Abstract
Background
The long pentraxin PTX3 is a key component of the humoral arm of innate immunity related to sepsis severity and mortality. We evaluated the clinical and prognostic significance of circulating PTX3 in the largest cohort ever reported of patients with severe sepsis or septic shock.
Design
Plasma PTX3 was measured on days 1, 2 and 7 after randomization of 958 patients to albumin or crystalloids for fluid resuscitation in the multicenter Albumin Italian Outcome Sepsis (ALBIOS) trial. We tested the association of PTX3 and its changes over time with clinical severity, prevalent and incident organ dysfunctions, 90-day mortality, and treatment.
Results
PTX3 was high at baseline (72 [33-186] ng/mL) and rose with the severity and number of organ dysfunctions (p<0.001) and the incidence of subsequent new failures. The PTX3 concentration dropped from day 1 to 7, but this decrease was less pronounced in patients with septic shock (p=0.0004). Higher concentrations of PTX3 on day 1 predicted incident organ dysfunctions. Albumin supplementation was associated with lower levels of PTX3 in patients with septic shock (p=0.005) but not in those without shock. In a fully adjusted multivariable model, PTX3 on day 7 predicted 90-day mortality. Smaller drops in PTX3 predicted higher 90-day mortality.
Conclusions
In severe sepsis and septic shock, early high PTX3 predict subsequent new organ failures, while a smaller drop in circulating PTX3 over time predicts an increased risk of death. Patients with septic shock show lower levels of PTX3 when assigned to albumin than to crystalloids.
Keywords: severe sepsis, septic shock, prognosis, pentraxin 3, albumin
Introduction
Despite recent improvements in their diagnosis and treatment, the incidence of severe sepsis and septic shock in hospitalized patients, as well as their outcomes remain unacceptably high [1]. Considering the variety of clinical phenotypes of severe sepsis and septic shock, a more accurate understanding of the underlying scenarios of the pathobiology of these clinical conditions would allow better targeted therapeutic strategies [2]. In this context, circulating biomarkers may improve early recognition and risk stratification, and therefore a targeted selection and monitoring of appropriate therapies [3].
The long pentraxin 3 (PTX3) is a key component of the humoral arm of the innate immune response. Primary pro-inflammatory signals (bacterial components, pathogen-associated molecular patterns, damage associated molecular patterns, IL-1, TNF-alpha) [4] induce the synthesis and release of PTX3 in various cell types such as endothelial cells, polymorphonuclear leukocytes (PMN), and monocytes/macrophages [5]. On account of its specific involvement in the immune system and inflammatory processes, and since it can be measured in blood, PTX3 has been characterized as a biomarker of disease severity and outcomes in different cardiovascular diseases, malignancies, and specific infections [6–11]. Since the first report [12] several studies have focused on the roles of elevated circulating PTX3 in sepsis and related complications such as shock. A recent systematic review of 11 studies concluded that circulating PTX3 has solid prognostic value in sepsis and correlates with organ dysfunction, but with limited specificity [8]. However, in most of these studies, patients with severe sepsis and septic shock (for whom early stratification and selection of more aggressive treatments could be vital) were a minority, as compared to the patients with less severe conditions (infection plus systemic inflammatory reaction syndrome, SIRS).
The present study aimed at verifying, on a large scale, the prognostic and clinical value of circulating PTX3 in patients with severe sepsis or septic shock, previously reported in smaller and heterogeneous cohorts of critically ill patients [13–16]. We assayed PTX3 in a large population of patients with severe sepsis and septic shock, enrolled in the ALBIOS trial [17] to address the following issues: first, do single or serial measurements of PTX3 have independent prognostic value? Second, are circulating PTX3 levels related with ongoing or future organ dysfunctions? Third, since the ALBIOS trial tested the efficacy of albumin replacement, and human albumin may have anti-inflammatory activity, does albumin given for fluid resuscitation affect circulating PTX3?
Materials and Methods
Study design
The Albumin Italian Outcome Sepsis (ALBIOS) trial was a multicenter, open-label, randomized, controlled trial on 1818 patients with severe sepsis or septic shock admitted to 100 Italian intensive care units (ICUs). Patients were randomly assigned to receive either 20% albumin and crystalloids or crystalloids alone for fluid resuscitation, from randomization until day 28 or discharge from the ICU, whichever came first. Albumin was targeted to achieve serum level of 30 g/L or more. Study design, inclusion and exclusion criteria, and main results have been published elsewhere [17]. The study was compliant with the 1975 Declaration of Helsinki as revised in 2008, and approved first by the Institutional Review Board of the Fondazione IRCCS Ca' Granda – Ospedale Maggiore Policlinico, Milan, Italy (coordinating center), and subsequently by the institutional review boards of all the other centers. Written informed consent or deferred consent was obtained from each participant.
Sample collection and PTX3 measurement
PTX3 was measured in 958 patients from the 40 centers that participated in a predefined biomarkers sub-study [18]. Venous blood samples were serially collected 1, 2 and 7 days after study enrolment (or at ICU discharge, whichever came first), centrifuged, and EDTA plasma was shipped on dry ice to a central repository and stored at -70°C until required. PTX3 was assayed, as previously described [19], with sandwich ELISA (detection limit 0.1 ng/mL, inter-assay variability from 8 to 10%) developed in-house, by personnel blind to patients’ characteristics. A pool of plasma from healthy subjects was assayed in each analytical session with PTX3 of 1.57±0.48 ng/ml (mean ± SD; CV 10%), in agreement with reported data for healthy subjects [20]. PTX3 was measured in 93 and 83% of the survivors on days 2 and 7, respectively.
Definitions
Severe sepsis was defined as a proved or suspected infection in at least one site, two or more signs of systemic inflammatory reaction, and at least one severe, acute sepsis-related organ dysfunction [21]. Organ function was assessed on a daily basis with the use of the Sequential Organ Failure Assessment (SOFA) score [22], which rates for each of five organ sub-components (respiratory, coagulation, liver, cardiovascular and renal systems) from 0 to 4, with higher scores indicating a greater degree of organ dysfunction. A cardiovascular SOFA score of 3 or 4 defines the presence of any dosages of either norepinephrine or epinephrine, or a higher dosage of dopamine (greater than 5.0 microgram/kg/min) to sustain mean arterial pressure. Septic shock at the time of randomization was defined as severe sepsis with a score of 3 or 4 for the cardiovascular component of the SOFA score [23]. New organ failures were defined as a change in each SOFA component during the study, to 3 or 4 from a baseline of 0, 1 or 2. Acute kidney injury was defined according to the risk, injury, failure, loss, and end-stage kidney injury (RIFLE) criteria [24], based on the daily incremental increases in serum creatinine from baseline. Clinical resolution of the primary infection, if any, was established by the treating physician according to standard clinical and microbiological findings. Acquired secondary infections were defined as new infections other than the primary infection responsible for the development of severe sepsis, arising during the study, based on microbiological and clinical assessments, and evaluated by the treating physician. Immunosuppression at enrolment was defined as previous pharmacological therapies or co-existent disease reducing the reactivity of the immune system towards an infection. Microorganism susceptibility to antibiotics was defined by a local microbiologist, unaware of the allocation group or treatment.
Statistical methods
Categorical variables are presented as proportions, and continuous variables as means (standard deviation) or medians (Q1-Q3), as appropriate. Differences in clinical characteristics according to tertiles of PTX3 concentration were compared by the Chi-square test or Fisher’s exact test for categorical variables; for continuous variables analysis of variance (ANOVA) or non-parametric Kruskal-Wallis test was adopted. For all analyses, PTX3 levels were transformed into the natural logarithm in order to satisfy the assumptions required by the models. We did multivariable linear regression analysis to identify the baseline characteristics independently associated with higher concentrations of PTX3 on day 1.
Repeated measures ANOVA for log-transformed PTX3 were used to assess the treatment effect over time in the overall population, and in the subgroups of patients with severe sepsis with or without shock. All measurements available at each time point for each patient were considered.
The relationship of PTX3 concentrations at each time (days 1, 2 and 7) with 90-day mortality was first described by median PTX3 levels (Q1-Q3) and analyzed by one-way ANOVA. Secondly, a univariate modified Poisson regression model was adopted to evaluate the prognostic value of PTX3, considered as a continuous variable. This model, indicated in case of a common outcomes (incidence >10%), gives a direct estimate of the relative risk (RR) with robust estimator for the variance. Finally, we adopted the multivariable modified Poisson regression model to evaluate the independent prognostic value of PTX3, accounting for the variables associated with the outcome. In addition to clinical variables, we also included into the multivariable models a cardiac biomarker (high sensitive cardiac troponin T, hs-cTnT), as well as an inflammatory biomarker (presepsin), previously investigated [18,25], having independent prognostic values. Kaplan-Meier survival analysis was done by tertiles of PTX3 concentrations on days 1, 2 and 7 and compared by the log-rank test. Normally distributed changes in PTX3 concentrations on days 2 and day 7 from day 1 were expressed as the difference in log-transformed levels and compared by ANOVA between survivors and patients who died. Unadjusted and adjusted modified Poisson Regression models were generated to assess potential associations between changes in PTX3 concentrations over time and 90-day mortality. All covariates in the multivariable models were measured at baseline and significantly associated with the outcome. Similarly, the prognostic value of PTX3 for the clinical outcomes was assessed by modified Poisson regression for categorical variables and by multivariable linear regression for continuous variables. A two-sided p value lower than 0.05 was deemed significant. All statistical analyses were done with SAS software 9.3 (SAS Institute, Cary, NC, USA). Reporting of the study conforms to CONSORT-revised and the broader EQUATOR guidelines [26].
Results
Clinical characteristics and PTX3 concentrations
The concentration of PTX3 on day 1 after randomization in the overall cohort of patients was elevated, all patients having higher than normal levels (>2 ng/mL) [20]. PTX3 levels on day 1 were associated with several demographic, clinical, hemodynamic and laboratory variables (Table 1).
Table 1. Clinical characteristics according to baseline PTX3 concentration.
| Characteristics | PTX3 | ||||
|---|---|---|---|---|---|
| All | T1 (3.1-41.0) | T2 (41.1-137.5) | T3 (137.6-9248.1) | P across tertiles | |
| No. (%) | 958 (100.0%) | 319 (33.3%) | 319 (33.3%) | 320 (33.4%) | |
| PTX3 – median [Q1-Q3] | 71.8 [32.9-186.3] | 24.5 [14.8-32.9 ] | 71.8 [51.2-103.2] | 244.2 [186.1-480.4] | -- |
| Age – year | 70.0 [58.0-78.0] | 67.0 [54.0–77.0] | 70.0 [57.0-77.0] | 71.0 [63.0–79.0] | 0.001 |
| Female sex – No. (%) | 395 (41.2%) | 124 (38.9%) | 124 (38.9%) | 147 (45.9%) | 0.11 |
| Body Mass Index – kg/m2 | 26.4 ±5.6 | 27.2 ± 6.2 | 26.4 ± 5.5 | 25.8 ± 4.8 | 0.007 |
| Reason for admission to ICU – No. (%) | |||||
| Medical | 540 (56.4%) | 167 (52.4%) | 183 (57.4%) | 190 (59.4%) | 0.10 |
| Elective surgery | 67 (7.0%) | 30 (9.4%) | 23 (7.2%) | 14 (4.4%) | |
| Emergency surgery | 351 (36.6%) | 122 (38.2%) | 113 (35.4%) | 116 (36.2%) | |
| Preexisting conditions – No. (%) | |||||
| Liver disease | 14 (1.5%) | 4 (1.3%) | 7 (2.2%) | 3 (0.9%) | 0.39 |
| COPD | 120 (12.5%) | 39 (12.2%) | 44 (13.8%) | 37 (11.6%) | 0.68 |
| Chronic renal failure | 42 (4.4%) | 12 (3.8%) | 17 (5.3%) | 13 (4.1%) | 0.59 |
| Immunodeficiency | 124 (12.9%) | 31 (9.7%) | 42 (13.2%) | 51 (15.9%) | 0.06 |
| Congestive or ischemic heart disease | 164 (17.1%) | 51 (16.0%) | 70 (21.9%) | 43 (13.4%) | 0.01 |
| SAPS II score | 46.7 ±15.6 | 40.9 ± 13.7 | 46.3 ± 14.4 | 53.1 ± 16.1 | <0.0001 |
| Physiological variables | |||||
| Heart rate – beats/min | 103.8 ± 20.8 | 98.8 ± 19.9 | 102.0 ± 19.9 | 110.7 ± 20.7 | < .0001 |
| Mean arterial pressure – mm Hg | 74.6 ± 15.0 | 78.4 ± 14.9 | 74.5 ± 14.7 | 70.9 ± 14.3 | < .0001 |
| Mean arterial pressure after 6 h – mm Hg | 78.3 ± 13.4 | 81.0 ± 13.5 | 78.8 ± 12.9 | 75.0 ± 13.2 | < .0001 |
| Central venous pressure – mm Hg | 9.9 ± 4.6 | 9.7 ± 4.4 | 9.7 ± 4.6 | 10.5 ± 4.9 | 0.07 |
| Urine output – ml/h | 60 [30–100] | 60 [40–100] | 70 [35–100] | 50 [20–100] | 0.002 |
| Central venous oxygen saturation | 72.6 ± 9.8 | 73.5 ± 9.1 | 72.5 ± 9.2 | 71.8 ± 10.9 | 0.12 |
| PaO2/FiO2 | 1.9 [1.3–2.7] | 2.0 [1.4–2.8] | 1.9 [1.3-2.7] | 1.9 [1.2-2.7] | 0.10 |
| Laboratory values | |||||
| Serum albumin – g/liter | 24.4 ± 6.2 | 24.7 ± 6.0 | 24.5 ± 6.5 | 24.2 ± 6.4 | 0.64 |
| Hemoglobin – g/dL | 10.9 ± 2.0 | 10.7 ± 2.0 | 11.0 ± 1.8 | 11.1 ± 2.1 | 0.04 |
| Serum lactate – mmol/L | 2.3 [1.4–4.0] | 1.7 [1.1–2.6] | 2.2 [1.5-3.7] | 3.4 [2.0-5.6] | < .0001 |
| Serum creatinine – mg/dL | 1.5 [0.9–2.5] | 1.1 [0.7–1.8] | 1.5 [0.9–2.5] | 1.9 [1.2–2.8] | < .0001 |
| White blood cells – 103/mm3 | 12.0 [5.7–18.6] | 12.7 [8.0–18.2] | 12.2 [6.4–19.2] | 9.1 [3.3–18.1] | < .0001 |
| Total serum bilirubin – mg/dL | 0.8 [0.5–1.5] | 0.8 [0.5–1.4] | 0.8 [0.5–1.5] | 0.9 [0.6–1.7] | 0.11 |
| Platelets – x 103/mm3 | 166 [102-237] | 174 [121-245] | 167 [104-247] | 148 [85–216] | 0.0003 |
| Circulating biomarkers on day 1 (median [Q1-Q3]) | |||||
| Presepsin – ng/L | 946 [492-1887] | 682 [426-1495] | 900 [486-1786] | 1213 [611-2334] | < .0001 |
| Hs-cTnT – ng/L | 50.4 [21.7-133.5] | 33.7 [14.8-76.0] | 50.0 [21.7-111.6] | 84.2 [36.5-217.0] | < .0001 |
| SOFA score [at baseline] | 7 [5-10] | 6 [4-8] | 7 [5-9] | 9 [7-10] | < .0001 |
| SOFA sub-score -respiration | 2 [2-3] | 2 [2-3] | 2 [2-3] | 2 [2-3] | 0.56 |
| SOFA sub-score -coagulation | 0 [0-1] | 0 [0-1] | 0 [0-1] | 1 [0-2] | 0.0006 |
| SOFA sub-score -liver | 0 [0-1] | 0 [0-1] | 0 [0-1] | 0 [0-1] | 0.13 |
| SOFA sub-score -cardiovascular | 3 [0-4] | 2 [0-3] | 3 [0-4] | 3 [1.5-4] | < .0001 |
| SOFA sub-score -kidney | 1 [0-2] | 0 [0-2] | 1 [0-2] | 2 [1-3] | < .0001 |
| Shock – No. (%) | 540 (56.4%) | 145 (45.5%) | 183 (57.4%) | 212 (66.3%) | <.0001 |
| Mechanical ventilation – No. (%) | 760 (79.3%) | 259 (81.2%) | 250 (78.4%) | 251 (78.4%) | 0.60 |
| Randomized to albumin– No. (%) | 474 (49.5%) | 173 (54.2%) | 155 (48.6%) | 146 (45.6%) | 0.09 |
| ICU mortality – No. (%) | 253 (26.4%) | 60 (18.8%) | 78 (24.5%) | 115 (35.9%) | < .0001 |
| 28-day mortality – No. (%) | 263/949 (27.7%) | 63/312 (20.2%) | 84/318 (26.4%) | 116/319 (36.4%) | < .0001 |
| 90-day mortality – No. (%) | 369/945 (39.1%) | 98/312 (31.4%) | 123/315 (39.1%) | 148/318 (46.5%) | 0.0005 |
| Positive blood culture – No. (%) | 271/852 (31.8%) | 64/286 (22.4%) | 88/281 (31.3%) | 119/285 (41.8%) | < .0001 |
| Antibiotics at randomization – No. (%) | 898 (93.7%) | 302 (94.7%) | 300 (94.0%) | 296 (92.5%) | 0.51 |
| Antibiotics 6 hours after randomization – No. (%) | 954 (99.6%) | 319 (100.0%) | 318 (99.7%) | 317 (99.1%) | 0.33 |
| Patients enrolled within 6 hours – No. (%) | 296 (30.9%) | 85 (26.7%) | 90 (28.2%) | 121 (37.8%) | 0.004 |
| Patients enrolled within 6-24 hours – No. (%) | 662 (69.1%) | 234 (73.3%) | 229 (71.8%) | 199 (62.2%) | |
| Time between enrolment and sampling on day 1 (h) | 20.1 ± 6.3 | 20.8 ± 6.2 | 20.4 ± 6.0 | 19.1 ± 6.5 | 0.0006 |
Kruskal-Wallis or Fisher’s tests, as appropriate.
Multiple linear regression analysis showed the strongest association of higher PTX3 concentrations with the following variables, by decreasing order of t-value: higher serum lactate (beta coefficient= 0.090±0.016, p<0.0001), shorter time between enrollment and sampling (-0.026±0.006, p<0.0001), higher heart rate (0.008±0.002, p<0.0001), presence of positive blood culture (0.35±0.08, p<0.0001), lower body mass index (-0.027±0.007, p<0.0001), higher SAPSII score (0.011±0.003, p=0.0005), higher hemoglobin (0.066±0.019, p=0.0007), higher SOFA score (0.0 ±0.02, p=0.03), lower white blood cell count (-0.009±0.004, p=0.04), and higher serum creatinine (0.06±0.03, p=0.04).
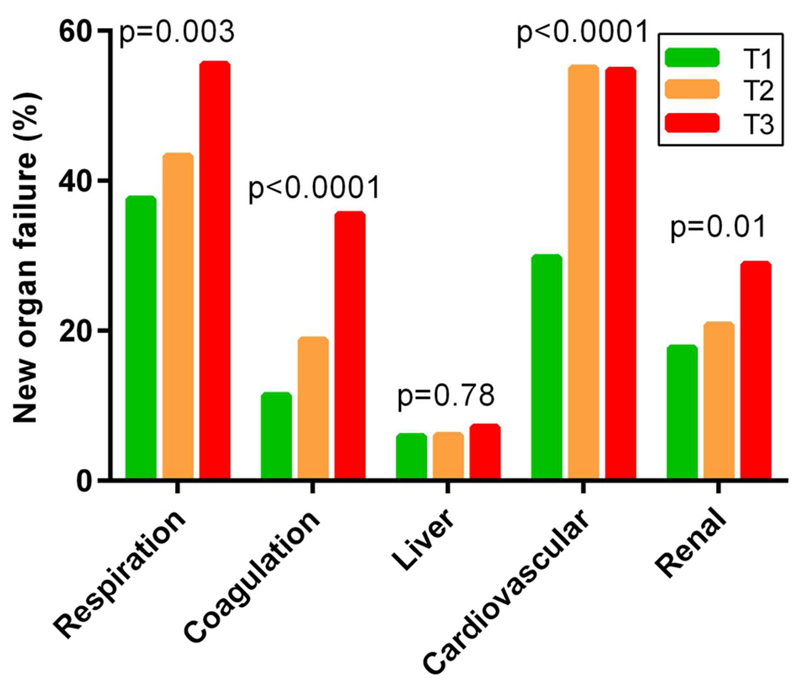
The concentration of PTX3 on day 1 paralleled the degree of the severity of organ dysfunctions, as denoted by the SOFA score (Figure S1), and the number of prevalent organ failures at enrolment (Figure S2). The PTX3 concentration was significantly higher in patients with urinary tract infection than those without urinary tract involvement, and lower in patients with lung infections than those without (Table S1). No significant association was observed in patients with specific infections originating in the abdomen or other sites. Patients with Gram-negative or Gram-positive infections had similar PTX3 levels (p=0.28 for site culture). Patients with depressed immune system function on day 1 (n=124) had higher PTX3 concentrations on day 1 (114.5 [40.4-229.2]) than those without (66.0 [31.8-181.7], p=0.002). Patients who had lower circulating levels of PTX3 on day 1 were more likely to achieve hemodynamic stability within the first 24 hours than those with higher levels (in particular, mean arterial pressure 65 mmHg or more, or serum lactate lower than 2 mmol/L). Accordingly, reduced use of vasoactive drugs was associated with lower levels of PTX3 on day 1 (Table S2). Finally, higher PTX3 levels on day 1 appeared to be associated with a greater probability of developing novel organ failures during the study, in particular coagulation, cardiovascular and renal (Figure 1).
Figure 1. PTX3 concentration and incident organ failures.
Relations between tertiles of PTX3 concentration on day 1 and incident organ failures. New organ failures were defined as a change in each component of the SOFA score during the study to 3 or 4 from 0, 1 or 2 at baseline. Number of patients without organ-specific failure at study entry: respiration (525), coagulation (862), liver (896), cardiovascular (392), renal (715). P across tertiles by chi-square test.
PTX3 levels during ICU stay
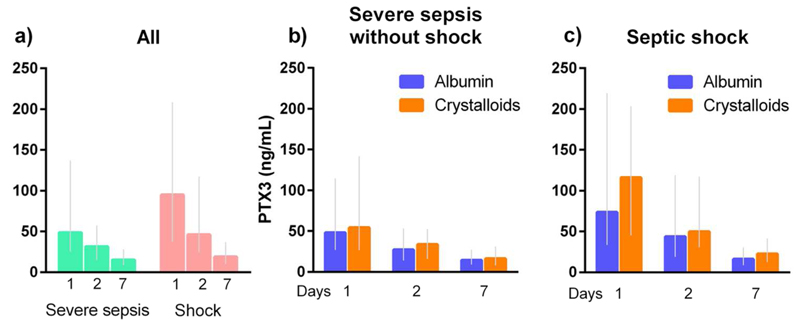
Patients with shock had higher PTX3 concentration than those without (p<0.0001) at all time-points (days 1, 2 and 7), and it appeared to drop more over time in the former than in the latter patients (p for interaction time by shock = 0.0004) (Figure 2a). Circulating PTX3 concentration was also consistently higher in patients randomized to crystalloids than in those assigned albumin (p=0.03), but this difference was limited to patients with septic shock (p=0.005), whereas there was no treatment effect in those with severe sepsis without shock (p=0.82). Of note, the time courses were not different in either sub-group (interaction time by treatment p=0.90 and 0.55, in severe sepsis without shock and in septic shock, respectively) (Figure 2b,c).
Figure 2. Time-course of PTX3 concentration by shock and randomized treatment.
Plasma PTX3 concentrations on days 1, 2 and 7 are reported in patients with the three time-points available, i.e. 311 with severe sepsis without shock (151 randomized to albumin, 160 to crystalloids) and 377 with septic shock (190 randomized to albumin, 187 to crystalloids). Data are shown as median [Q1-Q3] after stratification for sepsis severity alone (a) or sepsis severity and randomized treatment (b,c). Repeated measures analysis of variance were done on log-transformed concentrations. In all patients: time p<0.0001, shock p<0.0001, interaction time by shock p=0.0004; in patients with severe sepsis without shock: time p<0.0001, treatment p=0.82, interaction time by treatment p=0.90; in patients with septic shock: time p<0.0001, treatment p=0.005, interaction time by treatment p=0.55.
PTX3 and clinical outcomes
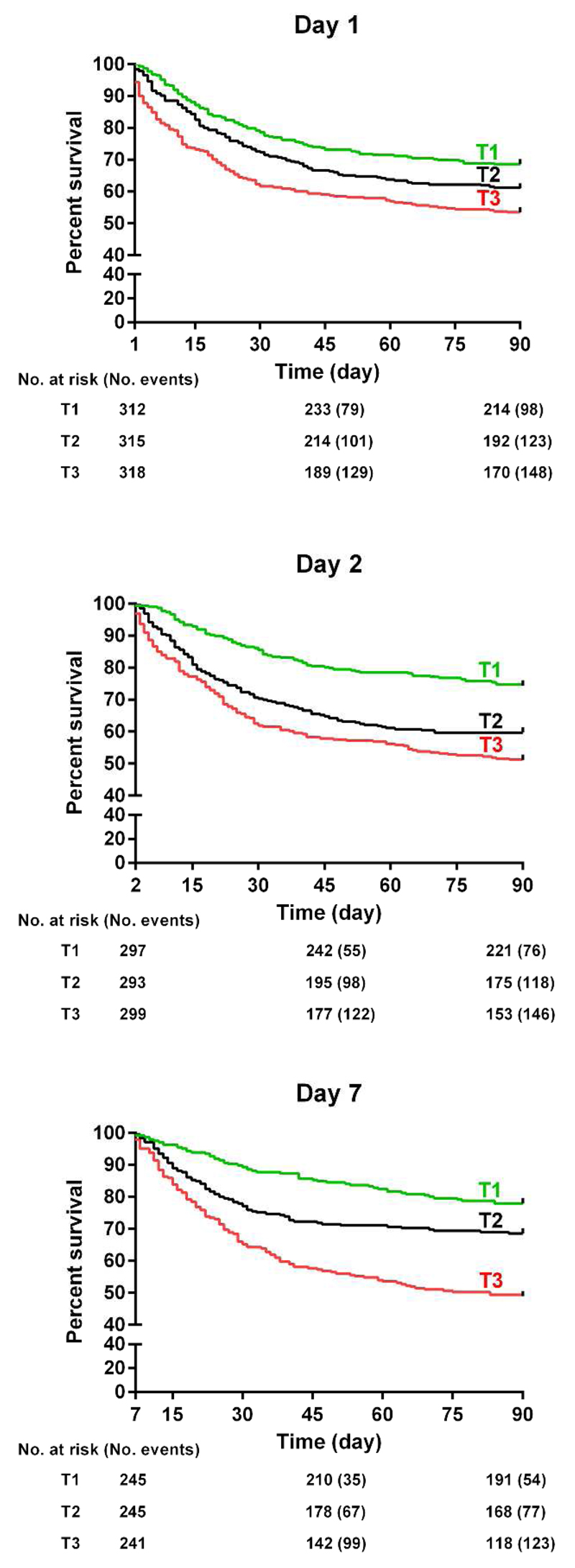
All-cause mortality in the ICU and at 90 days was respectively 26.4% and 39.0%. Clinical characteristics of the study population according to 90-day mortality are shown in Table S3. Plasma concentrations of PTX3 were consistently higher at each time point (days 1, 2 and 7) in those who died than in survivors after 90 days (p<0.0001, Figure S3). Survival analysis at 90 days with Kaplan-Meier curves by tertiles of PTX3 concentration on days 1, 2, and 7 is shown in Figure 3. There was a graded increase in the risk of death across tertiles of PTX3 levels (log rank test: p<0.0001 for the three time points). However, after adjustment for all confounders, including the circulating biomarkers hs-cTnT and presepsin, neither the PTX3 concentration on day 1 nor on day 2 was associated with increased 90-day mortality, and only PTX3 on day 7 showed a significant, independent predictive value (HR =1.13 [1.01-1.26], p=0.04). Finally, although higher PTX3 levels on day 1 appeared to be associated with clinical outcomes assessed at post-hoc analyses, after adjustment for clinical and laboratory variables, the only significant association was between PTX3 concentration on day 1 and acute kidney injury (Table S4).
Figure 3. Kaplan-Meier survival curves for 90-day mortality by tertiles of PTX3 on days 1, 2 and 7.
The number of patients at risk and events are shown below the curves. Tertiles of PTX3: day 1 (lower < 41.1, mid 41.1 to 137.5, upper > 137.5 ng/mL), day 2 (lower < 25.3, mid 25.3 to 54.6, upper >54.6 ng/mL), day 3 (lower < 11.8, mid 11.8 to 26.9, upper >26.9 ng/mL). P for log-rank test <0.0001 for all.
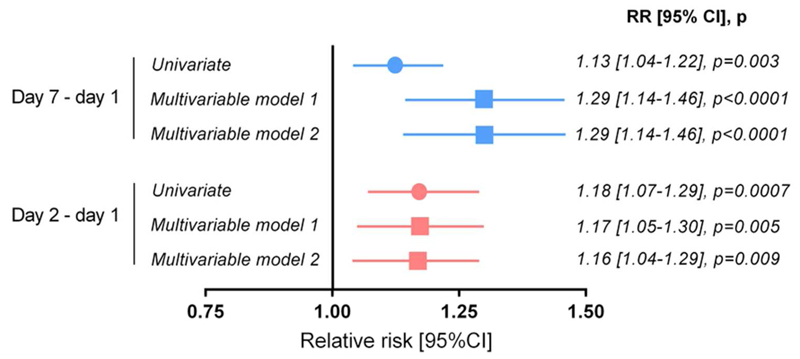
We then evaluated the predictive value of the changes of PTX3 concentrations from day 1 to day 2 or from day 1 to day 7. The prognostic models included demographic and clinical variables associated with 90-day mortality, and were also adjusted for baseline PTX3 concentrations. The drop over the first two days of PTX3 was more marked in survivors (-0.66±0.87 on a log scale) than in the patients who died (-0.45±0.84, p=0.0007), with similar differences in patients with or without shock. Changes in PTX3 from day 1 to day 2 and from day 1 to day 7 (Figure S4) were independently associated with 90-day mortality, after adjustment for clinical variables and two circulating biomarkers of inflammation and cardiac injury, presepsin and high-sensitive cardiac troponin T (Figure 4). PTX3 had a significant, but limited prognostic discrimination with area under the receiver operating characteristic (ROC) curves for 90-day mortality comprised between 0.57 and 0.60 (data not shown). Finally, there was no significant interaction between PTX3 concentration on day 1 and treatment allocation (albumin or crystalloids) on 90-day mortality (p=0.31).
Figure 4. Modified Poisson regression models for 90-day mortality by changes in PTX3 concentrations over time.
The relation between continuous changes in PTX3 concentrations between days 1, 2 or 7 was estimated with simple or adjusted modified Poisson regression models. Multivariable model 1 included the log-transformed concentration of PTX3 on day 1, age, sex, body-mass index, reason for ICU admission, SAPS II and SOFA scores, pre-existing conditions (liver disease, chronic obstructive pulmonary disease, chronic renal failure, immunodeficiency, congestive or ischemic heart disease), mean arterial pressure, fraction of inspired oxygen FiO2, diuresis, serum concentrations of lactate, albumin, bilirubin and creatinine, blood platelets, septic shock at randomization, mechanical ventilation, antibiotics at randomization, positive blood culture. Model 2 = model 1 plus plasma concentrations of presepsin and high-sensitive cardiac troponin T on day 1.
Discussion
In a large cohort of well-characterized patients with severe sepsis or septic shock in the ALBIOS trial: (1) the PTX3 concentrations on day 1 were extremely elevated, compared with other clinical conditions (cardiovascular diseases, cancer, autoimmune diseases) and mostly associated with hyperlactatemia, tachycardia, and lower body-mass index (BMI), (2) higher PTX3 on day 1 predicted incident new organ failures, (3) a slow decrease in PTX3, but not absolute levels on day 1 and 2, were independently associated with higher mortality, and (4) patients with septic shock assigned to albumin and crystalloids had lower PTX3 levels through days 1 to 7 as compared to those randomized to crystalloids alone. In addition, higher PTX3 on day 1 was associated with greater hemodynamic instability, as indicated by a smaller proportion of patients with adequate mean arterial pressure, or a greater need for vasoactive drugs during the first 24 hours. These findings may explain the close relationship between PTX3 and the number and degree of organ dysfunctions, as indicated by the SOFA scores for all its components.
Some of the findings of the current study confirm those previously reported [13], such as the higher concentrations of circulating PTX3 in patients with urinary tract infections, and the persistent higher PTX3 levels in non-survivors compared to survivors. Nonetheless, the 10-fold larger sample size and the presence of two randomly assigned strategies of fluid therapy (crystalloids with or without human albumin) meant we could confidently assess the behavior of PTX3 in different subgroups, as well the effect of albumin.
The strong associations of higher PTX3 and incident cardiovascular or renal failure are consistent with previous evidence of PTX3 involvement in acute coronary syndromes/acute myocardial infarction, on one side [6,27], and in chronic kidney disease on the other [28]. In experimental models of myocardial infarction and coronary artery disease, PTX3 appeared to have a significant protective role in the pathogenesis of myocardial injury [29,30]. Similarly, lower levels of circulating PTX3 appeared to be also associated with enhanced neutrophil recruitment, cell death, activation of coagulation cascades, and inflammatory responses in the lung in an experimental model of acute lung injury [31,32]. In different models of tissue repair, PTX3 deficiency was associated with greater deposition and persistence of fibrin [33]. These findings increase the difficulty of interpreting high circulating levels of PTX3 as a marker of injury or of a protective response of the body. They further highlight the complexity of the pathogenesis of sepsis and its difference from other diseases with putative inflammatory involvement, with appreciable imbalances between excessive activation of the immune system and an immune-paralysis state [2]. Along this line of reasoning, the higher PTX3 levels in patients with immunosuppression at the time of developing sepsis may be interpreted as an extreme, though ineffective, defensive response. It is worth mentioning that this “response” has been shown to be orchestrated by rapid release of PTX3 stored in PMN followed by an increase in its synthesis [34,35]. Although we did not do a differential leukocyte count, it is conceivable that the lower levels of PTX3 on day 1, together with higher counts of circulating leukocytes may indicate lower activation of PMN and ultimately less severe disease.
Lower PTX3 levels on day 1 were also associated with a greater probability of achieving hemodynamic stability during the first 24 hours of treatment, suggesting a possible role of PTX3 in the pathogenesis of microvascular alterations and endothelial dysfunction, as previously reported [36].
In relation to the prognostic value of early measurements of plasma PTX3, we found that changes in PTX3 over time, but not its single levels, independently predicted mortality, confirming preliminary findings in a smaller cohort of septic patients [13], and data from patients with chronic heart failure [7]. The prognostic value of PTX3 changes remained significant also when considering variations during the early phases of severe sepsis or septic shock, i.e. from day 1 to day 2. These results strongly suggest the clinical utility of serial monitoring of circulating PTX3, particularly during the early, “active” phase of this syndrome, when monitoring the response to therapy is so important.
In the ALBIOS trial, patients were randomized to receive either albumin and crystalloids or crystalloids alone for volume replacement [17]. Albumin replacement did not improve survival at 28 and 90 days in the overall population, but provided slight, significant hemodynamic advantages, as well as a survival advantage in the subgroup of patients with septic shock [17,37]. In the current study, PTX3 concentrations of patients with septic shock receiving albumin were significantly lower during the first seven days than in patients receiving only crystalloids, whereas there was no significant treatment effect in patients with severe sepsis without shock. In addition, there was no significant interaction on PTX3 concentration between time and the treatment. Nonetheless, the consistently lower levels of PTX3 in the first seven days, in association with albumin replacement, suggest an effect of exogenous albumin on circulating PTX3, either indirect or direct. First, a low PTX3 concentration in the albumin group may be simply an indirect marker of its anti-inflammatory effect [38], with a consequent potential improvement of organ function [39]. Second, although to the best of our knowledge this has never been investigated, we may hypothesize a direct binding of albumin to the PTX3 molecule, considering the extensive ligand ability of human albumin [40], leading to reduced PTX3 levels in patients with higher serum albumin concentrations.
Finally, since the anti-oxidant activity of human albumin, related to the free cysteine residue [40], reduces overall plasma oxidative stress [38], conceivably higher serum albumin concentrations protect PTX3 from redox-sensitive oligomerization [41], leading to faster clearance of PTX3 while in the monomeric state, as compared to patients not receiving albumin.
The present study has some limitations. First, it did not include all the patients enrolled in the ALBIOS trial, as only a subgroup of the participating centers was involved in the biomarker sub-study. Nonetheless, in the biomarker sub-study these centers enrolled the majority of the patients randomized in the main trial, guaranteeing representativeness of the disease and the entire cohort investigated. Second, as ALBIOS was a pragmatic trial, the first blood sample was drawn not at study enrolment, which would have created an undue workload in a critical phase of the trial (and patient treatment), but on average 20±6 hours later (on the morning of day 1), after completion of the diagnostic workup, optimization of treatments, and starting study treatment. Therefore, PTX3 levels on day 1 may have been affected by early study treatment. Nonetheless, the availability of plasma collections at further time points allows the evaluation of the time-course of the biomarkers with reasonable accuracy during the first, most critical phase of the clinical treatment.
Conclusions
The present study confirms in a large cohort of patients enrolled in a controlled, multicenter randomized clinical trial, that circulating PTX3 concentrations are elevated in severe sepsis and even higher in septic shock. They peak within the first day after diagnosis, and they tightly correlate with prevalent and incident organ failures. Impaired normalization of these levels in the first few days independently predict unfavorable clinical outcomes (hemodynamic instability, multi-organ dysfunction and failure) that may ultimately compromise survival. The lower levels of PTX3 in patients with septic shock treated with albumin are consistent with the survival benefit observed in this subgroup [18].
Supplementary Material
Acknowledgments (Permissions obtained)
The following investigators participated in the ALBIOS Biomarkers study group (all from Italy): Paola Bruzzone, Francesca Pagan and Riccarda Russo, Fondazione IRCCS Ca' Granda - Ospedale Maggiore Policlinico, Milano; Andrea Confalonieri, Chiara Abbruzzese and Beatrice Vergnano, Ospedale San Gerardo, Monza; Stefano Faenza, Antonio Siniscalchi and Elisabetta Pierucci, Policlinico Universitario S. Orsola Malpighi, Bologna; Andrea Noto, Angelo Pezzi and Paolo Spanu, A.O. San Paolo-Polo Universitario, Milano; Vieri Parrini and Roberto Oggioni, Ospedale del Mugello, Borgo San Lorenzo; Giovanni Stefano Pasetti, Maria Cinzia Casadio and Rosa Buontempo, Ospedale S. Giovanni di Dio, Orbetello Scalo; Sara Carrer, Francesca Piccoli and Tatiana Rizzi, Ospedale di Circolo, Rho; Anselmo Caricato, Monica La Sala and Alessandra Antonaci, Università Cattolica - Policlinico Universitario A. Gemelli, Roma; Paola Fassini, Silvia Paganini and Virginia Porta, Ospedale Civile, Legnano; Gabriella Moise, Silvia Marell and Mirella Furia, Ospedale "Città di Sesto San Giovanni", Sesto San Giovanni; Maria Cristina Urbano, Roberta Carobbi and Simona Poleni, Ospedale della Misericordia, Grosseto; Hassan Kandil, Andrea Ballotta and Fabrizio Bettini, IRCCS Policlinico San Donato, San Donato Milanese; Manlio Sanseverino, Alessandro Gatta and Francesca Cecchini, Ospedale Ceccarini, Riccione; Luca Guatteri and Gabriella Ciceri, Ospedale di Desio, Desio; Ferdinando Raimondi and Roberto Colombo, A.O. Luigi Sacco, Milano; Sandra Ferraris and Massimo Borelli, Ospedale di Treviglio, Treviglio; Valentina Bellato and Franco Cancellieri, Istituto Clinico Humanitas, Rozzano; Silvia Senni and Ester Bertocchi, Azienda Bolognini, Seriate; Paola Ferri and Gianpietro Moioli, Ospedale Maggiore, Crema; Andrea Fedele and Alexandra Molin, Azienda Ospedaliera Universitaria "San Martino", Genova; Giovanni Salati and Pierpaolo Salsi, Arcispedale S. Maria Nuova, Reggio Emilia; Emanuela Brunori and Daniele Elisei, Ospedale Macerata ASUR Marche, Macerata; Giuseppe Maggio and Federico Guardia Nicola, IRCCS Fondazione Policlinico” San Matteo” / Rianimazione 1, Pavia; Marco Cavana and Giacomo Morelli, Azienda USL “V. Parini” Valle d'Aosta, Aosta; Arturo Guarino and Michele Isetta, Azienda Ospedaliera "Villa Scassi", Sampierdarena; Giorgio Tulli and Valerio Mangani, Ospedale San Giovanni di Dio, Firenze; Nicola Rossi, Ospedale San Giuseppe - Milano Cuore, Milano; Marta Ferrari, IRCCS Fondazione Policlinico "San Matteo" / Rianimazione 2, Pavia; Francesco Bona, Istituto per la Cura e la Ricerca del Cancro, Candiolo; Monica Vay, ASO Universitaria S. Giovanni Battista di Torino – Molinette, Torino; Teresa Bartoli, Azienda Sanitaria Santa Maria Annunziata, Bagno a Ripoli; Mauro Gallo, A.O. Ordine Mauriziano, Presidio Ospedaliero "Umberto I", Torino; Katiuscia Vettoretto, Ospedale di Manerbio, Manerbio; Mauro Della Morte, Azienda Ospedaliera della Valtellina e della Valchiavenna, Sondalo; Enrico Boselli, Azienda Ospedaliera "Guido Salvini", Garbagnate; Daniela Puscio, Ospedale "Vito Fazzi", Lecce; Monia Bovo, ULSS 15 Alta Padovana, Camposampiero; Antonio Galzerano, Ospedale Santa Maria della Misericordia, Perugia; Manuela Carli, Ospedale del Ceppo, Pistoia; Giovanni Zagara, Azienda Ospedaliera “V. Cervello”, Palermo.
Source of the support: The ALBIOS trial was funded by grants from the Italian Medicines Agency (AIFA, grant FARM6JS3R5, 2006) and the Italian Ministry of Health (Ricerca Finalizzata 2011-2012, grant RF-2011-02348358). AM and BB were also supported by Fondazione CARIPLO (Contract No. 2015/0564) and European Research Council (Contract No. 669415).
Role of the sponsors: the funding sources had no role in the development of the research and manuscript.
Abbreviations
- ALBIOS
Albumin Italian Outcome Sepsis trial
- ANOVA
analysis of variance
- BMI
body-mass index
- hs-cTnT
high sensitive cardiac troponin T
- ICU
intensive care unit
- PMN
polymorphonuclear leukocytes
- PTX3
pentraxin-3
- RIFLE
risk, injury, failure, loss, and end-stage kidney injury
- RR
relative risk
- SD
standard deviation
- SIRS
systemic inflammatory reaction syndrome
- SOFA
Sequential Organ Failure Assessment
Footnotes
Conflicts of interest: Alberto Mantovani and Barbara Bottazzi are inventors of patents on pentraxin-3 and obtain royalties on related reagents. The other authors have nothing to disclose.
References
- 1.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Singer M. Biomarkers in sepsis. Curr Opin Pulm Med. 2013;19:305–9. doi: 10.1097/MCP.0b013e32835f1b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garlanda C, Jaillon S, Doni A, Bottazzi B, Mantovani A. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr Opin Immunol. 2016;38:39–44. doi: 10.1016/j.coi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 7.Latini R, Gullestad L, Masson S, et al. Pentraxin-3 in chronic heart failure: the CORONA and GISSI-HF trials. Eur J Heart Fail. 2012;14:992–9. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Qu X, Liu F, Wang C. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediators Inflamm. 2014;2014:421–9. doi: 10.1155/2014/421429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ristagno G, Varpula T, Masson S, et al. Elevations of inflammatory markers PTX3 and sST2 after resuscitation from cardiac arrest are associated with multiple organ dysfunction syndrome and early death. Clin Chem Lab Med. 2015;53:1847–57. doi: 10.1515/cclm-2014-1271. [DOI] [PubMed] [Google Scholar]
- 10.Infante M, Allavena P, Garlanda C, et al. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int J Cancer. 2016;138:983–91. doi: 10.1002/ijc.29822. [DOI] [PubMed] [Google Scholar]
- 11.Bastrup-Birk S, Munthe-Fog L, Skjoedt MO, et al. Pentraxin-3 level at admission is a strong predictor of short-term mortality in a community-based hospital setting. J Intern Med. 2015;277:562–72. doi: 10.1111/joim.12294. [DOI] [PubMed] [Google Scholar]
- 12.Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–7. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–9. doi: 10.1007/s00134-010-1752-5. [DOI] [PubMed] [Google Scholar]
- 14.Bastrup-Birk S, Skjoedt MO, Munthe-Fog L, Strom JJ, Ma YJ, Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 2013;8:e73119. doi: 10.1371/journal.pone.0073119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uusitalo-Seppälä R, Huttunen R, Aittoniemi J, et al. Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One. 2013;8:e53661. doi: 10.1371/journal.pone.0053661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuello F, Shankar-Hari M, Mayr U, et al. Redox state of pentraxin 3 as a novel biomarker for resolution of inflammation and survival in sepsis. Mol Cell Proteomics. 2014;13:2545–57. doi: 10.1074/mcp.M114.039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–21. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 18.Masson S, Caironi P, Fanizza C, et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015;41:12–20. doi: 10.1007/s00134-014-3514-2. [DOI] [PubMed] [Google Scholar]
- 19.Mauri T, Coppadoro A, Bombino M, et al. Alveolar pentraxin 3 as an early marker of microbiologically confirmed pneumonia: a threshold-finding prospective observational study. Crit Care. 2014;18:562. doi: 10.1186/s13054-014-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47:471–7. doi: 10.1515/CCLM.2009.110. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 24.Kellum JA, Bellomo R, Ronco C. The concept of acute kidney injury and the RIFLE criteria. Contrib Nephrol. 2007;156:10–16. doi: 10.1159/000102010. [DOI] [PubMed] [Google Scholar]
- 25.Masson S, Caironi P, Fanizza C, et al. Sequential N-Terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock. Crit Care Med. 2016;44:707–16. doi: 10.1097/CCM.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 26.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 27.Mjelva ØR, Pönitz V, Brügger-Andersen T, Grundt H, Staines H, Nilsen DW. Long-term prognostic utility of pentraxin 3 and D-dimer as compared to high-sensitivity C-reactive protein and B-type natriuretic peptide in suspected acute coronary syndrome. Eur J Prev Cardiol. 2015 Dec 3; doi: 10.1177/2047487315619733. pii: 2047487315619733. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Sjöberg B, Qureshi AR, Heimbürger O, et al. Association between levels of pentraxin 3 and incidence of chronic kidney disease in the elderly. J Intern Med. 2016;279:173–9. doi: 10.1111/joim.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salio M, Chimenti S, De Angelis N, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–64. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 30.Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 31.Deban L, Russo RC, Sironi M, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 32.Han B, Haitsma JJ, Zhang Y, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37:334–42. doi: 10.1007/s00134-010-2067-2. [DOI] [PubMed] [Google Scholar]
- 33.Doni A, Musso T, Morone D, et al. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J Exp Med. 2015;212:905–25. doi: 10.1084/jem.20141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maugeri N, Rovere-Querini P, Slavich M, et al. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J Immunol. 2011;187:970–9. doi: 10.4049/jimmunol.1100261. [DOI] [PubMed] [Google Scholar]
- 36.Carrizzo A, Lenzi P, Procaccini C, et al. Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/Matrix Metalloproteinase-1 pathway. Circulation. 2015;131:1495–505. doi: 10.1161/CIRCULATIONAHA.114.014822. [DOI] [PubMed] [Google Scholar]
- 37.Caironi P, Gattinoni L. Proposed benefits of albumin from the ALBIOS trial: a dose of insane belief. Crit Care. 2014;18:510. doi: 10.1186/s13054-014-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinlan GJ, Margarson MP, Mumby S, Evans TW, Gutteridge JM. Administration of albumin to patients with sepsis syndrome: a possible beneficial role in plasma thiol repletion. Clin Sci (Lond) 1998;95:459–65. [PubMed] [Google Scholar]
- 39.Dubois MJ, Orellana-Jimenez C, Melot C, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34:2536–40. doi: 10.1097/01.CCM.0000239119.57544.0C. [DOI] [PubMed] [Google Scholar]
- 40.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–90. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Cuello F, Shankar-Hari M, Mayr U, et al. Redox state of pentraxin 3 as a novel biomarker for resolution of inflammation and survival in sepsis. Mol Cell Proteomics. 2014;13:2545–57. doi: 10.1074/mcp.M114.039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.