Abstract
Nitrated polycyclic aromatic hydrocarbons (NPAHs) and heterocyclic PAHs (HPAHs) are recognized environmental pollutants. However, the health risks of NPAHs and HPAHs to humans and environmental systems are not well-studied. The developmental zebrafish (Danio rerio) model was used to evaluate the toxicity of a structurally diverse set of 27 NPAHs and 10 HPAHs. The individual activity of each compound towards the aryl hydrocarbon receptor (AHR), including the role of the AHR in observed toxicity, and genetic markers of oxidative stress and cardiac toxicity were evaluated. Zebrafish embryos were exposed from 6 to 120 hours post fertilization (hpf), to a broad concentration range of individual compounds, and evaluated for 22 developmental endpoints. The potential role of AHR was determined using the transgenic Tg(cyp1a:nls-egfp) reporter zebrafish line. All compounds were screened computationally through molecular docking using a previously developed AHR models of zebrafish isoforms 1A, 1B, and 2. Some compounds did not induce observable developmental toxic responses, whereas others produced statistically significant concentration-dependent toxicity. The tested compounds also exhibited a range of predicted AHR binding and cyp1a/GFP induction patterns, including cyp1a expression in the liver, vasculature, skin, and yolk, which we determined to be due to distinct isoforms of the AHR, using morpholino oligonucleotide knockdown. Furthermore, we investigated mRNA expression of oxidative and cardiac stress genes at 48 and 120 hpf, which indicated several potential mechanisms-of-action for NPAHs. Overall, we observed a range of developmental toxicities, cyp1a/GFP expression patterns, and gene expression profiles, suggestive of several potential mechanisms of action.
Keywords: nitrated polycyclic aromatic hydrocarbon, heterocyclic polycyclic aromatic hydrocarbon, aryl hydrocarbon receptor, zebrafish.
Polycyclic aromatic hydrocarbons (PAHs) are well-known and established environmental contaminants with a range of sources to the environment (Khalili et al., 1995; Yunker et al., 2002). Exposure to PAHs has been linked with a range of health effects, including cancer and developmental challenges (Bernstein et al., 2004; Hardin et al., 1992; Irigaray et al., 2007; Perera et al., 1998; Perera et al., 2005; Sydbom et al., 2001; WHO, 2015).
Despite the toxicity of PAHs, the overall toxicity or mutagenicity of complex environmental mixtures often cannot be explained by PAHs alone (Chibwe et al., 2015; Toftgård et al., 1985). This has led to an increasing interest in PAH derivatives, including oxygenated PAHs (OPAHs), nitrated PAHs (NPAHs) and heterocyclic PAHs (HPAHs), which contain one or more heteroatomic substitutions within or on the aromatic ring system. These PAH derivatives are less studied than the corresponding unsubstituted, or parent, PAHs, both with regards to toxicity and environmental occurrence.
PAH derivatives can occur in the environment from a range of primary and secondary sources, including biotic and abiotic transformation processes (Cochran et al., 2016; Li et al., 2015; Shailaja et al., 2006; Vicente et al., 2015; Wei et al., 2015). NPAHs are known to be formed through reactions with particulate-bound PAHs and NOx radicals during atmospheric transport (Arey et al., 1986; Jariyasopit et al., 2014; Zimmermann et al., 2013), as well as being common pollutants in diesel vehicle exhaust (Bamford et al., 2003; Schuetzle et al., 1982). Both NPAHs and HPAHs are also known constituents of petroleum-based products, such as sealcoat pavement sealant (Titaley et al., 2016; Wei et al., 2015).
Limited toxicological data exists for NPAHs and HPAHs, most of which has been focused on mutagenicity. Many NPAHs and HPAHs are highly mutagenic in the Ames Salmonella assay (Ball et al., 1984; Jariyasopit et al., 2013; Rosenkranz and Mermelstein, 1983), and several NPAHs are known or suspected human carcinogens (RI DEM, 2008; WHO, 2015).
Limited data exists for NPAHs and HPAHs with other acute or developmental toxicity endpoints. However, these studies have been limited in the number of compounds investigated, or the non-mortality endpoints observed, leading to a lack of comparable information across a large set of compounds (Dumont et al., 1979; Iwanari et al., 2002; Onduka et al., 2012; Peddinghaus et al., 2012).
Aromatic compounds, such as PAHs, are well-known and established to activate the aryl hydrocarbon receptor (AHR) pathway and, in many cases, the observed toxicity is AHR-dependent (Goodale et al., 2013; Knecht et al., 2013; Prasch et al., 2003). Binding of a ligand, such as a PAH, to the AHR results in up-regulation of numerous downstream genes, including the cytochrome P450s (cyp genes), which metabolize certain AHR ligands, including PAHs (Denison and Nagy, 2003; Miranda et al., 2006; Schmidt and Bradfield, 1996).
Other mechanisms of action for PAHs and PAH-containing mixtures have also been commonly observed, including oxidative stress and cardiac stress. Oxidative stress pathways are known to be linked with activation of the AHR pathway (Dalton et al., 2002), and AHR knockout mice have a reduced ability to generate an antioxidant response following exposure to TCDD, a known AHR agonist (Senft et al., 2002). Cardiac toxicity following exposure to 5-ring PAHs can be mediated by the AHR pathway, but these effects were AHR-independent for other structurally similar compounds (Brown et al., 2014; Incardona et al., 2011; Scott et al., 2011). Cardiac toxicity is also commonly observed following exposure to PAH-containing mixtures (Hicken et al., 2011; Jung et al., 2013).
Zebrafish (Danio rerio) are an increasingly popular model system for high-throughput screening of compounds for developmental toxicity and mechanistic investigations (Garcia et al., 2016). Zebrafish embryos develop rapidly and externally, with their major organs and systems formed within 5 days post-fertilization, and are amenable to cell culture techniques, including growth in 96-well tissue culture plates (Bugel et al., 2014; Jones et al., 2010; MacRae and Peterson, 2015; Noyes et al., 2015; Truong et al., 2014). Developing zebrafish embryos have a high degree of homology to humans, and this model has previously been used for the evaluation of PAH and OPAH toxicity (Brown et al., 2014; Huang et al., 2012; Incardona et al., 2004; Incardona et al., 2006; Knecht et al., 2013; Knecht et al., 2016).
The goal of this study was to determine the developmental toxicity of a suite of NPAHs and HPAHs using the embryonic zebrafish model. In addition to the developmental toxicity screen, potential mechanisms of action for NPAH and HPAH toxicity were investigated, including activation of the AHR pathway, oxidative stress, and cardiac stress.
MATERIALS AND METHODS
Fish Care and Husbandry
Adult zebrafish were maintained with a water temperature of 28° ±1 °C on a recirculating system with a 14h light: 10h dark photoperiod at the Sinnhuber Aquatic Research Laboratory (SARL). All experiments were conducted with the wild type Troptical 5D strain or Tg(cyp1a:nls-egfp) (background strain TL) zebrafish (Kim et al., 2013). Adult care and reproductive techniques were conducted according to the Institutional Animal Care and Use Committee protocols at Oregon State University (OSU). All 5D embryos used in exposures were collected following group spawning of adult zebrafish as described previously (Reimers et al., 2006). Embryos from the Tg(cyp1a:nls-egfp) reporter line were collected following incross or outcross small group spawns.
Chemicals and Developmental Exposures
Analytical-grade standards were obtained from several vendors, including Sigma (St. Louis, MO), AccuStandard (New Haven, CT) and Chiron AS (Trondheim, Norway). Dimethyl sulfoxide (DMSO) was obtained from Sigma. Two compounds, 7-nitrobenzo[k]fluoranthene and 3,7-dinitrobenzo[k]fluoranthene, were custom synthesized in-house at OSU (Jariyasopit et al., 2013). For a complete list of chemicals tested, see Supplementary Data (SD) 1. In total, 27 NPAHs, two amino PAHs (potential metabolites of NPAHs), and 10 heterocyclic PAHs were tested. Individual compounds were dissolved in DMSO to make stock concentrations of either 10 or 1 mM, dependent on solubility (concentrations are listed in SD 1). For the static exposures to zebrafish, compounds were dispensed into polystyrene 96-well plates pre-loaded with 100 µl of embryo media and individual zebrafish embryos, which had been enzymatically dechorionated and loaded into the plates using automation (Mandrell et al., 2012). Chemicals were dispensed into the plates using an HP D300 Digital Dispenser and shaken using pre-optimized protocols (Titaley et al., 2016; Truong et al., 2016). Two plates were treated with each chemical at a range of initial exposure concentrations (50, 35.6, 11.2, 5, 1.12 µM for chemicals with a 10 mM DMSO stock, or 5, 3.56, 1.12, 0.5, 0.1 µM for chemicals with a 1 mM DMSO stock), for a total number of 32 animals exposed to each concentration. 1% DMSO vehicle controls were also exposed on each plate. Following chemical addition, plates were moved to a temperature-controlled room (28 °C) and were placed on a custom-modified rotating shaker table overnight (Truong et al., 2016). Embryos were evaluated at 24 hpf for developmental progress, spontaneous movement, notochord malformations and mortality. At 120 hpf, embryos were screened for total mortality and a suite of morphological endpoints, including pericardial and yolk sac edemas, malformations of the eye, snout, jaw, pectoral and caudal fins, axial malformations, and touch response (Knecht et al., 2013; Truong et al., 2011). Compounds were bi-hierarchically clustered based on similar malformation profiles using the “aheatmap” function in the NMF R package, in order to better evaluate structure-activity relationships.
Cyp1a/GFP Reporter Fish
For CYP1A evaluation using the Tg(cyp1a:nls-egfp) transgenic reporter fish (Kim et al., 2013), 8–12 embryos per compound for all 39 compounds were exposed as described previously in section 2.2, to a single concentration selected to maximize malformations whereas minimizing mortality. Concentrations evaluated are listed in SD 1. Following chemical addition using the HP D300 Digital Dispenser, plates were shaken overnight as described in section 2.2. Embryos were evaluated following continuous chemical exposure at 48 (prior to liver development and metabolic capacity) and 120 hpf for cyp1a/GFP expression using a Keyence BZ-X700 fluorescence microscope (Keyence North America, Itasca, IL). The cyp1a/GFP reporter line harbors a fosmid construct with GFP under control of a recombineered zebrafish cyp1a promoter, and recapitulates cyp1a induction by TCDD with high sensitivity (Kim et al., 2013). Immunohistochemistry (IHC) was also performed for all compounds following exposure in the wild type 5D zebrafish from 6–120 hpf to validate results from the reporter line. Further information can be found in SD 2.
Antisense Morpholino Injections
Morpholinos designed against zebrafish AHR1A, AHR1B, and AHR2, as well as a standard injection control morpholino were purchased from Gene Tools (Philomath, OR), and sequences are provided in Table 1 (Gerlach et al., 2014; Goodale et al., 2012). Morpholinos were dissolved in ultrapure water, and heterozygous Tg(cyp1a:nls-egfp) transgenic reporter embryos were injected at the 1–2 cell stage with 2 nL of morpholino (concentrations give in Table 1) and 0.5% phenol red. Embryos developing normally with morpholino incorporation were exposed to single concentrations of selected compounds, representing each of the previously observed cyp1a/GFP expression patterns: 5-nitroacenaphthalene, 1,6-dinitropyrene, 1,3-dinitropyrene, 7-nitrobenzo[k]fluoranthene, 9-nitrophenanthrene, 7-nitrobenz[a]anthracene, 3-nitrofluoranthene, 3,7-dinitrodibenzo[k]fluoranthene, and DMSO. Embryos were chemically exposed from 6 to 48 or 6 to 120 hpf and imaged at 48 or 120 hpf, respectively, using a Keyence BZ-X700 fluorescence microscope, and evaluated for developmental toxicity and cyp1a expression.
TABLE 1.
Sequences of Morpholino Oligonucleotides Used, with Appropriate Injection Concentrations
| AHR Isoform | Sequence | Concentration |
|---|---|---|
| AHR1A | CTTTTGAAGTGACTTTTGGCCCGCA | 1.5 mM |
| AHR1B | ACACAGTCGTCCATGATTACTTTGC | 0.75 mM |
| AHR2 | TGTACCGATACCCGCCGACATGGTT | 0.75 mM |
| Control | CCTCTTACCTCAGTTACAATTTATA | 1.5 mM |
| AHR1B/AHR2 mixture | 0.75 mM each |
AHR Docking Predictions
Docking of individual compounds to in silico models of the Per-AHR/Arnt-Sim (PAS)-B domains of each of the zebrafish AHR isoforms, as well as human AHR, was performed as previously described (Bisson et al., 2009; Goodale et al., 2015; Perkins et al., 2014). Docking scores were assigned for each compound and AHR isoform, overall priority for docking to any AHR isoforms were assigned, and predictions were compared to in vivo cyp1a/GFP expression.
RNA Extraction
For RNA samples, dechorionated 5D embryos were exposed from 6 to 48 hpf or 6 to 120 hpf to a subset of NPAHs at the same concentration used for Tg(cyp1a:nls-egfp) transgenic reporter fish evaluations (SD 1). Compounds were selected for qPCR analysis in order to sample a range of developmental toxicities and AHR activation patterns: 5-nitroacenaphthalene, 1,6-dinitropyrene, 1,3-dinitropyrene, 7-nitrobenzo[k]fluoranthene, and vehicle control. Briefly, 48 hpf or 120 hpf embryos were rinsed in embryo media and four samples of 20 embryos each were collected on ice in snap-safe Eppendorf tubes with 0.5 mm zirconium oxide beads. 500 μl RNAzol was added and samples were homogenized with a Bullet Blender (Next Advance) for 3 min at speed 8, and then stored at –20 °C until RNA isolation.
For total RNA isolation, RNA was extracted via RNAzol/isopropanol precipitation as previously described (Knecht et al., 2013). RNA was quantified using a Synergy/Mxmicroplate reader (Biotek) with the Gen5 Take3 module to calculate 260/280 O.D. ratios and RNA concentration. RNA was stored at –80 °C until use.
Quantitative RT-qPCR
Gene expression of a suite of 21 AHR-related, cardiac stress-related and oxidative stress gene transcripts was assessed in whole-embryo homogenates for 6–48 hpf or 6–120 hpf exposures of single concentrations of selected compounds (listed in Section 2.4). Gene-specific primers (MWG Operon) are listed in SD 3. All qRT-PCR experiments were performed in 10 µl reactions consisting of 5 µl Power SYBR Green PCR one-step master mix (Applied Biosystems), 0.4 µl of each 10 mM primer in 0.1% Triton X, 4.2 µl H2O and 2 µg of cDNA in 0.1% Triton X, which were dispensed using the PCR program on the HP D300 Digital Dispenser. The temperature program consisted of 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 58 or 60 °C (dependent on primer set), with a final melt curve consisting of a 15 s hold at 95 °C, 2 min at 70 °C, and a 0.2 °C per minute ramp, with a final hold at 95 °C for 15 s.
Statistics and Data Analysis
Data analysis
Statistical analysis and data visualization for developmental toxicity screening was done using code developed in R, using the custom Zebrafish Analysis and Acquisition (ZAAP) program (Truong et al., 2014). Concentrations which induced 50 percent effect (EC50) were calculated by fitting dose-response data with a binomial logit regression using custom R scripts and the dose.p function (Haggard et al., 2016; R Core Team, 2013). Venn diagram representations of cyp1a/GFP expression patterns were generated using the Draw Venn Diagrams software from the Ghent University Bioinformatics Evolutionary Genomics group.
PCR statistics
qRT-PCR analysis was performed with StepOne software (Applied Biosystems), using the Δ ΔCT method with genes normalized to β-actin (Pfaffl, 2001). Four biological replicates of 20 embryos each were analyzed by comparing NPAH treated to DMSO control with a one-way ANOVA following normalization with β-actin, using SigmaPlot V11.0 software.
RESULTS
Developmental Toxicity Screen
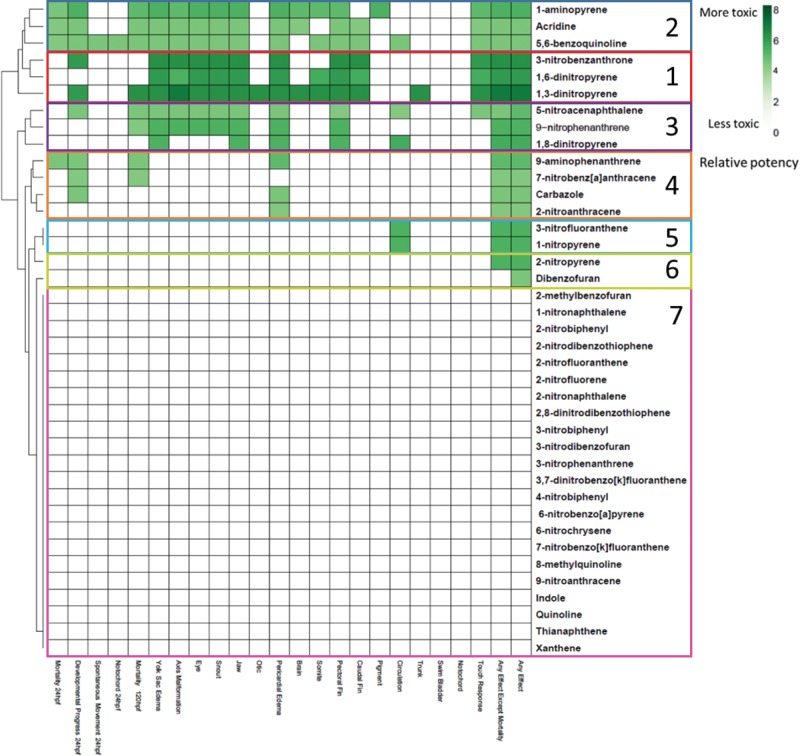
Concentration-dependent morphological responses were observed at 24 hpf and 120 hpf in the zebrafish embryos exposed to a dilution series of NPAHs and HPAHs, compared to the DMSO-exposed control, for which no significant developmental toxicity was observed. This data is presented in Figure 1 as a heatmap, showing the relative potency derived from the lowest effect level (LEL) value for each endpoint evaluated, as well as any effect including and excluding mortality, for each compound tested.
FIG. 1.
Heatmap displaying developmental toxicity of all 39 compounds investigated. Color scaling indicates relative potency, based on the lowest effect level (LEL) observed for a given compound and endpoint. Darker shades of green indicate a higher relative potency, and therefore a more toxic response, whereas lighter shades indicate a lower relative potency and less toxic response. White indicates that there was no significant observable LEL for that endpoint, or that values were incalculable due to high mortality. Compounds are vertically clustered based on the observation of similar developmental endpoints. Groups of compounds with similar toxicity profiles are indicated by boxes and group numbers.
Based on the clustering of compounds with similar developmental toxicity endpoints, several groups of compounds, with similar toxicity profiles and commonly observed endpoints, emerged. The most toxic group of compounds, as indicated by lowest effect levels at the lowest concentrations, were 3-nitrobenzanthrone, 1,6-dinitropyrene, and 1,3-dinitropyrene (labeled as group 1 in Figure 1), which caused the common endpoints of pericardial and yolk sac edemas and craniofacial malformations at 120 hpf with high potency. Similar endpoints, in addition to mortality at 24 hpf, were observed for 1-aminopyrene, acridine, and 5,6-benzoquinoline (group 2). However, for this group of compounds, these effects were observed at higher concentrations. The next group of compounds included 5-nitroacenaphthalene, 9-nitrophenanthrene, and 1,8-dinitropyrene (group 3), displayed a higher degree of lethality and a lower incidence of non-mortality endpoints, at relatively higher concentrations. The next group of chemicals (9-aminophenanthrene, 7-nitrobenz[a]anthracene, carbazole, and 2-nitroanthracene; group 4) resulted in stronger effects at 24 hpf, including developmental delay and mortality, as well as pericardial edema at 120 hpf. Defects in circulation, marked by pooling of blood in the body of the zebrafish embryos, was the only endpoint observed following exposure to 1-nitropyrene and 3-nitrofluoranthene (group 5). Two compounds (2-nitropyrene and dibenzofuran; group 6) had a significant effect for the “any effect” or “any effect including mortality” endpoints, but not for any of the individual endpoints. The remaining 22 compounds (group 7), greater than half of those tested, did not result in significant toxicity to the zebrafish embryos following exposure at the tested concentrations.
The EC50 values were calculated for compounds with observed toxicity and are shown in Table 2. Values ranged from 0.096 µM (1,3-dinitropyrene) to 44.8 µM (1-nitropyrene). Three compounds (2-nitroanthracene, 7-nitrobenz[a]anthracene, and dibenzofuran) had EC50 values estimated to be beyond the tested range of concentrations, and beyond the range of accuracy of the EC50 model.
TABLE 2.
EC50 (µM) Values for All Compounds, With Standard Error
| EC50 (µM) | Std Error | |
|---|---|---|
| NPAHs | –– | –– |
| 1-nitronaphthalene | –– | –– |
| 2-nitronaphthalene | –– | –– |
| 2-nitrobiphenyl | –– | –– |
| 3-nitrobiphenyl | –– | –– |
| 4-nitrobiphenyl | –– | –– |
| 3-nitrodibenzofuran | –– | –– |
| 5-nitroacenaphthalene | 13.41 | 0.78 |
| 2-nitrofluorene | –– | –– |
| 9-nitroanthracene | 222.13† | 189 |
| 9-nitrophenanthrene | 1.81 | 0.78 |
| 2-nitrodibenzothiophene | –– | –– |
| 3-nitrophenanthrene | –– | –– |
| 2-nitroanthracene | –– | –– |
| 2-nitrofluoranthene | –– | –– |
| 3-nitrofluoranthene | 2.04 | 0.85 |
| 1-nitropyrene | 44.82 | 8.34 |
| 2-nitropyrene | –– | –– |
| 7-nitrobenz[a]anthracene | 237.6† | 108 |
| 2,8-dinitrodibenzothiophene | –– | –– |
| 6-nitrochrysene | –– | –– |
| 3-nitrobenzanthrone | 0.2 | 0.17 |
| 1,3-dinitropyrene | 0.1 | 0.05 |
| 1,6-dinitropyrene | 0.88 | 0.22 |
| 1,8-dinitropyrene | 3.33 | 1.18 |
| 6-nitrobenzo[a]pyrene | –– | –– |
| 7-nitrobenzo[k]fluoranthene | –– | –– |
| 3,7-dinitrobenzo[k]fluoranthene | –– | –– |
| Amino PAHs | ||
| 1-aminopyrene | 1.85 | 0.74 |
| 9-aminophenanthrene | 8.68 | 0.87 |
| HPAHs | ||
| Indole | –– | –– |
| Quinoline | –– | –– |
| 2-Methylbenzofuran | –– | –– |
| Thianaphthene | –– | –– |
| 8-Methylquinoline | –– | –– |
| Carbazole | 15.15 | 0.96 |
| Dibenzofuran | 228.3a | 227.4 |
| 5,6-Benzoquinoline | 10.35 | 1.04 |
| Acridine | 13.49 | 0.96 |
| Xanthene | –– | –– |
Blank cells indicate insufficient data to calculate EC50.
Indicates calculated value is beyond the range of concentrations tested, and beyond the range of accuracy of the EC50 model.
CYP Expression as a Biomarker for AHR Pathway Activation
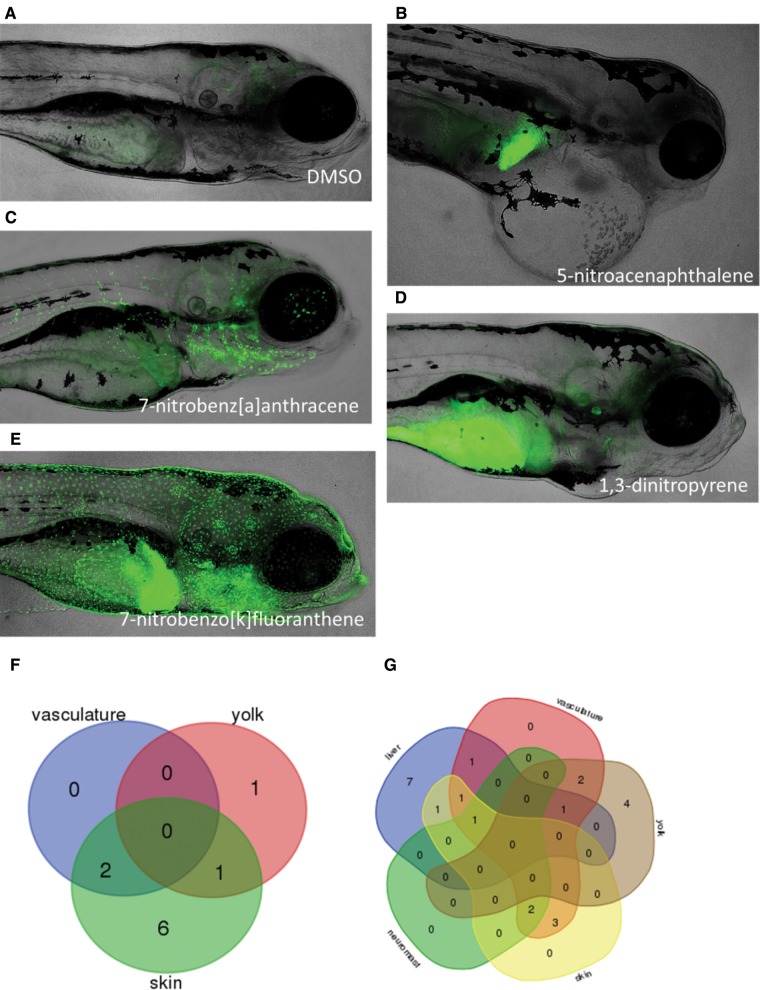
To investigate the potential role of AHR, we utilized the transgenic Tg(cyp1a:nls-egfp) zebrafish line to evaluate expression of cyp1a/GFP, a known biomarker for AHR activation. Several distinct cyp1a/GFP expression patterns were observed following exposure to each of the 39 individual compounds from samples collected at 48 and 120 hpf (Figure 2). Compared to the DMSO-exposed control in which no distinct cyp1a/GFP expression was observed (Figure 2A), cyp1a/GFP expression was observed in the liver (Figure 2B), vasculature (Figure 2C), yolk sac (Figure 2D), skin, and neuromasts (Figure 2E) following exposure to certain NPAHs. There were also a number of compounds for which no cyp1a/GFP expression was observed. Tissues displaying fluorescence, indicating cyp1a/GFP expression and activation of the AHR pathway, for all compounds tested are listed in Table 3. Several compounds also showed cyp1a/GFP expression in multiple tissues simultaneously. For example, expression in the neuromasts was always observed alongside expression in the skin. Unique and overlapping cyp1a/GFP expression patterns are shown in Venn diagrams, for expression at 48 hpf (Figure 2F) and 120 hpf (Figure 2G).
FIG. 2.
Selected representative images of cyp1a/GPF expression in Tg(cyp1a:nls-egfp) transgenic zebrafish following chemical exposures. Examples of cyp1a/GFP expression patterns in the DMSO-exposed animals (A), liver (B), vasculature (C), yolk sac (D) and skin, neuromasts and liver (E) following exposure to indicated NPAHs. Expression patterns observed at 48 hpf (F) and 120 hpf (G), showing unique and overlapping cyp1a/GFP expression patterns for all compounds where expression in one or more tissues was observed. All results are summarized in Table 3.
TABLE 3.
Summary of cyp1a/GFP expression Patterns Observed Using Tg(cyp1a:nls-egfp) Transgenic Zebrafish Following Individual Exposure to all NPAHs and HPAHs Studied, at 48 hpf and 120 hpf
| 48 hpf | 120 hpf | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPAHs | Liver | Vasculature | Yolk | Skin | Neuromast | None | Liver | Vasculature | Yolk | Skin | Neuromast | None |
| 1-nitronaphthalene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 2-nitronaphthalene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 2-nitrobiphenyl | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 3-nitrobiphenyl | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| 4-nitrobiphenyl | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 3-nitrodibenzofuran | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 5-nitroacenaphthalene | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| 2-nitrofluorene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 9-nitroanthracene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 9-nitrophenanthrene | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| 2-nitrodibenzothiophene | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| 3-nitrophenanthrene | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| 2-nitroanthracene | –– | –– | x | –– | –– | –– | –– | –– | x | –– | –– | –– |
| 2-nitrofluoranthene | –– | x | –– | x | –– | –– | x | x | x | –– | –– | –– |
| 3-nitrofluoranthene | –– | –– | –– | –– | –– | x | –– | x | x | –– | –– | –– |
| 1-nitropyrene | –– | –– | –– | –– | –– | x | –– | –– | x | –– | –– | –– |
| 2-nitropyrene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 7-nitrobenz[a]anthracene | –– | –– | –– | x | –– | –– | –– | x | –– | x | –– | –– |
| 2,8-dinitrodibenzothiophene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 6-nitrochrysene | –– | –– | –– | x | –– | –– | –– | x | –– | x | –– | –– |
| 3-nitrobenzanthrone | –– | –– | x | x | –– | –– | –– | –– | x | –– | –– | –– |
| 1,3-dinitropyrene | –– | –– | –– | –– | –– | x | –– | –– | x | –– | –– | –– |
| 1,6-dinitropyrene | –– | –– | –– | x | –– | –– | –– | x | –– | x | –– | –– |
| 1,8-dinitropyrene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 6-nitrobenzo[a]pyrene | –– | –– | –– | –– | –– | x | x | x | –– | x | –– | –– |
| 7-nitrobenzo[k]fluoranthene | –– | –– | –– | x | –– | –– | x | x | –– | x | x | –– |
| 3,7-dinitrobenzo[k]fluoranthene | –– | –– | –– | –– | –– | x | –– | x | –– | x | x | –– |
| Amino PAHs | –– | –– | –– | –– | –– | –– | –– | |||||
| 1-aminopyrene | –– | –– | –– | –– | –– | x | –– | x | x | –– | –– | –– |
| 9-aminophenanthrene | –– | –– | –– | –– | –– | x | x | –– | –– | –– | –– | –– |
| HPAHs | –– | –– | –– | –– | –– | –– | –– | –– | –– | –– | –– | –– |
| Indole | –– | –– | –– | x | –– | –– | –– | x | –– | x | x | –– |
| Quinoline | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 2-Methylbenzofuran | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| Thianaphthene | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 8-Methylquinoline | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| Carbazole | –– | –– | –– | –– | –– | x | x | x | –– | –– | –– | –– |
| Dibenzofuran | –– | –– | –– | –– | –– | x | –– | –– | –– | –– | –– | x |
| 5,6-Benzoquinoline | –– | –– | –– | x | –– | –– | x | –– | –– | x | –– | –– |
| Acridine | –– | x | –– | x | –– | –– | x | –– | –– | –– | –– | x |
| Xanthene | –– | –– | –– | –– | –– | x | x |
Tissues where cyp1a/GFP expression are observed are designated with an “x”.
Knock-Down of AHR to Elucidate its Role in Developmental Toxicity
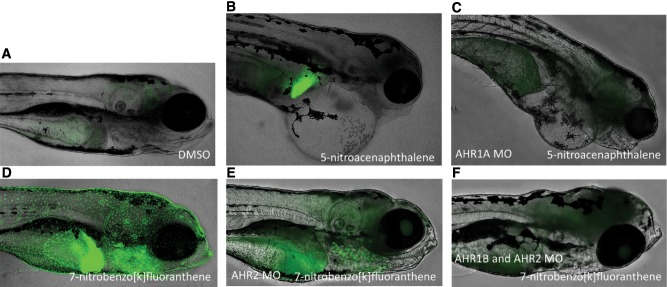
Embryos injected with any of the morpholinos did not display an increase in background developmental toxicity, compared to non-injected controls (data not shown). cyp1a/GFP expression in the livers of fish exposed to 5-nitroacenaphthalene, 9-nitrophenanthrene, and 7-nitrobenzo[k]fluoranthene was eliminated in AHR1A morphants (Figs. 3B–C), but did not result in a substantial decrease in observed developmental toxicity following exposure to 5-nitroacenaphthalene.
FIG. 3.
Selected representative images of cyp1a/GPF expression in Tg(cyp1a:nls-egfp) transgenic zebrafish following chemical exposures and morpholino oligonucleotide injections. Examples of cyp1a/GFP expression patterns in the DMSO-exposed animals (A), liver (B), absence liver expression in AHR1A morphants (C), skin, neuromasts and liver (D), near-complete elimination of skin, neuromast, and vascular expression in AHR2 morphants (E), and complete skin, neuromast, and vasculature expression elimination (F) following co-injection of the AHR2 and AHR1B morpholinos, following exposure to indicated NPAHs.
Decreased GFP expression in AHR2 morphants cyp1a/GFP reporter embryos exposed to 7-nitrobenzo[k]fluoranthene in the skin and neuromasts, and 7-nitrobenz[a]anthracene in the vasculature, indicating AHR2 dependence for cyp1a/GFP expression (Figs. 3D–E), at both 48 and 120 hpf. Expression in the skin and vasculature following exposure to 1,6-dinitropyrene and 3,7-dinitrobenzo[k]fluoranthene were also reduced in AHR2 morphants (not shown). Injection of the AHR1B morpholino alone did not visibly reduce cyp1a/GFP expression following exposure to any of the compounds investigated. However, co-injection of AHR2 and AHR1B morpholinos appeared to reduce GFP expression slightly more than the AHR2 morpholino alone (Figure 3F).
In Silico Docking to AHR Active Site Models
Of the set of 39 compounds investigated, 22 were predicted to dock to one or more isoforms of the zebrafish AHR. Table 4 shows the docking scores, (given in kilocalories/mol), for all three zebrafish AHR isoforms, as well as human AHR, along with the overall docking priority to the zebrafish AHR isoforms. The more negative the docking score, the higher the predicted binding and activation. Of the 22 compounds predicted to dock to one or more isoforms of the zebrafish AHR, 16 displayed in vivo cyp1a/GFP expression, indicative of AHR activation, for a success rate of 73%. Of the 23 compounds which showed in vivo cyp1a/GFP expression, 16 were predicted to dock to the AHR, for a success rate of 70%.
TABLE 4.
Predicted AHR Docking Scores (kilocalories/mol) for All Three Zebrafish AHR Isoforms, as Well as Human AHR
| Docking Scores | |||||
|---|---|---|---|---|---|
| NPAHs | Human | Zebrafish AHR2 | Zebrafish AHR 1B | Zebrafiah AHR1A | High Priority |
| 1-nitronaphthalene | –14.32 | –16.08 | –13.01 | –19.36 | |
| 2-nitronaphthalene | –13.43 | –14.78 | –18.03 | –17.97 | |
| 2-nitrobiphenyl | –11.83 | –15.5 | –19.13 | –15.43 | |
| 3-nitrobiphenyl | –14.94 | –17.73 | –18.01 | –15.42 | |
| 4-nitrobiphenyl | –18.12 | –12.71 | –14.19 | –17.2 | |
| 3-nitrodibenzofuran | –19.52 | –19.09 | –14.9 | –18.07 | † |
| 5-nitroacenaphthalene | –5.91 | –11.29 | –15.46 | –17.04 | † |
| 2-nitrofluorene | –12.97 | –9.1 | –9.72 | –17.07 | † |
| 9-nitroanthracene | –9.89 | –10.15 | –12.06 | –14.84 | † |
| 9-nitrophenanthrene | –3.08 | –12.25 | –15.91* | –15.72 | † |
| 2-nitrodibenzothiophene | –15.85 | –17.06 | –10.85 | –17.92 | † |
| 3-nitrophenanthrene | –12.91 | –15.17 | –16.31* | –16.34 | † |
| 2-nitroanthracene | –16.55 | –14.81 | –8.2 | –19.02 | † |
| 2-nitrofluoranthene | –8.33 | –12.79 | –15.25* | –12.43 | |
| 3-nitrofluoranthene | –9.12 | –7.91 | –16.55* | –17.14 | † |
| 1-nitropyrene | ND | –6.91 | –15.67* | –12.07 | † |
| 2-nitropyrene | –3.54 | –4.4 | –14.36* | –17.92 | |
| 7-nitrobenz[a]anthracene | –9.97 | –5.53 | –18.08* | ND | † |
| 2,8-dinitrodibenzothiophene | –15.14 | –18.19 | –10.85 | –17.31 | † |
| 6-nitrochrysene | –11.37 | –5.47 | –16.91* | –11.6 | † |
| 3-nitrobenzanthrone | –5.64 | –3.86 | –17.15* | –6.66 | † |
| 1,3-dinitropyrene | ND | ND | –15.13* | ND | |
| 1,6-dinitropyrene | ND | –5.71 | –15.85* | ND | |
| 1,8-dinitropyrene | –3.69 | –5.66 | –16.23* | –11.64 | |
| 6-nitrobenzo[a]pyrene | ND | –1.64 | –18.13* | ND | |
| 7-nitrobenzo[k]fluoranthene | –5.27 | ND | –16.05* | ND | |
| 3,7-dinitrobenzo[k]fluoranthene | –5.27 | ND | –16.05* | ND | |
| Amino PAHs | |||||
| 1-aminopyrene | ND | –5.3 | –15.06* | –11.09 | † |
| 9-aminophenanthrene | –7.77 | –10.99 | –11.89 | –21.81 | † |
| HPAHs | |||||
| Indole | –11.14 | –15.57 | –13.94 | –19.94 | † |
| Quinoline | –12.29 | –15.18 | –17.52 | –15.83 | |
| 2-Methylbenzofuran | –15.38 | –17.2 | –16.96 | –14.17 | |
| Thianaphthene | –13.89 | –12.07 | –14.46 | –17.7 | |
| 8-Methylquinoline | –7.85 | –13.23 | –12.8 | –17.29 | |
| Carbazole | –11.86 | –14.85 | –15.81 | –19.78 | † |
| Dibenzofuran | –15.02 | –15.82 | –14.07 | –16.12 | † |
| 5,6-Benzoquinoline | –14.18 | –11.97 | –14.57 | –20.46 | † |
| Acridine | –15.68 | –15.48 | –11.31 | –15.09 | † |
| Xanthene | –16.26 | –14.69 | –11.64 | –14.18 | † |
ND indicates the compound was not predicted to dock, or docked with non-favorable score/energy to the AHR active site model. Asterisk indicates that docking was predicted, but with an unfavorable pose in the active site, despite the promising binding energy. Compounds were classified as “high priority” for AHR activation (indicated by †) based on overall AHR binding favorability to the three zebrafish isoforms.
ND, not docked or docked with non favorable score/energy.
despite the promising number, docked with a non favorable pose (binding pattern).
Differential Gene Expression to Elucidate Other Potential Mechanisms of Action
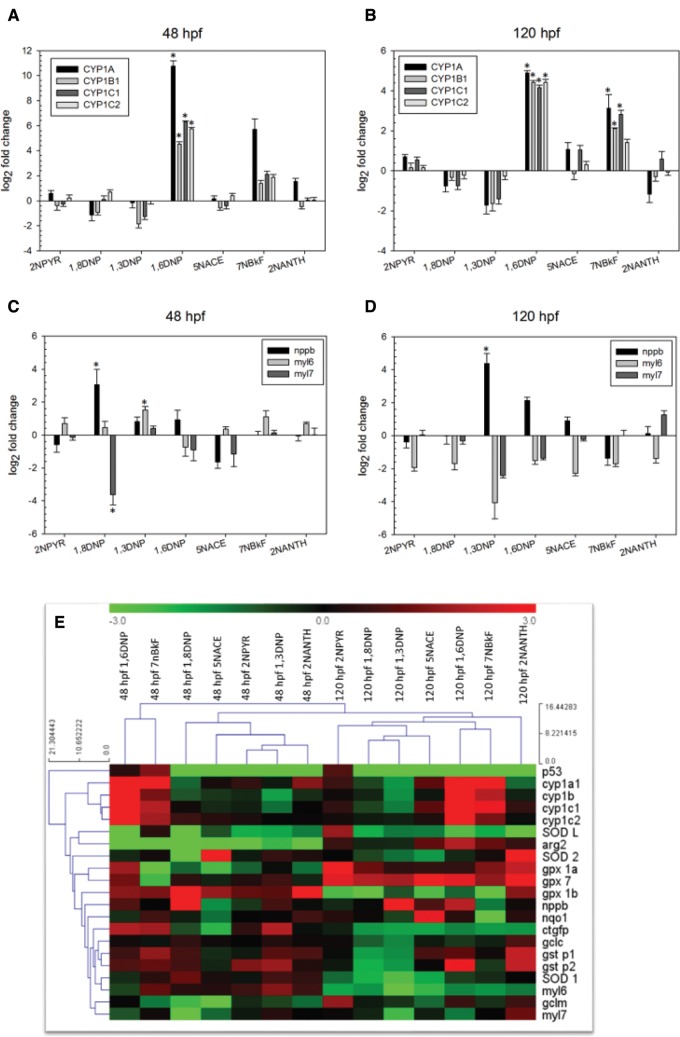
To further investigate other potential mechanisms of action for NPAHs, qPCR analysis was performed for a suite of 21 genes selected to investigate cardiac toxicity, oxidative stress, and additional potential mechanisms of action. Significantly increased expression of cyp1a, cyp1b1, cyp1c1, and cyp1c2 was observed following exposure to 5 µM 1,6-dinitropyrene at 48 and 120 hpf, and at 120 hpf following exposure to 50 µM 7-nitrobenzo[k]fluoranthene for cyp1a, cyp1b1, and cyp1c1 (Figure 4A–B). Expression of cyp1a1 at 48 hpf was increased by 1,963-fold following exposure to 1,6-dinitropyrene, compared to the DMSO exposed controls. In the genes related to cardiac stress, expression of nppb (natriuretic peptide b, which encodes a small peptide responsible for the regulation of homeostatic contractility and response to cardiac stress) was increased by 12-fold in animals exposed to 1.12 µM 1,8-dinitropyrene at 48 hpf, and 23-fold in animals exposed to 3.56 µM 1,3-dinitropyrene at 120 hpf. Additionally, expression of myl6 (cardiac myosin light chain 6, which encodes the essential cardiac myosin light chain) was significantly decreased by 0.9-fold in the 1,8-dinitropyrene exposed animals at 48 hpf, whereas expression of myl7 (cardiac myosin light chain 7, which encodes the regulatory cardiac myosin light chain) was significantly increased by 2-fold in animals exposed to 1,3-dinitropyrene at 48 hpf (Figure 4C–D). Other genes important in detoxification and cellular protection from oxidative stress were also differentially expressed following developmental exposure to NPAHs (Figure 4E, SD 3). These included members of the superoxide dismutase (sod) family (an antioxidant enzyme responsible for the dismutation of superoxide radical into molecular oxygen or hydrogen peroxide), glutathione transferases (gst, phase II enzymes responsible for the conjugation of reduced glutathione to xenobiotic substrates), and glutathione peroxidases (gpx, which protect against oxidative stress by reducing peroxides). 1,3-dinitropyrene at 48 hpf resulted in differential expression of the greatest number of genes (4), whereasarg2 (arginase) was the gene most commonly mis-expressed at both 48 hpf and 120 hpf.
FIG. 4.
Changes in gene expression induced by exposure to selected NPAHs. Graphs indicate changes in expression to selected cyp genes at 48 hpf (A) and 120 hpf (B), and in selected cardiac stress genes at 48 hpf (C) and 120 hpf (D). Asterisks indicate statistical significant at a P < 0.05 level, based on one-way ANOVA. All genes investigated are shown in the heatmap (E) for 48 and 120 hpf, where color scaling indicates increased or decreased expression.
DISCUSSION
Whereas unsubstituted PAHs are known to elicit a range of human and environmental health effects, NPAHs and HPAHs are not as thoroughly studied. Investigation of the developmental toxicity of these compounds, as well as potential contributing mechanisms of action, is important in order to more accurately determine potential human and environmental health hazards as a result of exposure. A range of developmental toxicity endpoints and cyp1a/GFP expression patterns were observed following NPAH or HPAH exposure. Selected compounds also resulted in a variety of differentially expressed genes, indicating multiple potential contributing mechanisms of action.
Differential Developmental Toxicity
Of the 39 compounds screened, 17 (44%) resulted in significant developmental toxicity of at least one observed endpoint. The profile of developmental toxicity malformations observed is similar to what has been previously observed with PAHs and oxygenated PAHs, as well as PAH-containing oil preparations (Goodale et al., 2013; Incardona et al., 2013; Jung et al., 2013; Knecht et al., 2013).
Comparing the calculated EC50 values with the log Kow, molecular weight, and water solubility of compounds tested did not result in any significant correlation (SD 1). However, experimentally derived values for the log Kow and water solubility do not exist for many of the compounds in this data set, resulting in a reliance on estimated values from the EPI Suite program (US EPA). Previous studies had shown relationships between the observed toxicity of N-heterocyclic (Schultz et al., 1980) and substituted anilines (Zok et al., 1991) with molecular weight, partition coefficients, and/or bioconcentration factors; however, experimentally determined values were available for the compounds under investigation in those studies. The reliability of our modeling would likely be improved if experimentally determined values had been available.
There is also significant uncertainly in the aqueous concentrations and uptake of these compounds during the assay. Sorption of hydrophobic analytes to the polystyrene plates commonly used for zebrafish testing is known to occur, and can result in significant analyte loss, with upwards of 50 percent of the analyte sorbing to the plastic rather than remaining in the aqueous exposure media (Chlebowski et al., 2016; Peddinghaus et al., 2012). Whereas the protocols have been optimized to minimize the sorptive losses which occur (Truong et al., 2016), and maximize the analyte concentration in the exposure solution, the actual analyte concentration remaining in the exposure media is uncertain. Furthermore, the availability and uptake of PAHs and PAH derivatives can be variable (Goodale et al., 2013), and is unknown for the NPAHs and HPAHs studied here.
Activation of the AHR Pathway and cyp1a Expression
Fluorescence indicating cyp1a expression and activation of one or more AHR isoforms was observed at 48 and/or 120 hpf for 23 of the 39 compounds tested, suggesting the involvement of AHR in the toxicity of at least some NPAHs and HPAHs. Other mechanisms for the induction of cyp1a, such as cellular stress, could potentially be contributing as well (Behrendt et al., 2010), but the selective elimination of cyp1a/GFP expression in AHR2 morphants suggests the AHR isoform dependence of cyp1a expression.
The near-complete inhibition of cyp1a/GFP expression in the vasculature and/or skin (and neuromasts, for 7-nitrobenzo[k]fluoranthene) of embryos exposed to 7-nitrobenzo[k]fluoranthene, 1,6-dinitropyrene, 7-nitrobenz[a]anthracene and 3,7-dinitrobenzo[k]fluoranthene, following knockdown of AHR2, indicates that AHR2 is the primary isoform responsible for expression in these tissues. Decreased toxicity was observed in 1,6-dinitropyrene exposed AHR2 morphants, indicating a role for AHR in the toxicity of this compound. No substantial change in toxicity was observed for the other three compounds investigated, which were all relatively non-toxic in the developmental toxicity screen. This is consistent with previous research on PAHs and oxygenated PAHs, where knockdown of AHR2 results in the inhibition of the skin or vasculature cyp1a/GFP expression patterns (Goodale et al., 2013; Incardona et al., 2011; Knecht et al., 2013). Expression of cyp1a has been observed previously in the neuromasts of transgenic Japanese Medaka (Oryzias latipes) following exposure to TCDD (Ng and Gong, 2013), although no further mechanistic information has been elucidated. Based on our results, the inhibition of cyp1a/GFP expression in the neuromasts, in AHR2 morphants, indicates AHR2 dependence of this expression pattern as well.
The decrease in cyp1a/GFP expression in AHR1A morphants is also consistent with previous research (Knecht et al., 2013), which implicated AHR1A as the dominant isoform present in the liver. Fluorescence in the liver was not observed in 48 hpf embryos, due to the majority of liver development occurring after 48 hpf (Ober et al., 2003). We did not observe a substantial decrease in toxicity in AHR1A morphants, indicating that the toxicity observed is not completely AHR-dependent.
cyp1a/GFP expression in the yolk of embryos exposed to 1,3-dinitropyrene or 3-nitrofluoranthene did not appear to decrease as a result of injection with any of the morpholinos. cyp1a/GFP expression in the syncytial layer of the yolk had been previously observed following exposure to 1,9-benz-10-anthrone (Goodale et al., 2015), although this was not determined to be due to a specific isoform of the AHR. Another isoform of cyp, cyp11a1, is expressed in the yolk syncytial layer (Goldstone et al., 2010; Hsu et al., 2002), although this isoform has not been linked with xenobiotic metabolism.
Overall, the in silico model for the AHR1A isoform had the highest predictive success rate, where eight of the 12 compounds with in vivo cyp1a/GFP expression were predicted to dock to the AHR, in most cases to the AHR1A with the highest affinity. In contrast, the predicted docking scores to the AHR2 isoform did not align well with the observed cyp1a/GFP expression patterns in the skin and vasculature. The AHR docking models were developed and refined using TCDD as the guide ligand for AHR2 and AHR1B, and leflunomide for AHR1A (Gerlach et al., 2014). Both TCDD and leflunomide are structurally distinct from PAHs. Whereas the AHR docking models performed adequately for the NPAHs and HPAHs studied here, there is room for further optimization of these models for PAHs and PAH derivatives, in particular the larger and more potent compounds. Binding to the different isoforms of the AHR appears to be the major factor contributing to the differential cyp1a/GFP expression patterns. Bioavailability and uptake for hydrophobic compounds can vary greatly and is not known for NPAHs and HPAHs, the larger and more hydrophobic compounds, such as 7-nitrobenzo[k]fluoranthene and the dinitropyrenes were among the most potent both with regards to cyp1a/GFP expression and developmental toxicity, suggesting that bioavailability and uptake is not necessarily a limiting factor for bioactivity of these compounds.
NPAHs Differentially Alter Gene Expression
PAH and oxygenated PAH exposures have been previously linked with alterations in the expression of genes related to phase I metabolism (cyp genes), Phase II metabolism and oxidative stress, and cardiac toxicity (Goodale et al., 2015; Huang et al., 2012; Incardona et al., 2004; Incardona et al., 2006; Knecht et al., 2013). Compared to oxygenated PAHs, fewer of the NPAHs studied resulted in significantly altered expression of the four cyp genes investigated. However, the induction was a similar order of magnitude. This could indicate that phase I metabolism by the cyp genes as a result of AHR activation is not a major detoxification pathway for many NPAHs. Whereas our data do suggest that the cyp metabolism pathways and phase II metabolism and oxidative stress could contribute to the toxicity of NPAHs, other mechanisms of action are likely also contributing. Metabolism and oxidative stress may be regulated, at least in part, by the AHR pathway, but further studies would be required to confirm this.
Cardiac toxicity has been previously observed following exposure to PAHs and PAH-containing products (Incardona et al., 2004; Incardona et al., 2014; Jung et al., 2013; McIntyre et al., 2016). We observed altered expression of each of the three genes investigated (nppb, myl6, and myl7) following exposure to 1,8-dinitropyrene and 1,3-dinitropyrene at 48 and 120 hpf, indicating that cardiac toxicity may play a role in the developmental toxicity of at least some NPAHs. Zebrafish exposed to these compounds also displayed pericardial edema and circulatory malfunction (indicated by pooling of blood in the body). However, these malformations were also observed following exposure to other compounds which did not alter expression of these cardiac genes. Of these two compounds, only 1,3-dinitropyrene also resulted in altered cyp1a expression, indicated by visual expression in the yolk sac, which indicates that the observed cardiac toxicity is, at least in part, not AHR-dependent. Previous work has shown a diversity in cardiac toxicity mechanisms among PAHs acting as AHR agonists (Brown et al., 2014). Therefore, investigation of a wider number of genes and toxicity phenotypes is necessary to more fully elucidate the role of cardiac toxicity in the observed developmental toxicity of NPAHs and HPAHs, as well as the role of the AHR pathway.
The gene most commonly differentially expressed following NPAH exposure was arg2, the gene encoding the protein arginase 2. Arginases catalyze the hydrolysis of the amino acid arginine to urea and ornithine, and has a role in nitric oxide and polyamine metabolism (Durante et al., 2007). Arginase expression is positively correlated to expression of nitric oxide synthase (NOS), and, therefore, increased production of nitric oxide. Nitric oxide plays regulatory roles on various physiological and pathophysiological properties, including vascular and neurological functions (Sousa et al., 2010). We observed significantly decreased expression of arg2 at 48 hpf following exposure to all seven NPAHs tested, and significantly increased expression at 120 hpf following exposure to 1,6-dinitropyrene, 5-nitroacenaphthalene, and 7-nitrobenzo[k]fluoranthene. Previous work (Goodale et al., 2015) showed that arg2 expression was significantly elevated by 1,9-benz-10-anthrone, but not by benz[a]anthracene-7,12-dione, in 48 hpf embryos, and was dependent on AHR2. Whereas we did not investigate AHR dependency of gene expression, two of the three compounds which had elevated arg2 expression at 120 hpf (1,6-dinitropyrene and 7-nitrobenzo[k]fluoranthene) also displayed AHR2-dependent cyp1a expression. However, since many of the compounds under investigation were not observed to be AHR2-agonists, the altered expression of arg2 at 48 hpf appears to be via an AHR2-independent mechanism.
CONCLUSION
NPAHs and HPAHs, like the more thoroughly-studied PAHs and oxygenated PAHs, are toxicologically a non-homogenous group of compounds in the developing zebrafish model, as summarized in SD 4. NPAHs and HPAHs can elicit a variety of developmental toxicity endpoints, at a range of concentrations, demonstrating potential for ecological and human health impacts. NPAH and HPAH exposure also resulted in a range of cyp1a/GFP expression patterns, indicative of differential activation of AHR isoforms. Select NPAHs caused altered gene expression of a diverse set of genes, suggesting contributions of several potential mechanisms of action, including cardiac stress, cyp metabolism pathways, and oxidative stress. When considering health risks from PAH-containing complex environmental mixtures, PAH derivatives, including NPAHs and HPAHs, should be included. Further research into the toxicity and mechanisms of action of these compounds is warranted.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank M. McIntosh for synthesizing standards, the SARL screen team for providing embryos, M. Geier and D. Haggard for assistance with R, and Professor Seok-Yong Choi of Chonnam National University for providing the Tg(cyp1a:nls-egfp) reporter zebrafish line. The authors declare that they have no potential conflicts of interest.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences (T32 ES000760, P42 ES016465, 1F31ES02037-01, 5F31ES026518-02, P30 ES025128).
REFERENCES
- Arey J., Zielinska B., Atkinson R., Winer, A.M., Ramdahl T., Pitts J.N. (1986). The formation of nitro-PAH from the gas-phase reactions of fluoranthene and pyrene with the OH radical in the presence of NO x. Atmos. Environ. 1967 20, 2339–2345. [Google Scholar]
- Ball L. M., Kohan M. J., Claxton L. D., Lewtas J. (1984). Mutagenicity of derivatives and metabolites of 1-nitropyrene: Activation by rat liver S9 and bacterial enzymes. Mutat. Res. Toxicol. 138, 113–125. doi: 10.1016/0165-1218(84)90033-8. [DOI] [PubMed] [Google Scholar]
- Bamford H.A., Bezabeh, D.Z., Schantz, M.M., Wise, S.A., Baker J.E. (2003). Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere 50, 575–587. [DOI] [PubMed] [Google Scholar]
- Behrendt L., Jönsson M. E., Goldstone J. V., Stegeman J. J. (2010). Induction of cytochrome P450 1 genes and stress response genes in developing zebrafish exposed to ultraviolet radiation. Aquat. Toxicol. 98, 74–82. doi: 10.1016/j.aquatox.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J.A., Alexis, N., Barnes, C., Bernstein, I.L., Nel, A., Peden, D., Diaz-Sanchez, D., Tarlo, S.M., Williams P.B., Bernstein J.A. (2004). Health effects of air pollution. J. Allergy Clin. Immunol. 114, 1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Bisson W.H., Koch, D.C., O’Donnell, E.F., Khalil, S.M., Kerkvliet, N.I., Tanguay, R.L., Abagyan, R., Kolluri S.K. (2009). Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J. Med. Chem. 52, 5635–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. R., Clark B. W., Garner L. V. T., Giulio R. T. D. (2014). Zebrafish cardiotoxicity: The effects of CYP1A inhibition and AHR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists. Environ. Sci. Pollut. Res. 22, 8329–8338. doi: 10.1007/s11356-014-3969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel S. M., Tanguay R. L., Planchart A. (2014). Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Curr. Environ. Health Rep. 1, 341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibwe L., Geier, M.C., Nakamura, J., Tanguay, R.L., Aitken, M.D., Simonich S.L.M. (2015). Aerobic bioremediation of PAH contaminated soil results in increased genotoxicity and developmental toxicity. Environ. Sci. Technol. 49, 13889–13898. doi: 10.1021/acs.est.5b00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski A. C., Tanguay R. L., Simonich S. L. M. (2016). Quantitation and prediction of sorptive losses during toxicity testing of polycyclic aromatic hydrocarbon (PAH) and nitrated PAH (NPAH) using polystyrene 96-well plates. Neurotoxicol. Teratol. 57, 30–38. doi: 10.1016/j.ntt.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran R.E., Jeong, H., Haddadi, S., Fisseha Derseh, R., Gowan, A., Beránek J., Kubátová A (2016). Identification of products formed during the heterogeneous nitration and ozonation of polycyclic aromatic hydrocarbons. Atmos. Environ. 128, 92–103. doi: 10.1016/j.atmosenv.2015.12.036. [Google Scholar]
- Dalton T. P., Puga A., Shertzer H. G. (2002). Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem. Biol. Interact. 141, 77–95. doi: 10.1016/S0009-2797(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Nagy S. R. (2003). Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Dumont J. N., Schultz T. W., Jones R. D. (1979). Toxicity and teratogenicity of aromatic amines toXenopus laevis. Bull. Environ. Contam. Toxicol. 22, 159–166. doi: 10.1007/BF02026923. [DOI] [PubMed] [Google Scholar]
- Durante W., Johnson F. K., Johnson R. A. (2007). Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 34, 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G. R., Noyes P. D., Tanguay R. L. (2016). Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 161, 11–21. doi: 10.1016/j.pharmthera.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C.V., Das S.R., Volz D.C., Bisson W.H., Kolluri S.K., Tanguay R.L. (2014). Mono-substituted isopropylated triaryl phosphate, a major component of Firemaster 550, is an AHR agonist that exhibits AHR-independent cardiotoxicity in zebrafish. Aquat. Toxicol. 154, 71–79. doi: 10.1016/j.aquatox.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone J.V., McArthur, A.G., Kubota, A., Zanette, J., Parente, T., Jönsson, M.E., Nelson, D.R., Stegeman J.J. (2010). Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 11, 643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale B.C., La Du J.K., Tilton S.C., Sullivan C.M., Bisson W.H., Waters K.M., Tanguay R.L. (2015). Ligand-specific transcriptional mechanisms underlie aryl hydrocarbon receptor-mediated developmental toxicity of oxygenated PAHs. Toxicol. Sci. 147, 397–411. doi: 10.1093/toxsci/kfv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale B.C., La Du J.K., Bisson W.H., Janszen D.B., Waters K.M., Tanguay R.L. (2012). AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS ONE 7, e29346. doi: 10.1371/journal.pone.0029346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale B.C., Tilton S.C., Corvi M.M., Wilson G.R., Janszen D.B., Anderson K.A., Waters K.M., Tanguay R.L. (2013). Structurally distinct polycyclic aromatic hydrocarbons induce differential transcriptional responses in developing zebrafish. Toxicol. Appl. Pharmacol. 272, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard D. E., Noyes P. D., Waters K. M., Tanguay R. L. (2016). Phenotypically anchored transcriptome profiling of developmental exposure to the antimicrobial agent, triclosan, reveals hepatotoxicity in embryonic zebrafish. Toxicol. Appl. Pharmacol. 308, 32–45. doi: 10.1016/j.taap.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A., Hinoshita F., Sherr D. H. (1992). Mechanisms by which benzo[a]pyrene, an environmental carcinogen, suppresses B cell lymphopoiesis. Toxicol. Appl. Pharmacol. 117, 155–164. doi: 10.1016/0041-008X(92)90232-H. [DOI] [PubMed] [Google Scholar]
- Hicken C.E., Linbo, T.L., Baldwin, D.H., Willis, M.L., Myers, M.S., Holland, L., Larsen, M., Stekoll, M.S., Rice, S.D., Collier, T.K., Scholz, N.L., Incardona J.P. (2011). Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc. Natl. Acad. Sci. 108, 7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-J., Hsiao P., Kuo M.-W., Chung B. (2002). Expression of zebrafish cyp11a1 as a maternal transcript and in yolk syncytial layer. Gene Expr. Patterns 2, 219–222. doi: 10.1016/S1567-133X(02)00059-5. [DOI] [PubMed] [Google Scholar]
- Huang L., Wang, C., Zhang, Y., Li, J., Zhong, Y., Zhou, Y., Chen, Y., Zuo Z. (2012). Benzo[a]pyrene exposure influences the cardiac development and the expression of cardiovascular relative genes in zebrafish (Danio rerio) embryos. Chemosphere 87, 369–375. doi: 10.1016/j.chemosphere.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Incardona J. P., Collier T. K., Scholz N. L. (2004). Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 196, 191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona J. P., Day H. L., Collier T. K., Scholz N. L. (2006). Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 217, 308–321. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Gardner L.D., Linbo T.L., Brown T.L., Esbaugh A.J., Mager E.M., Stieglitz J.D., French B.L., Labenia J.S., Laetz C.A.. et al. (2014). Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc. Natl. Acad. Sci. 111, E1510–E1518. doi: 10.1073/pnas.1320950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona J. P., Linbo T. L., Scholz N. L. (2011). Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol. Appl. Pharmacol. 257, 242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Swarts T.L., Edmunds R.C., Linbo T.L., Aquilina-Beck A., Sloan C.A., Gardner L.D., Block B.A., Scholz N.L. (2013). Exxon valdez to deepwater horizon: Comparable toxicity of both crude oils to fish early life stages. Aquat. Toxicol. 142–143, 303–316. doi: 10.1016/j.aquatox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Irigaray P., Newby J. A., Lacomme S., Belpomme D. (2007). Overweight/obesity and cancer genesis: More than a biological link. Biomed. Pharmacother. 61, 665–678. doi: 10.1016/j.biopha.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Iwanari M., Nakajima, M., Kizu, R., Hayakawa, K., Yokoi T. (2002). Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: Chemical-, cytochrome P450 isoform-, and cell-specific differences. Arch. Toxicol. 76, 287–298. doi: 10.1007/s00204-002-0340-z. [DOI] [PubMed] [Google Scholar]
- Jariyasopit N., McIntosh M., Zimmermann K., Arey J., Atkinson R., Cheong P.H.-Y., Carter R.G., Yu T.-W., Dashwood R.H., Massey Simonich S.L. (2013). Novel nitro-PAH formation from heterogeneous reactions of PAHs with NO2, NO3/N2O5, and OH radicals: Prediction, laboratory studies, and mutagenicity. Environ. Sci. Technol. 48, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariyasopit N., Zimmermann, K., Schrlau, J., Arey, J., Atkinson, R., Yu, T.-W., Dashwood, R.H., Tao, S., Simonich S.L.M. (2014). Heterogeneous reactions of particulate matter-bound PAHs and NPAHs with NO3/N2O5, OH radicals, and O3 under simulated long-range atmospheric transport conditions: Reactivity and mutagenicity. Environ. Sci. Technol. 48, 10155–10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. S., Panter G. H., Hutchinson T. H., Chipman J. K. (2010). Oxidative and conjugative xenobiotic metabolism in zebrafish larvae in vivo. Zebrafish 7, 23–30. doi: 10.1089/zeb.2009.0630. [DOI] [PubMed] [Google Scholar]
- Jung J.-H., Hicken, C.E., Boyd, D., Anulacion, B.F., Carls, M.G., Shim, W.J., Incardona J.P (2013). Geologically distinct crude oils cause a common cardiotoxicity syndrome in developing zebrafish. Chemosphere 91, 1146–1155. doi: 10.1016/j.chemosphere.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Khalili N. R., Scheff P. A., Holsen T. M. (1995). PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 29, 533–542. doi: 10.1016/1352-2310(94)00275-P. [Google Scholar]
- Kim K.-H., Park, H.-J., Kim, J.H., Kim, S., Williams, D.R., Kim, M.-K., Jung, Y.D., Teraoka, H., Park, H.-C., Choy, H.E.. et al. (2013). Cyp1a reporter zebrafish reveals target tissues for dioxin. Aquat. Toxicol. Amst. Neth. 134–135, 57–65. doi: 10.1016/j.aquatox.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Knecht A.L., Goodale B.C., Truong L., Simonich M.T., Swanson A.J., Matzke M.M., Anderson K.A., Waters K.M., Tanguay R.L. (2013). Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 271, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht A. L., Truong L., Simonich M. T., Tanguay R. L. (2016). Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish. Neurotoxicol. Teratol. 59, 27–34. doi: 10.1016/j.ntt.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang, C., Shen, H., Su, S., Shen, G., Huang, Y., Zhang, Y., Chen, Y., Chen, H., Lin, N.. et al. (2015). Concentrations and origins of nitro-polycyclic aromatic hydrocarbons and oxy-polycyclic aromatic hydrocarbons in ambient air in urban and rural areas in northern China. Environ. Pollut. 197, 156–164. doi: 10.1016/j.envpol.2014.12.019. [DOI] [PubMed] [Google Scholar]
- MacRae C. A., Peterson R. T. (2015). Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 14, 721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- Mandrell D., Truong L., Jephson C., Sarker M.R., Moore A., Lang C., Simonich M.T., Tanguay R.L. (2012). Automated zebrafish chorion removal and single embryo placement optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 17, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J.K., Edmunds R.C., Redig M.G., Mudrock E.M., Davis J.W., Incardona J.P., Stark J.D., Scholz N.L. (2016). Confirmation of stormwater bioretention treatment effectiveness using molecular indicators of cardiovascular toxicity in developing fish. Environ. Sci. Technol. 50, 1561–1569. doi: 10.1021/acs.est.5b04786. [DOI] [PubMed] [Google Scholar]
- Miranda C.L., Chung W.G., Wang-Buhler J.-L., Musafia-Jeknic T., Baird W.M., Buhler D.R. (2006). Comparative in vitro metabolism of benzo[a]pyrene by recombinant zebrafish CYP1A and liver microsomes from β-naphthoflavone-treated rainbow trout. Aquat. Toxicol. 80, 101–108. doi: 10.1016/j.aquatox.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Ng G. H. B., Gong Z. (2013). GFP transgenic medaka (Oryzias latipes) under the inducible cyp1a promoter provide a sensitive and convenient biological indicator for the presence of TCDD and other persistent organic chemicals. Plos One 8, e64334. doi: 10.1371/journal.pone.0064334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes P. D., Haggard D. E., Gonnerman G. D., Tanguay R. L. (2015). Advanced morphological-behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 1451, 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober E. A., Field H. A., Stainier D. Y. (2003). From endoderm formation to liver and pancreas development in zebrafish. Mech. Dev. 120, 5–18. [DOI] [PubMed] [Google Scholar]
- Onduka T., Ojima, D., Kakuno, A., Ito, K., Koyama J., Fujii K. (2012). Nitrated polycyclic aromatic hydrocarbons in the marine environment: Acute toxicities for organisms at three trophic levels. Jpn. J. Environ. Toxicol. 15, 1–10. doi: 10.11403/jset.15.1. [Google Scholar]
- Peddinghaus S., Brinkmann, M., Bluhm, K., Sagner, A., Hinger, G., Braunbeck, T., Eisenträger, A., Tiehm, A., Hollert, H., Keiter S.H. (2012). Quantitative assessment of the embryotoxic potential of NSO-heterocyclic compounds using zebrafish (Danio rerio). Reprod. Toxicol. 33, 224–232. doi: 10.1016/j.reprotox.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Perera F.P., Rauh V., Whyatt R.M., Tang D., Tsai W.Y., Bernert J.T., Tu Y.H., Andrews H., Barr D.B., Camann D.E.. et al. (2005). A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. NeuroToxicology 26, 573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Perera F.P., Whyatt, R.M., Jedrychowski, W., Rauh, V., Manchester, D., Santella, R.M., Ottman R. (1998). Recent developments in molecular epidemiology: a study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am. J. Epidemiol. 147, 309–314. [DOI] [PubMed] [Google Scholar]
- Perkins A., Phillips, J.L., Kerkvliet, N.I., Tanguay, R.L., Perdew, G.H., Kolluri, S.K., Bisson W.H. (2014). A structural switch between agonist and antagonist bound conformations for a ligand-optimized model of the human aryl hydrocarbon receptor ligand binding domain. Biology 3, 645–669. doi: 10.3390/biology3040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT–PCR. Nucl. Acids Res. 29, e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch A.L., Teraoka H., Carney S.A., Dong W., Hiraga T., Stegeman J.J., Heideman W., Peterson R.E. (2003). Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 76, 138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reimers M.J., La Du J.K., Periera C.B., Giovanini J., Tanguay R.L. (2006). Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol. Teratol. 28, 497–508. [DOI] [PubMed] [Google Scholar]
- RI DEM. (2008). Rhode Island Air Toxics Guideline.
- Rosenkranz H. S., Mermelstein R. (1983). Mutagenicity and genotoxicity of nitroarenes. Mutat. Res. Genet. Toxicol. 114, 217–267. doi: 10.1016/0165-1110(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Schmidt J. V., Bradfield C. A. (1996). Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 12, 55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schuetzle D., Riley, T.J., Prater, T.J., Harvey, T.M., Hunt D.F. (1982). Analysis of nitrated polycyclic aromatic hydrocarbons in diesel particulates. Anal. Chem. 54, 265–271. doi: 10.1021/ac00239a028. [Google Scholar]
- Schultz T. W., Cajina-Quezada M., Dumont J. N. (1980). Structure-toxicity relationships of selected nitrogenous heterocyclic compounds. Arch. Environ. Contam. Toxicol. 9, 591–598. doi: 10.1007/BF01056938. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Incardona, J.P., Pelkki, K., Shepardson, S., Hodson P.V. (2011). AhR2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat. Toxicol. 101, 165–174. doi: 10.1016/j.aquatox.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Senft A.P., Dalton, T.P., Nebert, D.W., Genter, M.B., Puga, A., Hutchinson, R.J., Kerzee, J.K., Uno, S., Shertzer H.G. (2002). Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic. Biol. Med. 33, 1268–1278. doi: 10.1016/S0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Shailaja M. S., Rajamanickam R., Wahidulla S. (2006). Formation of genotoxic nitro-PAH compounds in fish exposed to ambient nitrite and PAH. Toxicol. Sci. 91, 440–447. doi: 10.1093/toxsci/kfj151. [DOI] [PubMed] [Google Scholar]
- Sousa M. S. A., Latini F. R. M., Monteiro H. P., Cerutti J. M. (2010). Arginase 2 and nitric oxide synthase: Pathways associated with the pathogenesis of thyroid tumors. Free Radic. Biol. Med. 49, 997–1007. doi: 10.1016/j.freeradbiomed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Sydbom A., Blomberg, A., Parnia, S., Stenfors, N., Sandström T., Dahlén S.-E. (2001). Health effects of diesel exhaust emissions. Eur. Respir. J. 17, 733–746. [DOI] [PubMed] [Google Scholar]
- Titaley I.A., Chlebowski A., Truong L., Tanguay R.L., Massey Simonich S.L. (2016). Identification and toxicological evaluation of unsubstituted PAHs and novel PAH derivatives in pavement sealcoat products. Environ. Sci. Technol. Lett. 36, 234–242. doi: 10.1021/acs.estlett.6b00116. [PMC free article] [PubMed] [Google Scholar]
- Toftgård R., Franzén B., Gustafsson J.-., Löfroth G. (1985). Characterization of TCDD-receptor ligands present in extracts of urban particulate matter. Environ. Int. 11, 369–374. doi: 10.1016/0160-4120(85)90031-5. [Google Scholar]
- Truong L., Bugel S.M., Chlebowski A., Usenko C.Y., Simonich M.T., Simonich S.L.M., Tanguay R.L. (2016). Optimizing multi-dimensional high throughput screening using zebrafish. Reprod. Toxicol. 65, 139–147. doi: 10.1016/j.reprotox.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., Harper S. L., Tanguay R. L. (2011). Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. Clifton NJ 691, 271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., Reif D.M., St Mary L., Geier M.C., Truong H.D., Tanguay R.L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA Estimation Programs Interface Suite [TM] for Microsoft Windows. United States Environmental Protection Agency, Washington DC, USA.
- Vicente E. D., Vicente A. M., Bandowe B. A. M., Alves C. A. (2015). Particulate phase emission of parent polycyclic aromatic hydrocarbons (PAHs) and their derivatives (alkyl-PAHs, oxygenated-PAHs, azaarenes and nitrated PAHs) from manually and automatically fired combustion appliances. Air Qual. Atmosphere Health 9, 653. doi: 10.1007/s11869-015-0364-1. [Google Scholar]
- Wei C., Bandowe, B.A.M., Han, Y., Cao, J., Zhan C., Wilcke W. (2015). Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (alkyl-PAHs, oxygenated-PAHs, nitrated-PAHs and azaarenes) in urban road dusts from Xi’an, Central China. Chemosphere 134, 512–520. doi: 10.1016/j.chemosphere.2014.11.052. [DOI] [PubMed] [Google Scholar]
- WHO. (2015). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available at: http://monographs.iarc.fr/index.php. Accessed November 2, 2016. [Google Scholar]
- Yunker M.B., Macdonald, R.W., Vingarzan, R., Mitchell, R.H., Goyette, D., Sylvestre S. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 33, 489–515. doi: 10.1016/S0146-6380(02)00002-5. [Google Scholar]
- Zimmermann K., Jariyasopit N., Massey Simonich S.L., Tao S., Atkinson R., Arey J. (2013). Formation of nitro-PAHs from the heterogeneous reaction of ambient particle-bound PAHs with N2O5/NO3/NO2. Environ. Sci. Technol. 47, 8434–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zok S., Görge G., Kalsch W., Nagel R. (1991). Bioconcentration, metabolism and toxicity of substituted anilines in the zebrafish (Brachydanio rerio). Sci. Total Environ. 109, 411–421. doi: 10.1016/0048-9697(91)90196-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.