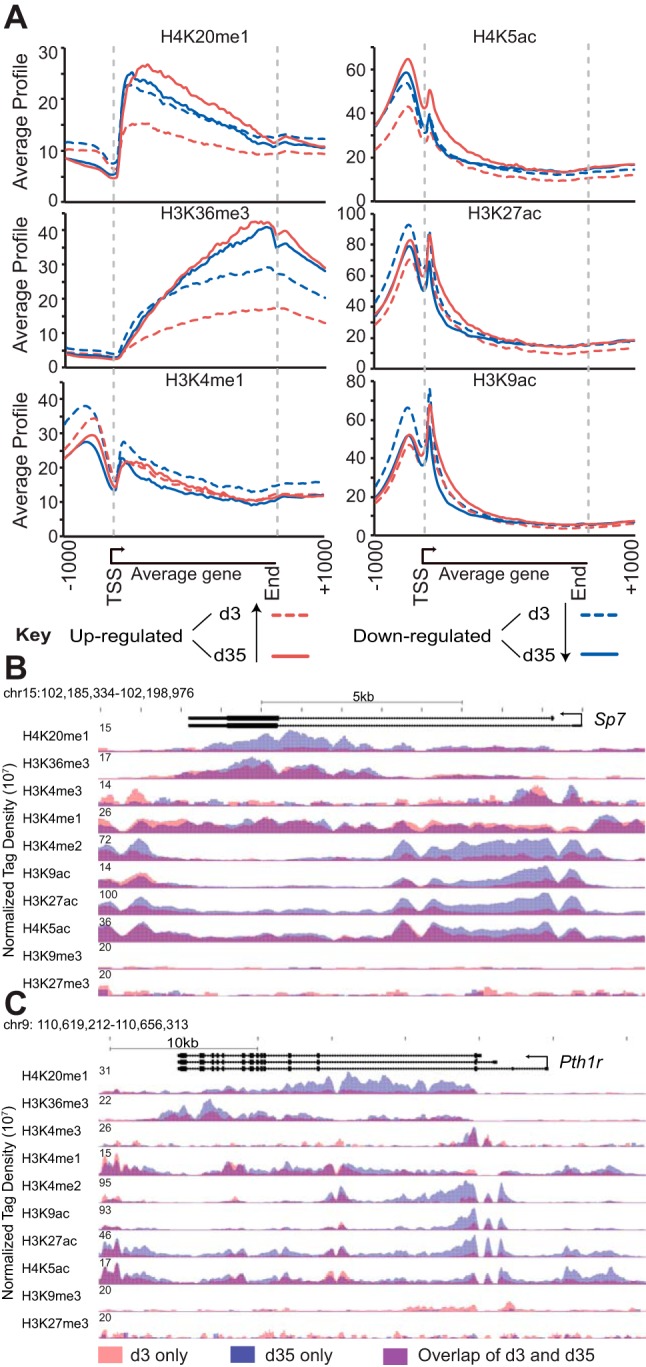
Osteoblasts are epigenetically engaged to become osteocytes. A, CEAS analysis of ChIP-seq histone levels in osteoblasts (d3, dashed lines) and osteocytes (d35, solid lines) for normalized gene profiles of genes up-regulated (red, ≥ 2 fold) or down-regulated (blue, ≤2-fold) during differentiation. Gene bodies were averaged to a 3-kb segment and displayed as an average gene. B, Overlaid ChIP-seq tag density profiles for histones around Sp7 and Pth1r (d3, red; d35, blue; overlap, purple), normalized to 107 tags. d3, day 3; d35, day 35; TSS, transcription start site.
Osteocytes display a unique 1,25(OH)2D3-sensitive transcriptome
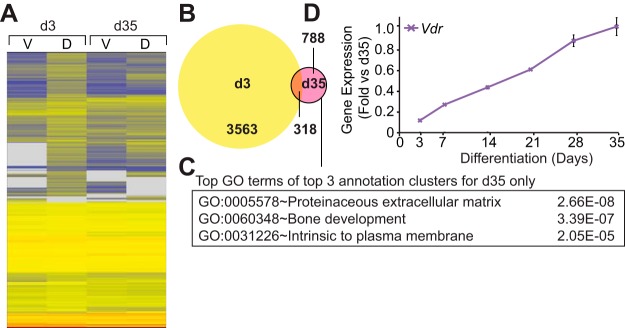
Changes to the transcriptome that occur during the osteoblast to osteocyte transition and the novel expression or reexpression of signaling pathway components, transcription factors, and chromatin regulators, together with associated epigenetic modifications that underlie these transcriptomic changes, are ultimately responsible for altered cell function. These changes may also presage differential response to local paracrine and systemic regulatory factors as well. To examine this phenomenon, we assessed the impact of a 24 hour treatment of 1,25(OH)2D3 on the transcriptomes of both day 3 and day 35 IDG-SW3 cells via RNA-seq analysis. That 1,25(OH)2D3 exerted a long-lasting effect on the phenotype of IDG-SW3 cells was established by showing that chronic treatment with the hormone strongly prevented progressive mineralization beginning on day 3 (Supplemental Figure 5). Genes regulated by 1,25(OH)2D3 ≥ 2 fold (95% confidence; moderated t test) at day 3 and day 35 were clustered using the Euclidean distance metric and visualized using the heat map documented in Figure 4A. As seen in the Venn diagram depicted in Figure 4B, the response to 1,25(OH)2D3 was much more profound on day 3 than on day 35. Accordingly, whereas the 1,25(OH)2D3-regulated transcriptome comprised 3881 genes on day 3, a much smaller and more restricted subset of 1106 genes was regulated by 1,25(OH)2D3 on day 35. Perhaps more surprising, only 318 of these genes were found to be regulated by 1,25(OH)2D3 in both osteoblasts and osteocytes despite the fact that the total number of genes expressed at each of these 2 stages of cell differentiation were similar. GO term analysis of the gene subset at day 35 included the bone development term (Figure 4C). Interestingly, this restriction in response to 1,25(OH)2D3 occurred following a 5-fold increase in VDR mRNA expression, as seen in Figure 4D; VDR protein levels were elevated as well during differentiation (Supplemental Figure 6). These analyses demonstrate that overall response to 1,25(OH)2D3 is different in osteoblasts and osteocytes despite the expression of an overlapping set of common genes.
Figure 4.
Osteocytes display a unique 1,25(OH)2D3-sensitive transcriptome. A, Heat map of regulated genes' expression in osteoblasts (d3) and osteocytes (d35) treated with vehicle (V) or 100 nM 1,25(OH)2D3 (D) for 24 hours. B, Venn diagram of 1,25(OH)2D3-regulated genes in the osteoblast (d3) and osteocyte (d35). C, The top GO term from the top 3 annotation clusters from DAVID are shown with associated P values. D, Gene expression (vehicle only) was normalized to β-actin levels and shown as fold change compared with d35 sample. Samples were analyzed in triplicate ± SEM. d3, day 3; d35, day 35.
The nature of the VDR/RXR cistrome in osteocytes
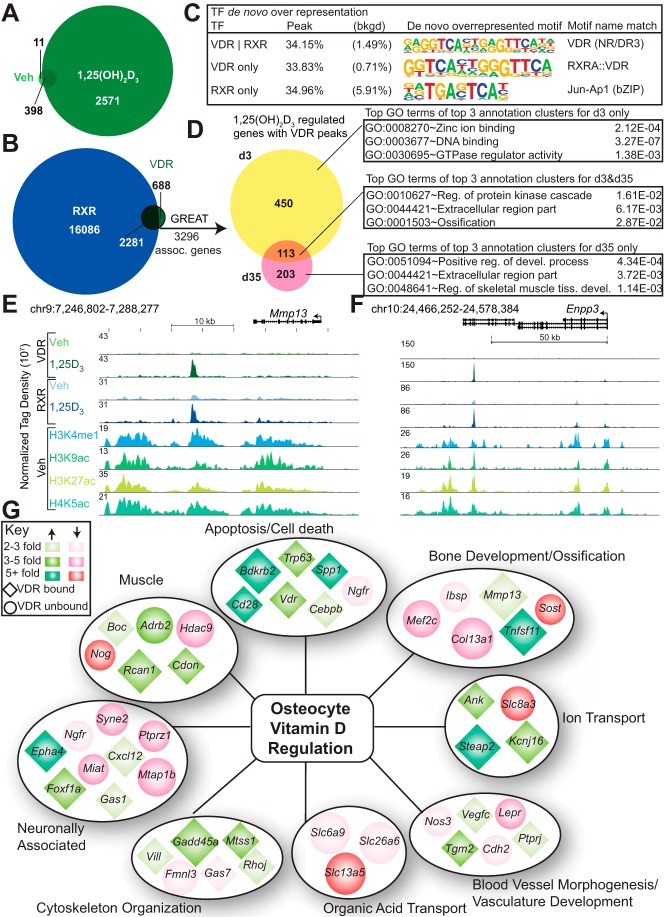
The actions of 1,25(OH)2D3 are mediated by the VDR, a classic secondary signal-dependent transcription factor (59) that binds to DNA following 1,25(OH)2D3 activation, recruits coregulatory complexes that may contain either histone acetyl transferases or HDACs, and modulates gene expression (60). Our previous studies using both ChIP-chip and ChIP-seq analysis indicate that binding of the VDR to DNA, together with the recruitment of cofactors, results in changes in acetylation density at both histones H3 and H4 (36, 44, 61). To further explore the role of the VDR in the osteocyte (day 35), we conducted a ChIP-seq analysis of VDR-binding sites (cistrome) in the absence and presence of 1,25(OH)2D3, quantitated the number of identified VDR-bound sites, and explored the features of this cistrome with respect to RXR binding. We also examined the alignment of these binding sites at regions of H3K4me1, H3K9ac, H4K5ac, and H3K27ac enrichment and searched for the presence of vitamin D-response elements (VDREs) as well. To gain a temporal/historical perspective on VDR binding activity, we also explored whether VDR was bound to sites near genes that were actively regulated by 1,25(OH)2D3 at the osteoblast stage but had become insensitive to the hormone following differentiation. Figure 5A provides a quantitative summary of the VDR cistrome showing that although few binding sites were occupied by the VDR in the absence of hormone, 2969 VDR sites were apparent at the osteocyte genome in the presence of the hormone. ChIP-seq analysis of the RXR cistrome revealed that although the cistrome for this protein was much larger than that of the VDR, RXR co-occupied 77% of the binding sites that were identified for the VDR in the presence of the hormone (Figure 5B). De novo motif analysis (Figure 5C) revealed that a bona fide consensus VDRE sequence was observed at 34% of these heterodimer occupied sites; a similar frequency (33%) was observed at sites that contained only the VDR. Consistent with previous cistromic analyses of the VDR (46, 62–64), the distribution of sites was both promoter proximal as well as promoter distal (introns, intergenic), the latter appearing much more prevalent than the former (data not shown). We then examined whether VDR/RXR-binding sites were located within regions enriched for the enhancer signature marks H3K4me1, H3K9ac, H4K5ac, and H3K27ac. As can be seen in Supplemental Table 5, the overlap between sites of VDR binding and these 4 marks was 93, 66, 89, and 86%, respectively, while much less for histone modifications that did not show enrichment at enhancers. Figure 5, E and F documents the overlap that exists between VDR and these enhancer signature marks specifically at the Mmp13 and Enpp1/3 loci, both of which are up-regulated by the hormone. 1,25(OH)2D3-induced VDR occupancy is generally associated with a concomitant enrichment of several of these histone marks (36, 61), although we did not evaluate that issue in this study. These findings indicate that VDR-binding sites are enriched for classic epigenetic marks of activation.
Figure 5.
The VDR/RXR cistrome in osteocytes is extensive and correlates with the expression of 1,25(OH)2D3-sensitive osteocytic genes. A, Venn diagram of VDR ChIP-seq peaks for osteocytes (d35) treated for 3 hours with vehicle or 100 nM 1,25(OH)2D3. B, Venn diagram of VDR and RXR ChIP-seq peaks in osteocytes (d35) treated for 3 hours with 100 nM 1,25(OH)2D3. C, De novo motif analysis for VDR only, VDR overlapping RXR peaks, and RXR-only peaks. D, The 2969 VDR peaks from panel B were associated with genes using GREAT. These genes were grouped according to their regulation by 1,25(OH)2D3 and the top GO terms from the top 3 annotation clusters from DAVID are shown with associated P values. ChIP-seq tracks in the osteocyte (d35) for the 1,25(OH)2D3-regulated genes (E) Mmp13, and (F) Enpp3, normalized to 107 tags. G, Regulated genes from panel D were manually grouped by category and classified according to their degree of regulation (green, up-regulated; red, down-regulated) and VDR occupancy at the locus (diamond, VDR bound; circle, VDR not bound). d35, day 35; TF, transcription factor; Veh, vehicle.
The VDR cistrome and the expression of 1,25(OH)2D3-sensitive osteocytic genes
In a final bioinformatic analysis, we contrasted the location of VDR-binding sites with gene cohorts in osteocytes that were either regulated, or had the potential to be regulated, by 1,25(OH)2D3. As depicted in Figure 5D, GREAT analysis (58) revealed that 2969 binding sites were located proximal to, and associated with, 3296 neighboring genes. Only 316 of these genes were actually regulated on day 35 by 1,25(OH)2D3 (up or down), however. Thus, the vast majority of the genes linked through GREAT are either not direct targets of these putative 1,25(OH)2D3-regulated enhancers or, despite nearby occupancy by VDR, are genes that are resistant to the actions of 1,25(OH)2D3 under the conditions described here. Interestingly, 450 genes found to be regulated in the osteoblast, but not in the osteocyte, still retained functional VDR-binding sites, suggesting that the genes may have had the potential to respond, but additional factors might be present that are influencing response to 1,25(OH)2D3. Figure 5G depicts an expanded plot of selected GO term-derived genes that were either induced or suppressed by 1,25(OH)2D3. This plot also documents whether VDR-binding sites were found adjacent to the specific gene. Strikingly, and in contrast to the many genes that are up-regulated by the hormone, the VDR is absent from most of those that are down-regulated by 1,25(OH)2D3. Indeed, we found that some 790 genes that were regulated by 1,25(OH)2D3 did not have adjacent VDR-binding sites. Interestingly, this feature includes the Sost gene the expression of which in IDG-SW3 cells on day 35 was down-regulated by the vitamin D hormone (Supplemental Table 1). As documented in the Supplemental Data, this down-regulation was confirmed at both the mRNA (Supplemental Figure 7A) and protein levels (Supplemental Figure 7) in IDG-SW3 cells and at the mRNA level in mouse L5 vertebrae in vivo following injection of 1,25(OH)2D3 as well (Supplemental Figure 7C). Importantly, ChIP-seq analysis of the Sost gene interval highlighted the presence of regulatory components via significant H3K4me1 and -me2 enrichment at the Sost promoter and at 2 enhancer regions located downstream of the gene (+20 kb and +45 kb) (Supplemental Figure 7D). The +45kb region in the mouse gene is homologous to the ECR5 region in the human gene (65, 66). Despite this, ChIP-seq analysis failed to identify a VDR-binding site(s) within the extended Sost locus. These results suggest that the suppression of Sost and perhaps other down-regulated genes as well may occur through a more complex VDR-dependent mechanism.
Regulation of the Hedgehog pathway coreceptors Boc and Cdon by 1,25(OH)2D3
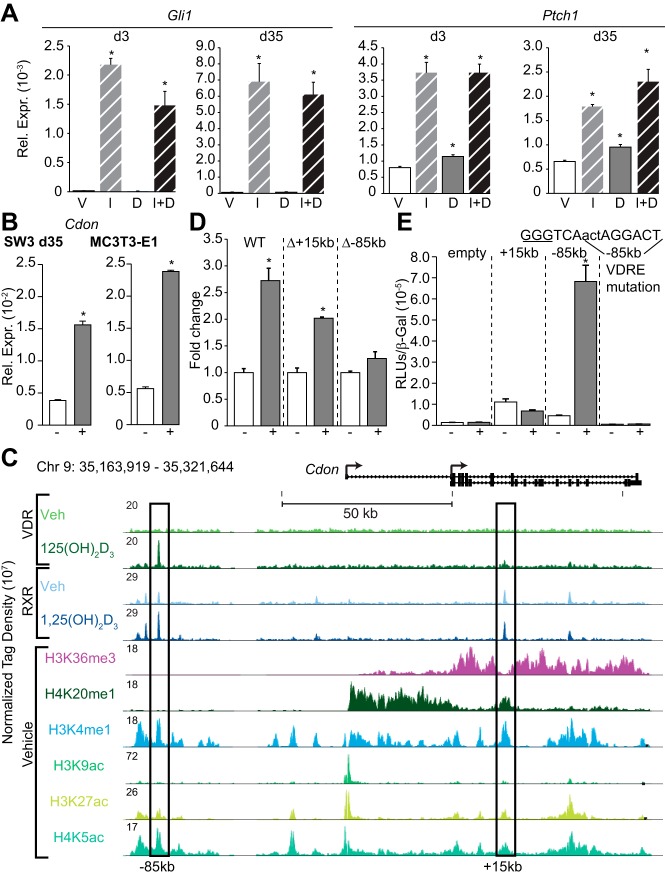
Transcriptome analysis during the osteoblast to osteocyte transition revealed the presence and up-regulation of several components of the Hedgehog pathway and that Boc and Cdon, coreceptors for this pathway (67), were both regulated by 1,25(OH)2D3. This pathway was indeed active in both osteoblasts and osteocytes, as assessed by the ability of Indian Hedgehog ligand (IHH) to up-regulate the classic Hedgehog target genes Gli1 and Ptch1 in IDG-SW3 cells at both 3 days and 35 days (Figure 6A) and in MC3T3-E1 osteoblasts (data not shown). Importantly, direct RT-PCR analysis confirmed that 1,25(OH)2D3 up-regulated Cdon expression in day 35 IDG-SW3 cells and established this up-regulation in MC3T3-E1 cells as well, as seen in Figure 6B. Close inspection of the ChIP-seq data tracks for both the VDR and RXR across the Cdon locus and for several key histone enhancer marks, as seen in Figure 6C, revealed the presence of potential binding sites for the VDR/RXR heterodimer at –85kb. The +15kb region served as a control region with RXR binding only. We therefore created a BAC reporter gene construct containing the extended Cdon locus together with BACs in which either the +15kb or −85kb segments were deleted, introduced these constructs stably into the MC3T3-E1 cell line, and examined reporter response to 1,25(OH)2D3. As documented in Figure 6D, deletion of the –85kb enhancer eliminated the 1,25(OH)2D3 response of the Cdon transcriptional reporter. Independent linkage of the +15 and –85kb enhancer segments to a heterologous TK promoter-reporter revealed further that the –85kb enhancer was capable of mediating response to 1,25(OH)2D3 following transient transfection into MC3T3-E1 cells and that this response was dependent upon an intact VDRE contained within (Figure 6E). This study demonstrates that Cdon is a direct target of 1,25(OH)2D3 action in osteoblasts and osteocytes and that the distal enhancer –85kb upstream of the Cdon transcriptional start site is functionally linked to this target gene. Parallel experiments were performed to dissect Boc regulation by 1,25(OH)2D3, which is primarily mediated through the +20kb region (Supplemental Figure 8).
Figure 6.
Regulation of the hedgehog pathway coreceptor encoding gene, Cdon, by 1,25(OH)2D3. A, Gene expression of cells treated with vehicle (V), 3 μg/mL IHH (I), 100 nM 1,25(OH)2D3 (D), or IHH + 1,25(OH)2D3 (I+D) were normalized to β-actin levels. Samples were analyzed in triplicate ± SEM (*, P < .05 vs vehicle). B, Cdon gene expression in IDG-SW3 and MC3T3–E1 cells treated with vehicle (−) or 100 nM 1,25(OH)2D3 (+) for 24 hours was normalized to β-actin levels and shown as fold change compared with d35 sample. Samples were analyzed in triplicate ± SEM (*, P < .05 vs vehicle). C, ChIP-seq tracks for Cdon, normalized to 107 tags, in the osteocyte (d35). D, BAC-Cdon-P2ALTN (WT, Δ+15kb, Δ-85kb) MC3T3–E1 stable cells treated with vehicle (−) or 100 nM 1,25(OH)2D3 (+) for 24 hours; relative light units (RLU) normalized to total protein and shown as fold change compared with vehicle ± SEM for a triplicate set of assays (*, P, .05 vs vehicle). E, pTK (empty), pTK-Cdon+15 kb, pTK-Cdon-85 kb region, or pTK-Cdon-85 kb VDRE mutated were transfected into MC3T3–E1 cells and treated with vehicle (−) or 100 nM 1,25(OH)2D3 (+). Each point represents relative light unit average normalized to β-gal ± SEM for a triplicate set of assays (*, P < .05 vs vehicle). Mutated sequence from GGG to AAA (underlined). d35, day 35; WT, wild type.
Discussion
Osteocytes have become the subject of intense research scrutiny recently, largely as a result of the development of new technological approaches that enable direct and informative studies of both osteocyte structure and function (1, 3). In the present work, we have used the IDG-SW3 cell line together with genome-wide approaches to explore features of osteocyte terminal differentiation from its osteoblast precursors in vitro (6) and from this have developed a model for osteoblast to osteocyte differentiation (Figure 7). We discovered that the striking changes in gene expression that accompany this temporally dependent transition are correlated directly with significant epigenetic alterations at histones H3 and H4, and that whereas these changes are largely ones of magnitude, they also include the development of novel histone marks as well. These changes in the epigenome that are apparent between the osteoblast and the osteocyte likely underlie the altered transcriptome responses that are observed between the early and late stage cells in the presence of 1,25(OH)2D3. Further mechanistic study revealed an extensive distribution of the VDR at 2969 sites across the osteocyte genome, sites that are contained within regions of H3K4me1, H3K9ac, H4K5ac, and H3K27ac enrichment, co-occupied by RXR, and frequently located many kilobases distal to the transcriptional start sites of previously annotated genes. Interestingly, whereas many of these sites are located adjacent to genes that are regulated by 1,25(OH)2D3, most the sites are not associated with genes that are actively regulated. This result highlights the difficulty of establishing a direct link between a distal regulatory region and the target gene the expression of which it controls (60). Nevertheless, we did establish such a linkage for both Boc and Cdon, 2 genes that encode coreceptors for the hedgehog signaling pathway that are up-regulated by 1,25(OH)2D3. Overall, these studies demonstrate that the osteoblast to osteocyte transition is accompanied by striking changes in gene expression that may occur, in part, as a result of qualitative and quantitative changes in epigenetic histone marks, especially those that are located within the regulatory regions of annotated genes. These progressive changes may contribute to altered cellular responsiveness to 1,25(OH)2D3 and perhaps to other regulators of the osteocyte phenotype.
Figure 7.
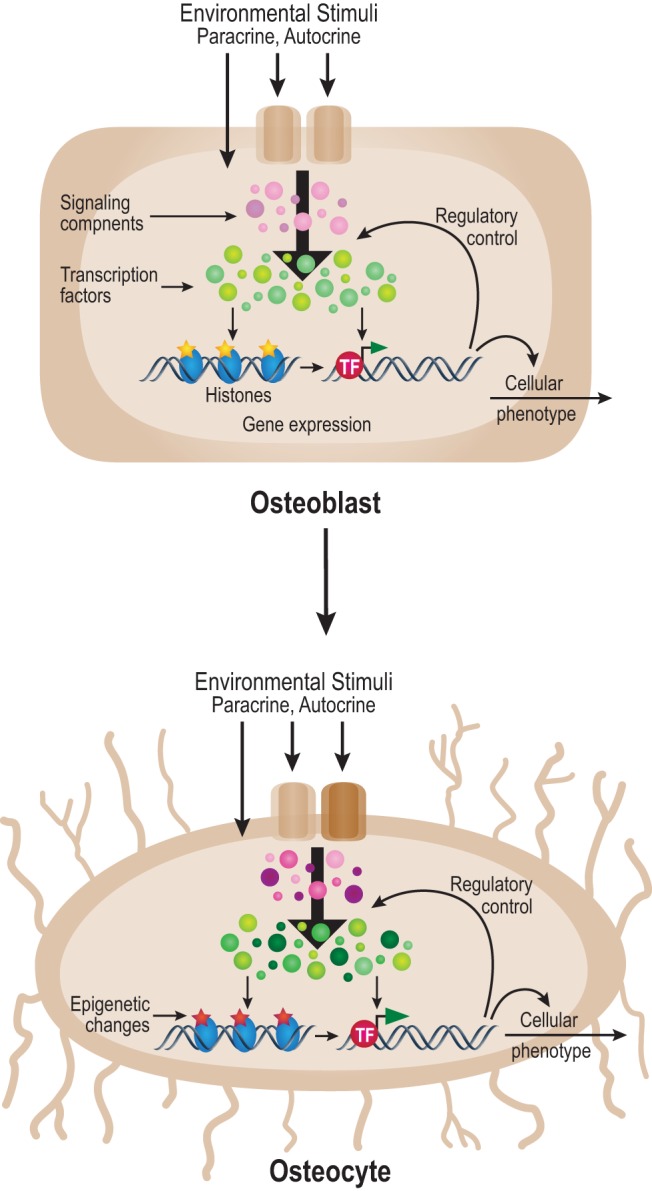
A model for cellular changes that direct the osteoblast to osteocyte phenotype. The osteoblast to osteocyte transition is achieved through preprogrammed genetic processes and changes in environmental inputs that induce alterations in signaling components (pink/purple), transcription factors (green), and chromatin regulators. These changes alter epigenetic patterns (yellow to red) across the genome that, together with direct transcription factor activity, results in changes in gene expression and cellular phenotype. TF, transcription factor.
Although a number of genes are uniquely activated or silenced during the osteoblast to osteocyte transition, the expression of the vast majority of the genes within the transcriptome were either unaffected by differentiation or simply up- or down-regulated from an established baseline. This latter result suggests that although the osteocyte is phenotypically unique, it retains significant regulatory features of its osteoblast precursor and perhaps its earlier mesenchymal progenitor as well. This conclusion is supported by the somewhat surprising observation that the epigenetic changes at the histones are largely ones of magnitude as opposed to the creation of new enhancers (or loss of residual enhancers) capable of conferring a change in the regulation of expression of specific genes de novo. It is worth noting that this distinction is highly subjective, particularly with respect to the appearance of new enhancers. Nevertheless, it seems likely that the potential to become osteoblasts as well as osteocytes is largely preprogrammed early in the osteoblast lineage.
A fundamental question is whether the relative epigenetic changes that are observed at histones in this study contribute to, or are simply a consequence of, changes that are noted with respect to gene expression. We do not address this issue directly here, although many previous studies of cellular differentiation, including those using early stem cells, immune cells, and other cell types, demonstrate directly that epigenetic changes precede changes in gene expression and therefore may underlie cellular programs of differentiation (68–72). It is worth noting here, however, that epigenetic modifications are highly dynamic as well, suggesting considerable flexibility during the differentiation process (73). If so, the nature of the inputs that drive the epigenetic changes that orchestrate the osteoblast to osteocyte transition is of considerable interest. It seems likely that they comprise both preprogrammed genetic inputs as well as environmental inputs (Figure 7), the latter triggered via the temporal and spatially dependent processes through which the osteoblast/early osteocyte becomes progressively embedded in mineralized matrix. Genetic preprogramming likely includes the timely expression of transcription, coregulatory, and chromatin-active factors the activities of which impact cell-specific gene networks and facilitate response to external factors as well. Indeed, our analyses show the altered expression of a wide variety of regulatory factors during the osteoblast to osteocyte transition that could be involved (74). They might include such factors as runt-related transcription factor 2 (RUNX2), OSX, and myocyte enhancer factor (MEF)2C, which auto-regulate their own expression and thus provide a forward-feed mechanism to accelerate the transition (75, 76). Environmental inputs might also influence the expression of these factors as well. Although much remains to be explored, the preliminary analyses documented here provide a basis for further, hypothesis-directed research into the nature of regulatory inputs and key signaling pathways and factors that are central to the differentiation process.
Because VDR levels appear to be up-regulated during osteocyte differentiation, our results suggest that epigenetic changes to the genome and/or changes in the expression of specific genes encoding regulatory factors may underlie an altered and dramatically restricted transcriptomic response to 1,25(OH)2D3. This change is unlikely to be limited to hormone response alone but extend to other external regulators as well. Certain histone modifications selectively alter chromatin structure and nucleosome position, both increasing as well as decreasing the accessibility of transcription factors such as the VDR, OSX, or MEF2C to specific DNA-binding sites within enhancers (77). Because VDR and other DNA binding proteins function largely to recruit coregulatory protein complexes that facilitate downstream changes in gene expression (epigenetic chromatin regulators, methylases, demethylases, readers, and erasers), alteration in the concentrations of these latter factors may also influence downstream regulation of gene expression. The recruitment of chromatin active complexes containing EZH2, on the other hand, may impose silencing marks such as H3K27me to histones, thus restricting the expression of specific genes (78). Other modifications directly influence higher-level chromatin organization, alter promoter function, or directly affect various features of the transcriptional process. Although the precise mechanisms related to osteocytes are not known, these potential activities appear to restrict 1,25(OH)2D3 action and reduce the number of genes sensitive to the vitamin D hormone. This finding is consistent with observations suggesting that different cell types display unique genetic responses to 1,25(OH)2D3 and that response to 1,25(OH)2D3 can be altered through selective activation of specific signaling pathways such as the TGFβ pathway (79).
The VDR cistrome in osteocytes comprises 2969 sites. Whereas the actual sites of VDR-binding are unique to the osteocyte genome, the general features of this cistrome are similar to that identified in other cell types. This includes the prevalence of VDRE-containing distal intergenic and intronic binding locations relative to annotated genes, co-occupancy of RXR at these sites, and the presence of enriched histone marks such as H3K4me1, H3K9ac, H4K5ac, and H3K27ac. A major question is the nature of the correlation that exists between VDR-binding sites and genes that are regulated by 1,25(OH)2D3. Interestingly, only 30% of the genes that are regulated by 1,25(OH)2D3 in the osteocyte were found to have VDR bound nearby. Several explanations are possible: 1) the binding sites are highly remote (perhaps mega bases from the target gene or on other chromosomes) and therefore, were not identified; 2) the genes are coregulated through enhancers that control the expression of other genes; 3) the genes are regulated via alternative mechanisms that may limit detection of the VDR, perhaps those that mediate suppression; and 4) the genes represent indirect targets of vitamin D action and are thus regulated directly by transcription factors the expression of which is, in turn, directly modulated by the VDR. Suppression mechanisms, such as the one that may be responsible for the down-regulation of the Sost gene by 1,25(OH)2D3, and possibly other regulatory factors, are particularly complex (80). For example, whereas Sost is similarly down-regulated by PTH, the mechanism through which at least 2 transcription factors, cAMP response element-binding protein and MEF2C, are involved remains elusive (81). Surprisingly, VDR binding activity can also be found at sites adjacent to genes that are not regulated by 1,25(OH)2D3. In these cases, it is possible that expression of the genes of interest are potentially modulated by 1,25(OH)2D3, but additional regulatory input may be required. This could explain the lack of induction of genes in the osteocyte that were previously regulated by 1,25(OH)2D3 in the osteoblast. Alternatively, the up- or down-regulation of a suppression factor or epigenetic modifications could prevent modulation by 1,25(OH)2D3. Finally, because of the distal nature of many regulatory elements, it is difficult to link an enhancer containing bound VDR directly to the regulation of a target gene without additional studies such as those conducted herein on the regulation of both Boc and Cdon expression.
The present studies coupling transcriptional output to the nearby presence of one or more VDR-binding sites identify a number of genes that are clear targets of 1,25(OH)2D3 action in both osteoblasts and osteocytes. With respect to osteocytes, these genes include recognizable targets such as Tnfsf11, Ank, Spp1, Enpp1, Mmp13, Cebpb, Vdr, and Fgf23 as well as novel targets such as Epha4, Gadd45a, Rcan1, Cd28, Trp63, Vegfc, Boc, Cdon, Sost, and Lepr. Interestingly, although Fgf23 was up-regulated by 1,25(OH)2D3, a VDR-binding region was not found within the near vicinity of this locus (data not shown). VDR-binding activity was also not detected near the Sost gene locus. Regardless of the strong relationship between VDR-binding sites and many 1,25(OH)2D3-regulated genes, the distal locations of most of the detected regulatory regions suggests that further studies are necessary to link the enhancers to the genes they regulate. Such studies using large recombinantly modified BAC clones comprising entire gene loci for the Vdr, Tnfsf11, and Cyp24a1 have been conducted (37, 42, 82). Nevertheless, we explored as novel examples the regulation of the hedgehog coreceptor genes, Boc and Cdon, by 1,25(OH)2D3. Using this BAC clone approach, we confirmed that the site of distal VDR binding −85 kb upstream of Cdon and the regulatory region at +20 kb downstream of Boc were indeed linked directly to the 1,25(OH)2D3-induced expression of the 2 coreceptors. Based upon the ability of Indian Hedgehog ligand (Ihh) to stimulate the expression of several known target genes in IDG-SW3 cells at day 35, we postulate that this pathway may target unique biologically active genes in osteocytes. The use of extended BAC clones or alternative methods using genomic enhancer deletion analysis will be necessary to establish a direct relationship between defined VDR-binding sites and additional target genes.
In summary, we have shown in these studies that the osteoblast to osteocyte transition results in a substantial change in the pattern of gene expression comprised, in part, of genes that are unique to either osteoblasts or osteocytes. These changes are accompanied by significant alterations in epigenetic histone marks that are likely to precede modifications to gene expression networks. These changes are likely influential in altering genetic responsiveness to 1,25(OH)2D3 that occurs via VDR binding across the osteocyte genome. We conclude that osteocyte differentiation is characterized by cellular changes at both the transcription factor and epigenomic levels and through altered response to a systemic ligand.
Acknowledgments
We thank members of the Pike laboratory for their helpful discussions and contributions to this manuscript; Ms Victoria Osinski for her technical assistance in the evaluation of Cdon and Boc regulation; and Dr Melda Onal for her technical assistance demonstrating Sost regulation in vivo. We also acknowledge members of the University of Wisconsin DNA Sequencing Facility in the University of Wisconsin Biotechnology Center.
This work was supported by grant DK-072281 (to J.W.P.) and Institute for Clinical and Translational Research UL1 RR025011 and GM102756 (to C.K.).
Current affiliation for K.A.B.: Maine Medical Center Research Institute, Scarborough, ME 04074.
Data Access: All sequencing data is publicly available through the GEO database: GSE54784.
Disclosure Summary: The authors declare no conflict of interest
Funding Statement
This work was supported by grant DK-072281 (to J.W.P.) and Institute for Clinical and Translational Research UL1 RR025011 and GM102756 (to C.K.).
Footnotes
- ANK
- ankyrin
- BAC
- bacterial artificial chromosome
- ChIP-seq
- chromatin immunoprecipitation coupled to high-throughput sequencing
- chr
- chromosome
- DMP1
- dentin matrix protein 1
- E11
- podoplanin
- ENPP
- ectonucleotide pyrophosphatase/phosphodiesterase
- FBS
- fetal bovine serum
- FGF
- fibroblast growth factor
- H3K
- histone 3 lysine
- HDAC
- histone deacetylase
- HRP
- horseradish peroxidase
- IFN
- interferon
- MEF
- myocyte enhancer factor
- MEPE
- matrix extracellular phosphoglycoprotein
- OSX
- osterix
- PBST
- PBS with 1% Tween 20
- PHEX
- phosphate-regulating gene with homologies to endopeptidases on the X chromosome
- RANKL
- receptor activator of NF-κB ligand
- RNA-seq
- RNA-sequencing
- RT
- room temperature
- RXR
- retinoid X receptor
- VDR
- vitamin D receptor
- VDRE
- vitamin D-response element.
References
- 1. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato M, Asada N, Kawano Y, et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18(5):749–758. [DOI] [PubMed] [Google Scholar]
- 5. Paic F, Igwe JC, Nori R, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45(4):682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26(11):2634–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16(3):319–327. [DOI] [PubMed] [Google Scholar]
- 8. Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2013;28(4):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang W, Harris MA, Heinrich JG, Guo D, Bonewald LF, Harris SE. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. [DOI] [PubMed] [Google Scholar]
- 11. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. [DOI] [PubMed] [Google Scholar]
- 12. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. [DOI] [PubMed] [Google Scholar]
- 13. Yang X, Matsuda K, Bialek P, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–398. [DOI] [PubMed] [Google Scholar]
- 14. Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem. 2013;288(35):25614–25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen ED, Schroeder TM, Bailey J, Gopalakrishnan R, Westendorf JJ. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J Bone Miner Res. 2008;23(3):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem. 2013;114(5):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: Effects on bone and mineral homeostasis. Bone. 2013;54(2):237–243. [DOI] [PubMed] [Google Scholar]
- 19. Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. [DOI] [PubMed] [Google Scholar]
- 20. Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–158. [DOI] [PubMed] [Google Scholar]
- 21. Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259–50272. [DOI] [PubMed] [Google Scholar]
- 22. Powell WF Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci U S A. 1971;68(4):803–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228(5273):764–766. [DOI] [PubMed] [Google Scholar]
- 25. Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971;230(5291):228–230. [DOI] [PubMed] [Google Scholar]
- 26. Norman AW. Evidence for a new kidney-produced hormone, 1,25-dihydroxycholecalciferol, the proposed biologically active form of vitamin D. Am J Clin Nutr. 1971;24(11):1346–1351. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto Y, Yoshizawa T, Fukuda T, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154(3):1008–1020. [DOI] [PubMed] [Google Scholar]
- 28. Lieben L, Masuyama R, Torrekens S, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nerenz RD, Martowicz ML, Pike JW. An enhancer 20 kilobases upstream of the human receptor activator of nuclear factor-κB ligand gene mediates dominant activation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2008;22(5):1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. [DOI] [PubMed] [Google Scholar]
- 31. Onal M, Galli C, Fu Q, et al. The RANKL distal control region is required for the increase in RANKL expression, but not the bone loss, associated with hyperparathyroidism or lactation in adult mice. Mol Endocrinol. 2012;26(2):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noda M, Vogel RL, Craig AM, Prahl J, DeLuca HF, Denhardt DT. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci USA. 1990;87(24):9995–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kriebitzsch C, Verlinden L, Eelen G, et al. 1,25-dihydroxyvitamin D3 influences cellular homocysteine levels in murine preosteoblastic MC3T3–E1 cells by direct regulation of cystathionine β-synthase. J Bone Miner Res. 2011;26(12):2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol. 2006;26(17):6469–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S, Yamazaki M, Shevde NK, Pike JW. Transcriptional control of receptor activator of nuclear factor-κB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol. 2007;21(1):197–214. [DOI] [PubMed] [Google Scholar]
- 36. Martowicz ML, Meyer MB, Pike JW. The mouse RANKL gene locus is defined by a broad pattern of histone H4 acetylation and regulated through distinct distal enhancers. J Cell Biochem. 2011;112(8):2030–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu Q, Manolagas SC, O'Brien CA. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26(17):6453–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galli C, Zella LA, Fretz JA, et al. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-κB ligand gene reduces bone remodeling and increases bone mass. Endocrinology. 2008;149(1):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barthel TK, Mathern DR, Whitfield GK, et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103(3–5):381–388. [DOI] [PubMed] [Google Scholar]
- 40. Meyer MB, Benkusky NA, Pike JW. The RUNX2 cistrome in osteoblasts: characterization, downregulation following differentiation and relationship to gene expression. J Biol Chem. 2014: 10.1074/jbc.M1114.552216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33(4):e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol. 2010;24(1):128–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szymczak-Workman AL, Vignali KM, Vignali DA. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb Protoc. 2012;2012(2):199–204. [DOI] [PubMed] [Google Scholar]
- 44. Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2006;20(6):1231–1247. [DOI] [PubMed] [Google Scholar]
- 45. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. [DOI] [PubMed] [Google Scholar]
- 46. Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol. 2012;26(1):37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 49. Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leng N, Dawson JA, Thomson JA, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29(8):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ji X, Li W, Song J, Wei L, Liu X. CEAS: cis-regulatory element annotation system. Nucleic Acids Res. 2006;34(Web Server issue):W551–W554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qing H, Ardeshirpour L, Pajevic PD, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. [DOI] [PubMed] [Google Scholar]
- 55. Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39(3):311–318. [DOI] [PubMed] [Google Scholar]
- 56. Ernst J, Kheradpour P, Mikkelsen TS, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. [DOI] [PubMed] [Google Scholar]
- 58. McLean CY, Bristor D, Hiller M, et al. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang DX, Glass CK. Towards an understanding of cell-specific functions of signal-dependent transcription factors. J Mol Endocrinol. 2013;51(3):T37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression [published online November 12, 2013]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res. 2005;20(2):305–317. [DOI] [PubMed] [Google Scholar]
- 62. Meyer MB, Goetsch PD, Pike JW. Genome-wide analysis of the VDR/RXR cistrome in osteoblast cells provides new mechanistic insight into the actions of the vitamin D hormone. J Steroid Biochem Mol Biol. 2010;121(1–2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meyer MB, Pike JW. Corepressors (NCoR and SMRT) as well as coactivators are recruited to positively regulated 1α,25-dihydroxyvitamin D3-responsive genes. J Steroid Biochem Mol Biol. 2013;136:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heikkinen S, Väisänen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39(21):9181–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collette NM, Genetos DC, Economides AN, et al. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci USA. 2012;109(35):14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Loots GG, Kneissel M, Keller H, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15(7):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429. [DOI] [PubMed] [Google Scholar]
- 68. Kuzmichev A, Margueron R, Vaquero A, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci USA. 2005;102(6):1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Komashko VM, Acevedo LG, Squazzo SL, et al. Using ChIP-chip technology to reveal common principles of transcriptional repression in normal and cancer cells. Genome Res. 2008;18(4):521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wong P, Hattangadi SM, Cheng AW, Frampton GM, Young RA, Lodish HF. Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood. 2011;118(16):e128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12(4):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hawkins RD, Hon GC, Lee LK, et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6(5):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishio Y, Dong Y, Paris M, O'Keefe RJ, Schwarz EM, Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. [DOI] [PubMed] [Google Scholar]
- 76. Ramachandran B, Yu G, Li S, Zhu B, Gulick T. Myocyte enhancer factor 2A is transcriptionally autoregulated. J Biol Chem. 2008;283(16):10318–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ong CT, Corces VG. Insulators as mediators of intra- and inter-chromosomal interactions: a common evolutionary theme. J Biol. 2009;8(8):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kirmizis A, Bartley SM, Kuzmichev A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18(13):1592–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim MS, Fujiki R, Murayama A, et al. 1α,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol. 2007;21(2):334–342. [DOI] [PubMed] [Google Scholar]
- 81. Leupin O, Kramer I, Collette N, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22(12):1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1α,25-dihydroxyvitamin D3. J Biol Chem. 2010;285(20):15599–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]