ABSTRACT
Growing evidence shows tumor-infiltrating neutrophils (TINs) involvement in tumorigenesis. The objective of this study is to assess the prognostic effect of TINs and its impact on adjuvant chemotherapy benefits in muscle invasive bladder cancer (MIBC). A total of 142 MIBC patients from Zhongshan Hospital, 119 MIBC patients from FUSCC, and 405 MIBC patients from TCGA cohort were enrolled in the study. TINs were evaluated by immunohistochemical staining of CD66b or the CIBERSORT method. Patients with high TINs had a significantly poorer overall survival (p = 0.001, p < 0.001, and p = 0.002, respectively) in the three sets. In the multivariate analysis, the presence of high TINs (HR = 2.122, p = 0.007; HR = 3.807, p < 0.001; HR = 2.104, p = 0.001; respectively) was identified as an independent prognostic factor for overall survival in the three sets. More importantly, Low TINs patients had significantly longer overall survival in patients without ACT in the three sets. Gene set enrichment analysis showed that lymphocyte activation (p < 0.001) and T cell activation (p = 0.008) were significantly enriched in the low TINs group. In addition, TINs were negatively correlated with CD8+ T cells, suggesting that the status of high-TINs was linked to the status of immunosuppression in MIBC. TINs could be used as independent prognostic factor. Low TINs identified a subgroup of MIBC patients who appeared to benefit from adjuvant chemotherapy. Incorporation of TINs into TNM system could further stratify patients with different prognosis.
KEYWORDS: Adjuvant chemotherapy, bladder cancer, overall survival, prognosis, tumor-infiltrating neutrophil
Introduction
One key feature of the tumor microenvironment is persistent inflammation and has been described as “wound that does not heal”.1 As the fifth most common cancer worldwide, urothelial carcinoma of the bladder (UCB) is one of the few tumors for which there is long-standing evidence of the efficacy of immunotherapy. Evidence-based guideline recommends radical cystectomy (RC) as the primary treatment of muscle invasive and high risk non-muscle invasive bladder cancer (MIBC) patients.2 Cisplatin-based combination chemotherapy is considered conventional first-line regimens for advanced UCB.3,4 However, meta-analyses of adjuvant treatment trials have shown a low effective rate of 22–25% reduction in risk of death with the combination of cisplatin-based adjuvant treatment.5,6 Therefore, there is an urgent need for a precise classification of UCB that can be used to better predict patient outcomes and treatment response.
Neutrophils are innate immune cells and essential during tissue damage and wound healing processes. In recent years, growing evidence shows that tumor-infiltrating neutrophils play important roles in cancer development, progression, and resistance to therapy7-10 A meta-analysis of the literature concluded that TINs are typically pro-tumor and are strongly associated with poorer prognosis in the majority of human cancers.11 Under different tumor microenvironment, neutrophils can be polarized into either an anti-tumoral (N1) or a pro-tumoral (N2) phenotype and show different functions.12 Indeed, three phenotypically distinct sub-pools of neutrophils with conflicting functions have been identified in the circulation of tumor-bearing mice and cancer patients, indicating high neutrophil plasticity.13 The main antitumor activity of neutrophils is linked to their cytotoxicity, an example of which was recently reported in melanoma where a sub-pool of tumor-associated neutrophil promotes cancer cell killing via nitric oxide release.14 Conversely, neutrophils have been extensively reported to promote tumor growth by influencing the tumor microenvironment using mechanism such as the promotion of angiogenesis.15,16 or the creation of a “safe” milieu where more immature neutrophils alongside with macrophages suppress antitumor immune responses.17 However, evidence of neutrophil plasticity predicts that many novel neutrophil-mediated functions are yet to be discovered.
In the present study, we evaluated the TINs in MIBC and explored its relation with clinical outcomes, especially in patients receiving adjuvant chemotherapy after surgery. These results may shed light on the importance of TINs in MIBC and provide a possible predictive system to evaluate outcomes for patients received adjuvant chemotherapy.
Results
Baseline clinical characteristics
The clinical data of the discovery set and validation sets are described in Table S1. In the discovery set and validation set (FUSCC), TINs were evaluated by immunohistochemical staining of CD66b. The CD66b positive staining represented TINs were located in the tumor tissues in a diffused manner (Fig. 1). The neutrophils infiltrated tumor tissues ranged from 0 to 151 cells/HPF, and 0 to 195 cells/HPF in the discovery set and validation set (FUSCC), respectively. In the validation set (TCGA), the CIBERSORT method,18 a computational method for inferring leukocyte representation in bulk tumor transcriptomes, was applied to TCGA database to analyze relationships between TINs and clinical outcomes. There were no strong relationships between TINs and clinicopathologic factors including patient age, gender, progression from NMIBC, pathological grade, pathological stage, and adjuvant chemotherapy (all p > 0.05) (Table S1).
Figure 1.
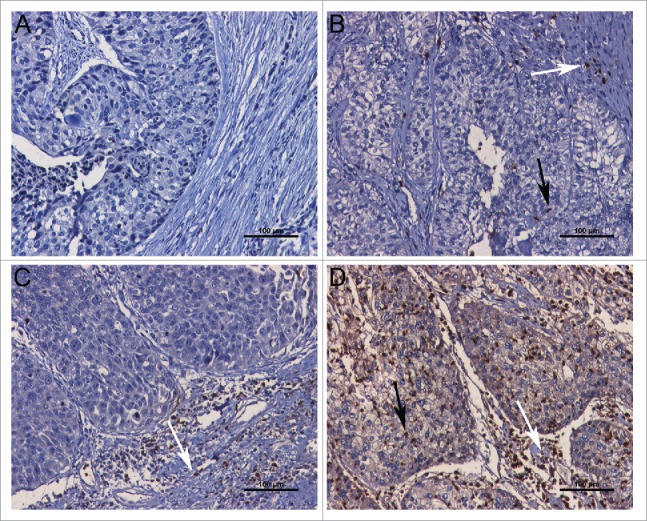
Representative microphotographs of CD66b staining. (A) Representative example of CD66b staining without intratumoral neutrophil and peritumoral neutrophil. (B) Representative example of CD66b+ staining with a small number of intratumoral neutrophil (black thin arrow) and peritumoral neutrophil (white thin arrow). (C) Representative example of CD66b+ staining with a large number of peritumoral neutrophil (white thin arrow), but without intratumoral neutrophil. (D) Representative example of CD66b+ staining with a large number of intratumoral neutrophil (black thin arrow) and peritumoral neutrophil (white thin arrow). Scale bars (black lines) = 100 um (magnification 400×).
Association of TINs with OS
Kaplan–Meier survival analysis was applied to compare overall survival (OS) according to TINs. In the discovery set and validation set (FUSCC), the cut-off was 15 cells/HP. In the validation set (TCGA), the cut-off was 0.0052 (relative leukocyte fraction). Patients with high TINs had a significantly poorer OS (p = 0.001, p < 0.001, and p = 0.002), than those with low level of TINs in the discovery set, validation set (FUSCC), and validation set (TCGA), respectively (Fig. 2). In the univariate Cox regression analysis, pathological stage and TINs were significantly associated with OS in all these sets (all p < 0.05) (Table 1). Those variables with a significant effect on OS were included in the multivariate analysis. The presence of high TINs was identified as an independent prognostic factor that was associated with OS (Discovery set: HR = 2.122, 95% CI 1.224–3.680, p = 0.007; validation set-FUSCC: HR = 3.807, 95% CI 1.876–7.725, p < 0.001; validation set-TCGA: HR = 2.104, 95% CI 1.354–3.268, p = 0.001) (Table 1).
Figure 2.
Kaplan–Meier analysis of OS in MIBC patients from the discovery set (A), validation set (FUSCC) (B), and validation set (TCGA) (C).
Table 1.
Univariate and multivariate Cox regression analyses for overall survival in the discovery and validation sets of MIBC patients.
| Overall survival |
||||||
|---|---|---|---|---|---|---|
| Discovery set |
Validation set (FUSCC) |
Validation set (TCGA) |
||||
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Univariate | ||||||
| Age | ||||||
| ≤ 60 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| >60 | 1.168 (0.734–1.860) | 0.512 | 1.712 (0.935–3.136) | 0.081 | 2.104 (1.253–3.534) | 0.005 |
| Gender | ||||||
| Female | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Male | 0.609 (0.332–1.116) | 0.109 | 0.937 (0.370–2.372) | 0.890 | 0.801 (0.531–1.208) | 0.290 |
| Pathological grade | ||||||
| PUNLMP+low grade | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| High grade | 2.610 (1.250–5.449) | 0.011 | 1.597 (0.495–5.144) | 0.433 | 21.526 (0.197–2350.656) | 0.200 |
| Pathological stage | ||||||
| II | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| III | 1.479 (0.914–2.393) | 0.111 | 3.629 (1.564–8.422) | 0.003 | 2.773 (1.473–5.223) | 0.002 |
| IV | 2.638 (1.113–6.249) | 0.028 | 6.901 (3.035–15.691) | <0.001 | 5.113 (2.798–9.345) | <0.001 |
| Adjuvant chemotherapy | ||||||
| No | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 0.819 (0.516–1.302) | 0.399 | 1.772 (1.123–2.794) | 0.014 | 0.620 (0.396–0.972) | 0.037 |
| TINs | ||||||
| Low | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| High | 2.332 (1.358–4.007) | 0.002 | 4.396 (2.179–8.869) | <0.001 | 1.985 (1.280–3.076) | 0.002 |
| Multivariate | ||||||
| TINs | ||||||
| Low | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| High | 2.122 (1.224–3.680) | 0.007 | 3.807 (1.876–7.725) | <0.001 | 2.104 (1.354–3.268) | 0.001 |
| Pathological grade | ||||||
| PUNLMP+low grade | 1.000 (reference) | |||||
| High grade | 2.347 (1.120–4.919) | 0.024 | ||||
| Pathological stage | ||||||
| II | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| III | 1.257 (0.771–2.047) | 0.359 | 3.023 (1.257–7.269) | 0.013 | 2.869 (1.522–5.409) | 0.001 |
| IV | 2.009 (0.843–4.787) | 0.115 | 5.815 (2.381–14.202) | <0.001 | 5.812 (3.141–10.755) | <0.001 |
| Age | ||||||
| ≤ 60 | 1.000 (reference) | |||||
| >60 | 1.808 (1.071–3.053) | 0.027 | ||||
| Adjuvant chemotherapy | ||||||
| No | 1.000 (reference) | 1.000 (reference) | ||||
| Yes | 1.040 (0.638–1.697) | 0.874 | 0.455 (0.284–0.730) | 0.001 | ||
Abbreviations: PUNLMP, papillary urothelial neoplasm of low malignant potential; CI, confidence interval; HR, hazard ratio.
Extension of the TNM stage prognostic model with TINs
To improve the prognostic accuracy for current prognostic model, we generated a predictive model for MIBC patients by combining tumor, node, metastasis (TNM) staging system and TINs. The C-index was 0.581 when assessed with TNM stage and improved to 0.617 when TINs were added in the discovery set (Table S2). The AIC was 637.80 when assessed with TNM stage and reduced to 636.20 when TINs were added in the discovery set (Table S2). The results were validated in the validation sets (Table S2).
Predictive value of TINs for ACT benefit
Previous researches have reported that increased populations of bone marrow-derived cells could promote tumor recovery and reduce chemotherapy efficacy and immunologic death.19-21 Inhibition of neutrophils recruitment into tumors by CXCR2 inhibitor could improve chemotherapy efficacy in breast cancer.21 Thus, we evaluated the benefit of platinum-based chemotherapy according to the level of TINs in patients who received adjuvant chemotherapy. For patients without ACT treatment (n = 72, 50.7%; n = 39, 32.8%; n = 300, 74%), the level of TINs was significantly associated with OS (p = 0.012, p = 0.007, and p = 0.006) in the discovery set, validation set (FUSCC), and validation set (TCGA), respectively (Fig. 3). Similarly, the association between TINs and OS was borderline significant in patients with ACT (n = 70, 49.3%, p = 0.069; n = 105, 25.9%, p = 0.108) in the discovery set and validation set (TCGA), respectively (Fig. 3). In addition, low TINs patients had significantly longer OS in patients without ACT in all these sets (Discovery set: HR = 2.736, 95%CI, 1.204–6.221, p = 0.016; validation set-FUSCC: HR = 4.937, 95%CI, 1.375–17.727, p = 0.014; validation set-TCGA: HR = 1.987, 95%CI, 1.207–3.271, p = 0.007). Similarly, low TINs had a longer, but insignificantly, OS in patients with ACT in the discovery set and validation set (TCGA) (HR = 1.931, 95%CI, 0.931–4.007, p = 0.077; HR = 2.104, 95%CI, 0.832 to 5.320, p = 0.116; respectively) (Table 2).
Figure 3.
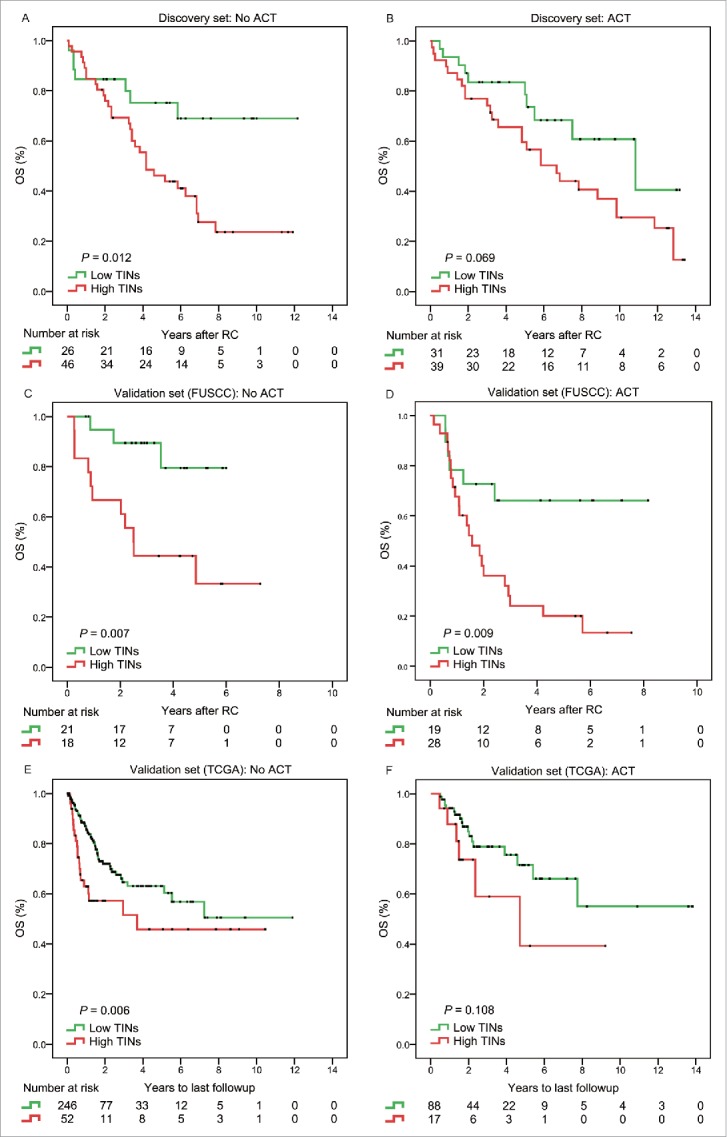
Subgroup analysis to assess predictive value of TINs for ACT benefit. Survival curves according to the level of TINs (high TINs vs. low TINs) in MIBC patients without ACT (A, C, E) and with ACT (B, D, F) from the discovery set and validation sets.
Table 2.
Hazard ratios for overall survival in the discovery and validation sets of MIBC patients with TINs according to tumor stage.
| Overall survival |
||||
|---|---|---|---|---|
| Patients |
TINs (High vs. Low) |
|||
| Variable | No. | % | HR (95% CI) | p |
| Discovery set | 142 | 100 | ||
| ACT | 70 | 49.3 | 1.931 (0.931–4.007) | 0.077 |
| No ACT | 72 | 50.7 | 2.736 (1.204–6.221) | 0.016 |
| Validation set (FUSCC) | 119 | 100 | ||
| ACT | 51 | 42.9 | 3.134 (1.263–7.772) | 0.014 |
| No ACT | 41 | 34.5 | 4.937 (1.375–17.727) | 0.014 |
| Validation set (TCGA) | 409 | 100 | ||
| ACT | 107 | 26.2 | 2.104 (0.832–5.320) | 0.116 |
| No ACT | 302 | 73.8 | 1.987 (1.207–3.271) | 0.007 |
Abbreviations: HR, Hazard Ratio; 95% CI, 95% confidence interval.
Cisplatin-containing combination chemotherapy has been the standard of care since the late 1980s.22 Subgroup analysis was performed to assess predictive value of TINs for gemcitabine/cisplatin (GC) chemotherapy benefit (Fig. S2). For patients with ACT-GC treatment, low TINs patients had significantly longer OS (p = 0.019) in the validation set (FUSCC). Similarly, low TINs had a longer, but insignificantly, OS in the discovery set and validation set (TCGA) (p = 0.112 and p = 0.221, respectively).
We next sought to investigate whether TINs increase or decrease after chemotherapy. CIBERSORT analysis for TINs was performed on matched pre- and post-chemotherapy MIBCs (n = 20) within Philadelphia neoadjuvant chemotherapy (NAC) cohort.23 The Philadelphia NAC cohort (n = 20 transurethral resections and 20 cystectomies) consisted of pre- and post-treatment tumors from patients enrolled in a Phase II clinical trial of neodjuvant dose dense MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin). According to the cut-off of the validation set-TCGA (0.0052/relative leukocyte fraction), there were 10% (n = 2) MIBCs with a decrease from high TINs to low TINs (Fig. S3).
Identification of TIN-associated biologic pathways and processes
Gene set enrichment analysis (GSEA)24 was used to identify relevant biologic processes and signaling pathway. We compared the gene expression profile of MIBC patients with high TINs and low TINs group. Interestingly, several immune-related pathways (p < 0.1 and NES > 1.5) were enriched in the low TINs group (Fig. S1), such as lymphocyte activation, T cell activation, immune effector process, regulation of T cell activation, positive regulation of lymphocyte activation, T cell differentiation, regulation of lymphocyte activation, and positive regulation of T cell activation. Among them, Fisher's exact test showed that lymphocyte activation (p < 0.001) and T cell activation (p = 0.008) were significantly enriched in the low TINs group (Table S3). These results suggested that the presence of low TINs was associated with positive regulation of lymphocyte activation in MIBC patients, which also explained that patients with low TINs had longer OS than those with high TINs.
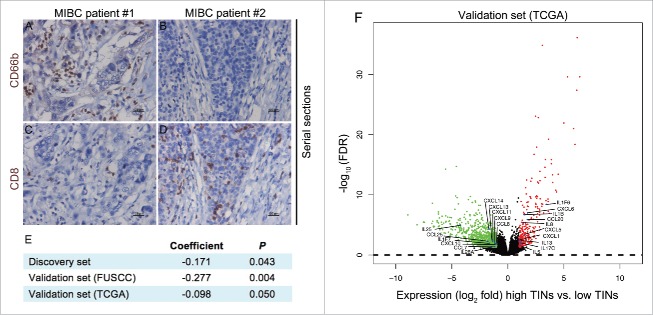
Given that the ability of neutrophils to influence CD8+ T cells has been suggested in cancer,12 we wanted to explore the association between TINs and CD8+ T cells in MIBC (Fig. 4). We found that TINs were negatively correlated with CD8+ T cells (Discovery set: r = −0.171, p = 0.043; validation set-FUSCC: r = −0.277, p = 0.004; validation set-TCGA: r = −0.098, p = 0.050.). In addition, we found that infiltration by CD8+ T cell was associated with improved OS (Fig. S4). These results suggested that high-TINs correlated with immunosuppression in the environment of MIBC (Fig. 4A–E).
Figure 4.
The association between TINs and CD8+ T cells in MIBC. (A–D) serial sections from MIBC samples immunohistochemically stained for CD66 and CD8+, scale bar = 100 mm. (E) Spearman correlation analysis for TINs and CD8+ T cells. (F) Volcano plot comparing the FDR versus fold-change for genes from high-TINs group relative to low-TINs group. Genes labeled in red or green are significantly differentially expressed.
Cytokines and chemokines in MIBC with high vs. low TINs
In addition to associated biologic processes and signaling pathway, we were also interested in cytokines and chemokines that may be enriched in high-TINs tumors. We found that the expression of numerous cytokines and chemokines was significantly increased in high-TINs tumors (Fig. 4F). High-TINs MIBC tumors were enriched for gene sets involved in neutrophil recruitment, including CXCL1, CXCL5, CXCL6, and IL8, as observed previously.7 In addition, MIBC tumors with high TINs exhibited significantly decreased interferon-stimulated chemokines (CXCL9, CXCL10, and CXCL11), which could control the migration of activated T cells and NK cells and enhance the anti-tumoral response.25-27 These results confirmed that high-TINs correlated with immunosuppression in the environment of MIBC.
Discussion
The TNM classification is the most common system for classifying the extent of cancer spread.28 Unfortunately, this traditional staging system narrowly focuses on the tumor cells without incorporating the effects of the host immune response.29 A growing body of research suggests that tumor-infiltrating neutrophils is involved in cancer development, progression, and resistance to therapy.7-10 Recent study showed that the CD66b+ neutrophil to CD8+ lymphocyte ratio could be used to predicted OS in bladder cancer patients after RC.30 However, strategies that improve the selection of patients most likely to benefit from chemotherapy would have more impact in this disease. Our research mainly focused on the impact of CD66b+ TINs on adjuvant chemotherapy benefits in MIBC patients. We found that MIBC patients with high TINs had a significantly shorter OS. Incorporation of TINs into TNM staging system could provide important prognostic information for the risk stratification of MIBC patients. Further, we assessed the relation between TINs and ACT. We found that among patients with ACT or without ACT, those with low neutrophils infiltration were easier to have longer OS compared with those with high TINs, indicating that TINs could be an important factor for predicting the efficiency of chemotherapy. This will be useful for better selection and management of patients who would receive ACT.
The success of anticancer chemotherapy is linked to a durable tumor-targeting immune response.31 The combination of immunotherapy and chemotherapy is a novel approach for tumor treatments.32 Various chemotherapy agents, including cisplatin, at low doses selectively inhibit regulatory and suppressor cells,25 which can be regarded as potential partners for checkpoint inhibitor immunotherapy in clinical development.33 Through GSEA, we found that the presence of low TINs was associated with positive regulation of lymphocyte activation in MIBC patients. In addition, TINs in MIBC were negatively correlated with antitumor immune cells (CD8+ T cell). What's more, MIBC tumors with high TINs exhibited significantly decreased interferon-stimulated chemokines (CXCL9, CXCL10, and CXCL11), which could control the migration of activated T cells and NK cells and enhance the anti-tumoral response.25-27 These results suggested that high-TINs correlated with immunosuppression in the environment of MIBC. As reported before, neutrophils played an immunosuppressive role by suppressing natural killer cell activity, resulting in a reduced antitumor response that allowed metastasis formation.34,35 Zhou et al. demonstrated that neutrophils recruited macrophages to promote hepatocellular carcinoma progression and resistance to sorafenib.36 Therefore, TINs might serve as an important prognostic factor in stratifying MIBC patients and selecting MIBC patients for the combination therapy with chemotherapy and immunotherapy.
The primary limitations of our study are its retrospective design and the relatively small sample size. In addition, the small cores sampled may not be representative of the whole tumor, which could bias the results. A multicenter, prospective study is needed to validate these results in a larger population in the future.
In conclusion, tumor-infiltrating neutrophils could be used as independent prognostic factor. Our results suggested that high-TINs correlated with immunosuppression in the environment of MIBC. Low TINs identified a subgroup of MIBC patients who appeared to benefit from adjuvant chemotherapy. Incorporation of TINs into TNM system could further stratify patients with different prognosis.
Materials and methods
Study population
The study enrolled three independent sets of MIBC patients. The discovery set including 142 MIBC patients underwent RC was obtained from Zhongshan Hospital (Shanghai, China) between 2002 and 2014. The validation set (FUSCC) including 119 MIBC patients underwent RC was obtained from Fudan University Shanghai Cancer Center (Shanghai, China) between 2008 and 2012. Pathologic data included histologic type, tumor grade according to the 2004 World Health Organization classification,37 tumor and nodal stage according to the 2009 TNM classification.38 No patient had received prior chemotherapy or radiotherapy.
Patients whose chemotherapy regimen was recorded received platinum-based treatment: GC, gemcitabine/carboplatin, gemcitabine/oxaliplatin, or MVAC (methotrexate/vinblastine/adriamycin/cisplatin). No patient had distant metastatic disease at the time of cystectomy. OS was defined as time from date of RC to a death from all causes. During the entire study period, the follow-up protocol comprised history, physical examination, urine cytology, and laboratory measurements every 3–4 month in the first year, semi-annually in the second year, and annually thereafter. The follow-up period ended in June 2016. All specimens were obtained from patients with informed consent approved by the Clinical Research Ethics Committee of Zhongshan Hospital (Shanghai, China).
Another validation set (TCGA) comprising 405 MIBC patients was obtained from TCGA database.39 Biospecimens were collected from patients diagnosed with muscle-invasive urothelial carcinoma undergoing surgical resection with either transurethral resection or RC. No patient had received prior chemotherapy or radiotherapy.
Patients whose chemotherapy regimen was recorded received platinum-based treatment: GC, gemcitabine/carboplatin, gemcitabine/oxaliplatin, or MVAC. The CIBERSORT method,18 a computational approach for inferring leukocyte representation in bulk tumor transcriptomes, was applied to TCGA database to analyze associations between clinical outcomes and TINs. The cutoff of TINs for survival analysis was determined by Cutoff Finder.40 The Philadelphia NAC cohort data was downloaded from the GEO (GSE48277).23
Immunohistochemistry
Immunohistochemical staining was performed on tissue microarray (TMA), which was established with formalin-fixed, paraffin embedded surgical specimens. Immunohistochemistry were performed according to the methods previously applied41 with appropriate antibodies after control staining (anti-CD66b antibody, Clone G10F5, BD Biosciences, diluted 1/100; anti-CD8+, IR623, DAKO, ready-to-use). The negative control sections were treated equally with the primary antibody omitted. The number of neutrophils per field was estimated using Image Pro plus 6.0 (Media Cybernetics Inc., Bethesda, MD). Identical settings were used for each photograph. Positive stainings were calculated under high magnification filed (HPF, 400×). The intensity of neutrophils or CD8+ T cell was recorded as the mean number of CD66b or CD8+ positive/HPF from three randomized field, respectively. The immunostaining of CD66b and CD8+ was evaluated by two pathologists blinded to the clinical information. The cutoff of TINs or CD8+ T cell for survival analysis was determined by Cutoff Finder.40 The cutoff from the discovery set was applied to the validation set (FUSCC).
Gene set enrichment analysis (GSEA)
GSEA performed by MSigDB24,42 was used to identify the pathways that were significantly enriched between low TINs and high TINs tumor samples.
1,000 random sample permutations were performed. If a gene set had a positive enrichment score, the majority of its members had higher expression accompanied with higher risk score, and the set was termed “enriched”.
Differential expression analysis
Differential gene expression was analyzed using edgeR43 Significantly up and downregulated genes between high TINs and low TINs tumor samples were defined as expressed genes with FDR-adjusted p-value ≤ 0.05 and fold change of at least 2x.
Statistical analysis
The chi-square test or Fisher exact was used for categorical variables, and the t-test or Wilcoxon rank-sum test for continuous variables. Analysis of the correlation between neutrophils and other immune cells in MIBC was made by Spearman correlation. Kaplan–Meier analysis was used to determine OS. Log-rank test was used to compare survival between subgroups. The Cox proportional hazards regression model was applied to perform univariate and multivariate analyses, and those parameters that demonstrated a statistically significant effect on OS in the univariate analysis were included in the multivariate analysis. Harrell's index of concordance (C-index) and Akaike information criterion (AIC) were calculated to compare the accuracy of the prognostic models. Statistical analyses were performed with SPSS, version 20.0 (IBM, Armonk, NY), Stata SE, version 13.0 (Stata, College Station, TX), and R software packages, version 3.1.2 (The R Foundation for Statistical Computing, http://www.r-project.org/). A two-sided p value of less than 0.05 was considered to be statistically significant for all reports.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by grants from National Natural Science Foundation of China (31270863, 81372755, 81402085, 81471621, 81472227 and 81671628) and Program for New Century Excellent Talents in University (NCET-13-0146). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
References
- 1.Dvorak HF, Flier J, Frank H. Tumors - wounds that do not heal - similarities between tumor stroma generation and wound-healing. N Engl J Med 1986; 315:1650-9; PMID:3537791; http://dx.doi.org/ 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A; European Association of Urology . EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Euro Urol 2014; 65:778-92; PMID:24373477; http://dx,.doi.org/9215826 10.1016/j.eururo.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 3.Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, Loehrer PJ Sr, Trump D. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol 1997; 15:2564-9; PMID:9215826 [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, Witjes JA, Spina M, van Groeningen CJ, Duclos B et al.. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Euro J Cancer 2006; 42:50-4; PMID:16330205; http://dx.doi.org/ 10.1016/j.ejca.2005.08.032 [DOI] [PubMed] [Google Scholar]
- 5.Bono AV, Goebell PJ, Groshen S, Lehmann J, Studer U, Torti FM et al.. Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data. Euro Urol 2005; 48:189-201; PMID:15939530; http://dx.doi.org/ 10.1016/j.eururo.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK et al.. Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. Euro Urol 2014; 66:42-54; PMID:24018020; http://dx.doi.org/27282249 10.1016/j.eururo.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 7.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16:431-46; PMID:27282249; http://dx.doi.org/ 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 8.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S et al.. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150-; PMID:27381735; http://dx.doi.org/ 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012; 33:949-55; PMID:22425643; http://dx.doi.org/ 10.1093/carcin/bgs123 [DOI] [PubMed] [Google Scholar]
- 11.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. Plos One 2014; 9:e98259; PMID:24906014; http://dx.doi.org/19732719 10.1371/journal.pone.0098259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16:183-94; PMID:19732719; http://dx.doi.org/ 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV et al.. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015; 10:562-73; PMID:25620698; http://dx.doi.org/ 10.1016/j.celrep.2014.12.039 [DOI] [PubMed] [Google Scholar]
- 14.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z et al.. MET is required for the recruitment of anti-tumoural neutrophils. Nature 2015; 522:349-53; PMID:25985180; http://dx.doi.org/ 10.1038/nature14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A 2006; 103:12493-8; PMID:16891410; http://dx.doi.org/ 10.1073/pnas.0601807103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A 2008; 105:2640-5; PMID:18268320; http://dx.doi.org/ 10.1073/pnas.0712185105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, DuBois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013; 24:631-44; PMID:24229710; http://dx.doi.org/ 10.1016/j.ccr.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman AM, Liu CL, Green MR, Gentles AJ, Feng WG, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015; 12:453-7; PMID:25822800; http://dx.doi.org/ 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev 2011; 25:2465-79; PMID:9060447; http://dx.doi.org/22039576 10.1101/gad.180331.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA et al.. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1:54-67; PMID:22039576; http://dx.doi.org/ 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE et al.. A CXCL1 Paracrine network links cancer chemoresistance and metastasis. Cell 2012; 150:165-78; PMID:22770218; http://dx.doi.org/ 10.1016/j.cell.2012.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellmunt J, Petrylak DP. New therapeutic challenges in advanced bladder cancer. Semin Oncol 2012; 39:598-607; PMID:23040256; http://dx.doi.org/ 10.1053/j.seminoncol.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 23.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL et al.. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014; 25:152-65; PMID:24525232; http://dx.doi.org/ 10.1016/j.ccr.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al.. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102:15545-50; PMID:16199517; http://dx.doi.org/ 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al.. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/ 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10kD (IP-10): A novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 1997; 8:207-19; PMID:9462486; http://dx.doi.org/ 10.1016/S1359-6101(97)00015-4 [DOI] [PubMed] [Google Scholar]
- 27.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997; 61:246-57; PMID:9060447 [PubMed] [Google Scholar]
- 28.Sobin LH, et al. TNM classification of malignant tumors. UICC International Union Against Cancer. 7th edn. 2009, Oxford. [Google Scholar]
- 29.Bindea G, Mlecnik B, Fridman W-H, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol 2010; 22:215-22; PMID:20207124; http://dx.doi.org/ 10.1016/j.coi.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Furuya K, Nakamura M, Sakamaki K, Osaka K, Ito H, Ito Y, Izumi K, Ohtake S, Miyoshi Y et al.. Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder cancer patients after radical cystectomy. Bmc Cancer 2016; 16:185; PMID:26944862; http://dx.doi.org/23890065 10.1186/s12885-016-2219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:23890065; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 32.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015; 3:436-43; PMID:25941355; http://dx.doi.org/ 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook AM, Lesterhuis WJ, Nowak AK, Lake RA. Chemotherapy and immunotherapy: mapping the road ahead. Curr Opin Immunol 2016; 39:23-9; PMID:26724433; http://dx.doi.org/ 10.1016/j.coi.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 34.Sceneay J, Chow MT, Chen A, Halse HM, Wong CSF, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ et al.. Primary tumor hypoxia recruits CD11b(+)/Ly6C(med)/Ly6G(+) immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 2012; 72:3906-11; PMID:22751463; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3873 [DOI] [PubMed] [Google Scholar]
- 35.Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK et al.. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 2016; 6:630-49; PMID:27072748; http://dx.doi.org/ 10.1158/2159-8290.CD-15-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016; 150:1646-58; PMID:26924089; http://dx.doi.org/ 10.1053/j.gastro.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 37.Sauter G AF, Amin M et al.. Tumours of the urinary system: non-invasive urothelial neoplasias In: Eble JN, Sauter G, Epstein Jl, et al. (eds). WHO classification of classification of tumors of the urinary system and male genital organs. Lyon: IARCC Press, 2004:29-34 [Google Scholar]
- 38.Sobin LH GM, Wittekind C, editors. TNM classification of malignant tumors. ed. 7 Hoboken, NJ: Wiley-Blackwell; 2009:262-5 [Google Scholar]
- 39.Weinstein JN, Akbani R, Broom BM, Wang W, Verhaak RGW, McConkey D et al.. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507:315-22; http://dx.doi.org/ 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budczies J, Klauschen F, Sinn BV, Gyoerffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: A comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. Plos One 2012; 7:e51862; PMID:23251644; http://dx.doi.org/12808457 10.1371/journal.pone.0051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Zhu Y, An H, Liu Y, Lin Z, Wang G, Xu J. Clinical significance of tumor-derived IL-1beta and IL-18 in localized renal cell carcinoma: Associations with recurrence and survival. Urol Oncol 2015; 33:68.e9-16; PMID:25241276; http://dx.doi.org/12808457 10.1016/j.urolonc.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 42.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J et al.. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34:267-73; PMID:12808457; http://dx.doi.org/ 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- 43.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26:139-40; PMID:19910308; http://dx.doi.org/ 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.