Abstract
IL-1R2 was the first decoy receptor to be described. Subsequently receptors which act as pure decoys or scavengers or trigger dampening of cytokine signaling have been described for cytokines and chemokines. Here we review the current understanding of the mode of action and significance in pathology of the chemokine atypical receptor ACKR2, the IL-1 decoy receptor IL-1R2 and the atypical IL-1 receptor family IL-1R8. Decoy and scavenger receptors with no or atypical signaling have emerged as a general strategy conserved in evolution to tune the action of cytokines, chemokines and growth factors.
Introduction
Cytokines are the key mediators of the inflammatory response being responsible for the recruitment to the inflammatory site and immune cell activation. Cytokines are also orchestrating the correct development of the adaptive immune response determining immune response polarization, tolerance and memory [1].
Cytokine activity need to be tight regulated for the correct development of the immune response. Indeed the robust responses necessary to fight pathogens need to be controlled for the termination of immune responses to avoid inflammation-induced tissue damage and autoimmunity.
Several mechanisms of negative regulation of the cytokine system have been described acting both at transcriptional and post transcriptional levels. One of these latter mechanism of regulation is represented by cytokine receptors that have been called decoys. The concept of a “receptor” was originally formulated by Langley in the ‘20s. It includes ligand recognition with high specificity and signaling, or being part of a recognition and signaling concept. Decoy receptors are able to recognize certain inflammatory cytokines with high affinity and specificity, but are structurally incapable of signaling or they signal through pathways different to the canonical receptors with whom they share ligands. They are negative regulators because they act as molecular traps for the agonist and for signaling receptor components or they function as scavenger receptor driving cytokines to intracellular degradative compartments [2].
The first decoy receptor identified was the interleukin-1 type II receptor (IL-1RII) [3] and subsequently decoy receptors have been identified in many cytokine receptor families such as the tumor necrosis factor receptor (TNFR), IL-1R, IL-10R and IL-6R families. Moreover, atypical receptors with scavenger function have been identified in the chemokine system. Therefore, the use of decoy and scavenger receptors is a general strategy of regulation of primary pro-inflammatory cytokines and chemokines to fine-tune and regulate innate and adaptive immunity [4] .
Decoy and scavenger receptors in the chemokine system
Chemokines are cytokines mainly involved in leukocyte chemoattraction acting through a distinct family of G protein coupled receptors (GPCR) [5]. Among chemokine receptors both functional and structural decoy receptors that negatively regulate chemokine function have been identified.
Under exposure to pro- and anti-inflammatory stimuli, inflammatory chemokine receptors (such as CCR2) can become functional decoy receptors meaning that they are unable to elicit migration while they are still able to sequester and scavenge inflammatory chemokines. These functional decoy receptors are uncoupled from G proteins while maintain the ability to internalize and degrade the ligand promoting the resolution of the inflammation [6]. The precise mechanism by which this uncoupling is happening was not fully investigated, but several negative regulators of G protein signaling have been found [7].
Soluble and seven-transmembrane domain chemokine decoy receptors have been found in virus and parasites and represent an important strategy to modulate leukocyte recruitment and subvert the immune response of the host [4].
In addition a subgroup of structural decoy chemokine receptors called atypical chemokine receptors (ACKRs) have been identified in vertebrate genomes [8]. They are supposed to be evolved from canonical chemokine receptors from which they differ especially in the aminoacid composition of intracellular motifs relevant for signal transduction [9].
ACKRs, upon ligand engagement, do not elicit migration or conventional signaling responses. Indeed they are unable to couple to G proteins while they are still able to activate other intracellular pathways such as β-arrestin dependent ones. ACKRs regulate inflammatory and immune reactions in several ways, including by acting as decoy receptors and scavenger receptors that modulate chemokine bioavailability by transporting chemokines to intracellular degradative compartments or, in the case of polarized cells, to the opposite side of the cell monolayer. ACKRs can also modulate the chemokine system by regulating the expression or signaling of other canonical chemokine receptors [10]. These receptors are now officially nomenclated and the ACKR subfamily includes: ACKR1, previously called Duffy Antigen Receptor for Chemokines (DARC); ACKR2, also known as D6 or CCBP2; ACKR3, also called CXC-chemokine receptor 7 (CXCR7) or RDC1; ACKR4, previously called CC-chemokine receptor-like 1 (CCRL1) and also known as CCX-CKR. Two other molecules (CCRL2 and PITPNM3) could be included in the ACKR family with the names 'ACKR5' and 'ACKR6' [11].
In the last years we have focused our attention on one of these receptors, ACKR2, and we have contributed in the characterization of its function as a negative regulator of inflammation. Here we will describe in details ACKR2 structure and function.
ACKR2 (D6 or CCBP2)
ACKR2 was cloned in 1997 by two groups [12, 13]. ACKR2 is able to bind almost all inflammatory CC chemokines, ligands of the canonical chemokine receptors from CCR1 to CCR5 [14]. In humans the ACKR2 gene is located on chromosome 3p21.3, a region that includes a cluster of chemokine receptor genes. It shares high homology sequence with CC chemokine receptors but having selected mutations in the intracellular motifs important for signal trasduction [9]. In particular both murine and human D6 lacked the canonical DRYLAIV motif, which was found in all other cloned chemokine receptors and is important for G protein signaling and migration. We therefore to set out to test the hypothesis that ACKR2/D6 was a decoy receptor [15].
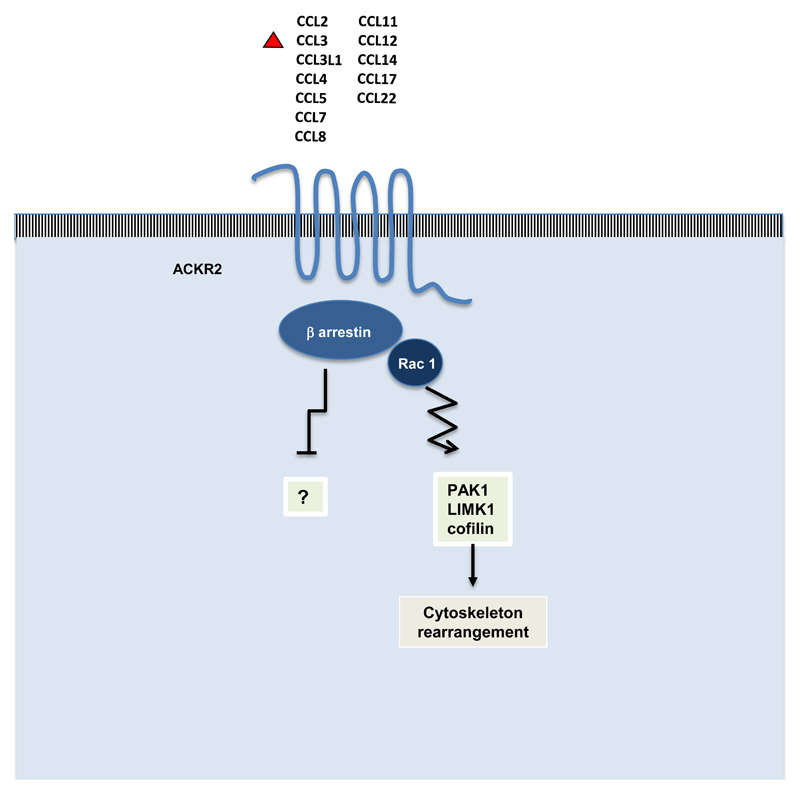
ACKR2 is a constituively internalizing receptor and in steady state most of the molecules are present in intracellular recycling compartment. After ligand binding ACKR2 does not induce leukocyte migration while it activates a β arrestin-dependent pathway that increases the number of receptors on the cell surface to optimize chemokine uptake and delivery to lysosomal compartments [16, 17] (Fig. 1). For this reason ACKR2 is not a pure decoy receptor because it is able to drive an intracellular signalling that is devoted to the optimization of its scavenger function. This property appear to be shared by the 4 ACKRs.
Figure 1. Ligand recognition and atypical signalling by the chemokine decoy and scavenger receptor ACKR2.
ACKR2 binds with high affinity inflammatory CC chemokines; it signals through β-arrestin and the small GTPase Rac1 activating cytoskeleton rearrangement in order to optimize the chemokine scavenging activity. It is not known if the interaction of ACKR2 with β-arrestin could have an inhibitory function on other signalling pathways.
ACKR2 is expressed by lymphatic endothelial cell [18], by trophoblasts in the placenta [19] and by some leukocytes such as alveolar macrophages [20] and innate-like B cells [21].
ACKR2 KO mice have increased number of circulating inflammatory monocytes [22] and defects in lymphatic vessel density and function [23] compared to WT mice. When challenged with inflammatory stimuli ACKR2 KO exibith exacerbated inflammatory reactions in barrier tissues such as the skin, lung, gut and placenta that result in worse pathology [10]. ACKR2 KO have also defect in cardiac remodelling after myocardial infarction [24] and are not able to control infectious diseases such as Mycobacterium tubercolosis [25]. All these phenotypes found in ACKR2 KO mice are mainly reconducted to lack of chemokine clearance, increased infiltration of inflammatory cells and lack of inflammation resolution. In addition it was found that ACKR2 expressed by leukocytes restrict their inflammatory phenotype by inhibiting neutrophil migration [26] [27]and regulating macrophage cytokine production and efferocytosis [28].
Finally, in the cancer context ACKR2 acts as a tumor suppressor gene inhibiting inflammation that fuel cancer in mouse models of chemically induced skin tumors [29] and in colon cancer [30]. In Kaposi sarcoma ACKR2 is expressed by tumor spindle cells and is downregulated in more advanced states by the oncogenic pathway KRas/Braf/MEK/ERK [31].
Unexpectedly it was found that ACKR2 deletion can be protective in several disease models that often involve adaptive immunity: ACKR2 KO mice are resistant to experimental autoimmune encephalomyelitis ([32]), have reduced graft versus host disease (GVHD) [22] and reduced renal inflammation in a model of diabetic nephropathy [33]. This protection to autoimmune or immune mediated diseases was explained by a defect in dendritic cell migration or increased myeloid derived suppressor cells. It has to be underlined that Hansell et al have recently reported that in four models of autoimmune diseases ACKR2 KO mice are not protected by disease development, not confirming previously published papers. They have also found that lack of ACKR2 is not affecting T cell priming while increased interleukin-17 (IL-17) production [27].
All these data indicate that considering ACKR2 only as an anti-inflammatory molecule can be overly simplistic. Indeed ACKR2 is able to scavenge CC chemokines such as CCL17 and CCL22 that are able to recruit Th2 and Treg cells and are important in the generation of chronic immune responses [14]. For this reason, ACKR2 deletion or inhibition could produce complex results depending on the pathological context.
Few data are available on the expression and regulation of ACKR2 in humans. In the preeclampatic placentas ACKR2 expression is lower than in normal placentas [34]. Elevated ACKR2 expression was found in skin of psoriatic patients around psoriatic plaques and in patients’ peripheral blood leukocytes [35]. ACKR2 was also expressed by human vascular tumors and in Kaposi sarcoma were a negative correlation was found with disease progression rate [31]. Finally ACKR2 is expressed by human colon carcinomas and its downregulation correlates to more invasive tumors [36].
Decoy receptors of the IL-1 system
The IL-1 system is involved in protective host responses in infections and inflammation, as well as in the activation of innate and adaptive lymphoid cells [37, 38]. The deregulated or excessive activation of these receptors is the potential cause of dangerous and detrimental local or systemic inflammatory reactions, as well as autoimmune or allergic responses. The system consists in receptors (collectively called ILR) and accessory proteins (AcP) and their ligands (IL-1α and IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ), as well as negative regulators, which include antagonists (IL-1Ra, IL-36Ra), decoy receptors (e.g. IL-1R2), scavengers (e.g IL-1R2 and IL-18BP), dominant negative molecules (IL-1R2), miRNAs and other mechanisms, acting extracellularly or intracellularly. Here, we will focus on the decoy receptor IL-1R2, the first decoy receptor identified that served to define the decoy paradigm, and on IL-1R8, a novel negative regulator of inflammatory and adaptive responses.
1. The decoy receptor IL-1R2
IL-1R2 was identified in the '90s as a highly conserved receptor localized in the cluster of IL-1R family members [39, 40] and acting as an IL-1 decoy receptor [3, 41] (Fig. 2). IL-1R2 shares 28% aminoacid homology with the extracellular portion of IL-1R1, but differs for the absence of a TIR domain and for the presence of only a short 29 amino acid-long cytoplasmic tail. The protein is glycosylated and consists of a 386 amino acid-long protein with a molecular mass of 68 kD. It also exists as a soluble form, generated by enzymatic cleavage, in particular by the metalloproteinase ADAM17 [42–44], or by alternative splicing [45].
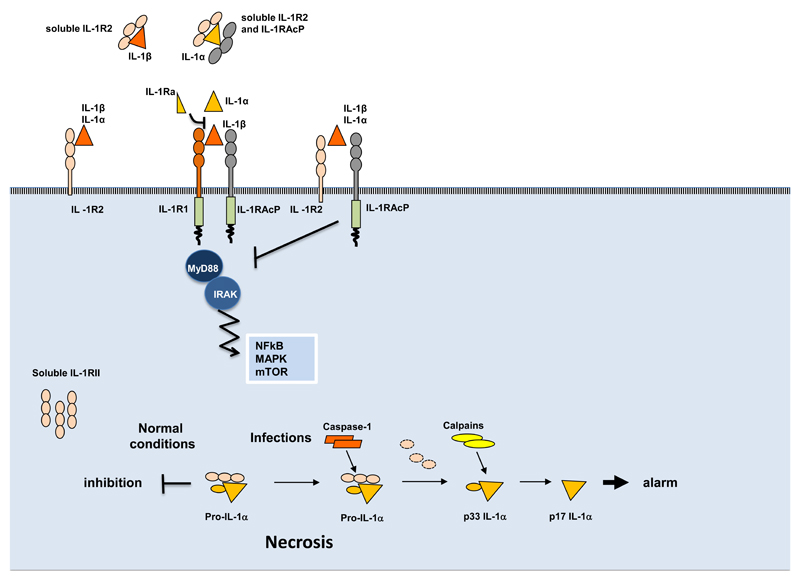
Figure 2. Mechanisms of negative regulation mediated by IL-1R2.
In membrane-bound or soluble form IL-1R2 captures IL-1β and IL-1α with high affinity, but not IL-1Ra; it interacts with IL-1RAcp inhibiting the formation of a signalling receptor complex, and with soluble IL-1RAcP, which increases the affinity for IL-1α and IL-1β. In cytosol soluble form, IL-1R2 interacts with pro-IL1α preventing its pro-inflammatory activity during necrosis. In infectious conditions, caspase-1 cleaves IL-1R2 and allows the secretion of IL-1α.
IL-1R2 binds IL-1α and IL-1β without inducing signalling, thus acting as a molecular trap for the agonists of the signalling IL-1R1 [3, 46]. IL-1R2 also forms a complex with IL-1 and IL-1RAcP, exerting a dominant-negative effect by sequestering IL-1RAcP [47–49]. In addition, soluble IL-1R2, normally present in the circulation at high concentrations, binds pro-IL-1β and blocks its processing by caspase-1 [50], as well as IL-1α and IL-1β [46, 51]. Finally, soluble IL-1R2 localizes in the cytosol of IL-1R2 expressing cells [52] where it interacts with pro-IL-1α, preventing its cleavage, and tuning IL-1α-dependent sterile-inflammation during necrosis [53] (Fig. 2).
The anti-inflammatory role of IL-1R2 was demonstrated in in vivo studies, including chronic skin inflammation [54], arthritis [55–57], endometriosis [58], and heart transplantation [59] or autoimmune myocarditis by Th17 cells [60]. Recently, IL-1R2-deficient mice have been generated and the actual in vivo role of IL-1R2 was demonstrated in a model of collagen-induced arthritis [61]. In mice, IL-1R2 was highly expressed in neutrophils, but no effects of IL-1R2-deficiency were observed in this cell type. In contrast, even if low expression was observed in monocytes and macrophages, the expression of inflammatory mediators in response to IL-1 was greatly enhanced in IL-1R2-deficient macrophages [61].
In humans, IL-1R2 is expressed at very high levels by a limited set of cell types, including monocytes, macrophages (in particular IL-4 or IL-13 activated M2 macrophages), neutrophils and B cells [3, 46, 62, 63]. Several anti-inflammatory mediators induce IL-1R2 expression, such as glucocorticoid hormones, prostaglandins, aspirin, Th2 cytokines (IL-4 and IL-13), IL-10 and IL-27 [3, 62, 64–69], suggesting that induction of IL-1R2 contributes to the anti-inflammatory effect of these mediators. Recently, fasting has been shown to induce IL-1R2 expression and IL-1 resistance in mice [70]. In contrast, pro-inflammatory and chemotactic molecules inhibit IL-1R2 expression while increasing the expression of the signalling complex. Among these are bacterial lipopolysaccharide (LPS) [71], interferon γ (IFNγ) [67], chemoattractants, reactive oxygen intermediates, TNFα and phorbol myristate acetate [42, 72, 73].
In the mouse, expression of IL-1R2 has been described in DC [74], CD4+ T cells [75], T regulatory cells [76, 77], monocytes [78], neutrophils [79]. IL-1R2 was described in different macrophagic cells, including atherosclerosis-associated macrophages [80], microglial cells [81, 82], and osteoclasts [83].
Increased blood concentrations or local expression of IL-1R2 have been detected in a wide range of human disorders, and it has been proposed that this increase may reflect the activation of endogenous pathways of negative regulation of inflammation. For instance, circulating levels of soluble IL-1R2 increase in critically ill patients with infectious conditions such as sepsis, acute meningococcal infection, experimental endotoxemia, operative trauma, necrotizing enterocolitis in preterm infants, and acute respiratory distress syndrome [84], often correlating with the severity of the disease [69, 85, 86]. IL-1R2 is expressed by basal epithelial cells of the skin in normal conditions and is upregulated in psoriasis [87]. IL-1R2 is upregulated in some tumors, including pancreatic ductal adenocarcinoma [88], prostatic cancer, benign prostatic hyperplasia [89] and ovarian cancer [90]. IL-1R2 increased levels were detected in sera of multiple sclerosis patients [91], in the synovial fluid and plasma of rheumatoid arthritis patients, negatively correlating with the severity of disease [92, 93], or in the cerebrospinal fluid of patients with Alzheimer’s disease, reflecting disease progression [94].
Genome-wide association studies identified several candidate genes potentially involved in inflammatory bowel disease pathogenesis, including IL-1R2 [95]. In addition, IL-1R2 was upregulated in remission compared with active Ulcerative colitis and controls and was proposed to act as a homeostatic regulator during remission of ulcerative colitis [96].
2. IL-1R8
IL-1R8, also known as TIR8 or SIGIRR, is a negative regulator of ILRs and TLRs [38] (Fig. 3). The human IL-1R8 gene is located on chromosome 11, band p15.5 and comprises 10 exons spanning about 11.7 kb [97]. The coded protein is 410 amino acids long with peculiar features compared to the other IL-1R family members: IL-1R8 consists in a single extracellular Ig domain, whereas the other ILRs have three, a transmembrane domain, a cytoplasmic TIR domain and a 95aa C terminal tail, longer than all the other C terminal tails of ILRs. Moreover, in the IL-1R8 TIR domain two conserved residues, Ser447 and Tyr536, are replaced by Cys222 and Leu305, suggesting unconventional signaling transduction activity of the molecule [97, 98]. Murine IL-1R8 is localized on chromosome 7, band F4 and comprises 9 exons spanning about 4 kb. The murine protein is 409 amino acids long and structural characteristics similar to the human one. Both human and murine IL-1R8 present complex N- and O- glycosylations in the extracellular domain, that play a crucial role for the biological function of the molecule [99]. IL-1R8 nucleotide and protein sequences as well as the pattern of expression are conserved among vertebrates, from chicken to humans [100]. This receptor is expressed by epithelial cells in kidney, digestive tract, liver, and lung, in endothelial cells and in leukocytes, in particular monocytes, B and T-lymphocytes, dendritic cells and NK cells [97, 101]. Inflammatory conditions, such as colitis, bacterial infections and stimulation with TLR ligands, down-modulate IL-1R8 mRNA and protein expression, but the mechanisms of regulation are still unclear [102–104]. It has been shown that LPS-dependent p38 activation reduced the binding to the IL-1R8 promoter of SP1, a transcription factor involved in IL-1R8 transcription [102, 105]. Other molecules affecting IL-1R8 expression include the vasoactive intestinal peptide that upregulated IL-1R8 in Pseudomonas aeruginosa infected cornea in mice [106], and Lactobacillus jensenii, a probiotic microorganism, that upregulated IL-1R8 expression via TLR2 in porcine Payer’s antigen presenting cells [107]. Finally, amyloid β decreased the expression of IL-1R8 in microglia through a mechanism possibly involving the PI3 kinase/Akt axes and the transcription factor peroxisome proliferator-activated receptor (PPAR)γ [108].
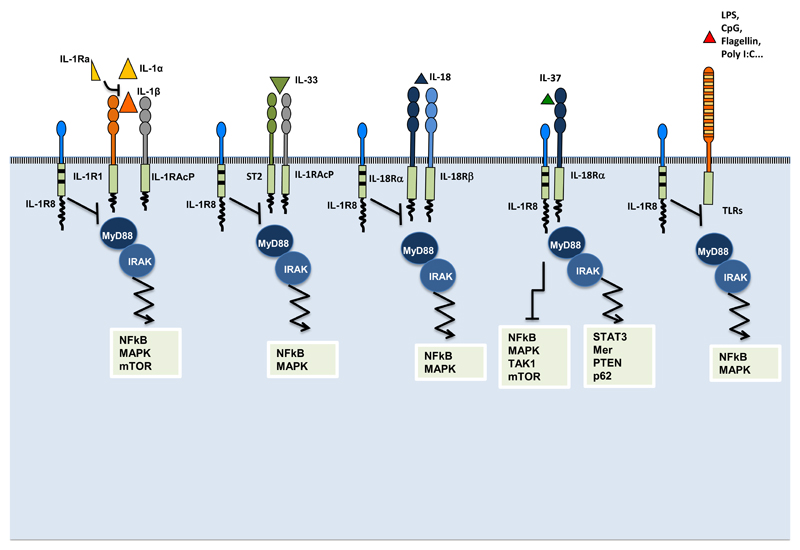
Figure 3. Mechanisms of negative regulation mediated by IL-1R8.
IL-1R8 inhibits IL-1R1, IL-18 and IL-33 signaling by competing with MyD88 and IRAK recruitment at the TIR domain, thus dampening the signaling pathway leading to NF-kB activation. In addition, in T and epithelial cells, IL-1R8 inhibits IL-1-dependent activation of the mTOR pathway and of MAPK, thus controlling cell proliferation and survival. IL-1R8 is also a co-receptor of IL-18Rα for IL-37, necessary for the activation of an anti-inflammatory signaling pathway that involves inhibition of MAPKs, NF-κB and TAK1, the pseudo-starvational effects on the mTOR pathway, and the activation of STAT3, Mer, PTEN and p62. IL-1R8 also inhibits the activation of TLR-dependent signaling pathways leading to MAPK and NF-kB activation.
The mechanism through which IL-1R8 carries out the inhibition of ILR and TLR signaling is still not completely elucidated. IL-1R8 dampens the activation of IL-1R1, IL-1R5/IL-18Ra, IL-1R4/ST2, TLR4, TLR7, TLR9, TLR3 and TLR1/2 [98, 109–112]. IL-1R8 inhibits the dimerization of IL-1R1 and IL-1RAcP induced by IL-1 stimulation (or the dimerization of IL-1R4/ST2 and IL-33R induced by IL-33 stimulation) with its extracellular domain, whereas its intracellular TIR domain binds the TIR domains of adapter molecules such as MyD88, IRAK and TRAF6 [109, 110, 112]. On the contrary, in order to inhibit the TLR4 or TLR7 signaling, the intracellular TIR domain of IL-1R8 is sufficient [110, 112]. In this case, IL-1R8 binds the TLRs through the BB-loop, present in all TIR domain-containing molecules, and blocks their dimerization as well as MyD88 dimerization [113, 114]. Different studies show that IL-1R8 can prevent the dimerization of other TIR containing adapter molecules such as Mal, TRAM and TRIF, but not through the BB-loop [114, 115]. Moreover, IL-1R8 interacts with c-jun and mTOR transcription factors in Th17 cells and intestinal epithelial cells [116, 117].
Very recently it was demonstrated that IL-1R8 binds IL-37, an anti-inflammatory cytokine induced by TLRs and cytokines in blood mononuclear cells, macrophages and epithelial cells. The binding produces the formation of the tripartite complex IL-37/IL-18Rα/IL-1R8 [118]. Therefore IL-1R8 is now considered a co-receptor of IL-1R5/IL-18Rα for IL-37, necessary for the activation of an anti-inflammatory signaling pathway. This interaction triggers multiple intracellular mechanisms leading to modulation of inflammation, which include the inhibition of MAPKs and NF-κB, the pseudo-starvational effects on the mTOR pathway, inhibition of TAK1 and Fyn, and the activation of STAT3, Mer, PTEN and p62 [118, 119] (Fig. 3).
Thus, the negative regulatory activity of IL-1R8 is mediated through different modes of action: IL-1R8 interferes with the TIR domain signalosome formation (with its intracellular domain) and prevents the dimerization between receptors and accessory proteins (by both its extracellular and intracellular domains), thus blocking the signal transduction, or it acts as co-receptor for IL-37 activating an anti-inflammatory program in the cell.
The relevance of IL-1R8 in negatively regulating inflammation has been demonstrated in different pathological conditions, ranging from infections to sterile inflammation and cancer.
In different models of infection (Mycobacterium tuberculosis, Pseudomonas aeruginosa, Candida albican, Aspergillus fumigatus), IL-1R8 deficient mice developed an exaggerated systemic inflammatory response characterized by increased levels of cytokines (mainly IL-1) and leukocyte infiltration in tissues resulting in increased susceptibility to the infection and tissue damage [104, 120, 121]. In agreement with mouse models, a case-population study demonstrated that the presence of three SNPs in IL-1R8 gene correlated with an increased susceptibility to tuberculosis [122]. In contrast, in two different models of infection (urinary tract infection induced by Escherichia coli and pneumonia induced by Streptococcus pneumoniae), IL-1R8 deficient mice showed decreased bacterial load and damage. Thus, in these cases, an increased and fast inflammatory response protected the host [123, 124]. Finally, in the model of Citrobacter rodentium infection IL-1R8 deficient mice were more susceptible compared to wild type mice, because the strong and early inflammatory response rapidly induced the depletion of the commensal microflora in the gut, thus favoring the colonization by the pathogen [125]. These studies underline that IL-1R8 is crucial for the maintenance of the equilibrium between protection against infections and dangerous tissue damaging inflammation.
IL-1R8 is also involved in the development of autoimmunity. For instance, IL-1R8 controls Th17 differentiation, expansion and effector functions, by modulating IL-1 signaling in these cells. IL-1R8 deficient mice were more susceptible to experimental autoimmune encephalomyelitis due to an exaggerated Th17 response in the CNS [116]. IL-1R8 deficiency was also associated to an increased susceptibility to psoriasis due to hyperactivation of γδT cell response [126]. Interestingly, downregulation of IL-1R8 gene expression was observed in patients with psoriasis. IL-1R8 protects mice in different models of rheumatoid arthritis, by suppressing the IL-1 and TLR2 mediated tissue damage [115]. Moreover, different in vivo studies showed the involvement of IL-1R8 in the pathogenesis of systemic lupus erythematosus. IL-1R8 regulates B cell proliferation and DC activation after auto antigens exposure, that stimulates TLR7 and TLR9 [127, 128]. These data are supported by the observation that patients affected by SLE had a reduced frequency of IL-1R8+ CD4+ T cells and in a Chinese population the presence of a genetic variant of the molecule correlated with the susceptibility to SLE [129, 130]. Finally, IL-1R8 was shown to inhibit the Th2 response by blocking IL-1R5/ST2 activation thus controlling allergic inflammatory response. In a model of allergic pulmonary inflammation induced by OVA, IL-1R8-deficiency was associated with increased leukocyte infiltration in lungs and increased levels of IL-5, IL-4 and IgE, suggesting an exacerbated Th2 response [109].
In a model of sterile inflammation of the kidney (ischemia and reperfusion injury), in which DAMPs activate TLR4 and TLR2 in neutrophils and macrophages, in IL-1R8-deficient mice the renal injury was more severe than in wild type mice due to an excessive myeloid cell activation, leukocyte recruitment and cytokine release in the organ [131]. In the brain IL-1R8 protects mice against β-amyloid peptide-induced TLR2 signaling, resulting as a potential therapeutic candidate for Alzheimer’s disease related pathology [108].
Since inflammation has clearly emerged as a novel hallmark of cancer, IL-1R8 function was investigated in tumor development. In particular, in a model of colitis-associated colon cancer, IL-1R8 played a protective role in mice by regulating intestinal permeability, in situ release of chemokines and cytokines and preventing an exceeding cell survival and proliferation mediated by NF-kB activation [120, 132]. In a genetic model of intestinal cancer, in which Apcmin/+ mice spontaneously develop adenomatous polyposis syndrome, IL-1R8-deficiency worsened polyposis by increasing the activation of the Akt-mTOR axis, known to be crucial in tumor initiation [117]. The relevance of these data has recently been shown in humans, where colorectal cancer lesions were shown to express lower levels of IL-1R8 compared to healthy tissues, as a consequence of the expression of a dominant negative isoform lacking exon 8 [99]. This isoform showed an aberrant TIR domain, an increased cytoplasmic retention, a reduced glycosylation and the capability to directly antagonize the full length IL-1R8, resulting in the suppression of the receptor regulatory activity [99]. Finally, IL-1R8 was demonstrated to slow down the initiation and progression of chronic lymphocytic leukemia (CLL) in TCL1 mice through still poorly defined molecular mechanisms [133].
Concluding remarks
Atypical receptors with decoy or decoy and scavenger function have been identified in different classes of cytokines, chemokines and growth factors, as illustrated here using IL-1R2, ACKR2 and IL-1R8 as prototypic examples. Decoy receptors have therefore emerged as a general strategy to regulate the action of cytokines, chemokines and growth factors, conserved in evolution from drosophila to man. In addition to regulating inflammation, there is evidence that the atypical receptor ACKR2 plays a role under homeostatic conditions, for instance in shaping chemokine gradients during lymphatic vessel development. A key aspect of inflammation is the regulation of resolution. Evidence suggests that the molecules discussed here play a key role in promoting resolution of inflammation. Indeed, non-resolving smouldering inflammation is an essential component of the tumor microenvironment [134]. A better understanding of the role of atypical decoy and scavenger receptors may pave the way to innovative strategies to control inflammation and cancer-related inflammation.
Acknowledgements
Alberto Mantovani is supported by Associazione Italiana per la Ricerca sul Cancro; European Commission (ERC Advanced Grant); Fondazione Cariplo; Italian Ministry of Health.
References
- [1].Mantovani A. Cytokines and their receptors. In: Ratcliffe MJH, editor. Encyclopedia of Immunobiology, Vol.2, Molecular Immunology. Elsevier; Amsterdam: 2016. pp. 438–604. [Google Scholar]
- [2].Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends in immunology. 2001;22(6):328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- [3].Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261(5120):472–5. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- [4].Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature reviews. Immunology. 2006;6(12):907–18. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- [5].Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci (Landmark Ed) 2009;14:540–51. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- [6].D'Amico G, Frascaroli G, Bianchi G, Transidico P, Doni A, Vecchi A, Sozzani S, Allavena P, Mantovani A. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nature immunology. 2000;1(5):387–91. doi: 10.1038/80819. [DOI] [PubMed] [Google Scholar]
- [7].Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172(11):7031–42. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- [8].Bonecchi R, Savino B, Borroni EM, Mantovani A, Locati M. Chemokine decoy receptors: structure-function and biological properties. Current topics in microbiology and immunology. 2010;341:15–36. doi: 10.1007/82_2010_19. [DOI] [PubMed] [Google Scholar]
- [9].Daiyasu H, Nemoto W, Toh H. Evolutionary Analysis of Functional Divergence among Chemokine Receptors, Decoy Receptors, and Viral Receptors. Frontiers in microbiology. 2012;3:264. doi: 10.3389/fmicb.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nature reviews. Immunology. 2013;13(11):815–29. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- [11].Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, Rot A, Sozzani S, Thelen M. New nomenclature for atypical chemokine receptors. Nature immunology. 2014;15(3):207–8. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- [12].Bonini JA, Martin SK, Dralyuk F, Roe MW, Philipson LH, Steiner DF. Cloning, expression, and chromosomal mapping of a novel human CC-chemokine receptor (CCR10) that displays high-affinity binding for MCP-1 and MCP-3. DNA and cell biology. 1997;16(10):1249–56. doi: 10.1089/dna.1997.16.1249. [DOI] [PubMed] [Google Scholar]
- [13].Nibbs RJ, Wylie SM, Yang J, Landau NR, Graham GJ. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. The Journal of biological chemistry. 1997;272(51):32078–83. doi: 10.1074/jbc.272.51.32078. [DOI] [PubMed] [Google Scholar]
- [14].Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, Van Damme J, et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004;172(8):4972–6. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- [15].Fra AM, Locati M, Otero K, Sironi M, Signorelli P, Massardi ML, Gobbi M, Vecchi A, Sozzani S, Mantovani A. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J Immunol. 2003;170(5):2279–82. doi: 10.4049/jimmunol.170.5.2279. [DOI] [PubMed] [Google Scholar]
- [16].Bonecchi R, Borroni EM, Anselmo A, Doni A, Savino B, Mirolo M, Fabbri M, Jala VR, Haribabu B, Mantovani A, Locati M. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood. 2008;112(3):493–503. doi: 10.1182/blood-2007-08-108316. [DOI] [PubMed] [Google Scholar]
- [17].Borroni EM, Cancellieri C, Vacchini A, Benureau Y, Lagane B, Bachelerie F, Arenzana-Seisdedos F, Mizuno K, Mantovani A, Bonecchi R, Locati M. beta-arrestin-dependent activation of the cofilin pathway is required for the scavenging activity of the atypical chemokine receptor D6. Science signaling. 2013;6(273):ra30 1–11. S1–3. doi: 10.1126/scisignal.2003627. [DOI] [PubMed] [Google Scholar]
- [18].Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158(3):867–77. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A, Lauri E, Agostinis C, Bulla R, et al. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007;104(7):2319–24. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bazzan E, Saetta M, Turato G, Borroni EM, Cancellieri C, Baraldo S, Savino B, Calabrese F, Ballarin A, Balestro E, Mantovani A, et al. Expression of the atypical chemokine receptor D6 in human alveolar macrophages in COPD. Chest. 2013;143(1):98–106. doi: 10.1378/chest.11-3220. [DOI] [PubMed] [Google Scholar]
- [21].Hansell CA, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, Goodyear CS, Nibbs RJ. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117(20):5413–24. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Savino B, Castor MG, Caronni N, Sarukhan A, Anselmo A, Buracchi C, Benvenuti F, Pinho V, Teixeira MM, Mantovani A, Locati M, et al. Control of murine Ly6C(high) monocyte traffic and immunosuppressive activities by atypical chemokine receptor D6. Blood. 2012;119(22):5250–60. doi: 10.1182/blood-2011-10-388082. [DOI] [PubMed] [Google Scholar]
- [23].Lee KM, Danuser R, Stein JV, Graham D, Nibbs RJ, Graham GJ. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. The EMBO journal. 2014;33(21):2564–80. doi: 10.15252/embj.201488887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Recalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, et al. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2206–13. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- [25].Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A, Sireci G, Nebuloni M, Caceres N, Cardona PJ, Dieli F, et al. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med. 2008;205(9):2075–84. doi: 10.1084/jem.20070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rot A, McKimmie C, Burt CL, Pallas KJ, Jamieson T, Pruenster M, Horuk R, Nibbs RJ, Graham GJ. Cell-autonomous regulation of neutrophil migration by the D6 chemokine decoy receptor. J Immunol. 2013;190(12):6450–6. doi: 10.4049/jimmunol.1201429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hansell CA, MacLellan LM, Oldham RS, Doonan J, Chapple KJ, Anderson EJ, Linington C, McInnes IB, Nibbs RJ, Goodyear CS. The atypical chemokine receptor ACKR2 suppresses Th17 responses to protein autoantigens. Immunology and cell biology. 2015;93(2):167–76. doi: 10.1038/icb.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pashover-Schallinger E, Aswad M, Schif-Zuck S, Shapiro H, Singer P, Ariel A. The atypical chemokine receptor D6 controls macrophage efferocytosis and cytokine secretion during the resolution of inflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(9):3891–900. doi: 10.1096/fj.11-194894. [DOI] [PubMed] [Google Scholar]
- [29].Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. The Journal of clinical investigation. 2007;117(7):1884–92. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, Mantovani A, et al. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 2010;59(2):197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- [31].Savino B, Caronni N, Anselmo A, Pasqualini F, Borroni EM, Basso G, Celesti G, Laghi L, Tourlaki A, Boneschi V, Brambilla L, et al. ERK-dependent downregulation of the atypical chemokine receptor D6 drives tumor aggressiveness in Kaposi sarcoma. Cancer immunology research. 2014;2(7):679–89. doi: 10.1158/2326-6066.CIR-13-0202. [DOI] [PubMed] [Google Scholar]
- [32].Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3-/- mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176(7):4399–409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- [33].Zheng S, Coventry S, Cai L, Powell DW, Jala VR, Haribabu B, Epstein PN. Renal Protection by Genetic Deletion of the Atypical Chemokine Receptor ACKR2 in Diabetic OVE Mice. Journal of diabetes research. 2016;2016:5362506. doi: 10.1155/2016/5362506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cho GJ, Lee ES, Jin HM, Lee JH, Kim YS, Oh MJ, Seol HJ, Hong SC, Kim HJ. Placental expression of D6 decoy receptor in preeclampsia. Obstetrics & gynecology science. 2015;58(5):333–9. doi: 10.5468/ogs.2015.58.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Singh MD, King V, Baldwin H, Burden D, Thorrat A, Holmes S, McInnes IB, Nicoll R, Shams K, Pallas K, Jamieson T, et al. Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. The American journal of pathology. 2012;181(4):1158–64. doi: 10.1016/j.ajpath.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Langenes V, Svensson H, Borjesson L, Gustavsson B, Bemark M, Sjoling A, Quiding-Jarbrink M. Expression of the chemokine decoy receptor D6 is decreased in colon adenocarcinomas. Cancer immunology, immunotherapy : CII. 2013;62(11):1687–95. doi: 10.1007/s00262-013-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140(6):935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Copeland NG, Silan CM, Kingsley DM, Jenkins NA, Cannizzaro LA, Croce CM, Huebner K, Sims JE. Chromosomal location of murine and human IL-1 receptor genes. Genomics. 1991;9(1):44–50. doi: 10.1016/0888-7543(91)90219-5. [DOI] [PubMed] [Google Scholar]
- [40].Morrison RN, Young ND, Nowak BF. Description of an Atlantic salmon (Salmo salar L.) type II interleukin-1 receptor cDNA and analysis of interleukin-1 receptor expression in amoebic gill disease-affected fish. Fish & shellfish Immunol. 2012;32(6):1185–90. doi: 10.1016/j.fsi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- [41].McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, Grubin CE, Wignall JM, Jenkins NA, Brannan CI, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. The EMBO journal. 1991;10(10):2821–32. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Orlando S, Sironi M, Bianchi G, Drummond AH, Boraschi D, Yabes D, Mantovani A. Role of metalloproteases in the release of the IL-1 type II decoy receptor. The Journal of biological chemistry. 1997;272(50):31764–9. doi: 10.1074/jbc.272.50.31764. [DOI] [PubMed] [Google Scholar]
- [43].Lorenzen I, Lokau J, Dusterhoft S, Trad A, Garbers C, Scheller J, Rose-John S, Grotzinger J. The membrane-proximal domain of A Disintegrin and Metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II. FEBS lett. 2012;586(8):1093–100. doi: 10.1016/j.febslet.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [44].Uchikawa S, Yoda M, Tohmonda T, Kanaji A, Matsumoto M, Toyama Y, Horiuchi K. ADAM17 regulates IL-1 signaling by selectively releasing IL-1 receptor type 2 from the cell surface. Cytokine. 2015;71(2):238–45. doi: 10.1016/j.cyto.2014.10.032. [DOI] [PubMed] [Google Scholar]
- [45].Liu C, Hart RP, Liu XJ, Clevenger W, Maki RA, De Souza EB. Cloning and characterization of an alternatively processed human type II interleukin-1 receptor mRNA. The Journal of biological chemistry. 1996;271(34):20965–72. doi: 10.1074/jbc.271.34.20965. [DOI] [PubMed] [Google Scholar]
- [46].Re F, Sironi M, Muzio M, Matteucci C, Introna M, Orlando S, Penton-Rol G, Dower SK, Sims JE, Colotta F, Mantovani A. Inhibition of interleukin-1 responsiveness by type II receptor gene transfer: a surface "receptor" with anti-interleukin-1 function. J Exp Med. 1996;183(4):1841–50. doi: 10.1084/jem.183.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lang D, Knop J, Wesche H, Raffetseder U, Kurrle R, Boraschi D, Martin MU. The type II IL-1 receptor interacts with the IL-1 receptor accessory protein: a novel mechanism of regulation of IL-1 responsiveness. J Immunol. 1998;161(12):6871–7. [PubMed] [Google Scholar]
- [48].Malinowsky D, Lundkvist J, Laye S, Bartfai T. Interleukin-1 receptor accessory protein interacts with the type II interleukin-1 receptor. FEBS lett. 1998;429(3):299–302. doi: 10.1016/s0014-5793(98)00467-0. [DOI] [PubMed] [Google Scholar]
- [49].Laye S, Lundkvist J, Bartfai T. Human/mouse interleukin-1 receptor/receptor accessory protein interactions in IL-1beta-induced NFkappaB activation. FEBS letters. 1998;429(3):307–11. doi: 10.1016/s0014-5793(98)00537-7. [DOI] [PubMed] [Google Scholar]
- [50].Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 1995;92(5):1714–8. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18(1):87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- [52].Kawaguchi Y, Nishimagi E, Tochimoto A, Kawamoto M, Katsumata Y, Soejima M, Kanno T, Kamatani N, Hara M. Intracellular IL-1alpha-binding proteins contribute to biological functions of endogenous IL-1alpha in systemic sclerosis fibroblasts. Proc Natl Acad Sci U S A. 2006;103(39):14501–6. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38(2):285–95. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rauschmayr T, Groves RW, Kupper TS. Keratinocyte expression of the type 2 interleukin 1 receptor mediates local and specific inhibition of interleukin 1-mediated inflammation. Proc Nat Acad Sci U S A. 1997;94(11):5814–9. doi: 10.1073/pnas.94.11.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bessis N, Guery L, Mantovani A, Vecchi A, Sims JE, Fradelizi D, Boissier MC. The type II decoy receptor of IL-1 inhibits murine collagen-induced arthritis. Eur J Immunol. 2000;30(3):867–75. doi: 10.1002/1521-4141(200003)30:3<867::AID-IMMU867>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [56].Dawson J, Engelhardt P, Kastelic T, Cheneval D, MacKenzie A, Ramage P. Effects of soluble interleukin-1 type II receptor on rabbit antigen-induced arthritis: clinical, biochemical and histological assessment. Rheumatology. 1999;38(5):401–6. doi: 10.1093/rheumatology/38.5.401. [DOI] [PubMed] [Google Scholar]
- [57].Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, Abramson SB, Amin AR. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. The Journal of biological chemistry. 2000;275(51):40307–15. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- [58].Khoufache K, Bondza PK, Harir N, Daris M, Leboeuf M, Mailloux J, Lemyre M, Foster W, Akoum A. Soluble human IL-1 receptor type 2 inhibits ectopic endometrial tissue implantation and growth: identification of a novel potential target for endometriosis treatment. The American journal of pathology. 2012;181(4):1197–205. doi: 10.1016/j.ajpath.2012.06.022. [DOI] [PubMed] [Google Scholar]
- [59].Simeoni E, Dudler J, Fleury S, Li J, Pagnotta M, Pascual M, von Segesser LK, Vassalli G. Gene transfer of a soluble IL-1 type 2 receptor-Ig fusion protein improves cardiac allograft survival in rats. Eur J Cardiothorac Surg. 2007;31(2):222–8. doi: 10.1016/j.ejcts.2006.10.042. [DOI] [PubMed] [Google Scholar]
- [60].Chang H, Wang Y, Wu W, Li G, Hanawa H, Zou J. Hydrodynamics-based delivery of an interleukin-1 receptor II fusion gene ameliorates rat autoimmune myocarditis by inhibiting IL-1 and Th17 cell polarization. Int J Mol Med. 2013;31(4):833–40. doi: 10.3892/ijmm.2013.1276. [DOI] [PubMed] [Google Scholar]
- [61].Shimizu K, Nakajima A, Sudo K, Liu Y, Mizoroki A, Ikarashi T, Horai R, Kakuta S, Watanabe T, Iwakura Y. IL-1 receptor type 2 suppresses collagen-induced arthritis by inhibiting IL-1 signal on macrophages. J Immunol. 2015;194(7):3156–68. doi: 10.4049/jimmunol.1402155. [DOI] [PubMed] [Google Scholar]
- [62].Colotta F, Saccani S, Giri JG, Dower SK, Sims JE, Introna M, Mantovani A. Regulated expression and release of the IL-1 decoy receptor in human mononuclear phagocytes. J Immunol. 1996;156(7):2534–41. [PubMed] [Google Scholar]
- [63].Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- [64].Spriggs MK, Nevens PJ, Grabstein K, Dower SK, Cosman D, Armitage RJ, McMahan CJ, Sims JE. Molecular characterization of the interleukin-1 receptor (IL-1R) on monocytes and polymorphonuclear cells. Cytokine. 1992;4(2):90–5. doi: 10.1016/1043-4666(92)90042-p. [DOI] [PubMed] [Google Scholar]
- [65].Re F, Muzio M, De Rossi M, Polentarutti N, Giri JG, Mantovani A, Colotta F. The type II "receptor" as a decoy target for interleukin 1 in polymorphonuclear leukocytes: characterization of induction by dexamethasone and ligand binding properties of the released decoy receptor. J Exp Med. 1994;179(2):739–43. doi: 10.1084/jem.179.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Colotta F, Re F, Muzio M, Polentarutti N, Minty A, Caput D, Ferrara P, Mantovani A. Interleukin-13 induces expression and release of interleukin-1 decoy receptor in human polymorphonuclear cells. The Journal of biological chemistry. 1994;269(17):12403–6. [PubMed] [Google Scholar]
- [67].Dickensheets HL, Donnelly RP. IFN-gamma and IL-10 inhibit induction of IL-1 receptor type I and type II gene expression by IL-4 and IL-13 in human monocytes. J Immunol. 1997;159(12):6226–33. [PubMed] [Google Scholar]
- [68].Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF-alpha and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J Immunol. 2010;185(11):7047–56. doi: 10.4049/jimmunol.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Daun JM, Ball RW, Burger HR, Cannon JG. Aspirin-induced increases in soluble IL-1 receptor type II concentrations in vitro and in vivo. J Leukoc Biol. 1999;65(6):863–6. doi: 10.1002/jlb.65.6.863. [DOI] [PubMed] [Google Scholar]
- [70].Joesting JJ, Moon ML, Gainey SJ, Tisza BL, Blevins NA, Freund GG. Fasting Induces IL-1 Resistance and Free-Fatty Acid-Mediated Up-Regulation of IL-1R2 and IL-1RA. Front Immunol. 2014;5:315. doi: 10.3389/fimmu.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Penton-Rol G, Orlando S, Polentarutti N, Bernasconi S, Muzio M, Introna M, Mantovani A. Bacterial lipopolysaccharide causes rapid shedding, followed by inhibition of mRNA expression, of the IL-1 type II receptor, with concomitant up-regulation of the type I receptor and induction of incompletely spliced transcripts. J Immunol. 1999;162(5):2931–8. [PubMed] [Google Scholar]
- [72].Colotta F, Orlando S, Fadlon EJ, Sozzani S, Matteucci C, Mantovani A. Chemoattractants induce rapid release of the interleukin 1 type II decoy receptor in human polymorphonuclear cells. J Exp Med. 1995;181(6):2181–6. doi: 10.1084/jem.181.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Orlando S, Matteucci C, Fadlon EJ, Buurman WA, Bardella MT, Colotta F, Introna M, Mantovani A. TNF-alpha, unlike other pro- and anti-inflammatory cytokines, induces rapid release of the IL-1 type II decoy receptor in human myelomonocytic cells. J Immunol. 1997;158(8):3861–8. [PubMed] [Google Scholar]
- [74].Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, Contassot E, Bachmann MF, French LE, Oxenius A, Kundig TM. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proc Natl Acad Sci U S A. 2011;108(44):18055–60. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Iwai H, Inaba M. Fetal thymus graft prevents age-related hearing loss and up regulation of the IL-1 receptor type II gene in CD4(+) T cells. J Neuroimmunol. 2012;250(1–2):1–8. doi: 10.1016/j.jneuroim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [76].Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113(21):5125–33. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mercer F, Kozhaya L, Unutmaz D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PloS one. 2010;5(1):e8639. doi: 10.1371/journal.pone.0008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Drevets DA, Schawang JE, Mandava VK, Dillon MJ, Leenen PJ. Severe Listeria monocytogenes infection induces development of monocytes with distinct phenotypic and functional features. J Immunol. 2010;185(4):2432–41. doi: 10.4049/jimmunol.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Martin P, Palmer G, Vigne S, Lamacchia C, Rodriguez E, Talabot-Ayer D, Rose-John S, Chalaris A, Gabay C. Mouse neutrophils express the decoy type 2 interleukin-1 receptor (IL-1R2) constitutively and in acute inflammatory conditions. J Leuk Biol. 2013;94(4):791–802. doi: 10.1189/jlb.0113035. [DOI] [PubMed] [Google Scholar]
- [80].Pou J, Martinez-Gonzalez J, Rebollo A, Rodriguez C, Rodriguez-Calvo R, Martin-Fuentes P, Cenarro A, Civeira F, Laguna JC, Alegret M. Type II interleukin-1 receptor expression is reduced in monocytes/macrophages and atherosclerotic lesions. Biochim Biophys acta. 2011;1811(9):556–63. doi: 10.1016/j.bbalip.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [81].Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN. Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem. 2002;83(4):754–63. doi: 10.1046/j.1471-4159.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- [82].McNamee EN, Ryan KM, Kilroy D, Connor TJ. Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1beta-induced neurotoxicity. Eur J Pharmacol. 2010;626(2–3):219–28. doi: 10.1016/j.ejphar.2009.09.054. [DOI] [PubMed] [Google Scholar]
- [83].Trebec DP, Chandra D, Gramoun A, Li K, Heersche JN, Manolson MF. Increased expression of activating factors in large osteoclasts could explain their excessive activity in osteolytic diseases. J Cell Biochem. 2007;101(1):205–20. doi: 10.1002/jcb.21171. [DOI] [PubMed] [Google Scholar]
- [84].Kovach MA, Stringer KA, Bunting R, Wu X, San Mateo L, Newstead MW, Paine R, Standiford TJ. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir Res. 2015;16:29. doi: 10.1186/s12931-015-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, Leung KT, Poon TC, Lee KH, Li K, Fok TF, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PloS one. 2012;7(5):e36977. doi: 10.1371/journal.pone.0036977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Muller B, Peri G, Doni A, Perruchoud AP, Landmann R, Pasqualini F, Mantovani A. High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: association of high decoy receptor levels with glucocorticoid administration. J Leukoc Biol. 2002;72(4):643–9. [PubMed] [Google Scholar]
- [87].Debets R, Hegmans JP, Croughs P, Troost RJ, Prins JB, Benner R, Prens EP. The IL-1 system in psoriatic skin: IL-1 antagonist sphere of influence in lesional psoriatic epidermis. J Immunol. 1997;158(6):2955–63. [PubMed] [Google Scholar]
- [88].Ruckert F, Dawelbait G, Winter C, Hartmann A, Denz A, Ammerpohl O, Schroeder M, Schackert HK, Sipos B, Kloppel G, Kalthoff H, et al. Examination of apoptosis signaling in pancreatic cancer by computational signal transduction analysis. PloS one. 2010;5(8):e12243. doi: 10.1371/journal.pone.0012243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ricote M, Garcia-Tunon I, Bethencourt FR, Fraile B, Paniagua R, Royuela M. Interleukin-1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer. 2004;100(7):1388–96. doi: 10.1002/cncr.20142. [DOI] [PubMed] [Google Scholar]
- [90].Laios A, O'Toole SA, Flavin R, Martin C, Ring M, Gleeson N, D'Arcy T, McGuinness EP, Sheils O, Sheppard BL, JJ OL. An integrative model for recurrence in ovarian cancer. Mol cancer. 2008;7:8. doi: 10.1186/1476-4598-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F, Drulovic J. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207(1–2):101–6. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [92].Arend WP, Malyak M, Smith MF, Jr, Whisenand TD, Slack JL, Sims JE, Giri JG, Dower SK. Binding of IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J Immunol. 1994;153(10):4766–74. [PubMed] [Google Scholar]
- [93].Jouvenne P, Vannier E, Dinarello CA, Miossec P. Elevated levels of soluble interleukin-1 receptor type II and interleukin-1 receptor antagonist in patients with chronic arthritis: correlations with markers of inflammation and joint destruction. Arthritis Rheum. 1998;41(6):1083–9. doi: 10.1002/1529-0131(199806)41:6<1083::AID-ART15>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [94].Garlind A, Brauner A, Hojeberg B, Basun H, Schultzberg M. Soluble interleukin-1 receptor type II levels are elevated in cerebrospinal fluid in Alzheimer's disease patients. Brain Res. 1999;826(1):112–6. doi: 10.1016/s0006-8993(99)01092-6. [DOI] [PubMed] [Google Scholar]
- [95].Keita M, AinMelk Y, Pelmus M, Bessette P, Aris A. Endometrioid ovarian cancer and endometriotic cells exhibit the same alteration in the expression of interleukin-1 receptor II: to a link between endometriosis and endometrioid ovarian cancer. J Obstet Gynaecol Res. 2011;37(2):99–107. doi: 10.1111/j.1447-0756.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- [96].Mora-Buch R, Dotti I, Planell N, Calderon-Gomez E, Jung P, Masamunt MC, Llach J, Ricart E, Batlle E, Panes J, Salas A. Epithelial IL-1R2 acts as a homeostatic regulator during remission of ulcerative colitis. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Thomassen E, Renshaw BR, Sims JE. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine. 1999;11(6):389–99. doi: 10.1006/cyto.1998.0452. [DOI] [PubMed] [Google Scholar]
- [98].Lech M, Garlanda C, Mantovani A, Kirschning CJ, Schlondorff D, Anders HJ. Different roles of TiR8/Sigirr on toll-like receptor signaling in intrarenal antigen-presenting cells and tubular epithelial cells. Kidney Int. 2007;72(2):182–92. doi: 10.1038/sj.ki.5002293. [DOI] [PubMed] [Google Scholar]
- [99].Zhao J, Bulek K, Gulen MF, Zepp JA, Karagkounis G, Martin BN, Zhou H, Yu M, Liu X, Huang E, Fox PL, et al. Human Colon Tumors Express a Dominant-Negative Form of SIGIRR That Promotes Inflammation and Colitis-Associated Colon Cancer in Mice. Gastroenterology. 2015;149(7):1860–1871 e8. doi: 10.1053/j.gastro.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Riva F, Polentarutti N, Tribbioli G, Mantovani A, Garlanda C, Turin L. The expression pattern of TIR8 is conserved among vertebrates. Vet Immunol Immunopathol. 2009 doi: 10.1016/j.vetimm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [101].Polentarutti N, Rol GP, Muzio M, Bosisio D, Camnasio M, Riva F, Zoja C, Benigni A, Tomasoni S, Vecchi A, Garlanda C, et al. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw. 2003;14(4):211–8. [PubMed] [Google Scholar]
- [102].Kadota C, Ishihara S, Aziz MM, Rumi MA, Oshima N, Mishima Y, Moriyama I, Yuki T, Amano Y, Kinoshita Y. Down-regulation of single immunoglobulin interleukin-1R-related molecule (SIGIRR)/TIR8 expression in intestinal epithelial cells during inflammation. Clinical and experimental immunology. 2010;162(2):348–61. doi: 10.1111/j.1365-2249.2010.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Batliwalla FM, Li W, Ritchlin CT, Xiao X, Brenner M, Laragione T, Shao T, Durham R, Kemshetti S, Schwarz E, Coe R, et al. Microarray analyses of peripheral blood cells identifies unique gene expression signature in psoriatic arthritis. Mol Med. 2005;11(1–12):21–9. doi: 10.2119/2006-00003.Gulko. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, et al. Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect Immunity. 2012;80(1):100–9. doi: 10.1128/IAI.05695-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ueno-Shuto K, Kato K, Tasaki Y, Sato M, Sato K, Uchida Y, Sakai H, Ono T, Suico MA, Mitsutake K, Tokutomi N, et al. Lipopolysaccharide decreases single immunoglobulin interleukin-1 receptor-related molecule (SIGIRR) expression by suppressing specificity protein 1 (Sp1) via the Toll-like receptor 4 (TLR4)-p38 pathway in monocytes and neutrophils. The Journal of biological chemistry. 2014;289(26):18097–109. doi: 10.1074/jbc.M113.532093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jiang X, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Vasoactive Intestinal Peptide Downregulates Proinflammatory TLRs While Upregulating Anti-Inflammatory TLRs in the Infected Cornea. J Immunol. 2012;189(1):269–78. doi: 10.4049/jimmunol.1200365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Villena J, Suzuki R, Fujie H, Chiba E, Takahashi T, Tomosada Y, Shimazu T, Aso H, Ohwada S, Suda Y, Ikegami S, et al. Immunobiotic Lactobacillus jensenii Modulates the Toll-Like Receptor 4-Induced Inflammatory Response via Negative Regulation in Porcine Antigen-Presenting Cells. Clin Vacc Immunol. 2012;19(7):1038–53. doi: 10.1128/CVI.00199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Costello DA, Carney DG, Lynch MA. alpha-TLR2 antibody attenuates the Abeta-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav Immun. 2015;46:70–9. doi: 10.1016/j.bbi.2015.01.005. [DOI] [PubMed] [Google Scholar]
- [109].Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, Li X. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182(5):2601–9. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nature immunology. 2003;4(9):920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- [111].Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101(10):3522–6. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. The Journal of biological chemistry. 2005;280(26):25233–41. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- [113].Gong J, Wei T, Stark RW, Jamitzky F, Heckl WM, Anders HJ, Lech M, Rossle SC. Inhibition of Toll-like receptors TLR4 and 7 signaling pathways by SIGIRR: a computational approach. J Struct Biol. 2010;169(3):323–30. doi: 10.1016/j.jsb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [114].Guven-Maiorov E, Keskin O, Gursoy A, Nussinov R. A Structural View of Negative Regulation of the Toll-like Receptor-Mediated Inflammatory Pathway. Biophysical journal. 2015;109(6):1214–26. doi: 10.1016/j.bpj.2015.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Drexler SK, Kong P, Inglis J, Williams RO, Garlanda C, Mantovani A, Yazdi AS, Brennan F, Feldmann M, Foxwell BM. SIGIRR/TIR-8 is an inhibitor of Toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 2010;62(8):2249–61. doi: 10.1002/art.27517. [DOI] [PubMed] [Google Scholar]
- [116].Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, Xiao H, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32(1):54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Xiao H, Yin W, Khan MA, Gulen MF, Zhou H, Sham HP, Jacobson K, Vallance BA, Li X. Loss of single immunoglobulin interlukin-1 receptor-related molecule leads to enhanced colonic polyposis in Apc(min) mice. Gastroenterology. 2010;139(2):574–85. doi: 10.1053/j.gastro.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC, Ellisdon AM, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nature immunology. 2015;16(4):354–65. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- [119].Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, Kim S, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(8):2497–502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007;179(5):3119–25. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- [121].Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, et al. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180(6):4022–31. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- [122].Horne DJ, Randhawa AK, Chau TT, Bang ND, Yen NT, Farrar JJ, Dunstan SJ, Hawn TR. Common polymorphisms in the PKP3-SIGIRR-TMEM16J gene region are associated with susceptibility to tuberculosis. J Infect Dis. 2012;205(4):586–94. doi: 10.1093/infdis/jir785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Leemans JC, Butter LM, Teske GJ, Stroo I, Pulskens WP, Florquin S. The toll interleukin-1 receptor (IL-1R) 8/single Ig domain IL-1R-related molecule modulates the renal response to bacterial infection. Infect Immunity. 2012;80(11):3812–20. doi: 10.1128/IAI.00422-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Blok DC, van Lieshout MH, Hoogendijk AJ, Florquin S, de Boer OJ, Garlanda C, Mantovani A, van't Veer C, de Vos AF, van der Poll T. Single immunoglobulin interleukin-1 receptor-related molecule impairs host defense during pneumonia and sepsis caused by Streptococcus pneumoniae. Journal of innate immunity. 2014;6(4):542–52. doi: 10.1159/000358239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sham HP, Yu EY, Gulen MF, Bhinder G, Stahl M, Chan JM, Brewster L, Morampudi V, Gibson DL, Hughes MR, McNagny KM, et al. SIGIRR, a Negative Regulator of TLR/IL-1R Signalling Promotes Microbiota Dependent Resistance to Colonization by Enteric Bacterial Pathogens. PLoS Pathog. 2013;9(8):e1003539. doi: 10.1371/journal.ppat.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Russell SE, Stefanska AM, Kubica M, Horan RM, Mantovani A, Garlanda C, Fallon PG, Walsh PT. Toll IL-1R8/Single Ig IL-1-Related Receptor Regulates Psoriasiform Inflammation through Direct Inhibition of Innate IL-17A Expression by gammadelta T Cells. J Immunol. 2013;191(6):3337–46. doi: 10.4049/jimmunol.1300828. [DOI] [PubMed] [Google Scholar]
- [127].Lech M, Kulkarni OP, Pfeiffer S, Savarese E, Krug A, Garlanda C, Mantovani A, Anders HJ. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med. 2008;205(8):1879–88. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lech M, Skuginna V, Kulkarni OP, Gong J, Wei T, Stark RW, Garlanda C, Mantovani A, Anders HJ. Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis. J Pathol. 2010;220(5):596–607. doi: 10.1002/path.2678. [DOI] [PubMed] [Google Scholar]
- [129].Wang DY, Su C, Chen GM, Pan HF, Wang FM, Liu GL, Hao L, Wang DG, Ye DQ. The decreased frequency of SIGIRR-positive CD4+ T cells in peripheral blood of patients with SLE and its correlation with disease activity. Mol Biol Rep. 2015;42(2):423–30. doi: 10.1007/s11033-014-3783-4. [DOI] [PubMed] [Google Scholar]
- [130].Zhu Y, Wang DG, Yang XK, Tao SS, Huang Q, Pan HF, Feng CC, Ye DQ. Emerging role of SIGIRR rs7396562(T/G) polymorphism in systemic lupus erythematosus in a Chinese population. Inflammation. 2014;37(5):1847–51. doi: 10.1007/s10753-014-9916-z. [DOI] [PubMed] [Google Scholar]
- [131].Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F, Garlanda C, Mantovani A, Anders HJ. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol. 2009;183(6):4109–18. doi: 10.4049/jimmunol.0900118. [DOI] [PubMed] [Google Scholar]
- [132].Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26(4):461–75. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- [133].Bertilaccio MT, Simonetti G, Dagklis A, Rocchi M, Rodriguez TV, Apollonio B, Mantovani A, Ponzoni M, Ghia P, Garlanda C, Caligaris-Cappio F, et al. Lack of TIR8/SIGIRR triggers progression of chronic lymphocytic leukemia in mouse models. Blood. 2011;118(3):660–9. doi: 10.1182/blood-2011-01-329870. [DOI] [PubMed] [Google Scholar]
- [134].Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]