Abstract
Fasiglifam (TAK-875), a Free Fatty Acid Receptor 1 (FFAR1) agonist in development for the treatment of type 2 diabetes, was voluntarily terminated in phase 3 due to adverse liver effects. A mechanistic investigation described in this manuscript focused on the inhibition of bile acid (BA) transporters as a driver of the liver findings. TAK-875 was an in vitro inhibitor of multiple influx (NTCP and OATPs) and efflux (BSEP and MRPs) hepatobiliary BA transporters at micromolar concentrations. Repeat dose studies determined that TAK-875 caused a dose-dependent increase in serum total BA in rats and dogs. Additionally, there were dose-dependent increases in both unconjugated and conjugated individual BAs in both species. Rats had an increase in serum markers of liver injury without correlative microscopic signs of tissue damage. Two of 6 dogs that received the highest dose of TAK-875 developed liver injury with clinical pathology changes, and by microscopic analysis had portal granulomatous inflammation with neutrophils around a crystalline deposition. The BA composition of dog bile also significantly changed in a dose-dependent manner following TAK-875 administration. At the highest dose, levels of taurocholic acid were 50% greater than in controls with a corresponding 50% decrease in taurochenodeoxycholic acid. Transporter inhibition by TAK-875 may cause liver injury in dogs through altered bile BA composition characteristics, as evidenced by crystalline deposition, likely composed of test article, in the bile duct. In conclusion, a combination of in vitro and in vivo evidence suggests that BA transporter inhibition could contribute to TAK-875-mediated liver injury in dogs.
Keywords: bile acid inhibition, biliary excretion, drug induced liver injury, biomarkers.
Fasiglifam (TAK-875) is a small molecule agonist of the Free Fatty Acid Receptor 1 (FFAR1), which is also known as G-protein-coupled receptor 40 (GPR40) (Negoro et al., 2010), that was developed for the treatment of type 2 diabetes (Burant et al., 2012; Leifke et al., 2012; Naik et al., 2012). Clinical data indicated that there was a benefit for patients who received TAK-875 based on improved glycemic parameters (Kaku et al., 2013). However, the development activities of TAK-875 were voluntarily terminated in 2013 due to liver safety concerns (Kaku et al., 2015, 2016). This outcome presented an opportunity to investigate potential mechanisms of the toxicity with animal models. The hypothesis tested in the nonclinical studies was that TAK-875 alters bile acid (BA) homeostasis, which may be a contributing factor to drug induced liver injury (DILI).
Bile is comprised of a combination of bile acids (BAs), phospholipids (such as phosphatidylcholine), protein, cholesterol, fatty acid, and conjugated bilirubin (Farina et al., 2009; Nicolaou et al., 2012). BAs are synthesized from cholesterol in the liver through a series of enzymatic steps (Russell, 2003). The primary bile acids in humans are cholic acid (CA) and chenodeoxycholic acid (CDCA) (Zhang et al., 2011). These 2 BAs undergo extensive modification that includes conjugation (with either taurine or glycine), reduction, dehydrogenation, oxidation, and cleavage to create a diverse BA pool (Chiang, 2013). In humans, most BAs in bile are conjugated to glycine (Monte et al., 2009). BAs are exported from the liver into the bile and then secreted into the intestinal tract to aid in nutrient absorption (Chiang, 2009). As a part of enterohepatic circulation, BAs are reabsorbed in the ileum of the small intestine, enter the bloodstream, and return to the liver (Hofmann, 2009). Some BAs are modified by bacterial enzymes in the intestine, which alters their rate of elimination or reabsorption (Ridlon et al., 2006). In humans, this enterohepatic circulation loop runs 4–12 times a day and can recycle 95% of BAs (Chiang, 2013).
Movement into and out of hepatocytes is largely mediated by specific transport proteins because most BAs cannot undergo passive diffusion due to their ionic charges (Dawson et al., 2009). Detailed mechanisms of BA transport are extensively reviewed elsewhere (Alrefai and Gill, 2007; Chiang, 2013; Rodrigues et al., 2014). Briefly, BA uptake into hepatocytes is mediated by Na+ taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptides (OATPs), while export is mediated by the bile salt export pump (BSEP) and multidrug resistance-associated proteins (MRPs) (Halilbasic et al., 2013). Both BSEP and MRP2 export BAs to the bile canaliculus while MRP3 and MRP4 transport to the blood (Morgan et al., 2010; Yang et al., 2013). Unconjugated BAs can act as detergents that can cause liver injury if their concentration gets too high (Monte et al., 2009). One protective mechanim is to conjugate the BAs to glycine or taurine in the liver as a means to promote canalicular transport (Attili et al., 1986). There are other transporters, such as apical sodium-dependent bile salt transporter (ASBT) and organic solute transporter (OST)α/β, that also have a role in hepatic bile acid transport (Yang et al., 2013).
Mutations of the human BSEP gene can lead to progressive hepatic cholestasis (Noe et al., 2005; Stieger et al., 2007) and increase the susceptibility to drug-induced cholestasis (Lang et al., 2007). BSEP knockout mice develop a mild form of cholestasis (Wang et al., 2001) but other transporters are able to compensate and mitigate the severity of the disease (Lam et al., 2005; Wang et al., 2009). Chemicals that inhibit BSEP can cause cholestatic injury (Dawson et al., 2012). From a broader perspective, inhibition of BA transporter activity appears to be a contributing factor to DILI (Aleo et al., 2014; Fattinger et al., 2001; Kostrubsky et al., 2006). One recent model has established a link between the level of BSEP inhibition and the clinical exposure to predict whether the drug could cause DILI (Morgan et al., 2013).
Initial in vitro evidence that TAK-875 could inhibit multiple BA transporters prompted a more thorough mechanistic investigation. The results of this study describe nonclinical in vitro and in vivo findings about the effects of TAK-875 on BA homeostasis in animals. We hypothesized that TAK-875 inhibition of BA transporters may be a contributing factor to drug-induced liver effects in animals. Both TAK-875 and the primary glucuronide metabolite TAK-875-Glu were in vitro inhibitors of multiple BA transporters. Rats and dogs administered TAK-875 daily for 2 weeks had dose-dependent increases in the level of serum BAs. Additionally, the BA composition of dog bile was also significantly altered by TAK-875. This work demonstrates that TAK-875-mediated transporter inhibition alters BA homeostasis in animals, which in turn may be a contributing factor to DILI.
MATERIALS AND METHODS
Materials
TAK-875 and TAK-875-Glu were synthesized internally at Takeda Pharmaceuticals.
Cell Based Assays
Single donor cryopreserved human hepatocytes (lot HH1031) were obtained from In Vitro ADMET Laboratories (IVAL; Columbia, MD). Cells were thawed in UCRM media (IVAL) and plated in HQM media (IVAL) at 25,000 cells/well in collagen coated, clear-bottom 96-well plates and incubated overnight at 37 °C with 5% CO2. Four hours after plating, the media was replaced with HQM that contained 0.1% DMSO or test compounds dissolved in DMSO. Duplicate cell cultures were treated with 0.1–100 μM of TAK-875 or TAK-875-Glu. For cell viability assays, the CellTiter-Glo kit (Promega, Madison, WI, USA) was used to measure intracellular ATP after 6 or 24 h of incubation with the test compound. For glutathione quantification assays, the GSH-Glo kit (Promega) was used after 6 h of incubation. For both assays, luminescence was quantified using a Victor3 V Plate Reader (Perkin-Elmer, Waltham, MA). Data were analyzed in Prism 5 (Graphpad Software, La Jolla, CA), and the least-squares method was used to determine the concentration causing lethality for 50% of cells (LC50).
A Seahorse XFe96 Analyzer (Seahorse Bioscience, North Billerica, MA) was used to measure the mitochondrial effects of TAK-875 and TAK-875-Glu on hepatocytes. Cryopreserved primary human hepatocytes from a single donor were thawed and cultured on XF 96-well cell culture microplates (Seahorse Biosciences) in triplicate. The oxidative phosphorylation and glycolysis stress test media (Seahorse Biosciences) were prepared following manufacturer’s instructions using protein-free media. Cells were maintained in a CO2-free incubator for 1 h at 37 °C prior to measuring the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) 3 times over a 28 min span to establish a baseline. Compounds were added and the OCR and ECAR were measured in triplicate samples over a 42 min period. Data was analyzed with the XF Wave (v 2.3) software (Seahorse Bioscience). Results for the final measurement were normalized as a percentage of DMSO control wells.
For the glucose and galactose (Gluc-Gal) assay, HepG2 human hepatocellular carcinoma cells were plated on a 96-well cell culture microplate in DMEM (ThermoFisher Scientific, Cambridge, MA) supplemented with 10% fetal bovine serum (ThermoFisher Scientific) and cultured overnight. The next day, the medium was replaced with medium containing 25 mM glucose or 10 mM galactose. Cells were dosed in triplicate with either vehicle (0.5% DMSO) or a range of TAK-875 or TAK-875-Glu concentrations (in 0.5% DMSO) and incubated for 72 h at 37 °C. Cells were stained with Hoechst 33342 (Life Technologies, Carlsbad, CA) and viability was determined with an automated fluorescent cellular imager, ArrayScan VTI (ThermoFisher Scientific).
Transporter Assays
For transporters assay, membrane vesicles prepared from baculovirus-infected Sf9 insect cells that over expressed human transporters were purchased from GenoMembrane, Inc. (Yokohama, Japan). These included vesicles for BSEP (GM0005), MRP2 (GM0001), MRP3 (GM0021), and MRP4 (GM0012). HEK293 cells transfected with vectors containing OATP1B1 or OATP1B3 were obtained from ADME & Tox. Research Institute (Sekisui Medical Co., Tokyo, Japan). Radiolabeled substrates for the BSEP and NTCP assay ([3H]-taurocholate) and for the MRP2, MRP3, MRP4, OATP1B1, and OATP1B3 assays ([3H]Estradiol-17β-d-glucuronide) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, Massachusetts). For the NTCP assay, the radiolabeled substrate ([3H]-taurocholate) was purchased from American Radiolabeled Chemicals (St. Louis, MO).
The BSEP and MRP2/3/4 assays were performed as described elsewhere (van Staden et al., 2012). Reaction incubation times were the following: BSEP, 1 min; MRP2, 2 min; MRP3, 15 min; MRP4, 15 min. Radioactivity was measured with a Tri-Carb 3100TR liquid scintillation counter (PerkinElmer) for the BSEP and MRP2 assays, and with a MicroBeta2 2450 (PerkinElmer) for MRP3 and MRP4 assays. The OATP1B1 and OATP1B3 assays were performed as described elsewhere (Oshida et al., 2016). The reactions were terminated after 2 min, cells were washed in PBS, lysed with 0.1 M sodium hydroxide, and the radioactivity measured with a Hionic-Fluor scintillator (PerkinElmer). The NTCP assay was performed essentially as described (Swift et al., 2010). Cryopreserved primary human hepatocytes were obtained from IVAL and were plated at 200,000 cells/well on a 48-well plate following manufacturer’s instructions. Three hours after plating the cells were washed twice with Hanks' Balanced Salts solution (HBSS) media (ThermoFisher Scientific) and incubated in HBSS media. Sodium-free buffer was used as a negative control. Uptake was initiated by adding HBSS containing 100 nM [3H]taurocholic acid in the absence or presence of multiple concentrations of TAK-875 or TAK-875-Glu. The assays were terminated after a 2 min incubation. The cells washed and lysed with 0.5% Triton X-100, and the radioactivity of cell lysates was assessed with a MicroBeta2 2450 detector.
Rat Studies
Ten-week-old male Sprague Dawley rats were purchased from Charles River Laboratories (Charleston, SC) and acclimated to their surroundings for 5 days. The environmental conditions of the animal facility were: a 12:12 h light:dark cycle; a temperature of 18–29°C; a relative humidity of 50 ± 20%; and ventilation changes of ≥10 per hour. Animals were supplied with an autoclaved pellet diet (Lab Diet® #5002, PMI Nutrition International LLC, St. Louis, MO) and ad libitum with reverse osmosis-filtered water. Twenty rats each received a 10 ml/kg oral gavage of vehicle (0.5% methylcellulose), 200 or 600 mg/kg TAK-875 for 14 consecutive days. Food was removed from the cage to fast animals the night before blood collection and returned at 6 h post-dose to minimize food effects. Whole blood was collected from 5 rats via the lateral tail vein in each dose group at predose, 1, 3, and 6 h on days 1, 7, and 14. Blood was collected from the vena cava from all animals 24 h after the final dose. To obtain serum, blood was collected into tubes containing no anticoagulant, allowed to clot, and centrifuged at 4 °C. Serum samples were analyzed with an AU680 Chemistry System (Beckman Coulter, Danvers, MA) by Idexx Laboratories (Grafton, MA). This system was used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), blood urea nitrogen (BUN), and total bile acids. For plasma, blood was collected into tubes containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 4 °C. Livers were collected at euthanasia fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4–6 μm, mounted on glass slides, stained with hematoxylin and eosin, and analyzed with an Olympus BX51 light microscope (Tokyo, Japan) for histopathology assessment by a board-certified veterinary pathologist. Euthanasia consisted of an intramuscular injection of an anesthetic cocktail (ketamine HCl [75 mg/kg], xylazine [2.5 mg/kg], and acepromazine [2.5 mg/kg]) and exsanguinations were performed in accordance with accepted American Veterinary Medical Association guidelines. One animal from both the vehicle-dosed (day 6) and from the 600 mg/kg TAK-875-dosed group (day 7) underwent unscheduled euthanasia due to moribund conditions that were not considered to be test article-related. All animal experiments for this study were conducted in accordance with Millennium Pharmaceutical’s Institutional Animal Care and Use Committee Guidelines.
Dog Studies
Ten-month-old male Beagle dogs were purchased from Marshal BioResources (North Rose, NY, USA) and acclimated to their surroundings for 13 days. The environmental conditions of the animal facility were: a 12:12 h light:dark cycle; a temperature of 18–29°C; a relative humidity of 50 ± 20%; and ventilation changes of ≥10 per hour. Animals were supplied with PMI Nutrition International Certified Canine Chow (Lab Diet® #5007, PMI Nutrition International LLC) and ad libitum with reverse osmosis-filtered water. The food ration was offered up to 2 h after dosing, and as an exception, animals were fed following the 6-h blood collection time point on Days 1, 7, and 14, and then fasted overnight prior to the 24-h time point. Six dogs each received a 5 ml/kg oral gavage of vehicle (0.5% methylcellulose), 40, 150, or 600 mg/kg TAK-875 for 14 consecutive days. Whole blood was collected from every animal at predose, 1, 3, 6, and 24 h after doses on days 1, 7, and 14 via venipuncture of the jugular vein. Whole blood was processed using the method described for rats. Serum samples were analyzed with an Olympus AU640e Chemistry Immuno Analyzer (Olympus) by Charles River Laboratories (Spencerville, OH). Bile was collected from the gall bladder at scheduled euthanasia. Liver samples were processed as described for rats. Euthanasia consisted of an injection with sodium pentobarbital followed by exsanguination. One dog that received 150 mg/kg TAK-875 underwent unscheduled euthanasia on day 8 for humane reasons due to a dose administration error. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals. All animal experiments for this study were conducted in accordance with Charles River Laboratories’ Institutional Animal Care and Use Committee Guidelines.
Toxicokinetic Analysis
The concentration of TAK-875 in the plasma samples was determined using a qualified liquid chromatography with tandem mass spectrometry (LC/MS/MS) method. A protein precipitation extraction method was used for sample preparation. Briefly, a Sciex API-4000 mass spectrometer (AB Sciex, Concord, Ontario, Canada) equipped with a Agilent 1200 LC System (Agilent Technologies, Santa Clara, CA) and a LEAP autosampler (LEAP Technologies, Carrboro, NC) used a reverse-phase gradient method running on an ACE C18 column (2.0 mm ID 2.0 × 50 mm; particle size 3 μm, [Advanced Chromatography Technologies, Aberdeen, Scotland]) for the analyte separation. The mobile phases used were in (A) 95:5 (v:v) water:acetonitrile and (B) 50:50 (v:v) methanol:acetonitrile, and both were supplemented with formic acid (0.1%, volume-to-volume ratio [v:v]). TAK-875 was ionized under a positive ion spray mode and detected through the multiple-reaction monitoring of a mass transition pair at a mass-to-charge ratio (m/z) of 525.6/465.2. Calibration curves of TAK-875 were established using standards, and the peak area ratios of the analyte against the internal standard (glyburide; Sigma-Aldrich, St. Louis, MO) were used to quantify samples. Toxicokinetic analysis of the individual plasma concentration data was performed using Phoenix WinNonlin, v.6.3 (Pharsight Corp, Mountain View, CA). Kinetic parameters were estimated using a noncompartmental model. The area under the curve during 24 h (AUC0–24) was calculated using the linear trapezoidal rule.
Individual Bile Acid Analysis
The concentration of individual BAs in serum samples was determined by LC/MS/MS. Samples were prepared by extracting a 50 μl aliquot of serum with 200 μl acetonitrile containing 0.1% formic acid. The sources of the standards for the 21 different BAs are included in the Supplementary Information. BAs are either unconjugated, taurine (T)-conjugated, or glycine (G)-conjugated. The following BAs were measured: unconjugated BAs: UDCA, ursodeoxycholic acid; HDCA, hyodeoxycholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; CA, cholic acid; aMCA, alpha-muricholic acid; bMCA, beta-muricholic acid; LCA, lithocholic acid; T-conjugated BAs: TCA, taurodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; THDCA, taurohyodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; TLCA, taurolithocholic acid; TCA, taurocholic acid; TaMCA, tauro-alpha-muricholic acid; TbMCA, tauro-beta-muricholic acid; and G-conjugated BAs: GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; GLCA, glycolithocholic acid.
For LC/MS/MS, an Eksigent MicroLC 200 system equipped with a QTRAP 5500 (AB Sciex) mass spectrometer and a LEAP autosampler running on a ACQUITY UPLC BEH C18 column (1.0 mm ID 2.1 × 50 mm; particle size 1.7 μm [Waters Corporation, Milford, MA]) at a flow rate of 50 µL/min for the analyte separation. The mobile phases used were (A) 25 mM ammonium acetate (pH 4) and (B) 10mM ammonium acetate in 90:10 (v:v) water:acetonitrile. The LC gradient used in this study is as follows (time [minutes], %B): 0, 32%; 1.6, 32%; 1.62, 38%; 6, 45%; 9, 45%; 13, 90%; 15.1, 90%. The gradient condition then returned to 32% B for equilibration. Internal standards using individual BAs were added before the precipitation. Calibration curves of BAs were established using standards for each analyte, and the peak area ratios of the analyte to internal standard were used to quantify samples.
Statistical Analyses
Two different types of statistical analyses were utilized that depended on the type of data analyzed. First, comparisons were made between the means of treatment versus control groups with student t-tests (unpaired and 2-tailed). Second, the relationship between TAK-875 plasma concentrations and serum total BA concentrations in rats and dogs were assessed using Pearson's correlations. Statistical analyses were performed with GraphPad Prism (v6.07; GraphPad Software, La Jolla CA).
RESULTS
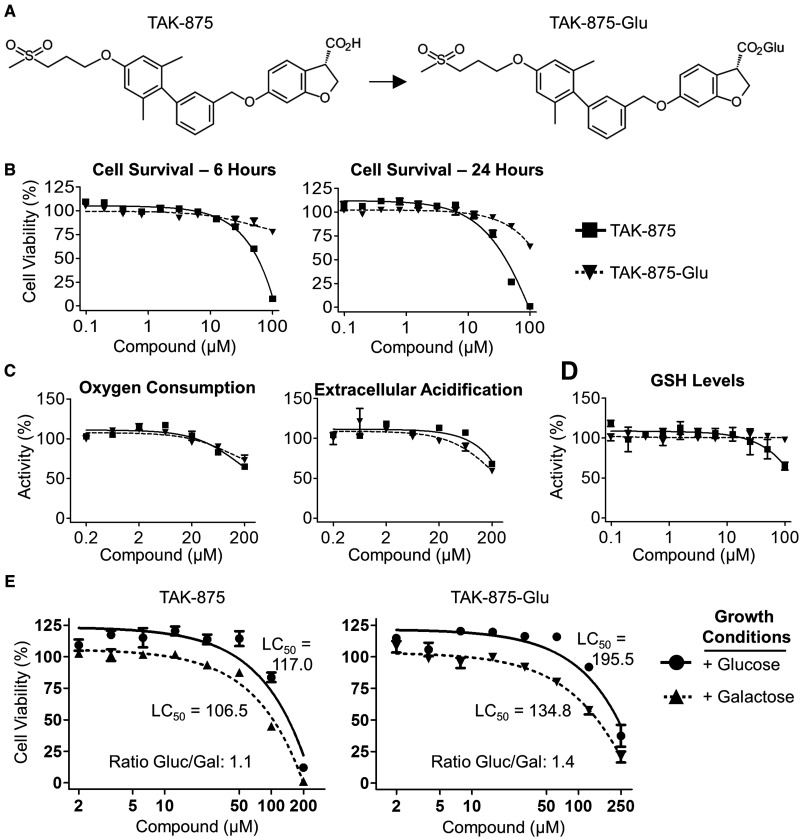
The pharmacokinetic (PK), pharmacodynamic (PD), and safety profiles of TAK-875 were established in rats and dogs before the compound entered the clinic (Negoro et al., 2010). Glucuronidation to TAK-875-Glu (Figure 1a) is the primary elimination pathway of TAK-875 in rats, dogs and humans (manuscript in preparation). Therefore, both TAK-875 and TAK-875-Glu were characterized in cell-based assays to identify potential toxicities. Primary human hepatocytes were treated with a range of compound concentrations (0.1–100 µM) that bracketed the 4.4 µM maximal plasma concentration (Cmax) of a single 50-mg dose in humans (Mayer et al., 2014). Cells tolerated ≤25 µM of either compound at 6 and 24 h of treatment (Figure 1b). The mitochondrial effects of the compounds were assessed with a Seahorse XFe96 Analyzer. There were no effects on oxygen consumption or extracellular acidification at compound concentrations ≤20 µM (Figure 1c). Additionally, there was no evidence of glutathione depletion (Figure 1d) or production of reactive oxygen species (Supplementary Figure 1) that was indicative of reactive oxygen stress. Human HepG2 cells were then used to determine whether there were effects on glycolysis or mitochondrial oxidative phosphorylation by growing cells in media that contained either glucose (Gluc) or galactose (Gal) as an energy source (Marroquin et al., 2007). The half maximal lethal concentration (LC50) was determined for cells treated with either TAK-875 or TAK-875-Glu (Figure 1e). The Gluc/Gal ratio was essentially the same between culture conditions, which was below the 2.0 threshold considered for a positive result (Swiss et al., 2013).
FIG. 1.
TAK-875 and the primary metabolite TAK-875-Glu were evaluated in cell-based assays. A, The structures of TAK-875 and its primary metabolite TAK-875-Glu. B–D, Primary human hepatocytes were treated with the indicated concentrations of TAK-875 (box with solid line) or TAK-875-Glu (triangle with dashed line). Values are normalized to appropriate DMSO controls. B, Cells were treated with compounds for either 6 (left) or 24 (right) hours and survival was determined by intracellular ATP quantification. C, A Seahorse XFe96 Analyzer was used to assess mitochondrial effects of the indicated compounds on oxygen consumption (left) and extracellular acidification of the media (right). D, Levels of glutathione (GSH) after a 6 h incubation with compounds. E, Human HepG2 cells were incubated in media that contains glucose (Gluc) or galactose (Gal) as the only energy source. A nonlinear fit of the data was used to determine the LC50. Error bars indicate standard error of the mean.
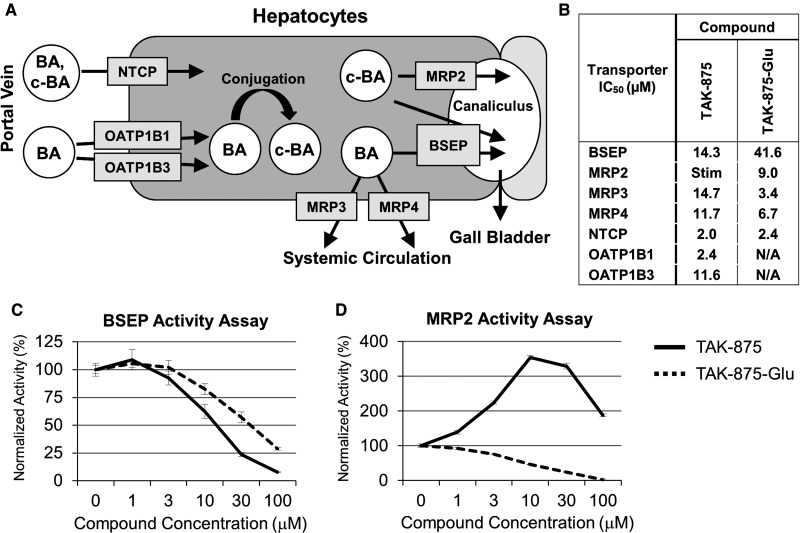
The maintenance of BA homeostasis is a complicated process that involves numerous BA transport proteins (Chiang, 2013; Rodrigues et al., 2014). A schematic diagram of the principal influx (NTCP, OATPs) and efflux (BSEP, MRPs) transporters is shown in Figure 2a. Influx transporters include NTCP, OATP1B1 and OATP1B3, and efflux transporters include BSEP, MRP2, MRP3, and MRP4. The ability of TAK-875 and TAK-875-Glu to inhibit the human version of these transporters was evaluated in vitro and the corresponding half maximal inhibitory concentration (IC50) values were determined (Figure 2b). Both compounds inhibited human BSEP activity in a membrane vesicle assay (Figure 2c). Interestingly, TAK-875 stimulated then inhibited human MRP2 activity, while TAK-875-Glu was only inhibitory at the concentrations tested (Figure 2d). Taken together, both TAK-875 and TAK-875-Glu were inhibitors of multiple BA transporters at low micromolar concentrations.
FIG. 2.
TAK-875 and TAK-875-Glu are in vitro inhibitors of BA transporters. A, Diagram of bile acid (BA) and conjugated BA (c-BA) influx and efflux into a hepatocyte. Once inside a hepatocyte, a BA can undergo conjugation with glycine or taurine. BAs and c-BAs are transported either to the bile duct canaliculus or back into the bloodstream. Figure modeled after Morgan et al. (2010). B, The IC50’s of TAK-875 and TAK-875-Glu against human BA transporters. Stim = a stimulatory effect was observed; N/A = assay was not performed. C, Human BSEP activity in the presence of TAK-875 (solid lines) or TAK-875-Glu (dashed lines). D, Human MRP2 activity. Error bars indicate standard error of the mean.
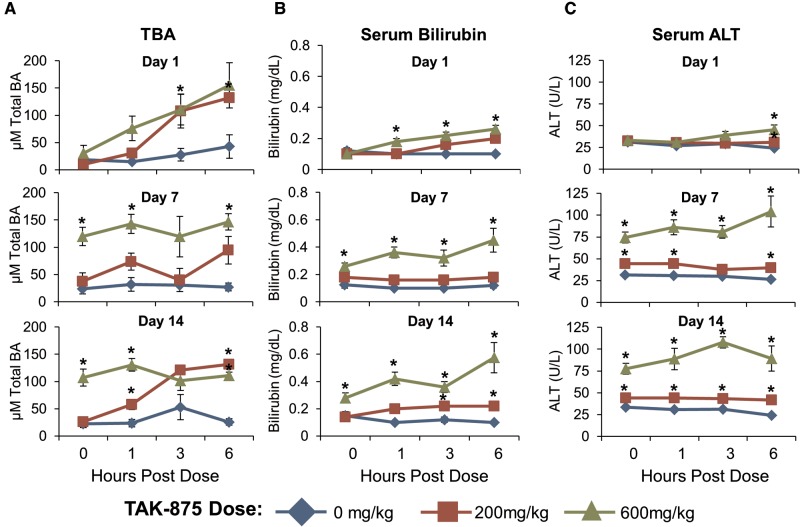
Animal studies were conducted to determine whether there was an actual in vivo relationship between the transporter assay data and effects on BAs in vivo. Rats received daily oral administration of 0, 200, or 600 mg/kg TAK-875 for 14 days. Fasted blood was collected on days 1, 7, and 14 for biomarker and TK analysis. The time of maximal plasma concentration (Tmax) for TAK-875 occurred at 6 h postdose for all groups and days (Supplementary Table 1). There were significant effects on total BA (TBA) levels within 3 h of TAK-875 dosing (Figure 3a). For the 600 mg/kg group, levels of TBA remained elevated throughout the 14 day study, even in samples collected 24 h after the previous dose. The serum levels of 2 biomarkers of liver injury, bilirubin (Figure 3b) and alanine aminotransferase (ALT) (Figure 3c), were elevated in the 600 mg/kg-dosed animals after the first week. However, other serum markers of liver damage remained at baseline (Supplementary Table 2) and there was no correlative microscopic evidence of liver injury (data not shown). Additionally, there was no significant change in the amount of TBA in the feces of rats that received TAK-875 for 2 weeks (Supplementary Figure 2), or in the livers of rats that received 2 doses of TAK-875 (Supplementary Figure 3). Taken together, TAK-875 clearly affected the serum BA levels in rats but the ALT elevation was asymptomatic as there was no clear evidence of liver damage. The complete raw dataset for rats is presented in Supplementary Table 3.
FIG. 3.
TAK-875 caused an increase in serum TBA and markers of liver damage in rats. Animals received a daily oral dose of 0, 200, or 600 mg/kg TAK-875 for 14 consecutive days. Serum from fasted rats was collected at predose (0 h) and 1, 3, and 6 h post-dose on days 1, 7, and 14. A, Group mean serum TBA levels. B, Serum bilirubin levels. C, Serum ALT levels. Error bars indicate standard error of the mean. An asterisk indicates a statistically significant (P < .05) difference between treatment and control groups.
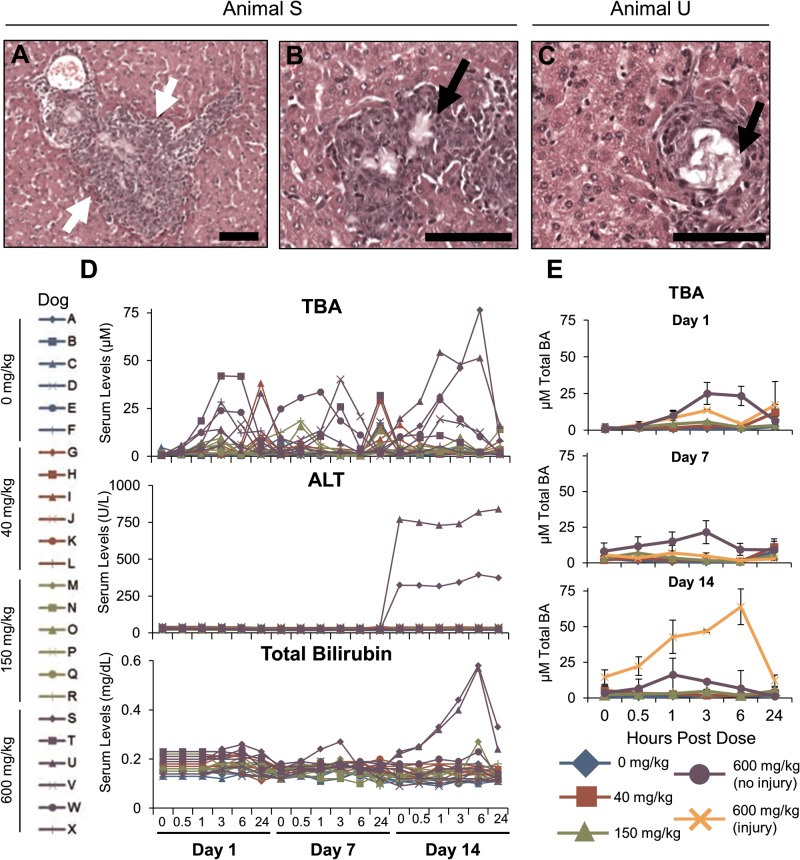
Dogs received daily oral administration of 0, 40, 150, and 600 mg/kg TAK-875 for 14 days. The TAK-875 Tmax was approximately 1–3 h postdose (Supplementary Table 4). Two of the 6 dogs at the 600 mg/kg level developed liver injury between day 7 and 14. The damage was characterized by granulomatous inflammation with neutrophils around an intralesional crystalline deposition (Figure 4a, white arrows). Biliary hyperplasia and fibroplasia were also present. Crystal formation within areas of granulomatous inflammation (black arrows) can be seen in both dogs “S” (Figure 4b) and “U” (Figure 4c). Similar crystal depositions collected from a previous dog study were identified by LC/MS as precipitated TAK-875 and its metabolite TAK-875-Glu (manuscript in preparation). No other dog had TAK-875-related microscopic changes. On day 14, the 2 dogs with liver injury had the highest serum levels of TBA, ALT, and bilirubin measured over the course of the study (Figure 4d). The mean serum TBAs are shown for groups 0, 40, 200, 600 (dogs T, V, W and X), and 600 mg/kg (dogs S and U) to illustrate the magnitude of changes (Figure 4e). Changes in other serum markers of liver damage are shown in (Supplementary Table 5), and the complete data set is presented in Supplementary Table 6.
FIG. 4.
TAK-875 caused liver injury in dogs. Animals received a daily oral dose of 0, 40, 150, or 600 mg/kg TAK-875 for 14 consecutive days. Livers were examined for microscopic damage, and black bars indicate 100 micrometers. A, Histology from dog “S” that received 600 mg/kg TAK-875. White arrows indicate granulomatous inflammation with neutrophils around intralesional crystalline. B, Dog “S” liver. Black arrows indicate areas of granulomatous inflammation. C, Liver from the 600 mg/kg dog “U” showing the injury. D, Serum from 24 fasted dogs (left, labeled “A” through “X”) was collected predose (0 h) and at 0.5, 1, 3, 6, and 24 h post-dose on days 1, 7 and 14. Individual values (right) are shown for bilirubin (top), ALT (middle) and TBA (lower). (E) Group mean serum TBA for the 6 dogs that received 0, 40, and 150 mg/kg TAK-875. The 600 mg/kg group was further divided into those that did not have liver injury (dogs T, V, W, and X) and those that did (dogs S and U). Error bars indicate standard error of the mean.
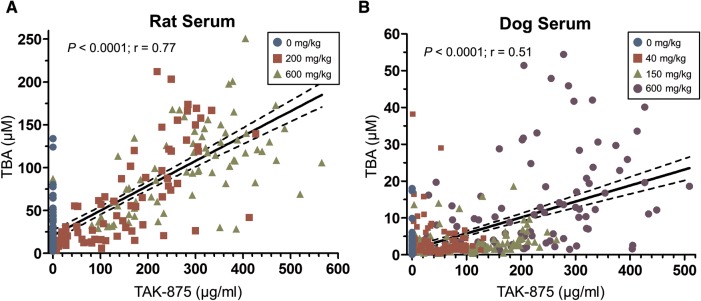
The PK/PD relationship between the plasma concentration of TAK-875 and serum TBA was then assessed in both species. This analysis combined every time-matched plasma and serum sample across all dose groups. Measurements from vehicle-dosed animals were included to account for the natural variation of BA levels. A Pearson correlation coefficient analysis was conducted to determine the statistical significance (P) and the correlation coefficient (r) of the relationship. There was a strong correlation between TAK-875 concentrations and TBA levels for rats (Figure 5a;r = 0.77, P < .0001) and dogs (Figure 5b;r = 0.51, P < .0001). Thus, higher concentrations of TAK-875 are associated with increased levels of TBA in both animal species.
FIG. 5.
Serum total bile acid levels are correlated to TAK-875 plasma concentration. For each sample, the TAK-875 plasma concentration was plotted against the time-matched serum TBA concentration. A linear regression line was generated by minimizing the sum of squares. The dotted line represents the 95% confidence band around the linear regression line. A Pearson's correlation coefficient analysis was conducted to determine the statistical significance (P) and the correlation coefficient (r) of the relationship. A, Rat; 234 plasma and serum samples that were collected across the entire study were analyzed. B, Dog; 426 plasma and serum samples.
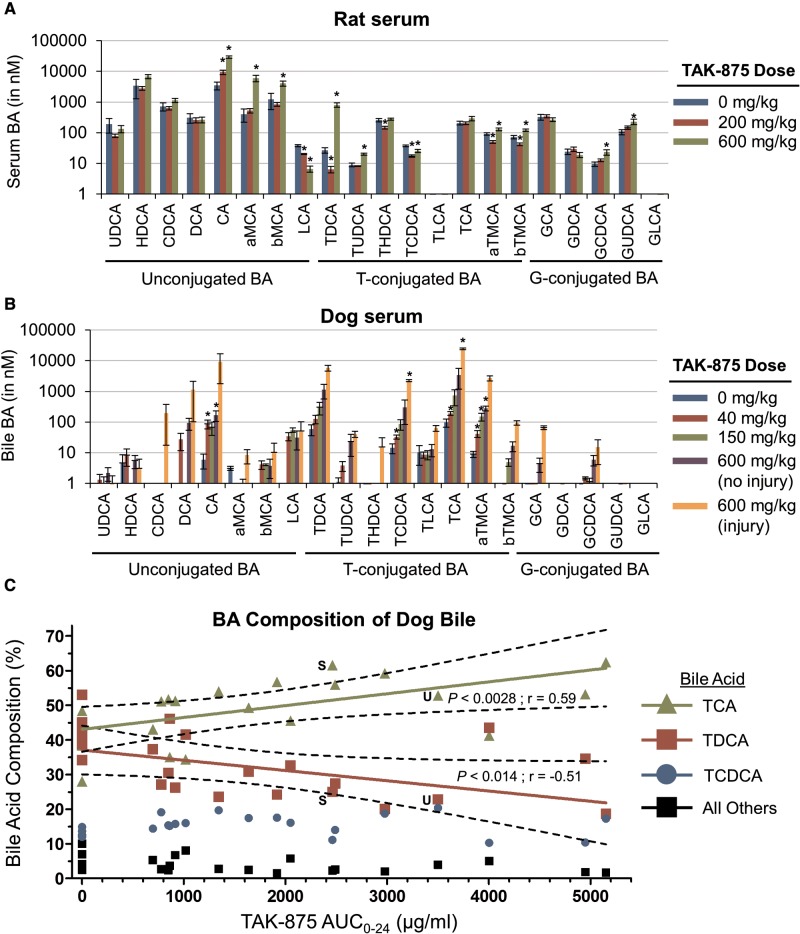
Rat and dog serum samples were then analyzed by LC/MS/MS for 21 different BAs. This extensive data set is included as time-matched samples for both rats (Supplementary Table 3) and dogs (Supplementary Table 6). Analysis of the data revealed that the levels of many BAs changed in relation to the TAK-875 dose (Supplementary Figs. 4–9). At the end of the rat study, there was a significant 8-fold increase of CA in 600 mg/kg TAK-875 group versus control animals (Figure 6a). In dogs, there was a significant 250-fold increase in TCA in the two 600 mg/kg dogs with liver injury versus controls (Figure 6b), and a 7-fold increase versus the 600 mg/kg no-injury group. Bile was also collected from dogs and analyzed for individual BAs. Three BAs (TCA, TDCA and TCDCA) comprise 95% of BA in dog bile (Figure 6c). Interestingly, TAK-875 caused a significant increase in the proportion of TCA (upper line) in the bile that corresponded to a decrease in TDCA (lower line). The percentages of TCA and TDCA for the 2 dogs with liver injury, S and U, are labeled. By a Pearson correlation coefficient analysis, these dose-dependent trends were significant for both TCA (P < .0028) and for TDCA (P < .014). When these bile BA data are alternatively analyzed by dose, the 600 mg/kg group has 2.4-fold more TCA than TDCA (Supplementary Table 7). Taken together, TAK-875 has clear BA effects on serum levels in rats and dogs, and in the bile of dogs.
FIG. 6.
TAK-875 caused changes in the levels of individual BAs from rats and dogs. Serum or bile was collected from animals at the indicated time points and the levels of 21 individual bile acids were determined using LC/MS/MS. A–B, Bile acids are grouped by type of modification (unconjugated, taurine [T]-conjugated, or glycine [G]-conjugated). The doses and groups are displayed to the right of graphs. Asterisks indicate a statistically significant (P < .05) difference in the means of treatment groups versus the control group. A, Rat serum was collected 24 h after the day 14 dose. B, Dog serum collected 6 h after the day 14 dose. The 600 mg/kg dose group was further divided into the 4 dogs without liver damage (no injury) and the 2 dogs with the liver damage (injury). C, Dog bile collected from animals 24 h after the day 14 dose. The proportion of each BA within the total BA pool was normalized for each animal. Plasma TK data was used to determine the day 14 exposure. The percentage of TCA, TDCA, and TCDCA are as shown; the remaining 18 BAs were combined into one value (All Others). A linear regression line was generated by minimizing the sum of squares. The dotted line shows a 95% confidence internal. A Pearson's correlation coefficient analysis was conducted to determine the statistical significance (P) and the correlation coefficient (r). Only statistically significant relationships (P < .05) are shown. The TCA and TDCA data points for dogs “S” and “U” are labeled.
DISCUSSION
The definitive cause for the liver effects seen in the TAK-875 clinical program remains unknown. Two of 6 dogs that received 600 mg/kg developed a liver injury that was characterized by crystalline deposition in the biliary duct with biliary hyperplasia and fibroplasia. In comparison, rats had an asymptomatic increase in serum ALT but no correlative microscopic evidence of liver damage. The key common finding between both species was a TAK-875 effect on the level of serum BAs.
Bile acid (BA) transporter inhibition can be identified by elevated serum BAs (Rodrigues et al., 2014). There was a clear relationship between TAK-875 plasma concentrations and serum TBA levels in both the rat (Figure 5a) and dog (Figure 5b). The first dose of 600 mg/kg TAK-875 caused a 5-fold increase of TBA in rats (Figure 3a) and an approximately 10-fold increase in dogs (Figure 4e) within a few hours of administration. TAK-875 and TAK-875-Glu inhibit multiple human BA transporters at similar low micromolar concentrations (Figure 2a). Similarly, TAK-875 was shown to be an inhibitor of multiple rat hepatobilliary transporters (Li et al., 2015). Taken together, the effects on serum TBA appear to be a direct result of BA transporter inhibition.
Repeated administration of 600 mg/kg TAK-875 caused crystalline depositions in the biliary tract of 2 dogs. The elevation of the serum markers of cholestasis, ALP and GGT, are consistent with this type of injury. Crystallization of TAK-875 and its primary metabolite TAK-875-Glu was also observed in 9-month repeat dose studies in dogs with 150 mg/kg TAK-875 (data not shown). Yet in rats, crystal formation was not observed in a 6-month rat study at doses up to 200 mg/kg (data not shown). In bile duct-cannulated studies, a single dose of 200 mg/kg TAK-875 did not impair bile flow in either rats or dogs (data not shown). Key biological and physiological differences between the species may explain the discrepancy. First, the rate of bile flow (normalized against body weights) is approximately 7-fold higher in rats than dogs (Davies and Morris, 1993). Second, while rats and dogs predominantly have taurine-conjugated BAs, their respective BA pools have different compositions (Suzuki et al., 2013; Washizu et al., 1990). And finally, rats naturally have very high levels of BSEP activity (Jemnitz et al., 2010) that may have made them less susceptible to TAK-875-mediated transporter inhibition. These and/or other potentially unknown factors may explain why the rat tolerated high doses of TAK-875 while dogs did not.
The mechanism behind the dramatic change in the bile BA composition of dogs administered TAK-875 is unknown. Both TCA and TDCA were at equal proportions in vehicle-dosed animals, but TAK-875 caused an increase in TCA with a corresponding decrease in TDCA (Figure 6c). Both TCA and TDCA are transported by BSEP, intestinal bacteria convert TCA to TDCA, and they are then reabsorbed in the gut as part of enterohepatic circulation (Kosters and Karpen, 2008; Legido-Quigley et al., 2011). The 2 dogs with liver injury had 2.5-fold more TCA than TDCA, but so did other dogs without injury (Supplementary Table 7), which suggests this is not the case of TCA accumulating at toxic levels. Unfortunately, bile was not analyzed for other parameters such as fatty acids, protein or bilirubin that could have provided insight into how the compound affected the bile. Future studies could also utilize bile duct cannulation to characterize the TAK-875 effects on bile using samples collected around the known plasma Cmax.
A recent model related to the plasma steady state concentration (Css) of a drug and its inhibition of BSEP to liver injury has been reported (Morgan et al., 2013). This approach concluded that compounds with a low BSEP IC50 and a high Css/BSEP IC50 ratio are at a higher risk for DILI (Morgan et al., 2013). The Css was 7.7 μM for a 50 mg dose of TAK-875 (Leifke et al., 2012), and the in vitro BSEP IC50 was 14.3 μM (Figure 2b). This Css/BSEP IC50 ratio of 0.53 puts TAK-875 in the high risk zone using the Morgan et al. classification system. TAK-875 also stimulated MRP2 activity (Figure 2d). This is interesting given that multiple compounds that caused DILI were similarly found to be both BSEP inhibitors and MRP2 stimulators (Morgan et al., 2013). Screening of compounds for the inhibition/stimulation effects on BA transporters may be a useful first step in de-risking future programs.
Translating animal findings from nonclinical studies to human outcomes is extraordinarily difficult. One drawback of the TAK-875 hepatobilliary transporter hypothesis was much of the significant serum BA changes required doses of 600 mg/kg. For example, dogs had a TAK-875 plasma AUC0–24 of 3800 μg*h/ml after 14 days of dosing at this concentration (Supplementary Table 4). Humans were administered TAK-875 (fasiglifam) at 25 and 50 mg in phase 3 clinical trials that were ultimately discontinued due to concerns about liver safety (Kaku et al., 2015, 2016). By comparison of exposures, the 600 mg/kg dogs had an exposure approximately 40- to 100-fold greater than humans who received repeat doses of 25 or 50 mg TAK-875 (AUC0–24 of 40.3 and 100.3 μg*h/ml, respectively) (Leifke et al., 2012). Unfortunately, there is no published information about BA levels in humans who received TAK-875. In conclusion, while TAK-875 BA transporter inhibition may have contributed to the liver injury of dogs, it is unclear whether these effects are relevant to humans at the clinical therapeutic doses.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rachel Peters, Shylah Wyllie, Danielle Markham, Adam Brown, Russ Naven, and Johnny Yang for experimental help and analysis; John Marcinak, Neila Smith, Mitchell Friedman, Christopher Northey, Prabhakar Viswanathan, Gail Cruz, Cindy Xia, Sean Ottinger, and Juliana Oliveira for protocol design and helpful discussions; Cyprotex (Watertown, MA) for high content screening work; and IVAL for hepatocyte assay development.
FUNDING
All authors were compensated employees of Takeda Pharmaceutical Company Limited when this research was conducted.
REFERENCES
- Aleo M. D., Luo Y., Swiss R., Bonin P. D., Potter D. M., Will Y. (2014). Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology 60, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Alrefai W. A., Gill R. K. (2007). Bile acid transporters: Structure, function, regulation and pathophysiological implications. Pharm. Res. 24, 1803–1823. [DOI] [PubMed] [Google Scholar]
- Attili A. F., Angelico M., Cantafora A., Alvaro D., Capocaccia L. (1986). Bile acid-induced liver toxicity: Relation to the hydrophobic-hydrophilic balance of bile acids. Med. Hypotheses 19, 57–69. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Viswanathan P., Marcinak J., Cao C., Vakilynejad M., Xie B., Leifke E. (2012). TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411. [DOI] [PubMed] [Google Scholar]
- Chiang J. Y. (2009). Bile acids: Regulation of synthesis. J. Lipid Res. 50, 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J. Y. (2013). Bile acid metabolism and signaling. Compr. Physiol. 3, 1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Morris T. (1993). Physiological parameters in laboratory animals and humans. Pharm. Res. 10, 1093–1095. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Lan T., Rao A. (2009). Bile acid transporters. J. Lipid Res. 50, 2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S., Stahl S., Paul N., Barber J., Kenna J. G. (2012). In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab. Dispos. 40, 130–138. [DOI] [PubMed] [Google Scholar]
- Farina A., Dumonceau J. M., Lescuyer P. (2009). Proteomic analysis of human bile and potential applications for cancer diagnosis. Expert Rev. Proteomics 6, 285–301. [DOI] [PubMed] [Google Scholar]
- Fattinger K., Funk C., Pantze M., Weber C., Reichen J., Stieger B., Meier P. J. (2001). The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: A potential mechanism for hepatic adverse reactions. Clin. Pharmacol. Ther. 69, 223–231. [DOI] [PubMed] [Google Scholar]
- Halilbasic E., Claudel T., Trauner M. (2013). Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 58, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F. (2009). The enterohepatic circulation of bile acids in mammals: Form and functions. Front. Biosci. (Landmark Ed.) 14, 2584–2598. [DOI] [PubMed] [Google Scholar]
- Jemnitz K., Veres Z., Vereczkey L. (2010). Contribution of high basolateral bile salt efflux to the lack of hepatotoxicity in rat in response to drugs inducing cholestasis in human. Toxicol. Sci. 115, 80–88. [DOI] [PubMed] [Google Scholar]
- Kaku K., Araki T., Yoshinaka R. (2013). Randomized, double-blind, dose-ranging study of TAK-875, a novel GPR40 agonist, in Japanese patients with inadequately controlled type 2 diabetes. Diabetes Care 36, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku K., Enya K., Nakaya R., Ohira T., Matsuno R. (2015). Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: A randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes. Metab. 17, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku K., Enya K., Nakaya R., Ohira T., Matsuno R. (2016). Long-term safety and efficacy of fasiglifam (TAK-875), a GPR40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: A 52-week open-label phase III study. Diabetes Obes. Metab. 18, 925–929. [DOI] [PubMed] [Google Scholar]
- Kosters A., Karpen S. J. (2008). Bile acid transporters in health and disease. Xenobiotica 38, 1043–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrubsky S. E., Strom S. C., Kalgutkar A. S., Kulkarni S., Atherton J., Mireles R., Feng B., Kubik R., Hanson J., Urda E., et al. (2006). Inhibition of hepatobiliary transport as a predictive method for clinical hepatotoxicity of nefazodone. Toxicol. Sci. 90, 451–459. [DOI] [PubMed] [Google Scholar]
- Lam P., Wang R., Ling V. (2005). Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44, 12598–12605. [DOI] [PubMed] [Google Scholar]
- Lang C., Meier Y., Stieger B., Beuers U., Lang T., Kerb R., Kullak-Ublick G. A., Meier P. J., Pauli-Magnus C. (2007). Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genomics 17, 47–60. [DOI] [PubMed] [Google Scholar]
- Legido-Quigley C., McDermott L., Vilca-Melendez H., Murphy G. M., Heaton N., Lindon J. C., Nicholson J. K., Holmes E. (2011). Bile UPLC-MS fingerprinting and bile acid fluxes during human liver transplantation. Electrophoresis 32, 2063–2070. [DOI] [PubMed] [Google Scholar]
- Leifke E., Naik H., Wu J., Viswanathan P., Demanno D., Kipnes M., Vakilynejad M. (2012). A multiple-ascending-dose study to evaluate safety, pharmacokinetics, and pharmacodynamics of a novel GPR40 agonist, TAK-875, in subjects with type 2 diabetes. Clin. Pharmacol. Ther. 92, 29–39. [DOI] [PubMed] [Google Scholar]
- Li X., Zhong K., Guo Z., Zhong D., Chen X. (2015). Fasiglifam (TAK-875) inhibits hepatobiliary transporters: A possible factor contributing to fasiglifam-induced liver injury. Drug Metab. Dispos. 43, 1751–1759. [DOI] [PubMed] [Google Scholar]
- Marroquin L. D., Hynes J., Dykens J. A., Jamieson J. D., Will Y. (2007). Circumventing the Crabtree effect: Replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol. Sci. 97, 539–547. [DOI] [PubMed] [Google Scholar]
- Mayer M., Nudurupati S., Peng X., Marcinak J. (2014). Evaluation of the pharmacokinetics and safety of a single oral dose of fasiglifam in subjects with normal or varying degrees of impaired renal function. Drugs R D 14, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte M. J., Marin J. J., Antelo A., Vazquez-Tato J. (2009). Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 15, 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. E., Trauner M., van Staden C. J., Lee P. H., Ramachadran B., Eschenberg M., Afshari C. A., Qualls C. W., Lightfoot-Dunn R., Hamdeh H. K. (2010). Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 118, 485–500. [DOI] [PubMed] [Google Scholar]
- Morgan R. E., van Staden C. J., Chen Y., Kalyanaraman N., Kalanzi J., Dunn R. T. 2nd, Afshari C. A., Hamadeh H. K. (2013). A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol. Sci. 136, 216–241. [DOI] [PubMed] [Google Scholar]
- Naik H., Vakilynejad M., Wu J., Viswanathan P., Dote N., Higuchi T., Leifke E. (2012). Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of the GPR40 agonist TAK-875: Results from a double-blind, placebo-controlled single oral dose rising study in healthy volunteers. J. Clin. Pharmacol. 52, 1007–1016. [DOI] [PubMed] [Google Scholar]
- Negoro N., Sasaki S., Mikami S., Ito M., Suzuki M., Tsujihata Y., Ito R., Harada A., Takeuchi K., Suzuki N., et al. (2010). Discovery of TAK-875: A potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 1, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou M., Andress E., Zolnericks J. K., Dixon P. H., Williamson C., Linton K. J. (2012). Canalicular ABC transporters and liver disease. J. Pathol. 226, 300–315. [DOI] [PubMed] [Google Scholar]
- Noe J., Kullak-Ublick G. A., Jochum W., Stieger B., Kerb R., Haberl M., Mullhaupt B., Meier P. J., Pauli-Magnus C. (2005). Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J. Hepatol. 43, 536–543. [DOI] [PubMed] [Google Scholar]
- Oshida K., Shimamura M., Seya K., Ando A., Miyamoto Y. (2016). Identification of transporters involved in beraprost sodium transport in vitro. Eur. J. Drug. Metab. Pharmacokinet. doi: 10.1007/s13318-016-0327-4. [DOI] [PubMed] [Google Scholar]
- Ridlon J. M., Kang D. J., Hylemon P. B. (2006). Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259. [DOI] [PubMed] [Google Scholar]
- Rodrigues A. D., Lai Y., Cvijic M. E., Elkin L. L., Zvyaga T., Soars M. G. (2014). Drug-induced perturbations of the bile acid pool, cholestasis, and hepatotoxicity: Mechanistic considerations beyond the direct inhibition of the bile salt export pump. Drug Metab. Dispos. 42, 566–574. [DOI] [PubMed] [Google Scholar]
- Russell D. W. (2003). The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174. [DOI] [PubMed] [Google Scholar]
- Stieger B., Meier Y., Meier P. J. (2007). The bile salt export pump. Pflugers Arch. 453, 611–620. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kaneko R., Nomura M., Naito H., Kitamori K., Nakajima T., Ogawa T., Hattori H., Seno H., Ishii A. (2013). Simple and rapid quantitation of 21 bile acids in rat serum and liver by UPLC-MS-MS: Effect of high fat diet on glycine conjugates of rat bile acids. Nagoya J. Med. Sci. 75, 57–71. [PMC free article] [PubMed] [Google Scholar]
- Swift B., Pfeifer N. D., Brouwer K. L. (2010). Sandwich-cultured hepatocytes: An in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab. Rev. 42, 446–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiss R., Niles A., Cali J. J., Nadanaciva S., Will Y. (2013). Validation of a HTS-amenable assay to detect drug-induced mitochondrial toxicity in the absence and presence of cell death. Toxicol. In Vitro 27, 1789–1797. [DOI] [PubMed] [Google Scholar]
- van Staden C. J., Morgan R. E., Ramachandran B., Chen Y., Lee P. H., Hamadeh H. K. (2012). Membrane vesicle ABC transporter assays for drug safety assessment. Curr. Protoc. Toxicol. Chapter 23, Unit 23.5. [DOI] [PubMed] [Google Scholar]
- Wang R., Chen H. L., Liu L., Sheps J. A., Phillips M. J., Ling V. (2009). Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology 50, 948–956. [DOI] [PubMed] [Google Scholar]
- Wang R., Salem M., Yousef I. M., Tuchweber B., Lam P., Childs S. J., Helgason C. D., Ackerley C., Phillips M. J., Ling V. (2001). Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. U. S. A. 98, 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washizu T., Ikenaga H., Washizu M., Ishida T., Tomoda I., Kaneko J. J. (1990). Bile acid composition of dog and cat gall-bladder bile. Nihon Juigaku Zasshi 52, 423–425. [DOI] [PubMed] [Google Scholar]
- Yang K., Kock K., Sedykh A., Tropsha A., Brouwer K. L. (2013). An updated review on drug-induced cholestasis: Mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J. Pharm. Sci. 102, 3037–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. K., Guo G. L., Klaassen C. D. (2011). Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One 6, e16683.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.