Abstract
The humoral arm of innate immunity is complex and includes various molecules that serve as markers of inflammation with complementary characteristics, such as the short pentraxins C-reactive protein (CRP) and serum amyloid P (SAP) and the long pentraxin PTX3. There is a growing amount of evidence – including mouse and human genetics – that suggests that PTX3 is essential in conferring host resistance against selected pathogens and, moreover, that it plays a dual antagonistic role in the regulation of inflammation. Dissection of such a yin-and-yang role of pentraxins in immunity and inflammation is timely and significant as it may pave the way for better clinical exploitation against various diseases.
Trends
The long pentraxin PTX3 is an essential component of humoral innate immunity and plays a role in the regulation of inflammation.
PTX3 has complex effects on the vasculature, including an interaction with the angiogenic growth factor FGF2 and the regulation of vessel wall tone.
By modulating complement-driven inflammation, PTX3 acts as an oncosuppressor gene in mice and selected human tumors.
By interacting with provisional matrix components, PTX3 contributes to the orchestration of wound healing and tissue repair/remodeling.
PTX3 and the related pentraxins C-reactive protein (CRP) and serum amyloid P (SAP) can exert dual roles in inflammation and antimicrobial resistance, by either exerting a protective function or amplifying tissue damage.
Dissection of the yin–yang role of pentraxins in immunopathology may pave the way towards better exploitation of these molecules as envisaged disease markers and candidate therapeutic agents.
The Humoral Arm of Innate Immunity: Pentraxins
The innate immune response is the first line of defense against invading microbes and tissue damage and comprises both a cellular and a humoral arm. Sensing of microbes and tissue injury through pattern recognition molecules (PRMs) (see Glossary) triggers a complex response in the organism that includes the production of inflammatory cytokines, the activation of the acute phase response, and leukocyte recruitment and polarization 1, 2. This response has general significance in defense and orchestration of tissue repair; however, it is potentially part of the pathogenic mechanisms associated with the original cause of a particular disease – for instance, in sepsis or in chronic inflammatory diseases such as arthritis.
The cellular arm of innate immunity comprises cell-associated PRMs located in different cellular compartments (plasma membrane, endosomes, cytoplasm) belonging to different molecular classes, such as the toll-like receptors (TLRs), the nucleotide-binding oligomerization domain (NOD), and RIG-like receptors, as well as the scavenger receptors. The humoral arm of innate immunity includes biochemically heterogeneous molecules such as the classic short pentraxins CRP) and SAP, the long pentraxin PTX3, complement recognition molecules such as C1q and ficolins, and the collectins. The activation of such molecules represents an important component of the host response against invading microbes or tissue injury [3]. These fluid-phase PRMs harbor antibody-like properties, recognizing microbial moieties, exhibiting opsonic activity, and activating and regulating the complement cascade [4], as well as interacting with extracellular matrix (ECM) components [5]. The expression of humoral PRMs is induced following infection or injury in various cell types and with different kinetics, thus providing a continuous presence of these molecules both in the circulation and in tissues [3]. The liver supports the expression and production of short pentraxins systemically. Other cell types, particularly macrophages, dendritic cells (DCs), and endothelial cells (ECs), produce PTX3 in a gene expression-dependent fashion [4]. Finally, neutrophils act as a reservoir of ready-made PTX3, rapidly released within minutes to sites where tissue damage or microbial stimulation are occurring, thus representing a primary source of soluble PRMs [6].
The rapid production of pentraxins at the systemic level or within tissues has been shown to correlate with the severity of various clinical conditions, such as cardiovascular diseases 7, 8, 9. This in turn appears to sustain their high levels of expression and consequently has often raised the question of their precise role in a given disease: are they simple markers, innocent bystanders, or players in disease pathogenesis 7, 8, 9? In particular, CRP is a widely used biomarker of inflammation in humans; however, lack of strict evolutionary conservation between mouse and human has precluded the use of straightforward genetic approaches to explore its functions in vivo 10, 11. By contrast, gene-targeted mice have allowed us to define the role of PTX3 in innate immunity and inflammation as a predecessor to antibody functions and an active player in tissue remodeling 5, 12, 13, 14.
Even if most animal studies on the long pentraxin PTX3 are supported by human genetic findings suggesting that PTX3 is important in conferring host resistance to infection 15, 16, a prevalent concept has surfaced: depending on the disease context, the cellular source, or the levels of protein released, PTX3 may contribute to disease pathogenesis 12, 17, 18, 19. Consequently, this review focuses on the multifaceted and yin–yang role of PTX3 in humoral innate immunity, microbial defense, and regulation of inflammation as well as in tissue remodeling and repair. This concept is presented at an exciting moment, when key players in innate immunity, the pentraxins, have emerged as being capable of exerting potential contradictory roles in health and disease. This is an opportune time to begin making a greater effort to elucidate the exact roles of PTX3 in disease pathogenesis and to better dissect its precise mechanisms of action in a context-specific manner.
PTX3: Gene and Protein
Pentraxins are conserved multimeric proteins characterized by the presence of a conserved eight-amino-acid sequence, the ‘pentraxin domain’, in their carboxy terminus [4]. Based on the primary structure of the protomer, pentraxins have been divided into short pentraxins, including CRP and SAP, and long pentraxins, such as the prototype long pentraxin PTX3 [4]. CRP and SAP are approximately 25-kDa proteins organized in five identical subunits arranged with pentameric radial symmetry 3, 4. CRP and SAP are the main acute phase proteins produced by human and murine liver, respectively [3]. They act as players in the innate immune response by regulating the complement system, recognizing pathogens, and interacting with Fcγ receptors (FcγRs), thus favoring cytokine secretion and phagocytosis of microorganisms by immune cells [20].
PTX3 was the first long pentraxin identified [4], followed by other long pentraxins including guinea pig apexin, neuronal pentraxin (NP) 1, NP2, neuronal pentraxin receptor (NPR), and PTX4 [21]. The gene encoding PTX3 is localized to chromosome 3 in humans and mice and comprises three exons encoding the leader signal peptide, the N-terminal domain, and the C-terminal pentraxin domain [4]. The expression of PTX3 is mainly induced by inflammatory stimuli such as inflammatory cytokines [tumor necrosis factor alpha (TNFα) and IL-1β] and damage-associated molecular patterns (DAMPs) or microbial moieties. In particular, IL-1 is a major inducer of local PTX3 production in sterile tissue damage, such as in mouse models of acute myocardial infarction and in 3-methylcholanthrene (3-MCA)-induced carcinogenesis 12, 22. In skin-wound healing, TLR sensing and IL-1 amplification are involved in the expression and production of PTX3 [5]. In urinary tract infections (UTIs) mediated by uropathogenic Escherichia coli (UPEC), PTX3 production by human and murine uroepithelial cells has been shown to be under the control of the TLR4/MyD88 signaling pathway [14]. Accordingly, the human and murine PTX3 gene promoters have potential binding sites for many inflammatory transcription factors, including PU.1, AP-1, NF-κB, Sp-1, and NF-IL-6 23, 24. The PI3K/Akt axis and JNK have also been shown to activate PTX3 transcription [14]. In addition, epigenetic mechanisms have been implicated in the regulation of human PTX3 expression, since methylation of a PTX3 enhancer and a PTX3 promoter has been deemed responsible of PTX3 gene silencing in human colorectal and esophageal cancer cell lines [12].
The protein is a multimer with a complex quaternary structure comprising two tetramers linked by interchain bridges to form an octamer of 340 kDa. The protomer comprises 381 amino acids including a 17-amino-acid signal peptide, an N-terminal domain unrelated to any known protein, and a C-terminal pentraxin domain homologous to the short pentraxins CRP and SAP [25]. A single N-glycosylation site localized in the C-terminal domain at Asn220 is occupied by core-fucosylated and sialylated complex-type oligosaccharides [26], which have been shown to modulate the interaction of human PTX3 with complement components such as human C1q [26], factor H [27], and ficolin-1 [28] and to be required for influenza virus recognition [29] and binding to the human and murine adhesion molecule P-selectin in vitro and in vivo [30].
Various cell types, including DCs, monocytes, macrophages, epithelial cells, ECs, fibroblasts, and adipocytes, produce PTX3 on stimulation with inflammatory cytokines (e.g., TNFα, IL-1β), TLR agonists and microbial moieties [e.g., lipopolysaccharide (LPS), Klebsiella pneumoniae outer membrane protein A (KpOmpA), other pathogens such as UPEC and fungal Aspergillus fumigatus] [14] (Figure 1 ). Neutrophils store PTX3 in specific granules and rapidly release it on encounter with microorganisms or on cell stimulation due to infection, such as in aspergillosis, or with sterile tissue damage, as in the case of human acute myocardial infarction 6, 31. PTX3 mRNA is transcribed only in myeloid precursors (promyelocytes and myelocytes/metamyelocytes) and not in resting mature cells in mice and humans [6].
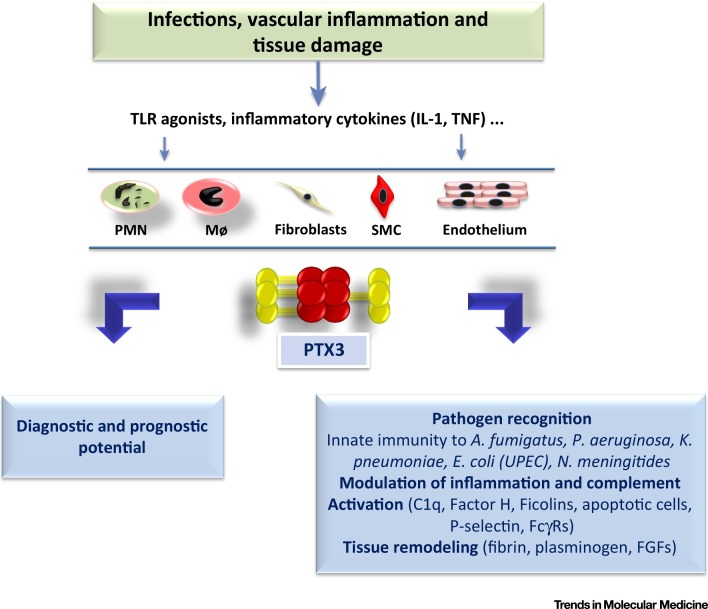
Figure 1.
Schematic View of the Functional Roles of PTX3. After injury or infection, proinflammatory cytokines and microbial moieties induce PTX3 production by neutrophils (PMNs), macrophages (MΦs), and mesenchymal cells [fibroblasts, endothelium, smooth muscle cells (SMCs)]. Once released, PTX3 becomes a potential diagnostic and prognostic marker of inflammation and tissue damage. By interacting with various microbial or endogenous ligands, it is an important player in innate immunity and the regulation of inflammation as well as tissue remodeling and repair.
PTX3 in Innate Immunity
The binding of PTX3 to select microorganisms, including bacteria, fungi, and viruses, leads to the activation of various antimicrobial effector mechanisms [3] (Figures 1 and 2 ). In addition, PTX3 behaves as an immunoregulatory molecule; it can interact with P-selectin, which results in the modulation of neutrophil recruitment, but it can also interact with components of the complement cascade and tune complement activation 30, 32. During infection, such as in the case of human sepsis, PTX3 blood levels rapidly increase from approximately 2 ng/ml to 200–800 ng/ml, correlating with both infection and disease severity and predicting patient survival 16, 33, 34, 35, 36 (Figure 1). Importantly, although PTX3 has long been demonstrated to exert a protective role during microbial infections, it has now emerged as a potential player in contributing to immunopathology under specific circumstances.
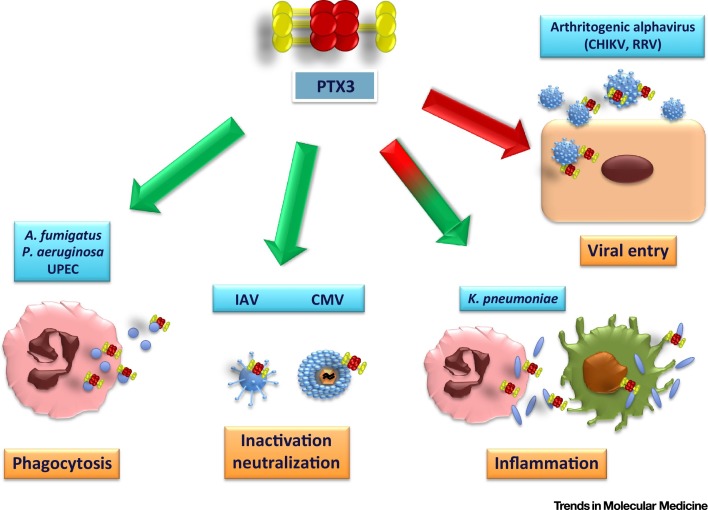
Figure 2.
Role of PTX3 in Innate Immunity. The binding of PTX3 to select microorganisms, including bacteria, fungi, and viruses, leads to the activation of various antimicrobial effector mechanisms, such as phagocytosis and viral neutralization and inactivation (green arrows). However, it can also lead to potentially dangerous inflammatory reactions or even facilitate viral entry (red arrows). Abbreviations: UPEC, uropathogenic Escherichia coli; IAV, influenza A virus; CMV, cytomegalovirus; CHIKV, Chikungunya virus; RRV, Ross River virus.
For instance, in vitro specific binding of PTX3 to fungal components has been observed for A. fumigatus [37], Paracoccidioides brasiliensis, and zymosan [38]. In particular, in vivo studies have revealed that global PTX3-deficient mice are more susceptible to A. fumigatus infection due to defective recognition of conidia by alveolar macrophages and DCs and to the induction of an impaired T helper (Th) 1 lymphocyte response [37]. Intravenous (i.v.) or intraperitoneal (i.p.) treatment with recombinant PTX3 alone or in combination with antifungal agents such as amphotericin B or voriconazole has resulted in therapeutic outcomes such as the reduction of fungal burden in murine and rat models of pulmonary aspergillosis 6, 37, 39, 40 and by favoring phagocytosis. PTX3 is stored in neutrophil granules and in response to microbial recognition has been found in human neutrophil extracellular traps (NETs), suggesting that PTX3 is involved in the antimicrobial activity of NETs [6]. In this same study, the recognition and elimination of A. fumigatus conidia by PTX3-deficient neutrophils was poorly efficient, but opsonization of conidia by PTX3 reversed this phenotype 6, 39. Furthermore, neutrophil adoptive transfer protected PTX3-deficient mice from aspergillosis and reduced fungal burden, revealing that neutrophil-associated PTX3 exerted major control of A. fumigatus infection [6]. PTX3 has also been reported to increase recognition and phagocytosis of microbes in an FcγRII- and complement-dependent manner, as evidenced from studies with C1q-, C3-, and FcγR-deficient mice that displayed impaired phagocytosis of conidia [39]. In particular, the binding of PTX3-opsonized conidia to FcγRII (which acts as a pentraxin receptor) [20] induced inside-out activation of CD11b, resulting in facilitated phagocytosis of C3b-opsonized conidia by human neutrophils [39]. In addition, other in vitro studies have also shown that the interaction and heterocomplex formation of PTX3 with ficolin-2, and, of PTX3 with mannose-binding lectin (MBL) on fungal surfaces, could increase complement deposition on the same surfaces of A. fumigatus and Candida albicans, respectively 41, 42.
Zymosan has also been shown to induce the expression of PTX3 in mouse peritoneal macrophages in vitro [38]. PTX3, in turn, by binding to zymosan particles as well as to the yeast form of P. brasiliensis, was found to induce their aggregation and phagocytosis by macrophages (in high numbers) through a Dectin-1-dependent mechanism, as demonstrated using blocking anti-Dectin-1 antibodies [38].
Moreover, SNPs and haplotypes in the human PTX3 gene have been associated with high susceptibility to invasive aspergillosis following allogeneic hematopoietic stem-cell transplantation [43]. Since then, a link between PTX3 genetic variants and susceptibility to mold infections has been confirmed for more than 1000 patients in the Swiss Organ Transplantation Cohort [44] as well as in a small cohort of lung transplantation patients [45]. This indicates that the results obtained with PTX3 in mouse models of fungal infections can be translated to human scenarios.
PTX3 can also bind various bacteria, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Neisseria meningitidis, and UPEC 13, 14, 37, 39, 46. In particular, PTX3 has been reported to behave as an opsonin of P. aeruginosa and UPEC, facilitating their recognition and ingestion by human and mouse phagocytes 14, 39 (Figure 2). Accordingly, in acute and chronic models of P. aeruginosa lung infection in mice PTX3 has exhibited therapeutic outcomes such as reduced bacterial burden and inflammation 47, 48. In addition, in UTIs, uroepithelial cells have been found to rapidly express PTX3 in response to UPEC infection in mice and humans, with PTX3 augmenting phagocytosis by, and phagosome maturation in, peripheral blood neutrophils [14]. In agreement with these findings, global PTX3-deficient mice displayed a defective capacity to clear bacteria concomitant with an exacerbated inflammatory response, as evidenced by increased production of inflammatory mediators and leukocyte recruitment in the urinary tract [14].
PTX3 binds to outer membrane vesicles (OMVs) from N. meningitidis and to three selected meningococcal molecules (GNA0667, GNA1030, and GNA2091) [13]. Moreover, global PTX3-deficient mice have shown a defective antibody response in vaccination protocols using N. meningitidis OMV, while coadministration of PTX3 was found to increase antibody responses relative to controls. Importantly, administration of PTX3 reversed the defective humoral responses to vaccination with OMV of PTX3-deficient mice but also protected infant rats from infection when they were challenged with N. meningitidis [13]. These results indicate that even if a direct effect of PTX3 in adaptive immune responses has not been described so far, the PTX3-dependent facilitated recognition of microbial components by antigen-presenting cells results in improved adaptive immune responses.
The relevance in humans of the results obtained in bacterial infection animal models has been confirmed by several genetic studies showing a clear association between specific PTX3 genetic variants and augmented susceptibility to Mycobacterium tuberculosis pulmonary infection [49], acute pyelonephritis and cystitis [14], or P. aeruginosa lung infection in cystic fibrosis patients [50].
Regarding viral infections, PTX3 has been proposed to play a protective role in defense against viruses such as human and murine cytomegalovirus (CMV) and influenza virus type A (IVA) 29, 51 (Figure 2). Increased susceptibility to CMV infection and to specific strains of influenza virus infections have been observed in global Ptx3 −/− mice 29, 51. In these cases, PTX3 was found to exert a protective role in the host by binding human and murine CMV, which reduced viral entry into permissive cells and DCs and induced interferon regulatory factor 3 (IRF3) activation in in vitro experiments [51]. Accordingly, intraperitoneal injection of PTX3 resulted in therapeutic efficacy against primary CMV infection and CMV reactivation in hematopoietic stem-cell transplantation experiments in mice, as indicated by the observed reduced viral load and tissue damage and increased animal survival [51]. PTX3 also recognizes specific strains of H3N2-subtype IAV by interacting with viral envelope hemagglutinin and neuraminidase glycoproteins through a sialic acid residue on its glycosidic moiety [29]. In this manner, human and murine PTX3 were shown to act as ‘receptor decoys’ for the virus, preventing viral spread and infection by specific IAV strains [29]. In line with these data, Ptx3 −/− animals were more susceptible to H3N2 infection than wild-type mice and administration of PTX3 resulted in a protective effect, as evidenced by increased survival and reduced viral burden [29]. By contrast, PTX3 did not result in antiviral effects against seasonal or pandemic H1N1 and other H3N2 IAV strains because specific amino acid residues of individual viral HA sequences led to a lack of interacting ability with the sialylated residue of PTX3 52, 53. Hence, these data suggested selective pressures on HA leading to viral escape from the neutralizing activity of PTX3.
Another study has reported that PTX3 exerts a protective role in defense against coronavirus murine hepatitis virus strain 1 (MHV-1) in vitro and in vivo [54]. PTX3 was shown to bind to MHV-1 with resulting reduced infectivity of cells in culture. In the same study, global PTX3-deficient mice were more susceptible than their wild-type counterparts to MHV-1 infection [54]. Administration of PTX3 led to protection against MHV-1, because reduced lung injury and inflammation and accelerated viral clearance were observed [54].
Although collectively these results have demonstrated a protective role of PTX3 during microbial invasion, there are other studies where PTX3 has been found to promote immunopathology in a context-specific manner (Figure 2). In a model of K. pneumoniae infection in mice, PTX3 overexpression was reported to play antagonistic roles depending on the bacterial concentrations used to infect animals [17]. With high bacterial loads, transgenic mice expressing multiple copies of PTX3 under the control of its own promoter displayed faster lethality, reduced lung infiltration of neutrophils, and increased bacterial dissemination in blood compared with wild-type animals [17]. By contrast, with lower K. pneumonia pulmonary inocula PTX3 overexpression conferred protection on mice by increasing the expression of proinflammatory cytokines and the recruitment of neutrophils into lung tissues and by promoting bacterial phagocytosis [17]. KpOmpA, a conserved major component of the outer membrane of Enterobacteriaceae, is one of the few characterized microbial PTX3 ligands [46]. In the mouse air pouch and footpad swelling model, KpOmpA has been found to induce PTX3 production and PTX3, in turn, can amplify TLR2-dependent inflammatory responses such as leukocyte recruitment induced following KpOmpA recognition of the scavenger receptors lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) and scavenger receptor expressed by ECs I (SREC-I) [46]. These results suggest that K. pneumoniae-induced, amplified PTX3-dependent inflammatory responses either play protective roles for the host or might contribute to immunopathology, depending on microbial load. This underlies the relevance of balanced and finely tuned inflammatory responses and innate resistance to this type of bacterial infection (Figure 2). Although future research might further clarify whether these type of findings could be influenced by particular experimental conditions, or whether the results hold true for different pathogen infections, the fact remains that PTX3 plays a complex role in immunoregulation.
Furthermore, a pathogenic role of PTX3 has been demonstrated in arthritogenic alphavirus infections induced by Chikungunya virus (CHIKV) and Ross River virus (RRV) [55] (Figure 2). For instance, the expression of PTX3 mRNA in peripheral blood leukocytes and PTX3 levels in serum have been found to be increased during the acute phase of alphavirus infection both in patients and in animal models [55]. Increased PTX3 expression was associated not only with enhanced viral load but also with disease severity based on temperature, pulse rate, and platelet counts [55]. A murine model of acute RRV infection revealed a delay in disease progression, as shown by increased animal strength and hind-leg paralysis as well as fast recovery in global Ptx3 −/− mice compared with wild-type controls. In addition, reduced leukocyte recruitment, expression of inflammatory mediators, and viral replication were reported with PTX3 deficiency. It was proposed that this phenotype could be explained by early RRV and CHIKV viral entry and replication, which were promoted by the binding of PTX3 to alphavirus, through still undefined mechanisms [55]. Interestingly, this suggested that PTX3 might potentially be exploited by viruses to facilitate an increase in viral entrance and replication in host cells [55] (Figure 2).
PTX3 in Tissue Remodeling
In addition to its involvement in immunoregulation, PTX3 has been implicated in various other biological processes. For instance, it has been postulated that PTX3 also contributes to tissue remodeling under physiological and inflammatory conditions (Figure 1). The involvement of PTX3 in physiological tissue remodeling was first identified in PTX3-deficient infertile female mice [56]. This phenotype was reported to be due to defective assembly of the hyaluronan (HA)-rich matrix that forms around oocytes in preovulatory follicles, a process that is absolutely required for fertilization in vivo [56]. PTX3 produced by cumulus cells during the preovulatory phase was found to interact through its N-terminal domain with two HA-binding proteins, TNFα-induced protein 6 (TNFAIP6 or TSG-6) and the serum proteoglycan inter-α-trypsin inhibitor (IαI), which are major functional proteins of the cumulus ECM, thus providing structural integrity to the cumulus matrix 56, 57. PTX3 is thus required to facilitate murine female fertility.
PTX3 has also been found to bind various fibroblast growth factors (FGFs) via its N-terminal extension, including FGF2, FGF6, FGF8b, FGF10, and FGF17 58, 59, 60, inhibiting FGF-dependent EC proliferation in vitro and angiogenesis in vivo in an animal model using chick embryo chorioallantoic membranes [61]. In addition, PTX3 has been demonstrated to inhibit FGF2-dependent smooth muscle cell (SMC) proliferation and suppressed the mitogenic and chemotactic activity exerted by FGF2 on these cells [62]. It has been therefore suggested that PTX3 produced by ECs and inflammatory cells, which are a major source of PTX3, may affect the autocrine and paracrine activity of FGFs on endothelium and SMCs, providing, in turn, a mechanism for finely tuning the neovascularization processes and restenosis of carotid arteries in rodent models of arterial balloon injury 63, 64.
Additional evidence has indicated that PTX3 is involved in modulating inflammation and tissue damage in animal models of sterile injury 22, 65. For example, in a murine model of cardiac ischemia/reperfusion injury, global PTX3 deficiency was associated with increased tissue damage and neutrophil infiltration in the myocardium [22]. Since higher deposition of complement C3 was observed in the infarct area of PTX3-deficient mice relative to controls [22], it was hypothesized that this phenotype could be attributed to the potential defective regulation of complement activation via Factor H [27]. Surface-bound PTX3 was found to enhance the recruitment of Factor H, which retained its cofactor activity leading to C3b cleavage [27]. This indicated that PTX3 participates in the localization of functionally active Factor H in sites of tissue damage [27]. Moreover, PTX3-deficiency in apolipoprotein E knockout mice (ApoE mice) was associated with increased atherosclerosis and macrophage accumulation within atherosclerotic plaques as well as with more pronounced inflammatory profiles in vascular walls, as revealed by the increased expression of inflammatory mediators and macrophage recruitment within plaques [65]. In addition to regulating complement, PTX3 has been reported to selectively bind P-selectin via its N-linked glycosidic moiety, thus inhibiting leukocyte rolling on endothelium and providing a negative feedback loop that prevents excessive P-selectin-dependent recruitment of neutrophils and tissue damage in murine models of acute lung injury, pleurisy, and mesenteric inflammation [30] as well as in post-ischemic acute kidney injury [66].
Recently PTX3 was demonstrated to play a nonredundant role in tissue repair, through a novel mechanism [5]. In various murine models of tissue damage, including skin-wound healing, chemically induced sterile liver and lung injury and arterial thrombosis, global PTX3 deficiency was associated with increased clot formation as well as with fibrin and collagen deposition/persistence in sites of tissue damage. Under these conditions, macrophages and mesenchymal cells produced PTX3 in response to TLR activation and amplification by IL-1, localizing to the pericellular matrix of macrophages and mesenchymal remodeling cells [5]. Moreover, PTX3-deficient mesenchymal remodeling cells exhibited defective pericellular fibrinolysis in vitro and impaired directional collective migration in the provisional fibrin-rich inflammatory matrix in mice in vivo. It was proposed that this phenotype resulted from the interaction of the PTX3 N-terminal domain with fibrin and plasminogen proteins at an acidic pH and that this occurred in vivo as a consequence of cell metabolic adaptation under conditions of tissue damage-associated hypoperfusion and hypoxia [5]. Furthermore, this work argued that the tripartite interaction between PTX3, fibrin, and plasminogen at sites of tissue repair facilitated plasminogen-dependent fibrinolysis [5]. Deposited fibrin in damaged tissues acts as a provisional matrix component and its timely remodeling is essential for normal tissue repair [67]. Consequently, the phenotype of Ptx3 −/− mice described by Doni et al. [5] demonstrates that the PTX3-dependent promotion of fibrin-rich inflammatory matrix remodeling contributes to tissue repair. Along the same lines, in another murine study PTX3-deficient mesenchymal stromal cells were found to be impaired in their ability to promote skin-wound healing and were also associated with defective pericellular fibrinolysis and cell migration through fibrin [68]. These studies provide further evidence of the interaction between humoral pattern recognition molecules and ECM components and underscore the evolutionary link that exists between microbial and ECM recognition in the humoral arm of innate immunity 3, 69.
Regarding the involvement of PTX3 in inflammatory processes, other studies have provided interesting data. For example, in a model of mouse cardiomyocyte death resulting from coxsackievirus B3 (CVB3) viral infection, global PTX3 deficiency increased heart injury, as evidenced by increased serum creatinine kinase activity and cardiomyocyte apoptosis, albeit without affecting viral titers [70] and through a poorly defined mechanism. In this context, it was proposed that the catalytic activity of the immunoproteasome that prevents exacerbation of CVB3-induced myocardial destruction regulated the timely availability of certain factors (ERK1/2 and p38) for controlling PTX3 mRNA expression and protein production during the infection [70]. This suggested that the cardioprotective function of immunoproteasome-dependent PTX3 expression was a crucial mechanism of stress-induced damage response in myocardial inflammation during CVB3 viral infection [70].
From a different perspective, PTX3 has also been shown to play a protective role in various brain disorders. For example, in a murine model of limbic seizure PTX3 synthesis was induced in the brain, exerting a protective role in seizure-induced neurodegeneration as evidenced by a lower number of degenerating neurons in wild-type mice compared with PTX3-deficient mice [71]. In cerebral ischemia mouse models, PTX3 production was induced in neurons and glia, and although PTX3 deficiency did not affect acute ischemic brain injury it did, however, compromise blood–brain barrier integrity and resolution of brain edema during recovery as well as impairing glial scar formation and neurogenesis 72, 73. Thus, PTX3 has been proposed to support blood–brain barrier integrity [74].
PTX3 induction in association with tissue damage has also been identified in other pathological contexts [70] (Figure 3 , Key Figure). For instance, in a mouse model of superior mesenteric artery ischemia and reperfusion, PTX3 deficiency was associated with inhibition of local and remote inflammation and tissue injury, whereas genetic PTX3 overexpression or i.v. PTX3 administration worsened tissue injury and lethality 75, 76. Accordingly, in another murine study, PTX3 overexpression resulted in increased inflammatory responses and a faster decline in a model of ventilator-induced lung injury [77]. PTX3 overexpression also led to increased hypertrophic responses and ventricular dysfunction following increased pressure overload in a murine cardiac injury model [78]. More recently, PTX3 has been found to induce mouse EC dysfunction by inhibiting acetylcholine-evoked vasorelaxation in an in vitro model of the vascular reactivity of resistance vessels and by inducing morphological changes in ECs [79]. These effects were reported to be mediated by a P-selectin/matrix metalloproteinase-1-dependent pathway leading to impaired phosphorylation of eNOS and nitric oxide production in vitro [79]. In agreement, i.v. PTX3 administration caused hypertension in wild-type animals but not in P-selectin-deficient mice [79]. These results suggest that PTX3 may have a possible direct role on blood pressure homeostasis and endothelial function.
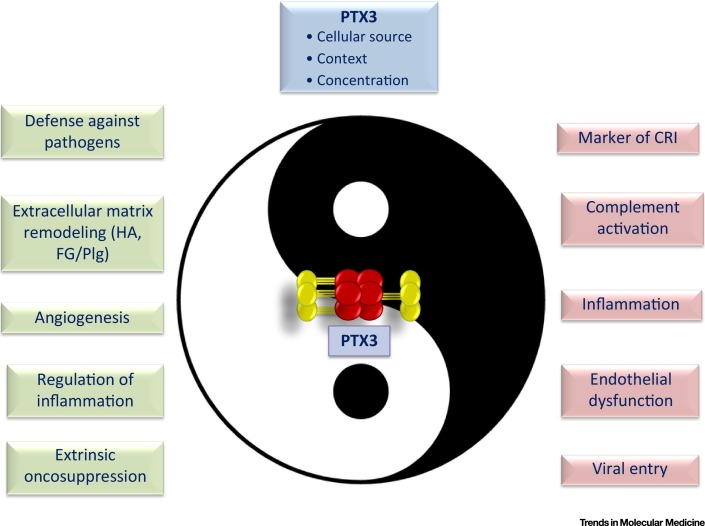
Figure 3.
Key Figure: The Yin–Yang of PTX3 in Innate Immunity, Inflammation, Tissue Damage, and Cancer
Depending on the tissue context, cellular source, and levels of production, PTX3 can either play a protective role in a host or contribute to impaired cell/tissue homeostasis and disease pathogenesis. Abbreviations: CRI, cancer-related inflammation; HA, hyaluronic acid; FG, fibrinogen; Plg, plasminogen.
PTX3 in Cancer
Inflammation is an essential component of tumor microenvironments in cancer, sustaining tumor development and growth [80]. However, the role of the humoral arm of the innate immune system in cancer is poorly understood.
Various reports have associated increased plasma levels of CRP with cancer risk 81, 82, 83. Furthermore, elevated local or systemic PTX3 levels have been observed for several cancers, including glioma, liposarcoma, lung cancer, prostate carcinoma, pancreatic carcinoma, and breast cancer bone metastases, that correlate with either grade of malignancy or a poor prognosis 18, 84, 85, 86, 87, 88. Gene expression profiling has identified PTX3 as one of the expressed genes associated with the stromal response/ECM signature and poor prognosis in human ovarian cancer [89]. In addition, PTX3 genetic variants have been associated with increased PTX3 plasma levels and a risk of developing hepatocellular carcinoma in hepatitis C virus-infected subjects [90].
To investigate the role of PTX3 as a marker of cancer-related inflammation and malignant transformation and/or an active player in pathogenesis, our group addressed the susceptibility of PTX3-deficient mice to mesenchymal and epithelial carcinogenesis in models of 3-MCA-induced carcinogenesis and 7,12-dimethylbenz [α] anthracene/terephthalic acid (DMBA/TPA)-induced skin carcinogenesis [12]. In these models global PTX3 deficiency was associated with more pronounced cancer-related inflammation relative to controls, displayed a higher number of tumor-infiltrating macrophages that showed upregulated expression of genes associated with macrophage M2-like polarization, as well as increased angiogenesis, secretion of proinflammatory cytokines, and complement activation (with increased C3 and lower Factor H localization in tumor tissues in addition to higher C5a levels in tumor homogenates) [12] (Figure 4 ). Moreover, PTX3-deficient tumors were also characterized by enhanced genetic damage as indicated by increased Trp53 mutations, oxidative DNA damage, and expression of DNA damage markers, in line with the hypothesis that inflammation contributes to genetic events that can lead to cancer and to the genetic instability observed in tumors [91]. Importantly, C3 deficiency in mice or inhibition through a blocking antibody of the chemokine CCL2 led to a reduction in tumor macrophages, an outcome that was sufficient to revert the increased susceptibility to chemically induced carcinogenesis of PTX3-deficient mice. These results indicate that, at least in mice, PTX3 exerts a protective role in carcinogenesis and that this effect is based on the regulation of complement activation [12] (Figure 3). The potential protumoral role of the complement system has also been suggested by a recent study showing that deficiency in key components of the complement pathway (e.g., C3, C5, C5a receptor) can be linked to colitis-associated colon cancer resistance in mice [92]. However, in specific mouse models, cancer-related inflammation was found to be complement independent 93, 94, indicating that complement activation may contribute to cancer-related inflammation depending on the tissue context and driving molecular pathways.
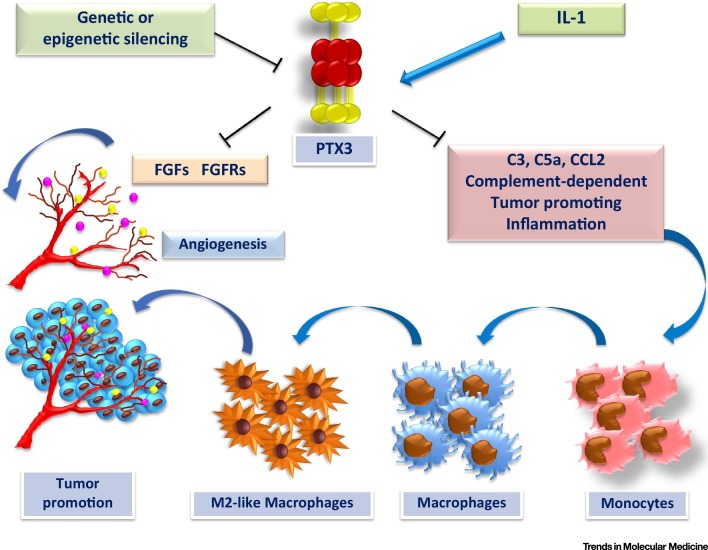
Figure 4.
PTX3 as an Extrinsic Oncosuppressor in Cancer. In cancer, PTX3 gene and protein expression is controlled by the presence of inflammatory mediators (e.g., IL-1) and/or by genetic and epigenetic mechanisms. PTX3 regulates complement-dependent tumor-promoting inflammation in a host, including macrophage infiltration and cytokine production, as well as fibroblast growth factor (FGF)-dependent angiogenesis, which can lead to genetic instability and tumor promotion.
The functional relevance of PTX3 in human cancer has been further strengthened by results showing that the PTX3 promoter and regulatory regions of the locus are highly methylated in human mesenchymal and epithelial tumors, in contrast to normal healthy tissue, and, furthermore, that such PTX3 methylation can result in silencing of PTX3 protein expression [12]. PTX3 promoter hypermethylation and reduced PTX3 expression have also been reported in human esophageal squamous cell carcinomas [95]. Taken together, these studies indicate that PTX3, which is an essential component of humoral innate immunity and a regulator of complement activation, also acts as an extrinsic oncosuppressor gene in mouse and man. Moreover, its oncosuppressive role appears to act at the level of complement-mediated, macrophage-sustained, tumor-promoting inflammation inhibition [95].
Of note, since PTX3 binds certain FGFs (i.e., FGF2, FGF8), impairing the interaction with their cognate receptors 60, 61, PTX3 plays a protective role in inhibiting tumor growth by regulating FGF-dependent effects as well 60, 96 (Figure 4). PTX3 overexpression, under the control of the endothelium-specific Tie2/Tek transcription regulatory sequences, has been associated with reduced malignant growth of FGF2-dependent mouse TRAMP-C2 prostate cancer and B16 melanoma as well as reduced melanoma metastasis and cancer-associated angiogenesis [96]. Furthermore, PTX3 has also been shown to impair cell proliferation and epithelial–mesenchymal transition (EMT) in human melanoma cells in vitro [60].
However, contrary to the reports describing a protective role of PTX3 against cancer, other studies have suggested a protumorigenic effect (Figure 3). PTX3 has been reported to promote in vitro migration and invasion of human pancreatic tumor cells, breast carcinoma, and head and neck squamous cell carcinoma, as well as macrophage chemotaxis, by still poorly defined molecular mechanisms 82, 84, 97, 98. In another study, PTX3 silencing was found to promote the gastric cancer cell migratory potential, the recruitment of macrophages, and their subsequent binding to gastric cancer cells [98]. In addition, the transcription factor CCAAT/enhancer-binding protein delta (CEBPD) was shown to activate PTX3 transcription by directly binding to its promoter region in response to cisplatin or 5-fluorouracil treatment in human and murine M2 macrophages and cancer-associated fibroblasts. As a result, PTX3 was found to promote the growth, metastasis, and invasion of drug-resistant human breast cancer cells in immunodeficient mice [19].
These murine studies and the reports showing a positive correlation between PTX3 expression and poor prognosis in specific human cancers suggest that the tumor-promoting or tumor-suppressing role of PTX3 might very likely depend on tissue, cancer type, and cellular source (e.g., cancer cells versus tumor-associated macrophages or fibroblasts) and contradict the assumption that PTX3 plays a unique role in cancer (Figures 3 and 4). However, it should be emphasized that hard genetic data in mice and humans demonstrate that this molecule is a bona fide cancer gene acting as an extrinsic oncosuppressor. Further research should help clarify these collective context-dependent findings.
Concluding Remarks
Pentraxins are a phylogenetically conserved superfamily of humoral PRMs exerting common essential functions in innate responses to pathogens and in tissue repair. CRP is a widely used biomarker of inflammation in humans. However, the lack of strict evolutionary conservation between mouse and human has precluded the use of straightforward genetic approaches to explore its functions in vivo. By contrast, gene-targeting mouse studies have allowed better definition of the functional role of PTX3 in innate immunity, inflammation, and tissue remodeling. In addition, genetic and epigenetic data are consistent with the hypothesis that PTX3 exerts similar functions in humans. From the results described here it has been inferred that, on selective binding of conserved microbial molecules, PTX3 generally exhibits protective antibody-like functions such as complement activation, opsonization of microbes, and glycosylation-dependent regulation of inflammation. Furthermore, studies in tissue repair models have shown that PTX3-dependent promotion of fibrin-rich inflammatory matrix remodeling contributes to tissue repair and underline the evolutionary link that exists between microbial and ECM recognition in the humoral arm of innate immunity. Thus, genetic evidence in mouse and human indicates that PTX3 is essential for resistance against selected pathogens and that it also promotes tissue repair. The described findings strongly support the promising potential of PTX3 in prophylaxis and therapy against infectious diseases in specific clinical settings. Of note, the regulation of complement activation also appears to be an essential mechanism of action for the oncosuppressive role of PTX3 that has been recently described, suggesting that novel therapeutic approaches could be envisioned.
However, several issues remain (see Outstanding Questions). For example, it is important to take into account that PTX3 could potentially lead to harmful consequences in an organism under certain circumstances. Consequently, when considering the exploitation of PTX3 by viruses (such as alphaviruses) to facilitate viral entry into host cells [55] (Figures 2 and 3), the interaction of PTX3 with collectins and ficolins, which can lead to potent synergic complement activation [32], as well as its potential to induce endothelial dysfunction [79] and inflammation 19, 76 (Figure 3), future clinical trials involving PTX3 administration (or blockade) should carefully assess the possibility of generating unbalanced inflammatory responses and other adverse clinical outcomes. Finally, a call is made to strive to achieve a better understanding of the multiple mechanistic pathways in which PTX3 is involved. Deciphering more clearly the multifaceted functional roles of PTX3 in physiology may facilitate the development of targeted therapeutic approaches in various clinical conditions.
Outstanding Questions.
What is the impact of PTX3 as a genetic or circulating protein marker on disease management for specific clinical conditions? In what immediate conditions can PTX3 be assessed?
What is the mechanistic basis of the oncosuppressive role of PTX3 in various forms of cancer? Does PTX3 play differential or redundant roles in diverse forms of cancer? What is the role of complement in carcinogenesis and human cancer?
What is the role of PTX3 in antiviral resistance? What impact does it have in mediating antiviral defense versus facilitating viral entry? For which different types of virus does PTX3 facilitate viral entry?
What is the therapeutic potential of PTX3 in specific microbial infections (e.g., Aspergillus fumigatus, Pseudomonas aeruginosa)?
What is the mechanistic basis for the observed antagonistic effects of PTX3 in response to varying bacterial burdens during an infection and is there a threshold? Would this phenomenon apply to different pathogens? Is there an underlying functional advantage in response to these different scenarios for the host?
Acknowledgments
The financial support of the European Commission (ERC-PHII), Ministero della Salute (Ricerca Finalizzata, RF-2013-02355470), Cluster Alisei (MEDINTECH CTN01_00177_962865), Italian Cystic Fibrosis Research Foundation, and Italian Association for Cancer Research (AIRC, AIRC 5 × 1000) is gratefully acknowledged.
Glossary
- Arthritogenic alphaviruses
include viruses such as CHIKV and RRV. They are enveloped, positive-sense, single-stranded RNA viruses that are usually transmitted by mosquitoes and cause rheumatic disease.
- C1q
subcomponent of complement C1. C1q has binding sites for antibodies and initiates the complement cascade via the classical pathway.
- Collectins
oligomeric proteins containing a C-terminal carbohydrate recognition domain linked to a collagen-like region through an alpha-helical hydrophobic neck region and an N-terminal region. They have the capacity to interact with carbohydrates and lipids exposed on pathogen surfaces. They also bear opsonic activity and can activate the lectin pathway of the complement system.
- Complement cascade
a multimolecular biologic process sustained by more than 20 proteins. These molecules act in concert and in a specific sequence called the complement cascade. Three different pathways (i.e., the classical pathway, alternative pathway, and lectin pathway) can be activated. The terminal pathway of the complement cascade leads to the formation of a membrane-attack complex on target surfaces, which induces cell lysis.
- Conidia
also called chlamidospores or mitospores; asexual, nonmotile spores of a fungus. They develop at the tip of specialized hyphae in fungi called conidiophores and are formed by the abstriction at the top of a hyphal branch. This process induces the separation and release of a mature spore by the formation of a septum.
- Cumulus cells
a cluster of cells forming the cumulus oophorus, which is a follicular mass surrounding the oocyte in the ovarian follicle.
- Damage-associated molecular patterns (DAMPs)
also known as danger-associated molecular patterns or endogenous alarmins; host cell-derived molecules released by stressed cells and necrotic cells (e.g., HMGB1, IL-1α). DAMPs can engage receptors (e.g., TLRs) to induce and sustain the inflammatory response.
- Fcγ receptors (FcγRs)
receptors for the Fc (tail) region of IgG. Based on their structure, they comprise FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). FcγRs participate in the regulation of the immune response. Besides their capacity to recognize IgG, FcγRs can act as receptors for opsonins (e.g., pentraxins).
- M2-like polarization
in response to external signals, macrophages can undergo M1 activation (also called classical activation) on stimulation by TLR ligands and IFNγ or M2 activation (also called alternative activation) on stimulation by IL-4/IL-13. The M2 or M2-like polarization state has been associated with high production of IL-10, low production of IL-12, and resolution or smoldering of chronic inflammation.
- Neutrophil extracellular traps (NETs)
extracellular fibrillary networks formed and released by activated neutrophils. NETs comprise DNA and histones and contain a set of proteins from neutrophil granules (e.g., MPO, neutrophil elastase, PTX3). NETs have the capacity to trap microbes and favor their elimination.
- Nucleotide-binding oligomerization domain (NOD) and RIG-like receptors
intracellular sensors of microbial moieties and DAMPs associated with cell stress or viruses, respectively.
- Opsonic activity
opsonization is a process by which a molecule (i.e., opsonin) interacts with microbes leading to facilitated microbial recognition and elimination by phagocytosis.
- Pattern recognition molecules (PRMs)
a set of germline-encoded receptors used to discriminate self- versus non-self and modified-self antigens. Based on their localization, PRMs have been divided into cell-associated receptors (e.g., TLRs) and soluble molecules (e.g., collectins, pentraxins).
- Restenosis
common adverse event in endovascular procedures; involves the recurrence of narrowing of a blood vessel, usually an artery.
- Toll-like receptors (TLRs)
a family of PRMs characterized by an extracellular leucine-rich domain and a cytoplasmic domain sharing homology with the receptor for IL-1 and the toll protein of Drosophila. TLRs are cell-associated molecules located in cytoplasmic membranes or in the membranes of endosomal vesicles. They sense molecules in extracellular compartments and in the lumen of endosomal vesicles.
References
- 1.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 2.Garlanda C. Negative regulatory receptors of the IL-1 family. Semin. Immunol. 2013;25:408–415. doi: 10.1016/j.smim.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Bottazzi B. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 4.Garlanda C. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 5.Doni A. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J. Exp. Med. 2015;212:905–925. doi: 10.1084/jem.20141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaillon S. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casas J.P. C-reactive protein and coronary heart disease: a critical review. J. Intern. Med. 2008;264:295–314. doi: 10.1111/j.1365-2796.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 8.Wensley F. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norata G.D. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc. Med. 2010;20:35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Pepys M.B. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfield G.M. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. U. S. A. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonavita E. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Bottazzi B. Recognition of Neisseria meningitidis by the long pentraxin PTX3 and its role as an endogenous adjuvant. PLoS ONE. 2015;10:e0120807. doi: 10.1371/journal.pone.0120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaillon S. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Garlanda C. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr. Opin. Immunol. 2016;38:39–44. doi: 10.1016/j.coi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaillon S. The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int. Arch. Allergy. Immunol. 2014;165:165–178. doi: 10.1159/000368778. [DOI] [PubMed] [Google Scholar]
- 17.Soares A.C. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Infante M. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int. J. Cancer. 2016;138:983–991. doi: 10.1002/ijc.29822. [DOI] [PubMed] [Google Scholar]
- 19.Chi J.Y. Targeting chemotherapy-induced PTX3 in tumor stroma to prevent the progression of drug-resistant cancers. Oncotarget. 2015;6:23987–24001. doi: 10.18632/oncotarget.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez de la Torre Y. Evolution of the pentraxin family: the new entry PTX4. J. Immunol. 2010;184:5055–5064. doi: 10.4049/jimmunol.0901672. [DOI] [PubMed] [Google Scholar]
- 22.Salio M. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 23.Altmeyer A. Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor/interleukin-1-inducible pentraxin protein. J. Biol. Chem. 1995;270:25584–25590. doi: 10.1074/jbc.270.43.25584. [DOI] [PubMed] [Google Scholar]
- 24.Basile A. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-κB in tumor necrosis factor-α and interleukin-1β regulation. J. Biol. Chem. 1997;272:8172–8178. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 25.Inforzato A. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J. Biol. Chem. 2008;283:10147–10161. doi: 10.1074/jbc.M708535200. [DOI] [PubMed] [Google Scholar]
- 26.Inforzato A. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–11551. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 27.Deban L. Binding of the long pentraxin PTX3 to Factor H: interacting domains and function in the regulation of complement activation. J. Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 28.Gout E. M-ficolin interacts with the long pentraxin PTX3: a novel case of cross-talk between soluble pattern-recognition molecules. J. Immunol. 2011;186:5815–5822. doi: 10.4049/jimmunol.1100180. [DOI] [PubMed] [Google Scholar]
- 29.Reading P.C. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 30.Deban L. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 31.Maugeri N. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J. Immunol. 2011;187:970–979. doi: 10.4049/jimmunol.1100261. [DOI] [PubMed] [Google Scholar]
- 32.Inforzato A. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin. Immunol. 2013;25:79–85. doi: 10.1016/j.smim.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J. Clin. Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 34.Muller B. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit. Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Wagenaar J.F. Long pentraxin PTX3 is associated with mortality and disease severity in severe leptospirosis. J. Infect. 2009;58:425–432. doi: 10.1016/j.jinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Mauri T. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–629. doi: 10.1007/s00134-010-1752-5. [DOI] [PubMed] [Google Scholar]
- 37.Garlanda C. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 38.Diniz S.N. PTX3 function as an opsonin for the Dectin-1-dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 39.Moalli F. Role of complement and Fcγ receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 40.Gaziano R. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 2004;48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y.J. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J. Biol. Chem. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y.J. Ficolin-1–PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J. Immunol. 2013;191:1324–1333. doi: 10.4049/jimmunol.1300382. [DOI] [PubMed] [Google Scholar]
- 43.Cunha C. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- 44.Wojtowicz A. PTX3 Polymorphisms and invasive mold infections after solid organ transplant. Clin. Infect. Dis. 2015;61:619–622. doi: 10.1093/cid/civ386. [DOI] [PubMed] [Google Scholar]
- 45.Cunha C. PTX3-based genetic testing for risk of Aspergillosis after lung transplant. Clin. Infect. Dis. 2015;61:1893–1894. doi: 10.1093/cid/civ679. [DOI] [PubMed] [Google Scholar]
- 46.Jeannin P. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Moalli F. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J. Immunol. 2011;186:5425–5434. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 48.Jaillon S. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J. Immunol. 2013;191:1873–1882. doi: 10.4049/jimmunol.1201642. [DOI] [PubMed] [Google Scholar]
- 49.Olesen R. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 50.Chiarini M. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun. 2010;11:665–670. doi: 10.1038/gene.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bozza S. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 52.Job E.R. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J. Immunol. 2010;185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 53.Job E.R. A single amino acid substitution in the hemagglutinin of H3N2 subtype influenza A viruses is associated with resistance to the long pentraxin PTX3 and enhanced virulence in mice. J. Immunol. 2014;192:271–281. doi: 10.4049/jimmunol.1301814. [DOI] [PubMed] [Google Scholar]
- 54.Han B. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab. Invest. 2012;92:1285–1296. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foo S.S. Role of pentraxin 3 in shaping arthritogenic alphaviral disease: from enhanced viral replication to immunomodulation. PLoS Pathog. 2015;11:e1004649. doi: 10.1371/journal.ppat.1004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salustri A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 57.Scarchilli L. PTX3 interacts with inter-α-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J. Biol. Chem. 2007;282:30161–30170. doi: 10.1074/jbc.M703738200. [DOI] [PubMed] [Google Scholar]
- 58.Camozzi M. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J. Biol. Chem. 2006;281:22605–22613. doi: 10.1074/jbc.M601023200. [DOI] [PubMed] [Google Scholar]
- 59.Presta M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J. Cell. Mol. Med. 2007;11:723–738. doi: 10.1111/j.1582-4934.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronca R. Long pentraxin-3 as an epithelial-stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J. Pathol. 2013;230:228–238. doi: 10.1002/path.4181. [DOI] [PubMed] [Google Scholar]
- 61.Rusnati M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–99. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 62.Camozzi M. Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler. Thromb. Vasc. Biol. 2005;25:1837–1842. doi: 10.1161/01.ATV.0000177807.54959.7d. [DOI] [PubMed] [Google Scholar]
- 63.Leali D. Fibroblast growth factor-2 antagonist and antiangiogenic activity of long-pentraxin 3-derived synthetic peptides. Curr. Pharm. Des. 2009;15:3577–3589. doi: 10.2174/138161209789206962. [DOI] [PubMed] [Google Scholar]
- 64.Alessi P. Anti-FGF2 approaches as a strategy to compensate resistance to anti-VEGF therapy: long-pentraxin 3 as a novel antiangiogenic FGF2-antagonist. Eur. Cytokine Netw. 2009;20:225–234. doi: 10.1684/ecn.2009.0175. [DOI] [PubMed] [Google Scholar]
- 65.Norata G.D. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 66.Lech M. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013;83:647–661. doi: 10.1038/ki.2012.463. [DOI] [PubMed] [Google Scholar]
- 67.Bugge T.H. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 68.Cappuzzello C. Mesenchymal stromal cell-derived PTX3 promotes wound healing via fibrin remodeling. J. Invest. Dermatol. 2016;136:293–300. doi: 10.1038/JID.2015.346. [DOI] [PubMed] [Google Scholar]
- 69.Hanington P.C., Zhang S.M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J. Innate Immun. 2011;3:17–27. doi: 10.1159/000321882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paeschke A. The immunoproteasome controls the availability of the cardioprotective pattern recognition molecule pentraxin3. Eur. J. Immunol. 2016;46:619–633. doi: 10.1002/eji.201545892. [DOI] [PubMed] [Google Scholar]
- 71.Ravizza T. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience. 2001;105:43–53. doi: 10.1016/s0306-4522(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Grande B. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J. Cereb. Blood Flow Metab. 2014;34:480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Grande B. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J. Neuroinflammation. 2015;12:15. doi: 10.1186/s12974-014-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shindo A. Astrocyte-derived pentraxin 3 supports blood–brain barrier integrity under acute phase of stroke. Stroke. 2016;47:1094–1100. doi: 10.1161/STROKEAHA.115.012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Souza D.G. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. 2009;174:1309–1318. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Souza D.G. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am. J. Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Real J.M. Pentraxin 3 accelerates lung injury in high tidal volume ventilation in mice. Mol. Immunol. 2012;51:82–90. doi: 10.1016/j.molimm.2012.02.113. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki S. Long pentraxin PTX3 exacerbates pressure overload-induced left ventricular dysfunction. PLoS ONE. 2013;8:e53133. doi: 10.1371/journal.pone.0053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carrizzo A. Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway. Circulation. 2015;131:1495–1505. doi: 10.1161/CIRCULATIONAHA.114.014822. [DOI] [PubMed] [Google Scholar]
- 80.Mantovani A. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 81.Guo L. C-reactive protein and risk of breast cancer: a systematic review and meta-analysis. Sci. Rep. 2015;5:10508. doi: 10.1038/srep10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondo S. Clinical impact of pentraxin family expression on prognosis of pancreatic carcinoma. Br. J. Cancer. 2013;109:739–746. doi: 10.1038/bjc.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morizane C. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas. 2011;40:415–421. doi: 10.1097/MPA.0b013e3182021376. [DOI] [PubMed] [Google Scholar]
- 84.Choi B. Elevated pentraxin 3 in bone metastatic breast cancer is correlated with osteolytic function. Oncotarget. 2014;5:481–492. doi: 10.18632/oncotarget.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diamandis E.P. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin. Cancer Res. 2011;17:2395–2399. doi: 10.1158/1078-0432.CCR-10-3024. [DOI] [PubMed] [Google Scholar]
- 86.Germano G. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 87.Locatelli M. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J. Neuroimmunol. 2013;260:99–106. doi: 10.1016/j.jneuroim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Stallone G. Pentraxin 3: a novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Cancer Res. 2014;74:4230–4238. doi: 10.1158/0008-5472.CAN-14-0369. [DOI] [PubMed] [Google Scholar]
- 89.Tothill R.W. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 90.Carmo R.F. Genetic variation in PTX3 and plasma levels associated with hepatocellular carcinoma in patients with HCV. J. Viral Hepat. 2016;23:116–122. doi: 10.1111/jvh.12472. [DOI] [PubMed] [Google Scholar]
- 91.Colotta F. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 92.Ning C. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1β/IL-17A axis. Mucosal Immunol. 2015;8:1275–1284. doi: 10.1038/mi.2015.18. [DOI] [PubMed] [Google Scholar]
- 93.Andreu P. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Visser K.E. Early neoplastic progression is complement independent. Neoplasia. 2004;6:768–776. doi: 10.1593/neo.04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang J.X. Aberrant methylation of the 3q25 tumor suppressor gene PTX3 in human esophageal squamous cell carcinoma. World J. Gastroenterol. 2011;17:4225–4230. doi: 10.3748/wjg.v17.i37.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ronca R. Long-pentraxin 3 derivative as a small-molecule FGF trap for cancer therapy. Cancer Cell. 2015;28:225–239. doi: 10.1016/j.ccell.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Chang W.C. PTX3 gene activation in EGF-induced head and neck cancer cell metastasis. Oncotarget. 2015;6:7741–7757. doi: 10.18632/oncotarget.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi B. Pentraxin-3 silencing suppresses gastric cancer-related inflammation by inhibiting chemotactic migration of macrophages. Anticancer Res. 2015;35:2663–2668. [PubMed] [Google Scholar]